Abstract
Objectives
To systematically evaluate the prodrome to mania in youth.
Methods
New-onset/worsening symptoms/signs of ≥ moderate severity preceding first mania were systematically assessed in 52 youth (16.2 ± 2.8 years) with a research diagnosis of bipolar I disorder (BD-I). Youth and/or caregivers underwent semi-structured interviews, using the Bipolar Prodrome Symptom Scale–Retrospective.
Results
The mania prodrome was reported to start gradually in most youth (88.5%), with either slow (59.6%) or rapid (28.8%) deterioration, while a rapid-onset-and-deterioration prodrome was rare (11.5%). The manic prodrome, conservatively defined as requiring ≥ 3 symptoms, lasted 10.3 ± 14.4 months [95% confidence interval (CI): 6.3–14.4], being present for ≥ 4 months in 65.4% of subjects. Among prodromal symptoms reported in ≥ 50% of youth, three were subthreshold manic in nature (irritability: 61.5%, racing thoughts: 59.6%, increased energy/activity: 50.0%), two were non-specific (decreased school/work functioning: 65.4%, mood swings/lability: 57.7%), and one each was depressive (depressed mood: 53.8%) or subthreshold manic/depressive (inattention: 51.9%). A decreasing number of youth had ≥ 1 (84.6%), ≥ 2 (48.1%), or ≥ 3 (26.9%) specific subthreshold mania symptoms (i.e., elation, grandiosity, decreased need for sleep, racing thoughts, or hypersexuality), lasting 9.5 ± 14.9 months (95% CI: 5.0–14.0), 3.5 ± 3.5 months (95% CI: 2.0–4.9), and 3.0 ± 3.2 months (95% CI: 1.0–5.0) for ≥ 1, ≥ 2, or ≥ 3 specific symptoms, respectively.
Conclusions
In youth with BD-I, a relatively lengthy, predominantly slow-onset mania prodrome appears to be common, including subthreshold manic and depressive psychopathology symptoms. This suggests that early clinical identification and intervention may be feasible in bipolar disorder. Identifying biological markers associated with clinical symptoms of impending mania may help increase chances for early detection and prevention before full mania.
Keywords: adolescents, bipolar disorder, children, clinical high risk, early recognition, mania, pediatric, prodrome
Early recognition and prevention in bipolar disorder (BD), particularly in youth, has gained considerable interest (1–16). The significant impairment and severity associated with BD underscores the importance of early identification and intervention (17–19). However, the concept of an identifiable clinical precursor state consisting of subsyndromal (i.e., prodromal) symptoms is still controversial for BD (20, 21). Numerous approaches have been proposed to identify those at highest risk for developing BD (3). These include studying offspring of patients with BD, focusing on youth with disorders frequently comorbid with BD [e.g., attention-deficit hyperactivity disorder (ADHD), disruptive behavior disorders] or preceding BD (i.e., major depressive disorder), assessing temperamental traits in patients with full BD, or, more recently, following patients with mania-like symptoms that are subthreshold to BD (3).
Since family history arguably is the most replicated risk factor for BD, many studies focused on bipolar offspring and described their psychiatric disorders and symptom constellations (17–19, 22–33). However, these studies are limited by their cross-sectional nature, often small samples, lack of systematic assessment of symptoms or consideration of severity or change in symptoms over time. Moreover, longitudinal studies of youth at familial high-risk for BD have generally reported low rates of conversion to BD (26–29, 30, 31, 33) and largely excluded patients without a first-degree relative with the disorder. Furthermore, a significant proportion of patients with BD report no clear family history of BD, thus limiting the generalizability of family high-risk studies. Studies investigating inter-episode symptoms prior to relapse (34, 35) may also have questionable generalizability, as it is unclear whether the time course and type of prodromal symptoms are identical with early signs of initial illness manifestation. While childhood depression and ADHD have been identified as risk factors or comorbid precursor states for BD, conversion usually occurs after many years, and most of these youth do not develop BD (36–39). Studies that assessed premorbid temperament and character traits in youth and adults with BD or their offspring (40–43) reported prevalent clusters of behavioral disinhibition, mood lability, and oppositionality (10, 33, 44–46,), but these observations may not necessarily be specific to BD. To date, relatively few studies have characterized the prodrome to BD in detail (for review, see 10, 11, 15). These have included studies of adults with BD or of caregivers of children with BD (47–49), or case series of patients enrolled into a program for people at risk for psychosis who developed BD (8, 9, 50). Across these studies, the following characteristics have been identified most frequently prior to a first or relapsing manic episode: depressed mood, changes in energy level, disturbance in sleep, anger dyscontrol, argumentativeness, irritability, mood swings/lability, reckless/dangerous behavior, emotional hypersensitivity, diminished concentration, psychomotor change, social isolation, deterioration in role functioning, increased anxiety, suicidal ideation or attempts, cyclothymic-hypersensitive temperament traits and psychotic symptoms. However, some studies were limited by the lack of a systematic assessment to confirm the BD diagnosis and all employed non-standardized approaches to obtaining information about the putative prodromal phase. These assessments of the BD prodrome included the use of unstructured interviews, chart reviews or mailed surveys, resulting in the reporting of symptom frequencies without knowledge of individual symptom duration, severity or impact.
The absence of reliable illness markers and information about the presence and timing of specific signs and symptoms that precede a first mania has limited the development of early interventions for high-risk samples of BD offspring who have familial risk and significant mood symptoms (51–55). Moreover, there is a paucity of data about which person (e.g., patient, parent, teacher, peer) is most likely to first notice the prodromal symptoms, which could inform the most effective psychoeducation strategies. Therefore, as a first step, and to guide future prospective studies of the bipolar prodrome, this study aimed to retrospectively, but comprehensively characterize subsyndromal symptoms preceding a first mania in youth with BD, utilizing the Bipolar Prodrome Symptom Scale-Retrospective (BPSS-R) (56). We hypothesized that a substantial number of youth would have experienced a prodrome of sufficient duration and severity with a relevant number of recognizable symptoms that emerged prior to their first syndromal manic episode allowing sufficient time for early identification and prevention efforts. We further hypothesized that some prodromal symptoms would be specific to BD, resembling subthreshold mania.
Material and methods
Subjects
Included in this study were 52 youth aged 7–21 years with a diagnosis of bipolar I disorder (BD-I). Subjects were drawn from 111 patients with a clinical diagnosis of BD-I who originally participated in the prospective [Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY)] study investigating the effects of newly initiated second-generation antipsychotic treatment in youth with psychotic, mood, and disruptive behavior disorders (57). The study was performed at inpatient and outpatient child psychiatric facilities located in a semi-urban, tertiary care psychiatric teaching hospital and was approved by the local Institutional Review Board. Legal guardians and patients ≥ 18 years of age gave informed written consent; youngsters aged 9–17 years gave written assent. In a first report on this sample, we compared the characteristics of patients with psychotic versus non-psychotic mania, exploring the overlap and differences between the prodrome to a first mania episode with psychosis and the prodrome to a first episode of schizophrenia based on retrospective studies in the literature (56).
Procedures
A semi-structured diagnostic interview was used to verify the chart diagnosis of BD. Most (n = 34) were administered The Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) (58) to be consistent with other ongoing studies at our center and to facilitate data-sharing if participants had already been assessed as part of the NIMH-funded “Treatment and Outcome of Early-Onset Bipolar Disorder” study (Principal Investigator: Vivian Kafantaris, M.D.). The Washington University in St. Louis K-SADS (WASH-U-K-SADS) (59) interview was implemented for patients recruited later (n = 18), as it was felt that the anchors were more child-specific. Patients and/or their caregivers were also questioned directly about the family history of mental illness, including BD-I and bipolar II disorder (BD-II), and the presence of comorbid disorders. These data were supplemented by information from charts and primary psychiatric care providers.
Patients with a confirmed diagnosis of BD-I and/or their caregivers independently underwent a semi-structured interview with the BPSS-R [(56); available from the first author upon request]. In brief, the BPSS-R was developed to identify youth or adults at clinical high-risk for mania. The BPSS-R was developed based on the DSM-IV criteria for mania and depression and available rating scales for BD and major depressive disorder in youth and in adults. In addition, the BPSS-R development was informed by a review of existing literature regarding risk factors and early symptoms of BD (3), published scales and interviews for the assessment of the psychotic prodrome and character traits, input from experts in the areas of the schizophrenia prodrome and BD, and open questioning of youth with BD and their caregivers regarding emerging subthreshold symptoms prior to the onset of a first syndromal bipolar manic, mixed, and depressive episode.
The BPSS-R systematically assesses the onset pattern, duration, severity and frequency of 39 symptoms and signs newly emerging or worsening during the period before a first major depressive and/or first manic episode. It includes subthreshold manic, depressive, general psychopathology and psychotic symptoms. Prodromal symptom severity was rated on an ordinal scale, with 0 = absent; 1 = mild (i.e., noticeable but not affecting functioning); 2 = moderate (i.e., clearly noticeable, may affect functioning somewhat); and 3 = severe (i.e., clearly noticeable, clearly affecting functioning). Symptom frequency was rated on an ordinal scale, with 0 = absent; 1 = infrequent (i.e., less than once a week); 2 = moderately frequent (i.e., a couple of times a week); 3 = very frequent (i.e., present more than 50% of the time); and 4 = static lifetime or character trait (which is not considered part of the dynamic prodrome). In case of clearly delineated, comorbid conditions that preceded the onset of the prodrome (i.e., symptoms limited to this disorder and without a more generalized dynamic worsening), only worsened symptoms during the prodromal phase are to be recorded.
Whenever possible, the patient and one caregiver were interviewed separately about prodromal symptoms. To increase reliability of the information, patients < 12 years of age were not interviewed (n = 4: aged 7, 8, 9, and 11 years), so that the information was based on caregiver report only. Moreover, patients were only interviewed if they had achieved sufficient stability, indicated by a Clinical Global Impression–Severity (CGI-S) (60) score of no more than ‘moderate’. In addition to the CGI-S, patients were also rated on the Children’s Global Assessment Scale (CGAS) (61). All interviews were conducted in person by the first author, a board certified child and adolescent psychiatrist, or by medical trainees at the MD or medical student level who were trained and supervised by the first author. All diagnoses were made or confirmed by the first author who also rated all patients on the CGI-S and CGAS.
The pattern of prodromal symptom onset and deterioration was evaluated using: (i) subjective responses to a multiple-choice question and (ii) a priori determined time cutoffs applied to the collected prodromal symptom data: patients/informants were asked: ‘During the time between the first noticeable symptoms and the first full-manic/mixed episode, did your/your child’s symptoms: (a) start gradually and slowly get worse; (b) start fairly gradually and quickly get worse; or (c) start all of a sudden, i.e., you were fine one day and sick the next?’. Answers translated into the following categories: a = gradual onset/slow deterioration; b = gradual onset/rapid deterioration; and c = rapid onset/rapid deterioration. The beginning of the onset phase was defined as the time at which the first newly prodromal symptom of at least moderate severity emerged. The beginning of the deterioration phase was defined as a clearly distinguishable cluster of newly emerging prodromal symptoms distal from the prodrome onset. We defined gradual onset as ≥ 4 months and rapid onset as < 4 months. While we recognize that four months is an arbitrary cutoff, we felt that this classification was conservative, erring on the side of caution, leaving sufficient time to detect an emerging prodrome, establish contact with appropriate health care services, and initiate appropriate intervention. Assuming that four weeks would be the minimum duration to intervene in patients displaying marked clinical deterioration, we defined slow deterioration as ≥ 4 weeks and rapid deterioration as < 4 weeks. Using these criteria, we derived in addition to the three predetermined patterns described above (i.e., multiple-choice responses a–c) a fourth prodromal pattern from the data set, i.e., rapid onset with continuous deterioration, characterized by emergence of most, if not all, prodromal symptoms < 4 weeks prior to full mania without relevant additional prodromal symptom additions until the full-manic episode. Prodrome duration and number of symptoms during the prodrome and at time of the first manic episode were calculated separately for each of the prodromal trajectories derived from the subjective and objective elicitation methods. Information about who noticed the prodromal mania symptoms first was elicited from the patients and/or their caregivers. Finally, we asked patients and caregivers to rate on a scale from 0–100% how certain they were that the information that they provided was accurate.
Data analysis
Although data were collected for the prodrome prior to first full depressive and manic episodes, for the purpose of this report, we focused only on the manic prodrome. We focused on the mania prodrome, as this phase is the defining period prior to the first diagnosis of BD where bipolar-specific preventive interventions need to be targeted. To assure clinical relevance of the prodromal symptoms and following the thresholds for positive psychotic symptoms used in the schizophrenia prodrome literature (62, 63), only attenuated symptoms of at least moderate severity were included in the analysis. Furthermore, to assure a dynamic character of emerging symptoms, we only considered as part of the prodrome those moderately or more severe symptoms that either emerged newly or worsened during the time preceding the first manic episode. Thus, we excluded symptoms that were reported as unchanged, life-long temperamental or character traits as well as preceding symptoms that were stable and could thus be related to comorbid psychiatric disorders. Although stable symptoms reported by patients and caregivers could be interpreted as a potentially useful proxy of background psychopathology against which true prodromal symptom frequency could be judged, we did not include these ratings due to concerns about confounding the targeted picture of a dynamic and evolving symptomatic BD prodrome. With the exception of prodrome duration and symptom prevalence in subgroup analyses, which were also calculated for patient and caregiver responses separately, patient-informant aggregates of data were used. Consistent with many studies of psychiatrically ill children and adolescents (17, 59) patient and parent/caregiver responses were integrated. In case of conflicting results, the positive response, higher (i.e., more severe) symptom rating and earlier symptom onset was counted. This method of counting the present, more severe and earlier rating is based on the assumption that patients and caregivers are differentially able to recognize either internalizing or externalizing symptoms better, and that symptoms might be forgotten, rather than invented.
We used descriptive statistics for the sample description and frequency and duration of reported prodromal symptoms. Moreover, we compared the frequency and duration of each of the prodromal symptoms in the group of patients who had a major depressive episode and a manic episode as their first illness polarity. For comparisons of categorical variables, chi-square tests (or Fisher’s Exact test when cell size was ≤ 5) were used. For continuous variables, ANOVAs were used. Drawing on results from a study that identified bipolar mania symptoms with high specificity for BD-I versus ADHD (i.e., elation, grandiosity, decreased need for sleep, racing thoughts, or hypersexuality) (64), we also analyzed the number of patients with at least one, two, or three of these ‘specific’ mania prodromal symptoms, calculating also the mean mania prodrome duration. In addition to analyses of the entire sample, we conducted subgroup analyses of the mania prodrome duration and the symptom prevalence, exploring the potential influence of five clinically relevant variables: patient versus guardian report; onset of the first manic episode at < 12 versus ≥ 12-years-old; first-degree family history of BD-I; comorbid oppositional defiant disorder (ODD)/conduct disorder (CD); comorbid ADHD. These subgroup analyses were conducted using three different, increasingly conservative definitions of the prodrome, i.e., requiring only one, two, or three prodromal symptoms to define the onset of the mania prodrome. Unless indicated otherwise, data are presented as mean ± standard deviation. Except for duration of the prodrome, where 95% confidence intervals (CI) were calculated, upper and lower limits are reported. Data were analyzed using JMP 5.0.1, 1989–2003, SAS Institute Inc. Significance levels were set at p < 0.05; analyses were two-tailed.
Results
Sample characteristics
Eighty interviews were conducted in 28 (53.9%) complete patient–informant pairs, 17 (32.7%) parent/guardians alone, and 7 (13.5%) youth alone, yielding information on 52 youth with BD. Two of the original 54 patients were excluded due to a research diagnosis of BD-II or bipolar disorder not otherwise specified (BD-NOS). Asking patients and caregivers to rate on a scale from 0–100% how certain they were that the information that they provided was accurate yielded the following estimate: 83.4% (95% CI: 77.4–89.9).
Patients were 16.2 ± 2.8-years-old at the time of the interview and had their first manic episode at age 13.4 ± 3.3 years (Table 1). The interview was conducted 2.8 ± 2.0 years after the first manic episode. The mean lag between the first full-manic episode and a community diagnosis of BD was 1.6 ± 2.0 years. In the 35 youngsters in whom interviews were conducted, the mean CGI-S score was 3.4 ± 1.5, and the mean CGAS score was 56.8 ± 14.0.
Table 1.
Demographic and illness characteristics of 52 youth with bipolar I disorder
| Characteristics | Frequency |
|---|---|
| Demographic information | |
| Age, years, mean ± SD [range]a | 16.2 ± 2.8 [7.9–20.9] |
| Sex, male/female, n (%) | 25 (48.1) / 27 (51.9) |
| Ethnicity/race, n (%)b | |
| White | 33 (64.7) |
| African American | 8 (15.7) |
| Hispanic | 4 (7.8) |
| Asian | 6 (11.8) |
| Illness characteristics | |
| CGI-S scale scorea,c | 3.4 ± 1.5 |
| CGAS score a,c | 56.8 ± 14.0 |
| Age at first diagnosis of BD, years, mean ± SD [range] | 14.5 ± 2.8 [6.5–18.9] |
| Age at first manic episode, years, mean ± SD [range] | 13.4 ± 3.3 [4.8–18.8] |
| Lag between mania and BD diagnosis, years, mean ± SD [range] | 1.6 ± 2.0 [0–6.1] |
| BD characteristics, n (%) | |
| BD with onset of mania before age 12 | 14 (26.9) |
| BD with major depression as the first mood episode | 20 (38.5) |
| First-degree family history of BDb | 15 (29.4) |
| First- or second-degree family history of BDb | 25 (49.0) |
| Comorbidities, n (%) | |
| Oppositional defiant disorder | 25 (48.1) |
| Attention-deficit hyperactivity disorder | 17 (32.7) |
| Substance use disorders | 12 (23.1) |
| Anxiety disordersd | 10 (19.2) |
| Learning disability | 4 (7.7) |
| No. of prodromal symptoms, mean ± SD [range] | |
| Self-report | 4.6 ± 2.1 [0–11] |
| Structured interview | 12.5 ± 5.1 [3–22]e |
| Combined structured interview plus free self-report symptoms | 13.1 ± 5.0 [4–22] |
SD = standard deviation; CGI-S = Clinical Global Impressions–Severity; CGAS = Children’s Global Assessment Scale; BD = bipolar disorder.
At time of interview.
Based on 51 patients with information (one patient was adopted).
Based on 35 interviewed patients.
Generalized anxiety disorder: n = 2, obsessive compulsive disorder: n = 4, anxiety disorder not otherwise specified: n = 2, posttraumatic stress disorder: n = 2.
p < 0.0001 comparing the number of prodromal symptoms elicited by the structures interview compared to the self-report method.
Using a free-report method to assess prodromal manic symptoms, patients and/or caregivers reported a mean of 4.6 ± 2.1 symptoms that were noted between the onset of any change in mental state and behavior and the first manic episode. This increased to 13.1 ± 5.0 symptoms, when answers to the semi-structured interview were added. Family members most often noticed the changes associated with prodromal onset first (80.8%), followed by patients themselves (46.1%), school personnel (46.1%), peers (40.4%), and health care personnel (8.8%) (total exceeds 100% due to simultaneous first recognition by several people). Altogether, 16 patients (30.7%) used marijuana (n = 10), alcohol (n = 8), or cocaine (n = 3) prior or during the mania prodrome. Of these, only four subjects (or their caregivers) reported that the substance use triggered or worsened symptoms occurring as part of the mania prodrome.
Duration and trajectory of the mania prodrome
In the full sample, the mean duration of the manic prodrome, consisting of at least one symptom of moderate severity, was 18.8 ± 19.2 (95% CI: 13.4–24.2) months. Using cutoff points of either at least two or at least three symptoms of at least moderate severity, the mean duration was 12.6 ± 16.8 (95% CI: 8.0–17.3) and 10.3 ± 14.4 (95% CI: 6.3–14.4) months, respectively. Using presence of at least moderately severe prodromal symptoms for ≥ 4 months as a threshold, 38 (73.1%) had at least one prodromal symptom present for at least four months, and 34 (65.4%) each had at least two or three prodromal symptoms present for ≥ 4 months. Requiring presence of ‘specific’ mania symptoms as part of the prodrome (i.e., elation, grandiosity, decreased need for sleep, racing thoughts, or hypersexuality) (58), at least one, two, or three of these symptoms were present in 84.6%, 48.1%, and 26.9% of youth, with an overall prodrome duration of 9.5 ± 14.9 (95% CI: 5.0–14.0), 3.5 ± 3.5 (95% CI: 2.0–4.9), and 3.0 ± 3.2 (95% CI: 1.0–5.0) months, respectively.
Table 2 depicts the duration of the subjectively determined onset and deterioration phases of the manic prodrome (based on responses to a multiple-choice question detailed above), including the mean number of symptoms during both prodromal phases and during the first syndromal manic episode. Using this methodology, the majority of patients (59.6%) reported a gradual symptom onset with slow deterioration, 28.8% had a gradual onset with rapid deterioration, and only 11.5% had a rapid symptom onset with rapid deterioration.
Table 2.
Prodromal symptom trajectories based on a priori selected multiple-choice question
| Manic prodrome onset and deterioration patterna |
n (%) | Total prodrome duration, months |
Total no. of prodromal symptoms ≥ moderate severity |
No. of symptoms at time of first full mania |
|||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Gradual onset with slow deterioration | 31 (59.6) | 21.0 ± 20.7 | 13.4–28.6 | 13.0 ± 5.3 | 11.0–15.0 | 20.4 ± 6.2 | 18.2–22.7 |
| Gradual onset with rapid deterioration | 15 (28.8) | 20.7 ± 17.5 | 11.0–30.4 | 12.5 ± 4.6 | 9.9–15.0 | 19.4 ± 4.6 | 16.8–21.9 |
| Rapid onset with rapid deterioration | 6 (11.5) | 2.8 ± 3.6 | −1.0 to 6.7 | 14.8 ± 4.9 | 9.7–19.9 | 20.5 ± 3.0 | 17.3–23.7 |
SD = standard deviation; CI = confidence interval.
Based on a multiple-choice question. Definition of onset and deterioration pattern based on multiple-choice question with three possible answers (see Methods) where patients/caregivers could take duration, frequency, and severity of symptoms into account.
Prodrome duration based on onset of at least one newly emerging prodromal symptom of at least moderate severity.
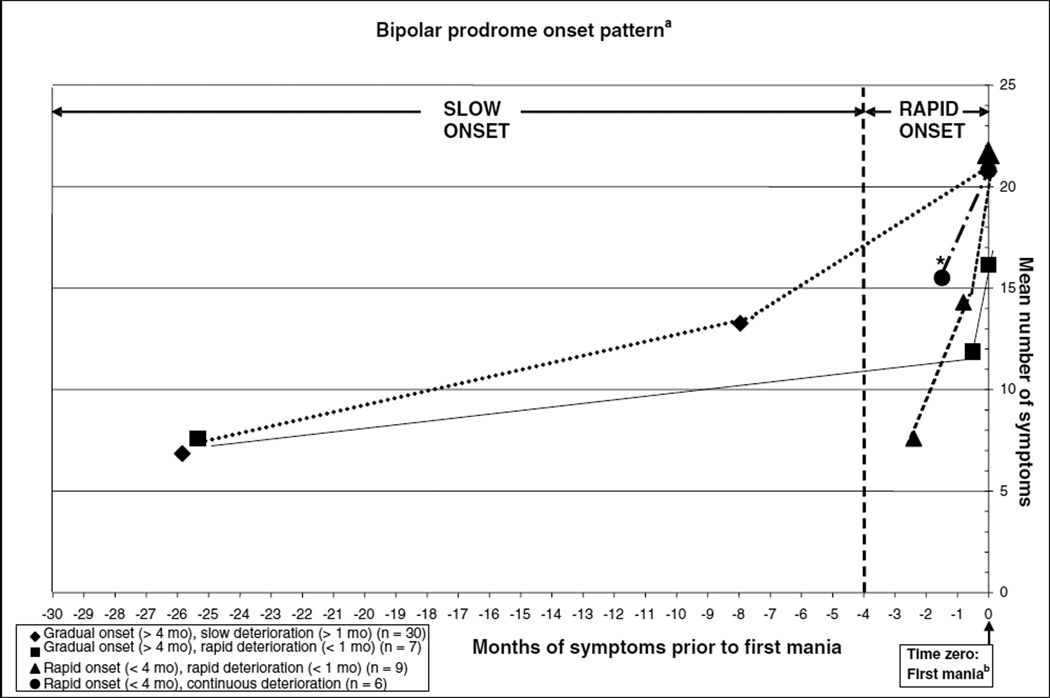
Figure 1 shows the timing of the objectively determined onset and deterioration phases of the manic prodrome (based on time cutoffs detailed above), including the mean number of symptoms during both prodromal phases and during the first syndromal manic episode. Using this pragmatic methodology, most frequently, symptoms during the prodrome had a gradual onset (≥ 4 months) with slow deterioration (≥ 1 month) (57.7 %), followed by rapid onset (< 4 months) with rapid deterioration (< 1 month) (17.3%), gradual onset (≥ 4 months) with rapid deterioration (< 1 month) (13.5%), and a fourth pattern that had emerged from the data (which had not been part of the multiple-choice answers), consisting of rapid onset (< 4 months) with continuous deterioration (11.5 %). Comparing the number of symptoms at the prodromal onset or deterioration phase or during the first full mania, only the number of prodromal symptoms during the onset phase differed across the four groups, being higher in patients with rapid prodrome onset with continuous deterioration compared to the other three groups [F(3,48) = 7.19, p = 0.0004]. This difference remained significant after adjusting for sex, age, race, and individual comorbidities [F(3,48) = 4.79, p = 0.0069].
Fig. 1.
Mean duration and number of prodromal symptoms during the mania prodrome onset and deterioration phase. mo = months.
aDefinition of onset pattern based on pragmatically, a priori defined duration threshold of four months for gradual versus rapid onset and of four weeks for slow versus rapid deterioration. The deterioration phase consists of a newly emerging symptom cluster after prodrome onset but before full mania criteria were met.
bTime zero = mean number of symptoms during the first manic episode.
*F(3,48) = 7.19, p = 0.0004 for number of symptoms during onset phase.
Individual symptom characteristics and duration of the mania prodrome
Table 3 shows the prevalence rates and percent ranks for symptoms present at any time during the prodrome and for those present for at least four months. In addition, the mean and median duration of individual prodromal symptoms is displayed. Among the top 13 ranking symptoms (present in more than 40% of patients) at any time during the prodrome, five each were either from the general psychopathology domain (drop in school/work functioning, mood swings/lability, anger outbursts/tantrums, social isolation, anxiety/nervousness) or the subthreshold mania domain (irritability/anger, racing thoughts, increased energy/activity, overtalkativeness, reckless/dangerous behavior), two each were from the overlapping domain of subthreshold manic or depressive symptoms (inattention/distractibility, psychomotor agitation) or subthreshold depressive symptom domain (depressed mood, anhedonia), and one was from the overlapping domain of subthreshold manic, depressive or psychotic symptoms (difficulty thinking clearly).
Table 3.
Reported symptoms and signs emerging during the bipolar mania prodrome
| Bipolar prodrome symptom/sign (≥ moderate severity) |
Total prevalence n (%) |
Percent rank |
Duration, months Mean ± SD |
Duration, months Median |
Present for ≥ 4 months n (%) |
Percent rank |
|---|---|---|---|---|---|---|
| Subsyndromal manic symptoms | ||||||
| Irritability or easily angered | 32 (61.5) | 2 | 8.0 ±11.7 | 3.0 | 15 (28.8) | 7 |
| Racing thoughts | 31 (59.6) | 3 | 7.5 ± 12.7 | 4.0 | 16 (30.8) | 5 |
| Increased energy or goal-directed activity | 26 (50.0) | 7 | 6.8 ± 14.0 | 2.5 | 11 (21.1) | 13 |
| Overly talkative | 22 (42.3) | 11 | 9.1 ± 16.9 | 3.5 | 11 (21.1) | 13 |
| Reckless or dangerous behavior | 21 (40.4) | 13 | 9.0 ± 10.9 | 6.0 | 13 (25.0) | 10 |
| Decreased need for sleep | 20 (38.5) | 16 | 5.2 ± 10.8 | 1.5 | 7 (13.5) | 21 |
| Overly cheerful or happy | 18 (34.6) | 19 | 6.8 ± 13.6 | 4.0 | 9 (17.3) | 16 |
| Overly self-confident | 12 (23.1) | 24 | 4.3 ± 6.7 | 1.6 | 4 (7.7) | 27 |
| Increased sexual energy | 10 (19.2) | 29 | 4.1 ± 3.8 | 3.0 | 5 (9.6) | 26 |
| Risky sexual behavior | 3 (5.8) | 35 | 4.8 ± 4.8 | 4.0 | 2 (3.8) | 32 |
| Subsyndromal manic or depressive symptoms | ||||||
| Decreased concentration/attention/memory | 27 (51.9) | 6 | 8.6 ± 13.9 | 4.0 | 15 (28.8) | 7 |
| Physically agitated | 25 (48.1) | 8 | 4.7 ± 5.7 | 2.0 | 10 (19.2) | 15 |
| Subsyndromal manic or psychotic symptoms | ||||||
| Grandiose ideas | 8 (15.7) | 32 | 1.9 ± 1.7 | 1.5 | 2 (3.8) | 32 |
| Subsyndromal psychotic symptoms | ||||||
| Strange or unusual (non-grandiose) ideas | 10 (19.2) | 29 | 1.4 ± 1.6 | 0.6 | 1 (1.9) | 35 |
| Suspiciousness | 12 (23.1) | 24 | 2.8 ± 4.8 | 0.7 | 3 (5.8) | 29 |
| Hallucinatory experiences | 12 (23.1) | 24 | 7.6 ± 15.4 | 2.0 | 3 (5.8) | 29 |
| Subsyndromal manic or psychotic or depressive symptoms | ||||||
| Difficulty thinking or communicating clearly | 21 (40.4) | 13 | 6.6 ± 12.9 | 3.0 | 7 (13.5) | 21 |
| Subsyndromal depressive symptoms | ||||||
| Depressed mood | 28 (53.8) | 5 | 12.5 ± 18.8 | 5.0 | 18 (34.6) | 3 |
| Anhedonia | 21 (40.4) | 13 | 9.2 ± 11.6 | 5.0 | 13 (25.0) | 10 |
| Insomnia | 19 (36.5) | 17 | 4.1 ± 4.9 | 2.0 | 8 (15.4) | 19 |
| Feeling worthless or guilty | 17 (32.7) | 20 | 11.2 ± 10.1 | 9.0 | 12 (23.5) | 12 |
| Thinking about suicide | 16 (30.8) | 21 | 5.7 ± 5.4 | 3.0 | 7 (14.0) | 21 |
| Tiredness or lack of energy | 13 (25.0) | 23 | 6.1 ± 4.0 | 5.5 | 9 (17.3) | 16 |
| Weight loss or ↓ in appetite | 12 (23.1) | 24 | 8.8 ± 21.1 | 1.5 | 4 (7.7) | 27 |
| Physically slowed down | 8 (15.7) | 31 | 6.4 ± 4.4 | 6.5 | 6 (11.5) | 24 |
| Weight gain or ↑ in appetite | 7 (13.5) | 33 | 12.1 ± 12.4 | 5.0 | 6 (11.5) | 29 |
| Attempting suicide | 6 (11.5) | 34 | 2.9 ± 5.1 | 0.5 | 1 (1.9) | 35 |
| Hypersomnia | 3 (5.8) | 35 | 9.3 ± 5.5 | 12.0 | 2 (3.8) | 32 |
| General psychopathology symptoms | ||||||
| Decreased school or work functioning | 34 (65.4) | 1 | 8.8 ± 10.6 | 4.5 | 19 (36.5) | 1 |
| Frequent mood swings/lability | 30 (57.7) | 4 | 9.6 ± 13.3 | 6.0 | 18 (35.3) | 3 |
| Anger or losing temper a lot | 25 (48.1) | 8 | 8.4 ± 9.8 | 5.0 | 15 (28.8) | 7 |
| Social isolation | 23 (44.2) | 10 | 14.6 ± 19.1 | 7.5 | 16 (31.4) | 5 |
| Anxiety or nervousness | 22 (43.1) | 11 | 7.3 ± 12.7 | 2.6 | 9 (17.6) | 16 |
| Oppositionality | 19 (36.5) | 17 | 13.1 ± 16.4 | 7.0 | 19 (36.5) | 1 |
| Obsessions or compulsions | 14 (26.9) | 22 | 7.2 ± 9.3 | 5.5 | 8 (15.4) | 19 |
| Ambivalence | 11 (21.1) | 28 | 7.2 ± 17.6 | 1.0 | 3 (5.8) | 29 |
Tied rankings are reported by giving the same rank to two symptoms and skipping the next rank.
Among the 15 most common prodromal symptoms with a rank of 13 or lower (giving symptoms that tied the same rank and skipping the next rank) that were present for at least four months in more than one of five patients, five were subthreshold variants of DSM-IV criteria for mania (i.e., irritability/anger, racing thoughts, increased energy/activity, overtalkativeness, reckless/dangerous behavior), five belonged to the general psychopathology domain (drop in school/work functioning, mood swings/lability, anger outbursts/tantrums, social isolation, anxiety/nervousness), two were subthreshold depressive in nature (depressed mood, anhedonia), two were either subthreshold manic or depressive (.inattention/decreased memory, physical agitation), and one was subthreshold manic, depressive or psychotic (difficulty thinking or communicating clearly). Prodromal symptom items with the longest durations were most often subthreshold depressive, with five items having mean durations of at least nine months (depressed mood, increased appetite/weight gain, worthlessness/guilt, hypersomnia, anhedonia). Four general psychopathology symptoms (social isolation, oppositionality, mood swings/lability, decreased school/work functioning), and two subthreshold mania symptoms (reckless/dangerous behavior, overtalkativeness) had average durations of nine months or longer. Since the standard deviations for the duration of individual prodromal symptoms were mostly large, we also calculated the median durations. Although general psychopathology and subthreshold depressive symptoms had median durations that were longer than those for subthreshold mania symptoms, five subthreshold mania symptoms (reckless/dangerous behavior, racing thoughts, overly cheerful/happy, inattention, risky sexual behavior) lasted for a median duration of ≥ 4 months (Table 3).
In exploratory analyses comparing patients with and without a full depressive episode prior to first mania on the frequency and duration of the 39 assessed symptoms, only racing thoughts was more frequent (78.9% versus 48.5%, p = 0.042) and risky sexual behaviors was more prolonged (1.5 ± 3.5 versus 0.1 ± 0.7 months, p = 0.038) during the mania prodrome in patients whose first polarity had been depression.
Subgroup analyses of mania prodrome duration and symptom characteristics
Of five subgroup analyses (Table 4), only the presence of ODD/CD was associated with a significantly longer prodrome duration using a minimum of ≥ 2 or ≥ 3 prodromal symptoms. There was a trend toward longer symptom recognition by caregivers compared to patients in the 28 complete patient–informant pairs, again, more pronounced in the prodrome characterized by ≥ 2 or ≥ 3 concurrent symptoms.
Table 4.
Subgroup analyses of the mania prodrome duration according to the number of concurrent symptoms
| Moderator variable | N | ≥ 1 prodromal symptom | p-value | ≥ 2 prodromal symptoms | p-value | ≥ 3 prodromal symptoms | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | |||||
| Informant8 | 0.12 | 0.097 | 0.067 | |||||||
| Patient | 28 | 12.2 ± 14.4 | 6.7–17.8 | 5.1 ±5.2 | 3.1–7.1 | 3.3 ±4.0 | 1.8–4.9 | |||
| Guardian | 28 | 16.7 ±21.3 | 8.4–25.0 | 11.5 ±18.9 | 4.2–18.8 | 9.9 ±17.6 | 3.1–16.8 | |||
| First-degree family history of BDb | 0.085 | 0.078 | 0.20 | |||||||
| Yes | 15 | 26.3 ± 20.7 | 14.9–37.7 | 19.3 ±20.1 | 8.2–30.4 | 14.6 ± 17.2 | 5.1–24.1 | |||
| No | 36 | 16.0 ±18.2 | 9.9–22.2 | 10.1 ±14.9 | 5.1–15.2 | 8.8 ±13.2 | 4.3–13.2 | |||
| Onset of mania before age 12 | 0.54 | 0.49 | 0.38 | |||||||
| Yes | 14 | 21.6 ±22.2 | 8.8–34.4 | 15.4 ±21.4 | 3.1–27.7 | 13.3 ±18.5 | 2.6–24.0 | |||
| No | 38 | 17.8 ±18.3 | 11.8–23.8 | 11.7 ±15.0 | 6.8–6.6 | 9.3 ±12.8 | 5.1–13.5 | |||
| Comorbid ODD/CD | 0.083 | 0.011 | 0.011 | |||||||
| Yes | 25 | 23.6 ±21.8 | 14.6–32.6 | 18.7 ±20.8 | 10.1–27.3 | 15.6 ±18.9 | 7.8–23.3 | |||
| No | 27 | 14.4 ± 15.6 | 8.2–20.5 | 7.1 ± 9.4 | 3.4–10.8 | 5.5 ±5.6 | 3.3–7.7 | |||
| Comorbid ADHD | 0.84 | 0.92 | 0.75 | |||||||
| Yes | 17 | 18.0 ±16.3 | 9.7–26.4 | 13.0 ±14.5 | 5.6–20.5 | 11.3 ± 11.9 | 5.2–17.4 | |||
| No | 35 | 19.2 ±20.8 | 12.1–26.3 | 12.5 ±18.0 | 6.3–18.7 | 9.9 ±15.7 | 4.5–15.3 | |||
SD = standard deviation; CI = confidence interval; BD = bipolar disorder; ODD = oppositional defiant disorder; CD = conduct disorder; ADHD = attention-deficit hyperactivity disorder.
Only complete pairs included in the matched-pair analyses.
Based on 51 patients with information (one patient was adopted).
Comparing the assessed prodromal symptoms across the five selected subgroup variables, only family history and ODD/CD comorbidity were associated with two symptoms each that were significantly different from patients without this characteristic: presence of a first-degree family member with BD-I was associated with a higher prevalence of increased sexual energy and oppositional defiant behavior in youth; and comorbid ODD/CD was associated with less feelings of euphoria and more worthlessness (Table 5). By contrast, informant type, an age of onset < 12 years, and comorbid ADHD were not noted to significantly influence symptom expression during the mania prodrome
Table 5.
Significant subgroup differences regarding prodromal symptom frequencies preceding a first manic episode
| Moderator variable | Subgroup | N | Symptom | % | p-value | Symptom | % | p-value |
|---|---|---|---|---|---|---|---|---|
| Informanta | Patient | 28 | Attenuated hallucinations | 0.0 | 0.019 | Anhedonia | 21.4 | 0.048 |
| Guardian | 28 | 12.9 | 46.6 | |||||
| First-degree family history of BDb | Yes | 15 | Increased sexual energy | 40.0 | 0.047 | Oppositional defiant behavior | 60.0 | 0.030 |
| No | 36 | 11.1 | 27.8 | |||||
| Onset of mania before age 12c | Yes | 14 | - | - | ||||
| No | 38 | - | - | |||||
| Comorbid ODD/CD | Yes | 25 | Feeling overly happy | 16.0 | 0.0091 | Feeling worthless | 48.0 | 0.024 |
| No | 27 | 51.9 | 18.5 | |||||
| Comorbid ADHDc | Yes | 17 | - | - | ||||
| No | 35 | - | - |
BD = bipolar disorder; ODD = oppositional defiant disorder; CD = conduct disorder; ADHD = attention-deficit hyperactivity disorder.
Only complete pairs included in the paired Mest analyses.
Based on 51 patients with information (one patient was adopted).
None of the prodromal symptoms reached statistical significance (p < 0.05).
Discussion
Our results suggest that many youth undergo a relatively lengthy and mostly non-acute prodromal symptom phase prior to the development of a first full-manic episode. In the majority of our sample (88.5%), the development of symptoms preceding a first manic episode was reported to have developed gradually, and the onset phase (defined as the initial presentation of prodromal symptoms separated from a period proximal to the first full-manic episode where either symptoms worsened and/or a cluster of additional prodromal symptoms occurred) was followed predominantly by a slow (59.6%), rather than rapid (28.8%) deterioration. By contrast, only a minority (11.5%) reported a rapid onset as well as deterioration pattern of their manic prodrome. Using four months as a minimum duration for gradual onset, 71.2% of patients had a gradual prodrome onset. Although these findings are still preliminary and need validation in larger samples, they suggest that the gradual onset in a majority of patients lends itself to early illness identification and, possibly, interventions (3, 6) to target early symptom manifestations. This is highlighted by our second and related finding, that the mean duration of the manic prodrome, using a conservative threshold of ≥ 3 concurrent symptoms of at least moderate severity, lasted for 10.3 months, and that 65.4% of patients had a mania prodrome consisting of at least three moderately severe concurrent symptoms that lasted ≥ 4 months. Although the prodrome duration in patients required to have at least one, two, or three ‘specific’ mania prodrome symptoms was shorter, the proportion of patients fulfilling these criteria and the 95% CIs of the prodrome duration indicate that a sizeable group of patients could be identified and receive early interventions, even when limiting the early recognition focus to generally accepted more specific symptoms as part of the mania prodrome. Of note, the prodromal symptom duration was considerably longer than that described in retrospective studies investigating the prodrome to a manic relapse (mean across studies: 21–29 days, range: 1–120 days) (35), suggesting that the mechanisms involved in a first mania episode might differ somewhat from those that occur in a patients whose brain has already undergone changes associated with mania. The third finding of this study is that patients and/or caregivers reported a mean of 13 symptoms for the entire prodromal period when asked structured questions, compared to recalling only 4.6 symptoms upon open questioning, underscoring the need for structured inquiries in patients with recent-onset mania and their caregivers. The reported symptoms consisted of a mixture of nonspecific general psychopathology and subthreshold depressive symptoms, as well as more specific subthreshold manic symptoms. Although drop in school/work functioning was the most frequently reported symptom and several other non-specific, general psychopathology symptoms were also reported frequently, seven of the 13 most common prodromal symptoms, present in at least 40% of the sample, were subsyndromal versions of DSM-IV mania criteria. Enthusiasm about the potential specificity of prodromal symptoms may be tempered by the fact that many of the reported subthreshold symptoms, even those overlapping with DSM-IV mania criteria (e.g., irritability, decreased concentration, agitation, reckless/dangerous behaviors, difficulty thinking, or communicating clearly), are relatively nonspecific. Nevertheless, the emergence of subthreshold accelerated mania symptoms, such as racing thoughts, increased energy/goal directed behavior and overtalkativeness in 59.6%, 50.0%, and 42.3% of patients, respectively, suggests that these may serve as more specific markers of emerging bipolarity. This finding is consistent with less detailed reports of pre-manic symptomatology (33, 47–49) and high frequencies of these suprathreshold symptoms in full mania across seven pediatric BD studies (65). This finding in the initial prodrome to a first mania episode is also consistent with findings from a recent study that followed patients with BD-NOS prospectively (66). Assessing the degree and predictors of conversion from BD-NOS to BD-I or BD-II, the authors found a sizeable conversion rate 45% after a mean of 58 weeks after intake. Diagnostic conversion was predicted by a parental history of manic symptoms, but also by an increased intensity of ‘hypomanic’/subyndromal symptoms (66).
While our results suggest that, in a sizeable proportion of subjects, the disease begins insidiously, with subthreshold expressions of the full symptom complement, we also identified a number of subthreshold depressive symptoms as part of the manic prodrome. Although a mania prodrome was only rated in the absence of a syndromal major depressive disorder, the impending development of mixed mania, mostly reported by patients and not by informants, may be partly responsible for the seemingly high prevalence of subthreshold depressive symptoms during a pre-manic prodrome. Nevertheless, depressive symptoms were also identified as part of a manic prodrome in three previous cohorts (33, 47–49).
In our sample, 70.6% did not have a first-degree family member with BD and 51.0% did not have a first- or second-degree member with BD. Nevertheless, the duration of the prodrome was predominantly non-acute and rich with symptoms that were impairing, underscored by the fact that almost two thirds of patients experienced a drop in school functioning. These findings are of great importance with regard to the potential for early interventions. Thus far, early intervention studies in BD, focusing on offspring of patients with BD or with mood disorders other than BD-I or BD-II, have yielded promising, but unclear long-term results (51–55). In these studies, most of our patients who ultimately developed BD-I would have been excluded. Therefore, future studies should broaden the focus to also include cohorts of high-risk individuals defined by clinical subthreshold symptoms. The high conversion rate in subjects with BD-NOS (66), which can be considered the most symptomatic and proximal prodrome to BD (67), further supports this research direction.
Exploratory subgroup analyses showed that the mania prodrome duration and symptom frequency mostly similar whether or not the patient or caregiver were interviewed and whether or not patients had a family history of BD-I, pre- or postpubertal onset of mania, or comorbid ODD/CD or ADHD. The duration of the manic prodrome was significantly longer only in patients with comorbid ODD/CD and when requiring at least two or at least three concurrent symptoms. In the 28 patient–guardian pairs, a trend was observed towards an earlier recognition of the prodrome by guardians, suggesting that educational campaigns for early recognition should target adults and youth. The overall symptom frequencies were mostly not different across subgroups. The only exceptions were a relative underreporting of hallucinations and anhedonia by patients compared to caregivers; an increased prevalence of hypersexuality (despite a more than one-year earlier age of onset) and oppositionality in patients with a first-degree family member with BD; and less euphoria and greater worthlessness in patients with comorbid ODD/CD. The greater tendency to display hypersexuality in patients with a strong familial risk for BD could represent a behavioral endophenotype for pediatric bipolarity (64). However, behavioral and environmental influences on childhood sexuality, possibly related to parental BD diagnosis (e.g., greater risk for overstimulation, lack of supervision, abuse, etc.) can also not be ruled out, pointing to a need for further research. The finding of relatively lower rates of euphoria and greater feelings of worthlessness in patients with comorbid ODD/CD is consistent with findings in patients exhibiting severe mood dysregulation. These patients most often fulfill criteria for ODD/CD (68, 69) and low self-esteem as well as feelings of worthlessness may also promote oppositional and conduct behaviors, independent of the underlying or ultimate mood disorder. Nevertheless, due to the small sample size, the significant results from the subgroup analyses are only hypothesis-generating and need to be examined further in future studies.
In addition to the prodromal symptom duration and pattern observed in our sample, there was a relatively short mean duration between first full-manic episode and the diagnosis of BD, which was 1.6 years. This is in contrast to the mean eight-year lag found in the study by Lish et al. (47) conducted in adults. The faster recognition in our early-onset BD sample might be explained by greater illness severity (70–73), by recruitment from a center with expertise in and active research focusing on the identification and treatment of pediatric bipolar disorder, or by the availability of caregivers and/or teachers who can bring patients to clinical attention countering some of the lack of illness insight often observed in patients. In addition, an increased public and mental health awareness of pediatric BD can also have played a role.
The results from this study clearly need to be interpreted within its limitations. These include its retrospective nature, modest sample size, enrollment of youth with BD-I with more severe illness expression and/or psychosis, and lack of a control group that would serve to determine the sensitivity and specificity of specific symptoms and symptom clusters for the development of BD. At the same time, this study aimed at characterizing the mania prodrome in patients with a verified BD-I diagnosis in order to assess if the BD prodrome is sufficiently symptomatic and lengthy in order to allow for early recognition efforts. Moreover, the focus on more severely ill youth with BD-I prescribed antipsychotic medications may strengthen the results, as arguably the more severely ill patients are, the more are early identification and intervention indicated. A further limitation is the lack of formal mood assessments at the time of the interview, which does not allow us to directly rule out that residual mood states could have influenced the recalled information. However, only youth with a CGI-S score of ≤ moderate were interviewed, the mean CGI-S score was 3.4 (mild–moderate), the CGAS score was 56.8 at the time of the interview, and information was integrated, taking also the caregiver report into account.
Further, although the retrospective assessments do not allow us to exclude the possibility that in some patients’ symptoms of comorbid disorders frequently observed in BD (72, 74) may have been misattributed as prodromal symptoms, the presence of subsyndromal, specific BD symptoms in a sizable proportion of our sample makes this less likely. We also cannot fully exclude the possibility that in some patients syndromal manic symptoms were erroneously rated as prodromal. However, this seems also unlikely, as clusters of at ≥ 2 or ≥ 3 symptoms were present for 12.6 and 10.3 months, respectively, prior to the full mania, and only 11.5% of patients were characterized by continuous symptom deterioration. In addition, although 65.4% of youngsters reported psychotic symptoms as part of their first full-manic episode, only one to three patients (2–6%) reported attenuated psychotic symptoms (except for difficulties communicating clearly, which was not specific enough to reliably represent thought disorder), which occurred mostly within the last month of the prodrome (56). A related question that requires further study is if and when some patients may have fulfilled criteria for hypomania or for BD-NOS (66, 75, 76) and whether BD-NOS represents an intermediate, late prodromal, phase, between attenuated, early prodromal symptoms and first syndromal mania, whether it can remit by itself, or whether it can also be a stable, intermediary outcome in certain patient subgroups. Unfortunately, we did not collect data about the presence, quality, and timing of hypomania or BD-II to assess the trajectory to BD-I in our sample. Finally, the possibility of different prodromal trajectories and durations in specific BD subgroups, suggested by the presence of different onset and deterioration patterns identified in our cohort, deserve further study. However, at least in the separate analysis of patients with a psychotic versus non-psychotic first manic episode, the overall mania prodrome duration was not significantly different (i.e., 1.7 ± 1.8 years with subsequent psychotic mania versus 1.9 ± 1.5 years without subsequent psychotic mania, p=0.70), although psychotic symptoms emerged late and relatively proximal to full mania (56).
Furthermore, compared to other pediatric BD samples (24, 71–74, 76), we observed a relatively low ADHD rate of only 32.7%. This may limit generalizability, possibly being due to a cohort effect in youth selected for second-generation antipsychotic use, as we found significantly lower rates of ADHD in patients with psychotic versus non-psychotic mania (14.7 versus 66.7%, p = 0.0001) (56). On the other hand, the frequency of ADHD in this study is consistent with studies (77, 78) consisting predominantly of adolescents and having equal sex distribution. By contrast, samples with predominance of male and prepubertal youth may bias the sample toward higher ADHD rates (24, 71–74, 76), supported by the fact that male participants in a lithium trial were significantly more likely to have ADHD (57.4%) than females (8.2%) (74). Finally, due to the lack of prospective data collection, we were unable to determine the temporal sequence of the development of mania and of frequently comorbid conditions, such as ADHD and internalizing or externalizing disorders. While one could consider these antecedent and comorbid conditions as part of a more broadly defined BD prodrome status (78), we distinguished disorders, which clearly preceded the emergence of other mania-related symptoms and which remained stable, from the clinical mania prodrome. Although future research may show that in certain patient subgroups ADHD-like and externalizing or internalizing disorder related presentations are biologically not separate from BD, current nosology considers these presentations as often related but distinct conditions. Additional research, integrating clinical, endophenotypic and genetic approaches (3, 10, 12, 13, 15, 16, 67), is sorely needed to clarify the underpinnings of these disorders to help distinguish between true biological unity, syndromal overlap, taxonometric overlap, or biological separateness of bipolar disorder from conditions that are currently considered comorbid.
In summary, findings from this study suggest that patients with early-onset BD experience a prodrome of considerable length, symptomatic richness and severity prior to first mania. The advantage of this study is the focus on mania-like symptoms in definite converters with the benefit of eliminating false positives. Clinicians should pay attention to the emergence and clustering of attenuated mania-like symptoms as constituting potentially the clinical prodrome of mania. While these findings need to be replicated through prospective studies, ideally in large multi-site studies that include and integrate biological markers and endophenotype related to the bipolar phenotype, this investigation provides strong evidence that early identification during an emerging mania prodrome is possible in a sizeable number of patients, setting the stage for targeted prevention efforts in both familial and non-familial BD. There is a crucial need for the field to find evidence-based ways to enrich potentially non-specific symptom expressions that may or may not progress to full mania with additional, either clinical or biological markers that will help increase the predictive power of clinical high-risk presentations for mania. Before treatment guidelines can be developed the sensitivity and specificity of specific symptoms and symptom constellations need to be clarified and the generalizability of these findings to other settings and populations needs to be established.
Acknowledgements
Supported in part by grant MH 61523-08 by the National Institute of Mental Health (NIMH) (BAC), The Feinstein Institute for Medical Research, and The Zucker Hillside Hospital National Institute of Mental Health (NIMH) Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH 074543-01 (Dr. Kane); and by grant MH60845 (NIMH) (VK).
Footnotes
Disclosures
CUC has received grant support from Bristol-Myers Squibb, the Feinstein Institute for Medical Research, Janssen/Johnson & Johnson, the National Institute of Mental Health (NIMH), the National Alliance for Research in Schizophrenia and Depression (NARSAD), and Otsuka; and has been a consultant and/or advisor to or has received honoraria from Actelion, Alexza, Bristol-Myers Squibb, Cephalon, Eli Lilly & Co., Genentech, Gerson Lehrman Group, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, Ortho-McNeill/Janssen/Johnson & Johnson, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Takeda, Teva, and Vanda. VK has received research support from AstraZeneca, Bristol-Myers Squibb, Forest Pharmaceuticals, Janssen, Eli Lilly & Co., Merck, and Pfizer. BB has received research grants from NIMH; and royalties for books from Random House, the American Psychiatric Association, Lippincott, and Williams & Wilkins. KDC has received research funding from GlaxoSmithKline and Merck; and is an unpaid consultant for GlaxoSmithKline, Merck, Bristol-Myers Squibb, and Eli Lilly & Co. MPD has received research support from AstraZeneca, Eli Lilly & Co., GlaxoSmithKline, Johnson & Johnson, Janssen, Lundbeck, Merck, Novartis, Otsuka, Pfizer, and Shire; and has received honoraria for lectures or consulting from Bristol-Myers Squibb, Merck, and Pfizer. MP has received grant support or honoraria (although none is current) from GlaxoSmithKline, Janssen, Abbott, and Bristol-Myers Squibb. MH, JBP, AMA, ES, DO, REC, MKS, and BAC do not have any conflicts of interest to report.
References
- 1.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 2.Berk M, Conus P, Lucas N, et al. Setting the stage: from prodrome to treatment resistance in bipolar disorder. Bipolar Disord. 2007;9:671–678. doi: 10.1111/j.1399-5618.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 3.Correll CU, Penzner JB, Lencz T, et al. Early identification and high risk strategies for bipolar disorder. Bipolar Disord. 2007;9:324–338. doi: 10.1111/j.1399-5618.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Hauser M, Pfennig A, Ozgürdal S, Heinz A, Bauer M, Juckel G. Early recognition of bipolar disorder. Eur Psychiatry. 2007;22:92–98. doi: 10.1016/j.eurpsy.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Juckel G, Correll CU, Leopold K, Pfennig A. Diagnosis and treatment in the early illness phase of bipolar disorders. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl. 5):50–54. doi: 10.1007/s00406-008-5009-z. [DOI] [PubMed] [Google Scholar]
- 6.Conus P, Ward J, Hallam KT, et al. The proximal prodrome to first episode mania - a new target for early intervention. Bipolar Disord. 2008;10:555–565. doi: 10.1111/j.1399-5618.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 7.Miklowitz DJ, Chang KD. Prevention of bipolar disorder in at-risk children: theoretical assumptions and empirical foundations. Dev Psychopathol. 2008;20:881–897. doi: 10.1017/S0954579408000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechdolf A, Nelson B, Cotton SM, et al. A preliminary evaluation of the validity of at-risk criteria for bipolar disorders in help-seeking adolescents and young adults. J Affect Disord. 2010;127:316–320. doi: 10.1016/j.jad.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Conus P, Ward J, Lucas N, et al. Characterisation of the prodrome to a first episode of psychotic mania: results of a retrospective study. J Affect Disord. 2010;124:341–345. doi: 10.1016/j.jad.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Luby JL, Navsaria N. Pediatric bipolar disorder: evidence for prodromal states and early markers. J Child Psychol Psychiatry. 2010;51:459–471. doi: 10.1111/j.1469-7610.2010.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. J Affect Disord. 2010;126:1–13. doi: 10.1016/j.jad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Pavuluri MN. Effects of early intervention on the course of bipolar disorder: theories and realities. Curr Psychiatry Rep. 2010;12:490–498. doi: 10.1007/s11920-010-0155-1. [DOI] [PubMed] [Google Scholar]
- 13.McNamara RK, Nandagopal JJ, Strakowski SM, DelBello MP. Preventative strategies for early-onset bipolar disorder: towards a clinical staging model. CNS Drugs. 2010;24:983–996. doi: 10.2165/11539700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Howes OD, Lim S, Theologos G, Yung AR, Goodwin GM, McGuire P. A comprehensive review and model of putative prodromal features of bipolar affective disorder. Psychol Med. 2010;14:1–11. doi: 10.1017/S0033291710001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leopold K, Ritter P, Correll CU, et al. Risk constellations prior to the development of bipolar disorders: rationale of a new risk assessment tool. J Affect Disord. 2012;136:1000–1010. doi: 10.1016/j.jad.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Brietzke E, Mansur RB, Soczynska JK, Kapczinski F, Bressan RA, McIntyre RS. Towards a multifactorial approach for prediction of bipolar disorder in at risk populations. J Affect Disord. 2012;140:82–91. doi: 10.1016/j.jad.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39:453–460. doi: 10.1097/00004583-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 18.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang K, Steiner H, Ketter T. Studies of offspring of parents with bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123:26–35. doi: 10.1002/ajmg.c.20011. [DOI] [PubMed] [Google Scholar]
- 20.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Correll CU, Hauser M, Auther AM, Cornblatt BA. Research in people with psychosis risk syndrome: a review of the current evidence and future directions. J Child Psychol Psychiatry. 2010;51:390–431. doi: 10.1111/j.1469-7610.2010.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapalme M, Hodgins S, LaRoche C. Children of parents with bipolar disorder: a metaanalysis of risk for mental disorders. Can J Psychiatry. 1997;42:623–631. doi: 10.1177/070674379704200609. [DOI] [PubMed] [Google Scholar]
- 23.Egeland JA, Shaw JA, Endicott J, et al. Prospective study of prodromal features for bipolarity in well Amish children. J Am Acad Child Adolesc Psychiatry. 2003;42:786–796. doi: 10.1097/01.CHI.0000046878.27264.12. [DOI] [PubMed] [Google Scholar]
- 24.Findling RL, Youngstrom EA, McNamara NK, et al. Early symptoms of mania and the role of parental risk. Bipolar Disord. 2005;7:623–634. doi: 10.1111/j.1399-5618.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 25.Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry. 2005;58:554–561. doi: 10.1016/j.biopsych.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Hillegers MH, Reichart CG, Wals M, Verhulst FC, Ormel J, Nolen WA. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord. 2005;7:344–350. doi: 10.1111/j.1399-5618.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- 27.Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord. 2007;9:828–838. doi: 10.1111/j.1399-5618.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 28.Duffy A. The early course of bipolar disorder in youth at familial risk. J Can Acad Child Adolesc Psychiatry. 2009;18:200–205. [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy A, Alda M, Hajek T, Sherry SB, Grof P. Early stages in the development of bipolar disorder. J Affect Disord. 2010;121:127–135. doi: 10.1016/j.jad.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birmaher B, Axelson D, Goldstein B, et al. Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS) Am J Psychiatry. 2010;167:321–330. doi: 10.1176/appi.ajp.2009.09070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diler RS, Birmaher B, Axelson D, et al. Dimensional psychopathology in offspring of parents with bipolar disorder. Bipolar Disord. 2011;13:670–678. doi: 10.1111/j.1399-5618.2011.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egeland JA, Endicott J, Hostetter AM, Allen CR, Pauls DL, Shaw JA. A 16-year prospective study of prodromal features prior to BPI onset in well Amish children. J Affect Disord. 2012;142:186–192. doi: 10.1016/j.jad.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Houston JP, Lipkovich IA, Ahl J, Rotelli MD, Baker RW, Bowden CL. Initial symptoms of manic relapse in manic or mixed-manic bipolar disorder: post hoc analysis of patients treated with olanzapine or lithium. J Psychiatr Res. 2007;41:616–621. doi: 10.1016/j.jpsychires.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- 36.Strober M, Carlson G. Bipolar illness in adolescents with major depression: clinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Arch Gen Psychiatry. 1982;39:549–555. doi: 10.1001/archpsyc.1982.04290050029007. [DOI] [PubMed] [Google Scholar]
- 37.Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry. 1994;33:461–468. doi: 10.1097/00004583-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158:125–127. doi: 10.1176/appi.ajp.158.1.125. [DOI] [PubMed] [Google Scholar]
- 39.Duffy A. The nature of the association between childhood ADHD and the development of bipolar disorder: a review of prospective high-risk studies. Am J Psychiatry. 2012;169:1247–1255. doi: 10.1176/appi.ajp.2012.11111725. [DOI] [PubMed] [Google Scholar]
- 40.Engstrom C, Brandstrom S, Sigvardsson S, Cloninger R, Nylander PO. Bipolar disorder: I. Temperament and character. J Affect Disord. 2004;82:131–134. doi: 10.1016/j.jad.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Kochman FJ, Hantouche EG, Ferrari P, Lancrenon S, Bayart D, Akiskal HS. Cyclothymic temperament as a prospective predictor of bipolarity and suicidality in children and adolescents with major depressive disorder. J Affect Disord. 2005;85:181–189. doi: 10.1016/j.jad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Mendlowicz MV, Akiskal HS, Kelsoe JR, Rapaport MH, Jean-Louis G, Gillin JC. Temperament in the clinical differentiation of depressed bipolar and unipolar major depressive patients. J Affect Disord. 2005;84:219–223. doi: 10.1016/j.jad.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 43.West AE, Schenkel LS, Pavuluri MN. Early childhood temperament in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Clin Psychol. 2008;64:402–421. doi: 10.1002/jclp.20471. [DOI] [PubMed] [Google Scholar]
- 44.Chang KD, Blasey CM, Ketter TA, Steiner H. Temperament characteristics of child and adolescent bipolar offspring. J Affect Disord. 2003;77:11–19. doi: 10.1016/s0165-0327(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 45.Hirshfeld-Becker DR, Biederman J, Henin A, et al. Clinical outcomes of laboratory-observed preschool behavioral disinhibition at five-year follow-up. Biol Psychiatry. 2007;62:565–572. doi: 10.1016/j.biopsych.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh MK, DelBello MP, Strakowski SM. Temperament in child offspring of parents with bipolar disorder. J Child Adolesc Psychopharmacol. 2008;18:589–593. doi: 10.1089/cap.2007.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 48.Egeland JA, Hostetter AM, Pauls DL, Sussex JN. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J Am Acad Child Adolesc Psychiatry. 2000;39:1245–1252. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Fergus EL, Miller RB, Luckenbaugh DA, et al. Is there progression from irritability/dyscontrol to major depressive and manic symptoms? A retrospective community survey of parents of bipolar children. J Affect Disord. 2003;77:71–78. doi: 10.1016/s0165-0327(02)00176-3. [DOI] [PubMed] [Google Scholar]
- 50.Thompson KN, Conus PO, Ward JL, et al. The initial prodrome to bipolar affective disorder: prospective case studies. J Affect Disord. 2003;77:79–85. doi: 10.1016/s0165-0327(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 51.Geller B, Cooper TB, Zimerman B, et al. Lithium for prepubertal depressed children with family history predictors of future bipolarity: a double-blind, placebo-controlled study. J Affect Disord. 1998;51:165–175. doi: 10.1016/s0165-0327(98)00178-5. [DOI] [PubMed] [Google Scholar]
- 52.Chang KD, Dienes K, Blasey C, Adleman N, Ketter T, Steiner H. Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry. 2003;64:936–942. doi: 10.4088/jcp.v64n0812. [DOI] [PubMed] [Google Scholar]
- 53.DelBello MP, Adler CM, Whitsel RM, Stanford KE, Strakowski SM. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry. 2007;68:789–795. doi: 10.4088/jcp.v68n0520. [DOI] [PubMed] [Google Scholar]
- 54.Findling RL, Frazier TW, Youngstrom EA, et al. Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry. 2007;68:781–788. doi: 10.4088/jcp.v68n0519. [DOI] [PubMed] [Google Scholar]
- 55.Miklowitz DJ, Chang KD, Taylor DO, et al. Early psychosocial intervention for youth at risk for bipolar I or II disorder: a one-year treatment development trial. Bipolar Disord. 2011;13:67–75. doi: 10.1111/j.1399-5618.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Correll CU, Penzner JB, Frederickson AM, et al. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr Bull. 2007;33:703–714. doi: 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of atypical antipsychotics during first-time use in children and adolescents. JAMA. 2009;302:1763–1771. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 59.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Government Printing Office. Department of Health, Education and Welfare publication ABM 76–338; 1976. pp. 223–241. [Google Scholar]
- 61.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 62.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 63.Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 64.Geller B, Zimerman B, Williams M, et al. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 65.Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 66.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50:1001–1016. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hauser M, Correll CU. Significance of prodromal symptoms of bipolar disorder in childhood and adolescence. Can J Psychiatry. 2014 doi: 10.1177/070674371305800106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brotman MA, Schmajuk M, Rich BA, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 69.Leibenluft E, Cohen P, Gorrindo T, Brook JS, Pine DS. Chronic versus episodic irritability in youth: a community-based, longitudinal study of clinical and diagnostic associations. J Child Adolesc Psychopharmacol. 2006;16:456–466. doi: 10.1089/cap.2006.16.456. [DOI] [PubMed] [Google Scholar]
- 70.Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3:202–210. [PubMed] [Google Scholar]
- 71.Carlson GA, Bromet EJ, Driessens C, Mojtabai R, Schwartz JE. Age at onset, childhood psychopathology, and 2-year outcome in psychotic bipolar disorder. Am J Psychiatry. 2002;159:307–309. doi: 10.1176/appi.ajp.159.2.307. [DOI] [PubMed] [Google Scholar]
- 72.Biederman J, Faraone SV, Wozniak J, Mick E, Kwon A, Aleardi M. Further evidence of unique developmental phenotypic correlates of pediatric bipolar disorder: findings from a large sample of clinically referred preadolescent children assessed over the last 7 years. J Affect Disord. 2004;82(Suppl. 1):S45–S58. doi: 10.1016/j.jad.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tillman R, Geller B, Bolhofner K, Craney JL, Williams M, Zimerman B. Ages of onset and rates of syndromal and subsyndromal comorbid DSM-IV diagnoses in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2003;42:1486–1493. doi: 10.1097/00004583-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 76.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kafantaris V, Coletti DJ, Dicker R, Padula G, Pollack S. Are childhood psychiatric histories of bipolar adolescents associated with family history, psychosis, and response to lithium treatment? J Affect Disord. 1998;51:153–164. doi: 10.1016/s0165-0327(98)00214-6. [DOI] [PubMed] [Google Scholar]
- 78.Kafantaris V, Coletti DJ, Dicker R, Padula G, Kane JM. Lithium treatment of acute mania in adolescents: a large open trial. J Am Acad Child Adolesc Psychiatry. 2003;42:1038–1045. doi: 10.1097/01.CHI.0000070247.24125.24. [DOI] [PubMed] [Google Scholar]