Abstract
Background
There is a disproportionately high smoking prevalence among individuals who are prone to depression. While depression has been conceptualized as a disorder of dysregulated positive affect and disrupted reward processing, little research has been conducted to determine the role of smoking in these processes among depression-prone smokers.
Methods
Depression-prone smokers (DP+; n = 34) and smokers not depression-prone (DP-; n=49) underwent two laboratory sessions, once while smoking abstinent and once while smoking ad-libitum, to assess the relative reinforcing value of smoking and reward sensitivity. Using experience sampling methods, participants completed self-report measures of subjective reward, positive affect, and negative affect across three days while smoking as usual and three days while smoking abstinent.
Results
DP+ were two times more likely to work for cigarette puffs versus money in a progressive ratio, choice task (OR 2.05; CI 95% 1.04 to 4.06, p=0.039) compared to DP-. Reward sensitivity as measured by the signal detection task did not yield any significant findings. Mixed models regressions revealed a 3-way interaction (depression group, smoking phase, and time) for subjective reward, negative affect and positive affect. For all three of these outcomes, the slopes for DP- and DP+ differed significantly from each other (p's < 0.05), and the effect of smoking (vs. abstinence) over time was greater for DP+ than DP- smokers (p's <0.05).
Conclusions
These findings indicate that the effects of smoking on reward and positive affect regulation are specific to DP+ smokers and highlight novel targets for smoking cessation treatment in this population.
Keywords: Smoking, depression, positive affect, negative affect, reward regulation, reward sensitivity
Introduction
About 30-60% of smokers seeking to enroll in smoking cessation programs have had at least one lifetime episode of Major Depressive Disorder (MDD) (1-5), compared to 15% of the general population (6). Further, half of smokers who enroll in smoking cessation programs have elevated depression symptoms (2, 4, 7-12). Smokers with a past history of depression and current symptoms of depression (i.e., depression-prone; DP+ smokers) tend to have more difficulty quitting and are at significantly higher risk of relapse than smokers not prone to depression (DP-) (7, 13, 14).
Negative affect has long been considered a critical factor in smoking maintenance among smokers in general, and depression-prone smokers specifically (15, 16). To date, smoking cessation interventions for DP+ smokers have focused on the management of negative affect through behavioral and anti-depressant therapy with little success (5, 17-24). Evidence indicates that targeting negative affect does not significantly improve smoking cessation rates or mitigate negative affect (17, 21, 23, 24), but can exacerbate negative affect and depression symptoms, and decrease the likelihood of quitting smoking (25).
While depression has been conceptualized as a disorder of dysregulated positive affect and disrupted reward processing (26), we know little about the role of smoking in these processes among DP+ smokers (24, 27). Behavioral Economic Theory (BET) provides a framework to integrate features of depression and the effects of smoking. According to BET the choice of one rewarding behavior (e.g., smoking) depends on the presence of alternative reinforcers and the reinforcing value of a drug can be enhanced, or reduced, based on the presence of alternative(s) (28-36). Of relevance, individuals prone to depression have fewer alternative reinforcers (37-39), derive less reward from natural reinforcers in their environment (26, 40-45), and as a result, have diminished positive affect (45-48).
In the context of fewer alternative reinforcers, smoking may be an easily available reinforcer with heightened reinforcing value for DP+ smokers (34, 49). DP+ smokers are two times more likely to rate smoking as their preferred activity (49), have greater smoking-induced dopamine release (50), and to find smoking more reinforcing than DP- smokers (51). Nicotine may also enhance the reinforcement derived from available alternative reinforcers. Pre-clinical models suggest that nicotine potentiates reward from available reinforcers by increasing the sensitivity of brain reward systems or the ability to experience pleasure (27, 52, 53), while nicotine withdrawal decreases reward sensitivity (54). In the context of fewer alternative reinforcers, smoking may be a critical reinforcer, while also increasing pleasure derived from available alternative reinforcers.
Research also indicates that nicotine increases positive affect (55-57), and smoking abstinence decreases positive affect, which predicts smoking relapse (24, 58-60). Nicotine's effect on positive affect may be especially important for DP+ smokers. Smokers with a history of MDD showed heightened positive affect to a positive mood induction when smoking (27). Although largely ignored, smoking may help regulate positive affect across time, rather than simply managing negative affect for DP+ smokers (48, 51, 61).
Supported by converging pre-clinical and clinical evidence, this study sought to provide initial evidence as to whether DP+ smokers compared to DP- smokers: (1) find smoking more reinforcing relative to other reinforcers; and (2) have greater changes in reward sensitivity, subjective reward from self-selected alternative reinforcers, and affect while smoking as usual versus abstinent. A better understanding of smoking's role among DP+ smokers may shed light on novel smoking cessation treatment targets for these smokers.
Methods and Materials
Study Participants
Cigarette smokers (n=83) were recruited from the community through print advertisements. Interested smokers completed a telephone screen assessing smoking history, depression status, medical and psychiatric conditions, and medication/drug use. Eligible smokers were between the ages of 18-65, who smoked at least 10 cigarettes a day for 6 months and could be classified into one of two depression status groups. DP- included smokers with no past history of major depression and no current depression symptoms. DP+ included smokers with both a past history of major depression and current depression symptoms; a group with disproportionate smoking burden and less success at quitting smoking (14, 62). Smokers who had a past history of MDD, but no current depression symptoms were excluded as were smokers with no past history of MDD with current depression.
Past history of major depression was determined by the Inventory to Diagnose Depression-Lifetime (IDD) (51, 63, 64). The IDD is a 22-item scale with response options ranging in symptom severity (0-4) and an additional question regarding the persistence of the symptom for > 2 weeks, resulting in a positive or negative history of depression. Current depressive symptoms were defined as a score > 16 on the 20-item CESD, which correlates with clinical ratings of depression and has high internal consistency (17, 65, 66). Both the IDD and the CESD were completed through a semi-structured telephone interview by masters-level trained psychologists to prevent ineligible participants from attending an intake visit.
Exclusion criteria included: pregnancy, lactation, chronic medical condition, current diagnosis or history of bipolar disorder, schizophrenia or substance abuse (other than nicotine) (27, 51, 60, 67), current or recent use of smoking cessation medications, antidepressant or antipsychotic medications. The exclusion criteria were assessed via self-report on the telephone screen with an objective assessment of smoking status, medication and drug use, and pregnancy at the intake visit. Participants provided written informed consent to a protocol approved by the University of Pennsylvania's Institutional Review Board. Participants then provided a carbon monoxide (CO) breath sample to verify smoking status and a urine sample for a drug screen (Instant Technologies, Inc. Norfolk, VA) and pregnancy. Participants who had a CO < 10 ppm or a positive urine drug screen for illicit drugs or psychotropic medication were excluded. The final sample consisted of 34 DP+ smokers and 49 DP- smokers. Table 1 characterizes the groups.
Table 1.
Descriptive characteristics of DP- (n=49) and DP+ (n=34) smokers at baseline.
| DP - | DP+ | Overall | P-value χ21df;t-test | ||||
|---|---|---|---|---|---|---|---|
| Covariate | Mean | SD | Mean | SD | Mean | SD | |
| Age | 41.02 | 14.17 | 34.47 | 13.86 | 38.34 | 14.33 | 0.04 |
| Smoking Rate (cigs/day) | 20.10 | 21.60 | 18.06 | 6.99 | 19.29 | 17.32 | 0.60 |
| Nicotine Dependence (FTND) | 5.61 | 1.79 | 5.85 | 1.58 | 5.71 | 1.70 | 0.50 |
| MNWS (Baseline) | 8.99 | 5.56 | 21.59 | 7.86 | 13.98 | 9.00 | <0.0001 |
| Positive Affect (PANAS) | 35.10 | 7.29 | 27.85 | 7.51 | 32.13 | 8.16 | <0.001 |
| Negative Affect (PANAS) | 13.57 | 3.75 | 25.11 | 8.75 | 18.30 | 8.47 | <0.001 |
| Complementary Reinforcers | 72.06 | 41.52 | 65.59 | 34.04 | 69.41 | 38.54 | 0.46 |
| Substitute Reinforcers | 31.65 | 23.43 | 20.09 | 19.53 | 22.00 | 31.84 | 0.02 |
| IDD | 5.83 | 7.96 | 40.42 | 7.12 | 19.52 | 18.63 | <0.0001 |
| CESD | 7.14 | 5.33 | 30.61 | 10.66 | 16.43 | 13.94 | <0.0001 |
| % | Count | % | Count | % | Count | ||
| Female | 27% | (13) | 47% | (16) | 35% | (29) | 0.06 |
| Race (White) | 37% | (18) | 44% | (15) | 40% | (14) | 0.50 |
| Attended College | 51% | (25) | 53% | (18) | 52% | (18) | 0.90 |
| Married | 12% | (6) | 18% | (6) | 14% | (5) | 0.50 |
| Employed | 53% | (26) | 68% | (23) | 59% | (20) | 0.18 |
Procedure
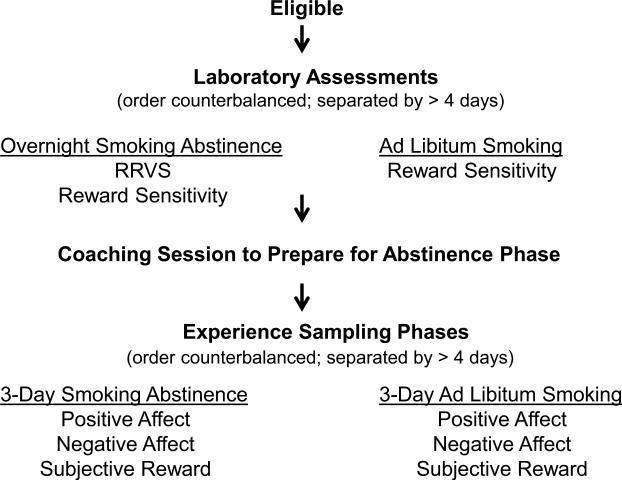
Eligible participants completed a baseline assessment of demographics, smoking history and mood and were scheduled for two morning laboratory assessment sessions. One was scheduled after overnight smoking abstinence (9 hours) and the second was scheduled while smoking as usual. The relative reinforcing value of smoking was assessed after smoking abstinence (to ensure motivation to respond) and reward sensitivity was assessed during both smoking abstinence and smoking ad-libitum. Participants received $30 compensation for each laboratory visit. Using experience sampling methods, participants then completed measures of subjective reward, positive affect, and negative affect via telephone across three days while smoking as usual and three days while smoking abstinent. Detailed procedures are described below and depicted in Figure 1.
Figure 1.
Flow chart of study visits and assessments.
Laboratory Assessments
A validated smoking choice paradigm permitted the evaluation of the relative reinforcing value of smoking (RRVS), which is the preference for smoking over other alternatives (67-70). In this paradigm, participants moved a computer mouse to hit targets on one of two computer screens: one to earn points toward smoking and one for money. Using a concurrent schedule (71, 72), participants could switch from working on one screen to the other as often as they wish. Participants were instructed to move the computer mouse to have the cursor hit the targets (either a $ or a cigarette). Consistent with relative reinforcement paradigms, the reinforcement schedule in the money-earning screen remained constant at a fixed ratio FR-25 (25 targets achieved to earn a point) while the reinforcement schedule for smoking increased (require more effort) with a progressive ratio schedule of PR-25x over 10 trials, such that 25, 50, 75, 100, 125, 150, 175, 200, 225, and 250 targets had to be achieved to earn a point (73, 74). The task was performed for 10 trials for a total of 10 points from which they earned either $0.25 for each point (i.e., up to $2.50 paid at the end of the procedure) or one puff of a cigarette for each point (i.e., up to 10 puffs, smoked at the end of the procedure to prevent satiation from influencing susequent responding), or any combination of both reinforcers. Participants then began a 1-hour wait in the lab to standardize session duration and ensure that responding was based on reinforcer preference.
A signal detection task was used to measure reward sensitivity. The participants’ goal was to determine, via key press, whether a short (11.5mm) or a long (13mm) mouth was presented on a previously mouth-less cartoon face for 100 ms (42, 75, 76). During each of the two 100-trial blocks, reward feedback (“Correct!! You win 5 cents”) was presented after 40 correct trials according to a controlled reinforcement schedule. One stimulus type (either the short or the long mouth) was randomly selected to be disproportionately rewarded (labeled the “rich stimulus”; 30 trials) for correct responses at a 3:1 ratio. The less-frequently rewarded stimulus was labeled the “lean stimulus” (10 trials). Participants were not informed of this reward disparity. Reward feedback for correct responses was given according to a pseudo-randomized schedule that determined which of the 10 lean stimulus and 30 rich stimulus trials of the 100 total trials were rewarded for correct identifications within a block. Reward feedback was displayed for 1500 ms and followed by a blank screen for 250 ms. For the entire task, each participant “earned” around $6 that was paid at the end of the session.
Experience Sampling
After the second laboratory session, participants received instructions for either a 3-day smoking abstinence phase or a 3-day smoking ad-lib phase. Each phase involved Experience Sampling, where participants were called via a study cell phone at 7 randomly determined times during a 14-hour day (e.g., 8am - 10pm) for a 1-2 minute, 12 question interview. Each interview assessed positive affect, negative affect, current activity, and the level of pleasure derived from the activity (subjective reward). The specific interview items are described under Subjective Outcomes, below.
The times of the 7 random assessments were computer generated and distributed evenly throughout the day (i.e., stratified block sampling) for each subject for each day of each phase. To promote compliance, participants received $5 for each phone call completed (a total of 21 calls for each phase) and a telephone number to call with issues (77, 78). The order of the abstinent and ad-lib phases was randomly determined for the depression status groups, and counterbalanced to minimize bias due to order effects. Both phases included one weekend and two week days of assessment, with over four days between phases. Consistent with the literature (78-80), compliance with the telephone calls was >80%. Participants who were unable to comply with study procedures were withdrawn from the study.
In preparation for the abstinence phase, participants received general instructions and a 20-minute coaching session on tips for remaining abstinent (81, 82). Participants refrained from smoking beginning 12:01 am on Day 1 of the 3-day Abstinence phase. During the 3-day abstinence phase, abstinence was verified daily via CO < 5 ppm and a Nic Alert saliva cotinine ≤ 1 (Nymox Pharmaceutical, Hackensack, NJ) (81, 83, 84). The NicAlert salivary cotinine test was performed on days 2 and 3, but not day 1 due to residual elevations from prior smoking. Participants received $100 for remaining smoke-free across the 3-day abstinence phase (81, 82, 84). Two DP+ and two DP- participants were unable to remain smoking abstinent and were withdrawn from the study.
Objective Outcomes
Relative reinforcing value of smoking was defined as the maximum amount of responding for smoking versus money across the 10 trials (73, 74). For the reward sensitivity task, response bias was calculated by a formula derived from a behavioral model of signal detection (85). Change in response bias from block 1 to 2 was used as the index of reward sensitivity (76). Outlying trials were removed following Bodgon and Pizzagalli (75).
Subjective Outcomes
Experience Sampling via brief phone interviews enabled us to capture prospective, random samples of smoking and abstinence related changes in affect and subjective reward from activities across smoking and abstinence days in the participants own environment in “real-time” avoiding retrospective recall (86, 87). Consistent with previous research, participants rated their positive affect (4 items: happy, relaxed, content, enthusiastic) and negative affect (5 items: sad, stressed, irritable, bored, tense) “at that moment” on a scale from 0=not at all to 4=extremely (45, 79, 88-90). Subjective reward from self-selected reinforcers was measured by asking participants to indicate what activity they were currently engaged in and how much they were enjoying that activity on a 5 point scale from 0=not at all to 4=very much (45, 91). These types of ecological momentary approaches produce valid and reliable assessments of affect and reward in substance using populations (45, 88-90, 92, 93).
Covariates
Baseline affect, nicotine dependence, and alternative reinforcers were measured to characterize the sample and to potentially control for their effects in the multivariate models. The Fagerstrom Test for Nicotine Dependence (FTND) is a 6-item measure with good internal consistency (α= .64) and high test-retest reliability (r = .88) (94). The Minnesota Nicotine Withdrawal Scale was used to measure withdrawal symptoms (95). The Positive and Negative Affect Schedule (PANAS) was used to measure positive and negative affect “over the past week” (1=not at all to 5= extremely (96) via two, 10-item scales with excellent psychometric properties (24, 27, 51, 96). Alternative Reinforcers were measured by the adapted Pleasant Events Schedule (PES) (39), which was designed to assess reinforcers that occur in an individual's natural environment. The 78-items were rated once in terms of frequency (0=none to 2=often) and once in terms of enjoyability (0=none to 2=very) over the past 30 days, yielding a frequency score, an enjoyability score, and the cross product is the reinforcement from the activity (39, 97-100).
Data Analysis
All analysis was conducted using Stata 13.0. Univariate statistics were generated to describe the study population. Preliminary bivariate analyses used contingency table methods (χ2) or t-tests as appropriate. Multivariate models used the Stata XT series of routines for population averaged repeated measures analysis (GEE), including mixed-effects regression for continuous measures, and mixed-effects logistic regression for binary outcomes.
Results
Descriptive Statistics
Table 1 presents the frequency distributions for all categorical variables, and the means and standard deviations for the continuous model variables. Consistent with prior work (14, 101-103), DP+ subjects were younger (35 years versus 41) and more were female (47% vs. 27%). Groups did not differ significantly on other characteristics, except for those associated with depression status (e.g., baseline affect, substitute alternative reinforcers). Table 2 provides univariate statistics for outcome variables by group and phase. Gender and age were considered in each model. Because they accounted for less than 1.5% combined variance in each model, they were not retained. The average effect sizes for the interactions described below are as follows: RRVS .60; positive affect .32; negative affect .66; and subjective reward .13. Reward sensitivity was an exploratory variable with an observed effect size of .10.
Table 2.
Univariate statistics for outcome variables by group and phase.
| DP- | DP+ | DP- | DP+ | |
|---|---|---|---|---|
| Outcome | Smoking | Abstinent | ||
| Mean SD | Mean SD | Mean SD | Mean SD | |
| RRVS | 1.03 (1.43) | 1.90 (2.66) | ||
| Response Bias | 0.13 (0.24) | 0.10 (0.15) | 0.12 (0.16) | 0.14 (0.16) |
| Positive Affect Day 1 | 12.52 (3.77) | 9.48 (3.39) | 12.15 (3.84) | 10.16 (3.62) |
| Positive Affect Day 2 | 12.32 (3.86) | 9.27 (3.42) | 12.17 (4.20) | 10.02 (3.64) |
| Positive Affect Day 3 | 12.38 (3.93) | 10.09 (3.74) | 12.10 (3.67) | 10.04 (3.82) |
| Negative Affect Day 1 | 7.41 (2.86) | 12.44 (5.77) | 7.08 (3.13) | 10.39 (5.08) |
| Negative Affect Day 2 | 7.18 (2.87) | 12.47 (5.68) | 7.43 (3.54) | 10.90 (5.31) |
| Negative Affect Day 3 | 6.91 (2.72) | 11.46 (5.78) | 7.29 (3.26) | 10.85 (5.37) |
| Subjective Reward Day 1 | 2.63 (1.23) | 1.85 (1.26) | 2.49 (1.15) | 1.80 (1.18) |
| Subjective Reward Day 2 | 2.40 (1.31) | 1.80 (1.27) | 2.47 (1.22) | 1.87 (1.26) |
| Subjective Reward Day 3 | 2.55 (1.24) | 2.10 (1.28) | 2.51 (1.15) | 1.93 (1.18) |
Multivariate Models
Relative Reinforcing Value of Smoking
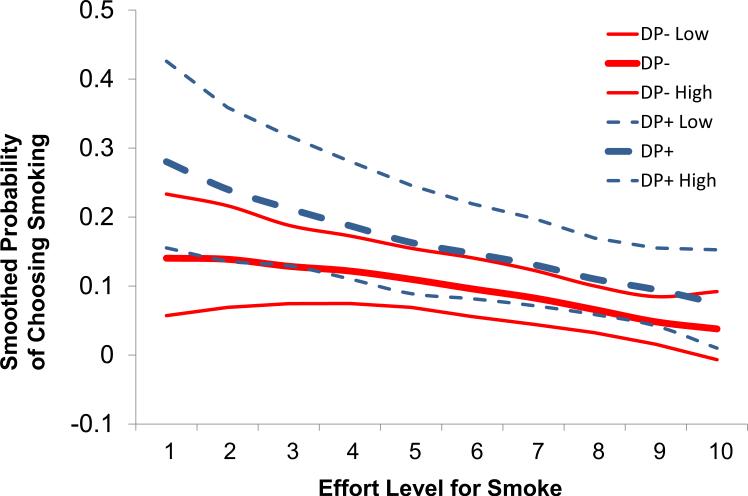
The choice to work for cigarette puffs versus money was modeled using logistic regression, with effort (i.e., number of responses) treated as a continuous variable, and depression group treated as a binary variable. We controlled for alternative reinforcers. As the effort required to earn a cigarette puff increased, the probability of working for cigarette puffs decreased (OR 0.86; CI 95% = 0.80 to 0.92), p<0.001). However, smokers in the DP + group were two times more likely to work for cigarette puffs than smokers in the DP- group, despite increasing levels of effort (OR 2.05; CI 95% = 1.04 to 4.06, p=0.039). Figure 2 depicts these findings.
Figure 2.
The probability of choosing smoking versus money as effort required to earn cigarette puff increased.
Reward Sensitivity
We tested reward sensitivity as measured by the signal detection task by modeling response bias using mixed models regression for the paired data. Neither depression status group nor smoking phase was predictive of response bias (p's > 0.24).
Subjective Reward, Positive Affect, and Negative Affect
The subjective reward derived from self-selected activities in the participants’ natural environment, positive affect and negative affect were modeled using mixed models regressions (Table 3; Figure 3). The models controlled for telephone call within day, and the difference in withdrawal scores between abstinent and smoking phases. This is important as the DP+ group's withdrawal symptoms were twice that of DP- when smoking (13.64 vs 6.64) and when abstinent (20.03 vs 9.72). Order of the smoking and abstinent phase was tested and found to be nonsignificant.
Table 3.
Mixed models regression of subiective reward, negative affect, and positive affect.
| Subiective Reward | |||
|---|---|---|---|
| Predictor | Coefficient (C195%) | P-Value | |
| DP - AND Abstinent | 0-Reference Group | ||
| DP - AND Smoking | 0.14 (−0.12 to 0.39) | p=0.284 | |
| DP + AND Abstinent | −0.37 (−0.74 to −0.01) | p=0.046 | Heterogeneity of slopes: |
| DP + AND Smoking | −0.79 (−1.15 to −0.42) | p=0.000 | χ2(3)=9.37, p=0.025 |
| Day by DP - AND Abstinent β1 | 0.02 (−0.06 to 0.10) | p=0.620 | |
| Day by DP - AND Smoking β2 | −0.03 (−0.12 to 0.05) | p=0.446 | Contrast (β3-β4)-(β1-β2): |
| Day by DP + AND Abstinent β3 | −0.04 (−0.14 to 0.06) | p=0.472 | χ2(1)=6.59, p=0.01 |
| Day by DP + AND Smoking β4 | 0.15 (0.05 to 0.25) | p=0.003 | |
| Negative Affect | |||
| DP - AND Abstinent | 0-Reference Group | ||
| DP - AND Smoking | 0.61 (−0.09 to 1.30) | p=0.087 | |
| DP + AND Abstinent | 2.73 (1.18 to 4.28) | p=0.001 | Heterogeneity of slopes: |
| DP + AND Smoking | 6.08 (4.53 to 7.63) | p=0.000 | χ2(3)=25.47, p<0.001 |
| Day by DP - AND Abstinent β1 | 0.06 (−0.17 to 0.29) | p=0.601 | |
| Day by DP - AND Smoking β2 | −0.29 (−0.52 to −0.06) | p=0.013 | Contrast (β3-β4)-(β1-β2): |
| Day by DP + AND Abstinent β3 | 0.30 (0.03 to 0.57) | p=0.033 | χ2(1)=4.470, p=0.035 |
| Day by DP + AND Smoking β4 | −0.60 (−0.87 to −0.33) | p=0.000 | |
| Positive Affect | |||
| DP - AND Abstinent | 0-Reference Group | ||
| DP - AND Smoking | 0.59 (0.01 to 1.16) | p=0.045 | |
| DP + AND Abstinent | −1.39 (−2.77 to −0.01) | p=0.048 | Heterogeneity of slopes: |
| DP + AND Smoking | −2.76 (−4.14 to −1.38) | p=0.000 | χ2(3)=10.67, p=0.014 |
| Day by DP - AND Abstinent β1 | 0.05 (−0.13 to 0.24) | p=0.578 | |
| Day by DP - AND Smoking β2 | −0.12 (−0.31 to 0.07) | p=0.207 | Contrast (β3-β4)-(β1-β2): |
| Day by DP + AND Abstinent β3 | −0.10 (−0.33 to 0.12) | p=0.376 | χ2(1)=8.20, p=0.004 |
| Day by DP + AND Smoking β4 | 0.33 (0.10 to 0.55) | p=0.004 | |
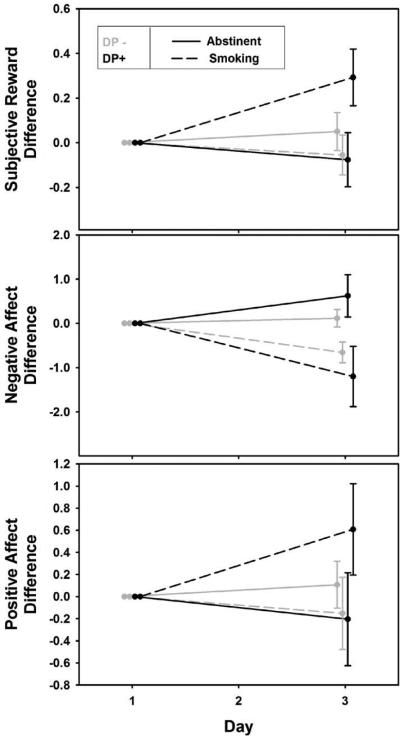
Figure 3.
Slopes reflecting change in subjective reward and affect across time and smoking or abstinent phase. Error bars reflect difference scores for day 3 minus day 1.
Depression group, smoking phase, and day were involved in a 3-way interaction for subjective reward. The interaction is best interpreted as a difference among slopes for the effect of day. For smokers in the DP- group, there was no significant change in subjective reward during the abstinence phase (p=0.62) or during the smoking phase (p=0.45). Although, subjective reward did not significantly decrease for DP+ during the abstinence phase (p=0.47), there was a significant increase during the smoking phase (p=0.003). The slopes for the depression groups differed significantly from each other (χ2(3)=9.37, p=0.025), and the effect of smoking versus abstinence over time was greater for DP+ than DP- (χ2(1)=6.59, p=0.01).
Similar to results for subjective reward, depression group, smoking phase, and day were involved in a 3-way interaction for positive affect as well as for negative affect. Positive affect did not change significantly for DP- during the abstinence phase (p=0.58) or the smoking phase (p=0.21). Positive affect did not change significantly for DP+ during the abstinence phase (p=0.40), but increased significantly during the smoking phase (p=0.004). The slopes for the two groups differed significantly from each other (χ2(3) =10.67, p=0.014), and the effect of smoking versus abstinence over time was greater for DP+ compared to DP- (χ2(1) =8.20, p=0.004).
For both groups, negative affect decreased during the smoking phase (DP-, p=0.013, and DP+, p<0.001). However, during the abstinence phase there was a significant increase for DP+ (p=0.03), but not for DP- (p=0.60). The slopes for the two groups differed significantly from each other (χ2(3)=25.47, p<0.001), and the effect of smoking versus abstinence over time was greater for DP+ than DP- (χ2(1)=4.470, p=0.035).
Discussion
The present study is the first to show that the relative reinforcing effects of smoking and the impact of smoking on reward and positive affect regulation are specific to DP+ smokers. Compared to DP- smokers, DP+ smokers have a heightened relative reinforcing value of smoking, and enhanced subjective reward from typical activities, and greater positive affect while smoking compared to abstinence. DP- smokers have little affective and reward dysregulation when abstinent, although both DP+ and DP- smokers show decreased negative affect while smoking. These findings suggest that DP+ smokers may be less able to sustain smoking abstinence because they not only lose smoking as an important reinforcer, but they also lose the enhanced positive affect, mitigated negative affect and pleasure from other reinforcers that accompany smoking. As such, the present study uncovers unique vulnerabilities to achieving and maintaining smoking abstinence and highlights novel targets for smoking cessation treatment in this population.
Previous research suggests that smokers with a history of MDD find smoking more reinforcing than smokers without a history of MDD (51). We extend these findings and show that DP+ smokers find smoking two times more reinforcing than other reinforcers compared to DP-smokers. The heightened relative reinforcing value of smoking for DP+ smokers highlights a significant reinforcer deficit upon quitting smoking that occurs within a context of already diminished reinforcers. Preliminary research suggests that behavioral activation smoking cessation treatment may allow DP+ smokers to overcome the loss of smoking as a reinforcer by promoting increased engagement in rewarding activities (104). However, behavioral activation alone may be insufficient to promote long-term smoking cessation among DP+ smokers as it does not appear to impact the enjoyment derived from typically rewarding activities in this population.
Indeed, the present findings highlight that DP+ smokers experience greater enjoyment from their daily activities while smoking ad libitum than while abstinent. For DP- smokers, subjective reward changed little regardless of smoking status. These differences in reward responsivity cannot be attributed to between-group differences in nicotine withdrawal symptoms. If smoking permits DP+ smokers to derive greater reward from typical reinforcers, smoking cessation treatment that includes behavioral skills to magnify or savor the enjoyment derived from daily reinforcers may help ameliorate the pleasure deficit (105, 106). These behavioral strategies derived from Positive Psychology focus on re-experiencing and dismantling the pleasurable aspects of daily events and have been shown to enhance positive mood in depression-prone populations (106). This is important because DP+ smokers have fewer reinforcers that are not linked to smoking. Recent studies in animals and humans suggest that bupropion and varenicline have reinforcement enhancing effects (107, 108), but surprisingly have not been fully evaluated in DP+ smokers (17, 20).
Evidence indicates that targeting negative mood does not significantly improve smoking cessation rates or mitigate negative mood for DP+ smokers (17, 21, 23, 24). Our results suggest that affective changes from smoking to abstinence are greater for DP+ smokers and that smoking helps to regulate positive affect, rather than simply reducing negative affect. Cognitive-behavioral treatment to lessen negative mood does not alter underlying reward-related substrates among persons with depression (109), which may offer a neurobiological account for its lack of effectiveness for smoking cessation among DP+ smokers. In contrast, behavioral therapy for depression that targets involvement in non-drug rewarding activities to promote positive mood increases neural responses to rewards (110). While there is debate as to whether positive and negative affect are distinct constructs or exist on opposite ends of an affective continuum, current research is focused on characterizing these dimensions to ultimately inform treatment development (111). For instance, targeting positive affect in DP+ smokers may have the added benefit of decreasing negative affect (106). Nevertheless, the present findings support a shift in smoking treatment for DP+ smokers from suppressing negative affect and smoking behavior to enriching positive affect and pleasure derived from alternative reinforcers such that smoking is a less attractive and reinforcing option (61).
Using a probabilistic reward task, we anticipated that the DP+ group would show a decreased response bias toward the reinforcing stimulus when abstinent, but not when smoking. This would have indicated reduced reward responsiveness while abstinent. Neither group of smokers developed a response bias. To detect a response bias in a community sample with elevated depression symptoms, we may have needed a greater number of learning trials versus the shortened version that was administered (76), more than overnight smoking abstinence, or a larger sample size to detect smaller effects. Although speculative, nicotine may help normalize disrupted reward learning that characterizes depression (112-114).
The present study focused on a population with disproportionately high smoking prevalence. Building on a strong theoretical and empirical foundation, its rigorous and ecologically valid experimental procedures included the first evaluation of (1) the relative reinforcing value of smoking, and (2) changes in affect and subjective reward in “real-time” in DP+ smokers’ natural environment. The findings provide initial support for novel smoking cessation treatment targets for a population that is often excluded from smoking cessation clinical trials. Despite these strengths, the limitations of the study should be acknowledged. While the CESD is a reliable and well-established self-report measure of current depression symptoms, we did not determine whether the DP+ participants met criteria for current MDD. Consequently, we cannot be sure that the present findings extend to smokers with past and present MDD. We also are unable to determine if similar relationships between affect and subjective reward exist for smokers who only have a past history of MDD or a current elevation in depression symptoms. Finally, participants were not seeking smoking cessation treatment, thus the findings may not fully generalize to those DP+ smokers who are motivated to quit smoking.
The present study advances smoking research in co-morbid populations by integrating unique deficits in depression with smoking's role in deficit amelioration. DP+ smokers experience a loss of smoking reinforcement, enhanced pleasure from other reinforcers, and positive mood upon smoking abstinence as well as increased negative affect. A smoking lapse could acutely restore these affective and reward-related functioning, which may then drive smoking relapse. This may help explain the difficulty sustaining smoking abstinence and the disproportionate smoking prevalence in this population. Novel behavioral smoking cessation interventions that consider the unique mechanisms that maintain smoking behavior in DP+ smokers may increase quitting success.
Acknowledgements
We wish to thank Dr. D.A. Pizzagalli for developing and providing the software and analysis code for the signal detection task. This study was supported by National Institute on Drug Abuse DA031946 (JAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Audrain-McGovern, Dr. Wileyto, Dr. Ashare, Ms. Cuevas, and Dr. Strasser reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Cinciripini PM, Tsoh JY, Wetter DW, Lam C, de Moor C, Cinciripini L, Baile W, Anderson C, et al. Combined effects of venlafaxine, nicotine replacement, and brief counseling on smoking cessation. Exp Clin Psychopharmacol. 2005;13:282–292. doi: 10.1037/1064-1297.13.4.282. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg D, Hall SM, Reus VI, Munoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3:389–395. [Google Scholar]
- 3.Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- 4.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 5.Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 6.SAMSHA Office of Applied Studies, National Survey on Drug Use and Health, Subsample. 2004.
- 7.Weinberger AH, Pilver CE, Desai RA, Mazure CM, McKee SA. The relationship of dysthymia, minor depression, and gender to changes in smoking for current and former smokers: longitudinal evaluation in the U.S. population. Drug Alcohol Depend. 2013;127:170–176. doi: 10.1016/j.drugalcdep.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall SM, Munoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, Hartz DT. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 9.Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 10.Levine MD, Marcus MD, Perkins KA. A history of depression and smoking cessation outcomes among women concerned about post-cessation weight gain. Nicotine Tob Res. 2003;5:69–76. doi: 10.1080/1462220021000060455. [DOI] [PubMed] [Google Scholar]
- 11.Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Tsoh JY, Hall SM. Depression and smoking: from the Transtheoretical Model of change perspective. Addict Behav. 2004;29:801–805. doi: 10.1016/j.addbeh.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger AH, Mazure CM, Morlett A, McKee SA. Two decades of smoking cessation treatment research on smokers with depression: 1990-2010. Nicotine Tob Res. 2013;15:1014–1031. doi: 10.1093/ntr/nts213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberger AH, Pilver CE, Desai RA, Mazure CM, McKee SA. The relationship of major depressive disorder and gender to changes in smoking for current and former smokers: longitudinal evaluation in the US population. Addiction. 2012;107:1847–1856. doi: 10.1111/j.1360-0443.2012.03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerman C, Audrain-McGovern J. Reinforcing effects of smoking: more than a feeling. Biol Psychiatry. 2010;67:699–701. doi: 10.1016/j.biopsych.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biol Psychiatry. 2010;67:707–714. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, Abrams D, Miller IW. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9:721–730. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, Baile WF. The effects of depressed mood on smoking cessation: mediation by postcessation self-efficacy. J Consult Clin Psychol. 2003;71:292–301. doi: 10.1037/0022-006x.71.2.292. [DOI] [PubMed] [Google Scholar]
- 19.Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K. A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. Am J Psychiatry. 2002;159:1731–1737. doi: 10.1176/appi.ajp.159.10.1731. [DOI] [PubMed] [Google Scholar]
- 20.Evins AE, Culhane MA, Alpert JE, Pava J, Liese BS, Farabaugh A, Fava M. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;28:660–666. doi: 10.1097/JCP.0b013e31818ad7d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72:563–570. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- 22.Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude-Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry. 2002;59:930–936. doi: 10.1001/archpsyc.59.10.930. [DOI] [PubMed] [Google Scholar]
- 23.Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein M, Mueller TI, Miller IW. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J Abnorm Psychol. 2002;111:670–675. doi: 10.1037//0021-843x.111.4.670. [DOI] [PubMed] [Google Scholar]
- 24.Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine Tob Res. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess ES, Brown RA, Kahler CW, Niaura R, Abrams DB, Goldstein MG, Miller IW. Patterns of change in depressive symptoms during smoking cessation: who's at risk for relapse? J Consult Clin Psychol. 2002;70:356–361. doi: 10.1037//0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes EE. Where's the fun in that? Broadening the focus on reward function in depression. Biol Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, Vanderveen J, Hedeker D. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 2008;196:461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- 28.Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- 29.Epstein LH, Bulik CM, Perkins KA, Caggiula AR, Rodefer J. Behavioral economic analysis of smoking: money and food as alternatives. Pharmacol Biochem Behav. 1991;38:715–721. doi: 10.1016/0091-3057(91)90232-q. [DOI] [PubMed] [Google Scholar]
- 30.Fisher EBJ. A behavioral-economic perspective on the influence of social support on cigarette smoking. In: Green L, Kagel JH, editors. Advances in behavioral economics, 3, Substance use and abuse. Alex Publishing Company; Norwood, NJ: 1996. [Google Scholar]
- 31.Green L, Fisher EB. Economic substitutability: Some implications for health behavior. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavior economics. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2000. [Google Scholar]
- 32.Green L, Freed DE. The substitutability of reinforcers. J of the Exper Analys of Behav. 1993;60:141–158. doi: 10.1901/jeab.1993.60-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 34.Perkins KA, Hickcox ME, Grobe JE. Behavioral economics of tobacco smoking. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavior economics. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2000. [Google Scholar]
- 35.Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology (Berl) 1995;118:250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- 36.Campbell UC, Carroll ME. Reduction of drug self-administration by an alternative non-drug reinforcer in rhesus monkeys: magnitude and temporal effects. Psychopharmacology (Berl) 2000;147:418–425. doi: 10.1007/s002130050011. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, Gortner E, Prince SE. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64:295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- 38.Lewinsohn PM, Amenson CS. Some relations between pleasant and unpleasant mood-related events and depression. J Abnorm Psychol. 1978;87:644–654. doi: 10.1037//0021-843x.87.6.644. [DOI] [PubMed] [Google Scholar]
- 39.MacPhillamy DJ, Lewinsohn PM. Depression as a function of levels of desired and obtained pleasure. J Abnorm Psychol. 1974;83:651–657. doi: 10.1037/h0037467. [DOI] [PubMed] [Google Scholar]
- 40.Dunn BD, Dalgleish T, Lawrence AD, Cusack R, Ogilvie AD. Categorical and dimensional reports of experienced affect to emotion-inducing pictures in depression. J Abnorm Psychol. 2004;113:654–660. doi: 10.1037/0021-843X.113.4.654. [DOI] [PubMed] [Google Scholar]
- 41.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: a biobehavioral study. J Abnorm Psychol. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- 44.Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. J Affect Disord. 1997;46:135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- 45.Wichers MC, Barge-Schaapveld DQ, Nicolson NA, Peeters F, de Vries M, Mengelers R, van Os J. Reduced stress-sensitivity or increased reward experience: the psychological mechanism of response to antidepressant medication. Neuropsychopharmacology. 2009;34:923–931. doi: 10.1038/npp.2008.66. [DOI] [PubMed] [Google Scholar]
- 46.Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103:103–116. [PubMed] [Google Scholar]
- 47.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21:461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- 49.Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry. 2003;160:316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- 50.Brody AL, Olmstead RE, Abrams AL, Costello MR, Khan A, Kozman D, Saxena S, Farahi J, et al. Effect of a history of major depressive disorder on smoking-induced dopamine release. Biol Psychiatry. 2009;66:898–901. doi: 10.1016/j.biopsych.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology (Berl) 2010;210:25–34. doi: 10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- 54.Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav. 2008;90:409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl) 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- 56.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 57.Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology (Berl) 1998;135:305–310. doi: 10.1007/s002130050514. [DOI] [PubMed] [Google Scholar]
- 58.al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl) 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- 60.Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- 61.Miller PG, Miller WR. What should we be aiming for in the treatment of addiction? Addiction. 2009;104:685–686. doi: 10.1111/j.1360-0443.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 62.Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, Borrelli B, Niaura R. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108:294–306. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmerman M, Coryell W. The inventory to diagnose depression, lifetime version. Acta Psychiatr Scand. 1987;75:495–499. doi: 10.1111/j.1600-0447.1987.tb02824.x. [DOI] [PubMed] [Google Scholar]
- 64.Zimmerman M, Coryell W, Corenthal C, Wilson S. A self-report scale to diagnose major depressive disorder. Arch Gen Psychiatry. 1986;43:1076–1081. doi: 10.1001/archpsyc.1986.01800110062008. [DOI] [PubMed] [Google Scholar]
- 65.Kinnunen T, Korhonen T, Garvey AJ. Role of nicotine gum and pretreatment depressive symptoms in smoking cessation: twelve-month results of a randomized placebo controlled trial. Int J Psychiatry Med. 2008;38:373–389. doi: 10.2190/PM.38.3.k. [DOI] [PubMed] [Google Scholar]
- 66.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 67.Perkins KA, Grottenthaler A, Wilson AS. Lack of reinforcement enhancing effects of nicotine in non-dependent smokers. Psychopharmacology (Berl) 2009;205:635–645. doi: 10.1007/s00213-009-1574-8. [DOI] [PubMed] [Google Scholar]
- 68.Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology (Berl) 2005;181:486–495. doi: 10.1007/s00213-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 69.Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 70.Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology (Berl) 2009;205:305–318. doi: 10.1007/s00213-009-1541-4. [DOI] [PubMed] [Google Scholar]
- 71.Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- 72.Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl) 2013;228:479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- 74.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hufford MR. Special methodological challenges and opportunities in ecological momentary assessment. In: Stone AA, et al., editors. The Science of Real-Time Data Capture. Oxford University Press, Inc.; New York, NY: 2007. pp. 54–75. [Google Scholar]
- 78.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. BMJ. 2002;324:1193–1194. doi: 10.1136/bmj.324.7347.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- 81.Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- 82.Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, Jain A. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2010;88:109–114. doi: 10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- 83.Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res. 2013;15:978–982. doi: 10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10:607–612. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 85.Macmillan NA, Creelman CD. Detection theory: a user's guide. 2nd ed. Lawrence Erlbaum Associates Publishers; Mahway NJ: 2005. [Google Scholar]
- 86.Freedman MJ, Lester KM, McNamara C, Milby JB, Schumacher JE. Cell phones for ecological momentary assessment with cocaine-addicted homeless patients in treatment. J Subst Abuse Treat. 2006;30:105–111. doi: 10.1016/j.jsat.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Shiffman S. Designing protocols for ecological momentary assessment. In: Stone AA, et al., editors. The Science of Real-Time Data Capture. Oxford University Press, Inc.; New York, NY: 2007. pp. 27–53. [Google Scholar]
- 88.Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM. Real-time craving and mood assessments before and after smoking. Nicotine Tob Res. 2008;10:1165–1169. doi: 10.1080/14622200802163084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weinstein SM, Mermelstein R. Relations between daily activities and adolescent mood: the role of autonomy. J Clin Child Adolesc Psychol. 2007;36:182–194. doi: 10.1080/15374410701274967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Snuggs S, Hajek P. Responsiveness to reward following cessation of smoking. Psychopharmacology (Berl) 2013;225:869–873. doi: 10.1007/s00213-012-2874-y. [DOI] [PubMed] [Google Scholar]
- 92.Gwaltney CJ, Bartolomei R, Colby SM, Kahler CW. Ecological momentary assessment of adolescent smoking cessation: a feasibility study. Nicotine Tob Res. 2008;10:1185–1190. doi: 10.1080/14622200802163118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weinstein SM, Mermelstein R, Shiffman S, Flay B. Mood variability and cigarette smoking escalation among adolescents. Psychol Addict Behav. 2008;22:504–513. doi: 10.1037/0893-164X.22.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 95.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 96.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 97.Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106:178–187. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Audrain-McGovern J, Rodriguez D, Epstein LH, Rodgers K, Cuevas J, Wileyto EP. Young adult smoking: what factors differentiate ex-smokers, smoking cessation treatment seekers and nontreatment seekers? Addict Behav. 2009;34:1036–1041. doi: 10.1016/j.addbeh.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Correia CJ, Simons J, Carey KB, Borsari BE. Predicting drug use: application of behavioral theories of choice. Addict Behav. 1998;23:705–709. [PubMed] [Google Scholar]
- 100.Van Etten ML, Higgins ST, Budney AJ, Badger GJ. Comparison of the frequency and enjoyability of pleasant events in cocaine abusers vs. non-abusers using a standardized behavioral inventory. Addiction. 1998;93:1669–1680. doi: 10.1046/j.1360-0443.1998.931116695.x. [DOI] [PubMed] [Google Scholar]
- 101.Jamal M, Does AJ, Penninx BW, Cuijpers P. Age at smoking onset and the onset of depression and anxiety disorders. Nicotine Tob Res. 2011;13:809–819. doi: 10.1093/ntr/ntr077. [DOI] [PubMed] [Google Scholar]
- 102.Peiper N, Rodu B. Evidence of sex differences in the relationship between current tobacco use and past-year serious psychological distress: 2005-2008 National Survey on Drug Use and Health. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1261–1271. doi: 10.1007/s00127-012-0644-0. [DOI] [PubMed] [Google Scholar]
- 103.Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005-2008. NCHS Data Brief. 2010:1–8. [PubMed] [Google Scholar]
- 104.MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Hopko DR, Zvolensky MJ, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duckworth LA, Steen TA, Seligman ME. Positive psychology in clinical practice. Ann Rev Clin Psychol. 2005;1:629–651. doi: 10.1146/annurev.clinpsy.1.102803.144154. [DOI] [PubMed] [Google Scholar]
- 106.Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60:410–421. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- 107.Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res. 2012;14:299–305. doi: 10.1093/ntr/ntr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perkins KA, Karelitz JL, Jao NC, Stratton E. Possible reinforcement enhancing effects of bupropion during initial smoking abstinence. Nicotine Tob Res. 2013;15:1141–1145. doi: 10.1093/ntr/nts224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 110.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]