Abstract
Background
Patients with severe combined immunodeficiency disease (SCID) who have matched sibling donors (MSD) can proceed to hematopoietic cell transplantation (HCT) without conditioning chemotherapy.
Objective
To determine whether the results of HCT without chemotherapy-based conditioning from matched unrelated donors (URD), either from volunteer adults or umbilical cord blood, are comparable to those from matched sibling donors (MSD).
Methods
A multicenter survey of SCID transplant centers in North America, Europe, and Australia to compile retrospective data on patients who have undergone unconditioned HCT, from either URDs (n = 37) or MSDs (n = 66).
Results
Most patients undergoing URD HCT (92%) achieved donor T-cell engraftment, compared to 97% for MSDs, however, estimated 5-year overall and event-free survival were worse for URD recipients (71% and 60%, respectively), compared to MSD recipients (92% and 89%, respectively; P <0.01 for both). URD recipients who received pre-HCT serotherapy had similar 5-year OS (100%) to MSD recipients. The incidences of Grade II-IV acute and chronic GVHD were higher in URD (50% and 39%, respectively), compared to MSD recipients (22% and 5%, respectively; P <0.01 for both). In the surviving patients, there was no difference in T-cell reconstitution at last follow-up between the URD recipients and MSD recipients, however MSD recipients were more likely to achieve B-cell reconstitution (72% vs. 17%; P <0.001).
Conclusion
Unconditioned URD HCT achieves excellent rates of donor T-cell engraftment similar to MSD recipients, and reconstitution rates are adequate. However, only a minority will develop myeloid and B cell reconstitution and attention must be paid to GVHD prophylaxis. This approach may be safer in children ineligible for intense regimens to spare potential complications of chemotherapy.
Keywords: Severe combined immunodeficiency, hematopoietic cell transplantation, sibling donors, unrelated donors, umbilical cord blood, conditioning, serotherapy
INTRODUCTION
Severe combined immunodeficiency (SCID) diseases are a heterogeneous group of genetic disorders that impair T and B cell function and, when untreated, lead to early death due to recurrent infections. Although gene therapy has had some success in recent years, and enzyme-replacement therapy can restore immunologic function for Adenosine Deaminase (ADA) deficient-SCID, the most successful treatment for SCID has been allogeneic hematopoietic cell transplantation (HCT).1 Due to the absence of functional T cells in patients with typical SCID, graft rejection is rare even when immunoablative conditioning is omitted.2 The major exception to this rule is in NK-positive SCID phenotypes, where NK cells have been shown to be capable of mediating rejection of HLA-mismatched donors unless maternal engraftment is present and the mother is used as the donor3–5.
An HLA-matched sibling donor (MSD) is always the preferred stem cell donor with overall survival rates exceeding 90%, especially when the transplant is performed before opportunistic infections develop.6, 7 MSD transplants are typically performed without any conditioning chemotherapy, because the immune system of a patient with SCID is generally incapable of mediating rejection of the donor stem cells.8 Unfortunately, most patients do not have MSDs, in which case either a haploidentical family member or an HLA-matched unrelated adult donor (URD) or Umbilical Cord Blood (UCB) unit can be utilized as the stem cell source.1, 9 Traditionally, many haploidentical HCTs have been performed without pre-HCT conditioning,3, 6, 10 while the majority of URD or UCB recipients have been conditioned with myeloablative doses of chemotherapy,7, 11, 12 making direct comparisons difficult.9
Given the advances in HLA-typing technology, we hypothesized that a closely matched URD or UCB could approximate the situation of a MSD, and thus be used to successfully transplant patients with SCID without the need for cytotoxic conditioning, thereby sparing the patients the potential risk of early and long-term complications of chemotherapy. However, reports describing URD or UCB HCTs for patients with SCID without the use of conditioning chemotherapy are few with small numbers.13–16 Therefore, to test this hypothesis we have assembled a cohort of 37 patients in North America, Europe, and Australia who have undergone unconditioned URD or UCB HCT for treatment of SCID, and compared their engraftment rates, complications, and immunologic outcomes to a contemporaneous cohort of 66 recipients of unconditioned MSD HCT transplanted at the same centers.
METHODS
Patient Identification
The Primary Immune Deficiency Treatment Consortium (PIDTC) consists of 33 centers in North America with a special interest in patients with primary immunodeficiencies (PID). The Inborn Errors Working Party of the European Blood and Marrow Transplant Society (IEWP-EBMT) consists of over 30 centers with specialty in HCT for patients with PID. A retrospective questionnaire-based analysis of 37 children with SCID who had undergone unrelated donor (adult or UCB) transplant without conditioning other than serotherapy was undertaken via a query of all members of the PIDTC and the IEWP-EBMT. Data from some URD13 and MSD17, 18 recipients have been previously published. URD HCTs were performed between July 1993 and November 2012 in 8 PIDTC and 8 IEWP-EBMT centers, with a median year of HCT of 2007. A contemporaneous control cohort of 66 non-conditioned MSD HCT recipients from the same centers was also identified, albeit with a median HCT year of 2002. When required, participating centers obtained informed consent from the parents prior to HCT to use blinded information for scientific purposes in accordance with the Declaration of Helsinki. Data were gathered and stored anonymously.
Summarized details of presenting characteristics are shown in Table I, while individual details can be found in the supplemental Tables IA (URD recipients) and IIA (MSD recipients). Patients with IL2RG, JAK3, or ADA defects were classified as “NK-negative,” and patients with RAG, DCLRE1C, or LIG4 mutations were classified as “NK-positive,” while actual numbers were used to classify those without a molecular diagnosis, using a threshold of > or <100 × 106/L. Twenty-nine URD recipients had NK-negative forms of SCID, including 21 with mutations of the interleukin receptor common gamma chain (IL2RG), and 7 with either mutations in the Adenosine Deaminase (ADA) gene or undetectable levels of ADA, and 1 undefined mutation. Eight patients had NK-positive forms of SCID, including 2 with RAG2 deficiency, 1 with DCLRE1C deficiency, 1 with LIG4 deficiency, and 3 with undefined mutations. Of the 66 MSD recipients, 48 had NK-negative SCID, including 20 with mutations in IL2RG, 3 with Janus Kinase 3 (JAK3) deficiency, 23 had ADA deficiency, and 2 undefined mutations. Eighteen had NK-positive forms of SCID, including 6 with mutations in a RAG gene, 5 with DCLRE1C mutations, and 7 with undefined mutations. All met the agreed definition of SCID from the PIDTC with very low T cell numbers and absent proliferative response to phytohemagglutinin (PHA).19 Both cohorts had an equal percentage of patients who met criteria for leaky SCID with reduced number of CD3+ T cells for age (>300 but < 1000/microliter up to 2 years), absence of maternal engraftment, and < 30% of lower limit of normal T cell function (as measured by response to phytohemagglutinin (PHA)).20
TABLE I.
Summary of clinical and immunologic characteristics at the time of SCID diagnosis
| Matched Sibling Donor | Unrelated Donor: Adult or Umbilical Cord Blood | |||||||
|---|---|---|---|---|---|---|---|---|
| IL2RG/JAK3 | ADA | Other° | Overall | IL2RG/JAK3 | ADA | Other° | Overall | |
| Genotype (number) | 20/3 (35%) | 23 (35%) | 20 (30%) | 66 | 21/0 (57%) | 7 (19%) | 9 (24%) | 37 |
| Leaky | 0 | 0 | 5 (20%) | 5 (8%) | 2 (10%) | 0 | 1 (11%) | 3 (8%) |
| Days at Diagnosis, median (range) | 130 (0–414) | 34 (0–490) | 108 (0–851) | 82 (0–851) | 184 (0–426) | 30 (11–200) | 109 (2–947) | 121 (0–947) |
| Gender (M/F) | 21/2 | 11/12 | 14/6 | 46/20 | 21/0 | 2/5 | 6/3 | 29/8 |
| Diagnostic Trigger: FH/NBS | 6 (26%) | 2 (9%) | 7 (35%) | 15 (23%) | 4 (19%) | 1 (14%) | 2 (22%) | 7 (19%) |
| Diagnostic Trigger: Infection | 17 (74%) | 21 (91%) | 13 (65%) | 50 (77%) | 17 (81%) | 6 (86%) | 7 (78%) | 30 (81%) |
| Immunologic Data, median (range) | ||||||||
| ALC (× 106/L) | 1610 (486–3102) | 250 (0–1540) | 975 (38–16054) | 759 (0–16054) | 1760 (272–8200) | 200 (60–380) | 1200 (210–3267) | 1100 (60–3267) |
| CD3 (× 106/L) | 15 (0–504) | 12 (0–455) | 112 (0–8076) | 20 (0–8076) | 10 (0–605) | 5 (0–56) | 17 (0–510) | 9 (0–605) |
| CD4 (× 106/L) | 12 (0–82) | 5 (0–134) | 73 (0–2935) | 12 (0–2936) | 2 (0–553) | 5 (0–31) | 0 (0–421) | 2 (0–553)* |
| CD8 (× 106/L) | 0 (0–490) | 5 (0–172) | 31 (0–5079) | 5 (0–5079) | 4 (0–194) | 0 (0–22) | 0 (0–49) | 3 (0–194) |
| B cells (× 106/L) | 1193 (336–3015) | 10 (0–1201) | 23 (0–1440) | NA | 1494 (5–7786) | 2 (0–22) | 20 (0–1486) | NA |
| NK cells (× 106/L) | 24 (0–215) | 52 (0–340) | 586 (5–7848) | NA | 20 (0–415) | 15 (0–88) | 445 (50–1200) | NA |
| PHA (% of control) | 0 (0–11) | 0 (0) | 0 (0–60) | 0 (0–60) | 0 (0–10) | 0 (0) | 0 (0–3) | 0 (0–10) |
| Presence of TME^ | 7/21 (33%) | 1/20 (5%) | 7/17 (41%) | 15/58 (26%) | 2/18 (11%) | 0/7 (0%) | 1/8 (13%) | 3/33 (9%) |
ADA, adenosine deaminase; FH, family history; NBS, newborn screen; ALC, absolute lymphocyte count; NK, Natural Killer; PHA, phytohemagglutinin; TME, transplacental maternal engraftment; NA, not applicable
°See methods for details
P <0.05 compared to sibling donor recipients
Not all patients tested
Two of 7 (29%) URD recipients and 7/23 (30%) MSD recipients received treatment with PEG-ADA before HCT, which was withdrawn 1–8 weeks prior to HCT. The median age at diagnosis for the URD recipients was 121 days (range, 0–947 days), while the MSD recipients were diagnosed at a median of 82 days of age (range, 0–851 days; P = 0.18). A similar percentage of URD recipients (19%) and MSD recipients (23%) were diagnosed by family history or newborn screen, while the others were brought to clinical attention due to the presence of 1 or more opportunistic infections. Whether the inciting infection had resolved by time of HCT was not able to be reliably determined. Transplacentally-transferred maternal T-cells were detected in 9% (3/33) of tested URD recipients and 15/58 (26%) of MSD recipients (P = 0.061). The URD and MSD recipients were very similar in terms of their immunologic data at the time of diagnosis, with the exception that the URD recipients had a statistically lower median number of CD4+ T cells (2 × 106/L; range 0–553 × 106/L) compared to the MSD recipients (12 × 106/L; range 0–2936 × 106/L; P = 0.04).
Transplantation Characteristics
Summarized details of transplantation characteristics are shown in Table II, while individual details can be found in the supplemental Tables IB (URD recipients) and IIB (MSD recipients). The MSD HCTs were almost exclusively done using BM as the stem cell source (97%), while 19 of the URD recipients received cells from an adult donor (16 from bone marrow (BM) and 3 from peripheral blood stem cells (PBSC) that were ex vivo CD34-selected), and an UCB unit was used in 18 patients (P < 0.0001). The median cell doses administered to the URD BM recipients were a TNC of 4.8 × 108/kg (range, 2–10 × 108/kg), CD34+ cells of 6.1 × 106/kg (range, 2.5–20 × 106/kg), and CD3+ cells of 4.5 × 107/kg (range, 3.4–72 × 107/kg). This did not significantly differ from the BM cell doses given to the MSD recipients: TNC of 5.5 × 108/kg (range, 0.7–12.6 × 108/kg), CD34+ cells of 8.4 × 106/kg (range, 0.8–29.1 × 106/kg), and CD3+ cells of 3.7 × 107/kg (range, 0.02–37.1 × 107/kg). URD UCB recipients received a median TNC of 13 × 107/kg (range, 4–26 × 107/kg), with CD34+ cells of 5 × 105/kg (range, 2–17 x105/kg) and CD3+ cells of 2.1 × 107/kg (range, 0.1–9.2 × 107/kg). HLA typing methods ranged over time from evaluating 6 loci (HLA-A,-B,-DR) in 35% of patients to 12 loci (HLA-A,-B, -C, DR, -DQ, -DP) in 16% of patients. All but one of the adult URDs and 14/18 UCB donors were fully matched by the criteria in place at the time of HCT (86%), while 3 of the sibling donors were mismatched at 1 locus, and the remaining 95% were fully matched (P = 0.13).
TABLE II.
Summary of unconditioned HCT characteristics
| Matched Sibling Donor | Unrelated Donor: Adult Umbilical Cord Blood | |||||||
|---|---|---|---|---|---|---|---|---|
| IL2RG/JAK3 | ADA | Other° | Overall | IL2RG/JAK3 | ADA | Other° | Overall | |
| Genotype (number) | 20/3 | 23 | 20 | 66 | 21/0 | 7 | 9 | 37 |
| Days from Diagnosis to HCT, median (range)° | 23 (1–153) | 27 (12–86) | 32 (12–1389) | 25 (1–1389) | 56 (25–281) | 31 (16–40) | 55 (16–111) | 49 (16–281)* |
| Donor Match^ | ||||||||
| “Complete” | 21 (91%) | 23 (100%) | 19 (95%) | 63 (95%) | 17 (81%) | 7 (100%) | 8 (89%) | 32 (86%) |
| ≥1 Known Mismatches | 2 (9%) | 0 | 1 (5%) | 3 (5%) | 4 (19%) | 0 | 1 (11%) | 5 (14%) |
| Stem Cell Source | ||||||||
| Bone Marrow | 21 (91%) | 23 (100%) | 20 (100%) | 64 (97%) | 9 (43%) | 1 (14%) | 6 (67%) | 16 (43%)* |
| Umbilical Cord Blood | 2 (9%) | 0 | 0 | 2 (3%) | 9 (43%) | 6 (86%) | 3 (33%) | 18 (49%)* |
| PBSC (TCD) | 0 | 0 | 0 | 0 | 3 (14%) | 0 | 0 | 3 (8%)* |
| Serotherapy Given | 0 | 0 | 2 (10%) | 2 (3%) | 10 (48%)* | 1 (14%) | 3 (33%) | 14 (38%)* |
| GVHD Prophylaxis Agents# | ||||||||
| Ex Vivo TCD | 4 (17%) | 0 | 1 (5%) | 5 (7%) | 3 (14%) | 0 | 0 | 3 (8%) |
| None | 16 (70%) | 17 (74%) | 6 (30%) | 39 (59%) | 1 (5%)* | 0 | 0 | 1 (3%)* |
| One | 2 (9%) | 6 (26%) | 5 (25%) | 13 (20%) | 3 (14%) | 2 (29%) | 4 (45%) | 9 (24%) |
| Two | 1 (4%) | 0 | 8 (40%) | 9 (14%) | 6 (29%)* | 4 (57%)* | 3 (33%) | 13 (35%)* |
| Three | 0 | 0 | 0 | 0 | 8 (38%)* | 1 (14%) | 2 (22%) | 11 (30%)* |
ADA, adenosine deaminase; PBSC, peripheral blood stem cells; TCD, ex vivo T cell depletion; GVHD, graft versus host disease
P <0.05 compared to sibling donor recipients
°See methods for details
°Excluding patients treated with ADA enzyme replacement therapy prior to HCT
As defined by era of HCT
Including Pre-HCT Serotherapy
The only conditioning utilized prior to HCT was serotherapy in the form of anti-thymocyte globulin (ATG) or alemtuzumab in 14/37 URD recipients (38%) and 2/66 MSD recipients (3%; P <0.0001), though this was typically administered for its ability to serve as prophylaxis against graft-versus-host disease (GVHD).21 Because no cytotoxic chemotherapy was utilized for any of these HCTs, we designate them as being ‘non-conditioned.’ Other pharmacologic GVHD prophylaxis regimens varied, and ranged from none, to a single calcineurin inhibitor (CI), such as cyclosporine (CSA) or tacrolimus, alone, to 2 agents (CI plus either methotrexate, corticosteroids, or mycophenolate mofetil (MMF)), to 3 agents. The majority (65%) of URD recipients received 2–3 agents, compared to only 14% of sibling HCTs (P <0.0001), while only 1 URD HCT was done without any GVHD prophylaxis (3%) compared to 59% of sibling donor HCTs (P <0.001). Acute and chronic GVHD were graded by the local investigators.
Chimerism and Immune Reconstitution
Chimerism was measured in peripheral blood mononuclear cells (PBMCs), T cells (CD3+), B cells (CD19+ or CD20+), and/or myeloid cells (CD15+ or CD33+). Donor T cell engraftment was defined as the detection of any amount of donor DNA in either whole blood or separated CD3+ cells. Given variations in detection limits of the various chimerism labs, 5% was set as the threshold amount of myeloid, or B cell chimerism required to be considered clinically relevant. NK cell chimerism was not evaluated. Immune reconstitution of T cell (CD3, CD4, CD8), naïve T cell (CD4/CD45RA), B cell, and NK cell numbers, quantitative immunoglobulins, and T-cell function (via proliferative response to PHA) were measured at approximately 1 year post-HCT and at most recent follow-up. Clinical freedom from gammaglobulin replacement therapy was utilized as a surrogate for B cell function. An effectively normal immune system was defined as a CD4 count >400 × 106/L,22 PHA >50% control, and freedom from gammaglobulin replacement.
Statistical Analysis
Descriptive statistics were calculated as median and range for quantitative variables or frequencies and percentiles for categorical variables. Comparisons between groups were done using the nonparametric Mann-Whitney test for quantitative variables and the Fisher’s exact test for categorical variables. Kaplan-Meier curves were used to analyze survival rates, with the log-rank test used to compare survival between different groups. Cumulative incidence functions considering death from any cause or the need for a conditioned second HCT as competing events were used to estimate other end points. Transplant-related mortality (TRM) was defined as any death from a cause (e.g. an infection) not present at the time of cell infusion. Age < 3.5 months was analyzed for effect of survival due to previous data that this is a biologically relevant time-point.6 Analyses were done using NCSS8 (Kaysville, Utah). Significant differences are defined as a P value ≤0.05.
RESULTS
Excluding patients who received ADA enzyme replacement therapy prior to HCT, patients were treated with HCT from a URD at a median of 49 days (range, 16–281 days) from diagnosis, with a median of 62 days for an adult URD compared to 42 days for a UCB unit (P = 0.08). This was significantly longer than the interval between diagnosis and HCT when a MSD was available, which took a median of only 25 days (range, 1–1389 days; P <0.001). Similarly, the median age at time of URD HCT was older at 182 days of life (range, 40–996 days), compared with a median of 132 days of life (range, 1–2240 days) at time of MSD HCT (P = 0.03).
Engraftment
All but one URD recipient (who died 9 days after HCT from ongoing infectious respiratory failure) and 1 MSD control (who died 16 days after HCT from CMV pneumonitis) survived at least 1 month following unconditioned HCT and were therefore considered evaluable for engraftment, need for second cell infusion, and GVHD. As shown in Table III, initial donor T cell engraftment was seen in 97% of MSD recipients, and 94% of URD recipients (P = 0.61). Of the URD recipients, the NK-negative patients universally engrafted donor T cells (28/28), compared to 75% (6/8) of NK-positive patients (P = 0.04).
TABLE III.
Clinical outcomes following unconditioned HCT
| Matched Sibling Donor | Unrelated Donor: Adult or Umbilical Cord Blood | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | NK-Negative | NK-Positive | Overall | NK-Negative | NK-Positive | No Serotherapy | With Serotherapy | |
| Donor T cell Engraftment^ | 63/65 (97%) | 45/46 (98%) | 18/19 (95%) | 34/36 (94%) | 28/28 (100%) | 6/8 (75%) | 22/22 (100%) | 12/14 (86%) |
| 2nd HCT Required^ | 10/65 (15%) | 5/46 (11%) | 5/19 (26%) | 7/36 (19%) | 4/28 (14%) | 3/8 (38%) | 3/23 (13%) | 4/14 (28%) |
| Unconditioned Boost | 6/65 (9%) | 3/46 (7%) | 3/19 (16%) | 3/36 (8%) | 3/28 (11%) | 0/8 (0%) | 2/23 (9%) | 1/14 (7%) |
| Conditioned | 4/65 (6%) | 2/46 (4%) | 2/19 (11%) | 4/36 (11%) | 1/28 (4%) | 3/8 (38%)° | 1/23 (4%) | 3/14 (21%) |
| TRM^ | 2/65 (3%) | 2/46 (4%) | 0/19 (0%) | 6/36 (17%)* | 3/28 (11%) | 3/8 (38%) | 6/22 (27%) | 0/14 (0%) |
| Acute GVHD^ | ||||||||
| Any Grade | 21/63 (33%) | 20/46 (43%) | 1/17 (6%)° | 22/34 (65%)* | 18/28 (64%) | 4/6 (67%) | 16/22 (73%) | 6/12 (50%) |
| Grade II–IV | 14/63 (22%) | 13/46 (28%) | 1/17 (6%) | 17/34 (50%)* | 14/28 (50%) | 3/6 (50%) | 14/22 (64%) | 3/12 (25%) |
| Grade III–IV | 3/63 (5%) | 3/46 (7%) | 0/17 (0%) | 8/34 (24%)* | 6/28 (21%) | 2/6 (33%) | 7/22 (32%) | 1/12 (8%) |
| Chronic GVHD^ | 3/62 (5%) | 2/45 (4%) | 1/17 (6%) | 11/28 (39%)* | 8/24 (33%) | 3/4 (75%) | 8/17 (47%) | 3/11 (27%) |
| Alive at last follow-up | 60/66 (91%) | 43/47 (92%) | 17/19 (89%) | 27/37 (73%)* | 22/29 (76%) | 5/8 (63%) | 13/23 (56%) | 14/14 (100%) |
| Alive without conditioning | 56/66 (85%) | 41/47 (87%) | 15/19 (79%) | 23/37 (62%)* | 21/29 (72%) | 2/8 (25%)° | 12/23 (52%) | 11/14 (79%) |
NK, Natural Killer; TRM, Transplant Related Mortality; GVHD, graft versus host disease
Of evaluable patients (see results)
P <0.05 compared to sibling donor recipients
°P <0.05 compared to NK-Negative phenotypes
A similar number of MSD recipients (15%) and URD recipients (19%) ultimately required additional infusions of donor cells to treat inadequate immune reconstitution (P = 0.78). This was done as a non-conditioned boost in 9% of MSD recipients and 8% of URD recipients (P = 1), while fully-conditioned second HCTs were eventually performed in 6% of MSD recipients and 11% of URD recipients (P = 0.45). Conditioned second HCTs were more common in NK-positive forms of SCID undergoing URD HCT (38% vs. 4% for NK-negative; P = 0.04). The use of serotherapy may have been associated with a higher need for second cell infusion (28% vs. 13% for no serotherapy) but did not reach statistical significance (P = 0.39).
Chimerism was not uniformly evaluated, though the majority of tested MSD recipients (86%, 38/44) and URD recipients (100%, 15/15) had high level (>90%) donor CD3+ engraftment (P = 0.32) (Table IV). At the time of last follow-up, B cell or myeloid chimerism >5% was detected in 48% of the MSD recipients, compared to 50% of the URD recipients (P = 1), with no significant differences between molecular subtypes. Patients who underwent conditioned second HCT were excluded from this analysis.
TABLE IV.
Summary of survivor’s immune reconstitution 1 year and at last follow-up following unconditioned HCT
| Matched Sibling Donor | Unrelated Donor: Adult or Umbilical Cord Blood | |||||||
|---|---|---|---|---|---|---|---|---|
| IL2RG/JAK3 | ADA | Other° | Overall | IL2RG/JAK3 | ADA | Other° | Overall | |
| # Evaluable; 1-year from HCT (+/− 1 mo) | 19 | 21 | 19 | 59 | 16 | 5 | 3 | 24 |
| CD3 (× 106/L) | 3428 (1751–9287) | 1008 (364–2500) | 744 (27–5698) | 1764 (27–9287) | 2153 (66–4673)* | 572 (540–2144) | 1414 (421–3233) | 1767 (66–4673) |
| CD4 (× 106/L) | 1766 (921–2653) | 405 (132–1330) | 330 (17–3773) | 585 (17–3773) | 1241 (21–3027) | 293 (272–530) | 853 (308–2221) | 692 (21–3027) |
| CD4/CD45RA (%) | 33 (17–78) | 14 (3–22) | 8 (1–82) | 21 (1–78) | 57 (9–94) | 55 (8–80) | 26 (19–32) | 55 (8–94) |
| CD8 (× 106/L) | 1180 (444–6302) | 370 (194–1114) | 298 (8–6646) | 764 (8–6646) | 770 (45–2350) | 207 (192–1503) | 541 (189–857) | 550 (45–2350) |
| B Cells (× 106/L) | 1200 (30–2571) | 50 (9–333) | 5 (0–1155) | NA | 280 (0–3602) | 25 (22–30) | 150 (11–333) | NA |
| NK Cells (× 106/L) | 142 (47–392) | 40 (0–602) | 260 (125–804) | NA | 52 (0–297) | 43 (10–78) | 251 (184–291) | NA |
| PHA (%) | 79 (8–100) | 76 (58–100) | 48 (16–100) | 59 (8–100) | 100 (69–100) | 86 (72–100) | 85 (85) | 100 (69–100) |
| Off gammaglobulin | 13 (68%) | 16 (76%) | 8 (47%) | 37 (63%) | 1 (6%) | 1 (20%) | 0 (0%) | 2 (8%)* |
| # Evaluable; At last follow-up | 20 | 20 | 17 | 57 | 16 | 5 | 2 | 23 |
| Time to last fu (years) | 5.5 (1–14.4) | 6.3 (2–17.1) | 7.5 (1.6–18.9) | 6.9 (1–18.9) | 4.3 (1–19) | 2.5 (1.1–12.3) | 2.8 (1.6–3.9) | 3.9 (1–19) |
| CD3 (× 106/L) | 2230 (1190–9287) | 1720 (578–4440) | 1000 (230–2606) | 1814 (230–9287) | 1864 (315–4210) | 1328 (593–6017) | 6091 (383–10790)* | 1574 (315–10790) |
| CD4 (× 106/L) | 1302 (458–2653) | 1065 (298–3330) | 432 (140–1640) | 944 (140–3330) | 943 (144–2330) | 506 (272–1105) | 3623 (247–6999)* | 840 (144–6999) |
| CD4/CD45RA (%) | 60 (17–80) | 13 (2–17) | 11 (3–71) | 19 (2–80) | 43 (4–94) | 8 (4–41) | 26 (0–51) | 35 (0–94) |
| CD8 (× 106/L) | 982 (468–6302) | 757 (220–2040) | 313 (140–1026) | 772 (220–2040) | 575 (80–1853) | 613 (199–4851) | 2007 (570–3443) | 580 (80–4851) |
| B Cells (× 106/L) | 460 (347–1879) | 180 (36–750) | 24 (0–395) | NA | 399 (0–3602) | 47 (30–137) | 1017 (338–1696) | NA |
| NK Cells (× 106/L) | 85 (0–392) | 131 (0–1025) | 115 (37–524) | NA | 29 (0–194) | 98 (10–363) | 306 (274–338) | NA |
| PHA (%) | 100 (66–100) | 100 (46–100) | 86 (47–100) | 100 (46–100) | 100 (65–100) | 72 (72) | 85 (85) | 100 (65–100) |
| Off gammaglobulin | 16 (80%) | 18 (90%) | 10 (59%) | 44 (77%) | 4 (25%) | 2 (40%) | 1 (50%) | 7 (30%)* |
| Donor Myeloid >5% | 7/16 (44%) | 8/15 (53%) | 7/15 (47%) | 22/46 (48%) | 6/13 (46%) | 2/4 (50%) | 1/1 (100%) | 9/18 (50%) |
P <0.05 compared to sibling donor recipients
°See methods for details
GVHD
As shown in Table III, despite the greater number of GVHD prophylaxis agents used in the URD recipients, the incidence of any grade acute GVHD was significantly higher: 22/34 (65%) evaluable URD recipients compared to 21/63 (33%) sibling donor recipients (P = 0.005). Grade II–IV aGVHD was seen in 17/34 (50%) URD recipients compared to only 14/63 (22%) sibling donor recipients (P < 0.01), while Grade III–IV aGVHD was seen in 8/34 (24%) URD recipients compared to only 3/63 (5%) sibling recipients (P = 0.02). For both the MSD recipients and the URD recipients, the year of HCT, the presence of known HLA mismatches, or the number of agents used for GVHD prophylaxis did not predict subsequent aGVHD (Supplementary Table III). However, the use of serotherapy in the URD recipients demonstrated a trend towards being protective from Grades II–IV aGVHD, with an incidence of 64% (14/22) in those not receiving serotherapy, compared to 25% in those given serotherapy (3/12; P = 0.07). For the URD recipients, there was no difference in the incidence of Grade II–IV aGVHD between recipients of BM (43%) vs. other stem cell sources (55%; P = 0.73), nor an impact of cell dose administered (data not shown). URD recipients with any grade aGVHD trended to have an increased incidence of developing B cell or myeloid chimerism (64%), including 2 patients with 100% donor chimerism in all cell lines, compared to those patients who never developed any aGVHD (14%; P = 0.07), likely due to a graft-vs.-marrow effect. This association of GVHD and donor chimerism was not seen in the MSD recipients, possibly due to a lower degree of alloreactivity and incidence of aGVHD.
Chronic GVHD was rare in the evaluable MSD patients (5%), and was significantly higher in the URD recipients (33%; P <0.001). For the URD recipients, there was no difference in the incidence of cGVHD between recipients of BM (38%) vs. UCB (38%) vs. PBSC (50%; P = 1), nor an impact of cell dose administered (data not shown). The use of serotherapy in URD recipients resulted in a lower incidence of cGVHD (27% vs. 47% in those not given serotherapy), however this did not reach statistical significance (P = 0.43).
Survival
Causes of death are listed in Supplemental Tables Ib and IIb. For the MSD recipients, this included progression of pre-existing problems/infections (n=4), probable sepsis (n=1), and GVHD (n=1). For the URD recipients, this included progression of pre-existing problems/infections (n=4), intraoperative complication (n=1), or GVHD (n=5), for a TRM of 3% in the MSD recipients compared to 17% in the URD recipients (P = 0.02). Possibly because most TRM was related to GVHD, in the URD recipients the use of serotherapy trended towards being protective from TRM (0%) compared to those that did not receive serotherapy (27%; P = 0.06), though this may also be due to preferential selection of serotherapy in patients without infection at time of HCT.
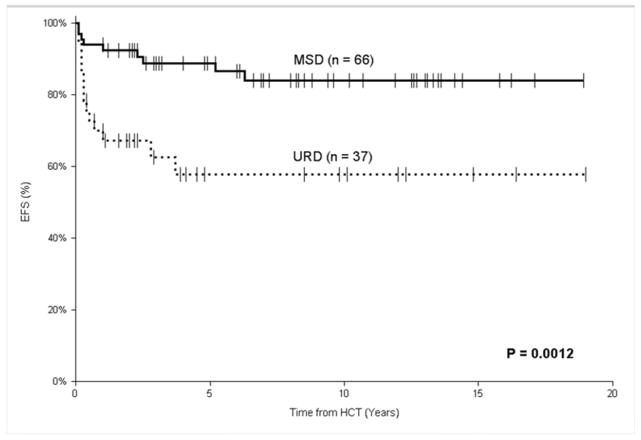
As shown in Figure I, of the MSD recipients, 57 patients are alive (median follow-up of 6.9 years; range, 1–18.9 years) without a conditioned second HCT, for an estimated 5-year EFS of 89% (95% CI, 81–97%). This was significantly better than the URD recipients, where only 23 patients are alive (median follow-up of 3.9 years; range 1–19 years) without a conditioned second HCT, for an estimated 5-year EFS of 60% (95% CI, 44–77%; P < 0.01). In the MSD recipients, 3 of 4 patients who underwent conditioned 2nd transplants were long-term survivors, as were all 4 URD recipients. Therefore, the estimated 5-year OS for the MSD recipients was 92% (95% CI, 85–98%), significantly better than for the URD recipients whose estimated 5-year OS was 71% (95% CI, 56–87%; P < 0.01). Amongst recipients of the same donor source, there was not a significant difference in OS between those with NK-negative and NK-positive SCID (Table III).
FIGURE I.
Event-free survival after unconditioned HCT for SCID comparing matched sibling donors (MSD) to unrelated donors (URD), with death and conditioned second HCT as events.
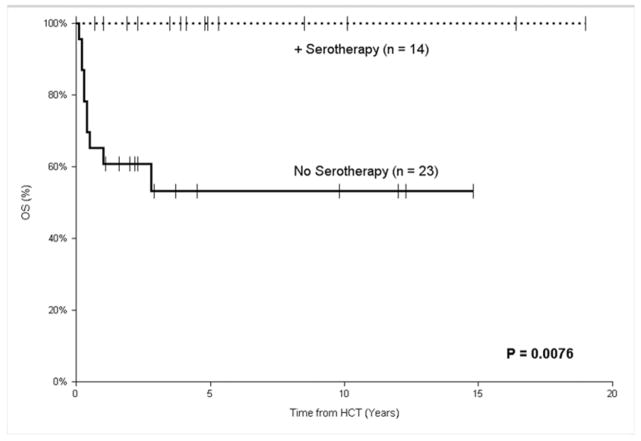
In the URD recipients, the use of serotherapy resulted in an estimated 5-year EFS of 71% (95% CI, 42–100%) compared to those not receiving serotherapy (38%; 95% CI, 13–62%), but this did not reach statistical significance (P = 0.18), likely because there was a slight trend towards more patients in the serotherapy group requiring a conditioned second transplant (21% vs. 4%; P = 0.14). However, because all re-transplanted patients survived, the use of serotherapy was associated with a significantly superior estimated 5-year OS of 100% (95% CI, 75–100%) compared to those patients that did not receive serotherapy (51%; 95% CI, 25–77%; P < 0.01; Figure II). Those receiving a URD HCT before 3.5 months of age had similar outcomes (5-year OS of 69%; 95% CI 39–99%; n = 12) compared to those transplanted after 3.5 months of age (5-year OS of 72%; 95% CI 54–90%; n = 25; P = 0.75), as did those diagnosed in the absence of infection (5-year OS of 75%; 95% CI 33–100%; n = 7) compared to those transplanted after an infection (5-year OS of 70%; 95% CI 54–86%; n = 30; P = 0.38). URD HCTs performed in the early period (1993 – 2007) had an estimated 3-year OS of 65% (95% CI, 42–87%), compared to 80% (95% CI, 62–98%) for those done more recently (P = 0.42). There was no difference in 5-year OS depending on the cell source of the unrelated donors.
FIGURE II.
Overall survival after unconditioned HCT for SCID using an unrelated donor comparing the use of pre-HCT serotherapy for GVHD prophylaxis to no serotherapy.
Immune Reconstitution
As shown in Table IV, T cell reconstitution was similar between the survivors of unconditioned HCT from a MSD and an URD. The median CD3 count at 1-year post-HCT was 1764 × 106/L (range, 27–9287 × 106/L) from a MSD compared to 1767 × 106/L (range 66–4673 × 106/L) from a URD (P = 0.55), and this lack of difference persisted at time of last follow-up (P = 0.57). Subgroup analysis demonstrated that IL2RG-defective patients had higher CD3 counts at 1-year post-HCT from a MSD compared to a URD (P < 0.01), though at time of last follow-up, the difference was less evident (P = 0.07). Similarly, there was no difference in the median CD4 count at either 1-year post-HCT (P = 0.58) or time of last follow-up (P = 0.74). At time of last follow-up, 4% (2/57) of MSD recipients have very poor CD4 reconstitution (<200 × 106/L) compared to 4% (1/23) URD recipients (P = 1), while 12% (7/57) of MSD recipients and 26% (6/23) of URD recipients have borderline (between 200–400 × 106/L) CD4 counts (P = 0.18). Naïve CD4/CD45RA counts were not always measured, but there was no apparent difference in the median percentage at the time of last follow-up between those undergoing MSD control HCT (19%; range, 2–80%) and URD recipients (35%; range, 0–94%; P = 0.51).
Despite the lack of difference in the percentage of patients with detectable myeloid or B cell chimerism between the MSD recipients and the URD recipients, there were significantly more MSD recipients with clinical freedom from gammaglobulin replacement at both 1-year post-HCT (63% vs. 8%; P <0.001) and the time of last follow-up (77% vs. 30%; P < 0.001). The URD recipients only achieved gammaglobulin replacement independence in the setting of donor myeloid or B cell chimerism, whereas these outcomes were not linked in the MSD recipients, possibly due to incomplete testing of lineage-specific chimerism in these patients (19% had neither myeloid or B cell chimerism tested) or testing methods with limited sensitivity for microchimerism. Patients with NK− forms of SCID undergoing MSD HCT were more likely to recover B cell function (85%; 35/41) compared to those with NK+ forms of SCID (56%; 9/16; P = 0.03), while there were too few NK+ patients undergoing URD HCT to compare. The URD stem cell source utilized did not impact on gammaglobulin independence (25% for non-UCB vs. 36% for UCB; P = 0.67)
An effectively normal immune system was seen in significantly more MSD recipients (72%; 41/57) compared to URD recipients (26%; 6/23) who survived without a conditioned second HCT (P < 0.001).
DISCUSSION
The data presented here represent the largest cohort of patients to be reported with SCID undergoing URD (including UCB) HCT without conditioning chemotherapy. Because most patients who undergo URD HCT do receive chemotherapy-based conditioning 23, the majority of this retrospective cohort is likely composed of a selected group of patients who were deemed too “sick” to undergo chemotherapy. Despite this selection bias, the majority of patients engrafted donor T cells (94%) and subsequently survived (5-year OS 71%), although some patients were not able to reverse their pre-HCT problems/infections in time. This survival rate is comparable to what has been reported in large multi-center trials for URD HCT (primarily with conditioning), with the IEWP-EBMT reporting a 10-year OS of 66%,10 and the PIDTC soon to report a 5-year OS of 64% (Pai S-Y, personal communication). Furthermore, T cell immune reconstitution in survivors of unconditioned URD HCTs was generally adequate and comparable to that seen in recipients of unconditioned MSD HCTs. We have therefore demonstrated that chemotherapy prior to URD (or UCB) HCT is not always required for patients with SCID due to deficiencies in IL2RG, ADA, and possibly even some NK+ genotypes, as long as the primary goal is donor T-cell reconstitution. Up to 20% of recipients of unconditioned HCTs require repeat HCTs, either as unconditioned boosts or with conditioning chemotherapy. While this is a limitation of this approach, the need for repeat HCTs has also been reported after conditioned HCTs.7
However, important differences remain between URD and MSD HCT, especially with respect to the high rates of GVHD seen after URD HCT, which contributed to 5 of the 6 deaths not related to progression of a pre-HCT problem. Of note, in many URD recipients, the development of GVHD was linked with the achievement of at least modest amounts of donor myeloid or B cell chimerism, likely from a direct immunologic attack on the recipients’ bone marrow.24, 25 While likely of ultimate benefit to the patient as it correlated with freedom from gammaglobulin replacement, this “graft-vs.-marrow” effect is not a reliable or safe method to achieve 100% donor chimerism. The high rate of GVHD seen in this cohort is likely multifactorial, as lower number of GVHD prophylaxis agents administered, the stem cell source, and the presence of known HLA-mismatches were not predictive for developing GVHD, though the failure to use pre-HCT serotherapy trended towards increased GVHD. We did find that the use of ATG was associated with an improved overall survival in the URD recipients. While this may be a direct protective effect from excessive alloreactivity, it is also possible that the use of serotherapy was simply a surrogate marker for “healthier” patients whose infections were under control at the time of HCT, as many clinicians avoid using serotherapy in an actively infected patient due to concerns of slower T cell recovery.
It should be noted that 43% of patients either received a known HLA-mismatched donor or underwent URD HCT in an era when HLA-typing was more limited than is currently employed, and that future analyses, confined to only to those with 10/10 (or 12/12) HLA allele-matched donors might demonstrate lower rates of GVHD. One potential method to decrease these rates of GVHD might be to utilize more aggressive in vivo T-cell depletion with alemtuzumab,26 though this could delay post-HCT T cell recovery27 and both URD recipients in this cohort who received alemtuzumab eventually underwent a fully-conditioned second HCT. Another possibility would be to utilize a relatively less-toxic pre-HCT chemotherapeutic agent, such as the monoclonal antibody rituximab or the non-alkylating fludarabine, with the goal of eliminating antigen-presenting host B cells that might contribute to the development of subsequent GVHD.28, 29 Furthermore, it should be noted that the majority of patients in this cohort underwent URD HCT after being diagnosed with one or more opportunistic infections, and it has been shown that the presence of certain infections (especially CMV30 and HHV-631) following HCT is linked to the subsequent development of GVHD, presumably via the release of inflammatory cytokines. Therefore, it is possible that this high rate of GVHD may become less of a problem as more patients are identified via newborn screening and proceed to well-matched HCT in the absence of a severe opportunistic infection. However, if indeed B cell recovery is linked to a graft-vs.-marrow effect, efforts to improve GVHD prophylaxis may subsequently result in decreased rates of complete T & B cell immunologic recovery.
In addition to GVHD and subsequent TRM leading to poorer survival for the URD recipients, the other major difference with survivors of unconditioned MSD HCT was the relative lack of freedom from clinical gammaglobulin replacement. Most surviving MSD recipients (77%) were able to stop gammaglobulin and 72% were considered to have “complete” immune reconstitution (to the limits of this retrospective analysis) with normal CD4+ T-cell counts and clinical gammaglobulin independence. Conversely, only 30% of surviving URD recipients were off gammaglobulin and only 26% had “complete” T and B cell reconstitution, though this degree of B cell recovery from an unconditioned URD HCT is similar to what has been reported for IL2RG-deficient patients undergoing non-conditioned haploidentical HCT (34%, also tightly linked to donor B cell engraftment).32 In the URD recipients, B cell recovery was tightly linked to myeloid engraftment, however, this was not the case for the MSD recipients, despite the lack of an agent to produce ‘space’ in the bone marrow niches. The exact mechanism behind this interesting dichotomy remains to be elucidated.
For patients with non-SCID primary immunodeficiencies, all of whom undergo pre-HCT conditioning chemotherapy, advances in HLA-typing during a relatively modern era of 2000–2005 have allowed URD HCT to produce equivalent outcomes to MSDs.10 However, for patients with SCID, the outcomes were worse for URD HCTs compared to MSDs.10 Some of this may be attributable to the delay in time from SCID diagnosis (typically via the presence of a severe opportunistic infection) to the time of HCT that is required for the unrelated donor search process (a median of 24 additional days for this cohort), during which time the patient’s infection may worsen. However another factor may potentially be the use of conditioning chemotherapy, which is used far more frequently in the recipients of URD HCT compared to MSD HCT, and appears in our cohort to be unnecessary to successfully achieve donor T-cell engraftment. Data from the PIDTC demonstrates that for patients with SCID transplanted from 2000–2009, the use of chemotherapy-based conditioning was associated with inferior survival when an active infection was present at the time of HCT (Pai S-Y, personal communication). Although advances in individualized drug dosing and other supportive care techniques have made the use of conditioning chemotherapy safer, there is still a finite risk of fatal end-organ toxicity, such as liver sinusoidal obstruction syndrome,33 which does not occur in non-conditioned HCTs. The only TRM in the URD recipients of this cohort resulted from GVHD or an unusual iatrogenic intra-operative complication. This implies that improved GVHD prophylaxis strategies could eventually enable non-conditioned HCT to become safer than a conditioned HCT.
This study is limited by the retrospective nature of the cohort, so it is possible that a positive selection bias was introduced by a lack of participation from centers that have performed unconditioned URD/UCB HCTs and found poor outcomes, though the majority of surveyed centers replied that they had no eligible patients to contribute to this cohort. Furthermore, we do not have data on the kinetics of T cell reconstitution, nor on some newer methods of monitoring the robustness of T cell reconstitution post-HCT, such as T cell receptor Vβ family diversity by spectratyping or T cell receptor excision circles, and it is possible that these may identify immune defects in the URD recipients not present in the MSD recipients. Finally, we did not directly compare the clinical outcomes and immune reconstitution in SCID patients undergoing URD HCT with or without pre-HCT conditioning because this was not the objective of this study and we believe that a significant selection bias may exist, with “healthier” patients allowed to receive conditioning chemotherapy and “sicker” patients given an unconditioned URD/UCB HCT as a last-ditch effort to salvage the patient.34 This selection bias can only be eliminated via either a randomized prospective clinical trial, or potentially by prospective analysis of sufficient numbers of uninfected patients diagnosed via family history or newborn screen, as the PIDTC is attempting in North America.23
In conclusion, our data demonstrate that in patients with SCID lacking MSDs, but in whom a well-matched adult URD or UCB can be located in a timely fashion, HCT can proceed without the use of conditioning chemotherapy in a fashion akin to common practice with a MSD, especially in the typical (non-leaky) NK-negative subtypes included in this cohort (i.e. mutations in IL2RG and ADA). If successful, such an approach would spare patients the potential short-term toxicity and long-term sequelae of conditioning chemotherapy. However, this approach is currently fraught with high rates of acute and chronic GVHD, so careful consideration of the optimal GVHD prophylaxis regimen (based on the severity of ongoing opportunistic infections) is required. In addition, while this approach can produce adequate long-term donor T-cell reconstitution, it has a much lower success rate of achieving normal B cell function (30%) than when conditioning chemotherapy is utilized (roughly 85%).8 Therefore, novel methods to improve donor myeloid and B cell engraftment are still needed, such as antibody-based targeted therapy of hematopoietic stem cells.35, 36 In those rare patients in which T-cell reconstitution is insufficient or those patients in which normal B cell function is desired, a subsequent conditioned HCT can still be considered in the future after the patient has recovered from their opportunistic infections and/or organ function has improved and matured.37
Supplementary Material
Clinical Implications.
Certain subtypes of SCID can develop donor T cell (and occasionally B cell) reconstitution following unrelated donor hematopoietic cell transplant without conditioning chemotherapy, thereby potentially sparing short and long-term toxicities.
Acknowledgments
Funding:
Data collection for this study was in-part facilitated through the CIBMTR (U24-CA76518; PI Horowitz MM) and the PIDTC Annual Scientific Workshop (R13 AI094943). The PIDTC is a part of the NIH Rare Diseases Clinical Research Network, with the DMCC at the University of South Florida. Funding and/or programmatic support for this project has been provided by Grant #1U54AI082973 from National Institute of Allergy and Infectious Diseases and the NIH Office of Rare Diseases Research, National Center for Advancing Translational Science. The views expressed are those of the authors and do not represent the position of the NIAID, ORDR/NCATS, NIH, or the US Government.
The authors thank Elizabeth Dunn and Jessica Carlson for their tireless efforts organizing the PIDTC.
Abbreviations
- SCID
Severe combined immunodeficiency
- ADA
Adenosine Deaminase
- HCT
hematopoietic cell transplantation
- MSD
HLA-matched sibling donor
- URD
unrelated donor
- UCB
umbilical cord blood
- IL2RG
interleukin receptor common gamma chain
- JAK3
Janus Kinase 3
- PHA
phytohemagglutinin
- ATG
anti-thymocyte globulin
- GVHD
graft-versus-host disease
- TRM
Transplant-related mortality
- OS
Overall survival
- EFS
Event-free survival
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher C. Dvorak, Email: dvorakc@peds.ucsf.edu.
Amel Hassan, Email: Amel.Hassan@gosh.nhs.uk.
Mary A. Slatter, Email: Mary.Slatter@nuth.nhs.uk.
Manfred Hönig, Email: Manfred.hoenig@uniklinik-ulm.de.
Arjan C. Lankester, Email: A.Lankester@lumc.nl.
Rebecca H. Buckley, Email: buckl003@mc.duke.edu.
Michael A. Pulsipher, Email: michael.pulsipher@hsc.utah.edu.
Jeffrey H. Davis, Email: Jdavis@cw.bc.ca.
Tayfun Güngör, Email: Tayfun.guengoer@kispi.uzh.ch.
Melissa Gabriel, Email: melissa.gabriel@health.nsw.gov.au.
Jacob H. Bleesing, Email: Jack.Bleesing@cchmc.org.
Nancy Bunin, Email: BuninN@email.chop.edu.
Petr Sedlacek, Email: Petr.Sedlacek@fnmotol.cz.
James A. Connelly, Email: jaconnel@med.umich.edu.
David F. Crawford, Email: David-Crawford@ouhsc.edu.
Luigi D. Notarangelo, Email: Luigi.Notarangelo@childrens.harvard.edu.
Sung-Yun Pai, Email: Sung-Yun.Pai@childrens.harvard.edu.
Jake Hassid, Email: jchassid@ucdavis.edu.
Paul Veys, Email: paul.veys@gosh.nhs.uk.
Andrew R. Gennery, Email: Andrew.gennery@newcastle.ac.uk.
Morton J. Cowan, Email: mcowan@peds.ucsf.edu.
References
- 1.Dvorak C, Cowan M. Hematopoietic stem cell transplantation for primary immunodeficiency disease. Bone Marrow Transplant. 2008;41(2):119–26. doi: 10.1038/sj.bmt.1705890. [DOI] [PubMed] [Google Scholar]
- 2.Sarzotti-Kelsoe M, Win C, Parrott R, Cooney M, Moser B, Roberts J, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114(7):1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dvorak C, Hung G, Horn B, Dunn E, Oon C, Cowan M. Megadose CD34(+) cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biol Blood Marrow Transplant. 2008;14(10):1125–33. doi: 10.1016/j.bbmt.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Murphy WJ, Kumar V, Bennett M. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J Exp Med. 1987;165(4):1212–7. doi: 10.1084/jem.165.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Z, Dunn E, Singh K, Khan I, Yannone S, Cowan M. A non-leaky Artemis-deficient mouse that accurately models the human severe combined immune deficiency phenotype, including resistance to hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(1):1–11. doi: 10.1016/j.bbmt.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 7.Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295(5):508–18. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 8.Haddad E, Leroy S, Buckley R. B-cell reconstitution for SCID: Should a conditioning regimen be used in SCID treatment? J Allergy Clin Immunol. 2013;131(4):994–1000. doi: 10.1016/j.jaci.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes J, Rocha V, Labopin M, Neven B, Moshous D, Gennery A, et al. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood. 2012;119(12):2949–55. doi: 10.1182/blood-2011-06-363572. [DOI] [PubMed] [Google Scholar]
- 10.Gennery A, Slatter M, Grandin L, Taupin P, Cant A, Veys P, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602–10. e1–11. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91(10):3646–53. [PubMed] [Google Scholar]
- 12.Slatter M, Brigham K, Dickinson A, Harvey H, Barge DJA, Bown N, Flood TJ, Cant AJ, Abinun M, Gennery AR. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. J Allergy Clin Immunol. 2008;121(2):361–7. doi: 10.1016/j.jaci.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya A, Slatter M, Chapman C, Barge D, Jackson A, Flood T, et al. Single centre experience of umbilical cord stem cell transplantation for primary immunodeficiency. Bone marrow Transplant. 2005;36(4):295–9. doi: 10.1038/sj.bmt.1705054. [DOI] [PubMed] [Google Scholar]
- 14.Taga T, Itoh E, Noda Y, Kato H, Maruo Y, Takano T, et al. Successful unrelated umbilical cord blood cell transplantation without conditioning for a neonate with severe combined immunodeficiency. Pediatr Transplant. 2011;15(7):E152–5. doi: 10.1111/j.1399-3046.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 15.Hassan A, Booth C, Brightwell A, Allwood Z, Veys P, Rao K, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120(17):3615–24. doi: 10.1182/blood-2011-12-396879. [DOI] [PubMed] [Google Scholar]
- 16.Janda A, Sedlacek P, Hönig M, Friedrich W, Champagne M, Matsumoto T, et al. Multicenter survey on the outcome of transplantation of hematopoietic cells in patients with the complete form of DiGeorge anomaly. Blood. 2010;116(13):2229–36. doi: 10.1182/blood-2010-03-275966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Marcaigh AS, DeSantes K, Hu D, Pabst H, Horn B, Li L, et al. Bone marrow transplantation for T-B-severe combined immunodeficiency disease in Athabascan-speaking native Americans. Bone Marrow Transplant. 2001;27(7):703–9. doi: 10.1038/sj.bmt.1702831. [DOI] [PubMed] [Google Scholar]
- 18.Heimall J, Keller M, Saltzman R, Bunin N, McDonald-McGinn D, Zakai E, et al. Diagnosis of 22q11.2 deletion syndrome and artemis deficiency in two children with T-B-NK+ immunodeficiency. J Clin Immunol. 2012;32(5):1141–4. doi: 10.1007/s10875-012-9741-9. [DOI] [PubMed] [Google Scholar]
- 19.Griffith L, Cowan M, Notarangelo L, Puck J, Buckley R, Candotti F, et al. Improving cellular therapy for primary immune deficiency diseases: recognition, diagnosis, and management. J Allergy Clin Immunol. 2009;124(6):1152–60. doi: 10.1016/j.jaci.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shearer W, Dunn E, Notarangelo L, Dvorak C, Puck J, Logan B, et al. Diagnosing SCID, leaky SCID, and Omenn syndrome: the primary immune deficiency treatment consortium experience. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant. 2005;35(3):225–31. doi: 10.1038/sj.bmt.1704758. [DOI] [PubMed] [Google Scholar]
- 22.Cavazzana-Calvo M, Carlier F, Le Deist F, Morillon E, Taupin P, Gautier D, et al. Long-term T-cell reconstitution after hematopoietic stem-cell transplantation in primary T-cell-immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood. 2007;109(10):4575–81. doi: 10.1182/blood-2006-07-029090. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak C, Cowan M, Logan B, Notarangelo L, Griffith L, Puck J, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the Primary Immune Deficiency Treatment Consortium prospective study 6901. J Clin Immunol. 2013;33(7):1156–64. doi: 10.1007/s10875-013-9917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvorak C, Sandford A, Fong A, Cowan M, George T, Lewis D. Maternal T-cell engraftment associated with severe hemophagocytosis of the bone marrow in untreated X-linked severe combined immunodeficiency. J Pediatr Hematol Oncol. 2008;30(5):396–400. doi: 10.1097/MPH.0b013e318168e7a0. [DOI] [PubMed] [Google Scholar]
- 25.Meuwissen H, Gatti R, Terasaki P, Hong R, Good R. Treatment of lymphopenic hypogammaglobulinemia and bone-marrow aplasia by transplantation of allogeneic marrow. Crucial role of histocompatiility matching. N Engl J Med. 1969;281(13):691–7. doi: 10.1056/NEJM196909252811302. [DOI] [PubMed] [Google Scholar]
- 26.Kottaridis P, Milligan D, Chopra R, Chakraverty R, Chakrabarti S, Robinson S, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–25. [PubMed] [Google Scholar]
- 27.Chiesa R, Gilmour K, Qasim W, Adams S, Worth A, Zhan H, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156(5):656–66. doi: 10.1111/j.1365-2141.2011.08994.x. [DOI] [PubMed] [Google Scholar]
- 28.Baran-Marszak F, Feuillard J, Najjar I, Le Clorennec C, Béchet J, Dusanter-Fourt I, et al. Differential roles of STAT1alpha and STAT1beta in fludarabine-induced cell cycle arrest and apoptosis in human B cells. Blood. 2004;104(8):2475–83. doi: 10.1182/blood-2003-10-3508. [DOI] [PubMed] [Google Scholar]
- 29.Schultz K, Paquet J, Bader S, HayGlass K. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16(2):289–95. [PubMed] [Google Scholar]
- 30.Larsson K, Aschan J, Remberger M, Ringdén O, Winiarski J, Ljungman P. Reduced risk for extensive chronic graft-versus-host disease in patients receiving transplants with human leukocyte antigen-identical sibling donors given polymerase chain reaction-based preemptive therapy against cytomegalovirus. Transplantation. 2004;77(4):526–31. doi: 10.1097/01.tp.0000109778.39235.f4. [DOI] [PubMed] [Google Scholar]
- 31.Pichereau C, Desseaux K, Janin A, Scieux C, Peffault de Latour R, Xhaard A, et al. The complex relationship between human herpesvirus 6 and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(1):141–4. doi: 10.1016/j.bbmt.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Buckley R, Win C, Moser B, Parrott R, Sajaroff E, Sarzotti-Kelsoe M. Post-Transplantation B Cell Function in Different Molecular Types of SCID. J Clin Immunol. 2013;33:96–110. doi: 10.1007/s10875-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan A, Church J, Cowan M, Agarwal R, Kapoor N, Kohn D, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140–50. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim VH-D, Murguia L, Schechter T, Grunebaum E, Roifman CM. Emergency treatment for ζ chain–associated protein of 70 kDa (ZAP70) deficiency. J Allergy Clin Immunol. 2013;131(4):1233–5. doi: 10.1016/j.jaci.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Straathof K, Rao K, Eyrich M, Hale G, Bird P, Berrie E, et al. Haemopoietic stem-cell transplantation with antibody-based minimal-intensity conditioning: a phase 1/2 study. Lancet. 2009;374(9693):912–20. doi: 10.1016/S0140-6736(09)60945-4. [DOI] [PubMed] [Google Scholar]
- 36.Czechowicz A, Kraft D, Weissman I, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–9. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savic R, Cowan M, Dvorak C, Pai S-Y, Pereira L, Bartelink I, et al. Effect of weight and maturation on busulfan clearance in infants and small children undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(11):1608–14. doi: 10.1016/j.bbmt.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.