Abstract
Background
Filaggrin is an epidermal protein that has a role in skin barrier function. Filaggrin loss-of-function FLG-LOF) mutations are a significant risk factor for eczema and atopy, but their association with food allergy (FA) is less clear.
Objective
We explored the longitudinal relationship between three common FLG-LOF mutations and FA using the Isle of Wight birth cohort.
Methods
FA diagnosis was based on recognised allergic reactions within 4 hours following exposure to known food allergens. Food allergen sensitization (FAS) was identified by skin prick test conducted between 1–18 years to a range of food allergens. Three filaggrin mutations were genotyped in 1150/1456 children (79%). The temporal relationships between FA, FAS and eczema in children with filaggrin mutations were explored using path analysis with total, direct and indirect effect models.
Results
There was a significant total effect of FLG-LOF mutations on the risk of FA in later childhood at ages 10 (OR: 31.46, 95%CI 2.86, >100) and 18 years (OR: 4.25, 95%CI 1.55, 11.61). Path analysis showed that there was no direct effect of FLG-LOF mutations on FA at any age, however an indirect effect was found on FA at all ages via eczema and FAS in the earlier years
Conclusion
FLG-LOF mutations are associated with FA in older children via eczema and FAS in their early childhood. Our results highlight a biologically plausible pathway, which suggests that skin barrier function is important in the development and persistence of FA.
Keywords: Food Allergy, Filaggrin, FLG-LOF, Food allergen sensitization, Path analysis, Prediction, Eczema
Introduction
The filaggrin (FLG) gene encodes a key epidermal protein (filament-aggregating protein), which plays a crucial role in maintaining the integrity of the skin (1–3). Loss-of-function mutations within the filaggrin gene (FLG-LOF) lead to reduced protein expression resulting in epidermal barrier dysfunction, making the skin more permeable to environmental allergens and increasing trans-epidermal water loss (4–6). The role of FLG-LOF variants in eczema has re-kindled the interest in the role of skin barrier dysfunction in the development of allergies.
According to the hypothesis proposed by Lack et al. (7, 8), exposure to food allergens by an oral route leads to tolerance, whereas cutaneous exposure leads to allergy. Animal studies have shown that allergen sensitization can occur via the cutaneous route via antigen presenting cells in the epidermis (9, 10). This occurs especially in the FLG-deficient state, and sensitization may be an important precursor to food and respiratory allergies. FLG-LOF mutations have been identified as a risk factor for allergic sensitization, atopic eczema and allergic rhinitis and asthma (only in the context of eczema), but their impact on food allergy has not yet been widely explored (11–15). To date, only one study has shown a significant association between FLG-LOF mutations and food allergy, which was limited to peanut allergy(16). Further studies are needed to corroborate the strength and consistency of the association and investigate this relationship with other types of food allergies.
We have previously investigated the time order sequence between FLG-LOF mutation, eczema and allergic sensitization (17), using the Isle of Wight (IOW) cohort, which showed that a combination of FLG-LOF and allergic sensitization in early life increases the risk of eczema in subsequent years (17). However, there have been no longitudinal studies exploring the interplay and time order relationships between FLG-LOF mutations, food allergy (FA), food allergen sensitization (FAS), and eczema. This information is vital in understanding the development of food allergies and to guide the evolution of strategies to restore skin barrier and prevent the development of sensitization and food allergies.
Methods
The Isle of Wight (IOW) birth cohort is an un-selected, whole population birth cohort established in 1989, and has been followed up prospectively for 18 years, with the aim of studying the natural history of allergic diseases and the influence of genetic and environmental factors on the development and progression of allergies (17–20). The study was approved by the local research ethics committee (06/Q1701/34). All children (n=1536) consecutively born on the Isle of Wight, UK between 1 January 1989 and 28 February 1990 were enrolled in the study and 1456 were available for further follow-up. The children were assessed at 1 year (n=1374, 94.4%), 2 (n=1231, 84.5%), 4 (n=1218, 83.7%), 10 (n=1373, 94.3%), and 18 years (n=1313, 90.2%).
Diagnostic criteria for food allergy
Definition of FA
We applied an a priori definition of food allergy based on the following criteria (Study Criteria):
A reaction to a recognised food allergen as defined by the European Union(21) and the Committee on toxicity of chemicals in foods, consumer products and the environment(22) (e.g. Cow’s Milk, Hen’s Egg, wheat, Soya, Peanuts, Other nuts, Fish, and Shell Fish).
-
The report of recognised allergic symptoms(23) such as :
localised symptoms: itching, sting/ burning of the lips/ mouth or throat, urticaria/ hives, angioedema
abdominal: nausea, vomiting, crampy/ colicky abdominal pain, diarrhoea
respiratory: wheeze, stridor, watery rhinitis, redness of eyes/ nose
skin: urticaria, itching, flushed skin, worsening eczema
systemic reaction: anaphylaxis
Temporal relationship: symptoms developing within 4 hours of food ingestion.
If criteria 1, 2, and 3 were fulfilled, children were designated as having a FA. Children with FA were further stratified based on the SPT results to the defined food allergen into subgroups: (FA+SPT Positive/ FA +SPT Negative, and FA + SPT Not available).
Eczema
Eczema was diagnosed based on the Hanifin and Rajka (24) criteria: itchy dermatitis lasting more than 6 weeks with characteristic morphology and distribution.
Food allergen sensitization (FAS
The skin prick test (SPT) was performed using standardised method and extracts (Alk-Abello, Horsholm, Denmark), towards a panel of common aero-allergens and food allergens. Food allergens included cows’ milk, hen’s egg, wheat, soya, cod and peanut. Food allergen sensitization (FAS) was defined as a positive reaction to one or more food allergens with a mean wheal diameter being ≥ 3mm greater than the negative control at 15 minutes. SPT was performed at 1 and 2 years in symptomatic children only and at 4, 10, and 18 years in all consenting participants.
Filaggrin genotyping
The FLG gene status was determined following extraction of DNA from the peripheral blood or saliva samples. Five polymorphisms (R501X, 2282del4, S3247X, 3702delG, and R2447X) leading to loss-of-function (LOF), prevalent in European populations, were genotyped as previously described (17). Children were classified as having FLG-LOF defect if they carry the minor allele for at least one of the 3 following FLG null variants: R501X, 2282del4, or S3247X.
Statistical analysis
SPSS (Version 19, IBM, USA) was used to prepare frequency tables and assess the prevalence of FA and FAS at each time point (1, 2, 4, 10 and 18 years). Significance of changes in FA prevalence rates over time (1, 4, 10 and 18 years) was tested using McNemar’s test for paired data and χ2/Fishers exact test for independent data. Path analysis (25) was used to explore the pathways leading to the development of FA in filaggrin-deficient individuals. We assessed the structure among multiple variables including allergic phenotypes such as eczema and FAS and decomposed the effects into total, direct, indirect effects (Mplus version 6) (26). In addition, we conducted separate pathways for FA and FAS. The detection of direct effects indicates the impact of a risk factor on an outcome that is not mediated by other variables. In contrast, indirect associations depicted the effect of a risk factor (X) on an outcome variable (Z) via an intervening variable (Y) such that X → Y → Z. The total effect of a risk factor is the combination of direct and indirect statistical relationships.
Results
The study population of the IOW birth cohort was dynamic, as many children participated at varying stages of the study, and not all children were seen at each time point (1, 2, 4, 10 and 18 years). Appendix 1(online repository) graphically depicts the availability of information regarding FA, FAS, and eczema at various stages of the study from 1 to 18 years. Since the 1 year and 2 year follow-up data on eczema and food allergy were collected in a relatively small time window, we have combined them for analytic purposes (Eczema 1 &2 years, FA 1&2 years).
Filaggrin Gene Analysis
The FLG genotype was determined in 1150 children (79%) of the cohort at 18 years. There were no significant differences between the characteristics (sex, eczema status and FA status) of the whole population and the genotyped population (Appendix 2, online repository). The overall FLG-LOF mutation frequency was 10.3% as reported previously (17).
Food allergy and FLG-LOF mutations
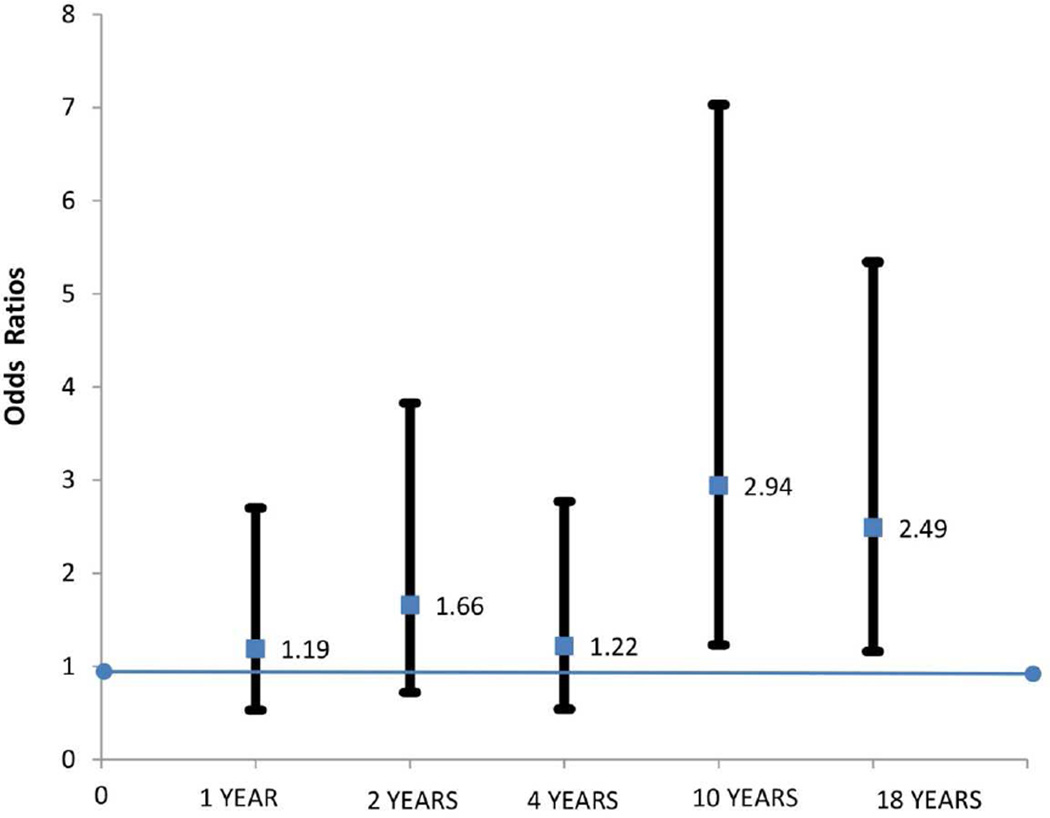
We used logistic regression analysis in the initial assessment of relationship between FLG-LOF mutations and FA at 5 time points. We found a significant association between FLG-LOF mutations and FA in the whole population IOW cohort at 10 years (OR: 2.9, 95%CI (1.2, 7.0), Fishers Exact p= 0.022) and 18 years (OR: 2.5, 95%CI (1.2, 5.3) FE p=0.032) Figure 1) but not at 1, 2 and 4 years.
Figure 1.
Odds of developing food allergy in children with FLG-LOF mutation at 1, 2, 4, 10 and 18 years of age. A significant association seen between FLG-LOF mutation and FA at ages 10 and 18 years (logistic regression)
Table 1 provides information on the prevalence of FA, FAS and eczema between 1–18 years. The longitudinal trend in prevalence of FA in the IOW cohort show relatively constant prevalence rates in early childhood, (5.3%, 4.4% and 5% at 1, 2, and 4 years, respectively), with a significant decline at 10 years (2.3%, p<0.001), followed by a significant rise at 18 years (4.1%, p=0.02) (Table 1). The association between FLG-LOF mutations and FA corresponds to these points of significant change in FA prevalence. No significant associations were seen in the earlier years. A significant increase in FAS was also seen at 18 years.
Table 1.
Prevalence of Food Allergy, Food allergen sensitisation and Eczema in the study population over 18 years
| Age | FA based on Study Criteria %, (95% CI) |
Prevalence of Food Allergen Sensitisation (based on SPT) %, (95% CI) |
Prevalence of Eczema %, (95% CI) |
|---|---|---|---|
| 1 year | 5.3 (4.2–6.7)% | ** | 11.7 (9.9–13.5)% |
| 2 years | 4.4 (3.4–5.7)% | ** | 19.0(16.7–21.2)% |
| 4 years | 5.0 (3.9–6.4)% | 3.2 (2.1–4.3)% | 12.1(10.3–13.9)% |
| 10 years | 2.3 (1.7–3.3)%* (p<0.001, Significant drop in prevalence) |
4.5 (3–5.4)% | 13.7(11.9–15.5)% |
| 18 years | 4.1(3.2–5.4)%* (p=0.024, Significant increase in prevalence) |
21.4(18.6–24.2)% * (p<0.001, Significant increase in sensitization) | 12.1(10.3–13.9)% |
SPT at 1 and 2 years limited to symptomatic children
Path analysis
We used path analysis (total, direct and indirect effects) to explore if the association between FLG-LOF mutations and FA was a direct effect, or it was an indirect effect secondary to the occurrence of eczema, or FAS. The relationship between FLG-LOF and FAS was not explored at 1 &2 years of age as only symptomatic children underwent skin prick testing and FAS data was not available in the whole cohort.
FLG-LOF, eczema, FAS, and FA pathways
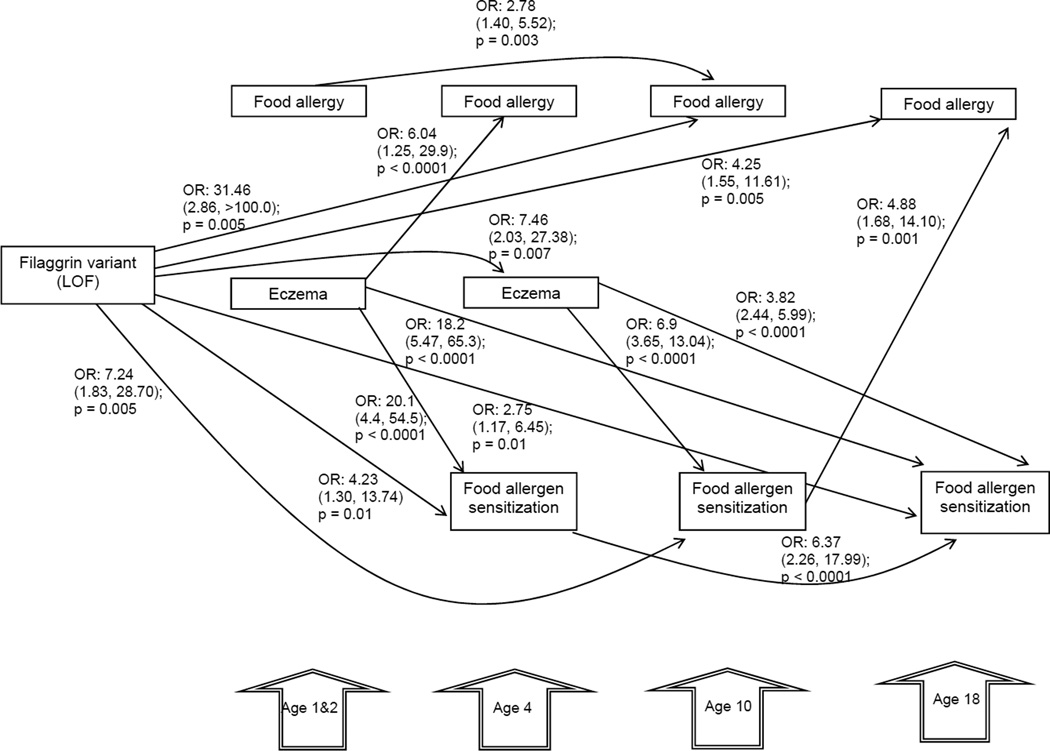
We found a significant total effect of FLG-LOF on FA at ages 10 years (OR: 31.46, 95% CI (2.86, >100) p = 0.005, and 18 years (OR: 4.25, 95% CI (1.55, 11.61), p = 0.005); after adjusting for gender (Figure 2). This significant association was not seen in the earlier years. We also found significant associations between FLG-LOF and FAS at 4 years (OR 4.23, 95% CI (1.3, 13.74) p=0.01); 10 years (OR 7.24, 95% CI (1.83, 28.7) p=0.005) and 18 years (OR 2.75, 95% CI (1.17, 6.45), p=0.01).
Figure 2.
Analytical path model exploring the total effects of FLG-LOF, eczema at ages 1&2 and 4 years on food allergy (FA) and food allergy sensitization (FAS) at 4, 10, and 18 years of age. The path coefficient (total effects) represents the Odds Ratios. Goodness of fit adjusted for degrees of freedom: 0.99; comparative fit index: 0.99; root mean square error of approximation: ≤ 0.06; deviation between covariance structure and the empirical covariance: Chi-squared/degree of freedom ≤2; p<0.05. Only significant direct paths are shown. The relationship between FLG-LOF and FAS was not explored at 1 &2 years of age as only symptomatic children underwent skin prick testing and FAS data not available in whole cohort.
Eczema at 1&2 years of age was associated with a total effect on FA at age 4 (OR 6.04, 95% CI (1.25, 29.9), p<0.0001) and FAS at ages 4 (OR 20.1, 95% CI (4.40, 54.50), p<0.0001) and 18 years (OR 18.2, 95% CI (5.47, 65.3), p<0.0001). In addition, eczema at age 4 was linked to FAS at ages 10 (OR 6.9, 95% CI (3.65, 13.04), p<0.0001) and 18 years (OR 3.82, 95% CI (2.44, 5.99), p<0.0001).
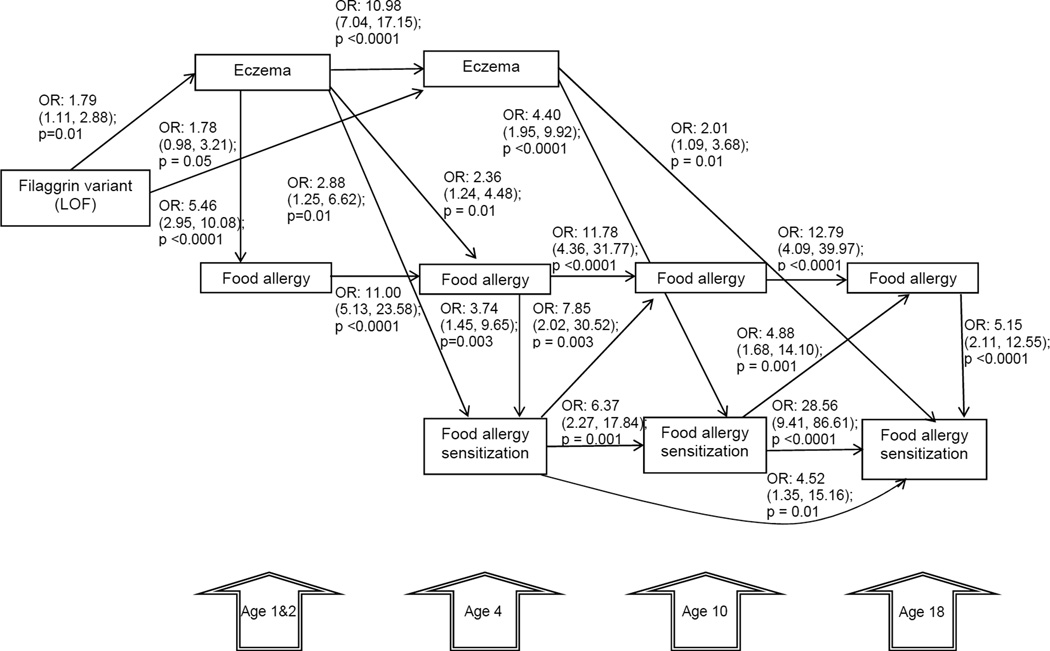
No direct effect of FLG-LOF on FA or FAS was found across all ages (Figure 3). A direct association was found between FLG-LOF with eczema at 1&2 years of age (OR 1.79, 95% CI (1.11, 2.88); p = 0.01) and 4 years (OR 1.78, 95% CI (0.98, 3.21); p= 0.05). Eczema at 1–2 was directly associated with food allergy at 1&2 years (OR 5.46, 95% CI (2.95, 10.08); p<0.0001) and 4 year (OR 2.36, 95% CI (1.24, 4.48); p=0.01) and FAS at age 4 years (OR: 2.88, 95% CI (1.25, 6.62); p=0.01) in FLG-LOF variant individuals. Eczema at 4 years was directly associated with FAS at 10 years (OR 4.40, 95%CI (1.95, 9.92); p<0.0001,) and 18 years (OR: 2.01, 95% CI 1.09, 3.68) p=0.01) in FLG-LOF variant individuals. Also, we found a direct effect of FAS at age 4 years on FA at age 10 years (OR 7.85, 95% CI (2.02, 30.52); p = 0.003). FAS at age 10 years was associated with FA at age 18 years (OR 4.88. 95% CI (1.68, 14.10); p = 0.001).
Figure 3.
Analytical path model exploring the direct effects of FLG-LOF, eczema at ages 1& 2 and 4 years on food allergy (FA) and food allergy sensitization (FAS) at 4, 10, and 18 years of age. The path coefficient (direct effects) represents the Odds Ratios. Goodness of fit adjusted for degrees of freedom: 0.99; comparative fit index: 0.99; root mean square error of approximation: ≤ 0.06; deviation between covariance structure and the empirical covariance: Chi-squared/degree of freedom ≤2; p<0.05. Only significant direct paths are shown. The relationship between FLG-LOF and FAS was not explored at 1 &2 years of age as only symptomatic children underwent skin prick testing and FAS data not available in whole cohort.
Early eczema in individuals with a FLG-LOF variant had an indirect effect on FAS and FA (Table 2). There was an indirect effect of FLG-LOF mutations through eczema at 1–2 years of age on FA at 1&2 years (OR 2.81, 95% CI (1.15, 6.86); p=0.02), FA at 4 years (OR 15.48, 95% CI (1.32, >100); p=0.02) and on FAS at 4 years (OR 2.18, 95% CI (0.99, 4.76); p=0.05). Further, FLG-LOF variants had an indirect effect on FA at age 10 (OR: 10.0; p = 0.03, CI 1.14, 87.0) through the occurrence of eczema at 1–2 years of age and FA at age 4 years. FLG-LOF mutation had an indirect effect via eczema at 4 years on FAS at 10 years (OR 4.49, 95% CI (1.44, 13.99); p=0.01) and 18 years (OR 2.38, 95%CI (1.19, 4.74); p=0.01), and FA at 18 years (OR: 21.93; 95% CI (1.50, >100); p = 0.02).
Table 2.
Significant results from indirect pathway analysis
| FLG status | 1 & 2 years | 4 years | 10 years | 18 years | ORa (95% CI) |
P value |
|---|---|---|---|---|---|---|
| FLG LOF → | Eczema → Food allergyb |
2.81 (1.15, 6.86) |
0.02 | |||
| FLG LOF → | Eczema → | Food allergyb | 15.48 (1.32, >100) |
0.02 | ||
| FLG LOF → | Eczema → |
Food sensitizationb |
2.18 (0.99, 4.76) |
0.05 | ||
| FLG LOF → | Eczema → | Food allergy → | Food allergyb | 10.0 (1.14, 87.0) |
0.03 | |
| FLG LOF → | → | Eczema → |
Food sensitizationb |
4.49 (1.44, 13.99) |
0.01 | |
| FLG LOF → | → | Eczema → | → |
Food sensitizationb |
2.38 (1.19, 4.74) |
0.01 |
| FLG LOF → | → | Eczema → | Food sensitization → | Food allergyb | 21.9 (1.50, >100.0) |
0.02 |
Only significant indirect paths are shown. Other models of the relationship between FLG-LOF mutations, eczema, food sensitization and food allergy were tested but did not have significant outcome.
OR represents the indirect association of FLG LOF on food allergy and food sensitization at different ages.
The outcomes of the indirect paths are in bold.
Discussion
This is the first study to explore the relationship between FLG-LOF mutations and the longitudinal trends in FA. Our study showed that FLG-LOF mutations were associated with FA and FAS at 10 and 18 years (total effects). Further exploration via path analysis suggested an association between FLG-LOF and eczema in younger children and the progression to FAS and FA in older children.
Filaggrin and food allergy
This is the first study to associate FLG-LOF with all causes of FA rather than with a specific food allergen. Brown SJ et al (16) described an association of FLG-LOF and peanut allergy in three different populations. Their study supports a relationship of FLG-LOF with peanut FA, but does not consider other types of FA. Our study demonstrated a significant association (total effect via path analysis) between FA and FLG-LOF mutations in older children and young adults (i.e., 10 and 18 years), but not during the earlier years. FA in early childhood are often due to egg and milk allergy, which tends to improve, while in older children and young adults, peanut and sea food allergies become more prevalent and tend to persist (Appendix 3, online repository). Our findings therefore further confirm the association of FLG-LOF with more persistent forms of food allergy
FLG-LOF Pathway analysis
The two most common pathways detected in this study are the following: (1) FLG-LOF mutations→Eczema→Food sensitization; (2) FLG-LOF mutations→Eczema→Food allergy. We found that FAS has a direct relationship with food allergy and the complex interplay between eczema and FAS in the pathway increases the odds of food allergies significantly in later life in filaggrin deficient individuals (FLG-LOF mutations→Eczema→Food allergen sensitization→Food allergy). These are biologically plausible pathways for a relationship between FLG-LOF and FA and are in keeping with current hypotheses on causality (7). This suggests that two different mechanistic pathways may be sequentially involved in the pathogenesis of FA: a barrier dysfunction caused by FLG-LOF and a barrier defect with associated inflammation caused by eczema leading to subsequent sensitization and immune response, all increasing the odds of developing FA at different time points (Fig 3, 4, and 5).
These results suggest a role for FLG-LOF mutations in the development of FA. The barrier defect associated with FLG deficient individuals makes them susceptible to development of cutaneous sensitization via antigen presenting cells and systemic atopic response (27–29). Peanut sensitization may occur via the topical application of peanut oil to the skin (8), and cutaneous exposure via presence of peanut allergen in the environment (30), and this process may be enhanced by a deficient barrier caused by FLG-LOF mutations or eczema (8). Animal studies also suggest that allergic sensitization can occur in the absence of cutaneous inflammation / eczema via the filaggrin deficient skin (10, 31).
Marenholz et al (11) reported that associations between FLG-LOF mutations and allergic sensitization/asthma were significant only in the presence of eczema. This supports the notion that presence of both inflammation and a defective barrier in eczema lead to the transcutaneous exposure to allergens and their subsequent sensitization and development of allergic disease. Further work by Marenholz et al (11) showed that in the presence of eczema and food sensitization, FLG-LOF mutation strongly predicted the development of childhood asthma, suggesting synergistic interaction between FLG-LOF variants and food sensitization, leading to the transition of allergic phenotype from eczema to asthma. These studies suggest that eczema plays an early and significant role in the progression of allergic phenotypes involved in the allergic march.
Ziyab et al (17), undertook a temporal sequence analysis to ascertain the time order sequence between eczema and allergic sensitization (both food and aero-allergens) with respect to FLG-LOF mutations in the IOW cohort. They found that FLG-LOF mutations and eczema increased the risk of subsequent allergic sensitization only in the first 10 years of life. Our study has looked into the complex interactions specifically looking at FAS and FA, and the results show that the risk of food sensitization is increased beyond 10 years via eczema in FLG-LOF mutations. FLG-LOF mutations has an indirect effect on FAS and FA in later childhood via the occurrence of eczema in earlier years, and this needs to be further explored by other longitudinal studies.
Flohr et al examined the relationship between FLG-LOF, atopic dermatitis, trans-epidermal water loss and food allergic sensitization in infancy (32). Their study found that children with eczema are more likely to be sensitized to food allergens independently of FLG-LOF status, severity of eczema and trans-epidermal water loss. They did not show a relationship between FLG-LOF and food sensitization, but instead severity of eczema was associated with FAS. Our study also showed a strong association between eczema (1&2 years, 4 years) and FAS at 4, 10 and 18 years, and lack of relationship between FLG-LOF and FA in the early years. The association between FLG-LOF and FA only becomes apparent in later childhood and adolescence. The effect of FLG-LOF may be important in the maintenance of food allergy but may play less of a role in FA or FAS events in infancy, where atopic dermatitis may adversely affect the skin barrier and contribute to allergic sensitisation and later development of FA in FLG deficient children
In summary, FLG-LOF mutations have an indirect effect on FA via eczema and FAS in the pathway. Our findings have discerned a relationship between FLG-LOF, eczema, FAS and FA. They provide further insight into the role of the skin barrier in the pathogenesis of FA.
Strengths and limitations
All consecutive children born in 1989 were enrolled into this birth cohort and there was no selection bias at recruitment. Data were gathered prospectively and the overall follow-up participation rates were high throughout the study period (84–94.3%), which ruled out a major bias due to loss-to-follow-up. The prevalence of eczema and allergic sensitization did not differ between the genotyped and full cohort data, supporting generalizability of the study findings. A unique aspect of our study is the repeated longitudinal assessment of individuals throughout childhood, where each child acts as their own control.
The Isle of Wight birth cohort is dynamic; some children did not participate at one time point, but re-joined at another. For this reason comparisons were made between two time points, where FA information on an individual was available at both time points (1–2, 2–4, 4–10, 10–18 years). We further explored this in a stable cohort where FA data was available at all five time points (stable cohort, Appendix 4, online repository) and our findings were similar.
In our study, strict symptom-based criteria were used to diagnose FA, as is common in a clinical setting. This method of diagnosis of FA is superior to FA surveys used in other population studies, but certainly not as accurate as studies using the ‘gold standard’ of double blind food challenges.
The measurement of SPT was performed at key stages, allowing FAS to be treated as a time-dependant covariate. The proportion of children that underwent SPT were 67.4%, 71.1% and 58.5% at 4, 10 and 18 years, respectively, which were much lower than the proportion of children that were followed-up at the same time periods. This reduced the availability of food sensitization data. We also only had information on SPT’s in symptomatic children at age 1 and 2, which limited the assessment of the relationship between allergic sensitization and food allergy in the early stages of childhood.
Conclusions
Our study is the first to investigate the relationship between FLG-LOF mutations, food allergen sensitisation, and food allergy longitudinally over 18 years (five exams), with each child acting as their own control in this process. We found statistically significant total effects of FLG-LOF mutations on FA at ages 10 and 18 years but not in early childhood, suggesting that FLG-LOF mutations may be associated with more persistent forms of FA in older children and young adults. We further explored this association via path-analysis by investigating effects of eczema and FAS in this pathway. FLG-LOF mutations had an indirect effect on FA in childhood by the occurrence of eczema and FAS in earlier years.
Impaired skin barrier function as a result of both eczema and FLG-LOF seems to be a crucial common factor in the pathogenesis of food allergy and improvement in the barrier function of skin in early childhood may influence the further development of allergy. This study has helped improve the understanding of pathways leading to FA which will help to develop targeted preventive and disease modifying strategies.
Supplementary Material
Clinical Implications/ Key messages.
Our study demonstrates the complex interactions between eczema, food sensitization and food allergy over time and the direct and indirect pathways predisposing to food allergy in individuals with filaggrin gene (FLG) variants. The associations (total effect) between FLG-loss of function (FLG-LOF) mutations and food allergy are stronger at 10 and 18 years than at earlier ages, suggesting that FLG-LOF mutations may be associated with more persistent forms of childhood food allergy. Pathway analysis showed significant and consistent relationship between filaggrin-loss of function, eczema and allergic sensitization to food in early years and food allergy in later childhood. This pathway provides a biologically plausible mechanism for the role of FLG in FA.
Acknowledgments
We would like to acknowledge the help of all the staff at The David Hide Asthma and Allergy Research Centre in undertaking the assessments of 1989 Isle of Wight birth cohort. We would also like to acknowledge the help of the participants and their families who helped us with this project over the last two decades.
Funding: This study was funded by the National Institutes of Health project grants R01-HL082925, R01-AI061471and R01-AI091905. The 10-year follow-up of this study was funded by National Asthma Campaign, UK (Grant No 364)
Abbreviations used
- CI
Confidence Interval
- FA
Food Allergy
- FAS
Food Allergen Sensitization
- FLG
Filaggrin
- FLG-LOF
Filaggrin Loss-of-function
- IOW
Isle of Wight
- SPSS
Statistical product and service solutions
- SPT
Skin Prick Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MEL generated the original hypothesis and all authors contributed to study design. SHA and RJK were responsible for all allergy phenotype data collection and SLE for genetic data collection. WK, SHA and JWH advised on analysis and interpretation of the genetic data. DV collated, analysed and interpreted the food allergy data and NSR analysed the path analysis. DV wrote the first draft of the manuscript, and all authors have seen and approved the final version of the report. MEL and SHA will serve as guarantors for its contents.
Conflict of interest statement
None of the authors have any conflicts of interests to declare.
References
- 1.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. J Invest Dermatol. Vol. 129. United States; 2009. Epidermal barrier dysfunction in atopic dermatitis; pp. 1892–1908. [DOI] [PubMed] [Google Scholar]
- 2.Rawlings AV, Harding CR. Dermatol Ther. Suppl 1. Vol. 17. Denmark: 2004. Moisturization and skin barrier function; pp. 43–48. [DOI] [PubMed] [Google Scholar]
- 3.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Nat Genet. Vol. 38. United States; 2006. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis; pp. 441–446. [DOI] [PubMed] [Google Scholar]
- 4.Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Nat Genet. Vol. 38. United States; 2006. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris; pp. 337–342. [DOI] [PubMed] [Google Scholar]
- 5.Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, et al. J Invest Dermatol. Vol. 128. United States; 2008. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum; pp. 2117–2119. [DOI] [PubMed] [Google Scholar]
- 6.Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. 2010;163(6):1333–1336. doi: 10.1111/j.1365-2133.2010.10068.x. [DOI] [PubMed] [Google Scholar]
- 7.Lack G. J Allergy Clin Immunol. Vol. 121. United States; 2008. Epidemiologic risks for food allergy; pp. 1331–1336. [DOI] [PubMed] [Google Scholar]
- 8.Lack G, Fox D, Northstone K, Golding J. N Engl J Med. Vol. 348. United States: Massachusetts Medical Society; 2003. Factors associated with the development of peanut allergy in childhood; pp. 977–985. 2003. [DOI] [PubMed] [Google Scholar]
- 9.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004;34(8):2100–2109. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 10.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41(5):602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marenholz I, Kerscher T, Bauerfeind A, Esparza-Gordillo J, Nickel R, Keil T, et al. An interaction between filaggrin mutations and early food sensitization improves the prediction of childhood asthma. J Allergy Clin Immunol. 2009;123(4):911–916. doi: 10.1016/j.jaci.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Weidinger S, O'Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, et al. J Allergy Clin Immunol. Vol. 121. United States; 2008. Filaggrin mutations, atopic eczema, hay fever, and asthma in children; pp. 1203–1209. e1. [DOI] [PubMed] [Google Scholar]
- 13.van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. doi: 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisgaard H, Simpson A, Palmer CN, Bonnelykke K, McLean I, Mukhopadhyay S, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5(6):e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuttelaar ML, Kerkhof M, Jonkman MF, Koppelman GH, Brunekreef B, de Jongste JC, et al. Allergy. Vol. 64. Denmark: 2009. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure; pp. 1758–1765. [DOI] [PubMed] [Google Scholar]
- 16.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of- function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127(3):661–667. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziyab AH, Karmaus W, Yousefi M, Ewart S, Schauberger E, Holloway JW, et al. PLoS One. Vol. 7. United States; 2012. Interplay of Filaggrin Loss-of-Function Variants, Allergic Sensitization, and Eczema in a Longitudinal Study Covering Infancy to 18 Years of Age; p. e32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108(2):E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 19.Roberts G, Zhang H, Karmaus W, Raza A, Scott M, Matthews S, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clin Exp Allergy. 2012;42(10):1501–1509. doi: 10.1111/j.1365-2222.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 20.Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. J Allergy Clin Immunol. Vol. 116. United States; 2005. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers; pp. 884–892. [DOI] [PubMed] [Google Scholar]
- 21.Food Allergen Labelling Food Standards agency. 2008 website: Crown Copyright; http://www.food.gov.uk/safereating/allergyintol/label/groups:[
- 22.HF W, PJ A. Committee on toxicity of chemicals in foods, consumer products and the environment: Allergic reactions to food and food ingredients. Crwon Publications. 2000 [Google Scholar]
- 23.Niggemann B. Allergy. Vol. 65. Denmark: 2010. When is an oral food challenge positive? pp. 2–6. [DOI] [PubMed] [Google Scholar]
- 24.Hanifin JMRG. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–47. [Google Scholar]
- 25.Lleras C. Encyclopedia of social measurement. Vol. 3. Pennysylvania: Elsevier; 2005. Path Analysis. [Google Scholar]
- 26.Muthen LKMB. Mplus User's guide. 7th edition 1998–2012. [Google Scholar]
- 27.TJ H. Skin Barrier function and allergic risk. Nat Genet. 2006:399–400. doi: 10.1038/ng0406-399. [DOI] [PubMed] [Google Scholar]
- 28.Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9(5):437–46. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanifin JM. J Invest Dermatol. Vol. 129. United States; 2009. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas; pp. 320–322. [DOI] [PubMed] [Google Scholar]
- 30.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. J Allergy Clin Immunol. Vol. 123. United States; 2009. Household peanut consumption as a risk factor for the development of peanut allergy; pp. 417–423. [DOI] [PubMed] [Google Scholar]
- 31.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17- dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124(3):485–93. doi: 10.1016/j.jaci.2009.05.042. 93 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flohr C, Perkin M, Logan K, Marrs T, Radulovic S, Campbell LE, et al. Atopic Dermatitis and Disease Severity Are the Main Risk Factors for Food Sensitization in Exclusively Breastfed Infants. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.