Abstract
Two hundred and thirty-two bacterial strains were isolated from the rhizospheric soil of Populus euphratica which is the dominant tree living in extreme arid regions in northwest China. Some strains with plant growth-promoting bacteria related metabolic characteristics were able to promote drought resistance in plants after inoculation. Ten strains with the greatest effects increased the dry weight of wheat shoots from 0.5 to 34.4 %, and the surface area of the root systems from 12.56 to 212.17 % compared to the control after drought treatment whereas no obvious promoting effect was observed in normal water conditions. These 10 strains were identified to be of the genera Pseudomonas, Bacillus, Stenotrophomonas and Serratia by 16S rRNA (rrs) gene sequence alignment. Among these strains, Serratia sp. 1-9 and Pseudomonas sp. 5-23 were the two most effective strains. Both of them produced auxin and the production increased significantly when cultured under simulated drought conditions which are inferred to be the most plausible mechanism for their plant growth-promoting effect under drought stress.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-014-0479-3) contains supplementary material, which is available to authorized users.
Keywords: Auxin, Drought stress, Plant drought-resistance promoting bacteria, Plant growth-promoting bacteria (PGPB), Populuseuphratica
Introduction
Plant growth-promoting bacteria (PGPB) exert beneficial effects on various plants and play an important role in plant growth promotion, biological control and also resistance to abiotic stress [1–3]. PGPB promote growth of plants in a variety of environments by direct and indirect mechanisms. Direct mechanisms have so far been identified and include: regulation or production of phytohormones [4], release of volatile compounds including 3-hydroxy-2-butanone (acetoin) and 2,3-butanediol that both affect plant signaling pathways [5], or enhancing plant uptake of nitrogen, phosphorous [6] and iron [7]. The indirectly beneficial effects act via suppression of deleterious microorganisms and plant pathogens mainly by production of antimicrobial metabolites and hydrolytic enzymes, competition for space and nutrients within the rhizosphere, inhibition of pathogen-produced enzymes or toxins, and triggering host induced systemic resistance [8].
Bacteria producing phytohormones such as auxins [2], cytokinins [9], gibberellins [10], and abscisic acid [11] were reported to improve the drought resistance of host plants. Several studies have identified PGPB which produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase and decrease ethylene levels of host plants as a method of conferring drought resistance [12, 13].
Populus euphratica is a dominant tree in arid area of northwest China and has strong resistance to drought and salt stresses. Due to the scarcity of wild forest, whether and how the microbes associated with P. euphratica contribute to the resistances of plants against severe environmental conditions still lack deep understanding. In this study, we cultured and isolated bacteria from the rhizosphere soil of the biggest wild P. euphratica forest located in the Tarim river basin of China. The metabolic characteristics related to PGPB including ACC deaminase activity, production of auxin, acetoin, siderophores, inorganic phosphate solubilizing trait, nitrogen fixation and arginine decarboxylase activity of these isolates were determined. The plant growth promoting and drought tolerance enhancing ability of those isolates were evaluated on wheat seedlings.
Materials and Methods
Soil Sample Collection
Soil samples from six sites were collected from the wild P. euphratica forest located in arid and semi-arid zones of the Tarim river basin (Xayar County, Aksu Prefecture, Xinjiang Uygur Autonomous Region), China. The latitude, longitude and relative place of the trees where soil samples were collected are listed in Table S2. Rhizospheric soil and non-rhizospheric soil within the forest were sampled at a depth of 30 cm by cylinder-shaped stainless steel soil sampler in June 2011. Water content and pH of the soil samples was also determined.
Isolation of the Bacterial Strains from Soil
The bacterial strains were isolated from the different soil samples on nutrient broth (NB) medium by serial dilution with sterilized distilled water H2O (three replicates for each dilution). Bacterial suspension were plated on NB plates and incubated for 48 h at 28 °C. On the basis of morphological differences, a total of 232 bacterial strains were isolated.
Screening for Metabolic Properties
ACC deaminase activity was determined for all of the isolated strains according to the protocol described by Belimov et al. [14]. The ability of bacteria to produce auxin was measured using the Salkowsky reaction [15]. Voges–Proskauer reaction was used to determine acetoin production [16]. The production of siderophores by the bacteria was determined according to the chrome azurol-S analytical method [17]. Phosphate solubilizing ability was proved by the transparent halo around colony on Pikovskaya’s agar medium containing the insoluble inorganic phosphate, Ca3(PO4)2 [18]. To test the growth in N-free media, bacteria were inoculated in Schatz medium without a nitrogen source (NH4NO3).
Inoculation of Wheat Seedlings with Bacteria and Plant Culture
The wheat seeds were surface-sterilized with 10 % HClO4 for 5 min and rinsed carefully with sterile water. Sterilizing effect and germination rate were also checked. Bacteria were grown in liquid NB medium for 24 h at 30 °C in a rotary shaker at 180 rpm, centrifuged, washed, and resuspended to 108 CFU ml−1 in sterile 10 mmol l−1 MgSO4 according to their CFU/OD600 correlation checked in advance. After the seeds were germinated on moistened filter gauze in the dark at 22 °C for 2 days, they were soaked in the bacterial suspension or sterile water (control) for 2 h, and then sown in plastic pots filled with 500 ml sand which was sterilized by autoclaving at 121 °C for 2 h. Five seeds were planted in each pot and five pots were used as replicates for each treatment.
The pots were cultivated in a growth chamber (ZRX-300D, Hangzhou Qianjiang Apparatus & Equipment CO., LTD, China) with a day/night cycle of 16/8 h at 25/22 °C and a photon flux density of 200 μmol quanta m−2 s−1. Plants were well watered for 5 days, and then not watered for 12 days. Then the roots and shoots of plants were washed in distilled water and dried at 60 °C for 72 h to obtain a dry weight.
In the initial plant experiments for 50 bacteria strains, to accelerate the screen process, the conditions for plant culture and drought treatment were the same as above while the OD600 of bacterial inoculum for inoculation was modified to 0.5 for each strain. The index of growth was counted by the following formula: Index = {Total dry weight (inoculated) − Total dry weight (uninoculated)}/Total dry weight (uninoculated). Plants were cultivated in sterile sand in the absence (control) or presence of strain inoculations. Ten seeds per pot without replicates were set for the first round of screening. Five seeds were planted in each pot and five pots were used as replicates for each treatment in the second round of plant experiment.
Strain Identification Through rrs Gene Sequencing
Template of bacterial genomic DNA for PCR reaction was roughly isolated according to the method described by Arciola et al. [19]. 100 μl of overnight culture prepared in 5 ml Tryptic Soy Broth at 37 °C with shaking was pelleted by centrifugation. Cell pellets were resuspended in 45 μl deionized water and 5 μl lysozyme solution (100 μg ml−1 in dH2O) and incubated for 10 min at 37 °C. 5 μl of proteinase K solution (100 μg ml−1 in dH2O) and 150 μl 0.1 mol l−1 Tris/HCl (pH 7.5) were added and the reaction was further incubated for 10 min at 37 °C. The products were heated for 5 min at 100 °C to inactive the enzymes and the supernatant was used as template for PCR. PCR for rrs genes was performed using the 27F-1492R primer set on thermal cycler (Techne TC-312, Techne and Techne Inc., England). The DNA amplification kit and Column DNA gel extraction kit were purchased from Sangon Biotech Co., Ltd., Shanghai, China. PCR product with correct size was purified and sequenced at Beijing Genomic Institute (Shanghai, China). Nucleotide sequence data were compared to publicly available sequences in GenBank using BLAST basic sequence alignment program to identify the bacterial strains.
Quantification of Root Surface Area
For root area quantification, the fresh roots of seedlings were photographed using a Kodak EasyShare C140 camera (Kodak China Inc., Shanghai, China). The root surface area was calculated based on the image analysis function of Adobe Photoshop CS5.
Effect of Polyethylene Glycol (PEG) Levels on Bacterial Growth and Auxin Production
To investigate the growth of bacteria under drought stress, PEG 6000 was added into the culture medium as an osmotic stress simulator [20]. Bacterial strains 1-9, 5-23 and 6-7 were cultivated at 30 °C in 1/10 strength 869 medium supplemented with 0 (set as control), 10, 20, or 30 % (w v−1) PEG [21]. The growth of bacterial strains was monitored by measuring the OD at 600 nm and compared to a control culture without PEG.
Strains 1-9 and 5-23 promote the plant growth under drought stress the best and they both produce auxin so the productions of auxin in medium supplemented with PEG were also investigated. Strains were cultured in medium supplemented with 2.0 mmol l-1 Trp and 0, 10, 20, or 30 % (w v-1) PEG and then the production of auxin were determined by the Salkowsky colorimetric method [22].
Statistical Analysis
The mean ± standard deviation for the replicates and the significance test among different treatments were analyzed based on student’s t test using the software DPS (Data Processing System) v. 7.05.
Results
Bacterial Colony Counting of Soil Samples
The basic soil properties were summarized in Table S2 along with the number of cultivatable bacterial for each sample. All of the soils were alkaline (pH > 8.0), with a low percentage of water content (2.16–7.05 %), and the number of cultivatable bacteria ranging from 5.8 × 106 to 2.2 × 108 CFU g−1. Using the data in Table S2, a highly significant association (P = 0.002, r2 = 0.929) between soil water content and total bacterial in CFU was observed by linear regression analysis. After morphological identification and purification, a total of 232 bacterial strains were isolated from the rhizospheric soils.
Determination of Metabolic Properties Related to PGPB
Among the 232 bacterial strains, 7 of them produced high ACC deaminase activities and 11 produced auxin at levels from 10.0 to 43.9 μg ml−1. The production of acetoin was detectable in 23 isolates, while only one strain was demonstrated to have the phosphate solubilizing ability. A large fraction of the isolates (approximately 74 % of total isolates) were able to produce detectable siderophores, while 18 strains produce high levels with the diameter of orange halos ranging from 6 to 22 mm. Finally, 50 strains with different PGPB related characteristics (Table S1) were selected as candidates for growth promoting studies on wheat seedlings against drought stress.
Initial Screen of PGPB by Plant Experiments
The inoculations of 50 selected strains displayed different influences on wheat seedlings growing under drought conditions (Fig. 1). Thirty-nine of them increased the dry weights of above-ground tissues (ranging from 2.2 to 64 %) compared to the control. The red circle on the bars represented the 10 strains which were selected as candidates for further experiments.
Fig. 1.
Influence of inoculation with 50 selected strains on wheat seedlings growing under drought condition compared to uninoculated control. (Color figure online)
Metabolic Characteristics and Identification of 10 Selected PGPB Strains
The most efficient 10 strains were selected based upon metabolic characteristics and plant growth-promoting performance in the initial screen, and they were further tested for their growth in N-free media. Their metabolic characteristics relating to plant growth promotion and taxonomy identified by rrs genes are summarized in Table 1. 60 % of them belong to the genera of Bacillus and Pseudomonas which are the most common PGPB according to previous reports.
Table 1.
Characteristics and identification of the selected 10 strains and their effects on the shoot dry weight of inoculated wheat plants under drought stress in fast screening
| Strain code | 16S rRNA gene identification | Sequence length (bp) | GenBank accession no. | Index of growth promotion (%)a | ACC deaminase (OD540) | Auxin (μg ml−1) | Acetoinb | Siderophore (mm)c | Growth in N-free mediad |
|---|---|---|---|---|---|---|---|---|---|
| 5-23 | Pseudomonas stutzeri (100 %) | 494 | KC832941 | 64.07 | – | 17.45 | – | – | ++ |
| 6-2 | Bacillus sp. (100 %) | 597 | KC832948 | 57.17 | – | – | +++ | – | – |
| 1-35 | Stenotrophomonas sp. (100 %) | 705 | KC832943 | 47.94 | – | – | – | + | + |
| 4-24 | Unconfirmed | – | – | 46.03 | – | 20.66 | – | – | – |
| 1-9 | Serratia sp. (100 %) | 716 | KC832940 | 37.13 | – | 10.55 | – | – | +++ |
| 6-12 | Bacillus cereus (100 %) | 693 | KC832946 | 36.33 | – | – | ++ | – | ++ |
| 6-7 | Serratia sp. (100 %) | 706 | KC832945 | 33.83 | – | – | ++ | – | – |
| 1-21 | Pseudomonas stutzeri (99 %) | 681 | KC832944 | 29.49 | – | – | + | + | + |
| 3-26 | Bacillus muralis (100 %) | 699 | KC832942 | 26.91 | – | – | – | +++ | ++ |
| 6-13 | Pseudomonas chlororaphis (100 %) | 646 | KC832939 | 10.58 | 0.604 | – | – | – | + |
aIndex of growth promotion = {Total dry weight (inoculated) − Total dry weight (uninoculated)}/Total dry weight (uninoculated). Ten seeds were used in each pot for each treatment with no replication in this fast screening step
bAcetoin production, colour reaction: +, pale red; ++, rose red; +++, dark red
cSiderophore production, the diameter of orange halos (mm): +, 4–8; ++, 8–16; +++, >16
dNitrogen fixation ability was determined by the growth of strains in N-free media for 7 days. +, OD600 of 0.1–0.5; ++, OD600 of 0.5–0.8; +++, OD600 of >0.8
Effect of Bacteria on Wheat Growth and Root Development Under Drought Stress
The 10 strains were re-tested with replicates to allow a statistical analysis of their effects on plant growth promotion and drought tolerance. Before drought treatment, the growths of inoculated and non-inoculated wheat seedlings were similar through visual observation. However, after 12 days drought treatment, the uninoculated control withered while 10 groups of inoculated wheat seedlings still presented strong vitality (Fig. 2). Correspondingly, all strains except for strain 1-35 increased the dry weight of shoots (5.7–34.4 %) significantly (P < 0.05) when compared to the uninoculated control after drought stress (Fig. 3). Among the strains of positive effects, 1-9, 5-23 and 4-24 were auxin-producing bacteria, but their effects on plant growth (34.4, 17.6, 12.73 % respectively) show inverse relation to their auxin production (10.55, 17.45, 20.66 μg ml−1 respectively). For siderophore-producing strains 1-21, 1-35, and 3-26, the siderophore production of 3-26 was the highest, and it increased the shoot dry weight the most (15.5 %).
Fig. 2.

Effects of 10 strains on the shoot growth of wheat seedlings under drought stress. a Growth appearance of seedlings before drought treatment. b Growth appearance of seedlings after drought treatment of 12 days
Fig. 3.
Shoot and root dry weight of wheat seedlings with or without inoculation of bacteria from 10 selected bacterial strains after drought treatment. a The first batch. b The second batch. Means sharing the same superscript are not significantly different (P < 0.05) from each other as determined by t test using DPS program (n = 5). (Empty bar) shoot, (dotted bar) root
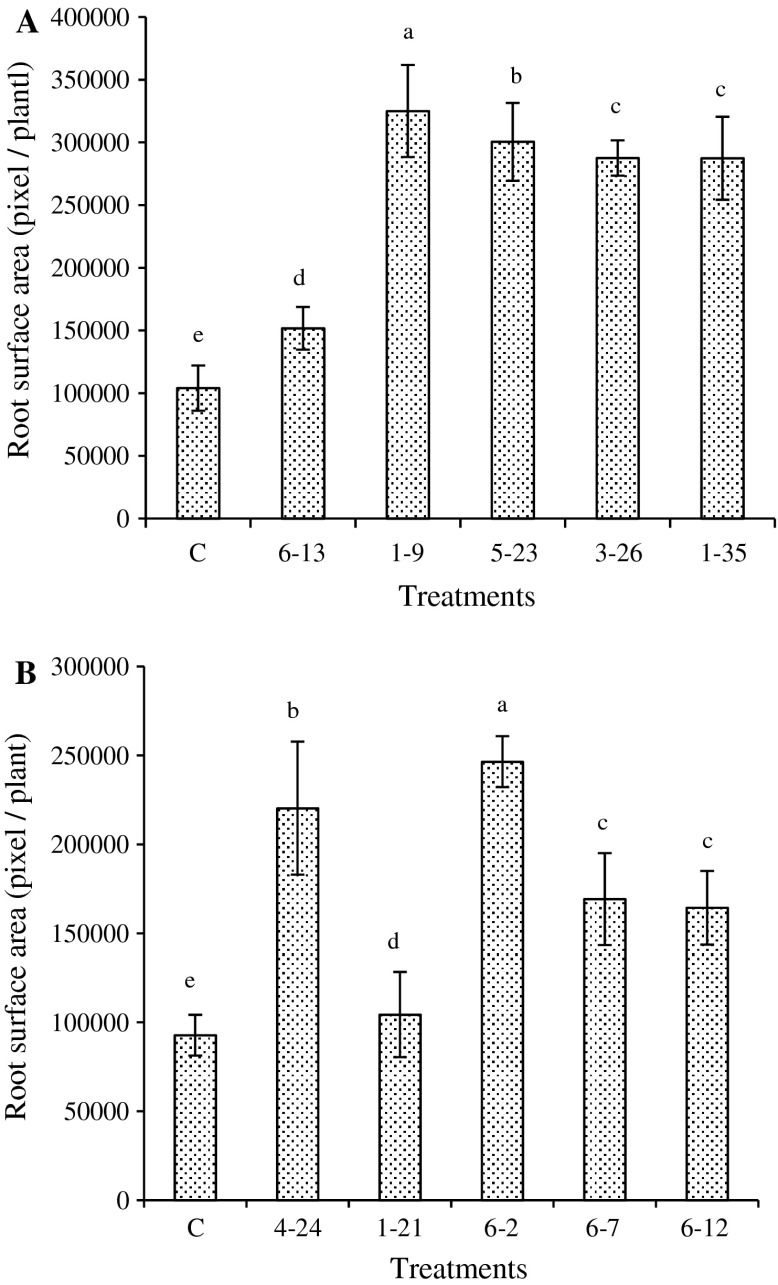
Although all of the 10 strains were able to promote root development and increase root dry weight when water supply was limited, only strains 1-9, 1-35, 6-2, 6-7, 6-12 conferred a statistically significant increase (P < 0.05) (Fig. 3). The plants from the inoculation groups did display much more vigorous root development in morphology than those from the non-inoculated control group (Figs. 4 and S2). Based on a combined comparison of the shoot/root dry weight and root surface areas, inoculations of strains 1-9, 5-23 and 6-7 were considered to increase plant biomass and root development the best (Figs. 4 and S2).
Fig. 4.
Root area of wheat seedlings with or without inoculation of bacteria from 10 selected bacterial strains after drought treatment. a The first batch. b The second batch. Means sharing the same superscript are not significantly different (P < 0.05) from each other as determined by t test using DPS program (n = 3)
Effect of PEG Levels on Bacterial Growth and Auxin Production
The three best performing strains were cultured in medium with PEG as dehydrating agent and the relation among auxin production, bacterial growth and PEG concentration were assessed. All the strains were sensitive to PEG of high concentration as the growth had been inhibited strongly (Fig. 5). Pseudomonas sp. 5-23 seemed to exhibit the highest tolerance to the osmotic stress as the growth was more vigorous than that of strain 1-9 and 6-7 in PEG of 20 and 30 % while the lag phase was longer.
Fig. 5.
Growth curve of Algoriphagus sp. 1-9, Pseudomonas stutzeri 5-23, and Serratia sp. 6-7 in 1/10 strength 869 medium supplemented with PEG (n = 3). PEG concentration: (opened square) 0, (filled square) 10 %, (filled circle) 20 % and (opened circle) 30 %
Of the three strains, only strains 1-9 and 5-23 produced auxin. Drought stress created by high PEG concentration in medium stimulated auxin production for both PGPB strains (Table 2). When PEG concentration was 30 %, auxin production reached the maximum. At different PEG concentration, auxin production of Pseudomonas sp. 5-23 was higher than that of Serratia sp. 1-9 after culture of 24 and 48 h. However the auxin productions of each strain didn’t increase obviously within the period from 24 to 48 h (Table 2). The enhanced auxin produced by these strains under drought stress conditions in vitro may occur in vivo within the colonized plant and it is likely to be the major cause of PGPB enhanced plant growth and root development during drought stress.
Table 2.
Auxin production (pg/cell) by strains 1-9 and 5-23 growing in medium with PEG (n = 3)
| % PEG | Serratia sp. 1-9 | Pseudomonas stutzeri 5-23 | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| 0 | 0.42 ± 0.07 | 0.42 ± 0.03 | ND | ND |
| 10 | 0.12 ± 0.00 | 0.93 ± 0.03 | 10.57 ± 1.27 | 11.57 ± 2.06 |
| 20 | 12.21 ± 0.86 | 19.72 ± 1.77 | 94.73 ± 20.37 | 103.35 ± 12.84 |
| 30 | 53.60 ± 2.09 | 54.31 ± 0.35 | 188.62 ± 30.12 | 215.50 ± 17.68 |
Discussion
Among the indices of bacterial metabolism related to plant growth promotion, it was found that auxin production may be the most important one for the discovery of plant drought-resistance promoting bacteria. Some reports confirmed that inoculation of indole-3-acetic acid (IAA)-producing PGPB resulted in increased root growth and/or enhanced formation of lateral roots and root hairs [23], which were also demonstrated in our study. Promotion of root growth results in a larger root surface, and therefore, has positive effect on water acquisition and nutrient uptake of plants which benefit the growth of bacteria in return. However, the effect of IAA on host plants depends on the active dose and plant susceptibility. According to our data on PGP characteristics, strains with a very high production of IAA did not show good effect on plant growth or drought resistance. Taghavi et al. surveyed the IAA productions of some PGPB strains associated with poplar and find they all produced IAA and the level varied from 2.38 to 29.39 μg ml−1. Among them the strain with the highest level of IAA production did not show significant promotion effect to the growth of poplar cuttings [24], which are consistent with our results.
So IAA production is an important index for PGPB while plant experiments still are necessary for correct evaluation. In our study, the auxin production of specific strains increased significantly in medium with PEG, while the increase may be not so dramatic when bacteria are not free-living but in symbiotic relationships with plants. In the other hand, the better development of plant root under drought stress after inoculation indicate the auxin production of bacteria should have increased but is still within the dose range beneficial for root development.
We also investigated the production of both siderophore and acetoin which are also believed to be beneficial for drought resistance for host plants [25]. We observed that acetoin-producing bacteria also had beneficial effects on plant growth and drought resistance in wheat as strains 6-2, 6-7 and 6-12 did dramatically improve plant dry weight and root area. As for the role of siderophore production, strain 3-26 producing higher level of siderophore performed excellent drought resistant properties to host plants. Siderophore producing bacteria were well known to increase the resistance of plant to pathogens [8] by providing the plants with additional iron. As for the abiotic stress, the role of siderophore production was demonstrated by the siderophore-over-producing mutant Kluyvera ascorbata SUD165/26 which was more efficient than wild type bacterium in protecting the plants against heavy metal stress [26].
Producing ACC deaminase was reported as an important mechanism for PGPB to resist stress for host plants [27], while in this study, most of the strains producing ACC deaminase didn’t play an effective role in promoting plant growth. It may be because the slow drying in this study did not promote ethylene production. Therefore, as gradual drought caused less ethylene production, the effect of ACC deaminase was less evident [28]. Nitrogen fixation was considered to be an important factor of PGPB for their effect to host plant. However, in our study, not all strains with potential nitrogen fixing capacity exhibited outstanding effects compared to other strains, which may because the early growth of wheat seedlings depends more on nutrients from seeds and the nitrogen is not a strict stress factor.
Bacillus and Pseudomonas seemed to be the two dominant PGPB genera in the rhizospheric community of P. phratica in this study. They both are the most reported PGPB genera and Bacillus sp. is commonly found in arid land as a consequence of their ability to form endospores that allow bacterial survival for extended time periods under adverse environmental conditions. At the same time, the high abundance of Pseudomonas sp. isolates in P. phratica grown in the barren and salty soil may possibly be explained by their diverse metabolisms and high tolerance to a variety of physical conditions.
In conclusion, some PGPB from wild P. phratica forest soil were found to be very helpful for wheat to resist drought stress. It is consistent with the recent reports of the dramatic effects of PGPB on the growth and drought tolerance of host plants. Timmusk et al. demonstrated the effect of bacterial priming on wheat drought stress tolerance enhancement, resulting in up to 78 % greater plant biomass and fivefold higher survivorship under severe drought [29]. Another interesting experiment inoculated pepper plants by bacterial isolates from plants cultivated under desert farming, and found the peppers developed larger root systems and exhibited a higher tolerance to water shortage, compared with untreated control [30]. Although the roles of them in sustainable growth of forest trees still lacked in-depth study, rhizospheric and endophytic microbes of trees should have contributed much for the survival of the symbiosis systems to resist various stresses especially for trees living in severe environments. And these PGPB strains have the potential applications in promoting drought resistance of host plant in agriculture and forestry.
Electronic supplementary material
Acknowledgments
This study was supported by the opening project of Xinjiang Production & Construction Crops Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin (No. BRZD1203). Great thanks to Dr. Jon Catterall for his careful suggestions on grammar and data analysis.
Contributor Information
Shanshan Wang, Email: wshanshanxyz@163.com.
Liming Ouyang, Phone: +86 21 64252257, Email: ouyanglm@ecust.edu.cn.
Xiangyang Ju, Email: juxiangyang@126.com.
Lili Zhang, Email: zhang63lyly@sina.com.
Qin Zhang, Email: jhtabszq@163.com.
Yanbin Li, Email: ydhant@163.com.
References
- 1.Carmen B, Roberto D. Soil bacteria support and protect plants against abiotic stresses. In: Shanker A, Venkateswarlu B, editors. Abiotic stress in plants-mechanisms and adaptations. Rijeka: InTech; 2011. [Google Scholar]
- 2.Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Dimkpa C, Weinand T, Asch F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 4.Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 5.Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS. Role of soil microorganisms in improving P nutrition of plants. Plant Soil. 2002;245:83–93. doi: 10.1023/A:1020663916259. [DOI] [Google Scholar]
- 7.Sharma A, Johri BN. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3 and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol Res. 2003;158:243–248. doi: 10.1078/0944-5013-00197. [DOI] [PubMed] [Google Scholar]
- 8.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arkhipova TN, Prinsen E, Veselov SU, Martinenko EV, Melentiev AI, Kudoyarova GR. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil. 2007;292:305–315. doi: 10.1007/s11104-007-9233-5. [DOI] [Google Scholar]
- 10.Joo GJ, Kim YM, Kim JT, Rhee IK, Kim JH, Lee IJ. Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. J Microbiol. 2005;43:510–515. [PubMed] [Google Scholar]
- 11.Figueiredo VB, Burity HA, Martinez CR, Chanway CP. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol. 2008;40:182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- 12.Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronoval VI, Davies WJ. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling. New Phytol. 2009;181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- 13.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004;166:525–530. doi: 10.1016/j.plantsci.2003.10.025. [DOI] [Google Scholar]
- 14.Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol Biochem. 2005;37:241–250. doi: 10.1016/j.soilbio.2004.07.033. [DOI] [Google Scholar]
- 15.Gordon SA, Weber RP. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barritt MM. The intensification of the Voges–Proskauer reaction by the addition of α-naphthol. J Pathol Bacteriol. 1936;42:44–54. doi: 10.1002/path.1700420212. [DOI] [Google Scholar]
- 17.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 18.Illmer P, Schinner F. Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem. 1992;24:389–395. doi: 10.1016/0038-0717(92)90199-8. [DOI] [Google Scholar]
- 19.Arciola CR, Collamati S, Donati E, Montanaro L. A rapid PCR method for the detection of slime-producing strains of Staphylococcus epidermidis and S. aureus in periprosthesis infections. Diagn Mol Pathol. 2001;10:130–137. doi: 10.1097/00019606-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Marulanda A, Barea JM, Azcon R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Regul. 2009;28:115–124. doi: 10.1007/s00344-009-9079-6. [DOI] [Google Scholar]
- 21.Mergeay M, Nies D, Schlegel HG, Gerits J, Charles P, Van Gijsegem F. Alcaligene seutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol. 1985;162:328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty U, Chakraborty B, Basnet M. Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J Basic Microb. 2006;46:186–195. doi: 10.1002/jobm.200510050. [DOI] [PubMed] [Google Scholar]
- 24.Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, Barac T, Vangronsveld J, van der Lelie D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol. 2009;75:748–757. doi: 10.1128/AEM.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA, Miransari M. Wheat (Triticum aestivum L.) growth enhancement by Azospirilum sp. under drought stress. World J Microbiol Biotechnol. 2011;27:197–205. doi: 10.1007/s11274-010-0444-1. [DOI] [Google Scholar]
- 26.Burd GI, Dixon DG, Glick BR. Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- 27.Sgroy V, Cassán F, Masciarelli O, Del Papa MF, Lagares A, Luna V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol. 2009;85:371–381. doi: 10.1007/s00253-009-2116-3. [DOI] [PubMed] [Google Scholar]
- 28.Morgan PW, Drew MC. Ethylene and plant responses to stress. Physiol Plant. 1997;100:620–630. doi: 10.1111/j.1399-3054.1997.tb03068.x. [DOI] [Google Scholar]
- 29.Timmusk S, Abd El-Daim IA, Copolovici L, Tanilas T, Kännaste A, Behers L, Nevo E, Gulaim S, Stenstrom E, Niinements U. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE. 2014;9:e96086. doi: 10.1371/journal.pone.0096086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marasco R, Rolli E, Vigani G, Borin S, Sorlini C, Ouzari H, Zocchi G, Daffonchio D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal Behav. 2013;8:e26741. doi: 10.4161/psb.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.