Abstract
We review here how diverse inherited and acquired abnormalities in epidermal structural and enzymatic proteins converge to produce defective permeability barrier function and antimicrobial defense in AD. Although best known are mutations in filaggrin (FLG), mutations in other member of the fused S-100 family of proteins (i.e., hornerin [hrn] and filaggrin 2 [flg-2]); the cornified envelope precursor (e.g., SPRR3); mattrin, encoded by Tmem79, which regulates the assembly of lamellar bodies; SPINK5, which encodes the serine protease inhibitor, LEKTI1; and the fatty acid transporter, FATP4, have all been linked to AD. Yet, these abnormalities often only predispose to AD; additional acquired stressors that further compromise barrier function; e.g., psychological stress, a low ambient humidity, or high pH surfactants, often are required to trigger disease. Th2 cytokines can also compromise barrier function by downregulating expression of multiple epidermal structural proteins, lipid synthetic enzymes and antimicrobial peptides. All of these inherited and acquired abnormalities converge on the lamellar body secretory system, producing abnormalities in lipid composition, secretion and/or extracellular lamellar membrane organization, as well as in antimicrobial defense. Finally, we briefly review therapeutic options that address this new pathogenic paradigm.
Keywords: antimicrobial peptides, atopic dermatitis, barrier function, ceramides, cytokines, filaggrin, kallikreins, lamellar bodies, lipid composition, pH, serine protease inhibitors, Th2 cells
INTRODUCTION
Basis for the Permeability Barrier in Normal Skin
7The epidermis generates a set of protective/defensive functions, mediated by its unique differentiation end-product, the stratum corneum (SC) 1, 2. As they migrate apically, keratinocytes acquire a series of differentiation-specific proteins 3–5 and lipids 6, 7 until cornification occurs. The SC comprises vertical stacks of anucleate corneocytes, embedded in an expanded extracellular matrix, replete with multiple stacks of broad, planar lamellar bilayers, enriched in ceramides, cholesterol, and free fatty acids (FFA) 8. These lamellar arrays of hydrophobic lipid species impede both the outward movement of water, and the inward bombardment of noxious environmental allergens and pathogens. A unique organelle, the epidermal lamellar body, delivers these lipids to the SC interstices as their precursors (e.g., glucosylceramides and phospholipids), along with a set of hydrolytic ‘lipid processing’ enzymes, including β-glucocerebrosidase, acidic sphingomylinase, secretory phospholipase A2 and steroid sulfatase 9 (Fig. 1). These enzymes generate a family of ceramides (Cer), essential and non-essential free fatty acids (FFA), as well as much of the cholesterol that is required for the supramolecular organization of these non-polar lipid species into mature lamellar membrane structures 10. In parallel, lamellar body-derived, desquamatory proteases and their inhibitors initiate the orderly digestion of corneodesmosomes (= transient intercellular rivets that connect adjacent corneocytes), a process that eventually leads to the desquamation of corneocytes from the skin surface 11, 12 (Fig. 1). Finally, at least two antimicrobial peptides, human β-defensin 2 and the carboxyterminal cathelicidin peptide, LL-37, are delivered to the SC intercellular domains through secretion of lamellar body contents 13–15. In fact, the permeability barrier and the antimicrobial barrier share many features that impede the colonization and invasion of pathogenic organisms, while simultaneously encouraging colonization by non-pathogenic ‘normal’ flora 16. Thus, the physicochemical characteristics of the SC; niche occupancy by resident normal flora; and secreted factors from the normal flora contribute to cutaneous antimicrobial defense. Because these two barriers share so many structural and biochemical features, perturbations in one function inevitably modify the other in parallel 15, 17. Thus, the epidermal lamellar body is a multifunctional organelle, whose contents influence not only permeability barrier status, but also SC cohesion/desquamation and antimicrobial defense.
Fig. 1. Multifunctional Impact of Secreted Lamellar Body Contents.
(Modified from106)
The Tight Junction (TJ) Controversy
How should we interpret an ever-expanding literature that proclaims a potential role for TJ in normal permeability barrier function 18, 19, as well as a potential role for abnormal TJ function in AD 20? We will attempt to navigate this heavily invested subject as follows: First, complex TJ structures, such as those found in the kidney and gastrointestinal tract, do not occur in adult keratinizing epithelia 21. Second, with the exception of these structures in renal collecting tubules, where they comprise multitiered sites of membrane fusion (‘zonulae occludentes’), TJ provide a relatively poor barrier against paracellular water movement. In the author’s opinion, confusion in the skin-related literature has occurred because ‘TJ proteins’ are widely equated with ‘TJ’ 18, 19. Doubtless, the apical-lateral plasma membranes of cells in the outer stratum granulosum of normal epidermis are heavily decorated with multiple TJ proteins, which form ‘kissing points’; i.e., ‘maculae occludentes’ 21. However, as noted above, these focal attachments do not comprise true zonulae occludentes, as occur in tubular epithelia. The most compelling evidence that these putative TJ play no direct role in the paracellular water barrier is that removal of SC lipids by gentle, external lipid solvent treatment completely abrogates the permeability barrier 22. While this observation likely also excludes a possible ‘back-up’ role for TJ-like structures in the water barrier, it still remains possible that these incomplete structures interdict the passage of larger xenobiotes, particularly when the overlying lipid-based barrier becomes defective, as occurs in AD.
Yet, these structures, though insufficient to contribute to the water barrier, are nonetheless critical for the development of barrier competence. Transgenic knock-out of the key TJ protein, claudin 1, results in a fatal, post-natal permeability barrier abnormality 23. Indeed, replete TJ are present early in epidermal development, but they become functionally incompetent later in fetal life in parallel with establishment of the lipid-based barrier 24. An acquired reduction in the expression of the TJ protein, claudin 1, has been reported in AD 20, and occludin protein levels decline in FLG-deficient human epidermis 25. It is possible that abnormalities in TJ proteins could result from the Th2 dominant milieu in AD, which simultaneously downregulates many other epidermal differentiation-linked proteins (see below).
Since adult epidermis does not generate the types of complex zonulae occludentes necessary to impede paracellular water movement, attention should be focused instead on the possible function(s) of the incomplete junctions (maculae occludentes) in normal epidermis; and how acquired defects in these focal connections could contribute to AD pathogenesis. These structures likely perform important ‘fence functions’ in adult epidermis, including polarizing the direction of lamellar body secretion towards the apex of the outermost granular layer 26.
Barrier Dysfunction in AD and Other Atopic Disorders
During the pre-genotype era, we and others proposed that the permeability barrier abnormality in AD is not merely an epiphenomenon, but rather the potential ‘driver’ of inflammation in AD (i.e., an ‘outside-to-inside’ view of disease pathogenesis) 27, 28, because i) the extent of the permeability barrier abnormality parallels severity of disease phenotype 29, 30; ii) clinically-uninvolved skin sites display significant barrier abnormalities 30; and iii) sustained barrier abnormalities, regardless of cause, inevitably stimulate a pro-inflammatory cytokine cascade that ‘recruits’ characteristic, disease-specific immunophenotypes 31, 32. Recent studies have shown that the cutaneous barrier abnormality is not only critical for the development of AD, but also other allergic disorders, including asthma, allergic rhinitis, and food allergies 33.
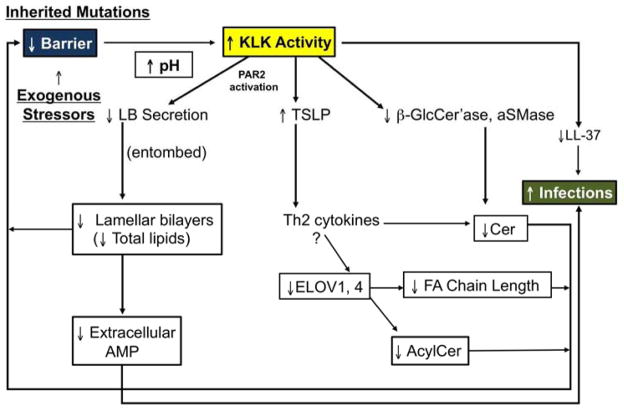
The barrier abnormality in AD leads to an increase in pH that activates serine proteases (kallikreins, KLK) in the outer epidermis, with widespread downstream and upstream consequences, as shown in Fig. 2. Yet, these mechanisms also stimulate a series of metabolic responses aimed at restoring permeability barrier homeostasis. Briefly, increased TNFα stimulates differentiation 34, while increased IL-1α enhances epidermal lipid synthesis 35. Nerve growth factor (NGF) and amphiregulin (AR) stimulate epidermal DNA synthesis; and several cytokines enhance antimicrobial peptide activation/production 36.
Fig. 2. Cytokine Cascade Leads To Multiple ‘Vicious Cycles’ in AD.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-1, interleukin-1; KLK, kallikrein; NF-κB, nuclear factor kappa B; TNFα, tumor necrosis factor alpha; TSLP, thymic stromal lymphopoietin
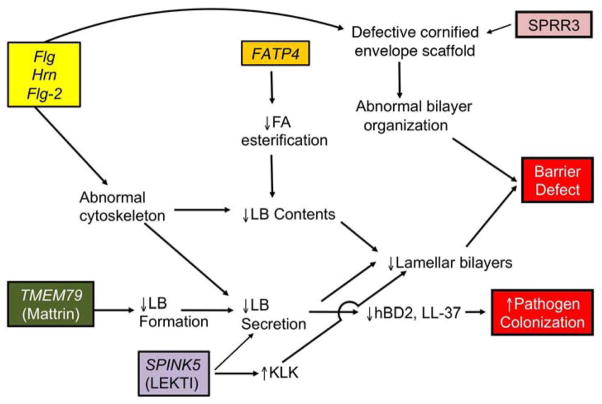
Related and Unrelated Mutations Impact Barrier Function in AD
Recent molecular genetic studies have fortified this ‘outside-to-inside-back- to-outside’ view of disease pathogenesis, because most of the recently-identified mutations associated with AD modify structural and enzymatic proteins that are required for normal barrier function.
Filaggrin
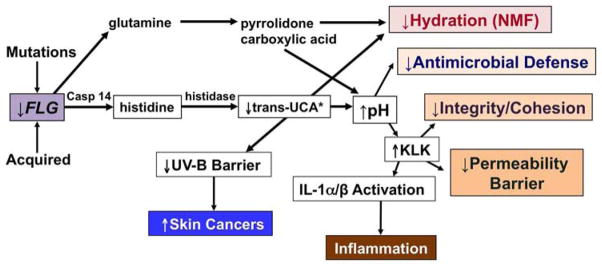
The initial molecular genetic evidence that a primary structural abnormality underlies the pathogenesis of AD derives from the strong association of loss-of-function mutations in the gene encoding filament aggregating protein (filaggrin, FLG) in AD 37–39. Up to 50% of certain northern European kindreds, and a substantial proportion of Asians, with AD reveal either single or double allele, loss-of-function mutations in the gene encoding FLG. Although more than 40 different FLG mutations have now been associated with AD 37, four predominate in northern and central Europeans 40, 41. The initial product of FLG translation is pro-FLG, a large, histidine-rich, highly cationic phosphoprotein, consisting of ten to twelve FLG repeats, connected by peptide segments enriched in hydrophobic amino acids 42, 43. Pro-FLG contains an amino-terminal sequence, including a calcium-binding A domain as well as a B domain of uncertain function. During cornification in normal, non-atopic humans, pro-FLG is dephosphorylated and proteolytically processed to FLG monomers 42. Then, as the water content of the SC declines in the mid-to-outer stratum corneum, FLG detaches from the cornified envelope, followed by its C-terminal proteolysis by caspase 14, bleomycin hydrolase, and other hydrolases into its constituent amino acids 44. These amino acids subsequently are further deiminated into polycarboxylic acids that account in part for SC hydration and acidification 45 (Fig. 3).
Fig. 3. Multiple Downstream Consequences of Filaggrin Deficiency in Atopic Dermatitis.
*Trans-urocanic acid (t-UCA) is the most potent endogenous UV-B filter in lightly-pigmented skin. Loss of t-UCA could account for the higher incidence of non-melanoma skin cancers in AD 45. (Modified from Elias & Williams, JID, 2013)
Decreased FLG expression results in a paucity of keratohyalin granules, a hallmark of ichthyosis vulgaris (IV) 46, the forme fruste of AD 47. Flg mutations exhibit an allele-dose effect, wherein heterozygous patients with IV show diminished FLG expression and a mild phenotype, as well as abnormalities in surface pH, hydration, and barrier function 25 (Fig. 3). But IV patients with homozygous and compound heterozygous FLG mutations exhibit more severe scaling 40, 48, and more pronounced abnormalities in stratum corneum structure and function 25 (Fig. 4), as well as a further propensity to develop AD 37, 40. Yet importantly, a substantial proportion of these double-allele IV patients still do not exhibit inflammation (i.e., AD), emphasizing the role of exogenous (acquired) stressors in AD pathogenesis (see below).
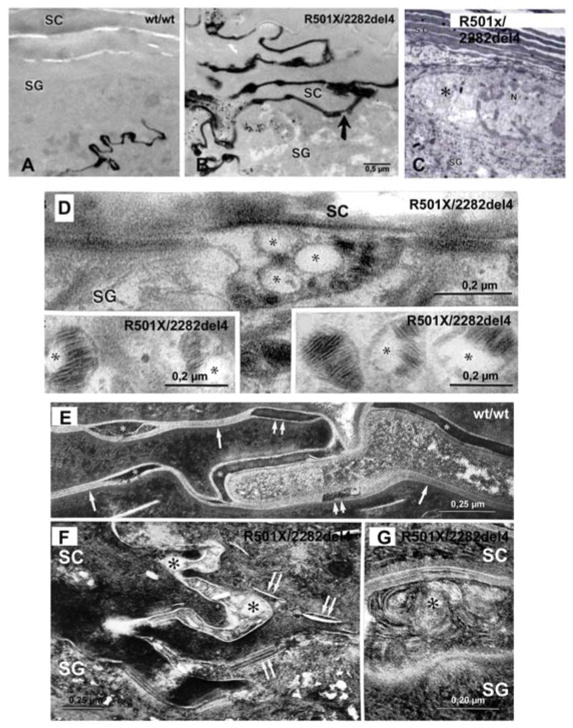
Fig. 4. Abnormalities That Lead to Paracellular Barrier Abnormality in FLG-Deficient Epidermis.
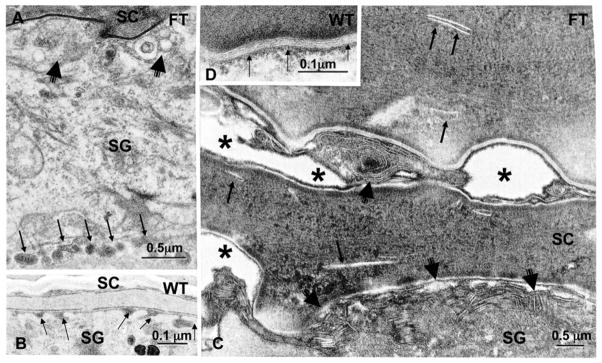
Lanthanum perfusion in double-allele ichthyosis vulgaris (IV) (B) vs. wild-type (A) human epidermis. C: Retraction of cytoskeleton (asterisks) in double-allele IV. D (+ inserts): Impaired loading and secretion of lamellar body cargo in double-allele IV. F–G: Post-secretory abnormalities in lamellar bilayer organization and maturation in double-allele IV (arrows). E: Normal (wild- type) human epidermis. RuO4 post-fixation (Modified from 25).
An acquired reduction in epidermal FLG expression also occurs in AD, independent of mutation status 49, due in part to Th2-induced down-regulation of a broad range of proteins associated with epidermal differentiation, including FLG 50,51. Moreover, IgE from AD patients auto-reacts against a variety of keratinocyte antigens, suggesting yet another ‘vicious cycle’ in AD 52. Thus, primary inherited barrier abnormalities in AD ultimately stimulate downstream paracrine mechanisms that likely further compromise permeability barrier function, completing an additional potential ‘outside-inside-outside’ pathogenic loop in AD 53 (Fig. 2).
Other Fused S-100 Proteins (Hornerin and Filaggrin-2)
The prevalence of FLG mutations in AD patients, though quite high in populations of northern European descent 37, does not account for many cases of AD in such northerners. It can be further assumed that almost all cases of AD in other populations will prove to be associated with other inherited abnormalities that compromise epidermal barrier function. Recent studies suggest an association of AD with mutations in two other members of the fused S-100 proteins; i.e., hornerin (hrn) and filaggrin 2 (Flg-2) 54, 55. Interestingly, FLG-2 mutations are linked to AD in African-Americans 56, 57. While filaggrin, hornerin, and FLG-2 are all differentiation-specific components of the corneocyte envelope 58–62, the specific functions of both hornerin and FLG-2 in normal epidermis remain unknown (Table 1). Hence, how defects in these proteins contribute to a putative barrier abnormality in AD also remains uncertain.
Table 1.
Consequences of Inherited Barrier Abnormalities in AD
| Structural Protein | Immediate Structural Consequences | Downstream Changes → Barrier Defect |
|---|---|---|
| S-100Type | ||
| ↓Filaggrin | Attenuated CE → Poor Scaffold | Bilayer disorganization |
| ↑SC pH → ↑KLK | Lamellar body entombment → Lamellar bilayers | |
| ↓SC hydration | ↑TEWL, ↑pro-inflammatory cytokines | |
| ↓Hornerin | ? Attenuated CE | ? |
| ↓Filaggrin-2 | ? Attenuated CE | |
| Other CE Precursors | ||
| SPRR3 | Attenuated CE → Poor Scaffold | |
| Enzyme Inhibitor | ||
| LEKTI 1 (Netherton syndrome) | ↑KLK activity | Destruction of corneodesmosomes, lipid processing enzymes, LL-37 |
| Lipid Metabolism | ||
| FATP4 | ↓FFA, FACoA, Esterified FA | ↓Glycerolipids; Detergent effects of excess FFA |
| Mattrin | ↓Lamellar body formation/secretion | ?↓Extracellular lipids |
Abbreviations: CE, cornified envelope; FATP4, fatty acid transport protein 4; FFA, free fatty acids; KLK, kallikreins, LEKTI, lympho-epithelial Kazal-type trypsin inhibitor; TEWL, transepidermal water loss
SPRR3 is a cornified envelope (CE) precursor protein that is virtually undetectable in normal skin 63. Several types of mutations in SPRR3, including an extra 24 base pair defect in the central domain, as well as additional in-frame deletions and insertions 64 have been associated not only with AD 65, but also with asthma 66. These mutations result in expression of SPRR3 at higher than normal levels in AD 64, 65, likely impacting barrier function through production of a CE scaffold that impairs the supramolecular organization of lamellar body-derived lipids into normal bilayer structures (Table 1). Ultrastructural studies show defective, thinner-than-normal CEs in AD, with decreased extracellular lipids and a poorly cohesive SC, associated with deficiencies not only in SPRR, but also in several other CE precursors in AD, perhaps due to a broad Th2-stimulated down-regulation of these proteins 67,50, 51.
TMEM79
Very recent studies have identified non-sense and mis-sense mutations in the gene, TMEM79, which encodes the protein, mattrin, in some Irish AD patients who lack FLG mutations 68. Mutations in the murine orthologue of this gene account for the flaky tail (ma/ma) strain of mice, which develop a spontaneous AD-like dermatitis 69. Mattrin localizes to the cytosol, and more specifically within the trans-Golgi network in the outermost cells of the granular layer 68, 69. Reductions in mattrin levels block the secretion of lamellar body contents, including desquamatory proteases, antiproteases 69, and likely lamellar-derived lipids. Indeed, defective lipid secretion has been demonstrated in flaky tail mice, bearing the mattrin mutation 70. Nonetheless, the association of this mutation with AD eloquently demonstrates that not only inherited deficiencies in structural proteins, but also that mutations which impair the delivery of lamellar body contents can predispose to disease.
Protease/Antiprotease Expression
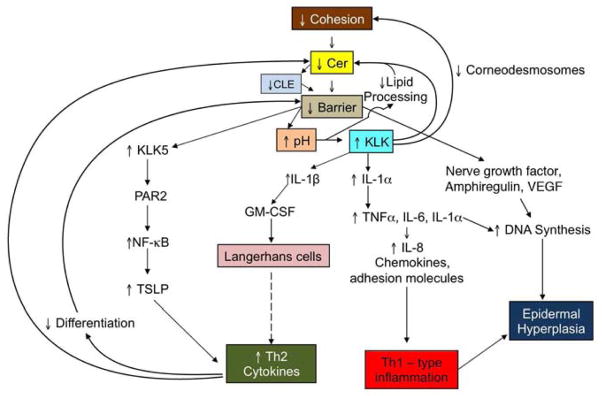
Inherited abnormalities that result in excessive serine protease (SP) (kallikreins, KLK) activity predisposes to severe AD, and more importantly, they provide unique insights into the pathogenesis of AD 71, 72 (Fig. 5). The most compelling demonstration for the role of excess SP activity in the pathogenesis of AD comes from Netherton syndrome (NS), an autosomal recessive disorder due to loss-of-function mutations in SPINK5, the gene encoding the SP inhibitor, lymphoepithelial Kazal-type trypsin inhibitor type 1 (LEKTI 1) 73. NS is characterized by a severe AD-like dermatosis, mucosal atopy, and anaphylactic reactions to food antigens. The extent of residual LEKTI expression in humans with NS correlates inversely with excess KLK activity within the outer epidermis 74, and unrestricted KLK activity provokes a severe permeability barrier defect, as well as dramatic thinning of the SC. Both defects can be attributed to KLK-dependent degradation of lipid-processing enzymes and corneodesmosome-constituent proteins, respectively 74. KLK-mediated degradation of the enzymes contributes to the depletion of Cer, a characteristic lipid abnormality in AD 75, 76 (see also below). Likewise, transgenic mice that over-express human KLK7 display a severe AD-like dermatosis 77. In NS, and likely also in AD, one of these KLKs; i.e., KLK5, or the SC tryptic enzyme, binds to the protease activated receptor, type 2 (PAR2), stimulating NFκB-dependent production of the pro-Th2 cytokine, thymic stromal lymphopoietin (TSLP) 78.
Fig. 5.
LESSONS FROM NETHERTON SYNDROME:
Proposed Roles for Increased pH and KLK Activation in Producing Lipid Abnormalities in AD
As a result of these divergent inherited associations, a broad view is emerging that virtually any inherited abnormality that leads to a sustained barrier abnormality can predispose to AD. For example, note the association of AD with loss-of-function mutations in the fatty acid transporter, FATP4, in patients with ichthyosis prematurity syndrome 79. Moreover, many patients with other inherited ichthyoses frequently report severe pruritus 80, although the inflammatory infiltrate in these patients (with the exception of NS) has not yet been characterized (Table 1). Yet, it would not be unreasonable then to query why diseases like psoriasis, which exhibit a well-known barrier abnormality 81, do not develop a Th-2-like immunophenotype. A perhaps too-simple explanation might be that allergen access is impeded by the tenacious scale in psoriasis.
How Unrelated Mutations in Epidermal Proteins Converge on the Lamellar Body Secretory System to Provoke a Barrier Abnormality in AD
We have shown that both reductions and loss of the cytosolic protein, filaggrin, lead to an extracellular permeability barrier defect, both in filaggrin-deficient IV 25, and in murine models of AD 70, 82. In all these settings, water loss accelerates via an extra- (para-) cellular pathway; i.e., through the extracellular matrix. The link between a defect in the cytosolic protein, Flg, and the extracellular permeability defect was clarified in patients with IV (Figs. 4A&B) 25. Both single- and double-allele patients demonstrate retraction of cytosolic keratin filaments into a perinuclear shell around nuclei of the stratum granulosum (Fig. 4C). This cytoskeletal abnormality appears to impact two cellular processes. First, it results in incomplete loading of cargo into nascent lamellar bodies, evidenced by ‘empty’ micro-vesicles within these organelles (Figs. 4D&E). The resulting deposition of non-lamellar contents in the intercellular spaces then leads to focal defects in the extracellular lamellar bilayer system (Figs. 4F&G), contributing to defective barrier function in IV. Second, the cytoskeletal abnormality impairs secretion of lamellar bodies, resulting in their partial entombment within corneocytes 25, 70 (Fig. 5). This latter pathogenic sequence is similar to that seen in patients with mutations in keratins 1 or 10, where cytoskeletal retraction results in entombment of lamellar bodies, and a paucity of lamellar bilayers 83.
Once inflammation is established; i.e., as IV transitions into AD, the pH of the SC increases still further, sufficient to activate a family of KLK in the outer epidermis 84. KLK activation has multiple negative consequences 72, including: i) the degradation of both corneodesmosomes, leading to a poorly cohesive SC, and ii) the destruction of extracellular lipid processing enzymes, β-glucocerebrosidase and acidic sphingomylinase 85. Loss of these two ceramide-generating enzymes (as well as their reduced activity at a high pH) results in failure of lamellar bilayer formation and maturation 25, 82, exactly as occurs in NS 74. A third downstream consequence of increased SP activity is generation of the primary cytokines, IL-1α and IL-1β from their 33kDa pro-forms in human SC 86, which are stored in large quantities in the cytosol of corneocytes 87, 88. This putative pH-induced increase in KLK activity generates the active, 17kDa forms of these cytokines, the first step in the cytokine cascade in AD, which includes downstream production of several additional cytokines, growth factors, chemokines, and adhesion molecules 31, 89, 90, including the TSLP-Th2 cytokine network, described above 78 (Fig. 2).
Excess KLK also activates the PAR2 receptor, which localizes to the plasma membrane of granular cells 74. Binding is followed by internalization of the PAR2 receptor, which then down-regulates lamellar body secretion, effectively entombing these organelles within the corneocyte cytosol 91, 92. Thus, even without allergen exposure, an Th2 immunophenotype likely can occur in AD, as described for NS 78 (Fig. 5)! Conversely, applications of either KLK or PAR2 inhibitors, or just acidification of the SC alone 93,94, prevents both the destructive effects of excess KLK activity and PAR2 internalization, normalizing lamellar body secretion and permeability homeostasis 91, 95. These studies demonstrate how multiple, pH-initiated steps in the secretion and post-secretory processing of lamellar body contents leads to AD (Fig. 6).
Fig. 6. How Inherited Abnormalities Converge to Produce Defective Permeability and Antimicrobial Barriers in AD.
Abbreviations: FA, fatty acid; Fatp4, fatty acid transport protein 4; Flg, filaggrin; hBD2, human beta-defensin 2; Hrn, hornerin; LB, lamellar body
Stressors That Further Aggravate Barrier Dysfunction Can Trigger AD (Fig. 5)
Alkaline soaps
In ichthyosis vulgaris (IV), even double-allele FLG mutations do not always suffice to provoke inflammation 25, 39, 96, but certain stressors can further aggravate the barrier abnormality; i.e., by provoking an incremental increase in the pH of the SC, leading to a further amplification of SP activity 72. Such a barrier-dependent increase in pH (and SP activity) likely accounts for the precipitation of AD following the use of neutral-to-alkaline soaps, a well-known exogenous stressor of clinical AD 97, 98.
Reduced Humidity as a Stressor
Prolonged exposure to a reduced environmental humidity, as occurs in radiant-heated homes in temperate climates during the winter, is also a well-known risk factor for AD 99. Under these conditions, transcutaneous water loss accelerates across a defective SC, aggravating the underlying permeability barrier abnormality, while also amplifying cytokine signaling of inflammation 72. Because FLG proteolysis is regulated by changes in external humidity 45, sustained reductions in environmental relative humidities likely further deplete residual FLG in single-allele FLG-deficient patients with AD.
Finally, sustained psychological stress (PS) aggravates permeability barrier function in otherwise normal humans 100, 101 and mice 102, 103. PS is also a widely-acknowledged precipitant of AD, but in the case of PS, however, the likely mechanism differs from either high pH surfactant use or decreased environmental humidities. Increased stress in experimental animals induces an increase in endogenous glucocorticoids (GC), which in turn alters permeability barrier homeostasis, SC cohesion and epidermal antimicrobial defense 15, 103, 104. The central role of GC has been demonstrated in two ways: first, exogenous systemic or topical GC recapitulate all of the above, stress-induced functional abnormalities 103, 105. Second, either blockade of GC production with the CRF inhibitor, antalarmin, or peripheral action, with the GC receptor inhibitor, mifeprostone (Ru486), prevent emergence of PS and GC-initiated functional abnormalities 104, 105. A GC-mediated inhibition of synthesis of the three key epidermal lipids that mediate barrier function; i.e., Cer, cholesterol, and FFA, accounts for the negative effects of PS 102, 105. Accordingly, a topical mixture of these three lipids largely normalized all of these functions in mice and humans, even in the face of ongoing PS or GC therapy 102, 106.
Basis for Lipid Abnormalities in AD
Global Decline in Lipids
Filaggrin-associated AD is characterized by profound abnormalities in lipid content, distribution, and lamellar membrane organization in lesional skin 107–109, which result in a paracellular barrier abnormality 25, 70, 82. A moderate impairment of lamellar body secretion, due to a cytoskeletal abnormality, results in entombment of substantial quantities of lamellar bodies within corneocytes, a feature that becomes more prominent once inflammation is established 82. In addition, KLK signaling of the PAR2 downregulates lamellar body secretion 92, likely providing an additional biochemical signal that accounts for entombment of these organelles in nascent corneocytes. Together, these abnormalities result in incomplete delivery of lamellar body-derived cargo, as well as a paucity of extracellular lamellar bilayers, leading to a global reduction in extracellular lipids 72, 108 (Fig. 6). Finally, not only extracellular lamellar bilayers, but also the covalently-bound lipids that form the corneocyte lipid envelope, decline in AD 110, further contributing to the barrier abnormality.
Sphingolipid Abnormalities in Atopic Dermatitis
The most distinctive hallmark of human AD is the repeatedly-noted, selective reduction in the Cer content of affected SC 75, 76. Several mechanisms likely contribute to the decrease in Cer. As noted above, the barrier-related increase in pH, and a pH-induced increase in KLK activity results in deactivation, and ultimately in accelerated degradation of the Cer-generating enzymes, acidic sphingomylinase and β-glucocerebrosidase (Fig. 5) 74. Moreover, the cytokine cascade, associated with AD and other inflammatory dermatoses with a barrier abnormality, upregulates production of interferon-γ, which downregulates epidermal synthesis of Cer 111. Furthermore, noting that neither sphingomyelin nor glucosylceramides accumulate in the SC of AD, Imokawa, et al. (2009) provided evidence that AD epidermis exhibits novel N-deacylation activities that degrade both sphingomyelin and glucosylceramides. However, the genes for these enzymes have not yet been identified in skin; hence, it remains possible that this deacylase activity could be of bacterial origin. Accordingly, several other microbial pathogens that are known to colonize AD elaborate acidic ceramidase activity 112, 113, which could further decrease Cer content. Yet, the sphingoid base content of the SC in AD is lower (not higher) than in normal SC 114, arguing against an important role for microbial ceramidases in producing Cer deficiency in AD. Finally, it should be noted that abnormalities in the ratio of sphingoid bases, specifically in sphingosine and sphinganine, could modify lamellar membrane permeability, thereby contributing to the barrier defect in AD 115.
Increased production of the Th2-derived cytokines, IL-4 and IL-13, also further contributes to the decrease in Cer in AD. In experimental animals, IL-4 not only down-regulates serine palmitoyl transferase, the rate-limiting enzyme of ceramide synthesis, but it also blunts the potential benefits of Th1-derived TNF-α on ceramide-generating enzymes 116, 117. Thus, while Th1 cytokines upregulate Cer production 118, the dominance of Th2 cytokines in AD likely overwhelms this Th1 response, with profound consequences for epidermal structure and function (Fig. 5).
Shorter N-Acyl Fatty Acids in AD
Researchers in Japan and The Netherlands recently reported that the sum of sphingoid bases plus the N-acyl fatty acids (FA) in ceramides declines, in parallel with a decline in the chain length of free and esterified fatty acids (FA) in lesional AD 119–121. These shorter-chain FA in turn produce abnormalities in lipid organization that likely compromise permeability barrier function in AD 119, 121, 122. The basis for these chain length abnormalities could prove to be reduced expression of two fatty acid elongases, ELOVL1 and ELOVL4, enzymes required to generate the very long chain N-acyl FA in Cer and FFA in AD 111 (Fig. 5). It is intriguing to speculate that the reduced levels of ELOVs could again prove to be an acquired abnormality due to elevated Th2 cytokines. Alternatively, elevated levels of interferon gamma (IFNγ) downregulate ELOV1 and 4 69, which could account for reduced N-acyl chain length 111. Finally, degradation of ELOVs by excessive KLK activity could at least in theory contribute to these abnormalities.
CONCLUSION: Clinical and Therapeutic Implications
Sustained antigen ingress through a defective barrier leads to a Th2-dominant infiltrate, which then becomes an additional cause of inflammation in AD (Fig. 2).While certain antigens, such as cat dander, preferentially trigger childhood AD in FLG-deficient patients 123, the worst offenders are mites and cockroach antigens, which themselves release and activate KLK, resulting in further damage to the barrier 124. Furthermore, the lipid-depleted barrier in AD may facilitate the penetration of water-soluble haptens, such as nickel. Indeed, nickel-induced, acute allergic dermatitis is more common in humans with FLG-deficient AD than in normals 125,126. Accordingly, correction of the barrier abnormality alone with measures that restore and correct lipid imbalance could prevent and/or ameliorate the barrier abnormality in AD, thereby reducing the inflammatory component in AD 98, 107, 108, 127. There is now emerging evidence that physiologic lipids, if delivered in sufficient quantities, and at appropriate molar ratios, are effective in the treatment of even moderate-to-severe AD, without any of the safety concerns surrounding glucocorticoids and immunomodulators 108*. Alternatively, those patients with AD due to either single-allele mutations in FLG, and/or acquired reductions in FLG, become potential candidates for strategies that either upregulate FLG expression 128,129; or enhance the transdermal delivery of FLG monomers to deficient skin 129. Yet, it must be noted that the latter approach, though very elegant in theory, may not be practical for a generalized disease such as AD.
Table 2.
Key Points
| 1 | A variety of unrelated mutations that compromise epidermal barrier function predispose to the development of atopic dermatitis (AD). |
| 2 | An acquired deficiency in filaggrin also occurs, independent of mutation status. |
| 3 | These mutations converge on the lamellar body secretory system, producing abnormalities in either lamellar body formation, secretion, or post-secretory processing that compromises extracellular lamellar bilayer structure |
| 4 | Theses secretory abnormalities account in large part for the distinctive lipid abnormalities in AD, which included a global decrease in barrier lipids; a further decline in ceramide content; and truncation of the chain lengths of free and esterified fatty acids. |
| 5 | The same pathogenic sequence compromising antimicrobial defense accounts at least in part for colonization by S. aureus and other pathogens in AD. |
| 6 | Based upon the above, rational therapy should address and correct filaggrin status, and/or the lipid abnormalities in AD. |
Acknowledgments
This work was supported by NIH grant AR019098, and by the Medical Research Service, Department of Veterans Affairs. These contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Abbreviations
- AD
atopic dermatitis
- AMP
antimicrobial peptides
- AR
amphiregulin
- Cer
ceramides
- FLG
filaggrin
- FFA
free fatty acids
- GC
glucocorticoids
- hBD
human β-defensins
- IFNγ
interferon gamma
- IV
ichthyosis vulgaris
- KLK
kallikreins
- LEKTI
lymphoepithelial Kazal-type trypsin inhibitor
- NGF
nerve growth factor
- NS
Netherton syndrome
- PAR2
protease activator type 2 receptor
- PS
psychological stress
- SP
serine protease
- SC
stratum corneum
- TJ
tight junction
- TSLP
thymic stromal lymphopoietin
Glossary
Diverse mechanisms converge on lamellar body secretion, producing the barrier abnormality in atopic dermatitis
- Lamellar body
Small, ovoid, membrane-bound, secretory organelle synthesized by keratinocytes as they reach the stratum spinosum. An intracellular pathway involving the Golgi apparatus produces them. Their lipid molecular contents display a plate like appearance when viewed through high magnification electron microscopy
- Ceramides
A group of amido sphingolipids (ex sphingomyelin and cerebrosides) formed by linking a fatty acid to a sphingoid base (C18H37NO2).
- Antimicrobial peptides
Components of the innate immune system capable of inserting into bacterial phospholipids that kill or slow microbial growth.
- Xenobiotes
Chemical compounds foreign to living organisms.
- Keratohyalin granules
Substance synthesized by free ribosomes in a keratinocyte as it passes through the epidermis. These granules expand and then interact with tonofilaments to aggregate keratin in corneocytes.
- S-100
Calcium binding proteins that modulate both intra- and extracellular processes in the epidermis, including keratinocyte differentiation.
- TSLP
An IL-7-like cytokine that activates dendritic cells to produce pro-Th2 chemokines and primes naïve T cells to differentiate into Th2 cells.
- pH
A scale measurement of acidity/alkalinity on which a value of 7 represents neutrality. Each unit of change represents a 100-fold change in acidity or alkalinity. Skin pH in a healthy adult ranges from 4.5 to 5.5. Washing skin with soap increases skin pH by approximately 3 units. Skin protease activity is enhanced at pH values above 7.5.
- Humidity
Typically expressed as relative humidity: The amount of water vapor in the air at a specified temperature and pressure relative to the total amount it could hold at those values.
Footnotes
Conflict of Interest Statement: Dr. Elias is a co-developer of this form of treatment for AD, currently licensed from the University of California to PuraCap Pharmaceutical, LLC. (Dr. Elias is a consultant to PuraCap).
The authors state no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125(2):183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 2.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 3.Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31(1):5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 4.Roop D. Defects in the barrier. Science. 1995;267(5197):474–5. doi: 10.1126/science.7529942. [DOI] [PubMed] [Google Scholar]
- 5.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Critical reviews in oral biology and medicine: an official publication of the American Association of Oral Biologists. 2000;11(4):383–408. doi: 10.1177/10454411000110040101. Epub 2001/01/02. [DOI] [PubMed] [Google Scholar]
- 6.Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.11.007. Epub 2013/11/23. [DOI] [PubMed] [Google Scholar]
- 7.Schurer NY, Elias PM. The biochemistry and function of stratum corneum lipids. Adv Lipid Res. 1991;24:27–56. doi: 10.1016/b978-0-12-024924-4.50006-7. [DOI] [PubMed] [Google Scholar]
- 8.Elias PM. Structure and function of the stratum corneum extracellular matrix. J Invest Dermatol. 2012;132(9):2131–3. doi: 10.1038/jid.2012.246. Epub 2012/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feingold KR. Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol. 2012;132(8):1951–3. doi: 10.1038/jid.2012.177. Epub 2012/07/17. [DOI] [PubMed] [Google Scholar]
- 10.Breiden B, Sandhoff K. The role of sphingolipid metabolism in cutaneous permeability barrier formation. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.08.010. Epub 2013/08/21. [DOI] [PubMed] [Google Scholar]
- 11.Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005;124(6):1099–110. doi: 10.1111/j.1523-1747.2005.23726.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin TK, Crumrine D, Ackerman LD, Santiago JL, Roelandt T, Uchida Y, et al. Cellular changes that accompany shedding of human corneocytes. J Invest Dermatol. 2012;132(10):2430–9. doi: 10.1038/jid.2012.173. Epub 2012/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124(2):394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 14.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74(2):180–2. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 15.Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128(4):917–25. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elias PM. The skin barrier as an innate immune element. Sem Immunopath. 2007;29(1):3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Martin M, Martin-Ezquerra G, Man MQ, Hupe M, Youm JK, Mackenzie DS, et al. Expression of epidermal CAMP changes in parallel with permeability barrier status. J Invest Dermatol. 2011;131(11):2263–70. doi: 10.1038/jid.2011.210. Epub 2011/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, et al. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol. 2002;81(5):253–63. doi: 10.1078/0171-9335-00244. Epub 2002/06/18. [DOI] [PubMed] [Google Scholar]
- 19.Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012;122(2):440–7. doi: 10.1172/JCI57416. Epub 2012/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773–86. e1–7. doi: 10.1016/j.jaci.2010.10.018. Epub 2010/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias PM, Goerke J, Friend DS. Mammalian epidermal barrier layer lipids: composition and influence on structure. J Invest Dermatol. 1977;69(6):535–46. doi: 10.1111/1523-1747.ep12687968. [DOI] [PubMed] [Google Scholar]
- 22.Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30(1):89–96. [PubMed] [Google Scholar]
- 23.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156(6):1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celli A, Zhai Y, Jiang YJ, Crumrine D, Elias PM, Feingold KR, et al. Tight junction properties change during epidermis development. Exp Dermatol. 2012;21(10):798–801. doi: 10.1111/j.1600-0625.2012.01573.x. Epub 2012/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber R, Elias PM, Crumrine D, Lin TK, Brandner JM, Hachem JP, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178(5):2252–63. doi: 10.1016/j.ajpath.2011.01.053. Epub 2011/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias PM, Cullander C, Mauro T, Rassner U, Komuves L, Brown BE, et al. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Invest Dermatol Symp Proc. 1998;3(2):87–100. doi: 10.1038/jidsymp.1998.20. [DOI] [PubMed] [Google Scholar]
- 27.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10(3):119–26. [PubMed] [Google Scholar]
- 28.Taieb A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis. 1999;41(4):177–80. doi: 10.1111/j.1600-0536.1999.tb06125.x. [DOI] [PubMed] [Google Scholar]
- 29.Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol. 2003;139(11):1417–22. doi: 10.1001/archderm.139.11.1417. [DOI] [PubMed] [Google Scholar]
- 30.Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75(6):429–33. doi: 10.2340/0001555575429433. [DOI] [PubMed] [Google Scholar]
- 31.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90(2):482–7. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30(4):535–46. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 33.Asai Y, Greenwood C, Hull PR, Alizadehfar R, Ben-Shoshan M, Brown SJ, et al. Filaggrin gene mutation associations with peanut allergy persist despite variations in peanut allergy diagnostic criteria or asthma status. J Allergy Clin Immunol. 2013;132(1):239–42. doi: 10.1016/j.jaci.2013.03.043. Epub 2013/05/21. NEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocr Rev. 1993;14(1):3–19. doi: 10.1210/edrv-14-1-3. Epub 1993/02/01. [DOI] [PubMed] [Google Scholar]
- 35.Ye J, Calhoun C, Feingold K, Elias P, Ghadially R. Age-related changes in the IL-1 gene family and their receptors before and after barrier abrogation. J Invest Dermatol. 1999;112:543. [Google Scholar]
- 36.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 37.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 38.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 39.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118(1):214–9. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 40.O’Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122(4):689–93. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin’s fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127(6):1282–4. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- 42.Presland RB, Rothnagel JA, Lawrence OT. Profilaggrin and the fused S100 family of calcium-binding proteins. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 111–40. [Google Scholar]
- 43.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123(1):23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 44.Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131(11):2233–41. doi: 10.1038/jid.2011.153. Epub 2011/06/10. [DOI] [PubMed] [Google Scholar]
- 45.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103(5):731–41. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 46.Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris: the filaggrin mutation disease. Br J Dermatol. 2013;168(6):1155–66. doi: 10.1111/bjd.12219. Epub 2013/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandilands A, O’Regan GM, Liao H, Zhao Y, Terron-Kwiatkowski A, Watson RM, et al. Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Invest Dermatol. 2006;126(8):1770–5. doi: 10.1038/sj.jid.5700459. [DOI] [PubMed] [Google Scholar]
- 48.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122(Pt 9):1285–94. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proksch E, Folster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43(3):159–69. doi: 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120(1):150–5. doi: 10.1016/j.jaci.2007.04.031. NEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2248–58. doi: 10.1038/jid.2008.74. NEW. [DOI] [PubMed] [Google Scholar]
- 52.Altrichter S, Kriehuber E, Moser J, Valenta R, Kopp T, Stingl G. Serum IgE autoantibodies target keratinocytes in patients with atopic dermatitis. J Invest Dermatol. 2008;128(9):2232–9. doi: 10.1038/jid.2008.80. [DOI] [PubMed] [Google Scholar]
- 53.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121(6):1337–43. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greisenegger EK, Zimprich F, Zimprich A, Gleiss A, Kopp T. Association of the chromosome 11q13.5 variant with atopic dermatitis in Austrian patients. Eur J Dermatol. 2013;23(2):142–5. doi: 10.1684/ejd.2013.1955. Epub 2013/04/06. [DOI] [PubMed] [Google Scholar]
- 55.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Mechin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131(4):1094–102. doi: 10.1016/j.jaci.2012.12.1566. Epub 2013/02/14. [DOI] [PubMed] [Google Scholar]
- 56.Toulza E, Mattiuzzo NR, Galliano MF, Jonca N, Dossat C, Jacob D, et al. Large-scale identification of human genes implicated in epidermal barrier function. Genome biology. 2007;8(6):R107. doi: 10.1186/gb-2007-8-6-r107. Epub 2007/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margolis DJ, Gupta J, Apter AJ, Ganguly T, Hoffstad O, Papadopoulos M, et al. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.015. Epub 2013/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henry J, Hsu CY, Haftek M, Nachat R, de Koning HD, Gardinal-Galera I, et al. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J. 2011;25(5):1567–76. doi: 10.1096/fj.10-168658. Epub 2011/02/02. [DOI] [PubMed] [Google Scholar]
- 59.Wu Z, Hansmann B, Meyer-Hoffert U, Glaser R, Schroder JM. Molecular identification and expression analysis of filaggrin-2, a member of the S100 fused-type protein family. PLoS One. 2009;4(4):e5227. doi: 10.1371/journal.pone.0005227. Epub 2009/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makino T, Takaishi M, Toyoda M, Morohashi M, Huh NH. Expression of hornerin in stratified squamous epithelium in the mouse: a comparative analysis with profilaggrin. J Histochem Cytochem. 2003;51(4):485–92. doi: 10.1177/002215540305100410. Epub 2003/03/19. [DOI] [PubMed] [Google Scholar]
- 61.Makino T, Takaishi M, Morohashi M, Huh NH. Hornerin, a novel profilaggrin-like protein and differentiation-specific marker isolated from mouse skin. J Biol Chem. 2001;276(50):47445–52. doi: 10.1074/jbc.M107512200. Epub 2001/09/27. [DOI] [PubMed] [Google Scholar]
- 62.Wu Z, Meyer-Hoffert U, Reithmayer K, Paus R, Hansmann B, He Y, et al. Highly complex peptide aggregates of the S100 fused-type protein hornerin are present in human skin. J Invest Dermatol. 2009;129(6):1446–58. doi: 10.1038/jid.2008.370. Epub 2008/11/21. [DOI] [PubMed] [Google Scholar]
- 63.Tesfaigzi J, Carlson DM. Expression, regulation, and function of the SPR family of proteins. A review. Cell biochemistry and biophysics. 1999;30(2):243–65. doi: 10.1007/BF02738069. Epub 1999/06/05. [DOI] [PubMed] [Google Scholar]
- 64.Kelsell DP, Byrne C. SNPing at the Epidermal Barrier. J Invest Dermatol. 2011;131(8):1593–5. doi: 10.1038/jid.2011.92. Epub 2011/07/15. [DOI] [PubMed] [Google Scholar]
- 65.Marenholz I, Rivera VA, Esparza-Gordillo J, Bauerfeind A, Lee-Kirsch MA, Ciechanowicz A, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131(8):1644–9. doi: 10.1038/jid.2011.90. Epub 2011/04/15. [DOI] [PubMed] [Google Scholar]
- 66.Epstein TG, LeMasters GK, Bernstein DI, Ericksen MB, Martin LJ, Ryan PH, et al. Genetic variation in small proline rich protein 2B as a predictor for asthma among children with eczema. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2012;108(3):145–50. doi: 10.1016/j.anai.2012.01.004. Epub 2012/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guttman-Yassky E, Suarez-Farinas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124(6):1235–44. e58. doi: 10.1016/j.jaci.2009.09.031. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 68.Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J Allergy Clin Immunol. 2013;132(5):1121–9. doi: 10.1016/j.jaci.2013.08.046. Epub 2013/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, et al. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J Allergy Clin Immunol. 2013;132(5):1111–20. e4. doi: 10.1016/j.jaci.2013.08.027. Epub 2013/09/26. [DOI] [PubMed] [Google Scholar]
- 70.Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009;124(3):496–506. e1–6. doi: 10.1016/j.jaci.2009.06.046. NEW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei medical journal. 2010;51(6):808–22. doi: 10.3349/ymj.2010.51.6.808. Epub 2010/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9(5):437–46. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29(2):175–8. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 74.Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126(7):1609–21. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- 75.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78(1):27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 76.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96(4):523–6. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 77.Muller FB, Huber M, Kinaciyan T, Hausser I, Schaffrath C, Krieg T, et al. A human keratin 10 knockout causes recessive epidermolytic hyperkeratosis. Hum Mol Genet. 2006;15(7):1133–41. doi: 10.1093/hmg/ddl028. [DOI] [PubMed] [Google Scholar]
- 78.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206(5):1135–47. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khnykin D, Ronnevig J, Johnsson M, Sitek JC, Blaas HG, Hausser I, et al. Ichthyosis prematurity syndrome: clinical evaluation of 17 families with a rare disorder of lipid metabolism. J Am Acad Dermatol. 2012;66(4):606–16. doi: 10.1016/j.jaad.2011.04.014. Epub 2011/08/23. [DOI] [PubMed] [Google Scholar]
- 80.Elias PM, Williams ML, Crumrine D, Schmuth M. Ichthyoses - clinical, biochemical, pathogenic, and diagnostic assessment. Basel: S. Kargar AG; 2010. p. 144. [Google Scholar]
- 81.Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. 1996;107(4):558–64. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- 82.Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128(1):79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmuth M, Yosipovitch G, Williams ML, Weber F, Hintner H, Ortiz-Urda S, et al. Pathogenesis of the permeability barrier abnormality in epidermolytic hyperkeratosis. J Invest Dermatol. 2001;117(4):837–47. doi: 10.1046/j.0022-202x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- 84.Voegeli R, Rawlings AV, Breternitz M, Doppler S, Schreier T, Fluhr JW. Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br J Dermatol. 2009;161(1):70–7. doi: 10.1111/j.1365-2133.2009.09142.x. [DOI] [PubMed] [Google Scholar]
- 85.Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121(2):345–53. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 86.Nylander-Lundqvist E, Back O, Egelrud T. IL-1 beta activation in human epidermis. J Immunol. 1996;157(4):1699–704. [PubMed] [Google Scholar]
- 87.Wood LC, Stalder AK, Liou A, Campbell IL, Grunfeld C, Elias PM, et al. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp Dermatol. 1997;6(2):98–104. doi: 10.1111/j.1600-0625.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 88.Kupper TS. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J Clin Invest. 1990;86(6):1783–9. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elias PM, Ansel JC, Woods LD, Feingold KR. Signaling networks in barrier homeostasis. The mystery widens. Arch Dermatol. 1996;132(12):1505–6. [PubMed] [Google Scholar]
- 90.Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128(5):1067–70. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Demerjian M, Choi EH, Man MQ, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol. 2009;18:643–9. doi: 10.1111/j.1600-0625.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126(9):2074–86. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- 93.Sakai T, Hatano Y, Zhang W, Fujiwara S. Defective maintenance of pH of stratum corneum is correlated with preferential emergence and exacerbation of atopic-dermatitis-like dermatitis in flaky-tail mice. J Dermatol Sci. 2014 doi: 10.1016/j.jdermsci.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Hachem JP, Roelandt T, Schurer N, Pu X, Fluhr J, Giddelo C, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J Invest Dermatol. 2010;130(2):500–10. doi: 10.1038/jid.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Houben E, Holleran WM, Yaginuma T, Mao C, Obeid LM, Rogiers V, et al. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006;47(5):1063–70. doi: 10.1194/jlr.M600001-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Weidinger S, Rodriguez E, Stahl C, Wagenpfeil S, Klopp N, Illig T, et al. Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J Invest Dermatol. 2007;127(3):724–6. doi: 10.1038/sj.jid.5700630. [DOI] [PubMed] [Google Scholar]
- 97.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118(1):3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- 98.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129(8):1892–908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 99.Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133(7):1706–9. doi: 10.1038/jid.2013.128. Epub 2013/06/14. [DOI] [PubMed] [Google Scholar]
- 100.Garg A, Chren MM, Sands LP, Matsui MS, Marenus KD, Feingold KR, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137(1):53–9. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- 101.Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117(2):309–17. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 102.Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124(3):587–95. doi: 10.1111/j.0022-202X.2005.23589.x. NEW. [DOI] [PubMed] [Google Scholar]
- 103.Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R367–72. doi: 10.1152/ajpregu.2000.278.2.R367. NEW. [DOI] [PubMed] [Google Scholar]
- 104.Denda M, Tsuchiya T, Hosoi J, Koyama J. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol. 1998;138(5):780–5. doi: 10.1046/j.1365-2133.1998.02213.x. NEW. [DOI] [PubMed] [Google Scholar]
- 105.Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1657–62. doi: 10.1152/ajpregu.00010.2006. NEW. [DOI] [PubMed] [Google Scholar]
- 106.Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120(3):456–64. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 107.Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41(3):282–95. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elias PM, Sun R, Eder AR, Wakefield JS, Man MQ. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Exp Rev of Dermatol. 2013;8(1):27–36. [Google Scholar]
- 109.Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Hogh JK, et al. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010;65(7):911–8. doi: 10.1111/j.1398-9995.2010.02326.x. Epub 2010/02/06. [DOI] [PubMed] [Google Scholar]
- 110.Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119(1):166–73. doi: 10.1046/j.1523-1747.2002.01833.x. NEW. [DOI] [PubMed] [Google Scholar]
- 111.Tawada C, Kanoh H, Nakamura M, Mizutani Y, Fujisawa T, Banno Y, et al. Interferon-gamma Decreases Ceramides with Long-Chain Fatty Acids: Possible Involvement in Atopic Dermatitis and Psoriasis. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.364. Epub 2013/09/07. NEW. [DOI] [PubMed] [Google Scholar]
- 112.Kita K, Sueyoshi N, Okino N, Inagaki M, Ishida H, Kiso M, et al. Activation of bacterial ceramidase by anionic glycerophospholipids: possible involvement in ceramide hydrolysis on atopic skin by Pseudomonas ceramidase. Biochem J. 2002;362(Pt 3):619–26. doi: 10.1042/0264-6021:3620619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohnishi Y, Okino N, Ito M, Imayama S. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clinical and diagnostic laboratory immunology. 1999;6(1):101–4. doi: 10.1128/cdli.6.1.101-104.1999. Epub 1999/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002;119(2):433–9. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 115.Loiseau N, Obata Y, Moradian S, Sano H, Yoshino S, Aburai K, et al. Altered sphingoid base profiles predict compromised membrane structure and permeability in atopic dermatitis. J Dermatol Sci. 2013;72(3):296–303. doi: 10.1016/j.jdermsci.2013.08.003. Epub 2013/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, et al. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol. 2013;22(1):30–5. doi: 10.1111/exd.12047. Epub 2012/11/24. [DOI] [PubMed] [Google Scholar]
- 117.Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008;128(5):1329–31. doi: 10.1038/sj.jid.5701138. [DOI] [PubMed] [Google Scholar]
- 118.Sawada E, Yoshida N, Sugiura A, Imokawa G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: an implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci. 2012;68(1):25–35. doi: 10.1016/j.jdermsci.2012.07.004. Epub 2012/08/14. [DOI] [PubMed] [Google Scholar]
- 119.Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130(10):2511–4. doi: 10.1038/jid.2010.161. Epub 2010/06/25. [DOI] [PubMed] [Google Scholar]
- 120.Park YH, Jang WH, Seo JA, Park M, Lee TR, Kim DK, et al. Decrease of ceramides with very long-chain fatty acids and downregulation of elongases in a murine atopic dermatitis model. J Invest Dermatol. 2012;132(2):476–9. doi: 10.1038/jid.2011.333. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 121.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53(12):2755–66. doi: 10.1194/jlr.P030338. Epub 2012/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131(10):2136–8. doi: 10.1038/jid.2011.175. Epub 2011/07/01. [DOI] [PubMed] [Google Scholar]
- 123.Bisgaard H, Simpson A, Palmer CN, Bonnelykke K, McLean I, Mukhopadhyay S, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5(6):e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, et al. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008;128(8):1930–9. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 125.Novak N, Baurecht H, Schafer T, Rodriguez E, Wagenpfeil S, Klopp N, et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol. 2008;128(6):1430–5. doi: 10.1038/sj.jid.5701190. [DOI] [PubMed] [Google Scholar]
- 126.Ross-Hansen K, Ostergaard O, Tanassi JT, Thyssen JP, Johansen JD, Menne T, et al. Filaggrin is a predominant member of the denaturation-resistant nickel-binding proteome of human epidermis. J Invest Dermatol. 2014;134(4):1164–6. doi: 10.1038/jid.2013.445. Epub 2013/10/26. NEW. [DOI] [PubMed] [Google Scholar]
- 127.Hon KL, Leung AK, Barankin B. Barrier Repair Therapy in Atopic Dermatitis: An Overview. Am J Clin Dermatol. 2013 doi: 10.1007/s40257-013-0033-9. Epub 2013/06/13. [DOI] [PubMed] [Google Scholar]
- 128.Otsuka A, Doi H, Egawa G, Maekawa A, Fujita T, Nakamizo S, et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J Allergy Clin Immunol. 2014;133(1):139–46. e10. doi: 10.1016/j.jaci.2013.07.027. Epub 2013/09/24. [DOI] [PubMed] [Google Scholar]
- 129.Stout TE, McFarland T, Mitchell JC, Appukuttan B, Timothy Stout J. Recombinant filaggrin is internalized and processed to correct filaggrin deficiency. J Invest Dermatol. 2014;134(2):423–9. doi: 10.1038/jid.2013.284. Epub 2013/06/25. NEW. [DOI] [PubMed] [Google Scholar]