Abstract
A total of 74 morphologically distinct bacterial colonies were selected during isolation of bacteria from different parts of tomato plant (rhizoplane, phylloplane and rhizosphere) as well as nearby bulk soil. The isolates were screened for plant growth promoting (PGP) traits such as production of indole acetic acid, siderophore, chitinase and hydrogen cyanide as well as phosphate solubilization. Seven isolates viz., NR4, NR6, RP3, PP1, RS4, RP6 and NR1 that exhibited multiple PGP traits were identified, based on morphological, biochemical and 16S rRNA gene sequence analysis, as species that belonged to four genera Aeromonas, Pseudomonas,Bacillus and Enterobacter. All the seven isolates were positive for 1-aminocyclopropane-1-carboxylate deaminase. Isolate NR6 was antagonistic to Fusarium solani and Fusarium moniliforme, and both PP1 and RP6 isolates were antagonistic to F. moniliforme. Except RP6, all isolates adhered significantly to glass surface suggestive of biofilm formation. Seed bacterization of tomato, groundnut, sorghum and chickpea with the seven bacterial isolates resulted in varied growth response in laboratory assay on half strength Murashige and Skoog medium. Most of the tomato isolates positively influenced tomato growth. The growth response was either neutral or negative with groundnut, sorghum and chickpea. Overall, the results suggested that bacteria with PGP traits do not positively influence the growth of all plants, and certain PGP bacteria may exhibit host-specificity. Among the isolates that positively influenced growth of tomato (NR1, RP3, PP1, RS4 and RP6) only RS4 was isolated from tomato rhizosphere. Therefore, the best PGP bacteria can also be isolated from zones other than rhizosphere or rhizoplane of a plant.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-014-0470-z) contains supplementary material, which is available to authorized users.
Keywords: PGPR, Host specificity, Rhizosphere, Tomato, Biofilm
Introduction
Plants, like other organisms, have evolved to associate with a variety of microbes. While most of these are neutral commensals, some are relevant to plants via pathogenesis, growth-promotion or disease-resistance. The two latter benefits to plants, sometime, are provided by a group of bacteria that effectively colonize plant roots, often referred to as plant growth promoting bacteria (PGPR) [1]. Although, the molecular mediators of the interaction between the plant and PGPR are not well-characterized, it has been recurrently found that PGPR act through at least one of the direct and/or indirect mechanisms [2].
Direct mechanisms of PGPR mainly include improved acquisition of nutrients such as nitrogen and phosphorus (biofertilization) and/or production of phytohormones such as indole-3-acetic acid (IAA), gibberellins, and cytokinins (biostimulation) [2, 3]. Other factors that contribute to biostimulation are volatile compounds such as 2,3-butanediol and acetoin, or the cofactor-pyrrolquinolinequinone. PGPR solubilize phosphate from either organic- or mineral-phosphates by producing enzymes such as phosphatases, phytases and C–P lyases or by secreting organic acids such as gluconic acid [7]. Certain PGPR can code for 1-aminocyclopropane-1-carboxylate (ACC) deaminase that reduces stress-ethylene levels and contributes to plant growth.
Indirect mechanisms principally include microbial antagonism/competition and/or enhancement of induced systemic resistance (ISR) and suppress the incidence of plant-diseases [2]. Antimicrobials such as hydrogen cyanide, phenazines, lipopeptide biosurfactants and siderophores are produced by PGPR [1]. A few PGPR produce hydrolytic enzymes such as chitinases, and glucanases to degrade fungal cell walls, elicit defence responses in plants and confer resistance to subsequent infections by pathogenic-bacteria, -fungi, and -viruses [4] or form biofilms on root surfaces that may protect roots against soil-borne bacterial and fungal pathogens [5].
In view of the importance of PGPR in improving plant-growth and development or tolerating multiple biotic and abiotic stresses, deployment of PGPR will help in developing ecofriendly practices for sustainable-agriculture. Here, we focused on bacteria from different parts (rhizoplane, phylloplane and rhizosphere) of Lycopersicon esculentum (tomato) plant as well as nearby bulk soil (here after referred to as non-rhizosphere). Selected bacterial isolates were screened for plant growth promoting (PGP) and root colonization traits. Seven bacterial isolates (NR4, NR6, RP3, PP1, RS4, RP6 and NR1), that exhibited multiple PGP activities, were identified based on 16S rRNA gene sequence analyses. We have reported that the PGP bacterial strain of Paenibacillus elgii responds positively to tobacco root exudates [6] and the root exudates of tobacco altered the cell wall components of Bacillus cereus in promoting root colonization [7]. It was not clear whether the PGPR exhibit host-specificity. To verify such a possibility, the PGP activity of the selected seven bacterial isolates from tomato was compared in tomato, groundnut, sorghum and chickpea.
Materials and Methods
Seed Material
Cicer arietinum (JG11), Arachis hypogaea (JL24) and Sorghum bicolour (SPV1414) were procured from International Crops Research Institute for the Semi-Arid-Tropics (ICRISAT), Hyderabad, INDIA. L. esculentum variety Arka Vikas was obtained from Indian Institute of Horticulture Research (IIHR), Bangalore, INDIA.
Phytopathogenic Fungal Cultures
Three broad-spectrum fungal pathogens (Fusarium solani, Fusarium moniliforme and Macrophomina phaseolina) were obtained from Prof. K. Satya Prasad, Department of Botany, Osmania University, Hyderabad, India, to test the antifungal activity of the bacterial isolates.
Sample Collection
Four tomato plants were randomly selected, uprooted from different locations of a field in Hyderabad, India and transferred to a sterile container. At the same time, non-rhizospheric soil was collected separately in sterile sample containers. Samples were stored at 4 °C until used.
Isolation and Selection of Bacteria
Bacteria were isolated from rhizosphere (RS), rhizoplane (RP), phylloplane (PP), and non-rhizosphere (NR) samples. For rhizospheric bacteria, 1 g of soil closely sticking to roots was suspended in 10 ml of 0.85 % (w/v) NaCl (saline), vortexed vigorously, serially diluted and plated on Luria–Bertani (LB) agar. For isolating rhizoplane bacteria, roots were thoroughly washed, suspended in saline and the resulting suspension was plated. Similarly, for phylloplane and non-rhizospheric bacteria, 1 g of leaves or non-rhizospheric soil were suspended in saline. The liquid portion of the suspension was serially diluted and plated. The plates were incubated at 37 °C for 24 h. Colonies with dissimilar morphology were selected for further tests.
Screening of Bacterial Isolates for Plant Growth Promotion (PGP) Traits [8]
Phosphate Solubilisation
Phosphate solubilization was assessed using Pikovskaya agar plates. Briefly, 10 μl of overnight culture was spot inoculated on plates, incubated at 37 °C for 6 days and observed for the zone of clearance.
Indole-3-acetic acid (IAA) production
Bacteria were grown at 37 °C for 72 h in LB broth (+0.1 % tryptophan) and centrifuged at 6,000×g for 30 min. Two ml of the supernatant was mixed with two drops of ortho-phosphoric acid and 4 ml of the Salkowski reagent (50 ml of 35 % of perchloric acid plus 1 ml of 0.5 M FeCl3). The intensity of the colour was measured at 530 nm to measure IAA.
Siderophore Production
Siderophore production was tested using Chrome Azurol S (CAS) agar plates. Briefly, 10 μl of overnight culture was spot inoculated on CAS agar plate that was divided into equal sectors and incubated at 37 °C for 12 days. Appearance of orange halos around the colonies on the blue coloured agar indicated siderophore production.
Chitinase Production
Chitinase assay was performed as described earlier [6]. Chitin plates were prepared with M9 agar medium amended with 1 % (w/v) colloidal chitin. The plates were divided into equal sectors; spot inoculated with 10 μl of overnight grown culture and incubated at 37 °C for 24–96 h. Zone of clearance around bacterial colonies indicated chitinase production.
Hydrogen cyanide (HCN) production
HCN production was assessed using nutrient agar supplemented with 0.44 % (w/v) of glycine. The agar surface, streak-inoculated with overnight culture, was overlayed with a Whatman filter paper (no. 1) soaked in filter sterile 2 % (w/v) sodium carbonate in 0.5 % (v/v) picric acid and incubated at 30 °C for 72 h. Change in colour of the filter paper from yellow to orange, red or brown indicated lesser, moderate or higher levels of HCN production, respectively.
ACC Deaminase Assay
The ability of the bacterial isolates to utilize ACC as a sole source of nitrogen was assayed [9], with minor modifications. The bacteria grown overnight in LB broth were collected and inoculated into Dworkin and Foster (DF) minimal salts medium containing ACC as the only nitrogen source. Cultures were incubated at 30 °C and 160 rpm for 48 h. Uninoculated DF-ACC medium served as control. Culture was then centrifuged at 6,000×g for 5 min. One hundred µl of the supernatant was diluted with 1 ml of DF medium, to which 2 ml of ninhydrin reagent (500 mg of ninhydrin and 15 mg of ascorbic acid dissolved in 60 ml of ethylene glycol) was added. The tubes were agitated and placed in a boiling water bath for 30 min. After boiling, the solution turned into purple colour. The boiled sample was kept at 30 °C for 10 min before its absorbance was measured at 570 nm. The DF medium supplemented with ACC served as a blank.
Antagonistic Activity Against Phytopathogenic Fungi
Antagonistic activity of the seven bacterial isolates against three soil-borne phytopathogenic fungi viz., F. solani, F. moniliforme and M. phaseolina was determined by dual culture technique. A fungal plug of 1 cm diameter was placed at the centre of potato dextrose agar plate, overnight grown bacterial cultures were then spot inoculated at an equi-distance of 3 cm from the central plug and the plates were incubated at 28 °C for 72 h.
Biofilm Formation In Vitro
Biofilm formation was tested during bacterial growth in borosilicate glass tubes [10]. Biomass attached to the glass surface was observed by staining with crystal violet, and quantified after dissolving the dye with 70 % ethanol and measuring absorbance at 590 nm.
Identification of Bacteria Based on 16S rRNA Gene Sequencing
Bacteria were grown in LB broth at 30 °C and 160 rpm for 12 h. Genomic DNA was isolated using a standard procedure [6] and 100 ng was used as the template for amplification of 16S rRNA gene in a thermocycler (Eppendorf Mastercycler Gradient, Germany) using universal primers: forward primer 27F (5′-GTTTGATCCTGGCTCAG-3′) and reverse primer 1489R (5′-TACCTTGTTACGACTTCA-3′). The PCR mixture contained 0.1 mM of each primer, 1X of PCR-buffer with 1.5 mM of MgCl2 (Sigma-Aldrich, USA), 10 mM of each dNTPs (Fermentas, USA), and 2 U of Taq DNA polymerase (Sigma- Aldrich, USA). The PCR products were analyzed on 1 % agarose gel and eluted for sequencing at Scigenome Pvt Ltd., Kerala, India. The resulting nucleotide sequences were searched for similar bacterial sequences using nucleotide–nucleotide basic local alignment search tool (BLASTn) provided by National Centre for Biotechnology Information (NCBI) database. Based on the highest degree of similarity, identity for bacterial isolates was assigned. GenBank accession numbers were also obtained by sequence deposition at NCBI.
Tomato root colonization
Tomato seeds were surface-sterilized using 2.4 % (v/v) sodium hypochlorite for 2–3 min, followed by 5–6 washes with sterile double distilled water and air-dried. Efficacy of surface sterilization protocol was tested by placing the surface sterilized seeds on LB agar and the plate was incubated at 37 °C for 24 h. The sterile seeds were soaked in bacterial suspension (108 CFUs/ml) for 45 min. Sterile LB broth served as a control. Then, the seeds were air dried, placed on ½ strength Murashige & Skoog (MS) medium and grown at 25 ± 2 °C with 12 h of light per day.
Scanning Electron Microscopy (SEM)
After 15 days of growth, roots were harvested, excised, suspended in 2 % glutaraldehyde and incubated for 30 min at room temperature (pre-fixation). Subsequently, roots were transferred to fresh 2 % glutaraldehyde and incubated overnight at 4 °C (post-fixation). The roots were washed with phosphate buffered saline (PBS). After washing, roots were transferred to 1 % osmium tetraoxide and incubated for 2 h at 4 °C. Then, roots were washed twice with PBS and gradually dehydrated using ethanol series (20, 30, 50, 70, 90, and 100 %) at 30 °C with 10 min gap between each wash-step. Finally, roots were kept overnight in 100 % ethanol at 4 °C. The samples were dried using a critical point drier. Then, the samples were mounted on brass stubs and sputter-coated with gold and viewed under SEM (Philips XL series, SEMTech solutions, North Billerica, US) to assess root colonization by bacteria.
Growth Responses of Tomato, Chickpea, Groundnut and Sorghum
The effect of seven bacterial isolates on growth of tomato (Arka Vikas), chickpea (JG11), groundnut (JL24) and sorghum (SPV1414) was assessed. Overnight grown cultures were concentrated by centrifugation at 6,000×g for 10 min, diluted in 0.05 M MgSO4 to a density of 108 CFUs/ml, and used for bacterization of surface sterilized seeds. The seeds were grown on ½ strength MS agar at 25 ± 2 ˚C with 12 h of light for 15 days. At the end of incubation, for each plant, root-length, shoot-height and total dry weight were measured.
Statistical Analyses
Appropriate data were analyzed for significant mean differences via either one-way or two-way ANOVA using JMP® statistical software (SAS Institute, Cary, NC, USA). Whenever required, multiple mean comparisons were performed using Tukey’s Honestly Significant Difference (Tukey’s HSD) post hoc tests. Statistical significance was determined at the critical α-level of 0.05.
Results
Isolation, selection and identification of bacteria
Bacteria were isolated from tomato-rhizoplane (23), -rhizosphere (21), -phylloplane (11) and from non-rhizosphere bulk soil (19), using culture-dependent standard plate method. Based on differential colony morphologies and biochemical tests (data not shown), a total of 74 bacterial isolates were selected. Of the 74 isolates, seven isolates that exhibited multiple plant growth promoting (PGP) activities (described below) were selected and tentatively identified using 16S rRNA gene sequencing to species level in majority of cases (Table 1) that belonged to four genera viz., Aeromonas, Pseudomonas, Bacillus, and Enterobacter.
Table 1.
Bacterial isolates and their identity based on 16S rRNA gene sequences
| Isolate | Source | NCBI strain | Similarity (%) | Taxonomic-class | GenBank accession no. |
|---|---|---|---|---|---|
| NR4 | Non rhizospheric soil | Aeromonas enteropelogenes | 99.85 | γ-proteobacteria | KF880833.1 |
| NR6 | Non rhizospheric soil | Pseudomonas aeruginosa | 99.87 | γ-proteobacteria | KF895389.1 |
| RP3 | Rhizoplane | Bacillus aerius | 100 | Bacilli | KF895392.1 |
| PP1 | Phylloplane | Enterobacter hormaechei | 99.89 | γ-proteobacteria | KF895390.1 |
| RP6 | Rhizoplane | Enterobacter cancerogenus | 99.71 | γ-proteobacteria | KF895391.1 |
| RS4 | Rhizosphere | Bacillus sonorensis | 99.41 | Bacilli | KF895394.1 |
| NR1 | Non rhizospheric soil | Bacillus sonorensis | 95.26 | Bacilli | KF895393.1 |
PGP traits of bacterial isolates
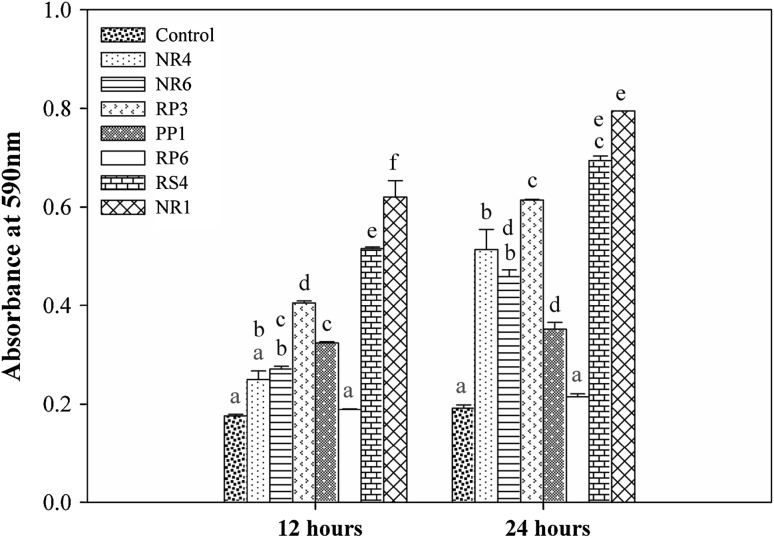
About 40, 49, 54, 24, 21 % of isolates were positive for phosphate solubilisation and production of IAA, siderophore, chitinase and HCN, respectively (data not shown). Seven isolates viz., NR4, NR6, RP3, PP1, RP6, RS4, and NR1, exhibited multiple PGP activities. However, the activities varied with the trait and the isolate (Table 2). Phosphate solubilisation was observed with all the isolates. While, NR6, RP3, PP1, and RS4 exhibited highest phosphate solubilisation, NR4 had lowest. IAA and siderophore were produced by all the seven isolates. HCN was produced by NR6 only. Chitinase was produced by NR4, RS4, and NR1. The seven isolates were screened for their ability to produce ACC deaminase and antagonize phytopathogenic fungi like F. solani, F. moniliforme and M. phaseolina (Table 2). All the seven isolates utilized ACC as a source of nitrogen suggesting that these isolates were positive for ACC deminase. None of the seven isolates were antagonistic to M. phaseolina. NR6 inhibited the growth of both F. solani and F. moniliforme. To a lesser extent, PP1 and RP6 were antagonistic to F. moniliforme. All isolates, except RP6, formed biofilm on glass surfaces (Fig. 1) although variably. After 24 h, except RP6, all isolates adhered significantly to the glass surface.
Table 2.
Plant growth promoting (PGP) traits of selected bacterial isolates
| PGP Trait | Bacterial isolate | ||||||
|---|---|---|---|---|---|---|---|
| NR4 | NR6 | RP3 | PP1 | RP6 | RS4 | NR1 | |
| Phosphate solubilisation | + | +++ | +++ | +++ | ++ | +++ | ++ |
| Production of | |||||||
| Indole 3-acetic acid (IAA) | ++ | ++ | ++ | +++ | +++ | ++ | ++ |
| Siderophore | ++ | +++ | ++ | ++ | ++ | + | + |
| Chitinase | + | − | − | − | − | +++ | +++ |
| Hydrogen cyanide (HCN) | − | + | − | − | − | − | − |
| 1-aminocyclopropane-1-carboxylate (ACC) deaminase | +++ | + | + | + | + | ++ | ++ |
| Antagonism in dual culture against | |||||||
| F. solani | − | +++ | − | − | − | − | − |
| F. moniliforme | − | +++ | − | + | + | − | − |
| M. phaseolina | − | − | − | − | − | − | − |
+, positive; −, negative result for the test. For phosphate solubilisation, siderophore production and chitinase production: +, zone of clearance <0.2 mm; ++, zone of clearance 0.2–0.4 mm; +++, >0.4 mm. For IAA production: +, absorbance <0.1; ++, absorbance between 0.1 and 0.3; ++, absorbance 0.3. For ACC deaminase production: +++ good; ++ medium; + slight. For antifungal assay: +, zone of inhibition <0.2 mm; ++, zone of inhibition between 0.2 and 0.4 mm; +++, zone of inhibition >0.4 mm
Fig. 1.
Biofilm formation by the bacterial isolates. Adherence to glass surface was used as a proxy for bacterial ability to form biofilm and it was quantified based on absorbance at 590 nm after staining the attached bacteria with crystal violet and re-dissolving the stain with ethanol. All values are means (n = 3) and vertical lines are ±1 standard error of the mean. The two groups (12 and 24 h) were analyzed separately using one-way ANOVA followed by Tukey’s HSD test for multiple mean comparisons, α = 0.05. Levels not connected by same letter are significantly different
Biofilm Formation and Tomato Root Colonization by PGP isolates
All isolates, except RP6, showed statistically significant adherence to glass surface (Fig. 1). After 24 h as compared to that after 12 h, higher amount of adherence by all isolates was observed. Consistent with the in vitro glass attachment assay, more numbers of NR1 and RS4 isolates were found to colonize tomato root surface (Fig. 2).
Fig. 2.
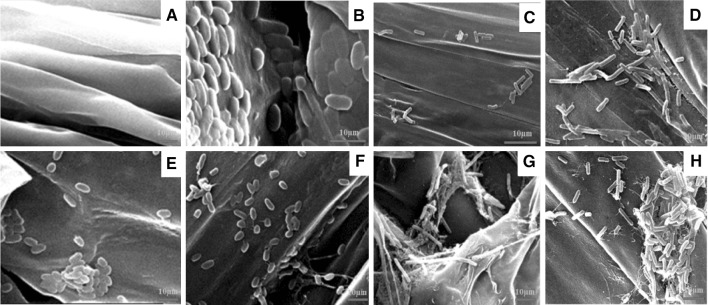
Scanning electron micrographs of bacterial isolates on tomato root surface: a control, b NR4, c NR6, d RP3, e PP1, f RP6, g RS4, and h NR1
Growth responses of tomato, groundnut, sorghum and chickpea to the selected bacterial isolates
Effect of NR4, NR6, RP3, PP1, RP6, RS4, or NR1 isolates on the growth of four different crop plants viz., tomato, groundnut, sorghum, and chickpea was assessed in terms of root length, shoot height, and dry weight of seedlings in response to seed bacterization (Fig. 3).
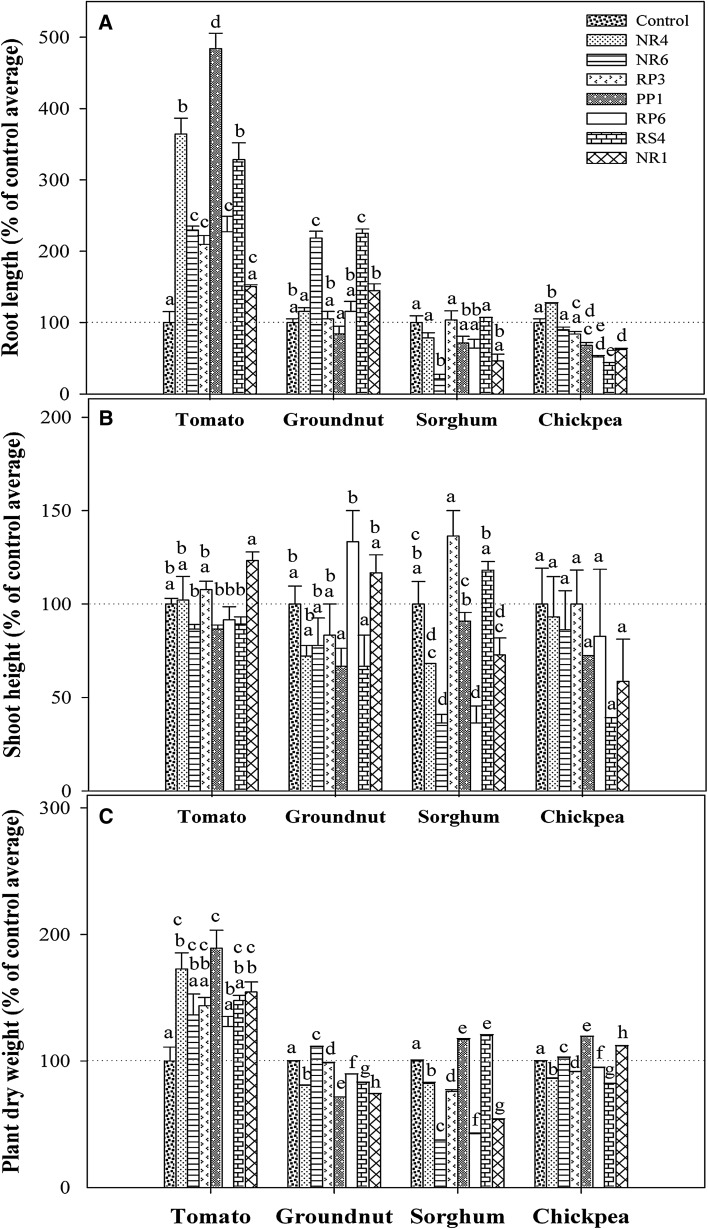
Fig. 3.
Influence of seven bacterial isolates on four crop plant responses: the root length (a), shoot height (b), and dry weight (c). The corresponding values were different for each plant; to compare the effects of a variety of isolates on different plants, actual values were converted to % of control values. Reported values are means (n = 3) and vertical lines are ±1 standard error of the mean. Using % of control values, two- way ANOVA along with Tukey’s HSD post hoc tests was performed for each of the growth response i.e., root length; shoot height; or dry weight using plant-type and isolate-type as two factors. In addition, the interaction between plant-type and isolate-type was tested. Subsequently, the group of bars i.e., the response of each plant treated with or without the isolates was separately analyzed using one-way ANOVA followed by Tukey’s HSD test for multiple mean comparisons, α = 0.05. Levels not connected by same letter are significantly different
The selected plants, with respect to root-length, responded differently to the seed-inoculated bacterial isolates (F = 506; p < 0.0001) (Fig. 3a). Tomato and groundnut exhibited different root-length response when compared to each other as well as to sorghum and chickpea. The response of sorghum and chickpea to seed bacterization was not significantly different. Interaction between plant-type and isolate-type was significant with root length response (F = 43; p < 0.0001) suggesting that different plants responded differently to different isolates. To reveal these differences, the response of each plant treated with or without the isolates was separately analyzed using one-way ANOVA followed by Tukey’s HSD post hoc tests. All the seven isolates, except NR1, as compared to the untreated-control, have significantly increased tomato root length. NR6 and RS4 have increased the root length of groundnut and rest of the isolates did not show this response. All of the isolates, except NR4 in case of chickpea, have failed to improve the length of roots of both sorghum and chickpea. Interestingly, isolates such as NR6 in case of sorghum, and PP1, RP6, RS4, NR1 with chickpea have significantly reduced the root length as compared to their respective controls.
A differential shoot-height plant-response to bacterial isolates (F = 3.5; p = 0.02) was observed (Fig. 3b). The response of tomato differed significantly from that of chickpea; while tomato response was similar to groundnut and sorghum, chickpea response was similar to groundnut and sorghum. The interaction between plant and isolate was significant with plant’s shoot height response (F = 4; p < 0.0001) suggesting that differences among plant-types varied among isolates. Subsequent one-way ANOVA test revealed that the shoot length of all plants was not significantly enhanced by the seven isolates. Except the significant negative effect of NR6 and RP6 on sorghum shoot height, shoot height of all plants was not affected by any of the isolate.
Dry weight response to seed bacterization varied with the plant (F = 242; p < 0.0001). Further, the dry weight of four plants in response to seed bacterization was statistically different from each other. The interaction between plant and isolate was significant with plant’s dry weight response (F = 19; p < 0.0001) suggesting that differences among plant-types varied among isolates (Fig. 3c). To reveal these differences, the response of each plant treated with or without the isolates was separately analyzed using one-way ANOVA followed by Tukey’s HSD post hoc tests. Three isolates NR4, PP1, and NR1, as compared to untreated-control have significantly enhanced dry weight of tomato plant. Similarly, slight but significant increase in dry weight of plant was observed in case of groundnut with NR4, sorghum with PP1 or RS4, and chickpea with NR4, PP1, or NR1. While, none of the isolates decreased the dry weight of tomato, NR4, RP3 and RP6 significantly reduced the dry weight of groundnut, sorghum, and chickpea. Similarly, significant reduction in dry weight was observed in case of groundnut with PP1, groundnut and chickpea with RS4, sorghum with NR6, and groundnut and sorghum with NR1.
Discussion
Isolation and characterization of bacteria that positively influence plant growth, development, and immunity facilitate the development of strategies for ecosystem friendly- and sustainable-agriculture [11, 12]. In the present study, a total of 74 different bacterial isolates from tomato plant were characterized for PGP activity, besides testing the host-specificity, if any, for the selected strains to promote growth. Bacteria were isolated from different parts of plant-rhizoplane, -rhizosphere, -phylloplane and from the bulk soil, to exploit their putative plant growth promotion activities [13, 14]. PGP microbes were mainly soil- and rhizosphere-dwelling communities, able to colonize plant roots in significant numbers and influence plant growth in a positive manner.
The selected potential strains showed PGP activities like phosphate solubilization; production of IAA, siderophore, chitinase and ACC-deaminase, besides inhibiting in vitro growth of fungal pathogens. All the seven selected strains solubilized phosphate in Pikovskaya agar plates. Solubilization of phosphate by rhizobacteria has been established as a possible mechanism for plant growth improvement [2]. The ACC deaminase producing bacteria promote plant growth by ameliorating plant growth inhibition encountered due to ethylene production [15]. Three out of eight tested ACC-deaminase producing Pseudomonas enhanced soybean growth [16]. On the other hand, the siderophores may directly stimulate the biosynthesis of other antimicrobial compounds by enhancing the bioavailabilty of these minerals to the bacteria [17]. In the present study, seven isolates viz., NR4, NR6, RP3, PP1, RP6, RS4, and NR1, exhibited multiple PGP activities including phosphate solubilization, production of indole 3-acetic acid (IAA), siderophore, chitinase and hydrogen cyanide (HCN). Rhizospheric bacteria from different crops belonging to genera Azotobacter, Pseudomonas and Bacillus produced IAA, HCN, siderophore and solubilized phosphate [8]. In addition, these bacteria exhibited antagonism against a variety of fungi including Aspergillus, Fusarium and Rhizoctonia species. Isolation of efficient PGPR strains was also achieved with multiple activities belonging to genera- Bacillus, Azotobacter, Pseudomonas and Rhizobium from different rhizospheric soil of chickpea [17]. These rhizobacterial isolates showed positive PGP characteristics including IAA, ammonia and siderophore production. Similarly studies were also conducted on ACC-deaminase and IAA-producing growth promoting bacteria from rhizospheric soil of tropical rice plants and reported six individual PGPR isolates to have a considerable impact on plant growth [18].
The production of chitinase by NR4, RS4 and NR1 and HCN by NR6 besides siderophore indicates the potential of the selected isolates as possible biocontrol agents. In vitro tests showed that NR6 was antifungal against F. solani and F. moniliforme. Extracellular chitinolytic enzymes produced by PGPR play a significant role in biocontrol of phytopathogenic fungi by lysing chitin which is a major constituent of fungal cell wall [19]. Antagonism against plant pathogens was due to production of siderophores, chitinase, antifungal antibiotics and HCN [1].
Rhizoplane or rhizosphere colonization by bacteria was essential to establish fruitful interactions between microbes and plants. Among the seven test strains, NR1 and RS4 were found to extensively colonize tomato root surface, while isolate RS4 produced extracellular matrix that enabled bacterial attachment to root surface. Effective root colonization by PGPR plays an important role in growth promotion. PGPR embedded in a matrix composed of exopolysaccharides, proteins, and sometimes DNA [20] efficiently colonize roots [21]. Root colonization in Arabidopsis thaliana by Paenibacilus polymyxa and Bacillus subtilis occurs in the form of a biofilm and confers biocontrol PGP activity [22]. Bacterial strain/plant genotype affinities and specificity in colonization patterns [23] were reported. Colonization of Bacillus amyloliquefaciens SQR9 (cucumber rhizosphere) and Bacillus subtilis N11 (banana rhizosphere) of their original host proved to be more effective as compared to the colonization of the non-host plant [24]. In the present study, the selected PGP bacterial strains showed varied ability to colonize the roots. The strains also showed variability in the capacity to form biofilm on glass surface. Although all the strains showed promising PGP traits like IAA production, phosphate solubilization, siderophore production, etc., these bacteria were unable to improve growth of plants other than tomato like groundnut, sorghum and chickpea. Compatibility of introduced PGPR with the host root exudates, therefore, could be critical for survival and establishment of the PGPR.
The phytostimulating rhizobacteria effectively enhance growth of a wide variety of host plants including crops and legumes through a combination of plant growth and plant health-improving mechanisms [25]. In the present study, tomato and groundnut were found to exhibit different root-length response when compared to each other as well as to sorghum and chickpea. PGP effects of bacteria depend on host plant genotypes [26, 27]. Thus, there exists an incongruity in establishing host specificity in case of PGP bacteria because the outcome of plant-PGP bacterial interaction depends on quantification and statistical analyses of plant growth parameters [28]. Individual PGPR strains may be crop specific, cultivar specific or non-specific for root colonization. Thus, with root colonization, individual PGPR strains exhibit host specificity for efficacy of growth promotion and biological control.
In present work, all the seven isolates, except NR1, significantly increased tomato root length. Growth promotion was less in non-host plants (groundnut, sorghum and chick pea). A discrepancy was observed in case of shoot-height plant response to bacterial isolates. The shoot length of all plants was not significantly enhanced by the seven isolates. Dry weight of bacterized seedlings also varied significantly. Remarkable enhancement in dry weight of host plant tomato was observed in comparison with non-host plants. The antifungal activity was dependent on the sugar and organic acid composition of root exudates [29]. Therefore, available nutrients also affect the ability of an introduced PGPR to colonize roots and perform their activity [30]. Genes of B. cereus were regulated by tomato seed exudates [31] suggesting requirement of host factors for growth of the bacteria. More recently, alteration of cell wall proteins of B. cereus was reported in response to tobacco root exudates but not to groundnut root exudates [7]. B. cereus sufficiently colonized tobacco roots so as to exert growth improvement but it failed to show positive effect on the growth of groundnut where the bacterial count on root surface decreased rapidly. The inefficiency of potential PGPR strains to significantly improve growth of sorghum, groundnut and chickpea may be due to difference in root exudate profile of these plants which render incompatibility of the bacterial strains to sufficiently colonize the roots.
The growth responses of test plants viz., tomato, groundnut, sorghum and chickpea to rhizobacterial treatments varied significantly with the plant-type and isolate-type. Majority of tomato isolates positively affected tomato plant growth and the response was either neutral or negative with rest of the test plants indicating that host specificity exists to certain extent, dependant largely on the test bacteria. Overall, our results suggest that it is not necessary that bacteria with PGP traits would always positively influence the growth of all plants but certain PGP bacteria may exhibit host-specificity. We need to select one or two isolates and perform more detailed analysis of the molecules involved in tomato-PGPR interaction to draw firm conclusions. Thus, a successful PGPR-plant genotype interaction/association would rely on a set of adaptation mechanisms which need to be fulfilled by both the test bacteria and the treated host plant to elicit maximum growth promotion effects for maintaining plant health and vigour.
Conclusions
The growth responses of tomato, groundnut, sorghum and chickpea to the treatment with PGP bacteria isolated from tomato varied with the plant-type and isolate-type. Majority of tomato isolates positively affected tomato plant growth, and the response was either neutral or negative with rest of the plants. Bacteria with PGP traits did not positively influence the growth of all plants, and certain PGP bacteria may exhibit host-specificity. Our study identified a few PGP bacteria that could be specific and non-specific to certain plants and this warrants further investigation. Bacterial strains isolated from tomato phylloplane, rhizoplane, or non-rhizosphere also had similar effect on tomato like the rhizosphere isolate.
Electronic supplementary material
Acknowledgments
We thank the International Atomic Energy Agency (IAEA) for a research grant to ARP. SD, VRVNR, SK, SR and VPR thank Department of Biotechnology (DBT), Government of India (GoI) for a post doctoral or research fellowship. We also thank the DBT-CREBB, DST-FIST and UGC-CAS programmes that provided infrastructure for this work. We thank Dr. Anil Singh for inputs in this work.
References
- 1.Dutta S, Podile AR. Plant growth promoting rhizobacteria (PGPR): the bugs to debug the root zone. Crit Rev Microbiol. 2010;36:232–244. doi: 10.3109/10408411003766806. [DOI] [PubMed] [Google Scholar]
- 2.Podile AR, Kishore GK. Plant-associated bacteria. In: Gnanamanickam SS, editor. Plant growth promoting rhizobacteria. Amsterdam: Springer; 2006. pp. 195–230. [Google Scholar]
- 3.Sashidhar B, Podile AR. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol. 2010;109:1–12. doi: 10.1111/j.1365-2672.2009.04654.x. [DOI] [PubMed] [Google Scholar]
- 4.Manjula K, Podile AR. Chitin-supplemented formulations improve biocontrol and plant growth promoting efficiency of Bacillus subtilis AF 1. Can J Microbiol. 2001;47:618–625. doi: 10.1139/cjm-47-7-618. [DOI] [PubMed] [Google Scholar]
- 5.O’ Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 6.Das SN, Dutta S, Anil K, Neeraja Ch, Sarma PVSRN, Srinivas V, Podile AR. Plant growth promoting chitinolytic Paenibacillus elgii responds positively to tobacco root exudates. J Plant Growth Regul. 2010;29:409–418. doi: 10.1007/s00344-010-9152-1. [DOI] [Google Scholar]
- 7.Dutta S, Rani TS, Podile AR. Root exudate-induced alterations in Bacillus cereus cell wall contribute to root colonization and plant growth promotion. PLoS One. 2013;8(10):e78369. doi: 10.1371/journal.pone.0078369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad F, Ahmad I, Khan MS. Screening of free living rhizospheric bacteria for their multiple plant growth promoting activities. Microbial Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Chang S, Lin L, Li Y, An Q. A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett Appl Microbiol. 2011;53:178–185. doi: 10.1111/j.1472-765X.2011.03088.x. [DOI] [PubMed] [Google Scholar]
- 10.Yousef CF, Travieso ML, Espinosa UM. Different, overlapping mechanisms for colonization of abiotic and plant surfaces by Pseudomonas putida. FEMS Microbiol Lett. 2008;288:118–124. doi: 10.1111/j.1574-6968.2008.01339.x. [DOI] [PubMed] [Google Scholar]
- 11.Indira Devi S, Talukdar NC, Sharma NC, Jeyaram K, Rohinikumar M. Screening of rhizobacteria for their plant growth promotion ability and antagonism against damping off and root rot diseases of broad bean (Vicia faba L.) Indian J Microbiol. 2011;51:14–21. doi: 10.1007/s12088-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vyas P, Rahi P, Chadha BS, Gulati A. Statistical optimization of medium components for mass production of plant growth-promoting microbial inoculant Pseudomonas trivialis BIHB 745 (MTCC5336) Indian J Microbiol. 2014;54(2):239–241. doi: 10.1007/s12088-013-0425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Kaur M. Antimicrobial activities of rhizobacterial strains of Pseudomonas and Bacillus strains isolated from rhizosphere soil of carnation (Dianthus caryophyllus cv. Sunrise) Indian J Microbiol. 2010;50:229–232. doi: 10.1007/s12088-010-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaki AA, Chaouche NK, Dehimat L, Milet A, Youcef-Ali M, Ongena M, Thonart P. Biocontrol and plant growth promotion characterization of Bacillus species isolated from Calendula officinalis rhizosphere. Indian J Microbiol. 2013;53:447–452. doi: 10.1007/s12088-013-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci. 2004;166:525–530. doi: 10.1016/j.plantsci.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA. Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrobials. 2011;3:34–40. [Google Scholar]
- 17.Joseph B, Patra RR, Lawrence R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.) Int J Plant Prod. 2007;1:141–152. [Google Scholar]
- 18.Bal HB, Nayak L, Das S, Adhya TK. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil. 2013;366:93–105. doi: 10.1007/s11104-012-1402-5. [DOI] [Google Scholar]
- 19.Podile AR, Prakash AP. Lysis and biological control of Aspergillus niger by Bacillus subtilis AF 1. Can J Microbiol. 1996;42:533–538. doi: 10.1139/m96-072. [DOI] [PubMed] [Google Scholar]
- 20.Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA. 2013;110:E1621–E1630. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Combes-Meynet E, Pothier JF, Moenne-Loccoz Y, Prigent-Combaret C. The Pseudomonas secondary metabolite 2,4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol Plant Microbe Interact. 2011;24:271–284. doi: 10.1094/MPMI-07-10-0148. [DOI] [PubMed] [Google Scholar]
- 22.Rudrappa T, Quinn WJ, Stanley-Wall NR, Bais HP. A degradation product of the salicylic acid pathway triggers oxidative stress resulting in down-regulation of Bacillus subtilis biofilm formation on Arabidopsis thaliana roots. Planta. 2007;226:283–297. doi: 10.1007/s00425-007-0480-8. [DOI] [PubMed] [Google Scholar]
- 23.Fan B, Carvalhais LC, Becker A, Fedoseyenko D, von Wiren N, Borriss R. Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates. BMC Microbiol. 2012;12:116. doi: 10.1186/1471-2180-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Wang X, Wang S. Addition of external organic carbon and native soil organic carbon decomposition: a meta-analysis. PLoS One. 2013;8:e54779. doi: 10.1371/journal.pone.0054779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bashan Y, De-Bashan LE. How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron. 2010;108:77–136. doi: 10.1016/S0065-2113(10)08002-8. [DOI] [Google Scholar]
- 26.Moutia JFY, Saumtally S, Spaepen S, Vanderleyden J. Plant growth promotion by Azospirillum sp. in sugarcane is influenced by genotype and drought stress. Plant Soil. 2010;337:233–242. doi: 10.1007/s11104-010-0519-7. [DOI] [Google Scholar]
- 27.Pedraza RO, Motok J, Salazar SM, Ragout AL, Mentel MI, Tortora ML, Guerrero Molina MF, Winik BC, Díaz Ricci JC. Growth-promotion of strawberry plants inoculated with Azospirillum brasilense. World J Microbiol Biotechnol. 2010;26:265–272. doi: 10.1007/s11274-009-0169-1. [DOI] [Google Scholar]
- 28.Drogue B, Dore H, Borland S, Wisniewski-Dye F, Prigent-Combaret C. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res Microbiol. 2012;163:500–510. doi: 10.1016/j.resmic.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Kravchenko LV, Azarova TS, Leonova-Erko EI. Root exudates of tomato plants and their effect on the growth and antifungal activity of Pseudomonas strains. Microbiology. 2003;72:37–41. doi: 10.1023/A:1022269821379. [DOI] [PubMed] [Google Scholar]
- 30.Miethling R, Wieland G, Backhaus H, Tebbe CC. Variation of microbial rhizosphere communities in response to crop species, soil origin, and inoculation with Sinorhizobium meliloti L33. Microb Ecol. 2000;40:43–56. doi: 10.1007/s002480000021. [DOI] [PubMed] [Google Scholar]
- 31.Dunn AK, Klimowicz AK, Handelsman J. Use of a promoter trap to identify Bacillus cereus genes regulated by tomato seed exudate and a rhizosphere resident, Pseudomonas aureofaciens. Appl Environ Microbiol. 2003;69:1197–1205. doi: 10.1128/AEM.69.2.1197-1205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.