Abstract
The current study was conducted to explore the potential of a phosphate solubilizing soil bacterium, Bacillus megaterium mj1212 for enhancing the growth of mustard plants. The newly isolated bacterial strain mj1212 was identified as B. megaterium using phylogenetic analysis and, its phosphate solubilization ability was shown by the clear zone formation on National Botanical Research Institute’s Phosphate medium. Moreover, the phosphate solubilization ability of B. megaterium mj1212 was enhanced by optimal culture conditions at pH 7.0 and 35 °C which might be due to the presence of malic and quinic acid in the culture medium. The beneficial effect of B. megaterium mj1212 in mustard plants was determined by an increasing shoot length, root length and fresh weight of plants. In the biochemical analysis revealed that chlorophyll, sucrose, glucose, fructose and amino acids (Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ilu, Leu, Tyr, Phe, Lys, His, Arg and Pro) were higher in B. megaterium mj1212 treated plants, when compared to their control. The result of present study suggests that B. megaterium mj1212 treatment could be act as phosphate biofertilizer to improve the plant growth.
Keywords: Bacillus megaterium, Biochemical, Mustard, Phosphate solubilization
Introduction
Phosphorus is a component of nucleic acids, phospholipids and ATP, involves in the regulation of various metabolic pathways in plants [1]. Plants can directly absorb phosphate from soil through root transporters; it is limited at low levels of available phosphate in rhizosphere [2]. Thus, the phosphate is additionally added to soil as fertilizers, but the frequent use of chemical fertilizers affects the soil nature and living organisms, which there is a need for alternative sources insert of chemical [3]. Some of the rhizobacteria are recognized as biofertilizers and it may be a potential alternative to chemical fertilizers [4]. The application of nitrogen fixing and phosphate solubilizing microorganisms promotes the uptake of plant nutrients and reduces the need of chemical fertilizers [5]. Rhizospheric bacteria are one of the promising agents of phosphate solubilizing microorganisms and their efficiency to plant growth promotion is well documented by several researchers [6]. Phosphate solubilizing bacteria secrete acid phosphatases and phytases, which are helpful to convert an insoluble form of phosphate into a soluble form that can be possibly taken up by the roots [7]. The production of organic acid by soil microbes is one of the known mechanisms responsible for phosphate solubilization [8]. Some of the bacteria belonging to Bacillus, Burkholderia, Enterobacter, Klebsiella, Kluyvera, Streptomyces, Pantoea and Pseudomonas genera, have been identified as phosphate solubilizing bacteria and widely investigated on crop plants to improve the crop yield [4].
In our previous study, we found Acinetobacter calcoaceticus promoted the plant growth by secretion of gibberellin and phosphate solubilization [9]. The current study was focused to elucidate the effect of Bacillus megaterium in mustard plants growth. B.megaterium is a gram positive and rod shaped bacterium, and produces endospores, plant growth promoting substances and inhibitory molecules [10]. Phosphate solubilization ability of B. megaterium was reported by several researcher [11], but optimal conditions for efficient phosphate solubilization of B. megaterium is still lacking. The mustard plants are grown in tropical and sub-tropical regions of the world and their seeds are used as a spice and to extract oil. The reports of B. megaterium induced growth promotion of mustard plants and their effects on photosynthetic pigments, carbohydrate and amino acid content are not available. The objectives of this study were to investigate the culture conditions for optimizing phosphate solubilizing capacity of B. megaterium mj1212 isolated from the soil and to evaluate their role on plant growth performance by determining chlorophyll, sugars and amino acids contents in mustard plants.
Materials and Methods
Isolation, Identification and Phylogenetic Analysis of B. magaterium mj1212
The rhizospheric soil samples from lettuces were collected from 10 cm depth of upper soil surface in a greenhouse located at Geongbuk Province, Republic of Korea. About 10 g soil was transferred to 250 ml flasks containing 100 ml sterile 0.85 % saline solution. The soil suspensions were serially diluted and 0.1 ml aliquots were inoculated on tryptic soy/agar (TSA; Merck Co., Germany) medium for bacterial culture and incubated for 48 h at 30 °C. The bacterial colonies were differentiated by their morphology and pigmentation and, the re-isolated colonies were separately cultured on fresh TSA medium. For long-term preservation, bacterial isolates were stored in 50 % glycerol at −80 °C.
In this study, the plant growth promoting soil bacterium mj1212 was isolated from rhizosphere soil and was identified by molecular phylogenetic analysis carried out on mj1212 after DNA extraction and subsequent sequencing of the 16S rDNA was performed as previously described by Kang et al. [9]. The isolated bacterial strain was identified on the basis of partial 16S ribosomal DNA (rDNA) sequence. The genomic DNA of bacterial strain was isolated and almost complete 16S rDNA was amplified using the 27F primer (5′-AGAGTTTGATC(AC)TGGCTCAG-3′) and 1492R primer (5′-CGG (CT) TACCTTGTTACGACTT-3′), which were complementary to the 5′ end and 3′ end of the prokaryotic 16S rDNA, respectively. The BLAST search program (http://www.ncbi.nlm.nih.gov/BLAST/) was used to compare the nucleotide sequence homology of this bacterial isolate. The selected sequences of various bacterial strains were analyzed by BLAST, presenting the highest sequence homology proportion, query coverage, and the lowest E values. The closely related sequences were aligned by ClustalW using MEGA version 5.0 software, and the neighbor-joining tree was generated using same software. Bootstrap replication (1,000 replications) was used for a statistical support for the nodes in phylogenetic tree. The sequence of other genera (Pseudomonasputida) was also used to determine the actual relationship among participating candidates in the group.
Phosphate Solubilization and Organic Acids Production Capacity of B. megaterium mj1212
B. megaterium mj1212 was inoculated on 0.5 % National Botanical Research Institute’s Phosphate media containing plates and flasks, and incubated for 4 days at 30 °C. The bacterial isolate formed the clarification halos on plates, which indicated phosphate solubilization and it was confirmed by testing the changes of pH in broth culture medium on daily basis [12]. To determine the organic acids production in culture medium, B. megaterium mj1212 was inoculated into tricalcium phosphate medium (pH 7.0) and cultured at 35 °C, and filtreted through 0.22 μm Millipore filter and 10 μl of filtrates were injected to High Performance Liquid Chromatography (Model: Waters 600E) equipped with a Refractive Index Detector (Model: Waters 410) and RSpak KC-811 column (8.0 × 300 mm) at 40 °C. The mobile phase was 0.1 % H3PO4 in H2O at 1.0 ml/min. The malic and quinic acid were quantified by values of respective standard peaks.
Plant Growth Promoting Effect of B. megaterium mj1212
Mustard seeds (Brassica juncea L.) were purchased from the Kyoungshin Seed Company and surface sterilized with NaOCl (5 %) and washed by autoclaved distilled water. The sterilized seeds were sown in autoclaved plastic pots containing soil in a green house at 30 ± 2 ºC. The isolated bacterial strain B. megaterium mj1212 was inoculated on tryptic soy broth for bacterial culture and incubated for 48 h at 30 °C. Five milliliters of 3 days old B. megaterium mj1212 culture (108 cfu/ml) was applied to pots containing 2 weeks old mustard plants. The shoot length, root length, and fresh weight were measured and, chlorophyll (by Chlorophyll Meter, Minolta Co., Ltd, Japan), content was analysed at 14 days (before bacterial inoculation), 21 days and 27 days (after bacterial inoculation) old plants.
Carbohydrate and Amino Acid Content in Mustard Plants
The sugar content such as fructose, glucose and sucrose were detected and quantified according to the method followed by Hinesley et al. [13]. Leaf samples were homogenized with liquid nitrogen and soluble sugars were extracted with 80 % (v/v) aqueous ethanol for three times. The supernatants were combined, and the ethanol was evaporated under vacuum. The residues were dissolved in water and the filtrate (10 ml) was injected to HPLC Waters system (Millipore Corp., Waters Chromatography, Milford, MA, USA) comprising sugar-pak column (300 mm, a model 600 controller) and, the sugar signals were detected by Waters 410 refractive index detector. The de-ionized water (50 mg Ca-EDTA/1L water) was used as mobile phase and flow rate was 0.5 ml/min at 90 °C. Fructose, glucose, and sucrose were quantified on the basis of peak areas and comparison with a calibration curve obtained with the corresponding standards. The amino acid composition of mustard plants was determined with an amino acid analyzer (L-8900, Hitachi, Japan) after hydrolysis of 50 mg sample with 0.02 N HCl at 110 °C for 24 h. The Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, Lys, His, Arg and Pro content were quantified by standard peak values.
Statistical Analysis
The experiment was arranged in a randomized block design and statistical analysis was performed using sigma plot software. The mean values were compared by Duncan’s multiple range test at p ≤ 0.05.
Results and Discussion
Phosphate Solubilizing Ability of Bacterial Isolate mj1212
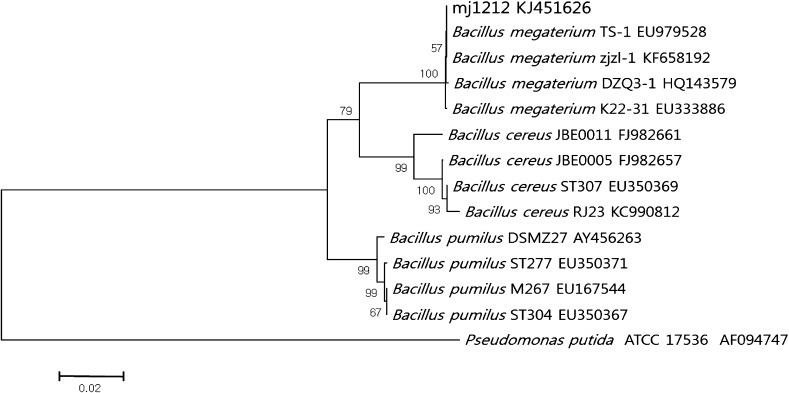
Rhizospheric soil microorganisms influence crop productivity in agricultural field by enhancing root growth and improving the nutrient availability in soil [14]. Numerous soil living rhizobacteria were identified their role on plant growth [4, 6]. In current study, the plant growth promoting bacterial isolate mj1212 sequence is accurately matched with B. megaterium (Fig. 1). The sequence of mj1212 was submitted to NCBI GenBank and was assigned Accession No. KJ451626. The result of phylogenetic analysis revealed that bacterial isolate mj1212 was identified as B. megaterium on the basis of sequence similarity.
Fig. 1.
Identification of bacterial isolate mj1212 by phylogenetic analysis. The analysis was carried out by using MEGA5 software
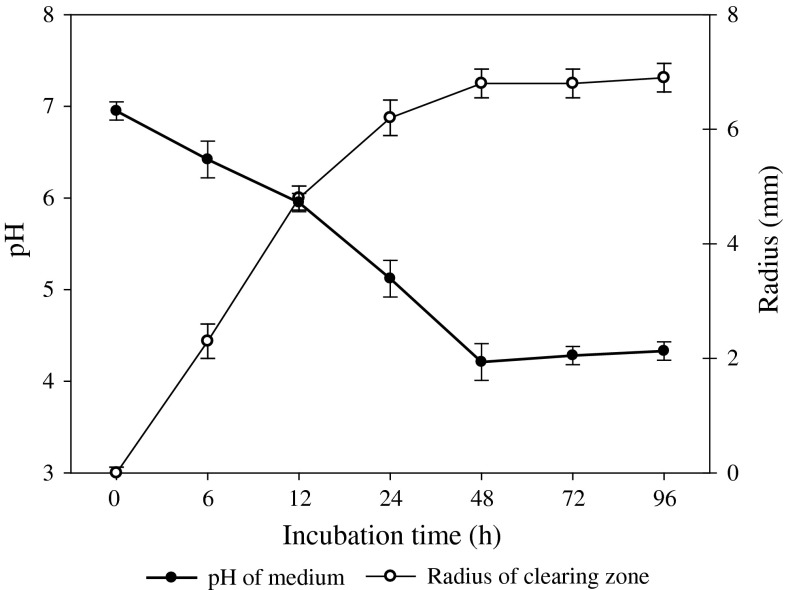
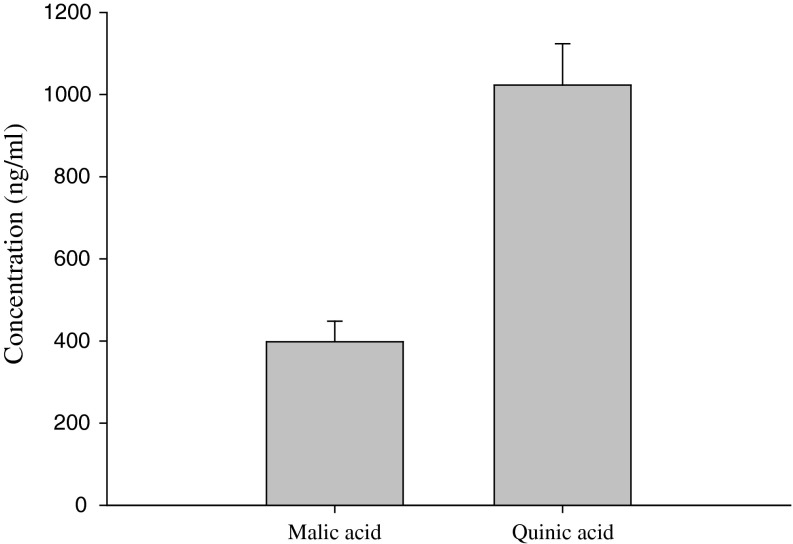
The characteristics of plant growth promoting bacteria are analysed by phosphate solubilization and secretion of plant growth promoting substances. The phosphate solubilizing ability of B. megaterium mj1212 was tested on plates containing phosphate (NBRIP) medium (Fig. 2). The bacterial medium pH was gradually declined and the clear zone (which indicated phosphate solubilization in the medium) was significantly increased by B. megaterium mj1212 during culture up to 96 days. Sandeep et al. [15] also found the several B. megaterium strains isolated from the various agroclimatic soils of Karnataka were effectively solubilized the insoluble phosphate in the culture medium. The presence of greater quantity of malic acid (~400 ng/ml) and quinic acid (~1,000 ng/ml) in the culture medium might be a supporting evidence of phosphate solubilization potential of B. megaterium mj1212 (Fig. 3). The organic acids have chelating properties [16] and additional supplementation of organic acids to soil enhances the plant phosphorus uptake [17]. Phosphate solubilizing B. megaterium mj1212 treatment could be support the plant growth promotion by their potential of production of organic acids.
Fig. 2.
Phosphate solubilization ability of Bacillus megaterium mj1212 on National Botanical Research Institute’s Phosphate (NBRIP) media plates
Fig. 3.
HPLC analysis of organic acids in culture filtrate of B. megaterium mj1212
Beneficial Effect of B. megaterium mj1212 on Plant Growth
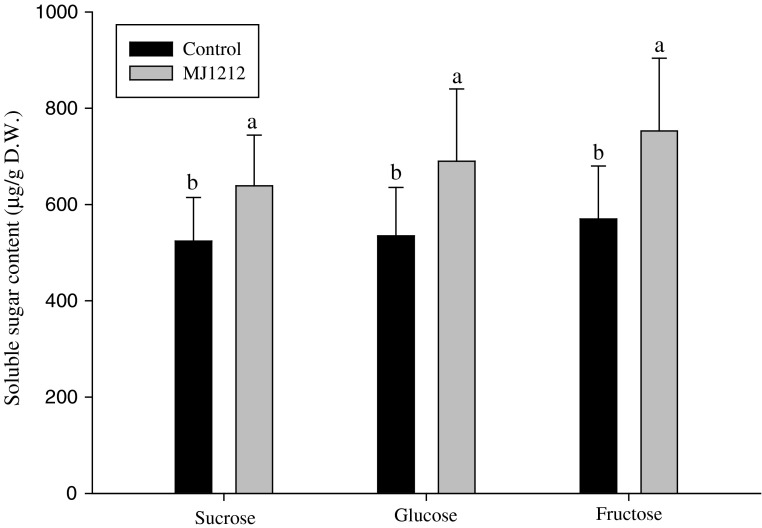
The mustard plants were grown in a greenhouse for evaluating the plant growth promoting effect of B. megaterium mj1212. The bacteria uninoculated plants showed low rate of plant growth, while B. megaterium mj1212 inoculation significantly increased the shoot length, root length and fresh weight of mustard plants (Table 1). Ortiz-Castro et al. [18] reported that the beneficial effect of B. megaterium on plant growth of Arabidopsis thaliana and Phaseolus vulgaris and showed the bacteria induced root growth enhancement. The photosynthetic pigment, chlorophyll content was also enhanced in plants treated with B. megaterium mj1212. The effect of B. megaterium on chlorophyll content was documented by Marulanda-Aguirre et al. [19], who observed an increased level of chlorophyll content in Lactuca sativa inoculated with B. megaterium. The higher level of chlorophyll content in B. megaterium treated plants when compared to their control could be indicating the positive interaction of B. megaterium. The bacterial interaction might trigger the chlorophyll related enzymes for chlorophyll synthesis and leads to increase the photosynthesis. Moreover, plant-bacterial interaction alters the carbohydrate metabolisms of plants. Sugars such as sucrose, glucose and fructose content were elevated in B. megaterium mj1212 applied plants (Fig. 4). The mono and disaccharides are major source of energy for all living organisms. The soil living bacteria help to plants in various ways including nutrient and water uptake from the soil. It activates various metabolic pathways to sucrose, glucose and fructose synthesis. In this study confirmed that the phosphate solubilizing B. megaterium mj1212 enhanced the photosynthetic pigments and subsequent process such as carbohydrate synthesis would be a reason for plant growth improvement.
Table 1.
Effect of B. megaterium mj1212 on growth parameters of mustard grown at 14, 21 and 27 days
| Treatments | Shoot length (cm/plant) | Root length (cm/plant) | Fresh weight (g/plant) | Chlorophyll (SPAD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 days | 21 days | 27 days | 14 days | 21 days | 27 days | 14 days | 21 days | 27 days | 14 days | 21 days | 27 days | |
| Control | 4.1 ± 0.5a | 6.4 ± 0.4b | 8.1 ± 0.3b | 2.8 ± 0.4a | 3.8 ± 0.4b | 7.8 ± 0.2b | 1.12 ± 0.21a | 2.00 ± 0.17b | 2.42 ± 0.317b | 27.2 ± 2.5a | 28.3 ± 1.3b | 29.5 ± 2.1b |
| mj1212 | 4.0 ± 0.3a | 8.1 ± 0.9a | 9.5 ± 0.6a | 2.9 ± 0.3a | 7.8 ± 0.3a | 9.5 ± 0.6a | 1.11 ± 0.26a | 2.53 ± 0.19a | 3.14 ± 0.24a | 27.9 ± 2.8a | 31.7 ± 1.1a | 32.7 ± 1.3a |
Fig. 4.
Influence of B. megaterium mj1212 on soluble sugar content in mustard leaves
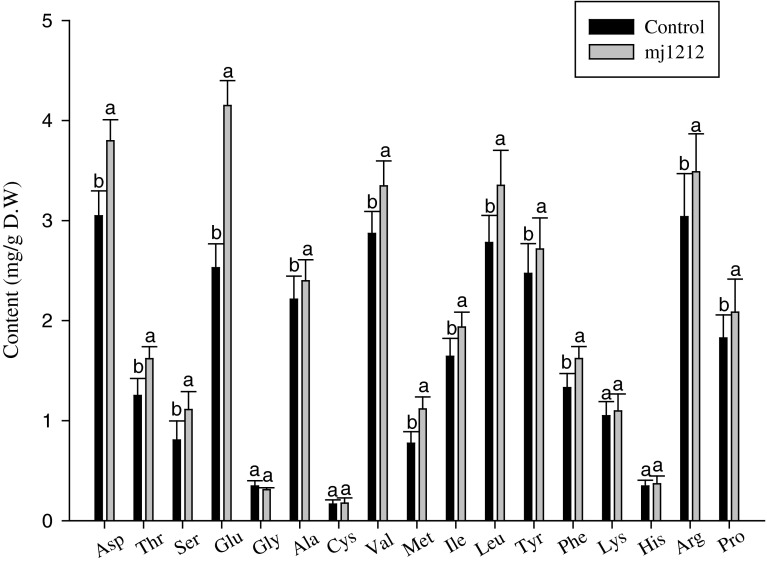
In addition, the amino acids were analyzed to determine the beneficial effects of phosphate solubilizing B. megaterium mj1212 and the results of this analysis revealed that B. megaterium mj1212 significantly influenced the amino acids by enhancing Asp, Thr, Ser, Glu, Ala, Val, Met, Ilu, Leu, Tyr, Phe, Lys, His, Arg and Pro content in mustard plants (Fig. 5). The uptake of nitrogen by plants in the form of amino acids has been determined as a widespread ecological phenomenon [20]. The microbial colonization in plants strongly influences amino acid uptake [21]. The rhizobacteria help to fix the atmospheric nitrogen in soil. The number of amino acid transporters have been identified in plants, which confirms that plants have require machinery for amino acids transport from soil into their roots [22]. However, soil bacteria induced amino acid changes were reported as scare. The B. megaterium mj1212 might produce the amino acids or fixes nitrogen to improve the mustard plant growth. In our previous experiment, we found A. calcoaceticus inoculation implicated on amino acids enhancement in cucumber plants [23]. In this study, Glu content was abundantly increased due to the B. megaterium mj1212 interaction. The plant growth beneficial bacterium B. megaterium mj1212 positively interacted with mustard plants and regulated the amino acid metabolism to promote the plant growth.
Fig. 5.
Effect of B. megaterium mj1212 application on plant amino acids
Conclusion
The results of current study have shown that B. megaterium mj1212 isolated from the rhizosphere soil have potential as a plant growth promoter to enhance the growth of mustard plant. Increase in growth was associated with organic acid production and phosphate solubilization capacity of B. megaterium mj1212. The bacterial interaction alters the biochemical pathways in plants by enhancing chlorophyll, glucose, fructose, sucrose and amino acids. The application of plant growth promoting rhizobacterium, B. megaterium mj1212 to soil could act as a bio-fertilizer for increasing plant growth and yield.
Acknowledgments
Authors wish to thank Korea Ministry of Environment which provided an Eco-Innovation Project for financial support.
Footnotes
Sang-Mo Kang and Ramalingam Radhakrishnan have contributed equally to this work.
References
- 1.Theodorou ME, Plaxton WC. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol. 1993;101:339–344. doi: 10.1104/pp.101.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidondo LF, Bompadre J, Pergola M, Silvani V, Colombo R, Bracamonte F, Godeas A. Differential interaction between two Glomus intraradices strains and a phosphate solubilizing bacterium in maize rhizosphere. Pedobiologia. 2012;55:227–232. doi: 10.1016/j.pedobi.2012.04.001. [DOI] [Google Scholar]
- 3.Reddy MS, Kumar S, Khosla B. Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger. Bioresour Technol. 2002;84:187–189. doi: 10.1016/S0960-8524(02)00040-8. [DOI] [PubMed] [Google Scholar]
- 4.Martınez-Viveros O, Jorquera M, Crowley D, Gajardo G, Mora M. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr. 2010;10:293–319. [Google Scholar]
- 5.Cakmakci R, Donmez MF, Erdogan U. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk J Agric For. 2007;31:189–199. [Google Scholar]
- 6.Richardson AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol. 2001;28:897–906. [Google Scholar]
- 7.Relwani L, Krishna P, Reddy MS. Effect of carbon and nitrogen sources on phosphate solubilization by a wild-type strain and UV-induced mutants of Aspergillus tubingensis. Curr Microbiol. 2008;57:401–406. doi: 10.1007/s00284-008-9212-y. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham JE, Kuiack C. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium billai. Appl Environ Microbiol. 1992;52:1451–1458. doi: 10.1128/aem.58.5.1451-1458.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang SM, Joo GJ, Hamayun M, Na CI, Shin DH, Kim HY, Hong JK, Lee IJ. Gibberellin production and phosphate solubilization by newly isolated strain of Acinetobacter calcoaceticus and its effect on plant growth. Biotechnol Lett. 2009;31:277–281. doi: 10.1007/s10529-008-9867-2. [DOI] [PubMed] [Google Scholar]
- 10.Gomathy M, Thangaraju M, Gunasekaran S, Gopal NO. Sporulation and regeneration efficiency of phosphobacteria (Bacillus megaterium var phosphaticum) Indian J Microbiol. 2007;47:259–262. doi: 10.1007/s12088-007-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han HS, Supanjani Lee KD. Effect of co- inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006;52:130–136. [Google Scholar]
- 12.King JE. The colorimetric determination of phosphorus. Biochem J. 1932;26:292–295. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinesley LE, Pharr DM, Snelling LK, Funderburk SR. Foliar raffinose and sucrose in four conifer species Relationship to seasonal temperature. J Am Soc Hortic Sci. 1992;117:852–855. [Google Scholar]
- 14.Tinker PB. The role of microorganisms in mediating and facilitating the uptake of plant nutrients from soil. Plant Soil. 1984;76:77–91. doi: 10.1007/BF02205569. [DOI] [Google Scholar]
- 15.Sandeep C, Thejas MS, Patra S, Gowda T, Venkat-Raman R, Radhika M, Suresh CK, Mulla SR. Growth response of ayapana on inoculation with Bacillus megaterium isolated from different soil types of various agroclimatic zones of Karnataka. J Phytol. 2011;3:13–18. [Google Scholar]
- 16.Kucey RMN, Janzen HH, Leggett ME. Microbially mediated increases in plant-available phosphorus. Adv Agron. 1989;42:199–228. doi: 10.1016/S0065-2113(08)60525-8. [DOI] [Google Scholar]
- 17.Hue NV. Effects of organic acids/anions on P sorption and phyto availability in soils with different mineralogies. Soil Sci. 1991;152:463–471. doi: 10.1097/00010694-199112000-00009. [DOI] [Google Scholar]
- 18.Ortiz-Castro R, Valencia-Cantero E, Lopez-Bucio J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav. 2008;3:263–265. doi: 10.4161/psb.3.4.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marulanda-Aguirre A, Azcon R, Ruiz-Lozano JM, Aroca R. Differential effects of a Bacillus megaterium strain on Lactuca sativa plant growth depending on the origin of the arbuscular mycorrhizal fungus coinoculated: physiologic and biochemical traits. J Plant Growth Regul. 2008;27:10–18. doi: 10.1007/s00344-007-9024-5. [DOI] [Google Scholar]
- 20.Kielland K. Amino acid absorption by arctic plants: implications for plant nutrition and nitrogen cycling. Ecology. 1994;75:2373–2383. doi: 10.2307/1940891. [DOI] [Google Scholar]
- 21.Whiteside MD, Garcia MO, Treseder KK. Amino acid uptake in arbuscular mycorrhizal plants. PLoS One. 2012;7:e47643. doi: 10.1371/journal.pone.0047643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jone DL, Healey JR, Willett VB, Farrar JF, Hodge A. Dissolved organic nitrogen uptake by plants an important N uptake pathway? Soil Biol Biochem. 2005;37:413–423. doi: 10.1016/j.soilbio.2004.08.008. [DOI] [Google Scholar]
- 23.Kang SM, Khan AL, Hamayun M, Shinwar ZK, Kim YH, Joo GJ, Lee IJ. Acinetobacter calcoaceticus ameliorated plant growth and influenced gibberellin and functional biochemical. Pak J Bot. 2012;44:365–372. [Google Scholar]