Abstract
Human platelets arise as subcellular fragments of megakaryocytes in bone marrow. The physiologic demand, presence of disease such as cancer, or drug effects can regulate the production circulating platelets. Platelet biology is essential to hemostasis, vascular integrity, angiogenesis, inflammation, innate immunity, wound healing, and cancer biology. The most critical biological platelet response is serving as “First Responders” during the wounding process. The exposure of extracellular matrix proteins and intracellular components occurs after wounding. Numerous platelet receptors recognize matrix proteins that trigger platelet activation, adhesion, aggregation, and stabilization. Once activated, platelets change shape and degranulate to release growth factors and bioactive lipids into the blood stream. This cyclic process recruits and aggregates platelets along with thrombogenesis. This process facilitates wound closure or can recognize circulating pathologic bodies. Cancer cell entry into the blood stream triggers platelet-mediated recognition and is amplified by cell surface receptors, cellular products, extracellular factors, and immune cells. In some cases, these interactions suppress immune recognition and elimination of cancer cells or promote arrest at the endothelium, or entrapment in the microvasculature, and survival. This supports survival and spread of cancer cells and the establishment of secondary lesions to serve as important targets for prevention and therapy.
Keywords: Platelet, TCIPA, Metastasis, Thrombosis, Extravasation, CTC
1 Historical perspectives
Twenty years ago when we first reviewed this topic [1], most researchers in the cancer field were skeptical about the concept that platelets played a major role as “first responders” in metastasis, despite our own pioneering work and that of others. In the time between, much of the early work has been corroborated, and remains substantiated as new techniques and investigators enter the field. In this review, we revisit the topic with the latest in accumulated evidence; evidence that clearly points to a role for platelet functions in the hemostatic microenvironment being subverted and employed by tumor cells to facilitate tumor progression, invasion, and metastasis.
1.1 Leeches, light microscopes, “Blutplättchen”, and bone marrow
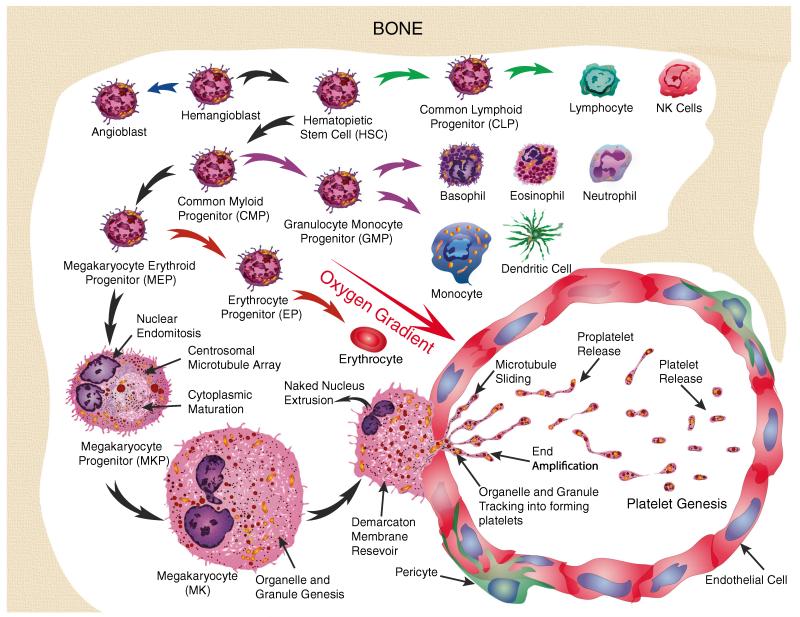
The vital ebb and flow of blood as a life force has always captivated humankind. Blood was considered to be the first of four essential “humors” by the ancient Greeks. The ancient Mesopotamian, Egyptian, Greek, Roman, Indian, and Chinese physicians practiced bloodletting and often used the application of leeches to treat diseases until the practice reached its height in the 1800s. The use of leeches continues to the present following the Food and Drug Administration-approved use of Hirudo medicinalis in modern medicine [2, 3]. The anticoagulant hirudin identified from leech saliva can help relieve venous insufficiency in skin and reattachment surgery [2, 3]. Despite keen medical interest in blood over the ages, the crucial cellular components that contribute to clot formation were not readily identified. Even after the invention of the light microscope in 1590 by Dutch spectacle makers, Zaccharias and Hans Janssen descriptions of blood cells were not immediately forthcoming. It was not until 1658 that Dutch biologist Jan Swammerdam identified red blood cells [4, 5]. Nevertheless, the key subcellular factors were not described until 1842. Within that year, the French physician Alfred Donne first described “particles” in the blood—red globules, white globules, and globulins (little globules) in a lecture to the Academie des Sciences of Paris. A month later, William Addison published hand drawings of platelet–fibrin thrombus referring to them as extremely minute granules [6]. In 1846, Gustav Zimmerman first studied anticoagulated blood by aspirating it into a potassium ferrocyanide solution describing “billions” of “Elementarkörperchen ” or “elementary corpuscles”. Subsequently in 1865, Schultze described the tiny spherules of the blood [7]. Then in 1882, Giulio Bizzozero suggested the term “Blutplattchen ” or “blood platelets” for these corpuscles [8, 9]. Hand-drawn images by Bizzozero from observations using a Hartmack light microscope showed fused platelets with stellate fibrin threads in freshly acquired blood samples as well as observations of their increased stickiness to damaged blood vessels [8, 9]. Rudolf Virchow first described clot formation within the blood vessels of a living animal in 1856 [10]. In 1869, Bizzozero also observed large bodies in bone marrow, irregular giant cells with a diameter of 25 to 65 μm, with a budding central nucleus that were likely platelet-producing megakaryocytes (MK)s, but their biological significance was unknown [11]. Platelet aggregates with bacteria were observed later by Osler and Schäfer in 1873 [12]. Then, although their existence had been known for some time, large rare marrow cells were first given the name “megakaryocyte” by Howell in 1890 and described by detailed camera lucida drawings [13]. Later, the connection between blood “plates” and their release from megakaryocytes was made by Wright in 1906 [14, 15]. We now know that mature megakaryocytes are among the scarcest (0.01–0.5 % of nucleated cells) and largest (50–100 μm) cells in human bone marrow and generate platelets (Fig. 1) [16, 17]. Nonetheless, many early hand-drawn observations of platelets and megakaryocytes using simple light microscopy remain remarkable testaments to the powers of keen observation.
Fig. 1.
Platelet genesis occurs in the bone marrow. Hemangioblasts initially undergo divergence to form two primary lineages of cells, either angioblasts or hematopoietic stem cells (HSC). HSC subsequently form common lymphoid progenitor (CLP) or common myeloid progenitor (CMP) cells. CLP give rise to lymphocytes and other lymphoid lineage cell types while CMP generate myeloid cell types. Granulocyte monocyte progenitor (GMP) lineages include basophils, eosinophils, neutrophils, monocytes, and dendritic cells. In contrast, megakaryocyte erythroid progenitors (MEP) give rise to megakaryocytes and erythroid cells. Megakaryocyte progenitor (MKP) cell progression involves nuclear endomitosis leading to polyploidy to as high as 128n. These changes are accompanied by centrosomal microtubule array formation and cytoplasmic maturation. The increase in DNA, cytoplasm, granule, and organelle formation significantly increases the size of megakaryocytes (MK) up to 65 μm in diameter in preparation for platelet genesis. MK migrates along an oxygen gradient and send platelet-generating processes into the lumen of bone marrow capillaries that fill along sliding microtubule tracks with organelles and granules. Platelets are formed by cytoplasmic end amplification followed by proplatelet release and maturation
1.2 Trousseau, thrombophlebitis, and occult cancer
The first known association between tumors and blood changes was around 1000 BC by the Indian surgeon, Sushruta in the Sanskrit text on surgery, Sushruta Samhita. This text describes six main types of tumors. In one, “Raktaja arbuda” is described as a tumor that enters the blood and compresses and constricts blood vessels.
It was not until Armand Trousseau in his 1865 lecture, “Phlegmasia alba dolens”, which is Latin for “inflammation white leg” or “milk leg” due to lack of blood in the extremity caused by deep vein thrombosis, that this condition was associated with cancer [18]. Trousseau’s lecture was the first description of migratory deep vein thrombosis as a prognostic indicator of a “deep-seated concealed cancer” associated with gastric malignancy [18]. It was based on series of case studies including one of his colleagues. Ironically, on 1 January 1867, Trousseau was stricken with the phlebitis syndrome that now bears his name in his upper left extremity [19]. Telling his student, Peter “I am lost, the phlebitis that has just appeared leaves me no doubt about the nature of my illness,” he thereby predicted having his own occult gastric cancer that caused his death a few months later [19].
Later in 1900, Osler and McCrae, among others around that time period, reported on the association of idiopathic phlebitis and gastric cancer [20]. Trousseau’s syndrome found greater acceptance in 1938 following a study by Sproul, where she performed 4,258 autopsies on patients with carcinomas [21]. She discovered multiple thromboses associated with 31.3 % of carcinoma cases in the body and tail of the pancreas, and 9.7 % in the head of the pancreas among other cancer sites [21]. Over the next several decades, the association with thrombophlebitis was discovered for numerous other cancers in case reports and cohort studies [22-39]. Some of these early studies associated thrombophlebitis with mucin-producing carcinomas [27, 31]. Later in 1973, extracts from mucinous adenocarcinoma caused intravascular coagulation and death in rabbits [40]. Thrombophlebitis associated with cancer is often migratory or recurrent [34]. Furthermore, affected veins do not consistently show tumor invasion or tumor emboli indicating the presence of cells is not required for thrombus formation [34]. Blood coagulation and fibrinolysis accompanies the development of lung carcinoma and other tumors [41, 34]. In rats, thromboplastic and fibrinolytic properties were observed in a transplantable rat tumor model [42-44]. Causes of hypercoagulability were thought by some to involve increased platelet adhesiveness [45]. Another analysis of 14,000 patient blood smears from patients bearing tumors of different types showed a significant increase in counts above 400,000 platelets/cubic milliliter [46]. The importance of tumor cell–platelet emboli formation and their contribution to the formation of metastasis was recognized in the late 1960s and early 1970s [47-50, 1, 51, 52]. The effects of thrombosis on metastasis were also reported based on early rabbit transplantable tumor models of Brown–Pearce carcinoma and the V2 carcinoma [53-57]. The importance of tumor cell-containing emboli to the formation of metastasis was first quantified using transplantable mouse tumors [58]. Tumor cell-induced platelet aggregation (TCIPA) was found to be critical for heterotypic aggregate formation, but could be inhibited by prostaglandins that prevent antiplatelet aggregation [59-63]. Subsequently, the role of shed tumor cell vesicles, now known as exosomes, on platelet aggregation was found to be influenced by the protease cathepsin B [64]. The light microscope revealed certain aspects of tumor cell–platelet interactions, but certain details would await improvements in technology. The invention of the scanning and transmission electron microscopes helped clarify many of the details of megakaryocyte and platelet structure as well as platelet–tumor cell interactions [65-74].
2 Megakaryocyte and platelet biology
Historically, the light microscope revealed aspects of tumor cell–platelet interactions. However, it was not until the invention of the scanning and transmission electron microscopes that details of megakaryocyte and platelet structure as well as platelet–tumor cell interactions were revealed [65-67, 75, 68, 69, 62, 72]. Unlike cancer cells, megakaryocytes are genetically stable and provide the genetic instructions along with the molecular and structural biomass for platelet genesis (Fig. 1). Platelets lack nuclei and the capacity for genetic adaptation. Intrinsically then, understanding the molecular, cellular, and systemic biology of megakaryocytes and platelets is an important starting point for understanding their role in cancer.
2.1 Megakaryocytes and platelet progenitors
2.1.1 Niches, riches, and bone marrow switches
Systemic, circulating/humoral, and microenvironmental factors in the bone marrow regulate physiologic responses to the body’s demand for platelets [76, 77, 69, 78]. Bone marrow exists in two primary forms, where the yellow bone marrow consists mostly of fat cells, and the red bone marrow contains the hematopoietic cell populations that generate platelets, as well as the red and white cells [79, 80]. Both forms of bone marrow contain extensive numbers of blood vessels and capillaries [81]. Microscopically, osteoblastic niches are spatially separated from vascular niches that provide a systemically enriched oxygen source within the bone marrow [82-85] (Fig. 1). The spatial separation of these niches effectively establishes a decreasing oxygen gradient [86, 87], as well as microenvironmental gradients of cytokines and chemokines, growth factors, and calcium in the osteoblastic niche. These gradients collectively regulate megakaryopoiesis or the formation of MK that produce platelets. Gradients of extracellular matrix proteins also provide migratory switches that drive MK toward the microvasculature during the later stages of platelet formation [88-91].
Megakaryopoiesis begins in the bone marrow with hematopoiesis, where hemangioblasts that are present mainly in bone marrow give rise to hematopoietic stem cells (HSCs) and multipotent progenitor cells (MPP) [92, 77, 78, 93]. Along the developmental cascade, a key separation in progenitor lineages occurs between the major lymphoid progenitors along with granulocytic progenitors versus the major erythromegakaryocytic progenitor cells [92, 77, 78, 93]. Common myeloid progenitor cells (CMP) give rise to megakaryocyte-erythroid progenitor (MEP) cells that are in turn delineated into megakaryopoietic progenitors (MKP) [94]. Ultimately, MKP undergo a complicated maturation process from immature to mature megakaryocytes before finally generating platelets [92, 77, 69, 95, 93].
3 Transcription factors, microRNAs, and hematopoiesis
Megakaryocyte lineage derivation is mediated by a series of transcription factors. Runt-related transcription factor 1 (RUNX1) is crucial for the entire hematopoietic process. In the case of RUNX1 haploinsufficiency as an example, thrombocytopenia and platelet dysfunction in addition to acute leukemia, are manifest by a fivefold reduction in platelet 12-lipoxygenase and a decrease in platelet production of 12(S)-HETE on agonist stimulation [96]. RUNX1 mutations also impact a number of cancers. For example, RUNX1 can work alone or in cooperation with the Ets transcription factor to promote PSA expression in prostate cancer [97]. The commitment and development of HSCs to form MEP and MK lineages relies on increases in erythroid transcription factor also known as GATA-binding factor 1 (GATA-1) and downregulation of PU.1 [98, 99]. Increased levels of GATA-1 along with Friend of GATA (FOG1) cofactor and the downregulation of Ikaros all help to generate MEP lineage cells [100-103]. As GATA-1 levels increase, diploid MKP that proliferate as part of the MEP stop dividing, but retain the capacity for DNA replication and cytoplasmic maturation. Continued RUNX1 production along with the loss of myosin heavy chain 10 (MYH10) expression stimulates the formation of immature MK [76, 104, 105]. The final stages of MK maturation as well as the initiation of platelet formation are stimulated by nuclear factor erythroid-derived 2 (NFE2) production [104, 106, 107]. NFE2 has also been shown to regulate thromboxane synthase (TXAS), a key enzyme in the regulation of platelet activation and aggregation during thrombogenesis, during MK maturation [108]. This complex cascade of changes in these transcription factors tightly regulates platelet genesis while priming them for their biological roles.
Many noncoding RNA molecules are involved in the genesis of megakaryocytes and platelets [109, 110]. In the bone marrow, microRNA (miR) 125a controls the size of the stem cell population by regulating HSC apoptosis [111]. At the MEP stage as well, miR150 negatively regulates c-myb production at the 3′UTR of the mRNA [112, 113]. MYB is a negative regulator of megakaryopoiesis and the resulting loss of protein expression stimulates MEP to differentiate into MK [114-116]. In other studies, loss of miR145 and increased expression of Fli-1A were reported in 5q-syndrome MDS, with anemia and thrombocytosis [117]. A variety of other miRNAs are also involved in the pathogenesis of myeloproliferative neoplasms [118, 119]. In contrast, there is likely to be significant potential in miRNA-based platelet therapies [120].
4 Surface receptors and megakaryopoiesis
A number of cell surface receptors serve as lineage markers while functioning to direct hematopoiesis then megakaryopoiesis in preparation for platelet genesis.
Certain cell surface receptors are retained by subpopulations of activated HSC that differentiate into immature and then mature MKP. These markers include stromal-derived factor-1 (SDF-1), CXC chemokine receptor type 12 (CXCR12), along with CXC chemokine receptor type 4 (CXCR4) and the thrombopoietin receptor, which is known as myeloproliferative leukemia virus oncogene (MPL) or cluster of differentiation 110 (CD110) [121]. MPL/CD110 levels significantly increase during the progressive maturation of MK in response to thrombopoietin (THPO/TPO) also known as megakaryocyte growth and development factor (MGDF), which is a key ligand in megakaryopoiesis [122-126].
Early clinical trials revealed that THPO administration caused temporary morphological changes in bone marrow constituents that mimicked chronic myeloproliferative diseases, but that these reversed within 3 months of discontinuing the treatment [127]. Administration of recombinant THPO can increase megakaryopoiesis and platelet production in subjects undergoing radiation and chemotherapy with no apparent impact on platelet aggregation. However, as some individuals developed neutralizing antibodies, this approach was abandoned in pursuit of alternate means to regulate Mpl [128]. With that said, a recent study that examined the effect of the hematopoietic microenvironment on leukemic stem cell survival found a strong correlation between acute myelogenous leukemia (AML) relapse and elevated levels of THPO and Mpl that may impact resistance to daunorubicin [129].
Along with MKP, the fibrinogen receptor known as integrin αIIbβ3 (GPIIb/IIIa or CD41) and von Willebrand factor (vWF) expression levels also significantly increase [130-134]. In contrast, CD34, CD45, CD150, Tie-2, and c-kit levels decrease once progenitors commit to the MK lineage [92, 135-138]. These changes in cell surface receptor profiles accompany a decrease in proliferation by MKP prior to the next stage of development involving endomitosis.
4.1 Got DNA? Got cytoplasm?
Endomitosis ensues with the reproduction of nuclear elements that ultimately results in polyploidy [77, 69]. This process involves diploid megakaryocytes that proceed through S phase and progress through mitotic anaphase. Anaphase progresses through the separation of paired sister chromosomes followed by cleavage furrow formation but halts at cytokinesis. Furrow regression then generates a tetraploid cell that reenters G1 arrest. This process continues until MK accumulate a DNA content of 4n, 8n, 16n, 32n, 64n, and even 128n in a single multilobed nucleus and then finally mature before beginning proplatelet formation [139]. This elaborate amplification of nuclear material helps support extensive organelle synthesis, as well as dramatic cytoplasmic maturation and expansion needed to produce platelets.
Cytoplasmic maturation in MK that accompanies endomitosis is designed to generate the extensive membrane, cytoskeletal, and organelle stores needed to support platelet production [69, 140, 141]. Structurally, this process forms a complex membrane network of tubules and cisternae termed either demarcation membrane system (DMS) or invaginated membrane system (IMS) [142, 143]. Membrane and cytoskeletal remodeling involves a Cdc42-interacting protein 4 (CIP4), which is an F-Bin–amphiphysin–Rvs (F-BAR) protein that localizes to membrane phospholipids through its BAR domain and interacts with Wiskott–Aldrich syndrome protein (WASP) via its SRC homology 3 (SH3) domain to coordinate platelet production [144]. Motor protein dynamin 3 (DNM3) participates in membrane rearrangements during megakaryopoiesis [145]. Structural proteins such as spectrin among others, form a two-dimensional lattice that stabilizes the IMS/DMS and becomes an important structural component of platelet precursors known as proplatelets [146].
5 Platelet granule formation
Alpha granule biogenesis leads to the formation of discrete populations as recently observed by three-dimensional electron tomography analysis [147]. These studies revealed that membrane-bound organelles display high variability in morphology, size, and luminal content that fall into three categories (Fig. 2)—(1) spherical granules that have both electron dense and electron lucent zones and 12 nm–vWF tubules; (2) 50-nm-wide tubular organelles containing membrane-bound proteins GLUT3 and αIIbβ3 integrin along with abundant fibrinogen, albumin, and some thromboglobulin; and (3) a population with 18.4-nm crystalline cross-striations. Other studies suggest that α-granule populations are differentially segregated in a zonal manner [148]. Many of the key granule vesicle trafficking proteins were discovered using samples from patients with α-granule deficiencies [149, 150]. Although the exact mechanism involved in forming the various α-granule subtypes remains unclear, some of the causative genes, NBEAL2, VPS33B, and VPS16B, have recently been identified [151-155].
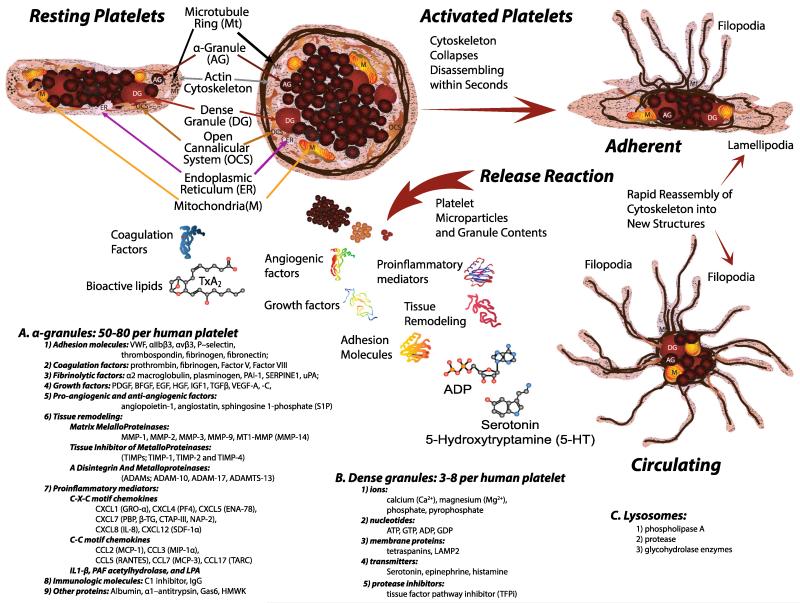
Fig. 2.
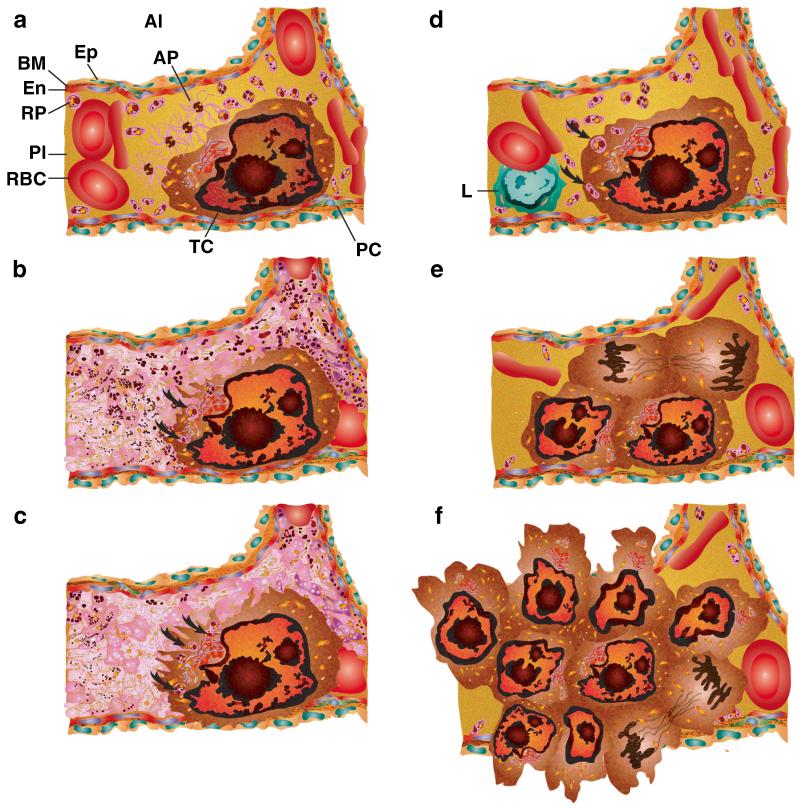
Resting platelets maintain their discoid shape using a structural ring of microtubules (Mt) and actin cytoskeleton. The plasma membrane is connected to an internal membrane reservoir called the open canalicular system (OCS). Platelets also contain organelles including endoplasmic reticulum (ER), and mitochondria (M). Resting platelets carry stores of bioactive contents in a granules (a), dense granules (b), and lysosomes (c) that are released following activation. Once activated, the platelet cytoskeleton collapses followed by extensive shape change depending upon the trigger stimulus. Lamellipodia are formed when in contact with flat surfaces such as extracellular matrix, and facilitate platelet migration. Filopodia that promote contact with other platelets or cells are formed by adherent and circulating platelets in suspension
Dense granule biogenesis, by contrast, involves a specialized mechanism that integrates secretory and the endocytic pathways [156]. Multidrug resistance protein 4 (MRP4) is involved in the transport of nucleotides and other factors into dense granules [157, 158]. Recent data suggest that Rab32 and Rab3 8 transmembrane proteins employ an internal secretory pathway through Golgi complexes and then endosomes to selectively target dense granules. Rab32 and Rab38 define a biosynthetic transport pathway from early endosome-associated tubules to maturing dense granules after they originate from multivesicular bodies. These studies used MEG-01 cells that were analyzed by transmission electron microscopy, spinning-disk confocal fluorescence microscopy of cells immunostained for lysosomal-associated membrane protein-2 (LAMP2) and MRP4, or live MEG-01 cells expressing the dense granule markers VMAT2-Cherry and LAMP2-GFP [156].
5.1 Getting in the blood
During this maturation process, MKs migrate from the osteoblastic to the vascular niche to elicit the final stages of platelet formation [159-161]. Collagen I is the primary protein in the osteoblastic niche involved in interactions between the extracellular matrix and α2β1 on the surfaces of MK cells [162-164]. These interactions with collagen I facilitate the differentiation of HSC to MK while inhibiting proplatelet production [165]. Maturing MKs migrate to the perivascular surfaces of bone marrow sinusoidal endothelial cells within vascular niches [166, 69, 167, 168]. The perivascular surfaces contain a number of extracellular matrix proteins including, vitronectin, collagen IV, laminin, fibronectin, fibrinogen, and vWF along with pericytes [169-173, 69, 174, 175]. Fibrinogen interactions with integrin αIIbβ3 receptors on the MK surfaces in particular help govern migration, maturation, and proplatelet formation [176, 177].
Once in contact with the perivascular surfaces of sinusoidal endothelial cells, mature MKs begin platelet genesis. The nucleus of MKs is extruded as platelet formation ensues [178]. MKs extend long-branching processes known as proplatelets into the lumens of bone marrow sinusoidal blood vessels. This process occurs by erosion of the MK pole involved in genesis. Centrosomes disassemble and microtubules made mainly of β1-tubulin translocate to the cell cortex accompanied by pseudopod formation as proplatelets begin forming [69, 78]. Podosomes protrude through basement membrane by degrading the perivascular matrix of sinusoidal vessels [167]. Proplatelet shafts fill with microtubule bundles then elongate to loop around and reenter the shaft, forming buds at the shaft tip [179]. Microtubule sliding drives shaft elongation as they polymerize their free ends in concert with dynein-driven sliding of overlapping microtubules [179, 69]. Microtubule sliding occurs at 4 to 5 μm/min to reach lengths of about 0.5 to 1.0 mm [179, 69]. Microtubule bundles provide physical tracks for the bidirectional movement of mitochondria and granules into proplatelet tips [180]. The formation of microtubule bundles depends upon the β1-tubulin isoform gene Tubb1 and the tubulin binding protein RanBP 10 [181, 173, 182, 183]. Proplatelets in vitro or within bone marrow sinusoids appear by intravital microscopy as dumbbell-shaped elongations or globules that shear off into the vascular lumen [181, 184, 69]. Intravascular proplatelets continue their morphogenesis to generate individual platelets aided by intravascular shear forces [185, 184, 69, 186]. The blood flow rate and fluid shear in bone marrow sinusoids is less than other areas of the body and is likely to influence platelet formation [181, 184, 187].
5.1.1 Platelet structural, molecular and cellular biology of mice or men
There are significant differences in resting platelet properties between mice and men [188]. The diameter of human platelets is 1.0 to 2.0 μm as compared to those that are smaller in the mouse, 0.5–1.0 μm [188]. Platelet counts are also significantly different ranging from 150 to 400×109/L in humans to 1,000–1,500×109/L in mice [188]. Similarly, platelet lifespan in human circulation is from 8 to 10 days whereas mouse platelets last for 3–4 days [188]. Likewise, the platelet alpha and dense granule number per platelet is higher in humans compared to mice [188].
6 Platelet proteomics
Platelets lack nuclei and robust protein synthesis machinery making them model candidates for proteomic analysis. Proteomic studies have revealed various numbers of proteins in resting platelets [189-194]. One study identified 1,507 proteins present in resting platelets 190 of which were membrane proteins and 262 phosphoproteins [192]. This approach has been useful in evaluating alterations in the proteome of platelet diseases [195, 196]. Comparative analyses of platelet-derived microparticles [197-199] produced upon activation also revealed proteome differences in their profile and product levels depending on the platelet stimulus applied [200]. Variations in the proteomic profiles present in activated platelets also vary depending on the treatment [201-203]. Many recent advances can be found in the “Platelet Web” at http://plateletweb.bioapps.biozentrum.uni-wuerzburg.de/plateletweb.php.
7 Platelet lipidomics and bioactive lipids
The role of bioactive lipids in platelet biology began with the study of prostaglandin endoperoxides [204]. The focus on platelet eicosanoid biology remained an important area of research throughout the next several decades [205-207]. Platelet activation not only stimulates the production of pro-aggregatory prostaglandin TxA2 but also various 12/15-lipoxygenase (LOX) products that mediate immune-regulatory activities, namely 12-HETE and 15-HETE [208, 209]. In particular the bioactive arachidonic acid metabolite, 12(S)-HETE, produced by platelets was found to contribute to endothelial cell retraction and extravasation [210, 211].
Lipids also contribute to platelet senescence [212], hemostasis, and other disease states [213-216]. Platelet phospholipids, sphingolipids, lysophosphatidic acid, and platelet-activating factor also play an important role in platelet regeneration therapy that involves platelet-rich plasma injections [217, 214, 218-220].
8 Platelet cytoskeleton and morphology
Ultrastructurally, resting platelets are organized into three zones: (1) a peripheral zone includes a fluffy glycocalyx surface coating, platelet membrane, and membrane cytoskeleton; (2) a sol–gel zone consists of a microtubule system, an open canalicular system (OCS), and the dense tubular system (DTS); (3) an organelle zone contains α-granules, dense granules, lysosomal granules, mitochondria, and glycogen granules [72, 73].
9 Dynamic microtubule rings, discoid shapes, and structural collapse
Morphologically, platelet maturation involves a cytoskeletal network of microtubules, actin, and spectrin among other proteins [221, 222, 181, 78, 74]. In resting platelets, a coil of microtubules forms around the platelet margin into a discoid shape that is modulated by RanBP10 [223, 224, 173, 225, 226] (Fig. 2). At any given time in resting platelets, 50 to 60 % of the total platelet heterodimeric tubulin β1, β2, β4, or β5 subunits are in the polymeric state. Time-lapse fluorescence studies of resting platelets either expressing end-binding protein-green fluorescent protein (EB3-GFP) chimeric fusion protein, or that incorporated end-binding protein 1 (EB1), which binds to polymerizing microtubule ends, revealed dynamic microtubule remodeling within the marginal band [225]. The resting platelet marginal band consists of multiple coils of stable and dynamically recycled microtubules that polymerizes in a bipolar fashion and shrinks with age or paclitaxel treatment [225]. Once stimulated by thrombin, adhesion, or other stimuli, the marginal band fragments leading to structural collapse of platelet disks. These fragmented microtubules then repolymerize from multiple nucleation centers, possibly of γ-tubulin rather than the centrosomes typical of nucleated cells [225]. This activation process helps initiate the axial growth of microtubule ends that bind EB1 [225]. Microtubules help form the core structure for the dynamic alterations that occur in the platelet cytoskeleton.
10 Resting platelet cytoskeleton
Along with microtubules, membrane composition and cytoplasmic actin scaffolding tightly regulate the structural morphology of platelets [227, 228] (Fig. 2). Resting platelet surfaces lack projections such as microvilli but are etched with plasma membrane pits that form the OCS of platelets [229, 230, 73]. The OCS contributes additional plasma membrane surface area of up to 420 % to platelets as they spread over exposed wounds or damaged endothelium [231]. The membrane cytoskeleton proteins include spectrin, adducin, filamin (FLN), actin, and vWFR. Around 2,000 tetramers of α and β spectrin (fodrin) molecules assemble into a heterodimeric two-dimensional lattice that is regulated by calpain, where each β spectrin free amino terminus can link to 2–5,000 actin filament ends into a space-filling structural latticework [232]. Nearly 8,000 α and β adducin molecules cap actin filament barbed ends and complex with spectrin in the resting platelet [232]. Similar to spectrin, nearly 12,500 filamin A, B, and C molecules complex with the GP1bα chain of the vWF receptor to strengthen the spectrin–actin network interactions with the plasma membrane [233]. Between 12 and 25,000 vWF receptor, molecules interact with GP1ba and bind 14-3-3 zeta protein, which influences signaling through PI3 kinase, adhesion under shear stress, and apoptosis [234-239]. Studies have suggested that the cytoskeleton is important for maintaining asymmetry in the lipid membranes (i.e., separation of cholinephospholipids from the aminophospholipids between the outer and inner leaflets, respectively). However, even when there is limited spectrin, the separation of phosphatidylserine and phosphatidylethanolamine from phosphatidylcholine is maintained until platelet activation (reviewed in [240]). Furthermore, flavonoids that alter membrane fluidity have been shown to impact platelet aggregation [241] , and the basis for platelet hypersensitivity in diabetics has also been demonstrated to be due to membrane fluidity [242] . In diabetic patients, platelets have increased procoagulant activity, aggregate more, show increased Ca2+ mobilization, and are insulin resistant [243]. Metformin is known to affect platelet function [244, 245]. It can reduce platelet aggregation and adhesion in addition to reducing hypercoagulability [246]. There are reports of high metformin doses contributing to bleeding in some patients [247] and platelet mitochondrial dysfunction has been observed in vitro [248].
11 Activated platelet cytoskeleton
The activated platelet undergoes extensive cytoskeletal changes depending upon the stimulus and lipid membrane asymmetry is lost on Ca2+ influx [227, 240]. Chloride channels, putatively anoctamin 6 (Ano6), have just been implicated in platelet procoagulant activity by affecting the Ca2+-dependent exposure of phosphatidylserine [249]. Once activated, platelet actin cytoskeleton rapidly disassembles within seconds and collapses resulting in less rigid spheroid morphology. Once disassembled, the actin subunits are rapidly reassembled into a variety of new structures such as filopodia and lamellipodia to dramatically generate new platelet shapes depending upon the external forces, extracellular signals, and physiologic requirements. Disengaging the membrane, cytoskeleton occurs as adducin complexes are released from the barbed ends of actin filaments thereby initiating actin latticework disassembly [232, 250, 251]. Adducin complex release is governed by calcium-calmodulin binding, phosphatidylinositides, Rho kinase, or protein kinase C (PKC)-mediated phosphorylation [232, 250, 251]. Signaling pathway involvement depends upon the initiating stimulus [232, 250, 251]. Concurrently, as actin filaments are disengaged, actin remodeling proteins gelsolin (20,000 molecules/platelet) and cofilin (100,000 molecules/platelet) are activated. Gelsolin is activated seconds after thrombin stimulation by increased cytosolic calcium and severs and caps actin filaments [252, 253]. Cofilin activation begins to plateau within 50 s following phosphorylation at serine 3. This leads to the disassembly of actin cytoskeletal filaments [254, 255]. Reassembly of actin units into new structures initiates within seconds by gelsolin inactivation. The barbed ends of actin fragments, provided by gelsolin severing, creates nucleation centers onto which the actin-related protein (Arp) 2/3 complex binds to initiate new filament genesis [256, 257]. The actin reassembly reaction is stopped by CapZ protein binding to the ends of new barbed filaments [258, 259]. These cytoskeletal responses and reorganization occur within seconds of wound exposure recognition. This is to help establish a physical meshwork of platelets and initiate thrombus formation. Speed is essential since this process must occur in dynamic waves to limit blood loss and begin repair under immense cardiovascular pressure.
12 Platelet degranulation
Microtubule and actin cytoskeleton disassembly that accompanies structural collapse is followed by platelet degranulation or release reaction [260, 261, 74] (Fig. 2). Various subsets of granules are released depending on the platelet activation stimulus [150, 200]. Various combinations of secretory mechanism proteins mediate the selectivity of exocytosis and degranulation [150]. These proteins include soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins [149, 262, 263]. The two primary subsets of SNARE proteins that distinguish platelet granules for secretion include synaptosomal-associated protein 23 (SNAP-23) that mediates membrane fusion, and vesicle-associated membrane protein (VAMP) 3, 7, and 8 that decorate subsets of granules [150, 264, 147]. A SNARE chaperone protein known as mammalian uncoordinated-13-4 (Munc13-4) mediates docking between granule and plasma membranes. Docking also involves interactions with Ras-related protein, Rab27a along with synaptotagmin-like protein 4-a/granuphilin-a (Slp4-a) [265, 266]. Syntaxin-11 remains inactive by Munc18b sequestering [267, 268]. Platelet activation causes a conformation change in Munc18b/syntaxin-11 complexes to form a four-helical protein bundle with SNAP-23 and VAMP during granule recruitment to platelet membranes and release. Granule release can occur through OCS membranes. Among other methods, time-lapse microscopy of spreading platelets revealed that VAMP-3 and VAMP-8-decorated granules localize to a centralized granulomere [264]. A collapsed microtubule ring surrounds the granulomere prior to granule release. In contrast, granules decorated with VAMP-7 localize at the platelet periphery followed by release [264]. These data illustrate differences in the granule population decoration that separate their release behavior.
13 Platelet granules
Platelet degranulation releases three major forms of storage granule products. (A) Alpha-granules are most numerous at 50–80 per human platelet and take up 10 % of the platelet volume [78] (Fig. 2). They also contain the greatest abundance of factors including the following: (1) adhesion molecules—vWF, αIIbβ3, αvβ3, P—selectin, thrombospondin, fibrinogen, and fibronectin; (2) coagulation factors—prothrombin, fibrinogen, factor V, and factor VIII; (3) fibrinolytic factors—α2 macroglobulin, plasminogen, PAI-1, SERPINE1, and urokinase plasminogen activator (uPA); (4) growth factors—PDGF, BFGF, EGF, HGF, IGF1, TGFβ, VEGF-A, and VEGF-C; (5) pro-angiogenic and anti-angiogenic factors—angiopoietin-1, angiostatin, and sphingosine-1-phosphate (S1P); (6) tissue remodeling matrix metalloproteinases—MMP-1, MMP-2, MMP-3, MMP-9, MT1-MMP (MMP-14), their tissue inhibitor of metalloproteinases (TIMPs; TIMP-1, TIMP-2, and TIMP-4), and a disintegrin and metalloproteinases (ADAMs; ADAM-10, ADAM-17, and ADAMTS-13) [269]. (7) Platelet α-granules contain a wide range of proinflammatory mediators such as C-X-C motif chemokines, including CXCL1 (GRO-α), CXCL4 (PF4), CXCL5 (ENA-78), CXCL7 (PBP, β-TG, CTAP-III, NAP-2), CXCL8 (IL-8), CXCL12 (SDF-1α) [270]. Among these, CXCL4 and CXCL7 are the most abundant [271, 272]. Platelet α-granule proinflammatory factors also include chemokine C-C motif ligands CCL2 (MCP-1), CCL3 (MIP-1α), and CCL5 (RANTES), CCL7 (MCP-3), and CCL17 (TARC) along with IL1-β, platelet-activating factor (PAF), acetylhydrolase, and lysophosphatidic acid (LPA) [149]; (8) immunologic molecules—C1 inhibitor and IgG; and (9) other proteins—albumin, α1-antitrypsin, growth arrest-specific 6 (Gas6), high-molecular-weight kininogen (HMWK) [273, 150]. (B) Dense granules by contrast are fewer in number at three to eight per human platelet and primarily contain small molecules and far fewer factors including the following: (1) ions—calcium, magnesium, phosphate, and pyrophosphate; (2) nucleotides—adenosine triphosphate (ATP), guanosine-5′-triphosphate (GTP), adenosine diphosphate (ADP), and guanosine diphosphate (GDP); (3) membrane proteins—tetraspanins and LAMP2; (4) transmitters—5-HT, epinephrine, and histamine; and (5) protease inhibitors—tissue factor pathway inhibitor (TFPi) [273, 150]. Both α-granule and dense granule release help to amplify secondary platelet responses and initiate wound repair. (C) Lysosome function in platelet biology and hemostasis is not well characterized. They release phospholipase A, protease, and glycohydrolase enzymes that may suggest a role in platelet responses and dissolution of clots [274-277]. As first responders, successive waves of platelet activation, adhesion, aggregation, and stabilization activate specific subsets of cytoskeletal changes depending on the stimulus and physiologic need to recognize, initiate, and secure vascular lesions.
14 Nitric oxide synthase
Endothelial cells express nitric oxide synthase (eNOS), which produces nitric oxide (NO) and maintains platelets in a resting state. Once produced, NO acts on NO-sensitive guanylyl cyclase (sGC) to produce cyclic GMP (cGMP) [278, 279]. cGMP then activates phosphodiesterase (PDE)2A and PDE5A, causing the degradation of cyclic AMP (cAMP) and cGMP, in contrast to inhibiting PDE3A. The phosphorylation of PDE5 also involves cGMP-dependent protein kinase (protein kinase G [PKG1]), which further activates the enzyme [280]. The net effect of cyclic nucleotide production, nitric oxide, and protein kinase activity is to maintain platelets in a resting state, which is in contrast to G protein-coupled receptor (GPCR)-mediated platelet activation.
15 Platelet G protein-coupled receptors
The activation signals that initiate Rho GTPase pathways among other biological responses by platelets involve multiple receptors and ligands (Fig. 3). These molecules fall into at least seven different families of receptors including GPCR, leucine repeat receptors (LRR), immunoglobulin (Ig) superfamily, integrins, C-type lectin receptors, tyrosine kinase (TK) receptors, and glycoproteins. These receptors act in concert to strike a balance between the initiation versus suppression of activation, aggregation, adhesion, amplification, coagulation, stabilization, and recruitment of platelets while executing their biological responses.
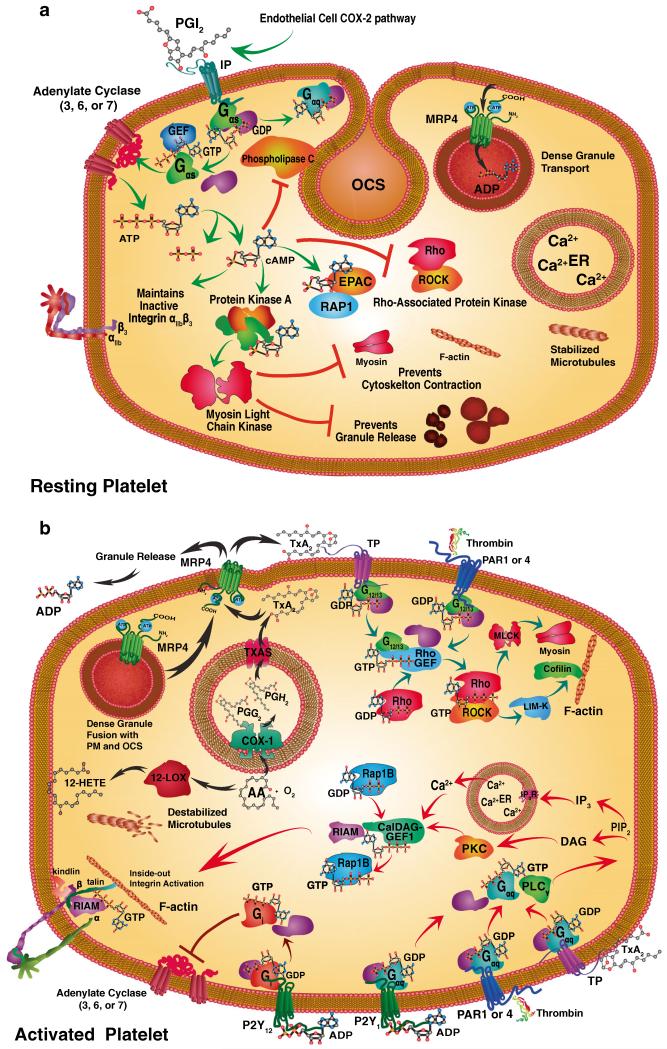
Fig. 3.
Specific signaling pathways maintain the resting platelet state (a) or initiate platelet activation (b). Prostacyclin (PGI2) is primarily produced via the COX-2 pathway by endothelial cells and binds to stimulatory Gαs-coupled and GTP-GDP exchange factor (GEF) proteins through prostacyclin receptors (IP, green arrows). The resting platelet state (a) is maintained by IP-mediated Gαs-protein coupled stimulation through the synthesis of cyclic-AMP (cAMP) by adenylate cyclase (AC 3, 6, or 7). cAMP elicits a variety of effects including: inhibition of phospholipase Cγ (PLCγ) and Rho/Rho-associated kinase (ROCK) pathway. cAMP also directly stimulates exchange protein directly activated by cAMP (EPAC) and Ras-related protein 1 (Rap1) signaling, along with PKA. Protein kinase A, in turn, phosphorylates cAMP myosin light chain kinase (MLCK) that maintains an inactive actin cytoskeleton and prevents granule release as well as maintaining integrins in an inactive state. Resting platelets also accumulate ADP in dense granules via granule membrane multidrug resistance protein 4 (MRP4). Calcium stores and microtubules also remain stabilized in resting platelets. Activating platelets by contrast can involve a variety of different signaling pathways depending on the stimulus (b). Thromboxane A2 is a potent activator of platelets through the G12/13 protein coupled thromboxane receptor (TP; teal arrows). Likewise, thrombin activates protease-activated receptors (PAR) 1 and 4 through G12/13 coupled proteins. Both G12/13 coupled receptors activate Rho/ROCK kinase activity leading to MLCK phosphorylation and thereby myosin activation, and LIM domain kinase (LIM-K) that phosphorylates cofilin, which triggers actin filament disassembly. These cytoskeletal changes are accompanied by the destabilization of microtubules. TP and PAR1,4 along with ADP activation of the purinergic G-protein coupled receptor (P2Y) act upon Gaq proteins (red arrows). This activity stimulates PLCγ and the release of phosphatidylinositol 4,5-bisphophonate (PIP2) and activation of IP3 in combination with diacyl-glycerol (DAG). These events trigger calcium release and protein kinase C (PKC) activation. Ca2+ and diacylglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEF-I) catalyzes GDP for GTP exchange in Rap1B. These events activate Rap1-GTP-interacting adapter molecule (RIAM) that stimulates talin and kindlin interactions between integrin β subunits and F-actin and integrin activation. A different inhibitory Gi protein coupled receptor, P12Y, is activated by ADP and inhibits AC 3, 6, or 7. ADP is released from platelet-dense granule fusion with the plasma membrane or OCS MRP4 that transports TXA2 and ADP. These activation events initiate the synthesis of 12-hydroxyeicosatetraenoic acid (12(S)-HETE) or TXA2 from arachidonic acid (AA). TXA2 is synthesized after conversion of AA and oxygen (O2) by cyclooxygenase 1 (COX-1) to prostaglandin G2 and then H2 (PGG2, PGH2) followed by the final conversion by thromboxane A synthase (TXAS)
Platelet GPCRs initiate key biological responses [281-283]. This receptor family consists of seven-transmembrane domain receptors [281-283]. Small GTPase proteins are bound to the cytoplasmic tails of inactive GPCR including (1) Gq alpha or Gq/11, which activates various phospholipase C isoforms such as, PLCγ2 or PLCβ, which in turn catalyzes production of second messengers diacyl glycerol (DAG)/inositol triphosphate (IP3) followed by stimulation of PKC, and, finally, increases intracellular calcium [284, 285]; (2) Gα12/13 alpha activates RhoA family cytoskeletal remodeling proteins [286]; (3) Gαi alpha inhibits the production of cAMP from ATP [287]; or (4) Gs alpha stimulates the cAMP pathway by activating protein kinase A (PKA) [287, 288]. Both Gα and Gγ protein subunits are post-translationally modified by the N-terminal covalent attachment of lipids, including being myristolated, palmitoylated, or prenylated, which enables them to anchor to the inner leaflet of the plasma membrane [289]. In resting platelets, GDP is bound to Gα subunit maintaining it in the inactive conformation. After ligand binding, GPCRs become activated through conformational changes [281-283]. This causes the disassociation of the Gα-subunit from its heterotrimeric Gβ, Gγ regulatory G protein complex [290, 291]. The Gα-subunit is then free to interact with a variety of guanine exchange factor (GEF) proteins that exchange GTP for GDP on the target. After downstream activation of a specific pathway, the target proteins interact with GTPase-activating proteins (GAP), which initiates hydrolysis of GTP to GDP and entry into a new signaling cycle. Platelet GPCRs include: (1) thrombin receptors—PAR1, PAR2, PAR3 (mouse), and PAR4; (2) ADP nucleotide receptors—P2Y1 and P2Y12; (3) prostaglandin receptors (PG)—thromboxane A2 (TxA2/prostanoid thromboxane receptor (TP)), prostacyclin (prostaglandin I2 (PGI2)/IP), and prostaglandin E2 (PGE2/EP1, EP3, and EP4); (4) lipid receptors—PAF and, lysophospholipid receptor (LPL-R); (5) chemokine receptors—C-X-C CXCR-4, CC chemokine receptors, CCR1, CCR3, and CCR4; (6) other receptors—vasopressin receptor (V1a), adenosine receptor (A2a), β2 adrenergic receptor (epinephrine, norepinephrine, and dopamine receptor), and serotonin (5-hydroxytryptamin, 5-HT2A) receptor [292, 293]. Specificity of downstream signaling depends on which combinatorial association of the Gα, Gβ, or Gγ heterotrimeric regulatory G protein complexes occurs with a given GPCR. This process is context-specific and typically involves crosstalk with other receptors and signaling pathways to elicit a specific platelet response. This is particularly true regarding the context and extent of platelet adhesion receptor engagement with a variety of receptors for extracellular matrix, other platelets, or specific cells. As a potential example, it is known that serotonin is elevated in breast cancer and that serotonin modulates breast involution through tight junction disruption, which becomes deranged in breast cancer [294]. Furthermore, exogenous addition of arachidonic acid (AA) can disrupt tight junctions [295]. In other systems, there is precedent for PLA2 stimulation and AA release after 5HTA or 5HT2A activation [296, 297]. Therefore, the potential exists for a continuous feedback loop where serotonin from breast cancer cells may activate platelets through 5HTA and this in turn may promote platelet release of AA and other metabolites that further disrupt tight junctions. These reactions then may influence angiogenesis as both serotonin and thromboxane have been shown to induce the shedding of platelet exosomes that induce smooth muscle proliferation [298].
16 Resting platelet G protein-coupled receptors
Platelets exhibit numerous GPCR-mediated checkpoints that maintain the resting state and involve cyclic nucleotide production and protein kinase activity (Fig. 3a). The most prominent of these is the prostacyclin receptor (IP receptor), which binds prostacyclin or PGI2 [299]. PGI2 is a metabolic derivative of the 20-carbon essential fatty acid AA. AA is converted primarily by the cyclooxygenase 2 (COX-2) to prostaglandin G2 and then H2 followed by conversion to prostacyclin by PGI2 synthase (PTGIS) in vascular endothelial cells [300]. PGI2 is one of the most potent inhibitors of platelet aggregation, a potent vasodilator, and moderator of anti-atherogenic control of vascular smooth muscle cell proliferation–migration–differentiation [301, 302]. IP receptors in resting platelets act through Gαs by stimulating adenylate cyclase (AC) to synthesize cAMP from ATP. Platelets express a number of different membrane-bound AC isoforms, including AC3, AC6, and AC7 [303, 304]. Provided that cAMP is not broken down by PDE, it activates either PKA [305, 288, 306] or exchange proteins activated by cAMP (Epac) [307, 308]. Platelet PKA exists as a tetramer of two regulatory subunits bound to catalytic subunits, RIα/β-C or RIIβ-C, that are inactive in resting platelets. The tetramer disassociates after cAMP binds to the regulatory subunits releasing the kinase to catalyze target protein phosphorylation. PKA phosphorylates Ras-related protein 1B (Rap1B) [309] among other proteins such as Rap1GAP2, TRPC6, glycoprotein Ib (GPIb)β, IP3RI-IRAG, and Gα13 to inhibit G-protein activity and Ca2+ release [288]. PKA also phosphorylates inhibitors of protein phosphatases PP1 and PP2A. This prevents the dephosphorylation of PKA and non-PKA targets. In addition, Epac proteins replace GTP for GDP in Rap to modulate kinase signaling.
Platelets exhibit numerous GPCR-mediated checkpoints that maintain the resting state and involve cyclic nucleotide production, nitric oxide, and protein kinase activity [310]. Resting platelets maintain basal levels of cAMP through AC [311, 312].
17 Activating G protein-coupled receptors
Many robust GPCR-mediated responses were first described by studying platelet agonists (Fig. 3b). The ADP response was the first studied in platelets [313]. Thrombin stimulation was also described early in platelets [314, 305]. The discovery of prostaglandin H2 (PGH2) [315] and TxA2 responses was also initially described in platelets [316]. We now know that ADP induces P2Y1, and thromboxane A2 stimulates TP, whereas thrombin acts through PAR1 or PAR4 [317]. In the case of thrombin activation, one of the primary initiators is tissue factor (TF), particularly in the case of tumor cells [318, 319, 317]. These platelet GPCRs, bind alpha regulatory subunits, Gq alpha or Gq/11. Upon the downstream activation of PLCγ2, or PLCβ, the production of second messengers DAG/IP3 stimulates PKC along with an increase in intracellular calcium. These events help the subsequent activation of Rap1 [309, 320]. This occurs through the combined activity of diacylglycerol-regulated guanine nucleotide exchange factor 1 (CalDAG-GEF1) together with Rap1-interacting adapter molecule (RIAM), a Rap1GTP adaptor protein [309, 320-323].
ADP can induce P2Y12 activation, which acts through inhibitory Gαi subunits. Gαi suppresses the generation of cAMP by AC in the plasma membrane. In some instances, Gαi-mediated inactivation of AC can block activation by Gαs-dependent GPCRs. The resting state of platelets is also influenced by PKA and PKG activation states, as well as by CalDAG-GEF 1 and Rap 1GTP adaptor protein status, and free Ca2+ levels [324, 320, 325].
18 Rho-induced morphological changes
Different morphological changes in the platelet cytoskeleton and degranulation predominantly involve specific Rho family GTPases [290, 291, 326-328] (Fig. 3b). Following activation, platelet RhoA influences actin contractility and shape change along with thrombus generation [329-331]. RhoA acts on multiple cytoskeletal proteins. In its GTP-bound form, Gα13 activates p115RhoGEF, thereby activating RhoA through its Gα13 switch region 1 (SRI) [332, 333]. This switch region of Gα13 interacts with p115RhoGEF activating Rho and then Rho-associated protein kinase (ROCK) [332, 333]. Once active, ROCK phosphorylates and inactivates the myosin light chain (MLC) phosphatase [334-336]. This increases the net MLC phosphorylation at serine 19 by myosin light chain kinase (MLCK) [337-339]. ROCK activation can also stimulate LIM-kinase (LIMK) to phosphorylate cofilin thereby regulating actin polymerization [340-342]. The end result of RhoA activation by GPCRs is cytoskeletal reorganization.
19 Cdc42-induced filopodia
Another Rho family protein, Cdc42 along with WASP and neuronal N-WASP induces Arp2/3-mediated actin branching to form filopodia and granule secretion [343-346]. In the absence of pre-existing actin filaments, IRSp53 initiates this process via its I-BAR domain by bending the membrane outward and binding Cdc42 along with its target proteins. These Cdc42 targets include a formin protein, mDia2, along with WASP/N-WASP. The resulting complexes stimulate actin polymerization. Filopodia also form from pre-existing lamellipodial actin filaments by clustering myosin X along WASP/Arp2/3-nucleation sites. The extension of filopodia follows by the addition of actin monomers (G-actin) onto polymerizing actin filaments (F-actin). As the filopodial process matures, vasodilator-stimulated phosphoprotein (VASP), myosin X, and mDia2 are localized to the tip. This occurs along with the dynamic movement of myosin X to facilitate delivery of proteins to the growing tip. The polymerization of actin occurs at mDia2 nucleation sites in cooperation with actin monomer delivery by VASP. Profilin binding presents actin monomers directly to mDia2. Process formation is catalyzed by Cdc42 and Rif stimulation of mDia2-facilitated actin polymerization. Cdc42 also initiates WASP/Arp2/3-driven polymerization. Platelet filopodia enhance interactions between other circulating platelets or cells, fibrin networks, and extracellular matrix proteins.
20 Raci-induced lamellipodia
Platelet spreading and lamellipodia formation are initiated by Rac1 [347, 346, 348, 320]. The physiologic demand for platelet coverage of exposed extracellular matrix among other triggers drives Rac1-mediated responses. These responses generally begin with cofilin-mediated severing of actin filaments, exposing barbed ends. Exposed filament barbs serve as nucleation sites for Arp2/3 binding and the formation of new filaments. During the extension process, Arp2/3 nucleation sites generate branches within the actin filament network. Rac1 GTPases then activate Arp2/3 along with WASP-family verprolin-homologous (WAVE) complexes at the membrane followed by formin-mediated actin complex extension. G-actin monomers are presented to formin by profilin during filament polymerization. The anticapping protein, VASP, also contributes to actin filament extension. In the absence Arp2/3 complexes, formin helps generate unbranched filaments. The respective events triggering RhoA, Cdc42, or Rac1 activity involves crosstalk with integrin, GPCR, LRR, or Ig receptors to initiate the specific platelet actin cytoskeletal morphological changes necessary to support either adhesion, aggregation, secretion, or thrombus formation.
21 Platelet extracellular matrix receptors
Platelets encounter various subendothelial proteins following vascular injury (Fig. 4). These factors include proteoglycans, laminin, fibronectin, and vitronectin along with various isoforms of collagen. vWF binds to exposed collagen I, III, and VI fibers. Additional vWF is recruited into the matrix network to form tethering fiber strands [349-353]. Platelet surface multimeric GPIb complexes form catch bond interactions with exposed tethering fiber strands [349-352, 354, 353]. The initiation of catch–slip tethering causes platelets to begin rolling behavior under the fluid shear stress caused by circulating blood [349-353]. Functional GPIb receptor complexes consist of four transmembrane proteins that include, from the outside in to the center, two 20-kDa GPIX subunits, two 26-kDa GPIbβ subunits, two 135-kDa GPIbα subunits, and one central 82-kDa GPV subunit [355, 354]. The GPIb receptor subunits belong to the LRR receptor family of proteins and are almost exclusively expressed in platelets [355, 354]. The loss of GPIb expression leads to an inherited bleeding disorder known as Bernard–Soulier syndrome [356]. Glycosphingolipid-enriched microdomains (GEM) support clustering of GPIb-IX-V multimeric complexes that interact with vWF multimers [357]. Interactions with vWF along with filamin and cytoskeletal proteins trigger multimeric GPIb-IX-V complex formation within GEM regions [357]. Outside-in signal transduction occurs through the interaction between cytoplasmic tails and calmodulin, filamin, 14-3-3ζ, PI3 kinase, actin-binding protein (ABP), and F-actin filaments [358, 234, 359, 238]. Src/Lyn family kinase activation by receptor protein-tyrosine phosphatase (RPTP), results in downstream signaling through spleen tyrosine kinase (Syk). Independent activation of Fc receptors FcγRIIA and FcR γ-chain stimulate Syk activity. Signals that converge on Syk can phosphorylate and activate p38 mitogen-activated protein kinase (p38MAPK). p38MAPK in turn can activate cytosolic phospholipase Aγ2 (cPLAγ2) and stimulate TxA2 synthesis [358, 234, 359, 238]. TxA2 or Syk can directly activate phospholipase Cγ2 (PLCγ2). IP3/DAG initiates the release of Ca2+ or PKC activation [358, 234, 359, 238]. Ca2+ release along with PKC stimulation, in turn, initiates crosstalk that causes inside-out activation of integrins to stabilize interactions with the exposed vessel wall. Tethering can also occur with ultralarge von Willebrand factor (ULVWF) and endothelial cells to attract platelets to these protein strands along with other cells such as leukocytes and potentially tumor cells [360-362].
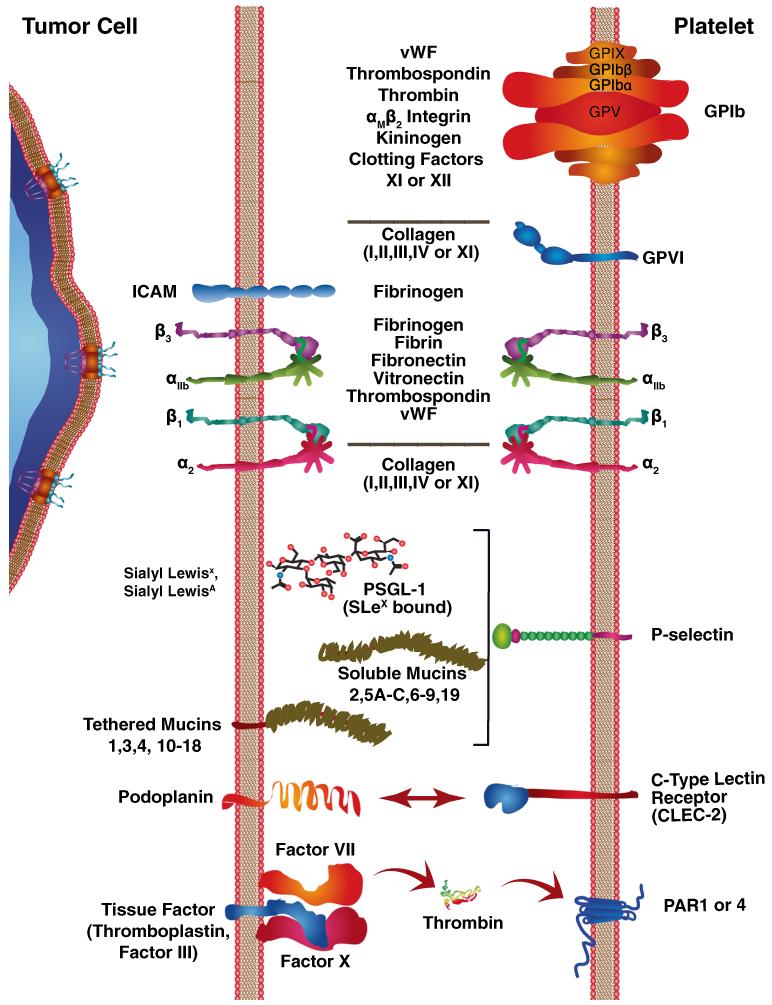
Fig. 4.
Tumor cells interact with platelets through a variety of receptors. Interactions between tumor cells and platelets can involve diverse types of cell–cell and extracellular matrix (ECM) proteins as intermediaries. Tumor cell invasion into the blood stream can expose extracellular matrix or trigger the expression of ultralarge von Willebrand factor (ULVWF) that trigger platelet rolling through tethering to collagen and other ECM proteins or endothelial cells. Platelet GPIb complexes recognize vWF tethers, along with thrombospondin, thrombin, αMβ2 integrin, kininogen, and clotting factors XI or XII. The GPIb complex consists of two sets of GPIX, GPIbβ, and GPIbα along with a centrally situated GPV protein. Subsequent stabilization of adhesive contacts with collagen exposed by tumor cells is mediated by GPVI. Tumor cell interactions with fibrinogen can also occur through intracellular adhesion molecule-1 (ICAM-1). Integrin heterodimers mediate numerous interactions with a variety of ECM proteins. The αIIbβ3 integrin is the most promiscuous and binds to fibrinogen, fibrin, fibronectin, vitronectin, thrombospondin, and vWF. Interactions with a variety of collagen molecules are mediated by α2β1. Interactions between tumor cells and cell surface carbohydrates can involve selectins. P-selectin binds to Sialyl Lewisx or Sialyl LewisxA; these interactions can also involve either tethered or soluble mucins. Similarly, carbohydrate interactions can involve C-type LECtin receptor-2 (CLEC-2) on platelets and podoplanin on tumor cells. Tissue factor expression by tumor cells can bind coagulation factor VII or X and trigger thrombin generation that activates PAR 1 or 4
22 Platelet integrin receptors, inside-out and outside-in signals
Integrin-mediated inside-out and outside-in signaling and/or crosstalk plays an essential role in the biologic responses of platelets (Fig. 3b). Platelets encounter numerous circulating ligands, cells, pathogens, and extracellular matrix ligands in the bloodstream depending upon context. In order to immediately respond, platelets express a wide variety of integrin receptors that engage context-specific ligands. Functional integrin receptors exist as heterodimers consisting of transmembrane glycoprotein α and β subunits. Resting platelets express low affinity or closed conformation integrins containing ligand-binding sites that are unexposed in a bent over conformation. Following activation, the α and β subunits project upright and shift to a high affinity or open state that efficiently binds ligands. Once rolling behavior is initiated by multimeric GPIb–complex interactions with vWF, crosstalk through release of Ca2+ or PKC activates a key stabilization integrin αIIbβ3 [363-366]. This inside-out activation process shifts αIIbβ3 integrins from a closed to an open state. In the open state, transmembrane 148-kDa subunit αIIb and 95-kDa subunit β3 heterodimers are very promiscuous and bind multiple RGD-containing ligands [363-366]. The list of RGD ligands includes fibrin, fibrinogen, fibronectin, vitronectin, thrombospondin, or vWF complexes. Active-state αIIbβ3 integrin complexes can subsequently recruit additional platelets, initiate platelet aggregation, stabilize adhesion to extracellular matrix, and promote thrombus formation. Abnormal αIIbβ3 integrin receptor expression is involved in Glanzmann’s thrombasthenia [367, 368]. This bleeding disorder was first described in 1918 by the Swiss pediatrician, Eduard Glanzmann [369]. Patients with this disorder exhibit hemorrhagic symptoms and “weak platelets” [369]. In addition to the promiscuous αIIbβ3, an array of more selective ligand binding integrins also stabilizes interactions with the vascular microenvironment. For example, the collagen receptor α2β1 is a key matrix-stabilizing integrin. The α2β1 heterodimer complex consists of a 150-kDa α2 chain and a 130-kDa β1 chain. Once activated, α2β1 stabilizes direct adhesive contacts to collagen that prompts lamellipodia formation and platelet spreading. In contrast, when platelets encounter circulating or extracellular matrix fibronectin they engage α5β1 integrin heterodimers. The α5β1 integrin response depends on context and the platelet activation state. The α5 (125-kDa subunit) and β1 (130-kDa subunit) heterodimer integrin complex binds more selectively to fibronectin RGD peptide sites in static conditions. Activation of α5β1 promotes platelet tyrosine phosphorylation, changes in calcium levels, and/or lamellipodia formation. Platelets also express α6β1 integrin receptors that selectively bind to laminin in the basement membrane. The laminin-binding 120-kDa α6 subunit combines with β1 subunits as an integrin complex. Adherence to laminin triggers PI3K and Cdc42 signaling pathways, which induce filopodia formation. The activation of α6β1 integrin receptors typically involves crosstalk with platelet collagen receptor glycoprotein VI (GPVI) to achieve stable adhesion.
23 Immunoglobulin receptor superfamily
A key platelet transmembrane GPVI belongs to the Ig receptor superfamily [370, 371] (Fig. 4). This 63-kDa glycoprotein is found exclusively in platelets and has two Ig extracellular domains that bind collagen. As a major collagen receptor, it recognizes glycine-proline-hydroxyproline-10-repeat sequences and the quaternary structure of collagen, which contributes to platelet-activating functions [372, 373]. Platelet recognition and activation involves GPVI monomers that shift from low affinity and signal transduction potential to multimers that gain high affinity and signal initiation potential for the periodic structure of collagen [357, 374]. GPVI associates with FcRγ homodimer adapter proteins containing an immunoreceptor tyrosine-based activation motif (ITAM) consisting of tandem YxxL amino acid motifs that are phosphorylated by Fyn and Lyn resulting in platelet activation [375-377]. Syk, through its SH2 domains, interacts with tyrosine-phosphorylated FcRγ. The downstream signaling pathway involves scaffold proteins linker for activation of T cells (LAT), and SH2-containing leukocyte protein of 76-kDa (SLP76), which organize signaling molecules into multiprotein complexes [357, 374]. These signaling complexes recruit Vav1/3, Bruton’s tyrosine kinase (BTk), PI3 kinase α/β, and other signaling molecules culminating in activation of PLCγ-2. PLCγ-2 in turn elevates intraplatelet Ca2+ levels and calcium and diacylglycerol regulated guanine exchange factor I (CalDAG-GEFI) activation [357, 374]. PLCγ-2 can also initiate DAG-mediated activation of PKC and signaling through the purinergic receptor P2Y12 [357, 374]. Both PLCγ-2, kinetically distinct, pathways converge on the small GTPase Rap1 that can initiate TxA2 synthesis, integrin activation, and granule secretion.
24 Platelet C-type lectin receptors
Platelet C-type LECtin-like receptor (CLEC-2/Aggrus) is another platelet receptor in addition to GPVI and GPIb-IX-V that initiates platelet activation through ITAM signaling and molecular multimerization [375, 357, 374] (Fig. 4). CLEC-2 binds to mucin glycoprotein podoplanin [378-380]. Podoplanin is expressed on cells of the lymphatic endothelia, type I lung epithelia, choroid plexus epithelia, kidney podocytes, lymph node stroma, along with cancer-associated fibroblasts (CAF) and tumor cells where it is found at the leading edge and is thought to potentiate migration and invasion [381, 378, 382, 383, 379, 380, 384]. Podoplanin is a heavily O-glycosylated type-1 transmembrane sialomucin glycoprotein [378, 357, 385, 386]. Multiple podoplanin molecules may interact with platelet CLEC-2 receptors triggering signal transduction [357, 387]. Two molecules of CLEC-2 are also known to bind to tetramers of the snake venom peptide rhodocytin to form a multimeric complex that stimulates signal transduction [388, 386, 389]. Multimerization of CLEC-2 activates the same ITAM-Syk-PLCγ-2 signaling cascade as GPVI [375, 357, 374].
25 Platelet P-selectin receptors
Platelet P-selectin was first described as membrane glycoprotein GMP-140 [390, 391] (Fig. 4). P-selectin/GMP-140 contains a “lectin” region, an “EGF” domain, nine complement binding protein-like tandem repeats, a transmembrane domain, and a short cytoplasmic tail. P-selectin in turn binds to P-selectin glycoprotein ligand (PSGL)-1 [392], neutrophil leukocyte-endothelial cell adhesion molecule 1 (LECAM-1) [393], endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) [394], and sialyl Lewis(x) oligosaccharide [395]. P-selectin interaction with a variety of immune cells and endothelial cells is important in mediating disease states such as inflammation, autoimmunity, pre-eclampsia, and wound healing [396-398]. The proinflammatory lipid metabolite, 12(S)-HETE, elevates the expression of platelet P-selectin [399]. Significant evidence supports the notion that platelet P-selectin also mediates tumor metastasis and thrombotic complications in cancer patients such as exemplified by Trousseau’s syndrome [400-403]. P-selectins along with Land E-selectins may initiate tethering and rolling of cells flowing past the luminal surfaces during the initial phases of intravascular adhesive interactions that are stabilized by other receptors [400, 401, 404-406, 402, 403].
26 Platelet activation, thrombus formation, and vascular shear
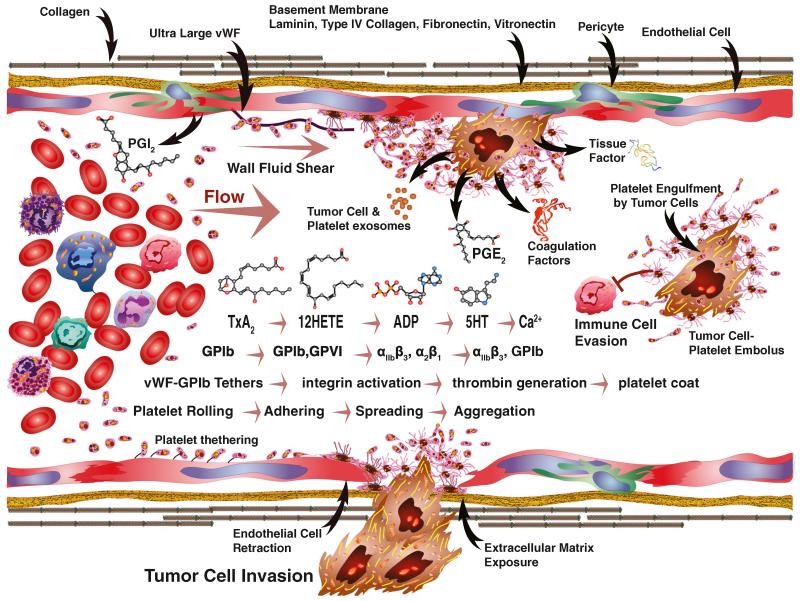
Thrombus formation involves multiple “first responder” platelet-mediated steps including rolling, adherence, spreading, migration, aggregation, and stabilization (Fig. 5). Fluid shear along with the discoid shape and the small size of platelets causes them to collect and contact the luminal walls of blood vessels. As they contact, normal intact endothelial cells that cover the luminal surfaces, various factors such as soluble nitric oxide, PGI2, and ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1/CD39)-mediated reduction of local purine nucleotides help maintain the resting state of circulating platelets [278, 407, 302, 279, 408]. Once injury-induced exposure of subendothelial matrix occurs, collagen fibrils cooperate in forming transient tethering bonds between matrix-bound vWF and platelet GPIb-IX-V receptor complexes [349-352, 354, 353]. These tethers are formed and broken to support rolling and slow platelet movement. Once movement slows, more intimate interactions between collagen fibers and platelet GPVI surface receptors triggers inside-out activation that shifts integrins from a closed to an open state [370, 371]. Activated α2β1 interactions with collagen and αIIbβ3 binding to fibrinogen initiate outside-in signaling that prompts platelet shape change and degranulation [363-366]. Degranulation releases α-granules and dense granules, which amplifies secondary platelet responses and initiates tissue repair. Platelets migrate over the subendothelial matrix isolating the exposed surfaces from the flowing blood, or into vascular beds, and initiate tissue repair [409-413]. A blanket of platelets begins to accumulate, but may form and release microemboli as reactive and protective surfaces simultaneously build up. Activated platelets serve as nucleation sites as build up proceeds. Nucleation is amplified through the release of prothrombotic granule contents and tissue factor-initiated coagulation [414]. The synthesis and release of TxA2, thrombin (FIIa), ADP, among other factors, recruits and activates additional platelets. This extends the platelet blanket. Platelets interact with adjacent platelets through αIIbβ3 receptors by binding fibrinogen and fibrin fibers [363-366]. Successive cycles of platelet activation, adherence, spreading, migration, aggregation, and stabilization form a thrombus that becomes enmeshed within a growing fibrin network and entraps other blood cells. Thrombotic plug formation is stabilized and counterbalanced by the disaggregation of platelets and fibrinolysis. Recruitment of fibroblasts and immune cells through platelet-initiated angiogenic repair mechanisms stimulates wound healing and resolution [410]. Exposure of subendothelial matrix along with the presentation of atherosclerotic plaques, foreign bodies, abnormal angiogenesis, or pathologic cells such as tumor cells in the circulation serve as robust triggers of platelet activation.
Fig. 5.
Tumor cell interactions with platelets and other circulating factors under fluid flow are complex. Fluid shear increases from the center of blood vessels toward the vessel walls. Prostacyclin (PGI2) produced by endothelial cells inhibits platelet activation. Various molecules produced by tumor cells or other sources can activate platelets as part of a cascade of events. Progressive stimulation by TxA2-12(S)-HETE-ADP-5HT and calcium release are events that fall within the small molecule cascade. The formation of vWF-GpIb tethers support cell rolling or ultralarge vWF molecule formation, attracts platelets, and promotes their binding. Along the same cascade, GPIb-GPVI begin stabilizing adhesion and triggering αIIbβ3 and α2β1 along with other activation followed by spreading, aggregation, and invasion. Within the same cascade, thrombin generation promotes platelet coat formation and embolus formation that can enable tumor cells to evade cell-mediated immunity. Tumor cell products include exosomes, PGE2, tissue factor, and coagulation factors that act as triggers for platelet activation. Endothelial cell retraction associated with tumor cell invasion exposes those basement membrane components such as laminin, type IV collagen, fibronectin, and vitronectin involved in the tumor cell–platelet interaction cascade
27 The cancer challenge to hemostasis
Cancer can exert significant challenges to all of the molecular, cellular, and biologic properties thus far described for platelets as “first responders”. Primary tumors produce many byproducts that stimulate angiogenesis and can enter the circulation (Fig. 6). Platelets can recognize and respond to circulating tumor by-products and initiate the coagulation cascade in the absence of intact cells. The system is challenged further as extracellular matrix is exposed during tumor cell invasion or as circulating cancer cells interact directly with platelets. As platelets fulfill their normal hemostatic function in the presence of cancer, they tend to initiate thrombotic events that can facilitate cancer progression.
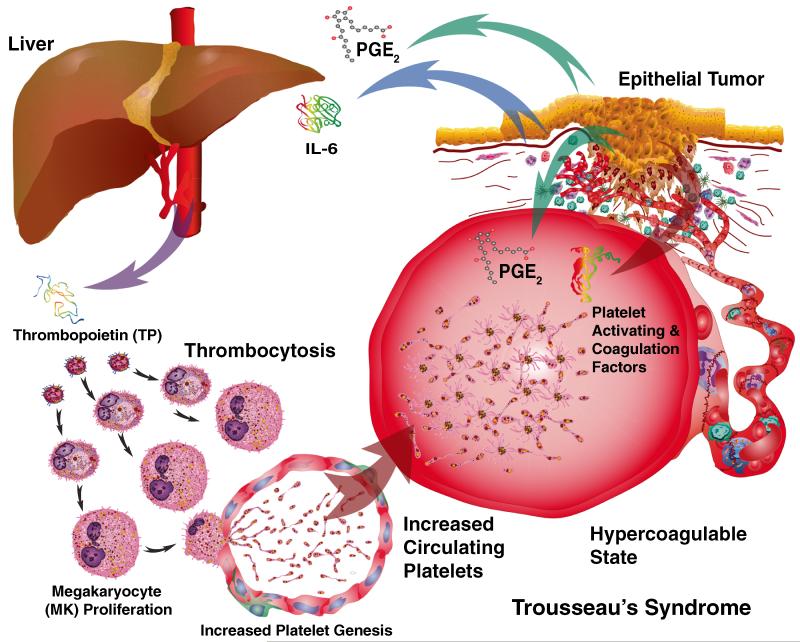
Fig. 6.
Trousseau’s syndrome is characterized by a hypercoagulable state. The production of IL6 by tumors stimulates the production of thrombopoietin by the liver. Thrombopoietin (THPO), in turn, stimulates thrombocytosis. The production of PGE2 by tumors also heightens the responsiveness of platelets. Platelet-activating and thrombin-stimulating factors produced by tumors also elevate the potential for thrombosis. The combination of increased numbers of circulating platelets along with circulating prothrombogenic factors creates the hypercoagulable state first described by Trousseau
28 Tumor by-products and thrombogenesis
The two major conduits for systemically distributing throughout the body the by-products and cells originating from tumors are the lymphatic and cardiovascular systems [415-417] (Fig. 6). These two vascular systems are interconnected through anastomoses at high endothelial venules (HEVs) in lymph nodes along with the larger collecting vessels of the thoracic duct and the jugular vein [415-417]. Tumor formation typically induces neoangiogenesis in both vascular systems that generally form abnormal vessels, which are leaky [418, 419, 406, 420]. Leaky vessels, in turn, facilitate the entry of tumor by-products into the cardiovascular circulation that can initiate venous thromboembolus (VTE) formation linked to Trousseau’s syndrome [317]. Tumors produce factors that activate the coagulation cascade to produce thrombin and activate PAR receptors on both platelets and tumor cells. Cancer procoagulants include thrombin produced by cancer cell-stimulated host cells, tumor tissue damage, inflammatory factors, tumor necrosis, mucin production, surgical trauma, chemotherapy-induced toxicity, and foreign bodies. Also, tissue factor on cancer cell surfaces binds to coagulation factor VIIa (FVIIa) and coagulation factor X (FX), activating the clotting pathway [406, 421, 317]. Once platelets and the clotting cascade are activated, thrombin serine protease activity alters fibrinogen to form a fibrin meshwork [406, 421, 317].
29 Thrombocytosis
Cancer-induced thrombocytosis significantly increases the number of circulating platelets [422, 1, 46]. This constitutes an important link to Trousseau’s syndrome, but the mechanisms responsible remained poorly understood until recently [423](Fig. 6). A multicenter study involving 619 patients with epithelial ovarian cancer was conducted to test associations between platelet counts and disease outcome [423]. Thrombocytosis along with elevated plasma levels of thrombopoietin and interleukin-6 (IL-6) is associated with advanced disease and shortened survival in ovarian cancer patients [423]. In accompanying orthotopic mouse models of ovarian cancer, tumor-derived IL-6 stimulated hepatic thrombopoietin synthesis and paraneoplastic induction of thrombocytosis [423]. These studies helped establish an important paracrine link between cancer and thrombocytosis. It has also been reported that there is an increase in the number of CD63-positive platelet microparticles in the circulation of ovarian cancer patients compared to those with benign tumors [424]. However, while these did reflect a procoagulant phenotype [425, 426], they did not, in and of themselves, discriminate benign from malignant tumors. Interestingly, the same group reported that menopause/climacteric was associated with fewer circulating platelet exosomes/microparticles [427]. Healthy individuals reportedly have a microvesicle concentration of 5–50 μg/ml [428].
30 Circulating tumor cells
The initial steps of hematogenous metastasis formation typically involve invasion of premalignant/cancer cells through the basement membrane and entry into blood vessels [429, 430]. The detection of circulating tumor cells (CTCs) in patient blood samples is associated with disease burden, metastatic relapse, and/or reduced survival in a variety of cancers [431] . Once in circulation, CTCs may encounter platelet-derived microparticles that harbor microRNA [428]. Likewise, platelets may encounter exosomes, or “oncosomes” [432] , derived from CTCs that harbor bioactive lipids such as 12(S)-HETE [433], which may serve to activate the platelets and begin the “ships passing in the night” cell-to-cell exchange as a result of extracellular vesicles [434] that leads to metastasis. The clinical outcome of these encounters remains to be determined, and offers a wealth of possibilities for therapeutic intervention.
Recent technological advances have enabled the isolation of CTCs from patients’ blood samples [435]. When coupled to new sequencing methods, these approaches can help better understand the metastatic process [436]. Understanding and tracing the lineage of cells that successfully enter the blood stream will help refine our ability to better target metastasis.
31 Tumor cell-induced platelet aggregation
CTCs activate and aggregate platelets, which correlate with their metastatic potential [48, 437, 1, 438] (Fig. 5). The heterotypic emboli that form following TCIPA enhance metastatic cell survival and dissemination. When CTCs become enveloped with platelets, they can evade the immune system as well as TNF-α-mediated cytotoxicity. Platelet coatings also shield CTCs from high fluid shear forces at the vascular wall. As heterotypic CTC emboli grow, entrapment in the microvasculature enhances their arrest rate. This process is facilitated by cell surface receptors on endothelial cells and a variety of immune cells. Adhesion of tumor emboli to the vascular endothelium as well as immune cells stimulates cytokine and growth factor production among other cellular responses.
32 Eicosanoids and tumor cell-induced platelet aggregation
Similar to wound site recognition, TCIPA triggers the production of TxA2 and platelet activation [439, 60, 440] (Fig. 5). Paclitaxel exerts an antiplatelet effect by interfering with TxA2 synthase [441]. In TCIPA, MRP4 moves from the dense granules to the platelet plasma membrane and transports TxA2 outside platelets [157, 158] (Fig. 3b). The release of TxA2 into the microenvironment activates TP receptors on platelets [442, 439, 60, 440, 443, 444]. Activation by Walker 256 carcinosarcoma cells, for example, induces arachidonic acid release followed by 12-lipoxygenase-mediated-conver-sion to 12(S)-HETE that further stimulates TCIPA [207]. TCIPA is inhibited by PGI2-mediated stimulation of IP receptors [445, 61, 440, 63]. PGI2-mediated inhibition of TCIPA is more effective than other classes of PGs including PGE1 or PGD2 as well as the PGI2 metabolite 6-keto-PGF1α [63]. In contrast, the proinflammatory/prooncogenic PGE2 and its enzymatic metabolite 13,14-dihydro-15-ketoPGE2 prevent PGI2 inhibition of TCIPA [63, 446]. PGI2-mediated signaling is also strong enough to arrest and reverse partial TCIPA. This inhibition serves as a potent antimetastatic agent [60, 445, 61, 440, 63]. The balance of eicosanoid metabolism in the vascular system is critical to the formation of tumor cell–platelet emboli and can be influenced by targeted therapy [447].
33 Tumor proteases
A variety of protease molecules such as thrombin are produced directly by tumor cells and stimulate PARs that initiate TCIPA [448-450] (Figs. 3b, 5, and 6). Thrombosis can also be triggered by neo-adjuvant and chemotherapy [451-453]. Other factors such as tissue factor act indirectly through thrombin generation, but tumor cells can also produce cancer procoagulant (CP) protein, which also stimulates TCIPA [454-456]. Another enzyme, cathepsin B is a cysteine protease that can be induced by 12(S)-HETE, and it also induces TCIPA when released from tumor cells [64]. MMPs produced by tumor cells can also act on platelet surface receptors and trigger activation [457-459]. In experiments using HT-1080 and MCF-7 cells, MMP-2 was released from both tumor cells and platelets that enhanced TCIPA [460, 459]. Further evaluation revealed that TCIPA involved proMMP-2 conversion to MMP-2 followed by MMP-14 activation [460]. Platelet and tumor activity is influenced by reciprocal feedback. For example, platelet granule products as well as 12(S)-HETE synthesized by platelets stimulate MMP-9 production by tumor cells and increase invasion [461, 462]. The regulation of MMP activity is modulated further by TIMPs that are produced by both tumor cells and released by platelets [463, 464]. Additional regulation of protease activity involves PAF [465, 466].
34 Tumor cell adhesion receptors
Tumor cell adhesion receptors play a vital role during the initiation and stabilization of plasma membrane interactions with platelets (Fig. 4). Similar to the initial platelet interactions with wounded blood vessels, GPIb-IX-V on both platelet and MCF7 tumor cell surfaces are among the first receptors engaged and can be upregulated on the tumor cell surfaces [460, 467] . Whether tethering via plasma vWF is involved during GPIb-IX-V-mediated platelet and MCF7 interactions remains unclear. A role for vWF in tethering is likely since the purified protein supports TCIPA induced by HT-1080 cells [468], which is further supported by TCIPA inhibition using anti-vWF antibody treatment [469, 467]. Also analogous to wounding, interactions between αIIbβ3 integrins on both platelet and tumor cell surfaces can help stabilize adhesive interactions [470, 75, 1, 469, 467]. Furthermore, RGD-containing proteins may act as a linker between the two plasma membranes [471-473]. Unlike collagen-platelet interactions, those with αIIbβ3 integrin may occur in the absence of platelet GPVI receptor engagement unless collagen fragments are present. Other integrin receptors are also likely to be involved in these heterotypic membrane interactions. The αvβ3 vitronectin receptor, for example, is very abundant on many cancer cell surfaces, but lower on platelets [474]. In the presence of plasma RGD linker proteins such as fibrinogen or vitronectin, αvβ3 membrane receptors can support tumor cell–platelet aggregate formation [474]. Another subset of heterotypic receptor interactions can potentially contribute to TCIPA when mucin-producing tumor cells are involved. In some instances, platelet CLEC-2 can bind to tumor cell podoplanin during heterotypic aggregate formation [378, 382, 383, 379, 380]. In other cases, P-selectins are also involved when mucin-producing tumor cells enter the blood stream and initiate TCIPA [475-479].
35 Tumor cell-induced platelet degranulation
The release of platelet α-granules in response to tumor cell stimuli profoundly influences biology in the cardiovascular system (Fig. 2). The release of vWF, αIIbβ3, αvβ3, P-selectin, thrombospondin, and fibronectin from platelet α-granules stimulates and strengthens adhesive interactions between tumor cells and exposed subendothelial extracellular matrix components. These adhesive interactions are accompanied by the release of α-granule prothrombin, fibrinogen, factor V, and factor VIII, contents that promote coagulation. Fibrinolytic factors that include A2 macroglobulin, plasminogen, PAI-1, SERPINE1, and uPA are also released from α-granules to counter fibrin meshwork formation and remodel fibrin clots.
Numerous growth factors, including PDGF, BFGF, EGF, HGF, IGF1, TGFβ, VEGF-A, and VEGF-C are released from α-granules during the formation of tumor cell–platelet emboli that stimulate tumor cell growth and angiogenesis. Additional α-granule contents such as angiopoietin-1 (ANGPT1), promote angiogenesis, and stabilization of endothelial cell junctions, which is counterbalanced by the release of antiangiogenic factors such as angiostatin. Platelet release of S1P depends on thromboxane [480] and has pleiotropic effects on angiogenesis, including the inhibition of sprouting angiogenesis through its action on the sphingosine-1-phosphate receptor (S1PR1).
As a part of their normal tissue remodeling properties, platelet α-granules release matrix metalloproteinases (MMP-1, MMP-2, MMP-3, MMP-9, MT1-MMP, and MMP-14), their TIMPs (TIMP-1, TIMP-2, and TIMP-4), and ADAMs (ADAM-10, ADAM-17, ADAMTS-13). Platelet α-granules along with pathologic contributions of these same classes of molecules by tumor cells, promote vascular remodeling, and invasion at secondary metastatic sites [269].
The simultaneous release of proinflammatory factors from platelet α-granules and those produced by tumor cells attracts a variety of immune cells to form tumor cell–platelet emboli [149]. Platelet α-granule release of C-X-C motif chemokines including CXCL1 (GRO-α), CXCL4 (PF4), CXCL5 (ENA-78), CXCL7 (PBP, β-TG, CTAP-III, NAP-2), CXCL8 (IL-8), and CXCL12 (SDF-1α), initiate and regulate microenvironmental and systemic immune responses during the formation of tumor cell–platelet emboli [270]. So also, MCP-1, CCL3 (MIP-1α), CCL5 (RANTES), CCL7 (MCP-3), and CCL17 (TARC), along with IL1-β3 and PAF acetyl hydrolase, released from platelet α-granules, initiate, amplify, or modulate immune responses to tumor cells [149, 270]. The concurrent release of lipids such as LPA stimulates platelet aggregation and metastasis [481]. Similarly, other molecules like C1 inhibitor, IgG, as well as albumin, α1-antitrypsin, Gas6, and HMWK also influence the inflammatory process during the formation of tumor cell emboli [273, 150].
The release of dense granules following tumor cell-induced platelet degranulation by contrast is less well understood. The release of platelet-dense granule components influences tumor cell-induced biological changes in the vascular microenvironment. The release of calcium and magnesium ions can promote platelet activation and aggregation. The release of nucleotides such as ADP activates platelets through P2Y1 and P2Y12 receptors [273, 150]. The dense granule release or engagement of membrane tetraspanins, CD9, CD63, CD151, Tspan9, and Tspan32, regulates cell surface interactions [482, 483]. CD9 is the most abundant platelet tetraspanin and engages Fcγ RIIA, a low-affinity receptor for IgG or GPVI collagen receptors during platelet interactions [482-484]. Tetraspanin CD63 interactions among others influence metastasis formation [485]. LAMP-2 is also present in platelet-dense granule membranes and has a similar distribution to CD63 [486]. The release of neurotransmitters 5-HT, epinephrine, and histamine can potentiate ADP-induced platelet activation, TCIPA, and aggregation [273, 150, 487]. Both a-granule and dense granule release helps to amplify both primary and secondary platelet responses as they interact with tumor cells. Tumor cells initiate successive waves of platelet activation, adhesion, aggregation, and degranulation during the formation of tumor cell platelet emboli [70].
36 Rabbit ears, ear chambers, V2 carcinoma, and tumor cell platelet emboli
The earliest accounts of cancer cell-containing thrombi found in circulation being associated with the spread of tumors were described in surgical pathology lectures by Billroth in 1878 [488]. Using the rabbit ear chambers to actively observe V2 carcinoma, Wood observed the dynamic arrest and formation of platelet thrombi along with the active migration of tumor cells from the vascular space to the perivascular space [54, 57]. The dynamic in vivo observations of Wood [54, 57] did not agree with those previously reported by Baserga and Safi-Otti in 1955 [489]. In their case, Baserga and Safi-Otti observed the intravascular arrest and proliferation of anaplastic carcinoma (T1SO) cells prior to extravasation. Both studies revealed tumor cell arrest in small arterioles in conjunction with thrombus formation [489, 54, 57]. As a primary difference, Baserga and Safi-Otti observed intravascular proliferation of arrested tumor cells [489]. This tumor growth occurred over several days and resulted in the destruction of the vessel, and subsequent tumor cell extravasation.
37 Tissue grinders, rat tumor cells, thromboemboli, and ultrastructure
Many details about the biology involved in tumor cell platelet emboli formation in vivo were revealed by ultrastructural analyses. Jones et al. first observed the formation of tumor cell platelet emboli along with formation of fibrin using electron microscopy and immunofluorescence after tail vein injection into Sprague–Dawley rats [490]. This is an allogeneic system that was described as free from immunologic effects during the short time frames examined. In follow-up studies using the same model system, early-stage emboli formation revealed the presence of neutrophils and lymphocytes as early as 2 min post-injection [491]. At 15 min, fibrin formation was present in emboli and remained from 3 to 6 h after injection even after the disappearance of platelets [491]. Similar observations were made by Warren and Vales [52]. This group also observed the release of platelet vesicles and adhesion to vessel walls in vitro [492]. The studies by Warren also described tumor cell migration through the endothelial layer from the adherent embolus that was restricted by the basement membrane of the endothelium [493]. Likewise, the injection of rat AH-130 ascites hepatoma cells also formed tumor cell platelet emboli in microvessels of the lung [68]. Arrest and extravasation of AH-130 cells was observed in lung arterioles and capillaries [68]. These model systems generally utilized tumor cells in suspension that were harvested from homogenized tumor sections. Residual connective tissue fragments may have contributed to promoting emboli formation, but by and large there was no evidence of collagen fragment or matrix protein association with tumor cell surfaces at the ultrastructural level.
38 Elutriation, mouse tumor cells thromboemboli, and more ultrastructure
Concerns over the homogeneity of tumor cell preparations led to the use of counterflow centrifugal elutriation to isolate pure tumor cell preparations of uniform size for tail vein injections [70]. Enzymatic digestion of minced tumor fragments using collagenase and trypsin inhibitors generates a heterotypic cell suspension. Subsequent counterflow centrifugal elutriation involves a specialized rotor chamber, where centrifugal forces generated outward are counterbalanced by drag forces from fluid flowing in the opposite direction to clean and purify cell preparations [494]. Elutriated tumor cells prepared in this fashion are more likely to retain the representative receptor expression profile and other intrinsic properties that are closer to the native tumor state (Figs. 7 and 8a-c). Tumor cells that are routinely passaged using trypsin over time are stripped of their surface components and are less likely to provide a true representation of the endogenous tumor. Tail vein injections of these pure tumor cell preparations into syngeneic mice enabled accurate characterization of the resulting intravascular biology in vivo [65, 70] (Figs. 7 and 8d, e). In these experiments, platelet-aggregating activities of Lewis lung carcinoma, B16 amelanotic melanoma, and 16C mammary adenocarcinoma resulted in tumor cell platelet emboli formation in the lungs [70]. Platelets began aggregating with tumor cells as early as 2 min followed by biphasic association with arrested tumor cells that peaked at 10–30 min and 4–24 h [70]. Ultrastructurally, tumor cells exhibited newly formed processes that became interdigitated with the platelet aggregate. Such processes were restricted to areas of contact with platelets and not endothelial cells or other blood elements (i.e., erythrocytes and polymorphonuclear leukocytes). Numerous tumor cell mitochondria were concentrated in the areas of greatest platelet-tumor cell process activity. At early time intervals (2–10 min), intravascular platelet degranulation occurred primarily in platelets associated with tumor cell processes. Tumor cells also engulfed platelet fragments in vivo [70]. Follow-up studies using the same models focused on tumor cell interactions with endothelial cells and the subendothelial matrix [67]. Platelet aggregation occurred from 1 to 4 h until a thrombus formed [67]. Around the 4-h time point, endothelial cells retracted exposing the subendothelial matrix in conjunction with the extension of tumor cell processes [67]. At about the 24-h time interval, thrombotic elements dissipated leaving the remaining attached tumor cells exposed to a reestablished circulation [67]. Mitoses were observed after 24 h with cell division and the development of intravascular tumor nodules. The final step in the extravasation sequence was dissolution of the basement membrane by the attached tumor cells [67]. In other mouse studies, platelet adhesive glycoprotein receptors and their counterparts expressed by tumor cells participated in TCIPA and served as an early step in the development of metastatic lesions [495]. These models are representative of experimental metastasis utilizing bolus tail-vein injection approaches. Spontaneous metastasis models are more representative of actual tumor cells shedding, which occurs a few cells at a time, into the blood stream. However, for practical reasons, it is difficult to examine the timing and progression of these thromboembolus formation steps using spontaneous metastasis models.
Fig. 7.
Sequence of events involved in tumor cell–platelet interactions during the arrest and extravasation of tumor cells. a The initial arrest often involves close contact between the plasma membranes of endothelial cell–platelets–tumor cells. Resting platelets (RP) associate with inactive endothelial cells (En) that are maintained in conjunction with pericytes (PC) while platelets are activated by interaction with tumor cells or their by-products in circulating plasma (Pl). b Tumor cell–platelet contacts initiate extensive platelet activation (AP) and aggregation with formation of tumor cell cytoplasmic extensions into platelet thrombus and uptake of platelet fragments by tumor cells. These interactions encompass the entire lumen of microvessels to the exclusion of other cells such as red blood cells (RBC). Platelet degranulation initially occurs in platelets closely associated with tumor cell surfaces. c Platelet degranulation becomes more extensive accompanied by additional platelet fragment engulfment by tumor cells and by endothelial cell retraction as tumor cells attach to subendothelial matrix and begin matrix degradation. d Platelet aggregates begin to disappear possibly by additional tumor cell uptake of platelet fragments. e Tumor cells begin to proliferate in capillaries. f Additional tumor cell proliferation occurs in conjunction with basement membrane (BM) degradation and invasion into the surrounding lung alveoli (Al)
Fig. 8.
Electron micrographs of early events in platelet–tumor cell interactions. a Elutriated W256 tumor cells in the plateau phase interacting with platelets in vitro at 10 min. Asterisked cells have lost surface microvilli (scanning electron micrograph; magnification, ×3,900). b Elutriated W256 tumor cells beginning shallow process formation (arrows) into platelets at midphase, and c deeper into platelet emboli at plateau phase (arrows) with engulfment of platelet fragments (open arrows) (transmission electron micrographs; magnifications—b ×3,000 and c ×4,300). d Interaction with and process penetration (arrows) of Lewis lung tumor cells into C57Bl/6 platelets 2 min post tail-vein injection, and e engulfing platelet fragments (arrows) at the plateau phase (transmission electron micrographs; magnifications—d ×6,800 and e ×12,000, scale bars 1 μm). a–c Reprinted from [59] with permission from S. Karger AG, Basel; d, e courtesy of Menter
39 Mouse tumor cells, platelet dynamics, and intravital microscopy
In contrast to rabbit ear chambers, intravital fluorescence microscopy performed on mice has revealed dynamic platelet-melanoma cell interactions [496]. Platelet depletion significantly reduced melanoma cell adhesion to the injured vascular wall in vivo [496]. Using a mouse model of hematogenous metastasis to the lung, platelet interactions and metastasis of B16 melanoma cells were decreased after treatment with mAb blocking the αIIbβ3 or αvβ3 integrin [496].
40 OK, so where to from here?
Collectively, these findings clearly illustrate the historical, predictive, and functional importance of platelets and hemostasis to cancer biology. Thrombocytosis and thrombogenesis are vital biologic and prognostic characteristics of cancer. These findings also illustrate how critical platelet-tumor cell thromboemboli formation is to metastasis. Many diagnostic as well as clinical and preventive translational targets have been identified. With these givens noted, numerous intervention points and unanswered questions remain open.
40.1 Detecting and preventing thrombogenesis?
Cancer is a heterogeneous disease with a significant risk of venous thrombosis depending on cancer types and stages, treatment measures, individual patient factors, and the specific population factors [497]. Once leaky vessels have been formed by aberrant angiogenesis in tumors, it is difficult to prevent the entry of tumor by-products into the circulation as associated with VTE and Trousseau’s syndrome. Early detection of tumor-specific or prothrombogenic biomarkers in the blood may be among various other options for routine screening, particularly in high-risk patients [498]. Aberrant TF expression is a characteristic of human cancer, metastatic potential, and neoangiogenesis. Similarly, uPA and urokinase plasminogen activator receptor (uPAR) can indicate proteolysis of the extracellular matrix in tumors. Measurement of TF and uPAR as biomarkers of cancer or neoangiogenesis in plasma may serve as diagnostic or prognostic risk factors [498].
Therapeutic management options for VTE in cancer patients remain limited [499]. Heparin monotherapy followed by long-term therapy with a vitamin K antagonist may be one option, but various clinical questions remain [500, 499]. Optimizing the duration of anticoagulant treatment for primary or recurrent VTE in conjunction with managing bleeding abnormalities is a key to success [499]. A consensus on clinical treatment recommendations is lacking due to the paucity of randomized controlled trials focused on cancer-associated thrombosis. The impact of new thrombin and factor Xa inhibitors in this setting remains to be established [500, 499].
40.2 Megakaryocytes as translational targets?
Influencing the biology of megakaryocytes or the production of platelets is a potential intervention point. Considering the potential link between tumor-derived IL-6 capacity to stimulate hepatic thrombopoietin synthesis and paraneoplastic induction of thrombocytosis, this is a potential intervention point [423]. Directly inhibiting IL-6 levels using anti-IL-6 therapy may be one option. These approaches have been explored in the treatment of chronic inflammatory disorders such rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) [501, 502]. Tocilizumab is one IL-6 inhibitor (IL-6i) licensed for RA and JIA, and other agents targeting either IL-6 or its receptor remain under investigation [502].
Directly targeting thrombopoietin synthesis or the development of thrombopoietin receptor (TPO-R) antagonists may be another approach. TPO-R agonists are available for treating thrombocytopenia. Two of these new agents, romiplostim and eltrombopag, are for use in patients with primary immune thrombocytopenia that is unresponsive to conventional treatments [503, 504]. No TPO-R antagonists are currently available.
There may be other intervention points for limiting IL-6 production. To some extent, IL-6 production may involve a feedback loop through the production of PGE2 in the tumor microenvironment since generation of the latter induces IL-6 synthesis [505]. Thus, nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclooxygenase-2 inhibitors (COXIBs) may help suppress thrombocytosis by preventing PGE2 synthesis [446].
Genetic interventions might be another possible approach in high-risk populations. However, to impact platelet biology requires molecular modification of megakaryocytes. Megakaryocytes are genetically more stable than tumor cells and therefore more reliable targets. Genetic interventions of this nature might include autologous bone marrow transplants. One could envision a potential scenario involving the extraction of patient bone marrow, isolation of MEP cells, and genetic manipulation prior to their delineation into MKP. Since platelets act as “first responders” and can actually migrate into the subendothelial matrix, this presents a useful targeting approach. Enhanced expression and packaging of shRNAs that suppress oncogene or enhance suppressor gene expression could then seek out circulating cancer cells or tumors. One foreseeable advantage of using autologous MEPs includes the terminal differentiation to MKPs that results in their loss once platelets are formed giving clinicians some control over biologic dosing.
41 NSAIDs and COXIBs inhibit cancer
NSAIDs and COXIBs effectively inhibit cancer formation and progression [446]. Aspirin covalently inactivates COX-1 more selectively than COX-2 but causes GI toxicity [446]. Population and clinical studies indicate that regular intake of aspirin and various other NSAIDs reduce the risk and incidence of various cancers [506-512]. Prospective cohort studies that involved 82,911 women enrolled in the Nurses’ Health Study [513] or 47,363 male health professionals [514] showed that regular long-term use of aspirin significantly reduced incidence of CRC. In other prospective studies, daily use of aspirin significantly reduced the incidence of colorectal adenomas, breast cancer, and lung cancer [515-520]. A recent international consensus statement indicates the benefits of aspirin use [521]. The advantage of using COX-1 selective agents, particularly aspirin, is the attenuation of TxA2 production by platelets. This approach prevents all of the platelet-mediated effects associated with their activation, aggregation, degranulation, and tumor cell platelet emboli formation. This reduces cardiovascular risk but unfortunately does not alleviate the GI toxicity or effectively prevent the production of proinflammatory/prooncogenic PGE2 [446].
In contrast, COXIBs are competitive and selective inhibitors of COX-2 but are associated with cardiovascular toxicity [446]. A number of case/control studies support the use of COXIBs in preventing colon cancer with fewer GI side effects [522, 515, 523-526]. However, the discovery of increased cardiovascular risk overshadowed these findings [522, 515, 523-526]. Similarly, lung cancer and prostate cancer incidence is also reduced through the use of COXIBs [527, 528]. Collectively, these studies illustrate the potential benefits associated with the use of COXIBs, particularly a reduction in cancer incidence in high-risk populations. Recently, Dovizio et al. showed that the induction of COX-2 overexpression by platelet-tumor cell cross-talk could be inhibited by disrupting galectin-3 function or collagen receptor-mediated platelet adhesion. Their study suggested that blocking physical interactions by targeting collagen binding sites could be an alternate means of affecting COX-2 [529].
In contrast to aspirin, the advantage of COXIBs is their suppressive effect on proinflammatory/prooncogenic PGE2 production by tumor cells. This reduces GI toxicity, but regrettably enhances cardiovascular risk by suppressing PGI2 production in endothelial cells, and by reducing the antiplatelet aggregation effects [446]. Since PGE2 and its enzymatic metabolite 13,14-dihydro-15-ketoPGE2 prevent PGI2 inhibition of TCIPA, this underscores the need for selective targeting of the PGE2 pathway [63, 446]. Although attempts have been made to selectively target the PGE2 pathway, more effective agents are needed [530, 531]. These new drugs could be used in conjunction with low-dose aspirin to eliminate the genesis and spread of many different cancers.
Ultimately, more targeted and selective approaches are needed to eliminate the proinflammatory, prooncogenic, and prometastatic effects of platelets in cancer biology while maintaining their important hemostatic role as “first responders”.
Acknowledgments
This study was supported by the following grants: CPRIT RP100969 to DGM, NCI CA177909 to AKS, and DoD W81XWH-11-1-0519 to KVH.
Contributor Information
David G. Menter, Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA; Department of Cancer Biology, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA
Stephanie C. Tucker, Bioactive Lipids Research Program, Department of Pathology, Wayne State University, 5101 Cass Ave. 430 Chemistry, Detroit, MI 48202, USA
Scott Kopetz, Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA.
Anil K. Sood, Gynecologic Oncology & Reproductive Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA; Department of Cancer Biology, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA; Center for RNA Interference and Non-Coding RNA, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA
John D. Crissman, Cancer Biology Division, Department of Pathology, School of Medicine, Wayne State University School of Medicine, 431 Chemistry Bldg., Detroit, MI 48202, USA
Kenneth V. Honn, Bioactive Lipids Research Program, Department of Pathology, Wayne State University, 5101 Cass Ave. 430 Chemistry, Detroit, MI 48202, USA; Cancer Biology Division, Department of Pathology, School of Medicine, Wayne State University School of Medicine, 431 Chemistry Bldg., Detroit, MI 48202, USA
References
- 1.Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Reviews. 1992;11(3-4):325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 2.Rados C. Beyond bloodletting: FDA gives leeches a medical makeover. FDA Consumer. 2004;38(5):9. [PubMed] [Google Scholar]
- 3.Winkel R, Tajsic N, Husum H, Schlageter M, Hanebuth G, Hoffmann R. Saphenous perforator flap. Operative Orthopädie und Traumatologie. 2013;25(2):152–161. doi: 10.1007/s00064-012-0198-z. [DOI] [PubMed] [Google Scholar]
- 4.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111(3):981–986. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinhubl SR. Historical observations on the discovery of platelets, platelet function testing and the first antiplatelet agent. Current Drug Targets. 2011;12(12):1792–1804. doi: 10.2174/138945011797635858. [DOI] [PubMed] [Google Scholar]
- 6.Addison W. On the colourless corpuscles and on the molecules and cytoblasts in the blood. London Med Gaz. 1842;30:144–152. [Google Scholar]
- 7.Schultze M. Ein heizbarer Objecttisch und seine Verwendung bei Untersuchungen des Blutes. Arch Mikrosc Anatomy. 1865;1:1–42. [Google Scholar]
- 8.Bizzozero J. Über einenneuen Formbestandtheil des Blutes und dessen Rolle bei der Thrombose und der Blutgerinnung. Virchows Archiv fur pathologische Anatomie und Physiologie und fur klinische Medizin. 1882;90:261–332. [Google Scholar]
- 9.de Gaetano G. A new blood corpuscle: an impossible interview with Giulio Bizzozero. Thrombosis and Haemostasis. 2001;86(4):973–979. [PubMed] [Google Scholar]
- 10.Virchow R. Gesammelte (ad) Handlungen zur wissenschaftlichen Medizin. Frankfurt Meidinger; 1856. [Google Scholar]
- 11.Bizzozero G. Sul midollo delle ossa. Tipografia Italiana; Napoli: 1869. [Google Scholar]
- 12.Osler W, Schäfer EA. über einige im Blute vorhandene bacterienbildende Massen. Centralbl Medicine Wissensch. 1873;11:577–578. [Google Scholar]
- 13.Howell WH. Observations upon the occurrence, structure, and function of the giant cells of the marrow. Journal of Morphology. 1890;4:117–130. [Google Scholar]
- 14.Wright JH. The origin and nature of the blood plates. Boston Medical and Surgical Journal. 1906;23:643–645. [Google Scholar]
- 15.Wright JH. The histogenesis of blood platelets. Journal of Morphology. 1910;21:263–278. [Google Scholar]
- 16.Nakeff A, Maat B. Separation of megakaryocytes from mouse bone marrow by velocity sedimentation. Blood. 1974;43(4):591–595. [PubMed] [Google Scholar]
- 17.Pease DC. An electron microscopic study of red bone marrow. Blood. 1956;11(6):501–526. [PubMed] [Google Scholar]
- 18.Trousseau A. Lectures on clinical medicine. Vol. 5. Hotel-Dieu, Paris: 1865. Phlegmasia alba dolens; pp. 281–332. Delivered at the. [Google Scholar]
- 19.Bariety M. Trousseau, 1801-1867. Mazenod; Geneva: 1947. pp. 234–235. [Google Scholar]
- 20.Osler W, McCrae T. Latent cancer of the stomach. Phil Medical Journal. 1900;5:245. [Google Scholar]
- 21.Sproul E. Carcinoma and venous thrombosis: the frequency of association of carcinoma in the body and tail of the pancreas with multiple venous thrombosis. American Journal of Cancer. 1938;34:566–585. [Google Scholar]
- 22.Edwards E. Migratory thrombophlebitis associated with carcinoma. The New England Journal of Medicine. 1949;240:1131–1135. doi: 10.1056/NEJM194906302402601. [DOI] [PubMed] [Google Scholar]
- 23.Gross FB, Jr., Jaehning DG, Coker WG. The association of migratory thrombophlebitis with carcinoma. North Carolina Medical Journal. 1951;12(3):97–101. [PubMed] [Google Scholar]
- 24.Henderson PH., Jr. Multiple migratory thrombophlebitis associated with ovarian carcinoma. American Journal of Obstetrics and Gynecology. 1955;70(2):452–453. doi: 10.1016/s0002-9378(16)37699-2. [DOI] [PubMed] [Google Scholar]
- 25.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(12):2362–2367. doi: 10.1161/ATVBAHA.110.207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarniou AP, Moreau A. Recurrent & migratory thrombophlebitis revealing a secondary cancer with mediastino-pulmonary form. Presse Médicate. 1959;67(27):1117–1118. [PubMed] [Google Scholar]
- 27.Jennings W, Russel W. Phlebothrombosis associated with mucin-producing carcinomas of the tail and body of the pancreas. Archives of Surgery. 1948;56:186–198. doi: 10.1001/archsurg.1948.01240010191009. [DOI] [PubMed] [Google Scholar]
- 28.Kenney W. The association of carcinoma in the body and tail of the pancreas with multiple venous thrombi. Surgery. 1943;14:600–609. [Google Scholar]
- 29.Linquette M, Mesmacque R, Fossati P, Luez G, Beghin B. Recurrent and migratory venous thromboses. Prog ress in Medical (Paris) 1964;92:689–698. [PubMed] [Google Scholar]
- 30.Mainoli S, Piccinelli O. Migratory thrombophlebitis and malignant tumors; migratory thrombophlebitis occurring during two cases of reticulosarcoma. La Riforma Medica. 1956;70(46):1330–1334. [PubMed] [Google Scholar]
- 31.McKay D, Wahle G. Disseminated thrombosis in colon cancer. Cancer. 1955;8:970–978. doi: 10.1002/1097-0142(1955)8:5<970::aid-cncr2820080520>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. British Journal of Cancer. 2010;102(Suppl I):S2–9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusbacher J. Migratory venous thrombosis and cancer. New York State Journal of Medicine. 1964;64:2166–2173. [PubMed] [Google Scholar]
- 34.Oster MW. Thrombophlebitis and cancer. A review. Angiology. 1976;27(10):557–567. doi: 10.1177/000331977602701002. [DOI] [PubMed] [Google Scholar]
- 35.Picard R, Horeau J, Guillon J, Robin C. Migratory thrombophlebitis & bronchopulmonary cancer. Bulletins et Mémoires de la Société Médicale des Hôpitaux de Paris. 1959;75(9-II):327–329. [PubMed] [Google Scholar]
- 36.Popesco I, Ciobanu V. Migratory thrombophlebitis as a manifestation of visceral cancer. La Semaine des Hôpitaux. 1958;34(1):26–30. [PubMed] [Google Scholar]
- 37.Rizzo JA. Migratory thrombophlebitis and visceral cancer. Revista de la Asociación Médica Argentina. 1956;70(825-826):236–238. [PubMed] [Google Scholar]
- 38.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Womack WS, Castellano CJ. Migratory thrombophlebitis associated with ovarian carcinoma. American Journal of Obstetrics and Gynecology. 1952;63(2):467–469. doi: 10.1016/s0002-9378(15)32851-9. [DOI] [PubMed] [Google Scholar]
- 40.Pineo GF, Regoeczi E, Hatton MW, Brain MC. The activation of coagulation by extracts of mucus: a possible pathway of intravascular coagulation accompanying adenocarcinomas. The Journal of Laboratory and Clinical Medicine. 1973;82(2):255–266. [PubMed] [Google Scholar]
- 41.Brugarolas A, Elias EG, Takita H, Mink IB, Mittelman A, Ambrus JL. Blood coagulation and fibrinolysis in patients with carcinoma of the lung. Journal of Medicine. 1973;4(2):96–105. [PubMed] [Google Scholar]
- 42.Peterson HI, Appelgren KL, Rosengren BH. Fibrinogen metabolism in experimental tumours. European Journal of Cancer. 1969;5(6):535–542. doi: 10.1016/0014-2964(69)90001-2. [DOI] [PubMed] [Google Scholar]
- 43.Peterson HI, Appelgren KL, Rosengren BH. Experimental studies on the mechanisms of fibrinogen uptake in a rat tumour. European Journal of Cancer. 1972;8(6):677–681. doi: 10.1016/0014-2964(72)90151-x. [DOI] [PubMed] [Google Scholar]
- 44.Peterson HI, Zettergren L. Thromboplastic and fibrinolytic properties of three transplantable rat tumours. Acta Chirurgica Scandinavica. 1970;136(5):365–368. [PubMed] [Google Scholar]
- 45.Moolten SE, Vroman L. The adhesiveness of blood platelets in thromboembolism and hemorrhagic disorders; measurement of platelet adhesiveness by the glass-wool filter. American Journal of Clinical Pathology. 1949;19(8):701–709. doi: 10.1093/ajcp/19.8.701. [DOI] [PubMed] [Google Scholar]
- 46.Levin J, Conley CL. Thrombocytosis associated with malignant disease. Archives of Internal Medicine. 1964;114:497–500. doi: 10.1001/archinte.1964.03860100079008. [DOI] [PubMed] [Google Scholar]
- 47.Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. International Journal of Cancer. 1973;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- 48.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proceedings of the National Academy of Sciences of the United States of America. 1968;61(1):46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gastpar H. Inhibition of “cancer cell stickiness” through bencylan-hydrogen fumarate (fluditate) Fortschritte der Medizin. 1973;91(33):1322–1328. [PubMed] [Google Scholar]
- 50.Hilgard P. The role of blood platelets in experimental metastases. British Journal of Cancer. 1973;28(5):429–435. doi: 10.1038/bjc.1973.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warren BA. Environment of the blood-borne tumor embolus adherent to vessel wall. Journal of Medicine. 1973;4(3):150–177. [PubMed] [Google Scholar]
- 52.Warren BA, Vales O. The adhesion of thromboplastic tumour emboli to vessel walls in vivo. British Journal of Experimental Pathology. 1972;53(3):301–313. [PMC free article] [PubMed] [Google Scholar]
- 53.Cliffton EE, Grossi CE. Effect of human plasmin on the toxic effects and growth of blood borne metastatis of the Brown-Pearce carcinoma and the V2 carcinoma of rabbit. Cancer. 1956;9(6):1147–1152. doi: 10.1002/1097-0142(195611/12)9:6<1147::aid-cncr2820090613>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JH, Woods JR. An in vitro study of fibrinolytic agents on V2 carcinoma cells and intravascular thrombi in rabbits. Bulletin of the Johns Hopkins Hospital. 1963;113:335–346. [PubMed] [Google Scholar]
- 55.Pearce L, Brown WH. Studies based on a malignant tumor of the rabbit: V. Metastases. Part 1. Description of the lesions with especial reference to their occurrence and distribution. The Journal of Experimental Medicine. 1923;38(4):347–366. doi: 10.1084/jem.38.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rous P, Kidd JG. The carcinogenic effect of a papilloma virus on the tarred skin of rabbits: I. Description of the phenomenon. The Journal of Experimental Medicine. 1938;67(3):399–428. doi: 10.1084/jem.67.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods JR. Experimental studies of the intravascular dissemination of ascitic V2 carcinoma cells in the rabbit, with special reference to fibrinogen and fibrinolytic agents. Bulletin der Schweizerischen Akademie der Medizinischen Wissenschaften. 1964;20:92–121. [PubMed] [Google Scholar]
- 58.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. Journal of the National Cancer Institute. 1970;45(4):773–782. [PubMed] [Google Scholar]
- 59.Honn KV, Menter D, Cavanaugh PG, Neagos G, Moilanen D, Taylor JD, et al. A review of prostaglandins and the treatment of tumor metastasis. Acta Clinica Belgica. 1983;38(1):53–67. doi: 10.1080/22953337.1983.11718906. [DOI] [PubMed] [Google Scholar]
- 60.Honn KV, Bockman RS, Marnett LJ. Prostaglandins and cancer: a review of tumor initiation through tumor metastasis. Prostaglandins. 1981;21(5):833–864. doi: 10.1016/0090-6980(81)90240-9. [DOI] [PubMed] [Google Scholar]
- 61.Menter D, Dunn J, Palazzo R, Tchen T, Taylor J, Honn K. Prostaglandins and cancer. Alan R. Liss; New York: 1982. Tumor cell induced platelet aggregation: inhibition by prostacyclin, thromboxane A2 and phosphodiesterase inhibitors. [Google Scholar]
- 62.Menter DG, Harkins C, Onoda J, Riorden W, Sloane BF, Taylor JD, et al. Inhibition of tumor cell induced platelet aggregation by prostacyclin and carbacyclin: an ultrastructural study. Invasion & Metastasis. 1987;7(2):109–128. [PubMed] [Google Scholar]
- 63.Menter DG, Onoda JM, Taylor JD, Honn KV. Effects of prostacyclin on tumor cell-induced platelet aggregation. Cancer Research. 1984;44(2):450–456. [PubMed] [Google Scholar]
- 64.Cavanaugh PG, Sloane BF, Bajkowski AS, Gasic GJ, Gasic TB, Honn KV. Involvement of a cathepsin B-like cysteine proteinase in platelet aggregation induced by tumor cells and their shed membrane vesicles. Clinical & Experimental Metastasis. 1983;1(4):297–307. doi: 10.1007/BF00121192. [DOI] [PubMed] [Google Scholar]
- 65.Crissman JD, Hatfield J, Schaldenbrand M, Sloane BF, Honn KV. Arrest and extravasation of B16 amelanotic melanoma in murine lungs. A light and electron microscopic study. Laboratory Investigation. 1985;53(4):470–478. [PubMed] [Google Scholar]
- 66.Crissman JD, Hatfield JS, Honn KV. Clinical and experimental morphologic parameters predictive of tumor metastasis. Progress in Clinical and Biological Research. 1986;212:251–267. [PubMed] [Google Scholar]
- 67.Crissman JD, Hatfield JS, Menter DG, Sloane B, Honn KV. Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Research. 1988;48(14):4065–4072. [PubMed] [Google Scholar]
- 68.Kinjo M. Lodgement and extravasation of tumour cells in blood-borne metastasis: an electron microscope study. British Journal of Cancer. 1978;38(2):293–301. doi: 10.1038/bjc.1978.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Machlus KR, Italiano JE., Jr. The incredible journey: from megakaryocyte development to platelet formation. The Journal of Cell Biology. 2013;201(6):785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menter DG, Hatfield JS, Harkins C, Sloane BF, Taylor JD, Crissman JD, et al. Tumor cell-platelet interactions in vitro and their relationship to in vivo arrest of hematogenously circulating tumor cells. Clinical & Experimental Metastasis. 1987;5(1):65–78. doi: 10.1007/BF00116627. [DOI] [PubMed] [Google Scholar]
- 71.Sloane BF, Rozhin J, Hatfield JS, Crissman JD, Honn KV. Plasma membrane-associated cysteine proteinases in human and animal tumors. Experimental Cell Biology. 1987;55(4):209–224. doi: 10.1159/000163420. [DOI] [PubMed] [Google Scholar]
- 72.White JG. A simple method for preservation of fine structure in blood cells. Thrombosis et Diathesis Haemorrhagica. 1967;18(3-4):745–753. [PubMed] [Google Scholar]
- 73.White JG, Krivit W. The canalicular system of blood platelets: apossible sarcoplasmic reticulum. The Journal of Laboratory and Clinical Medicine. 1967;49:60. [Google Scholar]
- 74.White JG, Krivit W. Changes in platelet microtubules and granules during early clot development. Thrombosis et Diathesis Haemorrhagica. Supplementum. 1967;26:29–42. [PubMed] [Google Scholar]
- 75.Grossi IM, Fitzgerald LA, Kendall A, Taylor JD, Sloane BF, Honn KV. Inhibition of human tumor cell induced platelet aggregation by antibodies to platelet glycoproteins Ib and IIb/IIIa. Proceedings of the Society for Experimental Biology and Medicine. 1987;186(3):378–383. doi: 10.3181/00379727-186-3-rc1. [DOI] [PubMed] [Google Scholar]
- 76.Bluteau D, Lordier L, Di Stefano A, Chang Y, Raslova H, Debili N, et al. Regulation of megakaryocyte maturation and platelet formation. Journal of Thrombosis and Haemostasis. 2009;7(Suppl 1):227–234. doi: 10.1111/j.1538-7836.2009.03398.x. [DOI] [PubMed] [Google Scholar]
- 77.Geddis AE. Megakaryopoiesis. Seminars in Hematology. 2010;47(3):212–219. doi: 10.1053/j.seminhematol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thon JN, Italiano JE. Platelet formation. Seminars in Hematology. 2010;47(3):220–226. doi: 10.1053/j.seminhematol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGrath K, Palis J. Ontogeny of erythropoiesis in the mammalian embryo. Current Topics in Developmental Biology. 2008;82:1–22. doi: 10.1016/S0070-2153(07)00001-4. [DOI] [PubMed] [Google Scholar]
- 80.Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicologic Pathology. 2006;34(5):548–565. doi: 10.1080/01926230600939856. [DOI] [PubMed] [Google Scholar]
- 81.Kelly PJ. Anatomy, physiology, and pathology of the blood supply of bones. The Journal of Bone and Joint Surgery. American Volume. 1968;50(4):766–783. doi: 10.2106/00004623-196850040-00015. [DOI] [PubMed] [Google Scholar]
- 82.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. European Cells & Materials. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 83.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nature Reviews. Immunology. 2008;8(4):290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 85.Oh IH, Kwon KR. Concise review: multiple niches for hematopoietic stem cell regulations. Stem Cells. 2010;28(7):1243–1249. doi: 10.1002/stem.453. [DOI] [PubMed] [Google Scholar]
- 86.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 87.Hevehan DL, Papoutsakis ET, Miller WM. Physiologically significant effects of pH and oxygen tension on granulopoiesis. Experimental Hematology. 2000;28(3):267–275. doi: 10.1016/s0301-472x(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 88.Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26(1):54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 89.Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends in Molecular Medicine. 2007;13(2):72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Lilly AJ, Johnson WE, Bunce CM. The haematopoietic stem cell niche: new insights into the mechanisms regulating haematopoietic stem cell behaviour. Stem Cells International. 2011;2011:274–564. doi: 10.4061/2011/274564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends in Immunology. 2011;32(7):315–320. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Deutsch VR, Tomer A. Advances in megakaryocytopoiesis and thrombopoiesis: from bench to bedside. British Journal of Haematology. 2013;161(6):778–793. doi: 10.1111/bjh.12328. [DOI] [PubMed] [Google Scholar]
- 93.Yu M, Cantor AB. Megakaryopoiesis and thrombopoiesis: an update on cytokines and lineage surface markers. Methods in Molecular Biology. 2012;788:291–303. doi: 10.1007/978-1-61779-307-3_20. [DOI] [PubMed] [Google Scholar]
- 94.Kanz L, Lohr GW, Fauser AA. Human megakaryocytic progenitor cells. Klinische Wochenschrift. 1987;65(7):297–307. doi: 10.1007/BF01745383. [DOI] [PubMed] [Google Scholar]
- 95.Tijssen MR, Ghevaert C. Transcription factors in late megakaryopoiesis and related platelet disorders. Journal of Thrombosis and Haemostasis. 2013;11(4):593–604. doi: 10.1111/jth.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaur G, Jalagadugula G, Mao G, Rao AK. RUNX1/core binding factor A2 regulates platelet 12-lipoxygenase gene (ALOX12): studies in human RUNX1 haplodeficiency. Blood. 2010;115(15):3128–3135. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fowler M, Borazanci E, McGhee L, Pylant SW, Williams BJ, Glass J, et al. RUNX1 (AML-1) and RUNX2 (AML-3) cooperate with prostate-derived Ets factor to activate transcription from the PSA upstream regulatory region. Journal of Cellular Biochemistry. 2006;97(1):1–17. doi: 10.1002/jcb.20664. [DOI] [PubMed] [Google Scholar]
- 98.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. The Journal of Experimental Medicine. 2005;201(9):1487–1502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nutt SL, Metcalf D, D’Amico A, Polli M, Wu L. Dynamic regulation of PU. 1 expression in multipotent hematopoietic progenitors. The Journal of Experimental Medicine. 2005;201(2):221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1(4):416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Chlon TM, Dore LC, Crispino JD. Cofactor-mediated restriction of GATA-1 chromatin occupancy coordinates lineage-specific gene expression. Molecular Cell. 2012;47(4):608–621. doi: 10.1016/j.molcel.2012.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dore LC, Chlon TM, Brown CD, White KP, Crispino JD. Chromatin occupancy analysis reveals genome-wide GATA factor switching during hematopoiesis. Blood. 2012;119(16):3724–3733. doi: 10.1182/blood-2011-09-380634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malinge S, Thiollier C, Chlon TM, Dore LC, Diebold L, Bluteau O, et al. Ikaros inhibits megakaryopoiesis through functional interaction with GATA-1 and NOTCH signaling. Blood. 2013;121(13):2440–2451. doi: 10.1182/blood-2012-08-450627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chagraoui H, Kassouf M, Baneijee S, Goardon N, Clark K, Atzberger A, et al. SCL-mediated regulation of the cell-cycle regulator p21 is critical for murine megakaryopoiesis. Blood. 2011;118(3):723–735. doi: 10.1182/blood-2011-01-328765. [DOI] [PubMed] [Google Scholar]
- 105.Lordier L, Bluteau D, Jalil A, Legrand C, Pan J, Rameau P, et al. RUNX1-induced silencing of non-muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nature Communications. 2012;3:717. doi: 10.1038/ncomms1704. [DOI] [PubMed] [Google Scholar]
- 106.Krumsiek J, Marr C, Schroeder T, Theis FJ. Hierarchical differentiation of myeloid progenitors is encoded in the transcription factor network. PLoS One. 2011;6(8):e22649. doi: 10.1371/journal.pone.0022649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takayama M, Fujita R, Suzuki M, Okuyama R, Aiba S, Motohashi H, et al. Genetic analysis of hierarchical regulation for Gata1 and NF-E2 p45 gene expression in megakaryopoiesis. Molecular and Cellular Biology. 2010;30(11):2668–2680. doi: 10.1128/MCB.01304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vitrat N, Letestu R, Masse A, Lazar V, Vainchenker W, Debili N. Thromboxane synthase has the same pattern of expression as platelet specific glycoproteins during human megakaryocyte differentiation. Thrombosis and Haemostasis. 2000;83(5):759–768. [PubMed] [Google Scholar]
- 109.Bray PF, McKenzie SE, Edelstein LC, Nagalla S, Delgrosso K, Ertel A, et al. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 2013;14:1. doi: 10.1186/1471-2164-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Edelstein LC, McKenzie SE, Shaw C, Holinstat MA, Kunapuli SP, Bray PF. MicroRNAs in platelet production and activation. Journal of Thrombosis and Haemostasis. 2013;11(Suppl 1):340–350. doi: 10.1111/jth.12214. [DOI] [PubMed] [Google Scholar]
- 111.Guo S, Lu J, Schlanger R, Zhang H, Wang JY, Fox MC, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu J, Guo S, Ebert BL, Zhang H, Peng X, Bosco J, et al. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Developmental Cell. 2008;14(6):843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117(19):5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carpinelli MR, Hilton DJ, Metcalf D, Antonchuk JL, Hyland CD, Mifsud SL, et al. Suppressor screen in Mpl−/− mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6553–6558. doi: 10.1073/pnas.0401496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Graaf CA, Kauppi M, Baldwin T, Hyland CD, Metcalf D, Willson TA, et al. Regulation of hematopoietic stem cells by their mature progeny. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21689–21694. doi: 10.1073/pnas.1016166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Metcalf D, Carpinelli MR, Hyland C, Mifsud S, Dirago L, Nicola NA, et al. Anomalous megakaryocytopoiesis in mice with mutations in the c-Myb gene. Blood. 2005;105(9):3480–3487. doi: 10.1182/blood-2004-12-4806. [DOI] [PubMed] [Google Scholar]
- 117.Kumar MS, Narla A, Nonami A, Mullally A, Dimitrova N, Ball B, et al. Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q-syndrome. Blood. 2011;118(17):4666–4673. doi: 10.1182/blood-2010-12-324715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hussein K, Dralle W, Theophile K, Kreipe H, Bock O. Megakaryocytic expression of miRNA 10a, 17-5p, 20a and 126 in Philadelphia chromosome-negative myeloproliferative neoplasm. Annals of Hematology. 2009;88(4):325–332. doi: 10.1007/s00277-008-0602-9. [DOI] [PubMed] [Google Scholar]
- 119.Lin J, Zhan R. Advance of studies on role of miRNA in hematopoietic regulation and myeloproliferative neoplasms. Zhongguo Shi Ym Xue Ye Xue Za Zhi. 2011;19(4):1071–1074. [PubMed] [Google Scholar]
- 120.Edelstein LC, Bray PF. Small RNAs as potential platelet therapeutics. Handbook of Experimental Pharmacology. 2012;210:435–445. doi: 10.1007/978-3-642-29423-5_17. [DOI] [PubMed] [Google Scholar]
- 121.Vigon I, Mornon JP, Cocault L, Mitjavila MT, Tambourin P, Gisselbrecht S, et al. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bartley TD, Bogenberger J, Hunt P, Li YS, Lu HS, Martin F, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77(7):1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 123.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 124.Kaushansky K. The mpl ligand: molecular and cellular biology of the critical regulator of megakaryocyte development. Stem Cells. 1994;12(Suppl 1):91–96. discussion 96-97. [PubMed] [Google Scholar]
- 125.Sohma Y, Akahori H, Seki N, Hori T, Ogami K, Kato T. Molecular cloning and chromosomal localization of the human thrombopoietin gene. FEBS Letters. 1994;353(1):57–61. doi: 10.1016/0014-5793(94)01008-0. [DOI] [PubMed] [Google Scholar]
- 126.Wendling F, Maraskovsky E, Debili N, Florindo C, Teepe M, Titeux M, et al. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994;369(6481):571–574. doi: 10.1038/369571a0. [DOI] [PubMed] [Google Scholar]
- 127.Douglas VK, Tallman MS, Cripe LD, Peterson LC. Thrombopoietin administered during induction chemotherapy to patients with acute myeloid leukemia induces transient morphologic changes that may resemble chronic myeloproliferative disorders. American Journal of Clinical Pathology. 2002;117(6):844–850. doi: 10.1309/09NP-3DFG-BLM9-E5LE. [DOI] [PubMed] [Google Scholar]
- 128.Neumann TA, Foote M. Megakaryocyte growth and development factor (MGDF): an Mpl ligand and cytokine that regulates thrombopoiesis. Cytokines, Cellular & Molecular Therapy. 2000;6(1):47–56. doi: 10.1080/13684730050515912. [DOI] [PubMed] [Google Scholar]
- 129.Dong-Feng Z, Ting L, Yong Z, Cheng C, Xi Z, Pei-Yan K. The TPO/c-MPL pathway in the bone marrow may protect leukemia cells from chemotherapy in AML patients. Pathology and Oncology Research. 2013 doi: 10.1007/s12253-013-9696-z. doi: 10.1007/s12253-013-9696-z. [DOI] [PubMed] [Google Scholar]
- 130.Cosgrove LJ, Sandrin MS, Rajasekariah P, McKenzie IF. A genomic clone encoding the alpha chain of the OKM1, LFA-1, and platelet glycoprotein IIb-IIIa molecules. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(3):752–756. doi: 10.1073/pnas.83.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fitzgerald LA, Poncz M, Steiner B, Rall SC, Jr., Bennett JS, Phillips DR. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- 132.Kostyak JC, Naik UP. Megakaryopoiesis: transcriptional insights into megakaryocyte maturation. Frontiers in Bioscience. 2007;12:2050–2062. doi: 10.2741/2210. [DOI] [PubMed] [Google Scholar]
- 133.Lanza F, Kieffer N, Phillips DR, Fitzgerald LA. Characterization of the human platelet glycoprotein IIIa gene. Comparison with the fibronectin receptor beta-subunit gene. The Journal of Biological Chemistry. 1990;265(30):18098–18103. [PubMed] [Google Scholar]
- 134.Levene RB, Williams NT, Lamaziere JM, Rabellino EM. Human megakaryocytes. IV. Growth and characterization of clonable megakaryocyte progenitors in agar. Experimental Hematology. 1987;15(2):181–189. [PubMed] [Google Scholar]
- 135.Majka M, Ratajczak J, Villaire G, Kubiczek K, Marquez LA, Janowska-Wieczorek A, et al. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT, and STAT proteins in normal human CD34+ cells, megakaryocytes, and platelets. Experimental Hematology. 2002;30(7):751–760. doi: 10.1016/s0301-472x(02)00810-x. [DOI] [PubMed] [Google Scholar]
- 136.Miyazaki H. Physiologic role of TPO in thrombopoiesis. Stem Cells. 1996;14(Suppl 1):133–138. doi: 10.1002/stem.5530140717. [DOI] [PubMed] [Google Scholar]
- 137.Monzen S, Takahashi K, Yoshino H, Kasai-Eguchi K, Kashiwakura I. Terminal maturation of megakaryocytes and platelet production by hematopoietic stem cells irradiated with heavy-ion beams. Radiation Research. 2011;176(1):8–16. doi: 10.1667/rr2392.1. [DOI] [PubMed] [Google Scholar]
- 138.Sumner R, Crawford A, Mucenski M, Frampton J. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene. 2000;19(30):3335–3342. doi: 10.1038/sj.onc.1203660. [DOI] [PubMed] [Google Scholar]
- 139.Zimmet J, Ravid K. Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Experimental Hematology. 2000;28(1):3–16. doi: 10.1016/s0301-472x(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 140.Thon JN, Italiano JE. Visualization and manipulation of the platelet and megakaryocyte cytoskeleton. Methods in Molecular Biology. 2012;788:109–125. doi: 10.1007/978-1-61779-307-3_9. [DOI] [PubMed] [Google Scholar]
- 141.Yamada E. The fine structure of the megakaryocyte in the mouse spleen. Acta Anatomica (Basel) 1957;29(3):267–290. doi: 10.1159/000141169. [DOI] [PubMed] [Google Scholar]
- 142.Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. Journal of Ultrastructure Research. 1968;24(5):412–433. doi: 10.1016/s0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- 143.Radley JM, Haller CJ. The demarcation membrane system of the megakaryocyte: a misnomer? Blood. 1982;60(1):213–219. [PubMed] [Google Scholar]
- 144.Chen Y, Aardema J, Kale S, Whichard ZL, Awomolo A, Blanchard E, et al. Loss of the F-BAR protein CIP4 reduces platelet production by impairing membrane-cytoskeleton remodeling. Blood. 2013;122(10):1695–706. doi: 10.1182/blood-2013-03-484550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang W, Gilligan DM, Sun S, Wu X, Reems JA. Distinct functional effects for dynamin 3 during megakaryocytopoiesis. Stem Cells and Development. 2011;20(12):2139–2151. doi: 10.1089/scd.2011.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel-Hett S, Wang H, Begonja AJ, Thon JN, Alden EC, Wandersee NJ, et al. The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood. 2011;118(6):1641–1652. doi: 10.1182/blood-2011-01-330688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Nispen TOT, Pannerden H, De Haas F, Geerts W, Posthuma G, Van Dijk S, Heijnen HF. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–1156. doi: 10.1182/blood-2010-02-268680. [DOI] [PubMed] [Google Scholar]
- 148.Kamykowski J, Carlton P, Sehgal S, Storrie B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet alpha-granules. Blood. 2011;118(5):1370–1373. doi: 10.1182/blood-2011-01-330910. [DOI] [PubMed] [Google Scholar]
- 149.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Reviews. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Current Opinion in Hematology. 2013;20(5):464–471. doi: 10.1097/MOH.0b013e3283632e6b. [DOI] [PubMed] [Google Scholar]
- 151.Albers CA, Cvejic A, Favier R, Bouwmans EE, Alessi MC, Bertone P, et al. Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nature Genetics. 2011;43(8):735–737. doi: 10.1038/ng.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nature Genetics. 2004;36(4):400–404. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]
- 153.Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, Zivony-Elboum Y, Gumruk F, Cetin M, et al. NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet alpha-granules. Nature Genetics. 2011;43(8):732–734. doi: 10.1038/ng.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kahr WH, Hinckley J, Li L, Schwertz H, Christensen H, Rowley JW, et al. Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nature Genetics. 2011;43(8):738–740. doi: 10.1038/ng.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Urban D, Li L, Christensen H, Pluthero FG, Chen SZ, Puhacz M, et al. The VPS33B-binding protein VPS16B is required in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2012;120(25):5032–5040. doi: 10.1182/blood-2012-05-431205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120(19):4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jedlitschky G, Greinacher A, Kroemer HK. Transporters in human platelets: physiologic function and impact for pharmacotherapy. Blood. 2012;119(15):3394–3402. doi: 10.1182/blood-2011-09-336933. [DOI] [PubMed] [Google Scholar]
- 158.Niessen J, Jedlitschky G, Grube M, Kawakami H, Kamiie J, Ohtsuki S, et al. Expression of ABC-type transport proteins in human platelets. Pharmacogenetics and Genomics. 2010;20(6):396–400. doi: 10.1097/FPC.0b013e32833997b0. [DOI] [PubMed] [Google Scholar]
- 159.Dhanjal TS, Pendaries C, Ross EA, Larson MK, Protty MB, Buckley CD, et al. A novel role for PECAM-1 in megakaryocytokinesis and recovery of platelet counts in thrombocytopenic mice. Blood. 2007;109(10):4237–4244. doi: 10.1182/blood-2006-10-050740. [DOI] [PubMed] [Google Scholar]
- 160.Mazharian A. Assessment of megakaryocyte migration and chemotaxis. Methods in Molecular Biology. 2012;788:275–288. doi: 10.1007/978-1-61779-307-3_19. [DOI] [PubMed] [Google Scholar]
- 161.Mazharian A, Thomas SG, Dhanjal TS, Buckley CD, Watson SP. Critical role of Src-Syk-PLC{gamma}2 signaling in megakaryocyte migration and thrombopoiesis. Blood. 2010;116(5):793–800. doi: 10.1182/blood-2010-03-275990. [DOI] [PubMed] [Google Scholar]
- 162.Reddi AH, Gay R, Gay S, Miller EJ. Transitions in collagen types during matrix-induced cartilage, bone, and bone marrow formation. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(12):5589–5592. doi: 10.1073/pnas.74.12.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sabri S, Jandrot-Perrus M, Bertoglio J, Farndale RW, Mas VM, Debili N, et al. Differential regulation of actin stress fiber assembly and proplatelet formation by alpha2beta1 integrin and GPVI in human megakaryocytes. Blood. 2004;104(10):3117–3125. doi: 10.1182/blood-2003-12-4398. [DOI] [PubMed] [Google Scholar]
- 164.Zou Z, Schmaier AA, Cheng L, Mericko P, Dickeson SK, Stricker TP, et al. Negative regulation of activated alpha-2 integrins during thrombopoiesis. Blood. 2009;113(25):6428–6439. doi: 10.1182/blood-2008-08-175356. [DOI] [PubMed] [Google Scholar]
- 165.Pallotta I, Lovett M, Rice W, Kaplan DL, Balduini A. Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis. PLoS One. 2009;4(12):e8359. doi: 10.1371/journal.pone.0008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kopp HG, Rafii S. Thrombopoietic cells and the bone marrow vascular niche. Annals of the New York Academy of Sciences. 2007;1106:175–179. doi: 10.1196/annals.1392.004. [DOI] [PubMed] [Google Scholar]
- 167.Schachtner H, Calaminus SD, Sinclair A, Monypenny J, Blundell MP, Leon C, et al. Megakaryocytes assemble podosomes that degrade matrix and protrude through basement membrane. Blood. 2013;121(13):2542–2552. doi: 10.1182/blood-2012-07-443457. [DOI] [PubMed] [Google Scholar]
- 168.Tavassoli M, Aoki M. Localization of megakaryocytes in the bone marrow. Blood Cells. 1989;15(1):3–14. [PubMed] [Google Scholar]
- 169.Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121(15):2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Diaz-Flores L, Jr., Gutierrez R, Madrid JF, Acosta E, Avila J, Diaz-Flores L, et al. Cell sources for cartilage repair; contribution of the mesenchymal perivascular niche. Frontiers in Bioscience (Scholar Edition) 2012;4:1275–1294. doi: 10.2741/s331. [DOI] [PubMed] [Google Scholar]
- 171.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histology and Histopathology. 2009;24(7):909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 172.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kunert S, Meyer I, Fleischhauer S, Wannack M, Fiedler J, Shivdasani RA, et al. The microtubule modulator RanBP10 plays a critical role in regulation of platelet discoid shape and degranulation. Blood. 2009;114(27):5532–5540. doi: 10.1182/blood-2009-04-216804. [DOI] [PubMed] [Google Scholar]
- 174.Mazhuga PM, Nosova LI. Proliferative characteristics of the endothelial cells and pericytes from the capillary vessels of rabbit bone marrow. Tsitologiia i Genetika. 1975;9(5):416–419. [PubMed] [Google Scholar]
- 175.Wang CH, Wang TM, Young TH, Lai YK, Yen ML. The critical role of ECM proteins within the human MSC niche in endothelial differentiation. Biomaterials. 2013;34(17):4223–4234. doi: 10.1016/j.biomaterials.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 176.Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, et al. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12819–12824. doi: 10.1073/pnas.202380099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood. 2006;108(5):1509–1514. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- 178.Lu XG, Zhu L, Wang WQ, Zhang XH, Zhao XY, Xu GB, et al. Morphological study on the megakaryocytes with nuclear extrusion and nucleocytoplasmic separation in four cases. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(6):1082–1085. [PubMed] [Google Scholar]
- 179.Hartwig JH, Italiano JE., Jr. Cytoskeletal mechanisms for platelet production. Blood Cells, Molecules & Diseases. 2006;36(2):99–103. doi: 10.1016/j.bcmd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 180.Richardson JL, Shivdasani RA, Boers C, Hartwig JH, Italiano JE., Jr. Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood. 2005;106(13):4066–4075. doi: 10.1182/blood-2005-06-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Italiano JE, Jr., Patel-Hett S, Hartwig JH. Mechanics of proplatelet elaboration. Journal of Thrombosis and Haemostasis. 2007;5(Suppl 1):18–23. doi: 10.1111/j.1538-7836.2007.02487.x. [DOI] [PubMed] [Google Scholar]
- 182.Schulze H, Dose M, Korpal M, Meyer I, Italiano JE, Jr., Shivdasani RA. RanBP10 is a cytoplasmic guanine nucleotide exchange factor that modulates noncentrosomal microtubules. The Journal of Biological Chemistry. 2008;283(20):14109–14119. doi: 10.1074/jbc.M709397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schwer HD, Lecine P, Tiwari S, Italiano JE, Jr., Hartwig JH, Shivdasani RA. A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Current Biology. 2001;11(8):579–586. doi: 10.1016/s0960-9822(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 184.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 185.Italiano JE, Jr., Bergmeier W, Tiwari S, Falet H, Hartwig JH, Hoffmeister KM, et al. Mechanisms and implications of platelet discoid shape. Blood. 2003;101(12):4789–4796. doi: 10.1182/blood-2002-11-3491. [DOI] [PubMed] [Google Scholar]
- 186.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. The Journal of Experimental Medicine. 2012;209(12):2165–2181. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. Journal of Leukocyte Biology. 1999;66(1):25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- 188.Schmitt A, Guichard J, Masse JM, Debili N, Cramer EM. Of mice and men: comparison of the ultrastructure of megakaryocytes and platelets. Experimental Hematology. 2001;29(11):1295–1302. doi: 10.1016/s0301-472x(01)00733-0. [DOI] [PubMed] [Google Scholar]
- 189.Di Michele M, Van Geet C, Freson K. Recent advances in platelet proteomics. Expert Review of Proteomics. 2012;9(4):451–466. doi: 10.1586/epr.12.31. [DOI] [PubMed] [Google Scholar]
- 190.Krishnan S, Gaspari M, Della Corte A, Bianchi P, Crescente M, Cerletti C, et al. OFFgel-based multidimensional LC-MS/MS approach to the cataloguing of the human platelet proteome for an interactomic profile. Electrophoresis. 2011;32(6-7):686–695. doi: 10.1002/elps.201000592. [DOI] [PubMed] [Google Scholar]
- 191.Premsler T, Lewandrowski U, Sickmann A, Zahedi RP. Phosphoproteome analysis of the platelet plasma membrane. Methods in Molecular Biology. 2011;728:279–290. doi: 10.1007/978-1-61779-068-3_19. [DOI] [PubMed] [Google Scholar]
- 192.Qureshi AH, Chaoji V, Maiguel D, Faridi MH, Barth CJ, Salem SM, et al. Proteomic and phospho-proteomic profile of human platelets in basal, resting state: insights into integrin signaling. PLoS One. 2009;4(10):e7627. doi: 10.1371/journal.pone.0007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Senis Y, Garcia A. Platelet proteomics: state of the art and future perspective. Methods in Molecular Biology. 2012;788:367–399. doi: 10.1007/978-1-61779-307-3_24. [DOI] [PubMed] [Google Scholar]
- 194.Zufferey A, Fontana P, Reny JL, Nolli S, Sanchez JC. Platelet proteomics. Mass Spectrometry Reviews. 2012;31(2):331–351. doi: 10.1002/mas.20345. [DOI] [PubMed] [Google Scholar]
- 195.Di Michele M, Van Geet C, Freson K. Proteomics to unravel platelet-related diseases and identify novel anti-platelet drugs. Current Medicinal Chemistry. 2012;19(27):4662–4670. doi: 10.2174/092986712803306312. [DOI] [PubMed] [Google Scholar]
- 196.Parguina AF, Rosa I, Garcia A. Proteomics applied to the study of platelet-related diseases: aiding the discovery of novel platelet biomarkers and drug targets. Journal of Proteomics. 2012;76:275–286. doi: 10.1016/j.jprot.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 197.Aatonen M, Gronholm M, Siljander PR. Platelet-derived microvesicles: multitalented participants in intercellular communication. Seminars in Thrombosis and Hemostasis. 2012;38(1):102–113. doi: 10.1055/s-0031-1300956. [DOI] [PubMed] [Google Scholar]
- 198.Hess MW, Siljander P. Procoagulant platelet balloons: evidence from cryopreparation and electron microscopy. Histochemistry and Cell Biology. 2001;115(5):439–443. doi: 10.1007/s004180100272. [DOI] [PubMed] [Google Scholar]
- 199.Siljander PR. Platelet-derived microparticles—an updated perspective. Thrombosis Research. 2011;127(Suppl 2):S30–33. doi: 10.1016/S0049-3848(10)70152-3. [DOI] [PubMed] [Google Scholar]
- 200.Shai E, Rosa I, Parguina AF, Motahedeh S, Varon D, Garcia A. Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. Journal of Proteomics. 2012;76:287–296. doi: 10.1016/j.jprot.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 201.Dowal L, Yang W, Freeman MR, Steen H, Flaumenhaft R. Proteomic analysis of palmitoylated platelet proteins. Blood. 2011;118(13):e62–73. doi: 10.1182/blood-2011-05-353078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schulz C, Leuschen NV, Frohlich T, Lorenz M, Pfeiler S, Gleissner CA, et al. Identification of novel downstream targets of platelet glycoprotein VI activation by differential proteome analysis: implications for thrombus formation. Blood. 2010;115(20):4102–4110. doi: 10.1182/blood-2009-07-230268. [DOI] [PubMed] [Google Scholar]
- 203.Wright B, Stanley RG, Kaiser WJ, Mills DJ, Gibbins JM. Analysis of protein networks in resting and collagen receptor (GPVI)-stimulated platelet sub-proteomes. Proteomics. 2011;11(23):4588–4592. doi: 10.1002/pmic.201100410. [DOI] [PubMed] [Google Scholar]
- 204.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Clarke RJ, Mayo G, Price P, FitzGerald GA. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. The New England Journal of Medicine. 1991;325(16):1137–1141. doi: 10.1056/NEJM199110173251605. [DOI] [PubMed] [Google Scholar]
- 206.Samuelsson B, Goldyne M, Granstrom E, Hamberg M, Hammarstrom S, Malmsten C. Prostaglandins and thromboxanes. Annual Review of Biochemistry. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- 207.Steinert BW, Tang DG, Grossi IM, Umbarger LA, Honn KV. Studies on the role of platelet eicosanoid metabolism and integrin alpha IIb beta 3 in tumor-cell-induced platelet aggregation. International Journal of Cancer. 1993;54(1):92–101. doi: 10.1002/ijc.2910540116. [DOI] [PubMed] [Google Scholar]
- 208.Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, et al. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. The Journal of Biological Chemistry. 2007;282(28):20151–20163. doi: 10.1074/jbc.M611776200. [DOI] [PubMed] [Google Scholar]
- 209.Morgan LT, Thomas CP, Kuhn H, O’Donnell VB. Thrombin-activated human platelets acutely generate oxidized docosahexaenoic-acid-containing phospholipids via 12-lipoxygenase. The Biochemical Journal. 2010;431(1):141–148. doi: 10.1042/BJ20100415. [DOI] [PubMed] [Google Scholar]
- 210.Chen YQ, Honn KV. Eicosanoid regulation of tumor cell-platelet and -endothelium interaction during arrest and extravasation. In: Nigam S, Honn K, Marnett L, Walden T Jr., editors. Developments in oncology. Vol. 71. Springer; New York: 1993. pp. 613–617. Eicosanoids and other bioactive lipids in cancer, inflammation and radiation injury. [Google Scholar]
- 211.Honn KV, Tang DG, Grossi I, Duniec ZM, Timar J, Renaud C, et al. Tumor cell-derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Research. 1994;54(2):565–574. [PubMed] [Google Scholar]
- 212.Ruebsaamen K, Liebisch G, Boettcher A, Schmitz G. Lipidomic analysis of platelet senescence. Transfusion. 2010;50(8):1665–1676. doi: 10.1111/j.1537-2995.2010.02584.x. [DOI] [PubMed] [Google Scholar]
- 213.Clark SR, Thomas CP, Hammond VJ, Aldrovandi M, Wilkinson GW, Hart KW, et al. Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):5875–5880. doi: 10.1073/pnas.1222419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dolegowska B, Lubkowska A, De Girolamo L. Platelet lipidomic. Journal of Biological Regulators and Homeostatic Agents. 2012;26(2 Suppl 1):23S–33S. [PubMed] [Google Scholar]
- 215.Hammad SM. Blood sphingolipids in homeostasis and pathobiology. Advances in Experimental Medicine and Biology. 2011;721:57–66. doi: 10.1007/978-1-4614-0650-1_4. [DOI] [PubMed] [Google Scholar]
- 216.Tam VC. Lipidomic profiling of bioactive lipids by mass spectrometry during microbial infections. Seminars in Immunology. 2013;25(3):240–248. doi: 10.1016/j.smim.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immunity & Ageing. 2013;10(1):23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. Journal of Biological Regulators and Homeostatic Agents. 2012;26(2 Suppl 1):35S–42S. [PubMed] [Google Scholar]
- 219.Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. Journal of Biological Regulators and Homeostatic Agents. 2012;26(2 Suppl 1):3S–22S. [PubMed] [Google Scholar]
- 220.Stanco D, Vigano M, Croiset SJ, De Girolamo L. Applications and limits of platelet-rich plasma in sports related injuries. Journal of Biological Regulators and Homeostatic Agents. 2012;26(2 Suppl 1):53S–61S. [PubMed] [Google Scholar]
- 221.Cimmino G, Golino P. Platelet biology and receptor pathways. Journal of Cardiovascular Translational Research. 2013;6(3):299–309. doi: 10.1007/s12265-012-9445-9. [DOI] [PubMed] [Google Scholar]
- 222.Italiano JE., Jr. Unraveling mechanisms that control platelet production. Seminars in Thrombosis and Hemostasis. 2013;39(1):15–24. doi: 10.1055/s-0032-1331157. [DOI] [PubMed] [Google Scholar]
- 223.Kenney DM, Linck RW. The cystoskeleton of unstimulated blood platelets: structure and composition of the isolated marginal microtubular band. Journal of Cell Science. 1985;78:122. doi: 10.1242/jcs.78.1.1. [DOI] [PubMed] [Google Scholar]
- 224.Kowit JD, Linck RW, Kenney DM. Isolated cytoskeletons of human blood platelets: dark-field imaging of coiled and uncoiled microtubules. Biology of the Cell. 1988;64(3):283–291. doi: 10.1016/0248-4900(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 225.Patel-Hett S, Richardson JL, Schulze H, Drabek K, Isaac NA, Hoffmeister K, et al. Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood. 2008;111(9):4605–4616. doi: 10.1182/blood-2007-10-118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Radley JM, Hartshorn MA. Megakaryocyte fragments and the microtubule coil. Blood Cells. 1987;12(3):603–614. [PubMed] [Google Scholar]
- 227.Hartwig JH. The platelet: form and function. Seminars in Hematology. 2006;43(1 Suppl 1):S94–100. doi: 10.1053/j.seminhematol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 228.Hartwig JH, Barkalow K, Azim A, Italiano J. The elegant platelet: signals controlling actin assembly. Thrombosis and Haemostasis. 1999;82(2):392–398. [PubMed] [Google Scholar]
- 229.Boyles J, Fox JE, Phillips DR, Stenberg PE. Organization of the cytoskeleton in resting, discoid platelets: preservation of actin filaments by a modified fixation that prevents osmium damage. The Journal of Cell Biology. 1985;101(4):1463–1472. doi: 10.1083/jcb.101.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.White JG. Interaction of membrane systems in blood platelets. The American Journal of Pathology. 1972;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]
- 231.Escolar G, Leistikow E, White JG. The fate of the open canalicular system in surface and suspension-activated platelets. Blood. 1989;74(6):1983–1988. [PubMed] [Google Scholar]
- 232.Barkalow KL, Italiano JE, Jr., Chou DE, Matsuoka Y, Bennett V, Hartwig JH. Alpha-adducin dissociates from F-actin and spectrin during platelet activation. The Journal of Cell Biology. 2003;161(3):557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hartwig JH, DeSisto M. The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. The Journal of Cell Biology. 1991;112(3):407–425. doi: 10.1083/jcb.112.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cranmer SL, Pikovski I, Mangin P, Thompson PE, Domagala T, Frazzetto M, et al. Identification of a unique filamin A binding region within the cytoplasmic domain of glycoprotein Ibalpha. The Biochemical Journal. 2005;387(Pt 3):849–858. doi: 10.1042/BJ20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14-3-3zeta protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood. 2005;106(6):1975–1981. doi: 10.1182/blood-2005-01-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gitz E, Koopman CD, Giannas A, Koekman CA, van den Heuvel DJ, Deckmyn H, et al. Platelet interaction with von Willebrand factor is enhanced by shear-induced clustering of glycoprotein Ibalpha. Haematologica. 2013;98(11):1810–1818. doi: 10.3324/haematol.2013.087221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li S, Wang Z, Liao Y, Zhang W, Shi Q, Yan R, et al. The glycoprotein Ibalpha-von Willebrand factor interaction induces platelet apoptosis. Journal of Thrombosis and Haemostasis. 2010;8(2):341–350. doi: 10.1111/j.1538-7836.2009.03653.x. [DOI] [PubMed] [Google Scholar]
- 238.Mangin P, David T, Lavaud V, Cranmer SL, Pikovski I, Jackson SP, et al. Identification of a novel 14-3-3zeta binding site within the cytoplasmic tail of platelet glycoprotein Ibalpha. Blood. 2004;104(2):420–427. doi: 10.1182/blood-2003-08-2881. [DOI] [PubMed] [Google Scholar]
- 239.Mu FT, Andrews RK, Arthur JF, Munday AD, Cranmer SL, Jackson SP, et al. A functional 14-3-3zeta-independent association of PI3-kinase with glycoprotein Ib alpha, the major ligand-binding subunit of the platelet glycoprotein Ib-IX-V complex. Blood. 2008;111(9):4580–4587. doi: 10.1182/blood-2007-09-111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89(4):1121–1132. [PubMed] [Google Scholar]
- 241.Furusawa M, Tsuchiy H, Nagayama M, Tanaka T, Nakaya KI, Iinumac M. Anti-platelet and membrane-rigidifying flavonoids in brownish scale of onions. Journal of Health Science. 2003;49(6):475–480. [Google Scholar]
- 242.Winocour PD, Bryszewska M, Watala C, Rand ML, Epand RM, Kinlough-Rathbone RL, et al. Reduced membrane fluidity in platelets from diabetic patients. Diabetes. 1990;39(2):241–244. doi: 10.2337/diab.39.2.241. [DOI] [PubMed] [Google Scholar]
- 243.Gerrits AJ, Gitz E, Koekman CA, Visseren FL, van Haeften TW, Akkerman JW. Induction of insulin resistance by the adipokines resistin, leptin, plasminogen activator inhibitor-1 and retinol binding protein 4 in human megakaryocytes. Haematologica. 2012;97(8):1149–1157. doi: 10.3324/haematol.2011.054916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.De Caterina R, Marchetti P, Bernini W, Giannarelli R, Giannessi D, Navalesi R. The direct effects of metformin on platelet function in vitro. European Journal of Clinical Pharmacology. 1989;37(2):211–213. doi: 10.1007/BF00558236. [DOI] [PubMed] [Google Scholar]
- 245.Gin H, Freyburger G, Boisseau M, Aubertin J. Study of the effect of metformin on platelet aggregation in insulin-dependent diabetics. Diabetes Research and Clinical Practice. 1989;6(1):61–67. doi: 10.1016/0168-8227(89)90058-2. [DOI] [PubMed] [Google Scholar]
- 246.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Annals of Internal Medicine. 2002;137(1):25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 247.Wiwanitkit V. Metformin high dosage and bleeding episode: a clinical case study. Indian Journal of Endocrinology and Metabolism. 2011;15(2):132–133. doi: 10.4103/2230-8210.81947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Protti A, Lecchi A, Fortunato F, Artoni A, Greppi N, Vecchio S, et al. Metformin overdose causes platelet mitochondrial dysfunction in humans. Critical Care. 2012;16(5):R180. doi: 10.1186/cc11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Harper MT, Poole AW. Chloride channels are necessary for full platelet phosphatidylserine exposure and procoagulant activity. Cell Death and Disease. 2013;4:e969. doi: 10.1038/cddis.2013.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gilligan DM, Sarid R, Weese J. Adducin in platelets: activation-induced phosphorylation by PKC and proteolysis by calpain. Blood. 2002;99(7):2418–2426. doi: 10.1182/blood.v99.7.2418. [DOI] [PubMed] [Google Scholar]
- 251.Tamarn S, Fukuta T, Kaibuchi K, Matsuoka Y, Shiku H, Nishikawa M. Rho-kinase induces association of adducin with the cytoskeleton in platelet activation. Biochemical and Biophysical Research Communications. 2005;332(2):347–351. doi: 10.1016/j.bbrc.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 252.Lind SE, Yin HL, Stossel TP. Human platelets contain gelsolin. A regulator of actin filament length. Journal of Clinical Investigation. 1982;69(6):1384–1387. doi: 10.1172/JCI110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang LL, Bryan J. Isolation of calcium-dependent platelet proteins that interact with actin. Cell. 1981;25(3):637–649. doi: 10.1016/0092-8674(81)90171-9. [DOI] [PubMed] [Google Scholar]
- 254.Bennett JS, Zigmond S, Vilaire G, Cunningham ME, Bednar B. The platelet cytoskeleton regulates the affinity of the integrin alpha(IIb)beta(3) for fibrinogen. The Journal of Biological Chemistry. 1999;274(36):25301–25307. doi: 10.1074/jbc.274.36.25301. [DOI] [PubMed] [Google Scholar]
- 255.Davidson MM, Haslam RJ. Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+ The Biochemical Journal. 1994;301(Pt 1):41–47. doi: 10.1042/bj3010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, et al. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. The Biochemical Journal. 1997;328(Pt 1):105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mahoney NM, Janmey PA, Almo SC. Structure of the profilin-poly-L-proline complex involved in morphogenesis and cytoskeletal regulation. Nature Structural Biology. 1997;4(11):953–960. doi: 10.1038/nsb1197-953. [DOI] [PubMed] [Google Scholar]
- 258.Barkalow K, Witke W, Kwiatkowski DJ, Hartwig JH. Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. The Journal of Cell Biology. 1996;134(2):389–399. doi: 10.1083/jcb.134.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nachmias VT, Golla R, Casella JF, Barron-Casella E. Cap Z, a calcium insensitive capping protein in resting and activated platelets. FEBS Letters. 1996;378(3):258–262. doi: 10.1016/0014-5793(95)01474-8. [DOI] [PubMed] [Google Scholar]
- 260.White JG. Exocytosis of secretory organelles from blood platelets incubated with cationic polypeptides. The American Journal of Pathology. 1972;69(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- 261.White JG, Estensen RD. Degranulation of discoid platelets. The American Journal of Pathology. 1972;68(2):289–302. [PMC free article] [PubMed] [Google Scholar]
- 262.Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95(3):921–929. [PubMed] [Google Scholar]
- 263.Marks MS. SNARing platelet granule secretion. Blood. 2012;120(12):2355–2357. doi: 10.1182/blood-2012-07-442756. [DOI] [PubMed] [Google Scholar]
- 264.Peters CG, Michelson AD, Flaumenhaft R. Granule exocytosis is required for platelet spreading: differential sorting of alpha-granules expressing VAMP-7. Blood. 2012;120(1):199–206. doi: 10.1182/blood-2011-10-389247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Fukuda M, Kanno E. Analysis of the role of Rab27 effector Slp4-a/Granuphilin-a in dense-core vesicle exocytosis. Methods in Enzymology. 2005;403:445–457. doi: 10.1016/S0076-6879(05)03039-9. [DOI] [PubMed] [Google Scholar]
- 266.Shirakawa R, Higashi T, Tabuchi A, Yoshioka A, Nishioka H, Fukuda M, et al. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. The Journal of Biological Chemistry. 2004;279(11):10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- 267.Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH, Whiteheart SW. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120(12):2493–2500. doi: 10.1182/blood-2012-05-430629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH, Whiteheart SW. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120(12):2484–2492. doi: 10.1182/blood-2012-05-430603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Santos-Martinez MJ, Medina C, Jurasz P, Radomski MW. Role of metalloproteinases in platelet function. Thrombosis Research. 2008;121(4):535–542. doi: 10.1016/j.thromres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 270.Gleissner CA, von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(11):1920–1927. doi: 10.1161/ATVBAHA.108.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. Journal of Leukocyte Biology. 2000;67(4):471–478. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- 272.Mellembakken JR, Solum NO, Ueland T, Videm V, Aukrust P. Increased concentrations of soluble CD40 ligand, RANTES and GRO-alpha in preeclampsia—possible role of platelet activation. Thrombosis and Haemostasis. 2001;86(5):1272–1276. [PubMed] [Google Scholar]
- 273.Fukami MH, Salganicoff L. Human platelet storage organelles. A review. Thrombosis and Haemostasis. 1977;38(4):963–970. [PubMed] [Google Scholar]
- 274.Emiliani C, Martino S, Orlacchio A, Vezza R, Nenci GG, Gresele P. Platelet glycohydrolase activities: characterization and release. Cell Biochemistry and Function. 1995;13(1):31–39. doi: 10.1002/cbf.290130108. [DOI] [PubMed] [Google Scholar]
- 275.Gordon JL. Blood platelet lysosomes and their contribution to the pathophysiological role of platelets. Frontiers of Biology. 1975;43(4):3–31. [PubMed] [Google Scholar]
- 276.Metzelaar MJ, Clevers HC. Lysosomal membrane glycoproteins in platelets. Thrombosis and Haemostasis. 1992;68(4):378–382. [PubMed] [Google Scholar]
- 277.Waite M, Griffin HD. The phospholipases A of lysosomes. Frontiers of Biology. 1976;45:257–305. [PubMed] [Google Scholar]
- 278.Dangel O, Mergia E, Karlisch K, Groneberg D, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. Journal of Thrombosis and Haemostasis. 2010;8(6):1343–1352. doi: 10.1111/j.1538-7836.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 279.Sabetkar M, Naseem KM, Tullett JM, Friebe A, Koesling D, Bruckdorfer KR. Synergism between nitric oxide and hydrogen peroxide in the inhibition of platelet function: the roles of soluble guanylyl cyclase and vasodilator-stimulated phosphoprotein. Nitric Oxide. 2001;5(3):233–242. doi: 10.1006/niox.2001.0343. [DOI] [PubMed] [Google Scholar]
- 280.Wilson LS, Elbatarny HS, Crawley SW, Bennett BM, Maurice DH. Compartmentation and compartment-specific regulation of PDE5 by protein kinase G allows selective cGMP-mediated regulation of platelet functions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(36):13650–13655. doi: 10.1073/pnas.0804738105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Audet M, Bouvier M. Restructuring G-protein-coupled receptor activation. Cell. 2012;151(1):14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 282.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annual Review of Pharmacology and Toxicology. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494(7436):185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 284.Stalker TJ, Newman DK, Ma P, Wannemacher KM, Brass LF. Platelet signaling. Handbook of Experimental Pharmacology. 2012;210:59–85. doi: 10.1007/978-3-642-29423-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zucker MB, Nachmias VT. Platelet activation. Arteriosclerosis. 1985;5(1):2–18. doi: 10.1161/01.atv.5.1.2. [DOI] [PubMed] [Google Scholar]
- 286.Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nature Medicine. 2003;9(11):1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- 287.Noe L, Peeters K, Izzi B, Van Geet C, Freson K. Regulators of platelet cAMP levels: clinical and therapeutic implications. Current Medicinal Chemistry. 2010;17(26):2897–2905. doi: 10.2174/092986710792065018. [DOI] [PubMed] [Google Scholar]
- 288.Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. Journal of Thrombosis and Haemostasis. 2012;10(2):167–176. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- 289.Rolfe BE, Worth NF, World CJ, Campbell JH, Campbell GR. Rho and vascular disease. Atherosclerosis. 2005;183(1):1–16. doi: 10.1016/j.atherosclerosis.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 290.Aslan JE, McCarty OJ. Rho GTPases in platelet function. Journal of Thrombosis and Haemostasis. 2013;11(1):35–46. doi: 10.1111/jth.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Collins C, Tzima E. RhoA goes global. Small GTPases. 2013;4(2):123–126. doi: 10.4161/sgtp.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Goggs R, Poole AW. Platelet signaling—a primer. Journal of Veterinary Emergency and Critical Care (San Antonio, Tex.) 2012;22(1):5–29. doi: 10.1111/j.1476-4431.2011.00704.x. [DOI] [PubMed] [Google Scholar]
- 293.Kauskot A, Hoylaerts MF. Platelet receptors. Handbook of Experimental Pharmacology. 2012;210:23–57. doi: 10.1007/978-3-642-29423-5_2. [DOI] [PubMed] [Google Scholar]
- 294.Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Research. 2009;11(6):R81. doi: 10.1186/bcr2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kundumani-Sridharan V, Dyukova E, Hansen DE, 3rd, Rao GN. 12/15-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction. The Journal of Biological Chemistry. 2013;288(22):15830–15842. doi: 10.1074/jbc.M113.453290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Research. 1997;768(1-2):43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 297.Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. Journal of Neurochemistry. 2003;86(4):980–991. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 298.Pakala R. Serotonin and thromboxane A2 stimulate platelet-derived microparticle-induced smooth muscle cell proliferation. Cardiovascular Radiation Medicine. 2004;5(1):20–26. doi: 10.1016/j.carrad.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 299.Dutta-Roy AK, Sinha AK. Purification and properties of prostaglandin E1/prostacyclin receptor of human blood platelets. The Journal of Biological Chemistry. 1987;262(26):12685–12691. [PubMed] [Google Scholar]
- 300.Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bunting S, Gryglewski R, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- 302.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperox-ides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 303.Boyanova D, Nilla S, Birschmann I, Dandekar T, Dittrich M. PlateletWeb: a systems biologic analysis of signaling networks in human platelets. Blood. 2012;119(3):e22–34. doi: 10.1182/blood-2011-10-387308. [DOI] [PubMed] [Google Scholar]
- 304.Dittrich M, Birschmann I, Mietner S, Sickmann A, Walter U, Dandekar T. Platelet protein interactions: map, signaling components, and phosphorylation groundstate. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(7):1326–1331. doi: 10.1161/ATVBAHA.107.161000. [DOI] [PubMed] [Google Scholar]
- 305.Lyons RM, Stanford N, Majerus PW. Thrombin-induced protein phosphorylation in human platelets. The Journal of Clinical Investigation. 1975;56(4):924–936. doi: 10.1172/JCI108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yamanaka M, Kume S, Kariya T, Tanabe A. cAMP-dependent protein kinase in human platelets and effect of prostaglandin E1 on its endogenous substrates (author’s transl) Nihon Ketsueki Gakkai Zasshi. 1979;42(3):541–542. [PubMed] [Google Scholar]
- 307.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396(6710):474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 308.Sand C, Grandoch M, Borgermann C, Oude Weernink PA, Mahlke Y, Schwindenhammer B, et al. 8-pCPT-conjugated cyclic AMP analogs exert thromboxane receptor antagonistic properties. Thrombosis and Haemostasis. 2010;103(3):662–678. doi: 10.1160/TH09-06-0341. [DOI] [PubMed] [Google Scholar]
- 309.Siess W, Winegar DA, Lapetina EG. Rap1-B is phosphorylated by protein kinase A in intact human platelets. Biochemical and Biophysical Research Communications. 1990;170(2):944–950. doi: 10.1016/0006-291x(90)92182-y. [DOI] [PubMed] [Google Scholar]
- 310.Mellion BT, Ignarro LJ, Ohlstein EH, Pontecorvo EG, Hyman AL, Kadowitz PJ. Evidence for the inhibitory role of guanosine 3′,5′-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981;57(5):946–955. [PubMed] [Google Scholar]
- 311.Marquis NR, Vigdahl RL, Tavormina PA. Platelet aggregation. I. Regulation by cyclic AMP and prostaglandin E1. Biochemical and Biophysical Research Communications. 1969;36(6):965–972. doi: 10.1016/0006-291x(69)90298-8. [DOI] [PubMed] [Google Scholar]
- 312.Salzman EW, Neri LL. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. Nature. 1969;224(5219):609–610. doi: 10.1038/224609a0. [DOI] [PubMed] [Google Scholar]
- 313.Salzman EW. ADP-platelet aggregation. Thrombosis et Diathesis Haemorrhagica. Supplementum. 1967;26:197–199. [PubMed] [Google Scholar]
- 314.Brodie GN, Baenziger NL, Chase LR, Majerus PW. The effects of thrombin on adenyl cyclase activity and a membrane protein from human platelets. The Journal of Clinical Investigation. 1972;51(1):81–88. doi: 10.1172/JCI106800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hamberg M, Svensson J, Wakabayashi T, Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Young A, Chapman O, Connor C, Poole C, Rose P, Kakkar AK. Thrombosis and cancer. Nature Reviews. Clinical Oncology. 2012;9(8):437–449. doi: 10.1038/nrclinonc.2012.106. [DOI] [PubMed] [Google Scholar]
- 318.Langer F, Bokemeyer C. Crosstalk between cancer and haemostasis. Implications for cancer biology and cancer-associated thrombosis with focus on tissue factor. Hamostaseologie. 2012;32(2):95–104. doi: 10.5482/ha-1160. [DOI] [PubMed] [Google Scholar]
- 319.van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2012;119(4):924–932. doi: 10.1182/blood-2011-06-317685. [DOI] [PubMed] [Google Scholar]
- 320.Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Desire L, Leblond B, et al. Rap1-Rac1 circuits potentiate platelet activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(2):434–441. doi: 10.1161/ATVBAHA.111.239194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tao L, Zhang Y, Xi X, Kieffer N. Recent advances in the understanding of the molecular mechanisms regulating platelet integrin alphαIIbbeta3 activation. Protein & Cell. 2010;1(7):627–637. doi: 10.1007/s13238-010-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Watanabe N. RIAM: bridge between Rap1 and integrin. Rinshō Ketsueki. 2010;51(6):377–383. [PubMed] [Google Scholar]
- 323.Wynne JP, Wu J, Su W, Mor A, Patsoukis N, Boussiotis VA, et al. Rap1-interacting adapter molecule (RIAM) associates with the plasma membrane via a proximity detector. The Journal of Cell Biology. 2012;199(2):317–330. doi: 10.1083/jcb.201201157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Stefanini L, Bergmeier W. CalDAG-GEFI and platelet activation. Platelets. 2010;21(4):239–243. doi: 10.3109/09537101003639931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Subramanian H, Zahedi RP, Sickmann A, Walter U, Gambaryan S. Phosphorylation of CalDAG-GEFI by protein kinase A prevents Rap1b activation. Journal of Thrombosis and Haemostasis. 2013;11(8):1574–1582. doi: 10.1111/jth.12271. [DOI] [PubMed] [Google Scholar]
- 326.Ridley AJ. Life at the leading edge. Cell. 2011;145(1):1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 327.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 328.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 329.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. The Journal of Cell Biology. 1999;144(4):145–154. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2012;119(4):1054–1063. doi: 10.1182/blood-2011-08-372193. [DOI] [PubMed] [Google Scholar]
- 331.Schoenwaelder SM, Hughan SC, Boniface K, Fernando S, Holdsworth M, Thompson PE, et al. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. The Journal of Biological Chemistry. 2002;277(11):14138–14146. doi: 10.1074/jbc.M200661200. [DOI] [PubMed] [Google Scholar]
- 332.Fujita A, Saito Y, Ishizaki T, Maekawa M, Fujisawa K, Ushikubi F, et al. Integrin-dependent translocation of p160ROCK to cytoskeletal complex in thrombin-stimulated human platelets. The Biochemical Journal. 1991;328(Pt3):169–115. doi: 10.1042/bj3280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Huang JS, Dong L, Kozasa T, Le Breton GC. Signaling through G(alpha)13 switch region I is essential for protease-activated receptor 1-mediated human platelet shape change, aggregation, and secretion. The Journal of Biological Chemistry. 2001;282(14):10210–10222. doi: 10.1074/jbc.M605678200. [DOI] [PubMed] [Google Scholar]
- 334.Calaminus SD, Auger JM, McCarty OJ, Wakelam MJ, Machesky LM, Watson SP. MyosinIIa contractility is required for maintenance of platelet structure during spreading on collagen and contributes to thrombus stability. Journal of Thrombosis and Haemostasis. 2001;5(10):2136–2145. doi: 10.1111/j.1538-7836.2007.02696.x. [DOI] [PubMed] [Google Scholar]
- 335.Getz TM, Dangelmaier CA, Jin J, Daniel JL, Kunapuli SP. Differential phosphorylation of myosin light chain (Thr)18 and (Ser)19 and functional implications in platelets. Journal of Thrombosis and Haemostasis. 2010;8(10):2283–2293. doi: 10.1111/j.1538-7836.2010.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ueda K, Ohta Y, Hosoya H. The carboxy-terminal pleckstrin homology domain of ROCK interacts with filamin-A. Biochemical and Biophysical Research Communications. 2003;301(4):886–890. doi: 10.1016/s0006-291x(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 337.Itoh K, Hara T, Shibata N. Diphosphorylation of platelet myosin by myosin light chain kinase. Biochimica et Biophysica Acta. 1992;1133(3):286–292. doi: 10.1016/0167-4889(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 338.Signorello MG, Giacobbe E, Passalacqua M, Leoncini G. The 2-arachidonoylglycerol effect on myosin light chain phosphorylation in human platelets. Biochimie. 2013;95(8):1620–1628. doi: 10.1016/j.biochi.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 339.Wraith KS, Magwenzi S, Aburima A, Wen Y, Leake D, Naseem KM. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood. 2013;122(4):580–589. doi: 10.1182/blood-2013-04-491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Leisner TM, Liu M, Jaffer ZM, Chernoff J, Parise LV. Essential role of CIB1 in regulating PAK1 activation and cell migration. The Journal of Cell Biology. 2005;170(3):465–416. doi: 10.1083/jcb.200502090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pandey D, Goyal P, Bamburg JR, Siess W. Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood. 2006;107(2):515–583. doi: 10.1182/blood-2004-11-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pandey D, Goyal P, Siess W. Lysophosphatidic acid stimulation of platelets rapidly induces Ca2+-dependent dephosphorylation of cofilin that is independent of dense granule secretion and aggregation. Blood Cells, Molecules & Diseases. 2001 doi: 10.1016/j.bcmd.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 343.Akbar H, Shang X, Perveen R, Berryman M, Funk K, Johnson JF, et al. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS One. 2011;6(1):e22111. doi: 10.1371/journal.pone.0022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Carpenter CL, Tolias KF, Couvillon AC, Hartwig JH. Signal transduction pathways involving the small G proteins rac and Cdc42 and phosphoinositide kinases. Advances in Enzyme Regulation. 1991;37:311–390. doi: 10.1016/s0065-2571(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 345.Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, et al. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. The Journal of Cell Biology. 1999;146(6):1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. The EMBO Journal. 1998;17(23):6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. The Journal of Biological Chemistry. 2005;280(41):39414–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Oda A, Miki H, Wada I, Yamaguchi H, Yamazaki D, Suetsugu S, et al. WAVE/scars in platelets. Blood. 2005;105(8):3141–3148. doi: 10.1182/blood-2003-04-1319. [DOI] [PubMed] [Google Scholar]
- 349.Coburn LA, Damaraju VS, Dozic S, Eskin SG, Cruz MA, McIntire LV. GPIbalpha-vWF rolling under shear stress shows differences between type 2B and 2M von Willebrand disease. Biophysical Journal. 2011;100(2):304–312. doi: 10.1016/j.bpj.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Colace TV, Diamond SL. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(1):105–113. doi: 10.1161/ATVBAHA.112.300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Fredrickson BJ, Dong JF, McIntire LV, Lopez JA. Shear-dependent rolling on von Willebrand factor of mammalian cells expressing the platelet glycoprotein Ib-IX-V complex. Blood. 1998;92(10):3684–3693. [PubMed] [Google Scholar]
- 352.Jackson SP, Mistry N, Yuan Y. Platelets and the injured vessel wall—“rolling into action”: focus on glycoprotein Ib/V/IX and the platelet cytoskeleton. Trends in Cardiovascular Medicine. 2000;10(5):192–191. doi: 10.1016/s1050-1738(00)00062-1. [DOI] [PubMed] [Google Scholar]
- 353.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. The Journal of Clinical Investigation. 2008;118(9):3195–3201. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. Journal of Thrombosis and Haemostasis. 2013;11(4):605–614. doi: 10.1111/jth.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Clemetson KJ. A short history of platelet glycoprotein Ib complex. Thrombosis and Haemostasis. 2001;98(1):63–68. [PubMed] [Google Scholar]
- 356.Bernard J, Soulier J. Sur une nouvelle variete de dystrophie thrombocytaire-hemorragipare congenitale. Semin Hop Paris. 1948;24:3211–3223. [PubMed] [Google Scholar]
- 357.Ozaki Y, Suzuki-Inoue K, Inoue O. Platelet receptors activated via mulitmerization: glycoprotein VI, GPIb-IX-V, and CLEC-2. Journal of Thrombosis and Haemostasis. 2013;11(Suppl 1):330–339. doi: 10.1111/jth.12235. [DOI] [PubMed] [Google Scholar]
- 358.Canobbio I, Balduini C, Torti M. Signalling through the platelet glycoprotein Ib-V-IX complex. Cellular Signalling. 2004;16(12):1329–1344. doi: 10.1016/j.cellsig.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 359.Gardiner EE, Arthur JF, Berndt MC, Andrews RK. Role of calmodulin in platelet receptor function. Current Medicinal Chemistry. Cardiovascular and Hematological Agents. 2005;3(4):283–281. doi: 10.2174/156801605774322283. [DOI] [PubMed] [Google Scholar]
- 360.Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. Journal of Thrombosis and Haemostasis. 2005;3(3):562–570. doi: 10.1111/j.1538-7836.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 361.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121(2):270–277. doi: 10.1182/blood-2012-07-442285. [DOI] [PubMed] [Google Scholar]
- 362.Desch A, Strozyk EA, Bauer AT, Huck V, Niemeyer V, Wieland T, et al. Highly invasive melanoma cells activate the vascular endothelium via an MMP-2/integrin alphavbeta5-induced secretion of VEGF-A. The American Journal of Pathology. 2012;181(2):693–705. doi: 10.1016/j.ajpath.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 363.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphαIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112(8):3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Kim C, Kim MC. Differences in alpha-beta transmembrane domain interactions among integrins enable diverging integrin signaling. Biochemical and Biophysical Research Communications. 2013;436(3):406–412. doi: 10.1016/j.bbrc.2013.05.115. [DOI] [PubMed] [Google Scholar]
- 365.Kim C, Lau TL, Ulmer TS, Ginsberg MH. Interactions of platelet integrin alphαIIb and beta3 transmembrane domains in mammalian cell membranes and their role in integrin activation. Blood. 2009;113(19):4747–4753. doi: 10.1182/blood-2008-10-186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Shattil SJ. The beta3 integrin cytoplasmic tail: protein scaffold and control freak. Journal of Thrombosis and Haemostasis. 2009;7(Suppl 1):210–213. doi: 10.1111/j.1538-7836.2009.03397.x. [DOI] [PubMed] [Google Scholar]
- 367.Nurden AT, Caen JP. An abnormal platelet glycoprotein pattern in three cases of Glanzmann’s thrombasthenia. British Journal of Haematology. 1974;28(2):253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- 368.Phillips DR, Jenkins CS, Luscher EF, Larrieu M. Molecular differences of exposed surface proteins on thrombasthenic platelet plasma membranes. Nature. 1975;257(5527):599–600. doi: 10.1038/257599a0. [DOI] [PubMed] [Google Scholar]
- 369.Glanzmann E. Hereditare hammorhagische thrombastehnie. Beitr Pathologie Bluplatchen J Kinderkt. 1918;88:113–141. [Google Scholar]
- 370.Clemetson KJ. Platelet activation: signal transduction via membrane receptors. Thrombosis and Haemostasis. 1995;74(1):111–116. [PubMed] [Google Scholar]
- 371.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. The Journal of Clinical Investigation. 1989;84(5):1440–1445. doi: 10.1172/JCI114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Asselin J, Knight CG, Farndale RW, Barnes MJ, Watson SP. Monomeric (glycine-proline-hydroxyproline)10 repeat sequence is a partial agonist of the platelet collagen receptor glycoprotein VI. The Biochemical Journal. 1999;339(Pt 2):413–418. [PMC free article] [PubMed] [Google Scholar]
- 373.Kehrel B, Wierwille S, Clemetson KJ, Anders O, Steiner M, Knight CG, et al. Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Blood. 1998;91(2):491–499. [PubMed] [Google Scholar]
- 374.Zahid M, Mangin P, Loyau S, Hechler B, Billiald P, Gachet C, et al. The future of glycoprotein VI as an antithrombotic target. Journal of Thrombosis and Haemostasis. 2012;10(12):2418–2427. doi: 10.1111/jth.12009. [DOI] [PubMed] [Google Scholar]
- 375.Bergmeier W, Stefanini L. Platelet ITAM signaling. Current Opinion in Hematology. 2013;20(5):445–450. doi: 10.1097/MOH.0b013e3283642267. [DOI] [PubMed] [Google Scholar]
- 376.Ezumi Y, Shindoh K, Tsuji M, Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. The Journal of Experimental Medicine. 1998;188(2):267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Watson SP, Asazuma N, Atkinson B, Berlanga O, Best D, Bobe R, et al. The role of ITAM- and ITIM-coupled receptors in platelet activation by collagen. Thrombosis and Haemostasis. 2001;86(1):276–288. [PubMed] [Google Scholar]
- 378.Navarro-Nunez L, Langan SA, Nash GB, Watson SP. The physiological and pathophysiological roles of platelet CLEC-2. Thrombosis and Haemostasis. 2013;109(6):991–998. doi: 10.1160/TH13-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107(2):542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 380.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. The Journal of Biological Chemistry. 2007;282(36):25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 381.Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thrombosis Research. 2012;129(Suppl 1):S30–37. doi: 10.1016/S0049-3848(12)70013-0. [DOI] [PubMed] [Google Scholar]
- 382.Ordonez NG. Value of podoplanin as an immunohisto-chemical marker in tumor diagnosis: a review and update. Applied Immunohistochemistry & Molecular Morphology. 2013 doi: 10.1097/PAI.0b013e31828a83c5. [DOI] [PubMed] [Google Scholar]
- 383.Pula B, Witkiewicz W, Dziegiel P, Podhorska-Okolow M. Significance of podoplanin expression in cancer-associated fibroblasts: a comprehensive review. International Journal of Oncology. 2013;42(6):1849–1857. doi: 10.3892/ijo.2013.1887. [DOI] [PubMed] [Google Scholar]
- 384.Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, et al. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One. 2013;8(8):e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Watson AA, Brown J, Harlos K, Eble JA, Walter TS, O’Callaghan CA. The crystal structure and mutational binding analysis of the extracellular domain of the plateletactivating receptor CLEC-2. The Journal of Biological Chemistry. 2007;282(5):3165–3172. doi: 10.1074/jbc.M610383200. [DOI] [PubMed] [Google Scholar]
- 386.Watson AA, O’Callaghan CA. Crystallization andX-ray diffraction analysis of human CLEC-2. Acta Crystallographica. Section F Structural Biology and Crystallization Communications. 2005;61(Pt 12):1094–1096. doi: 10.1107/S1744309105037991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Suzuki-Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. Journal of Thrombosis and Haemostasis. 2011;9(Suppl 1):44–55. doi: 10.1111/j.1538-7836.2011.04335.x. [DOI] [PubMed] [Google Scholar]
- 388.Suzuki-Inoue K, Inoue O, Ozaki Y. The novel platelet activation receptor CLEC-2. Platelets. 2011;22(5):380–384. doi: 10.3109/09537104.2011.556274. [DOI] [PubMed] [Google Scholar]
- 389.Watson AA, O’Callaghan CA. Molecular analysis of the interaction of the snake venom rhodocytin with the platelet receptor CLEC-2. Toxins (Basel) 2011;3(8):991–1003. doi: 10.3390/toxins3080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- 391.Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. The Journal of Cell Biology. 1985;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Zarbock A, Muller H, Kuwano Y, Ley K. PSGL-1-dependent myeloid leukocyte activation. Journal of Leukocyte Biology. 2009;86(5):1119–1124. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- 393.Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 394.Polley MJ, Phillips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, et al. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. The Journal of Cell Biology. 1992;117(4):895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Habets KL, Huizinga TW, Toes RE. Platelets and autoimmunity. European Journal of Clinical Investigation. 2013;43(7):746–757. doi: 10.1111/eci.12101. [DOI] [PubMed] [Google Scholar]
- 397.Kazmi RS, Cooper AJ, Lwaleed BA. Platelet function in pre-eclampsia. Seminars in Thrombosis and Hemostasis. 2011;37(2):131–136. doi: 10.1055/s-0030-1270339. [DOI] [PubMed] [Google Scholar]
- 398.Nurden AT. Platelets, inflammation and tissue regeneration. Thrombosis and Haemostasis. 2011;105(Suppl 1):S13–33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 399.Ozeki Y, Ito H, Nagamura Y, Unemi F, Igawa T. 12(S)-HETE plays a role as a mediator of expression of platelet CD62 (P-selectin) Platelets. 1998;9(5):297–302. doi: 10.1080/09537109876537. [DOI] [PubMed] [Google Scholar]
- 400.Borsig L. The role of platelet activation in tumor metastasis. Expert Review of Anticancer Therapy. 2008;8(8):1247–1255. doi: 10.1586/14737140.8.8.1247. [DOI] [PubMed] [Google Scholar]
- 401.Dammacco F, Vacca A, Procaccio P, Ria R, Marech I, Racanelli V. Cancer-related coagulopathy (Trousseau’s syndrome): review of the literature and experience of a single center of internal medicine. Clinical and Experimental Medicine. 2013;13(2):85–97. doi: 10.1007/s10238-013-0230-0. [DOI] [PubMed] [Google Scholar]
- 402.Kyriazi V, Theodoulou E. Assessing the risk and prognosis of thrombotic complications in cancer patients. Archives of Pathology & Laboratory Medicine. 2013;137(9):1286–1295. doi: 10.5858/arpa.2012-0490-RA. [DOI] [PubMed] [Google Scholar]
- 403.McEver RP. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconjugate Journal. 1997;14(5):585–591. doi: 10.1023/a:1018584425879. [DOI] [PubMed] [Google Scholar]
- 404.Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Gay LJ, Felding-Habermann B. Platelets alter tumor cell attributes to propel metastasis: programming in transit. Cancer Cell. 2011;20(5):553–554. doi: 10.1016/j.ccr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 406.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nature Reviews. Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Koziak K, Sevigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thrombosis and Haemostasis. 1999;82(5):1538–1544. [PubMed] [Google Scholar]
- 408.Zimmermann H. Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nature Medicine. 1999;5(9):987–988. doi: 10.1038/12419. [DOI] [PubMed] [Google Scholar]
- 409.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Platelets exit venules by a transcellular pathway at sites of F-met peptide-induced acute inflammation in guinea pigs. International Archives of Allergy and Immunology. 1998;116(3):188–195. doi: 10.1159/000023944. [DOI] [PubMed] [Google Scholar]
- 410.Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122(15):2550–2554. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 411.Lowenhaupt RW, Glueck HI, Miller MA, Kline DL. Factors which influence blood platelet migration. The Journal of Laboratory and Clinical Medicine. 1977;90(1):37–45. [PubMed] [Google Scholar]
- 412.Nathan P. The migration of human platelets in vitro. Thrombosis et Diathesis Haemorrhagica. 1973;30(1):173–177. [PubMed] [Google Scholar]
- 413.Schmidt EM, Munzer P, Borst O, Kraemer BF, Schmid E, Urban B, et al. Ion channels in the regulation of platelet migration. Biochemical and Biophysical Research Communications. 2011;415(1):54–60. doi: 10.1016/j.bbrc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 414.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. Journal of Thrombosis and Haemostasis. 2011;9(11):2251–2261. doi: 10.1111/j.1538-7836.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Fisher B, Fisher ER. Transmigration of lymph nodes by tumor cells. Science. 1966;152(3727):1397–1398. doi: 10.1126/science.152.3727.1397. [DOI] [PubMed] [Google Scholar]
- 416.Sleeman JP, Cady B, Pantel K. The connectivity of lymphogenous and hematogenous tumor cell dissemination: biological insights and clinical implications. Clinical & Experimental Metastasis. 2012;29(7):737–746. doi: 10.1007/s10585-012-9489-x. [DOI] [PubMed] [Google Scholar]
- 417.Sleeman JP, Nazarenko I, Thiele W. Do all roads lead to Rome? Routes to metastasis development. International Journal of Cancer. 2011;128(11):2511–2526. doi: 10.1002/ijc.26027. [DOI] [PubMed] [Google Scholar]
- 418.Baker M, Reynolds LE, Robinson SD, Lees DM, Parsons M, Elia G, et al. Stromal Claudin14-heterozygosity, but not deletion, increases tumour blood leakage without affecting tumour growth. PLoS One. 2013;8(5):e62516. doi: 10.1371/journal.pone.0062516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436(7050):456–458. doi: 10.1038/436456a. [DOI] [PubMed] [Google Scholar]
- 420.Kushner EJ, Bautch VL. Building blood vessels in development and disease. Current Opinion in Hematology. 2013;20(3):231–236. doi: 10.1097/MOH.0b013e328360614b. [DOI] [PubMed] [Google Scholar]
- 421.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(24):11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Bouvenot G, Escande M, Xeridat B, Simonin G, Boucoiran J, Delboy C. Thrombocytosis and cancer. Apropos of a chronological series of 100 patients. La Semaine des Hopitaux. 1977;53(36):1921–1925. [PubMed] [Google Scholar]
- 423.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. The New England Journal of Medicine. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Rank A, Liebhardt S, Zwirner J, Burges A, Nieuwland R, Toth B. Circulating microparticles in patients with benign and malignant ovarian tumors. Anticancer Research. 2012;32(5):2009–2014. [PubMed] [Google Scholar]
- 425.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96(10):3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 426.van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, et al. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thrombosis and Haemostasis. 2012;108(1):160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 427.Rank A, Nieuwland R, Roesner S, Nikolajek K, Hiller E, Toth B. Climacteric lowers plasma levels of platelet-derived microparticles: a pilot study in pre-versus postmenopausal women. Acta Haematologica. 2012;128(1):53–59. doi: 10.1159/000337327. [DOI] [PubMed] [Google Scholar]
- 428.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Fidler IJ. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Research. 1978;38(9):2651–2660. [PubMed] [Google Scholar]
- 430.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Research. 2010;70(14):5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bidard FC, Pierga JY, Soria JC, Thiery JP. Translating metastasis-related biomarkers to the clinic—progress and pitfalls. Nature Reviews. Clinical Oncology. 2013;10(3):169–179. doi: 10.1038/nrclinonc.2013.4. [DOI] [PubMed] [Google Scholar]
- 432.Morello M, Minciacchi VR, de Candia P, Yang J, Posadas E, Kim H, et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle. 2013;12(22):3526–3536. doi: 10.4161/cc.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Uchide K, Sakon M, Ariyoshi H, Nakamori S, Tokunaga M, Monden M. Cancer cells cause vascular endothelial cell (vEC) retraction via 12(S)HETE secretion; the possible role of cancer cell derived microparticle. Annals of Surgical Oncology. 2007;14(2):862–868. doi: 10.1245/s10434-006-9225-3. [DOI] [PubMed] [Google Scholar]
- 434.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological Reviews. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 435.Williams SC. Circulating tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(13):4861. doi: 10.1073/pnas.1304186110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumour evolution inferred by singlecell sequencing. Nature. 2011;472(7341):90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Green DL, Karpatkin S. Effect of cancer on platelets. Cancer Treatment and Research. 2009;148:17–30. doi: 10.1007/978-0-387-79962-9_2. [DOI] [PubMed] [Google Scholar]
- 438.Pearlstein E, Salk PL, Yogeeswaran G, Karpatkin S. Correlation between spontaneous metastatic potential, platelet-aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic-variant derivatives of a rat renal sarcoma cell line. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(7):4336–4339. doi: 10.1073/pnas.77.7.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Grignani G, Pacchiarini L, Almasio P, Pagliarino M, Gamba G, Rizzo SC, et al. Characterization of the plateletaggregating activity of cancer cells with different metastatic potential. International Journal of Cancer. 1986;38(2):237–244. doi: 10.1002/ijc.2910380214. [DOI] [PubMed] [Google Scholar]
- 440.Menter DG, Onoda JM, Moilanen D, Sloane BF, Taylor JD, Honn KV. Inhibition by prostacyclin of the tumor cell-induced platelet release reaction and platelet aggregation. Journal of the National Cancer Institute. 1987;78(5):961–969. [PubMed] [Google Scholar]
- 441.Lee JJ, Yu JY, Lee JH, Zhang WY, Kim TJ, Myung CS, et al. The protective effects of paclitaxel on platelet aggregation through the inhibition of thromboxane A2 synthase. Archives of Pharmacal Research. 2010;33(3):387–394. doi: 10.1007/s12272-010-0307-1. [DOI] [PubMed] [Google Scholar]
- 442.de Leval X, Benoit V, Delarge J, Julemont F, Masereel B, Pirotte B, et al. Pharmacological evaluation of the novel thromboxane modulator BM-567 (II/II). Effects of BM-567 on osteogenic sarcoma-cell-induced platelet aggregation. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2003;68(1):55–59. doi: 10.1016/s0952-3278(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 443.Pacchiarini L, Zucchella M, Milanesi G, Tacconi F, Bonomi E, Canevari A, et al. Thromboxane production by platelets during tumor cell-induced platelet activation. Invasion & Metastasis. 1991;11(2):102–109. [PubMed] [Google Scholar]
- 444.Tzanakakis GN, Krambovitis E, Tsatsakis AM, Vezeridis MP. The preventive effect of ketoconazole on experimental metastasis from a human pancreatic carcinoma may be related to its effect on prostaglandin synthesis. International Journal of Gastrointestinal Cancer. 2002;32(1):23–30. doi: 10.1385/IJGC:32:1:23. [DOI] [PubMed] [Google Scholar]
- 445.Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981;212(4500):1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- 446.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clinical Cancer Research. 2010;16(5):1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Kanazawa S, Yamaguchi K, Kinoshita Y, Muramatsu M, Komiyama Y, Nomura S. Gefitinib affects functions of platelets and blood vessels via changes in prostanoids balance. Clinical and Applied Thrombosis/Hemostasis. 2005;11(4):429–434. doi: 10.1177/107602960501100409. [DOI] [PubMed] [Google Scholar]
- 448.Gordon SG, Chelladurai M. Non-tissue factor procoagulants in cancer cells. Cancer Metastasis Reviews. 1992;11(3-4):267–282. doi: 10.1007/BF01307182. [DOI] [PubMed] [Google Scholar]
- 449.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet-cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. British Journal of Pharmacology. 2004;143(7):819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Schaffner F, Ruf W. Tissue factor and PAR2 signaling in the tumor microenvironment. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Arteriosclerosis Thrombosis and Vascular Biology. 2009;29(12):1999–2004. doi: 10.1161/ATVBAHA.108.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Kirwan CC, McDowell G, McCollum CN, Kumar S, Byrne GJ. Early changes in the haemostatic and procoagulant systems after chemotherapy for breast cancer. British Journal of Cancer. 2008;99(7):1000–1006. doi: 10.1038/sj.bjc.6604620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Levine MN. Adjuvant therapy and thrombosis: how to avoid the problem? Breast. 2007;16(Suppl 2):S169–174. doi: 10.1016/j.breast.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 453.Starakis I, Koutras A, Mazokopakis EE. Drug-induced thromboembolic events in patients with malignancy. Cardiovascular & Hematological Disorders Drug Targets. 2010;10(2):94–102. doi: 10.2174/187152910791292493. [DOI] [PubMed] [Google Scholar]
- 454.Anand M, Brat DJ. Oncogenic regulation of tissue factor and thrombosis in cancer. Thrombosis Research. 2012;129(Suppl 1):S46–49. doi: 10.1016/S0049-3848(12)70015-4. [DOI] [PubMed] [Google Scholar]
- 455.Falanga A, Consonni R, Marchetti M, Locatelli G, Garattini E, Passerini CG, et al. Cancer procoagulant and tissue factor are differently modulated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Blood. 1998;92(1):143–151. [PubMed] [Google Scholar]
- 456.Ogiichi T, Hirashima Y, Nakamura S, Endo S, Kurimoto M, Takaku A. Tissue factor and cancer procoagulant expressed by glioma cells participate in their thrombin-mediated proliferation. Journal of Neuro-Oncology. 2000;46(1):1–9. doi: 10.1023/a:1006323200001. [DOI] [PubMed] [Google Scholar]
- 457.Belloc C, Lu H, Soria C, Fridman R, Legrand Y, Menashi S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. International Journal of Cancer. 1995;60(3):413–417. doi: 10.1002/ijc.2910600324. [DOI] [PubMed] [Google Scholar]
- 458.Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. International Journal of Cancer. 2000;86(1):15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 459.Jurasz P, North S, Venner P, Radomski MW. Matrix metalloproteinase-2 contributes to increased platelet reactivity in patients with metastatic prostate cancer: a preliminary study. Thrombosis Research. 2003;112(1-2):59–64. doi: 10.1016/j.thromres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 460.Alonso-Escolano D, Strongin AY, Chung AW, Deryugina EI, Radomski MW. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. British Journal of Pharmacology. 2004;141(2):241–252. doi: 10.1038/sj.bjp.0705606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Dilly AK, Ekambaram P, Guo Y, Cai Y, Tucker SC, Fridman R, et al. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-kappaB. International Journal of Cancer. 2013;133(8):1784–1791. doi: 10.1002/ijc.28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Lindenmeyer F, Legrand Y, Menashi S. Upregulation of MMP-9 expression in MDA-MB231 tumor cells by platelet granular membrane. FEBS Letters. 1997;418(1-2):19–22. doi: 10.1016/s0014-5793(97)01336-7. [DOI] [PubMed] [Google Scholar]
- 463.Radomski A, Jurasz P, Sanders EJ, Overall CM, Bigg HF, Edwards DR, et al. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. British Journal of Pharmacology. 2002;137(8):1330–1338. doi: 10.1038/sj.bjp.0704936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Zhong J, Gencay MM, Bubendorf L, Burgess JK, Parson H, Robinson BW, et al. ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action and affect cell migration: a comparison between mesothelioma and mesothelial cells. Journal of Cellular Physiology. 2006;207(2):540–552. doi: 10.1002/jcp.20605. [DOI] [PubMed] [Google Scholar]
- 465.Axelrad TW, Deo DD, Ottino P, Van Kirk J, Bazan NG, Bazan HE, et al. Platelet-activating factor (PAF) induces activation of matrix metalloproteinase 2 activity and vascular endothelial cell invasion and migration. The FASEB Journal. 2004;18(3):568–570. doi: 10.1096/fj.03-0479fje. [DOI] [PubMed] [Google Scholar]
- 466.Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. The Journal of Biological Chemistry. 2006;281(5):2911–2922. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- 467.Oleksowicz L, Mrowiec Z, Schwartz E, Khorshidi M, Dutcher JP, Puszkin E. Characterization of tumor-induced platelet aggregation: the role of immunorelated GPIb and GPIIb/IIIa expression by MCF-7 breast cancer cells. Thrombosis Research. 1995;79(3):261–274. doi: 10.1016/0049-3848(95)00113-6. [DOI] [PubMed] [Google Scholar]
- 468.Jurasz P, Stewart MW, Radomski A, Khadour F, Duszyk M, Radomski MW. Role of von Willebrand factor in tumour cell-induced platelet aggregation: differential regulation by NO and prostacyclin. British Journal of Pharmacology. 2001;134(5):1104–1112. doi: 10.1038/sj.bjp.0704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. The Journal of Clinical Investigation. 1988;81(4):1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Chopra H, Timar J, Rong X, Grossi IM, Hatfield JS, Fligiel SE, et al. Is there a role for the tumor cell integrin alpha IIb beta 3 and cytoskeleton in tumor cell-platelet interaction? Clinical & Experimental Metastasis. 1992;10(2):125–137. doi: 10.1007/BF00114589. [DOI] [PubMed] [Google Scholar]
- 471.Timar J, Trikha M, Szekeres K, Bazaz R, Honn K. Expression and function of the high affinity alphαIIbbeta3 integrin in murine melanoma cells. Clinical & Experimental Metastasis. 1998;16(5):437–445. doi: 10.1023/a:1006533508560. [DOI] [PubMed] [Google Scholar]
- 472.Trikha M, Timar J, Lundy SK, Szekeres K, Cai Y, Porter AT, et al. The high affinity alphαIIb beta3 integrin is involved in invasion of human melanoma cells. Cancer Research. 1997;57(12):2522–2528. [PubMed] [Google Scholar]
- 473.Trikha M, Timar J, Zacharek A, Nemeth JA, Cai Y, Dome B, et al. Role for beta3 integrins in human melanoma growth and survival. International Journal of Cancer. 2002;101(2):156–167. doi: 10.1002/ijc.10521. [DOI] [PubMed] [Google Scholar]
- 474.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Iwamura T, Caffrey TC, Kitamura N, Yamanari H, Setoguchi T, Hollingsworth MA. P-selectin expression in a metastatic pancreatic tumor cell line (SUIT-2) Cancer Research. 1997;57(6):1206–1212. [PubMed] [Google Scholar]
- 476.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Pottratz ST, Hall TD, Scribner WM, Jayaram HN, Natarajan V. P-selectin-mediated attachment of small cell lung carcinoma to endothelial cells. The American Journal of Physiology. 1996;271(6 Pt 1):L918–923. doi: 10.1152/ajplung.1996.271.6.L918. [DOI] [PubMed] [Google Scholar]
- 478.Stone JP, Wagner DD. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. The Journal of Clinical Investigation. 1993;92(2):804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Varki A, Varki NM. P-selectin, carcinoma metastasis and heparin: novel mechanistic connections with therapeutic implications. Brazilian Journal of Medical and Biological Research. 2001;34(6):711–717. doi: 10.1590/s0100-879x2001000600003. [DOI] [PubMed] [Google Scholar]
- 480.Ulrych T, Bohm A, Polzin A, Daum G, Nusing RM, Geisslinger G, et al. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. Journal of Thrombosis and Haemostasis. 2011;9(4):790–798. doi: 10.1111/j.1538-7836.2011.04194.x. [DOI] [PubMed] [Google Scholar]
- 481.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. The Journal of Clinical Investigation. 2004;114(12):1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Goschnick MW, Jackson DE. Tetraspanins-structural and signalling scaffolds that regulate platelet function. Mini Reviews in Medicinal Chemistry. 2007;7(12):1248–1254. doi: 10.2174/138955707782795656. [DOI] [PubMed] [Google Scholar]
- 483.Haining EJ, Yang J, Tomlinson MG. Tetraspanin microdomains: fine-tuning platelet function. Biochemical Society Transactions. 2011;39(2):518–523. doi: 10.1042/BST0390518. [DOI] [PubMed] [Google Scholar]
- 484.Protty MB, Watkins NA, Colombo D, Thomas SG, Heath VL, Herbert JM, et al. Identification of Tspan9 as a novel platelet tetraspanin and the collagen receptor GPVI as a component of tetraspanin microdomains. The Biochemical Journal. 2009;417(1):391–400. doi: 10.1042/BJ20081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Sordat I, Decraene C, Silvestre T, Petermann O, Auffray C, Pietu G, et al. Complementary DNA arrays identify CD63 tetraspanin and alpha3 integrin chain as differentially expressed in low and high metastatic human colon carcinoma cells. Laboratory Investigation. 2002;82(12):1715–1724. doi: 10.1097/01.lab.0000044350.18215.0d. [DOI] [PubMed] [Google Scholar]
- 486.Israels SJ, McMillan EM, Robertson C, Singhory S, McNicol A. The lysosomal granule membrane protein, LAMP-2, is also present in platelet dense granule membranes. Thrombosis and Haemostasis. 1996;75(4):623–629. [PubMed] [Google Scholar]
- 487.Vanags DM, Rodgers SE, Duncan EM, Lloyd JV, Bochner F. Potentiation of ADP-induced aggregation in human platelet-rich plasma by 5-hydroxytryptamine and adrenaline. British Journal of Pharmacology. 1992;106(4):917–923. doi: 10.1111/j.1476-5381.1992.tb14435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Billroth T. Lectures on surgical pathology and therapeutics, a handbook for students and practitioners. II. The New Sydenham Society; London: 1878. [Google Scholar]
- 489.Baserga R, Saffiotti U. Experimental studies on histogenesis of blood-borne metastases. AMA Archives of Pathology. 1955;59(1):26–34. [PubMed] [Google Scholar]
- 490.Jones DS, Wallace AC, Fraser EE. Sequence of events in experimental metastases of Walker 256 tumor: light, immunofluorescent, and electron microscopic observations. Journal of the National Cancer Institute. 1971;46(3):493–504. [PubMed] [Google Scholar]
- 491.Chew EC, Wallace AC. Demonstration of fibrin in early stages of experimental metastases. Cancer Research. 1976;36(6):1904–1909. [PubMed] [Google Scholar]
- 492.Warren BA, Vales O. The release of vesicles from platelets following adhesion to vessel walls in vitro. British Journal of Experimental Pathology. 1972;53(2):206–215. [PMC free article] [PubMed] [Google Scholar]
- 493.Warren BA. Some aspects of blood borne tumour emboli associated with thrombosis. Zeitschrift fur Krebsforschung und Klinische Onkologie. Cancer Research and Clinical Oncology. 1976;87(1):1–15. doi: 10.1007/BF00285068. [DOI] [PubMed] [Google Scholar]
- 494.Banfalvi G. Cell cycle synchronization of animal cells and nuclei by centrifugal elutriation. Nature Protocols. 2008;3(4):663–673. doi: 10.1038/nprot.2008.34. [DOI] [PubMed] [Google Scholar]
- 495.Oleksowicz L, Dutcher JP. Adhesive receptors expressed by tumor cells and platelets: novel targets for therapeutic anti-metastatic strategies. Medical Oncology. 1995;12(2):95–102. doi: 10.1007/BF01676709. [DOI] [PubMed] [Google Scholar]
- 496.Lonsdorf AS, Kramer BF, Fahrleitner M, Schonberger T, Gnerlich S, Ring S, et al. Engagement of alphαIIbbeta3 (GPIIb/IIIa) with alphanubeta3 integrin mediates interaction of melanoma cells with platelets: a connection to hematogenous metastasis. The Journal of Biological Chemistry. 2012;287(3):2168–2178. doi: 10.1074/jbc.M111.269811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 498.Beleva E, Grudeva-Popova J. From Virchow’s triad to metastasis: circulating hemostatic factors as predictors of risk for metastasis in solid tumors. Journal of BUON. 2013;18(1):25–33. [PubMed] [Google Scholar]
- 499.Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood. 2013;122(14):2310–2317. doi: 10.1182/blood-2013-04-460162. [DOI] [PubMed] [Google Scholar]
- 500.Barsam SJ, Patel R, Arya R. Anticoagulation for prevention and treatment of cancer-related venous thromboembolism. British Journal of Haematology. 2013;161(6):764–777. doi: 10.1111/bjh.12314. [DOI] [PubMed] [Google Scholar]
- 501.Rajalingham S, Das S. Antagonizing IL-6 in ankylosing spondylitis: a short review. Inflammation & Allergy Drug Targets. 2012;11(4):262–265. doi: 10.2174/187152812800958979. [DOI] [PubMed] [Google Scholar]
- 502.Schoels MM, van der Heijde D, Breedveld FC, Burmester GR, Dougados M, Emery P, et al. Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statement. Annals of the Rheumatic Diseases. 2013;72(4):583–589. doi: 10.1136/annrheumdis-2012-202470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Homeida S, Ebdon C, Batty P, Jackson B, Kolade S, Bateman C, et al. New thrombopoietin receptor agonists for platelet disorders. Drugs of Today (Barcelona, Spain) 2012;48(4):293–301. doi: 10.1358/dot.2012.48.4.1740505. [DOI] [PubMed] [Google Scholar]
- 504.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annual Review of Medicine. 2009;60:193–206. doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 505.Hinson RM, Williams JA, Shacter E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(10):4885–4890. doi: 10.1073/pnas.93.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal antiinflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. British Journal of Cancer. 2009;100(3):551–557. doi: 10.1038/sj.bjc.6604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes & Control. 2006;17(7):871–888. doi: 10.1007/s10552-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 508.Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. Journal of the National Cancer Institute. 2009;101(4):256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17(2):55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 510.Jafari S, Etminan M, Afshar K. Nonsteroidal antiinflammatory drugs and prostate cancer: a systematic review of the literature and meta-analysis. Canadian Urological Association Journal. 2009;3(4):323–330. doi: 10.5489/cuaj.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Khuder SA, Herial NA, Mutgi AB, Federman DJ. Nonsteroidal antiinflammatory drug use and lung cancer: a metaanalysis. Chest. 2005;127(3):748–754. doi: 10.1378/chest.127.3.748. [DOI] [PubMed] [Google Scholar]
- 512.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Journal of the National Cancer Institute. 2008;100(20):1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 513.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Chan AT, Zauber AG, Hsu M, Breazna A, Hunter DJ, Rosenstein RB, et al. Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.02.045. doi:10.1053/j.gastro.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. The New England Journal of Medicine. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 516.Gallicchio L, McSorley MA, Newschaffer CJ, Thuita LW, Huang HY, Hoffman SC, et al. Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer. 2006;106(7):1443–1452. doi: 10.1002/cncr.21763. [DOI] [PubMed] [Google Scholar]
- 517.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. The New England Journal of Medicine. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 518.Shen J, Gammon MD, Terry MB, Teitelbaum SL, Neugut AI, Santella RM. Genetic polymorphisms in the cyclooxygenase-2 gene, use of nonsteroidal anti-inflammatory drugs, and breast cancer risk. Breast Cancer Research. 2006;8(6):R71. doi: 10.1186/bcr1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Slatore CG, Au DH, Littman AJ, Satia JA, White E. Association of nonsteroidal antiinflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiology, Biomarkers & Prevention. 2009;18(4):1203–1207. doi: 10.1158/1055-9965.EPI-08-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(1):148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. The Lancet Oncology. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 522.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. The New England Journal of Medicine. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 523.Baron JA, Sandler RS, Bresalier RS, Lanas A, Morton DG, Riddell R, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372(9651):1756–1764. doi: 10.1016/S0140-6736(08)61490-7. [DOI] [PubMed] [Google Scholar]
- 524.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, Kim K, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prevention Research. 2009;2(4):310–321. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. The New England Journal of Medicine. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 526.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England Journal of Medicine. 2005;352(11):1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 527.Harris RE, Beebe-Donk J, Alshafie GA. Reduced risk of human lung cancer by selective cyclooxygenase 2 (COX-2) blockade: results of a case control study. International Journal of Biological Sciences. 2007;3(5):328–334. doi: 10.7150/ijbs.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Pruthi RS, Derksen JE, Moore D, Carson CC, Grigson G, Watkins C, et al. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clinical Cancer Research. 2006;12(7 Pt 1):2172–2177. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 529.Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Munch G, et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Molecular Pharmacology. 2013;84(1):25–40. doi: 10.1124/mol.113.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Konya V, Marsche G, Schuligoi R, Heinemann A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacology & Therapeutics. 2013;138(3):485–502. doi: 10.1016/j.pharmthera.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Norberg JK, Sells E, Chang HH, Alla SR, Zhang S, Meuillet EJ. Targeting inflammation: multiple innovative ways to reduce prostaglandin E2. Pharmaceutical Patent Analyst. 2013;2(2):265–288. doi: 10.4155/ppa.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]