Abstract
Bacillus subtilis XF-1 has been used as a biocontrol agent of clubroot disease of crucifers infected by Plasmodiophora brassicae, an obligate pathogen. In order to maximize the growth inhibition of the pathogen, random mutagenesis using N-methyl-N′-nitro-N-nitrosoguanidine was applied to strain XF-1. The efficacy of 226 selected mutants was assessed against the growth of an indicator fungal pathogen: Fusarium solani using agar plate assay and the disruptive effects on the resting spores of P. brassicae. Four mutants exhibited inhibition activity significantly higher than the wild type. The cell extracts of these mutants and the XF-1 were subjected to matrix-assisted laser desorption ionization-time of flight mass spectra analysis, and three families of cyclic lipopeptides (CLPs) fengycin, surfactin and iturin were identified from the parental strain and the screened mutants. However, the relative contents and compound diversity changed after mutagenesis, and there was slight variation in the surfactin and fengycin. Notably, only 5 iturin components were discovered from the wild strain XF-1, but 13 were obtained from the mutant strains, and the relative CLPs contents of all mutant strains increased substantially. The results suggested that CLPs might be one of main biocontrol mechanisms of the clubroot disease by XF-1. The 4 mutants are far more effective than the parental strain, and they would be promising biocontrol candidates not only against P. brassicae but probably other plant diseases caused by fungi.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-014-0471-y) contains supplementary material, which is available to authorized users.
Keywords: Iturin, Fengycin, Surfactin, Cyclic lipopeptides, MALDI-TOF–MS
Bacillus subtilis XF-1 (XF-1), is a patented strain for controlling the clubroot disease of crucifers infected by Plasmodiophora brassicae, an obligate plant pathogen [1–3]. Isolated from the Chinese cabbage rhizosphere, XF-1 produced a diversity of cyclic lipopeptides (CLPs): fengycins, surfactins and iturins which proved to be antagonistic against a broad spectrum of bacterial and fungal phytopathogens. These CLPs, usually synthesized by nonribosomal peptide synthetases (NRPSs) [4], are also known as biocontrol agents for plant disease reduction [5]. In addition, they are also involved in the biofilm formation, colonization and cell motility of Bacillus and Pseudomonas [6], as well as in the systemic stimulation of immune system of the host plant [7]. Thus, XF-1 has great potentials in the environmental and phytopathogen control, but the application has been hampered by the low activity of the wild strain resulting in low yield of CLPs. Therefore, it is important to enhance the activity of the strain, while increasing CLPs production. Random mutagenesis by either physical or chemical means has been known as a useful tool for the improvement of biocontrol agents and/or antifungal metabolite producers [6].
In the present survey, we induced random chemical mutagenesis in XF-1 using N-methyl-N′-nitro-N-nitrosoguanidine (NTG) [8] in order to improve its antagonistic activity against P. brassicae. The efficacy of the mutants was screened based on their inhibition effects on the growth of a facultative indicator plant-pathogenic fungus: Fusarium solani on agar plates isolated from the rhizosphere of Panax notoginseng in Yunnan Province of China [1], and the disruptive effects on the resting spores of P. brassicae, extracted from the Chinese cabbage club root rot [3]. Subsequently, the CLPs were evaluated with Matrix-assisted laser desorption ionization-time of flight mass spectra analysis (MALDI-TOF-MS) [9] by comparing the parental strain with the screened mutants.
From 226 fungal colonies that showed decrease in growth diameters, 4 mutants with high activity were selected for further evaluation and they demonstrated significant differences (p < 0.01) when compared to the wild type (Fig. 1). The resting spores of P. brassicae were sub-spherical to spherical with well-defined spore wall in the control without B. subtilis [10]. However, after treated with the parental strain XF-1, and especially the mutants XF-1A, XF-1C, XF-1D, XF-1E, the resting spores became deformed or ruptured (Fig S1) [3] .
Fig. 1.
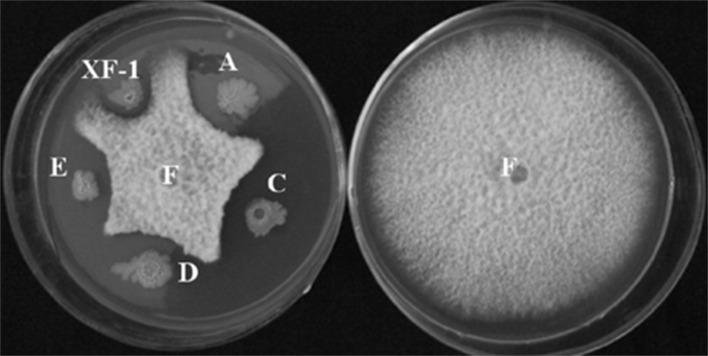
Antifungal activity of Bacillus subtilis strains against Fusarium solani (F) (wild type: XF-1; mutants XF-1C, XF-1D, XF-1E represented respectfully by A, C, D and E)
Mass spectra obtained from all strains showed very clear peak clusters (Figs.S2~6). Three families of CLPs: fengycin, surfactin and iturin could be observed in the mass spectra and all of CLPs detected are listed in Table 1. The diversity of the surfactin and fengycin families was very similar, but iturin family was very different: Only 5 iturin components were discovered from the wild strain XF-1, but 13 were obtained from the mutant strains. The relative content of three families varied, especially for the iturin family which increased by 3–10 times more than the original strain (Table 1), suggesting that the chemical mutagenesis could enhance the production of itutins, and greatly enriched the iturin constitution diversity.
Table 1.
CLPs from the parental strain and the screened mutants after chemical mutagenesis
| Mass peak (m/z) | Calcd MW | Molecular ions | Relative content (%) | ||||
|---|---|---|---|---|---|---|---|
| XF-1 | XF-1A | XF-1C | XF-1D | XF-1E | |||
| Surfactin (C12–C17) [11] | |||||||
| 1,032.578 | 993.636 | [M + K]+ | 0 | 0.85 | 0.34 | 0 | 0.4 |
| 1,030.668/1,046.608 | 1,007.652 | [M + Na]+/[M + K]+ | 0.73 | 4.39 | 2.68 | 2.58 | 2.36 |
| 1,044.646/1,060.626 | 1,021.668 | [M + Na]+/[M + K]+ | 6.53 | 11.04 | 8.44 | 8.09 | 4.79 |
| 1,058.67/1,074.643 | 1,035.683 | [M + Na]+/[M + K]+ | 11.3 | 9.84 | 8.29 | 8.57 | 3.67 |
| 1,088.656 | 1,049.699 | [M + K]+ | 1.09 | 0.85 | 0.93 | 0.9 | 0.21 |
| 1,102.652 | 1,063.714 | [M + K]+ | 0.21 | 0 | 0 | 0 | 0 |
| Relative content surfactins | 19.86 | 26.97 | 20.68 | 20.14 | 11.43 | ||
| Iturin A (C9–C15) [12] | |||||||
| 1,054.559 | 1,015.497 | [M + K]+ | 0 | 0 | 0.09 | 0.08 | 0 |
| 1,067.503 | 1,028.529 | [M + K]+ | 0 | 0 | 0.14 | 0.13 | 0 |
| 1,043.562/1,065.517/1,081.499 | 1,042.545 | [M + H]+/[M + Na]+/[M + K]+ | 0 | 10.91 | 5.58 | 5.34 | 2.02 |
| 1,057.579/1,079.549/1,095.517 | 1,056.560 | [M + H]+/[M + Na]+/[M + K]+ | 0 | 18.52 | 10.71 | 10.26 | 4.5 |
| 1,109.537 | 1,070.589 | [M + K]+ | 0 | 5.65 | 3.86 | 3.7 | 1.36 |
| 1,123.558 | 1,084.592 | [M + K]+ | 0 | 0.75 | 0.56 | 0.54 | 0 |
| 1,121.595 | 1,098.607 | [M + Na]+ | 0 | 0 | 0.27 | 0.26 | 0 |
| Iturin C (C16–C18) (With a double bond) [13] | |||||||
| 1,084.578 | 1,083.560 | [M + H]+ | 0 | 0.86 | 0 | 0 | 0.21 |
| 1,098.578 | 1,097.576 | [M + H]+ | 0.66 | 0 | 0.81 | 0.77 | 0.53 |
| 1,112.597 | 1,111.591 | [M + H]+ | 1.03 | 1.21 | 1.11 | 1.06 | 0.38 |
| Iturin C (15, 17–19) [4] | |||||||
| 1,096.631 | 1,057.544 | [M + K]+ | 0.23 | 0 | 0 | 0 | 0 |
| 1,138.574 | 1,099.591 | [M + K]+ | 0 | 0 | 0.12 | 0.12 | 0 |
| 1,136.549 | 1,113.607 | [M + Na]+ | 0.4 | 1.11 | 0.61 | 0.58 | 0.54 |
| 1,150.556 | 1,127.623 | [M + Na]+ | 0.7 | 0.78 | 0.34 | 0.32 | 0.32 |
| Relative content iturins | 3.02 | 39.79 | 24.2 | 23.16 | 9.86 | ||
| Fengycin A (C14–C19) or B (C12–C17) or C (C13–C18) or D (C13–C18) or S (C13–C18) [1, 3] | |||||||
| 1,435.135/1,473.711 | 1,434.765 | [M + H]+/[M + K]+ | 0.68 | 1.14 | 0.76 | 0.74 | 2.83 |
| 1,449.758/1,471.738/1,487.727 | 1,448.780 | [M + H]+/[M + Na]+/[M + K]+ | 0.6 | 2.79 | 2.84 | 2.72 | 9.48 |
| 1,463.774/1,485.757/1,501.742 | 1,462.796 | [M + H]+/[M + Na]+/[M + K]+ | 4.33 | 5.91 | 7.89 | 7.56 | 18.8 |
| 1,477.791/1,499.799/1,515.754 | 1,476.812 | [M + H]+/[M + Na]+/[M + K]+ | 5.92 | 3.85 | 3.89 | 3.64 | 8.5 |
| 1,491.801/1,513.812/1,529.77 | 1,490.827 | [M + H]+/[M + Na]+/[M + K]+ | 5.72 | 1.38 | 1.74 | 1.67 | 3.48 |
| 1,505.842/1,527.813/1,543.802 | 1,504.843 | [M + H]+/[M + Na]+/[M + K]+ | 6.02 | 0.64 | 0.59 | 0.57 | 0.78 |
| Relative content fengyicns | 23.27 | 15.71 | 17.71 | 16.9 | 43.87 | ||
The four mutant strains were obtained after random mutagenesis with NTG and the relative contents and compound diversity of CLPs (fengycin, surfactin and iturin) were enhanced. Since they showed greater inhibition effect on the growth of F. solaini and caused more resting spores of P. brassicae to become deformed and ruptured, they should be much better candidates than the parental strain as the biocontrol agent. It is of interest to note that 13 iturin components were discovered from the mutant strains in contrast to only 5 from the wild strain XF-1, suggesting that NTG mutagenesis could enhance the production of itutin, and greatly enriched the iturin constitution diversity, leading to the possible development of high yield iturin antibiotic strains of B. subtilis. The iturin family, encompassing iturin A and C, bacillomycin D, F, L and LC, and mycosubtilin are heptapeptide molecules with a β-amino fatty acid chain, comprised of 14–17 carbons and exhibit strong antifungal activity against a wide range of yeast and fungi [5], by forming small vesicles and by aggregating membrane-spanning particles to disrupt the plasma membrane. The iturin family compounds not only act as antibiotics, but also play an important role in the swarming/mobility behavior of production strain [13].
Electronic supplementary material
Acknowledgments
This research was supported by the Ministry of Agriculture of China, for Special Fund for the Agro-scientific Research in the Public Interest (2010029030).
Footnotes
Xing-Yu Li and Jing-Jing Yang contributed equally to this work.
Contributor Information
Xing-Yu Li, Email: lixingyushd@aliyun.com.
Yue-Qiu He, Phone: 86-871-6522-3233, Email: ynfh2007@163.com.
References
- 1.Li XY, Mao ZC, Wang YH, Wu YX, He YQ, Long CL. ESI LC-MS and MS/MS characterization of antifungal cyclic lipopeptides produced by Bacillus subtilis XF-1. J Mol Microbiol Biotechnol. 2012;22:83–93. doi: 10.1159/000338530. [DOI] [PubMed] [Google Scholar]
- 2.Guo SY, Mao ZC, Wu YX, Hao K, He PF, He YQ. Genome sequencing of Bacillus subtilis strain XF-1 with high efficiency in the suppression of Plasmodiophora brassicae. Genome Announc. 2013;1:e00066-13. doi: 10.1128/genomeA.00066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XY, Mao ZC, Wang YH, Wu YX, He YQ, Long CL. Diversity and active mechanism of fengycin-type cyclopeptides from Bacillus subtilis XF-1 against Plasmodiophora brassicae. J Microbiol Biotechnol. 2013;23:313–321. doi: 10.4014/jmb.1208.08065. [DOI] [PubMed] [Google Scholar]
- 4.Roongsawang N, Washio K, Morikawa M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int J Mol Sci. 2010;12:141–172. doi: 10.3390/ijms12010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Afsharmanesh H, Ahmadzadeh M, Majdabadi A, Motamedi F, Behboudi K, Javan-Nikkhah M. Enhancement of biosurfactants and biofilm production after gamma irradiation-induced mutagenesis of Bacillus subtilis UTB1, a biocontrol agent of Aspergillus flavus. Arch Phytopathol Plant Protect. 2013;46:1874–1884. doi: 10.1080/03235408.2013.780374. [DOI] [Google Scholar]
- 7.Desoignies N, Schramme F, Ongena M, Legrève A. Systemic resistance induced by Bacillus lipopeptides in Beta vulgaris reduces infection by the rhizomania disease vector Polymyxa betae. Mol Plant Pathol. 2013;14:416–421. doi: 10.1111/mpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HG, Luo W, Gu QY, Wang Q, Hu WJ, Yu XB. Acetone, butanol, and ethanol production from cane molasses using Clostridium beijerinckii mutant obtained by combined low-energy ion beam implantation and N-methyl-N-nitro-N-nitrosoguanidine induction. Bioresource Technol. 2013;137:254–260. doi: 10.1016/j.biortech.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 9.Athukorala SNP, Fernando WGD, Rashid KY. Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can J Microbiol. 2009;55:1021–1032. doi: 10.1139/W09-067. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SF, Strelkov SE, Feng J, Gossen BD, Howard RJ. Plasmodiophora brassicae: a review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol Plant Pathol. 2012;13:105–113. doi: 10.1111/j.1364-3703.2011.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathak KV, Keharia H. Characterization of fungal antagonistic bacilli isolated from aerial roots of banyan (Ficus benghalensis) using intact-cell MALDI-TOF mass spectrometry (ICMS) J Appl Microbiol. 2013;114:1300–1310. doi: 10.1111/jam.12161. [DOI] [PubMed] [Google Scholar]
- 12.Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microbial Cell Fact. 2009;8:63. doi: 10.1186/1475-2859-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falardeau J, Wise C, Novitsky L, Avis T. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol. 2013;39:869–878. doi: 10.1007/s10886-013-0319-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


