Abstract
We report herein application of an in situ material strategy to attenuate allograft T cell responses in a skin transplant mouse model. Functionalized peptidic membranes were used to impede trafficking of donor antigen-presenting cells (dAPCs) from skin allografts in recipient mice. Membranes formed by self-assembling peptides (SAPs) presenting antibodies were found to remain underneath grafted skins for up to 6 days. At the host-graft interface, dAPCs were targeted by using a monoclonal antibody that binds to a class II MHC molecule (IAd) expressed exclusively by donor cells. Using a novel cell labeling near-infrared nanoemulsion, we found more dAPCs remained in allografts treated with membranes loaded with aI-Ad than without. In vitro, dAPCs released from skin explants were found adsorbed preferentially on aI-Ad membranes. Recipient T cells from these mice produced lower concentrations of interferon-gamma cultured ex vivo with donor cells. Taken together, the data indicate that the strategy has the potential to alter the natural course of rejection immune mechanisms in stringent allogeneic models.
Keywords: Skin allograft, self-assembling peptides, EAK16-II, His-tags, protein formulation, injectable membranes
1. Introduction
The performance of an in situ forming fibrillar membrane in attenuating T cell responses toward allogeneic skin grafts was investigated. Human skin allografts are important biological dressings for temporary wound closure [1]. Patients with partial and full-thickness burns benefit from intact epidermis and dermis; together these structures serve as a protective barrier to minimize desiccation of the underlying exposed tissues, limit water evaporation, reduce bacterial contamination, relieve pain, and promote wound healing by accelerating re-epithelialization [1]. However, the heightened antigenicity of skin allografts drives powerful allospecific T cell responses in recipients [2].
Calcineurin inhibitors are the mainstay in managing allograft rejection [3–5]. These agents exert their immunosuppressive effects mainly by dampening the activation, proliferation and survival of all T cells through down-regulation of interleukin-2. Patients exposed to these drugs have increased risk of developing opportunistic infections because skin flora may contain antibiotic-resistant Staphylococcus aureus [6, 7]. Herein we propose to generate selective immuosuppression by exploiting a fundamental molecular difference between donor and recipient cells: class II MHC (MHC-II) molecules expressed by donor antigen-presenting cells (dAPCs).
Acute rejection is driven by mismatched class I and II MHC molecules expressed by donors and recipients whereby the latter mount potent T cell responses against skin allografts [8]. Allograft survival correlates with the density of resident dAPCs. Within hours following allogeneic skin transplantation, dAPCs residing within allografts begin migrating to recipient draining lymph nodes [9–11]. More than three-quarters of the resident dAPCs would egress from skins within three days [12]. Once in lymph nodes, dAPCs activate allospecific T cells via MHC and costimulatory molecules [2, 8]. Inside lymph nodes host CD4 helper T cells recognizing mismatched MHC-II molecules are activated to drive CD8 T cell differentiation into cytotoxic T cells (CTLs) (Fig. 1). Presentation of MHC-II antigens is critical because generation of CTLs is compromised without CD4 T cell participation. With visceral allografts, activation of CD4 and CD8 T cells is correlated with the frequency of dAPCs in lymph nodes [13–15]. Activated allospecific CD8 CTLs in turn migrate to the transplant site and damage graft parenchyma via recognition of MHC class I molecules [8]. The magnitude of the rejection depends on the qualitative and quantitative encounter between dAPCs and recipient T cells shortly after grafting [12].
Figure 1.
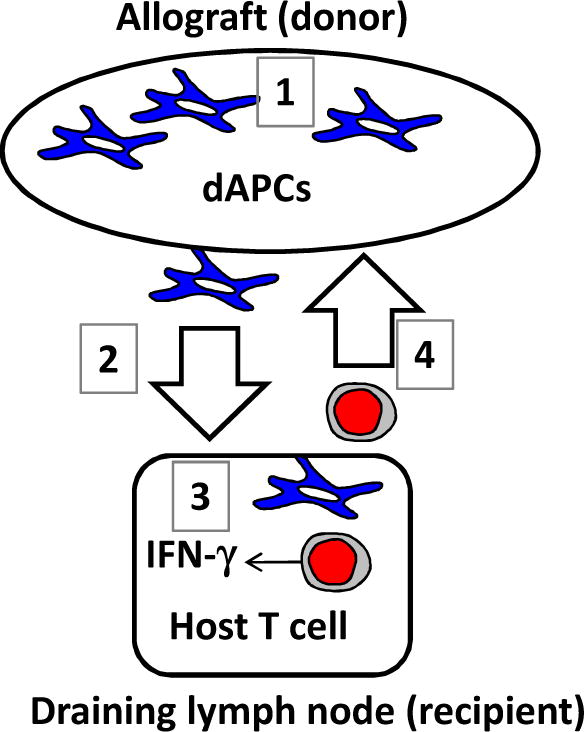
A generalized depiction of cellular mechanism of acute rejection of allografts. Shortly after the transplantation, dAPCs in the allograft are activated (1) and migrate towards draining lymph nodes (2). Host T cells in the draining lymph nodes are activated via direct allorecognition by dAPCs (3). Activated T cells will migrate to the allograft and mediate graft rejection (4).
Because acute rejection is a function of dAPCs accumulating in recipient lymph nodes, preclinical modalities have been devised to deplete dAPCs prior to transplantation. Typically the methods require systemic infusion of anti-leukocyte antibodies into donor animals before organ harvest [16–18]. While such preemptive strategies can be effective in delaying rejection of allografts in animal models, translation to humans is complicated by potential harms that can be done to the donors. Recognizing the unmet need, we envisaged a new strategy by which dAPCs trafficking can be impeded selectively after transplantation.
Previously we have reported an injectable platform by which retention of IgG molecules in local tissues can be enhanced using EAK16-II, a self-assembling peptide (SAP) with the sequence AEAEAKAKAEAEAKAK [19]. This and related SAPs are primarily utilized for their environmental responsiveness [20, 21]. These peptides undergo sol-gel phase transition at high ionic strengths (> 20 mM NaCl); in deionized water, EAK16-II can be injected into physiological environment to establish gels in situ. A system containing EAK16-II, its histidinylated analogue EAKIIH6, protein linkers (αH6-IgG and pAG) can localize model therapeutic IgG molecules in vivo through directional binding [19, 22]. Membranes loaded with antibodies can be established in vivo by subcutaneous injection. Such immobilization partially overcomes antibody clearance mechanisms in tumors, as evidenced by prolonged retention of IgG in mouse mammary and melanoma lesions [22]. In both tumor types, localized IgG remained in tumors significantly longer than free IgG.
In the present study, we characterize the system of materials designed to localize anti-MHC-II antibodies specific to dAPCs (Fig. 1). The rationale is that EAK16-II, its histidinylated analogue EAKIIH6, protein linkers, and anti-MHC-II antibodies spontaneously complex non-covalently upon mixing in syringes, with the resultant assembly localizes at the graft-host interface. dAPCs migrating toward draining lymph nodes would interact with the antibody-functionalized membranes, resulting in delayed and/or diminished encounter with T cells. The strategy was examined in the current study using an allogeneic mouse skin graft model. All MHC alleles between the donor and recipient are mismatched, rendering this a stringent model. Although delay in rejection was not expected, the work was aimed to characterize membranes presenting anti-I-Ad IgG (referred hereafter as “αI-Ad”) in vitro and in vivo to assess feasibility of the strategy. To this end, we investigated retention of IgG co-administered with the self-assembling components under normal and grafted skins. A nanoemulsion encapsulated with a fluorescent tracer was used to image dAPCs in vitro and in vivo. The immune responses were determined by analyzing production of IFN-γ from T cells in recipient mice. The data indicate that the platform has the potential to become a useful clinical tool by which skin graft rejection can be mitigated.
2. Methods and Materials
2.1. Materials
Peptides were custom synthesized by American Peptide Company (Sunnyvale, CA) at greater than 95% purity with termini acetylated or amidated. Peptides were reconstituted in sterile MilliQ water (18.2 MΩ at 25°C) at 5 mg/mL (3 mM, EAK16-II, AcNH-AEAEAKAKAEAEAKAK-CONH2) or 7.5 mg/mL (3 mM, EAKIIH6, AcNH-AEAEAKAKAEAEAKAKHHHHHH-CONH2). Rabbit anti-His-tag polyclonal antibody (αH6-IgG, 0.2 mg/mL) was obtained from AnaSpec, Inc. (San Jose, CA). Recombinant protein A/G (pAG) was obtained from Pierce Biotechnology (Rockford, IL) and reconstituted to 0.25 mg/mL in sterile deionized water. Anti-mouse I-Ad (MHC-II) antibody (αI-Ad, 0.5 mg/mL; IgG3κ, clone: 39-10-8) was purchased from Biolegend (San Diego, CA). Allophycocyanin conjugated anti-mouse MHC-II I-Ad antibody (0.2 mg/mL) was obtained from eBioscience (San Diego, CA). DyLight™ 800 conjugated goat anti-chicken IgG (IgG800) was obtained from KPL (Gaithersburg, MD) and reconstituted to 1 mg/mL in sterile deionized water. RPMI-1640 and fetal bovine serum (FBS) were obtained from HyClone (Logan, Utah). ACK lysing buffer and penicillin/streptomycin solutions were purchased from Lonza (Walkersville, MD). Perfluorocarbon (PFC), Perfluoro-15-Crown-5 Ether was purchased from Exfluor Research Corporation (Round Rock, TX). Lipophilic carbocyanine DiOC18(7) (“DiR”) was purchased from Life Technologies and used without further purification.
2.2. Skin transplantation
Six–to-eight week old female (certified-virus-free) BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Duquesne University Animal Care Facility. Animals were handled in accordance to protocols approved by the Duquesne University Institutional Animal Care & Use Committee. The transplant procedure was modified from a protocol described by Garrod et al. [23]. Briefly, the recipient C57BL/6 mice were anesthetized using isoflurane (induction: 5%; maintenance: 3%) and subsequently administrated with Buprenex at 0.1 mg/kg via the intraperitoneal route. Ear skin explants (dorsal layer) were prepared from BALB/c mice and transplanted onto graft beds (each ~0.5 cm2) prepared in the flank of C57BL/6 mice. Upon completion of the surgery, C57BL/6 mice were bandaged and returned to cage with their activities monitored regularly.
2.3. In vivo stability of IgG membrane in subcutaneous space and under the skin graft
Anti-chicken IgG labeled with a near infrared dye emitting at 800 nm (IgG800) was used as a model antibody to investigate the assembling and stability of the system in vivo. EAK16-II (100 μg in 20 μL) was mixed with 37.5 μg (in 5 μL) of EAKIIH6. In a second vial, αH6-IgG (2 μg), IgG800 (2 μg), and pAG (0.375 μg) were pipet mixed. The peptides and antibodies solution were withdrawn into the same insulin syringe (28 G’ needle) and injected into the subcutaneous space in footpad or the posterior flank of BALB/c mice. Fluorescence in the footpad was imaged ex vivo at 30 min, 6, 24, 48, and 72 hours. Excised footpads were stretched out on a 15×100 mm dish using scotch tapes for ex vivo imaging using an Odyssey Imager (Li-Cor, Inc., Lincoln, NE) and measured at an intensity of 1.0 with focal distances of 3.2 mm and 3.5 mm. The averaged signal of three blank footpads at each focal distance was subtracted as background. Data reported represent intensities averaged from the measurement performed at each focal distance.
Signal at the posterior flank was recorded in real time in vivo for up to 4 days. Anesthetization was induced using isoflurane (induction 5%, maintenance 3 %) for real time in vivo imaging. Abdominal hairs were removed prior to scanning. Mice were scanned prior to injection and monitored at the indicated times after injection using a Pearl Impulse imager (Li-Cor, Inc.). All images were collected using the same threshold and resolution (170 μm) settings. Oval-shaped regions of interest were defined at the site of injection using the contralateral flank as background. Fluorescence was quantified using the Impulse (2.0) software (Li-Cor, Inc.) with a standard deviation multiplier of 10 and a search limit of 100 pixels. To demonstrate the assembling and stability of the system under the skin graft, the same amount of peptides and antibodies solutions were mixed and administrated onto the graft bed of C57BL/6 mice before placement of the skin. Signal was monitored in real time using a Li-Cor Pearl Impulse Imager for up to 6 days as in the previous experiment.
2.4. Detection of I-Ad + dAPCs in draining lymph nodes
The capability of anti-I-Ad membrane to impede the trafficking of dAPCs to host draining lymph nodes was studied using flow cytometry. αI-Ad IgG with or without the self-assembling components were administrated onto the graft bed prior to the skin graft. Draining lymph nodes (inguinal, popliteal and axillary) were collected and pooled 1 day, 2 days, or 3 days after transplantation. Single cell suspensions were prepared by mechanical crushing and filtering through 70 μm nylon cell strainers. APCs (both donor and recipient) were enriched using BD IMag (BD Bioscience, San Jose, CA). APCs were re-suspended in 200 μL 3% FBS PBS and stained with Allophycocyanin conjugated anti-mouse MHC-II I-Ad antibody (4 μg/mL). Samples were analyzed using an Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ).
2.5 Preparation and characterization of near infrared dye-encapsulated PFC nanoemulsions
Nanoemulsions were produced using microfluidization by adapting our earlier described published procedures [24–30]. Briefly, the lipophilic payload, NIR dye (DiR) was dissolved at room temperature in Miglyol® 812N (synthetic hydrocarbon oil). This oil solution was mixed with PFC and surfactant mixtures to form a pre-emulsion which was then processed on a Microfluidizer M110S (Microfluidics Corp, Westwood, MA) to produce the final nanoemulsion. Droplet size and zeta potential were measured using dynamic light scattering on a Zetasizer Nano (Malvern, Westborough, PA). Measurements were taken after diluting the nanoemulsion in deionized water (1:40 v/v, made at 25°C) using 173° scattering angle with respect to the incident beam. The stability of the nanoemulsions was assessed by measuring the hydrodynamic diameter (Z average) and half width of polydispersity index at different time points (days). Zeta potential was measured at the same dilution using specialized zeta cells with electrodes following the manufacturer’s instructions. Li-Cor Odyssey was used to confirm labeling of the nanoemulsion with DiR. For the measurement, four dilutions were prepared with 100 μL of each dilution placed in a 96-well plate. The plates were scanned at 800 nm with intensity setting at 2.5 and the offset 3.0 mm with the resulting images analyzed using Li-Cor software.
2.6. Ex vivo labeling of skin explants with PFC nanoemulsions and imaging of cells in vitro and in vivo
Dorsal ear skin from BALB/c mice was collected and incubated with 4 mL of diluted with PBS PFC NIR nanoemulsion (2 mg/mL) in a 6-well plate for 2 h. Labeled ear skin was rinsed with PBS 3 times before grafting. After 2 days, recipient mice were scanned in Li-Cor Pearl Imager as previously described in the stability studies. For in vitro confocal microscopy, donor ear skin was incubated with 4 mL of serum free RPMI diluted PFC NIR nanoemulsion (2 mg/mL) overnight in incubator (2 skin grafts per sample). CCL21 (final concentration 10 ng/mL) and GM-CSF (final concentration 5 ng/mL) were added to each well to facilitate emigration of dAPCs. On the second day, adherent and suspended cells released from the ear explants were harvested and centrifuged to remove unincorporated nanoemulsion. The cells were then re-suspended in 200 μL serum free medium and added to the glass bottom culture dish with SAPs and linker proteins with or without αI-Ad stained with Congo red. The membrane-cell mixtures were fixed with 2% PFA for 0.5 h and washed twice with PBS containing 1% fetal bovine serum. Cells were then stained with 0.5 mL of 1 (μg/mL Hoechst 33342 for 10 min before washing twice with buffer. Anti-fade mounting solution (Prolong® Gold Antifade, Life Technologies) was added to cover slide and placed over the membrane. Images were collected using a Zeiss Axio Observer microscope equipped with the ApoTome 2 imaging system. Nuclear images were obtained with a DAPI filter set (excitation/emission: 335–383 nm/420–470 nm). αI-Ad or control membranes were imaged with a Cy3 filter set (excitation/emission: 538–562 nm/570–640 nm). Z-stack was used to generate three-dimensional (3D) images.
2.7. Allogeneic APC-T cells reaction
To demonstrate that systemic T cell responses were attenuated, splenic T cells from recipient C57BL/6 were cultured with naïve BALB/c APCs. Six days after transplantation, the spleen was collected from BALB/c mice to prepare naïve APCs. Single cell suspensions were prepared by dish crushing, hypotonic solution (ACK lysing buffer to remove erythrocytes) treatment and filtering through 70 μm nylon mesh strainers. Cells were cultured in 1 mL complete RPMI medium with 5 ng/mL GM-CSF at a density of 1×106 cells/mL overnight. Culture medium was gently pipetted to remove non-adherent cells. Recipient C57BL/6 mice were euthanized and single cell suspensions were prepared from spleen adjusted to 5×105 cells/mL. C57BL/6 splenic T cells (1×106 cells in 2 mL medium) were added into previously obtained naïve BALB/c APCs, and cultured for 48 h. Cell culture medium was centrifuged to remove debris. ELISA was used to quantify concentrations of IFN-γ (DuoSet, R&D Systems, Minneapolis, MN) produced by recipient C57BL/6 splenic T cells.
2.6. Statistical analysis
Statistical analysis was performed in GraphPad Prism 5.0. Data were analyzed using one way ANOVA and Tukey-Kramer multiple comparison (α=0.05) or one-tailed Student’s t-test (α=0.05) as indicated. Data shown represent mean and standard deviation.
3. Results
Mouse ear skin grafting is a robust and stringent method for measuring the magnitude of allogeneic T cell responses [31]. Excision of epidermal and dermal layers results in exposed underlying tissues that mimic debrided burnt skins [32]. The targets of the current strategy, dAPCs, are interspersed within skin layers of mouse and human [8]. Skin dAPCs include donor Langerhans’ cells (dLCs) in the epidermis and dermal dendritic cells (dDCs). dLCs and dDCs migrate in waves, with the trafficking complete within 4–5 days in rodent models [12]. LCs are immature DCs but promptly mature upon encountering alloantigens while migrating to secondary lymphoid tissues. The density of these cells per unit area is correlated with immunogenicity. For example, mouse tail skin, which contains few LCs, is significantly less antigenic than ear skins [12]. We postulated that SAPs could form stable structures at the host-graft interface and that anti-graft T cell responses could be attenuated by establishing membranes that capture dAPCs.
3.1. In vivo retention of IgG membranes in subcutaneous space
To determine the extent to which the system was able to localize antibodies in subcutaneous space in vivo, the fate of a NIR (λ=800nm) labeled IgG (hereafter “IgG800”) was tracked in BALB/c mice (Fig. 2 and 3). Retention was demonstrated by injecting the proteins and peptides into the footpads of BALB/c mice subcutaneously (Fig. 2). Fluorescence could be detected for at least 72 h when IgG800 was injected with the assembling components (“System+IgG800”), while IgG800 in saline (“free IgG”) was significantly lower at 6 h (p < 0.001) and near background after 24 h (p < 0.001). Without pAG, retention of IgG800 was significantly lower at 6 h (p < 0.01), 24 h (p < 0.001), 48 h (p < 0.001) and 72 h (p < 0.001) compared to mice received the antibody-membrane treatment. This confirmed previous results showing that the bioaffinity interactions involve pAG [22]. Further evidence of localization was supported by real-time optical measurements. When injected into the mouse flank, solutions containing IgG800 with the self-assembling components showed remarkable localization (Fig. 3). Strong fluorescence was detected at the injection site for at least 96 h, while relative low fluorescence was observed with IgG800 delivered in saline after 24 h. These in vivo results show that the injectable formulation was effective in retaining IgG locally.
Figure 2.
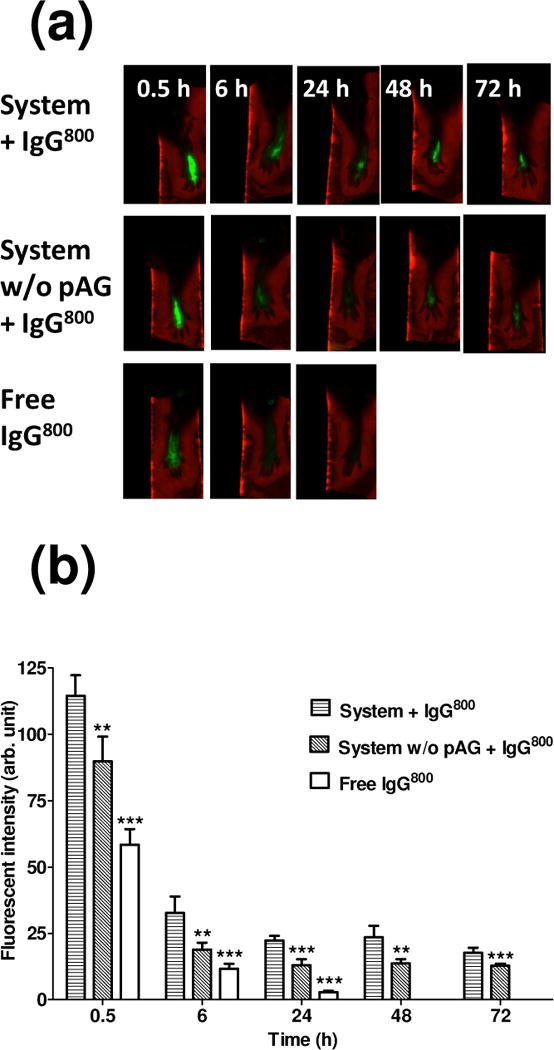
Stability of the antibody display system in BALB/c mouse footpads. (a) Representative ex vivo images of footpads injected with solution containing EAK16-II, EAKIIH6, αH6-IgG, pAG, and IgG800 (“System + IgG800”) or solution of EAK16-II, EAKIIH6, αH6-IgG, and IgG800 (“System w/o pAG + IgG800”) or IgG800 alone in PBS (“Free IgG800”). (b) Fluorescent intensity in footpads quantified using Odyssey. Statistical significance (compared to “System + IgG800”) was analyzed by one way ANOVA with Tukey’s multiple comparison (0.5 h, 6 h, and 24 h) or student’s t test (48 h and 72 h) (n=3–6, ***: p < 0.001, **: p < 0.01).
Figure 3.
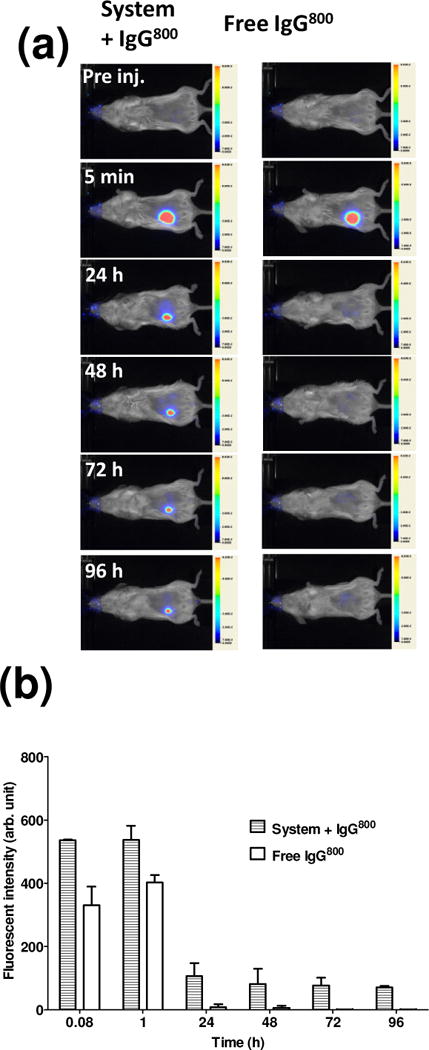
Real-time monitoring of model antibody localization in vivo. (a) Representative real time images of BALB/c mice injected with solution containing EAK16-II, EAKIIH6, αH6-IgG, pAG, and IgG800 (“System + IgG800”) or IgG800 alone in PBS (“Free IgG800”) at the lower flank. (b) Fluorescence at the site of injection (n=2). BALB/c mice were used to track stability due to lack of pigmentation (as in C57BL/6 mice). Once the kinetics in subcutaneous space was established, the study moved to C57BL/6 as hosts for allografts.
3.2. In vivo stability of IgG membranes under skin grafts
Having established that the self-assembly and bioaffinity interactions could take place in vivo in normal subcutaneous space, the ability of the system to localize at the site of allograft implantation was examined. Membrane components mixed with IgG800 were established in the graft bed immediately prior to placement of allogeneic skins. Fluorescence underneath the graft was monitored in live mice for 6 days (Fig. 4). IgG800 delivered with the assembling components showed superior localization; as early as 5 minutes after injection, local fluorescence registered at least two-fold higher in intensity than in mice received IgG800 without the components. Superior fluorescence was observed at all time-points monitored; fluorescence in mice that received the antibody in membrane were significantly higher on day 3 (p < 0.01) and day 6 (p < 0.01) when compared to those in mice that received IgG800 in saline. These results indicate that the assembling components provided a mechanism by which IgG were retained at the graft-host interface.
Figure 4.
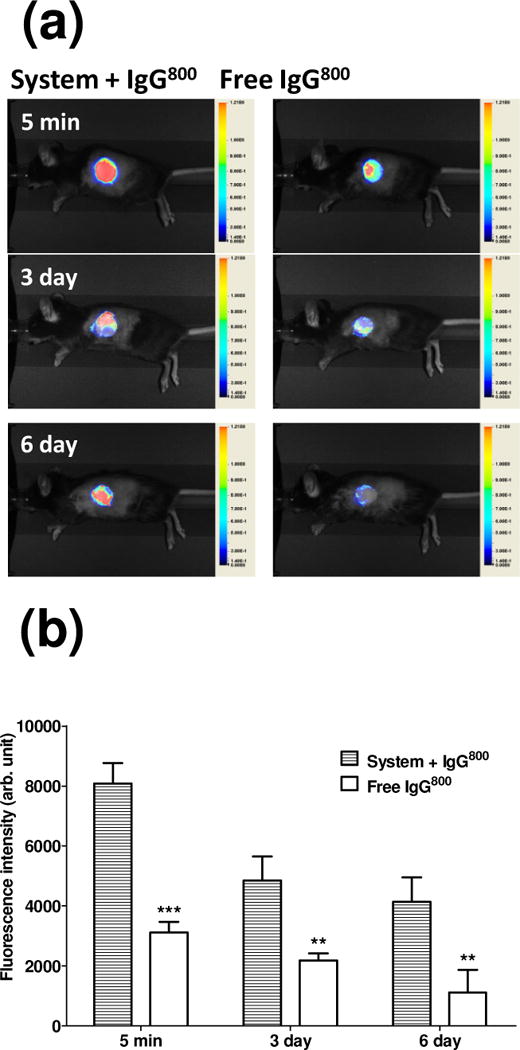
Real-time monitoring of model antibody in graft bed in vivo. (a) Representative real time images of recipient C57BL/6 administrated with solution containing EAK16-II, EAKIIH6, αH6-IgG, pAG, and IgG800 (“System + IgG800”) or IgG800 alone in PBS (“Free IgG800”) at the graft bed prior to the skin graft. (b) Fluorescence at the graft site. Student’s t test was used for statistical analysis at each time point (n=3–6, ***: p < 0.001, **: p < 0.01).
3.3. Detecting dAPCs in lymph nodes
To determine the extent to which dAPCs trafficking was impeded by membranes loaded with anti-I-Ad IgG (hereafter referred to as “αI-Ad”), draining lymph nodes (inguinal, axillary, and popliteal) of recipient mice were analyzed for the presence of I-Ad+ cells (Fig. 5). αI-Ad was placed on graft beds with or without the self-assembling components prior to placement of ear skins. At predetermined time points after transplantation, draining lymph nodes were excised from the euthanized mice. Lymph nodes from each mouse were pooled and stained for I-Ad+ cells. Flow cytometric analyses indicated that on day 2 αI-Ad membrane-treated C57BL/6 mice had 2.0% (±0.18) of dAPCs in draining lymph nodes, significantly lower (p < 0.05) than that of C57BL/6 mice received αI-Ad delivered in saline (3.5%±0.52; Fig. 5c). αI-Ad membrane-treated C57BL/6 mice showed lower (but not significantly different) frequency of I-Ad+ cells on day 3 (Fig. 5d). The two groups showed comparable frequencies of I-Ad+ cells on day 1 (Fig. 5b). There may be at least two reasons for observing reduced dAPCs in lymph nodes only on day 2. First, due to limited hydration and salt content, there might be a lag-time (though not evident in vitro) for complete assembly of the components in graft beds. Thus, upon placement of skin graft, dAPCs immediately migrated out (within the first hour) might have escaped the membrane barrier. The effect seen on day 2 might result from impeded trafficking from a second wave of emigrated dAPCs. Because egressed dAPCs likely express high densities of foreign MHC molecules, these cells would be killed by host allospecific T cells in lymph nodes.
Figure 5.
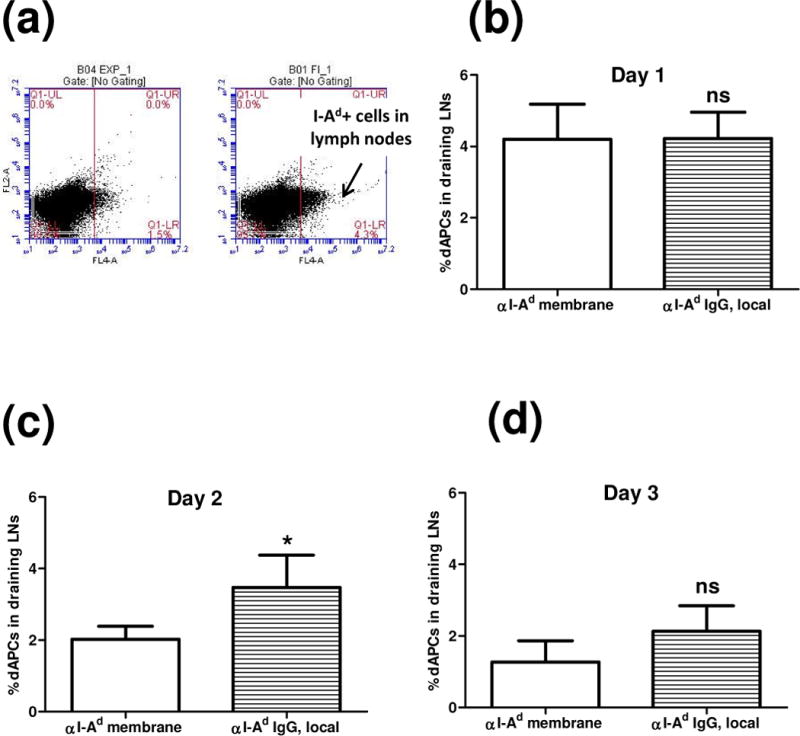
Detection of I-Ad dAPCs in draining lymph nodes. αI-Ad was administrated at the graft bed with the self-assembling components (“αI-Ad membrane”) or PBS (“αI-Ad IgG, local”) prior to the skin graft. Single cell suspensions enriched for APCs were prepared from draining lymph nodes of recipient C57BL/6 mice. Cells were stained with Allophycocyanin conjugated anti-mouse MHC-II I-Ad antibody, and flow cytometry was used to probe the frequency of I-Ad dAPCs. (a) Representative dAPCs frequency on day 2. Student’s t test was used to confirm the statistical difference on day 1(b, n=4, ns: not significant), day 2 (c, n=3-, *: p < 0.05), and day 3 (d, n=3, ns: not significant).
3.4 Tracing dAPCs with near infrared nanoemulsion
Given the uncertainties in tracking dAPCs in lymph nodes, we used a novel phagocytic cell-specific PFC nanoemulsion to image these cells at the graft-host interface. Similar formulations of the emulsion have been shown to label macrophages and dendritic cells with PFC droplets encapsulating near infrared dyes [25]. The data show that dAPCs in skin grafts can be labeled ex vivo; fluorescence in labeled ear explants correlated with the dose of the nanoemulsion used (Fig. S2). Upon grafting of the labeled skins, strong fluorescence was found in graft bed fortified with αI-Ad membrane (Fig. 6), suggesting retention of dAPCs in graft beds. Higher intensity of fluorescence was observed in graft beds with membranes loaded with αI-Ad than without (p<0.001). In a separate in vitro experiment, dAPCs egressed from skin explants were found on membranes loaded with αI-Ad (Fig. 7a) but not without (Fig. 7b). These observations suggest that the αI-Ad membrane retained skin-resident dAPCs.
Figure 6.
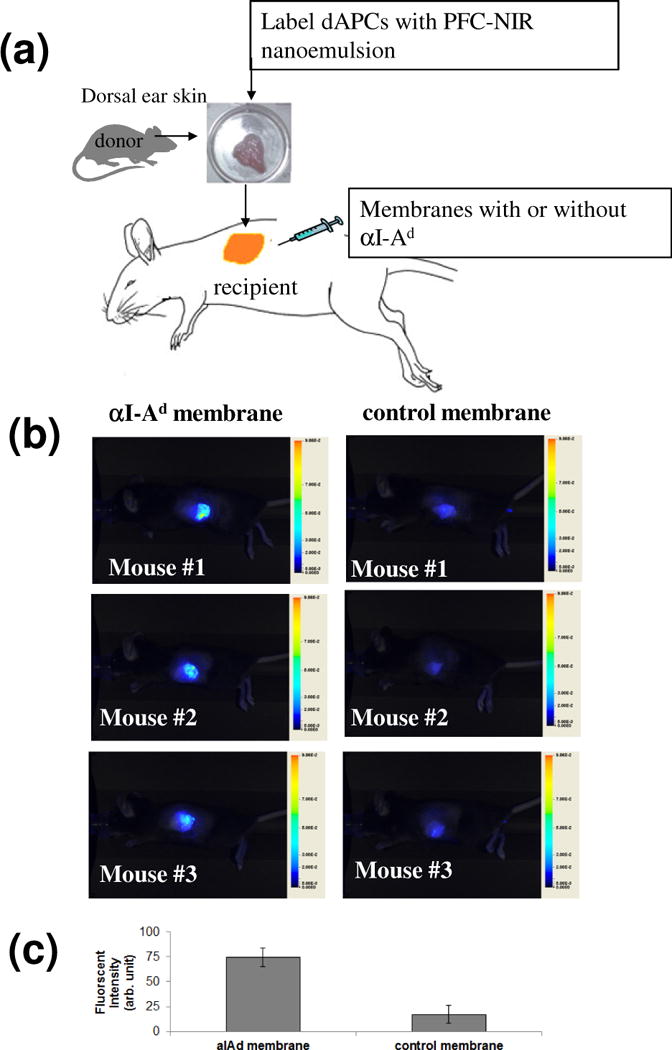
Immobilization of dAPCs on αI-Ad membrane in vivo. Dorsal ear skin from BALB/c mouse was incubated with 2 mg/mL PFC nanoemulsion to label dAPCs ex vivo. Membranes with αI-Ad IgG or without (control) were administrated on graft beds before placement of ear skins. Signal was monitored using a Li-Cor Pearl imager 2 days after transplantation. (a) Representative NIR images at day 2. (b) Student’s t test was used to confirm the statistical difference. n = 3, ****p< 0.001.
Figure 7.
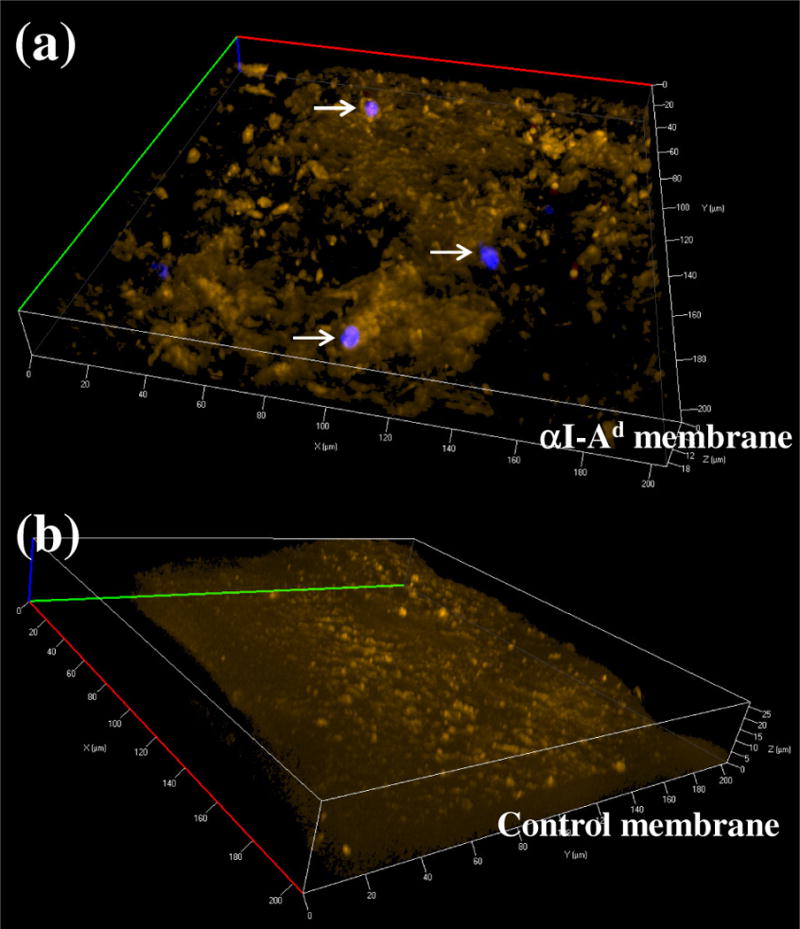
Immobilization of dAPCs on membrane imaged using confocal microscopy (40×). dAPCs were induced to egress using CCL21. Cells adsorbed on membranes with αI-Ad (“αI-Ad membrane”) or without (“Control membrane”) were stained for DNA (visualized using a DAPI filter) and fixed. Cells ingested with nanoemulsions were illuminated with DiR (red). Membranes were imaged with a Cy3 filter set (orange).
3.4. Suppression of T cell responses in recipient mice
We next hypothesized that reduced dAPCs trafficking would manifest in altered systemic T cell responses toward the allograft. To test this, T cells of recipient mice were analyzed ex vivo to evaluate immune reactivity toward donor antigens. Lymphocytes isolated from spleens harvested from transplanted animals were stimulated in vitro by culturing with fresh leukocytes from naïve donor mice (Fig. 8a). After 48 h, culture supernatants were analyzed using ELISA to determine the concentrations of IFN-γ, a characteristic cytokine produced by activated T cells in cell-mediated rejection. The results indicate that mice received αI-Ad membrane yielded T cells that were hypo-responsive. Splenic T cells recovered from mice received αI-Ad membrane produced 4.4 ng/ml (±1.7) IFN-γ, in contrast to 15.1 ng/ml (±3.4) and 22.1 ng/ml (±5.1) from mice received only αI-Ad in saline (“αI-Ad IgG, local”; p < 0.01) or SAPs (“EAK16-II/EAKIIH6”; p < 0.001), respectively. T cells from transplanted mice administered with the SAPs without αI-Ad produced high levels of IFN-γ. Low levels of IFN-γ were released from T cells from mice transplanted with isografts (C57BL/6 ear → C57BL/6 host) in which no rejection was expected (Fig. 8b). As expected, the treated transplanted skins were rejected comparably (scored based on gross necrosis) by day 9 (see supplemental data Fig. S3). This was not unexpected given the extent of MHC disparities. Overall, the differential IFN-γ produced by recipient T cells support the notion that T cell responses were attenuated specifically with local deposition of αI-Ad membranes.
Figure 8.
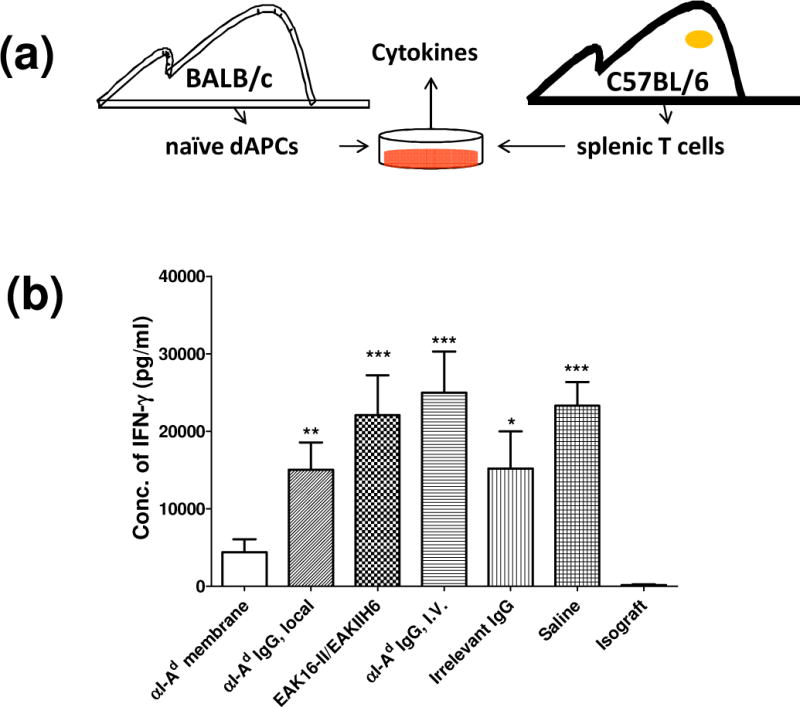
Concentration of IFN-γ produced by recipient C57BL/6 mouse splenic T cells cultured with naïve BALB/c APCs. Membranes or other treatments were established in graft beds prior to the skin graft: “αI-Ad membrane” (EAK16-II, EAKIIH6, αH6-IgG, pAG, and αI-Ad IgG), “αI-Ad IgG, local” (αI-Ad IgG in PBS), “EAK16-II/EAKIIH6” (SAPs without other components), “Irrelevant IgG” (EAK16-II, EAKIIH6, αH6-IgG, pAG, and anti-I-Ab IgG), and “Saline” (isotonic saline solution). “αI-Ad IgG, I.V.” represents αI-Ad IgG administrated through tail vein. “Isograft” refers to C57BL/6 to C57BL/6 transplantation. (a) Schematic of the experimental procedures. 7 days after the transplantation, T cells were prepared from spleen of recipient C57BL/6 mice and cultured with naïve BALB/c mouse APCs. The cell culture medium was collected by centrifugation and concentrations of IFN-γ were quantified by ELISA. (b) Concentration of IFN-γ produced by recipient C57BL/6 mouse splenic T cells. One way ANOVA with Tukey’s multiple comparison was used for statistical analysis (n≥3, compared to αI-Ad membrane group, ***: p < 0.001, **: p < 0.01, *: p < 0.05, ns: not significant).
4. Discussion
The two mouse strains tested here express distinct sets of class II MHC molecules: I-Ad and I-Ed (BALB/c), and I-Ab (C57BL/6). These antigenic mismatches drive a robust rejection. In C57BL/6 recipients, T cells exist to recognize I-Ad and I-Ed on dAPCs to develop into IFN-γ driven cytotoxic responses. These reactions are initiated and sustained by dAPCs (Fig. 1). When placed in vivo, dAPCs migrate from skin explants to stimulate recipient T cells in regional lymph nodes and ultimately populate the rest of the host. The extent of anti-graft T cell activation correlates with MHC-II+ dAPCs migrated to graft-draining lymph nodes [8]. Donor MHC antigens (released by dead graft cells) are also presented by graft-infiltrated host APCs. While this form of indirect recognition contributes to rejection [2], the initial rejection intensity largely depends on dAPCs.
Viability of skin allografts are difficult to sustain in allogeneic hosts owing to their stronger antigenicity. Attenuating acute rejection of skin-derived allogeneic antigens would improve the viability of such transplants functioning as wound dressings. Cytotoxic T cell responses constitute the major mechanisms of acute rejection. High doses of calcineurin inhibitors (cyclosporine A and tacrolimus) and glucocorticoids suppress the activation of all lymphocytes, not just allospecific T cells.[3, 4] An immune-compromised state is not conducive to healing in patients with burn wounds [32]; the incidence of opportunistic infections in burn victims receiving skin allografts and standard immunosuppressants is predictably high.[32]
Local-regional delivery of immunosuppressants was first reported by Billingham et al. in 1951 [33]. Using cortisone acetate in a topical formulation the investigators were able to prolong skin allograft survival in a rabbit model. Importantly, the same dose, which was relatively low, was ineffective when administered systemically. The next documented attempt came 35 years later when another group applied topical cyclosporine A formulated in DMSO to rats received skin allografts [34]. While long-term graft survival was attained, interpreting results of the topical formulations was complicated by the large dose used (5 mg per kg daily); indeed, the authors noted significant systemic drug absorption. In general, the rationale for topical delivery of calcineurin inhibitors and steroidal anti-inflammatory drugs are weakened by their lipophilicity and penetration into the vasculature of deeper tissues. Furthermore, Zhao and colleagues have reported bacterial infiltrates in skin allografts of rats received topical cyclosporine A and fluocinolone acetonide [35]. Thus topical or local drug delivery per se does not necessarily circumvent the toxicities of systemic immunosuppressants.
Rather than suppressing all host leukocytes, depleting dAPCs may impute a targeted mechanism to attain specific immunosuppression. The concept has been explored as potential anti-rejection therapies. Odling and coworkers treated donor skins ex vivo with low-dose ultraviolet light radiation or chemical carcinogens prior to grafting in mice to reduce dAPC activity [36, 37]. Depletion has also been done using anti-cell antibodies and genetically engineered knock-out mice defective in select populations of APCs [16–18]. Moreover, skin grafts live longer when lymphatic drainage is temporarily impeded [2, 38, 39].
In this paper we present evidence supporting a local strategy that builds upon SAPs to facilitate stringent allogeneic skin transplantation. The goal is to target unique cell-surface molecules on migratory donor immune cells. The design of the membrane is such that IgG would be bound via the Fc domain thereby precluding from activating FcγRs on leukocytes. But we recognize that dissociated IgG may elicit such cytotoxic mechanisms and eliminate dAPCs either in graft bed or in lymphatics. On the other hand, a fraction of the dAPCs would have their MHC-II blocked by the antibodies, thus inhibiting signal 1 in recognition by allospecific T cells. The data presented herein demonstrate the utility of an anti-MHC-II material system in mitigating skin allograft rejection. The anti-dAPCs membrane was shown to form in situ, in both normal skins and on transplant graft bed. The membranes remained in place for 6 days, exceeding the peak (5 days) egression of dAPCs from ear skins in vitro [12]. Taken together, the data show that anti-MHC-II membrane is stable at host-graft interface and can attenuate allogeneic T cell responses by neutralizing dAPCs in vivo.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grant AI081218, a C.U.R.E. award from the Pennsylvania Department of Health, and the Hunkele Dreaded Disease Fund (all to W.S.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burd A, Chiu T. Allogenic skin in the treatment of burns. Clin Dermatol. 2005;23:376–87. doi: 10.1016/j.clindermatol.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Benichou G, Yamada Y, Yun SH, Lin C, Fray M, Tocco G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3:757–70. doi: 10.2217/imt.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.First MR, Fitzsimmons WE. New drugs to improve transplant outcomes. Transplantation. 2004;77:S88–92. doi: 10.1097/01.tp.0000126934.97815.2e. [DOI] [PubMed] [Google Scholar]
- 4.Barshes NR, Goodpastor SE, Goss JA. Pharmacologic immunosuppression. Front Biosci. 2004;9:411–20. doi: 10.2741/1249. [DOI] [PubMed] [Google Scholar]
- 5.Paczek L, Pawlowska M, Krawczyk M, Rowinski W. New concepts in organ transplantation. Transplant Proc. 2004;36:1232–4. doi: 10.1016/j.transproceed.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 6.Kagan RJ, Robb EC, Plessinger RT. Human skin banking. Clin Lab Med. 2005;25:587–605. doi: 10.1016/j.cll.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Moscicka M, Olszewski WL, Zolich D. The effect of cyclosporine and tacrolimus on indigenous bacterial flora in human skin grafts. Transplant Proc. 2003;35:2361–2. doi: 10.1016/s0041-1345(03)00802-9. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–58. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- 9.Cumberbatch M, Fielding I, Kimber I. Epidermal Langerhans cell migration: signals and mechanisms. Adv Exp Med Biol. 1995;378:173–5. doi: 10.1007/978-1-4615-1971-3_38. [DOI] [PubMed] [Google Scholar]
- 10.Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, et al. Migration of langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66:462–70. [PubMed] [Google Scholar]
- 11.Richters CD, van Gelderop E, du Pont JS, Hoekstra MJ, Kreis RW, Kamperdijk EW. Migration of dendritic cells to the draining lymph node after allogeneic or congeneic rat skin transplantation. Transplantation. 1999;67:828–32. doi: 10.1097/00007890-199903270-00008. [DOI] [PubMed] [Google Scholar]
- 12.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–93. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung JJ, Zeevi A, Kaufman C, Paradis IL, Dauber JH, Hardesty RL, et al. Interactions between bronchoalveolar lymphocytes and macrophages in heart-lung transplant recipients. Hum Immunol. 1985;14:287–94. doi: 10.1016/0198-8859(85)90236-8. [DOI] [PubMed] [Google Scholar]
- 15.Sekine Y, Bowen LK, Heidler KM, Van Rooijen N, Brown JW, Cummings OW, et al. Role of passenger leukocytes in allograft rejection: effect of depletion of donor alveolar macrophages on the local production of TNF-alpha, T helper 1/T helper 2 cytokines, IgG subclasses, and pathology in a rat model of lung transplantation. J Immunol. 1997;159:4084–93. [PubMed] [Google Scholar]
- 16.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984;81:3864–8. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–7. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidler KM, Baker K, Woods K, Schnizlein-Bick C, Cummings OW, Sidner R, et al. Instillation of allogeneic lung antigen-presenting cells deficient in expression of major histocompatibility complex class I or II antigens have differential effects on local cellular and humoral immunity and on pathology in recipient murine lungs. Am J Respir Cell Mol Biol. 2000;23:499–505. doi: 10.1165/ajrcmb.23.4.4172. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Wen Y, George AM, Steinbach AM, Phillips BE, Giannoukakis N, et al. A peptide-based material platform for displaying antibodies to engage T cells. Biomaterials. 2011;32:249–57. doi: 10.1016/j.biomaterials.2010.08.083. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 21.Wen Y, Roudebush SL, Buckholtz GA, Goehring TR, Giannoukakis N, Gawalt ES, et al. Coassembly of amphiphilic peptide EAK16-II with histidinylated analogues and implications for functionalization of β-sheet fibrils in vivo. Biomaterials. 2014;35:5196–205. doi: 10.1016/j.biomaterials.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Kolonich HR, Kruszewski KM, Giannoukakis N, Gawalt ES, Meng WS. Retaining antibodies in tumors with a self-assembling injectable system. Mol Pharm. 2013;10:1035–44. doi: 10.1021/mp300504z. [DOI] [PubMed] [Google Scholar]
- 23.Garrod KR, Cahalan MD. Murine skin transplantation. J Vis Exp: JoVE. 2008 doi: 10.3791/634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel SK, Zhang Y, Pollock JA, Janjic JM. Cyclooxgenase-2 inhibiting perfluoropoly (ethylene glycol) ether theranostic nanoemulsions-in vitro study. PLoS One. 2013;8:e55802. doi: 10.1371/journal.pone.0055802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janjic JM, Patel SK, Patrick MJ, Pollock JA, DiVito E, Cascio M. Suppressing inflammation from inside out with novel NIR visible perfluorocarbon nanotheranostics. SPIE BiOS: Int Soc Opt and Photonics. 2013:85960L-L-12. [Google Scholar]
- 26.Patel SK, Williams J, Janjic JM. Cell Labeling for 19F MRI: New and Improved Approach to Perfluorocarbon Nanoemulsion Design. Biosensors. 2013;3:341–59. doi: 10.3390/bios3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Hanlon CE, Amede KG, O’Hear MR, Janjic JM. NIR-labeled perfluoropolyether nanoemulsions for drug delivery and imaging. J Fluor Chem. 2012;137:27–33. doi: 10.1016/j.jfluchem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SK, Patrick MJ, Pollock JA, Janjic JM. Two-color fluorescent (near-infrared and visible) triphasic perfluorocarbon nanoemuslions. J Biomed Opt. 2013;18:101312. doi: 10.1117/1.JBO.18.10.101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janjic JM, Srinivas M, Kadayakkara DK, Ahrens ET. Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J Am Chem Soc. 2008;130:2832–41. doi: 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]
- 30.Janjic JM, Shao P, Zhang S, Yang X, Patel SK, Bai M. Perfluorocarbon nanoemulsions with fluorescent, colloidal and magnetic properties. Biomaterials. 2014;35:4958–68. doi: 10.1016/j.biomaterials.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vries-van der Zwan A, Besseling AC, van der Pol MA, de Waal LP, Boog CJ. Specific tolerance induction and organ transplantation. Leuk Lymphoma. 1998;31:131–42. doi: 10.3109/10428199809057593. [DOI] [PubMed] [Google Scholar]
- 32.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billingham R, Krohn P, Medawar P. Effect of locally applied cortisone acetate on survival of skin homografts in rabbits. BMJ. 1951;2:1049. doi: 10.1136/bmj.2.4739.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai CS, Wesseler TA, Alexander JW, Babcock GF. Long-term survival of skin allografts in rats treated with topical cyclosporine. Transplantation. 1987;44:83–7. doi: 10.1097/00007890-198707000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Zhao XF, Alexander JW, Schroeder T, Babcock GF. The synergistic effect of low-dose cyclosporine and fluocinolone acetonide on the survival of rat allogeneic skin grafts. Transplantation. 1988;46:490–2. doi: 10.1097/00007890-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Odling KA, Halliday GM, Muller HK. Effects of low or high doses of short wavelength ultraviolet light (UVB) on Langerhans cells and skin allograft survival. Immunol Cell Biol. 1987;65(Pt 4):337–43. doi: 10.1038/icb.1987.38. [DOI] [PubMed] [Google Scholar]
- 37.Odling KA, Halliday GM, Muller HK. Enhanced survival of skin grafts depleted of Langerhans’ cells by treatment with dimethylbenzanthracene. Immunology. 1987;62:379–85. [PMC free article] [PubMed] [Google Scholar]
- 38.Barker CF, Billingham RE. The role of regional lymphatics in the skin homograft response. Transplantation. 1967;5(Suppl):962–6. doi: 10.1097/00007890-196707001-00026. [DOI] [PubMed] [Google Scholar]
- 39.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


