Abstract
CTLA-4 is a co-receptor that plays a pivotal role in regulating the threshold for T-cell activation. We recently reported that CTLA-4 ligation can over-ride the stop-signal induced by anti-CD3 ligation [Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop-signal by CTLA-4. Science 2006;313:1972]. While these studies compared CTLA-4 positive and negative T-cells from normal mice, little is known regarding the behaviour of T-cells from diseased Ctla4 deficient mice with auto-proliferative disease. In this study, we show that while activated wild-type and Ctla-4−/− T-cells have similar rates of motility, Ctla-4−/− T-cells show a marked resistance to the induction of a stop-signal by anti-CD3 ligation. By contrast, T-cells from normal mice and CD28 deficient mice underwent a normal slowing of motility in response to anti-CD3 ligation. Our findings identify a fundamental difference between normal versus CTLA-4−/− T-cells from diseased mice in the regulation of motility by anti-CD3 ligation. This dysregulation of motility may contribute to the tissue infiltration and the autoimmune disorder observed in Ctla-4−/− mice.
Keywords: CTLA-4, T-cells, TcR, Stop-signal
CD28 and CTLA-4 play central roles in determining the outcome of T-cell activation [1–3]. While CD28 can generate positive signals needed for T-cell proliferation, CTLA-4 expression and ligation impairs the response [1–3]. In this manner, CTLA-4 has been linked to the onset of several autoimmune disorders such as type 1 diabetes [4], and plays a central role in anergy induction [5]. Lentiviral induced CTLA-4 knock-down mice show a more rapid onset of diabetes [6]. Several mechanisms have been proposed to account for the molecular mechanism by which CTLA-4 generates inhibitory signals. These include ectodomain competition for CD28 binding to CD80 and CD86 [7], disruption of CD28 localization at the immunological synapse [8], modulation of phosphatases PP2A and SHP-2 [9,10] and interference with lipid raft expression [11]. CTLA-4 engagement of CD80 and CD86 on dendritic cells can also induce the release of indoleamine 2,3-dioxygenase (IDO) [12]. Recently, we demonstrated that anti-CTLA-4 increases integrin adhesion and induces the rapid polarization of T-cells [13,14]. CTLA-4 can also reverse the anti-TcR induced stop-signal needed for stable T-cell/APC conjugation [15]. A limitation on the interaction time between T-cell and APC would reduce the number of TcR ligation events and raise the threshold needed for a production T-cell response.
Two types of CTLA-4 negative cells can be studied, one population that is present in the normal peripheral compartment, and another that is derived from diseased CTLA-4 deficient (Ctla-4−/−) mice. Ctla-4−/− mice show a lympho-proliferative disorder with increased numbers of activated T-cells and autoimmune diseases with organ destruction [16,17]. Our previous study on the reversal of the TcR induced stop-signal was conducted using a combination of cell lines and primary T-cells from healthy, normal mice [15]. To date, the nature of motility in T-cells from diseased Ctla-4−/− deficient mice has not been examined. A question is whether Ctla-4−/− T-cells show any abnormalities in the regulation of motility by anti-CD3. In this study, we show that Ctla-4−/− T-cells fail to undergo the normal stop-signal in response to TcR ligation. This de-coupling of the TcR from the regulation of motility in Ctla4−/− T-cells was not observed in sorted CTLA-4 negative T-cells from normal mice or T-cells from CD28 deficient animals. This dysregulation of motility may contribute to the massive tissue infiltration and autoimmune disorder observed in Ctla-4−/− mice.
1. Results and discussion
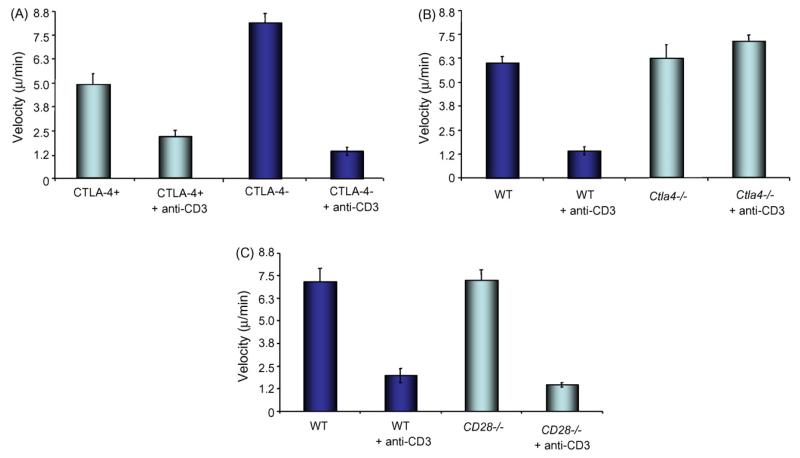
Previous studies have shown that TcR ligation causes a reduction in motility (i.e. stop-signal) needed for stable T-cell conjugate formation and proliferation [18–20]. To assess whether anti-CD3 can affect the motility of Ctla4−/− T-cells, CD4+ cells were isolated with L3T4-coated magnetic Dyna beads, and pre-activated with 3 μg/ml anti-CD3 (2C11) and 3 μg/ml anti-CD28 (PV1) for 3 days. 50,000 cells were then added to 3 μg/ml ICAM-1 coated glass-bottomed chamber wells in the presence or absence of 20 μg/ml anti-CD3 antibody. Cells were monitored using a Nikon Diaphot 300 microscope at 37 °C and photographed at 10 s intervals for 20 min. Individual cells were tracked using AQM Advance Image Analysis software, and their velocities calculated over the period of the experiment. As previously reported in mixed populations of T-cells[18–20], anti-CD3 can also slow the migration of sorted CTLA-4+ and CTLA-4− T-cells (alternately designated CTLA-4low or CTLA-4high) from normal mice (Fig. 1A). Cells were sorted using anti-CTLA-4 coated magnetic Dyna beads as previously described [15]. In this case, while CTLA-4 negative cells generally moved more quickly than CTLA-4 positive cells, as reported [15], anti-CD3 slowed the motility of both subsets. By contrast, anti-CD3 failed to induce a slowing of Ctla4−/− cells (i.e. KO) (Fig. 1B). Ctla-4−/− cells generally moved at the same or higher speeds than wild-type cells. The latter were the same age as the sorted CTLA-4+ and – T-cells (3–4 weeks old). The observation was made in three individual experiments and over the entire period of incubation (i.e. 20 min). The lack of an effect was also not due to the non-specific loss of a co-receptor since anti-CD3 induced normal slowing of motility in CD28−/− T-cells (Fig. 1C). The degree of inhibition of motility of WT versus CD28−/− cells was similar.
Fig. 1.
Panel A: anti-CD3 induces a stop-signal in sorted CTLA-4− and + primary T-cells. T-cells from normal mice were separated by sorting with anti-CTLA-4 beads as described [15], expanded using anti-CD3/CD28 stimulation, rested and stimulated with anti-CD3 and assessed for motility on plates coated with ICAM-1. Histogram shows motility of cells (μm/min). Panel B: anti-CD3 fails to induce a stop-signal for motility in T-cells from Ctla-4−/− mice. T-cells from CTLA-4 deficient mice expanded and exposed to anti-CD3 as described above and assessed for motility on plates coated with ICAM-1. Histogram shows motility of cells (μm/min). Panel C: anti-CD3 induces a stop-signal in T-cells from CD28−/− mice. Pre-activated primary CD4+ T-cells from CD28−/− mice were assessed for motility as described above. Error bars represent standard deviations (S.D.). p values were <0.05 and considered significant.
Overall, our findings demonstrate for the first time that Ctla4−/− T-cells from diseased animals differ from normal activated T-cells in that the TcR is de-coupled from its normal ability to induce a stop-signal for motility. Whether this altered behaviour of cells contributes to the induction of the autoimmune disease or is the consequence of the onset of autoimmune disease remains to be determined. The earlier observation of longer binding of CTLA-4 negative cells to APCs could allow reactivity to self-antigen [15]. Indeed, in our previous analysis, a subset of CTLA-4 negative cells remained bound to APCs for the full duration of the assay (i.e. hours). In the case of Ctla4−/− mice, this could lead to expansion of an hyper-activated subset of cells that become resistant with further TcR regulation. In turn, this could lead to chronic cytokine production and a cascade of inflammation and dys-regulated motility. Alternatively, the hyper-activation of cells via other mechanisms may create an environment where Ctla4−/− cells become hyper-activated and develop a resistance to TcR regulation. It will be interesting to assess the effect of certain cytokines and chemokines on the regulatory connection between the TcR and motility.
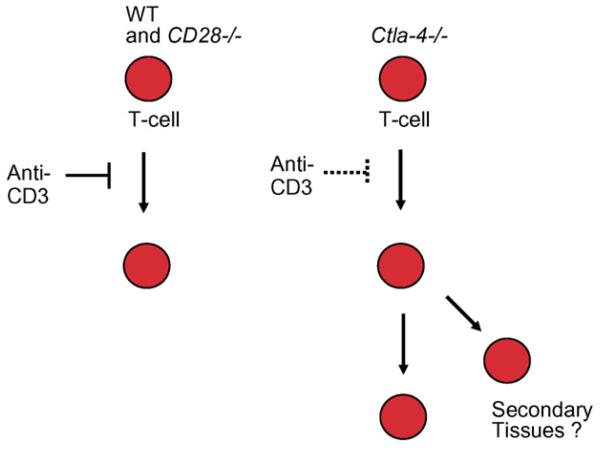
Ctla4−/− mice seem to have developed a mechanism by which T-cells function similar to normal CTLA-4 expressing T-cells under conditions of co-receptor ligation [15]. The major difference in the two systems is that CTLA-4 ligation occurs in a restricted environment with CD80/86 bearing presenting cells. By contrast, Ctla4−/− T-cells appear free to migrate in the absence of these normal regulatory mechanisms. In this manner, our finding may explain or aggravate the massive tissue infiltration that has been observed in CTLA-4 deficient mice. The inability to regulate motility may result in increased migration of T-cells to various tissues resulting in delocalized inflammatory responses and autoimmunity (see model Fig. 2). While T-cells need to be activated to express certain homing receptors for entry to tissue sites, the possible initial activation of T-cells prior to the development of a refractory state could induce sufficient receptor expression. Altered chemokine production and/or chemokine receptor expression may cooperate with TcR dysregulated cells to promote tissue infiltration. The disrupted CD3 stop-signal in Ctla-4−/− cells may increase the severity of the disease pathology in the Ctla-4−/− animals. Lastly, although Ctla4− T-cells occasionally exhibited slightly higher motility, this was not a consistent finding. It is therefore unlikely that the slight difference in basal motility of CTLA-4 positive and negative cells can in itself account for the difference in response to anti-CD3. Further studies will be needed to ascertain the cellular and molecular basis for the decoupling of the antigen-receptor from the regulation of motility in Ctla4−/− T-cells.
Fig. 2.
Model depicting the dysregulation of T-cell motility in CTLA-4−/− T-cells. T-cells from CTLA-4 deficient mice have escaped the regulatory effect of TcR ligation on T-cell motility. As a result, Ctla-4−/− T-cells are likely to exhibit more extensive migration patterns that may contribute to the massive tissue infiltration observed in Ctla-4−/− mice.
References
- [1].Alegre ML, Fallarino F. Mechanisms of CTLA-4-Ig in tolerance induction. Curr Pharm Des. 2006;12:149–60. doi: 10.2174/138161206775193046. [DOI] [PubMed] [Google Scholar]
- [2].Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- [3].Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA-4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–56. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- [4].Ueda H, Howson J, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA-4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- [5].Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- [6].Chen Z, Stockton J, Mathis D, Benoist C. Modeling CTLA4-linked autoimmunity with RNA interference in mice. Proc Natl Acad Sci USA. 2006;103:16400–5. doi: 10.1073/pnas.0607854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Masteller EM, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–27. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- [8].Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–13. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [9].Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr MG, Vander Heiden JP, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–22. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- [10].Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- [11].Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 potently inhibits cell surface raft expression in its regulation of T cell function. J Exp Med. 2001;194:1675–81. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- [13].Schneider H, Valk E, da Rocha Dias S, Wei B, Rudd CE. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for co-receptor function. Proc Natl Acad Sci USA. 2005;102:12861–6. doi: 10.1073/pnas.0505802102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wei B, da Rocha Dias S, Wang H, Rudd CE. CTL-associated antigen-4 ligation induces rapid T cell polarization that depends on phosphatidylinositol 3-kinase, Vav-1, Cdc42, and myosin light chain kinase. J Immunol. 2007;179:400–8. doi: 10.4049/jimmunol.179.1.400. [DOI] [PubMed] [Google Scholar]
- [15].Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- [16].Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- [17].Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- [18].Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- [19].Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–42. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- [20].Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, et al. In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–23. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]