Abstract
The co-receptor CD28 binds to several intracellular proteins including PI3 kinase, Grb-2, Gads and ITK. Grb-2 and PI3 kinase binding has been mapped to the pYMNM motif within the cytoplasmic tail of CD28 and has been shown to play a role in co-stimulation. In this study, we demonstrate that amongst the Grb-2 family adapter proteins, CD28 precipitated Grb-2 and specifically co-operated in the up-regulation of NFAT/AP-1 transcription. By contrast, Gads and Grap either failed or only weakly collaborated with CD28 ligation. Further, the loss of Grb-2 binding interferes with the ability of Vav1 to co-operate with CD28. Anti-CD28 ligation alone was capable for co-operating with Grb-2 or Grb-2-Vav1. Our findings define a pathway involving CD28 binding to Grb-2 and its co-operativity with Vav1 in the regulation of T-cell co-stimulation.
Keywords: CD28, Grb-2, Vav1, NFAT/AP-1
The co-stimulatory molecules CD28 and CTLA-4 determine the ultimate outcome of the immune response after ligation of the T cell receptor (TcR)–CD3 complex. Both bind to CD80 and CD86 and are required for optimal T cell activation [1-4]. In contrast to CD28, which enhances and sustains T cell responses, CTLA-4 potently inhibits T cell activation [5-8].
CD28 and CTLA-4 possess small cytoplasmic domains of 41 and 36 residues, respectively. Common to each is the presence of a YxxM consensus motif: a YMNM motif for CD28 and a YVKM motif for CTLA-4. Phosphorylation of the tyrosine creates conditions for the binding of one of two SH2 domains of the p85 subunit of the phosphatidylinositol 3-kinase (PI3K) [9-14]. Specificity is determined by residues adjacent to the tyrosine, specifically a methionine (M) in the plus 3 position. CD28 also carries an asparagine (N) in the plus 2 position (i.e., pYxNM) that is not found in CTLA-4. This is a signature residue for SH2 domain binding of the adaptor Grb-2 [15-17]. Co-precipitation and binding analysis have confirmed that CD28 associates with Grb-2 and its binding partner Son of sevenless (Sos) [15]. Binding has been shown to occur via the predicted YxNM motif as well as via SH3 domain binding to the C-terminal proline-based motif [16,17]. Grb-2 related Gads has also been reported to bind to CD28 at distinct sites from Grb-2 [18], while the src kinase p56lck has been reported to bind to a PxxP motif [19,20].
Interestingly, the loss of the asparagine needed for Grb-2 binding resulted in both impaired interleukin-2 (IL-2) production and phosphorylation of the guanine nucleotide exchange factor Vav1 [16]. Conversely, the insertion of the asparagine in the YxxM motif of ICOS confirmed an ability of this co-receptor to support IL-2 production [21]. Vav1 has a calponin homology (CH) domain, an acidic (Ac) motif, a zinc finger (ZF)-like region, two Src homology 3 (SH3) domains and an SH2 domain. The SH2 domain of Vav1 binds tyrosine residues within the adaptor SH2 domain-containing lymphocytic protein of 76-kDa (SLP-76) [22]. The DH domain has guanine nucleotide exchange factor (GEF) activity for the activation of the small GTPases Rac1 and Cdc42 [23]. Vav1 deficient T-cells show defects in TcR capping and in the induction of cytokine production [24,25]. Phosphorylation of Vav1 by CD28 depends on residues 173–181 of the receptor [12,16]. The CD28-Vav1 pathway operates independently of the CD28-PI3K pathway [26], although this may vary with different modes of ligation [27].
Grb-2 is an adaptor protein comprised of one SH2 domain flanked by two SH3 domains that bind to the exchange factor Sos, an activator of the GTPase p21ras [28]. The Grb-2 SH3 domain can bind constitutively at low levels to the same motif, whereas the phospho-YMNM motif facilitates tandem SH2–SH3 domain binding [14,16,17,29]. Grb-2 has been shown to bind to the CD28 YMNM motif with additional SH3 domain binding to proline residues [16,17]. Other members of the Grb-2 family include Gads (Grid) and Grap. Gads possesses an SH3–SH2–SH3 domain structure and also contains a unique proline/glutamine rich domain of unknown function [30,31]. In Jurkat cells, CD3/CD28 stimulation has been reported to lead to the recruitment of Gads to the CD28-YMNM motif [18]. Grap (Grb-2-related adaptor protein) is a Grb-2 like SH3–SH2–SH3 adaptor protein with expression restricted to lymphoid tissues. Grap, unlike Grb-2, has been reported to act as a negative regulator of TcR/CD3 mediated proliferation and IL-2 production [32].
In this study, we demonstrate that amongst the Grb-2 family adapter proteins, CD28 precipitated Grb-2 and specifically co-operated in the up-regulation of NFAT/AP-1 transcription. By contrast, Gads and Grap either failed or only weakly collaborated with CD28 ligation. The loss of Grb-2 binding interferes with the ability of Vav1 to co-operate with CD28. Our findings define a pathway involving CD28 binding to Grb-2 and its co-operativity with Vav1 in the regulation of T-cell co-stimulation.
Materials and methods
Cells and reagents
Jurkat T cells were cultured in RPMI 1640 supplemented with 5% fetal calf serum (FCS), 2 mM l-glutamine and 100 U/ml penicillin/streptomycin. Anti-CD3 (OKT3) was obtained from American Type Culture Collection, anti-CD28 (9.3) from Bristol-Myers Squibb. Anti-Vav1, anti-Grb-2 and anti-Flag antibodies were purchased from Upstate Biotechnologies, anti-myc antibody was from Invitrogen.
The pCMV3-myc-hVavWT and pCMV3-hVav-L213Q (GEF-inactive) plasmids were a kind gift from M. Turner (Babraham Institute).
Luciferase assay
For the luciferase assays, 0.5 × 106 Jurkat cells were incubated in 100 μl RPMI 1649 medium containing 5% fetal calf serum plus the appropriate antibodies for 6 h at 37 °C. Cells were lysed, and 3XNFAT/AP-1 luciferase activity was measured using a Luminat LB9507 luminometer. The luciferase activity was determined according to the luciferase assay system protocol from Promega. All luciferase assays were repeated at least three times.
Immunoprecipitation and immunoblotting
For immunoprecipitation, cells were lysed in ice-cold lysis buffer containing 1% Triton X-100 in 20 mM Tris–HCl, pH 8.3, 150 mM NaCl. The lysis buffer contained protease and phosphatase inhibitors. Postnuclear lysates were incubated for 1 h with the indicated antibody. Protein A–Sepharose beads (30 μl, Amersham Pharmacia) were added and incubated for 1 h at 4 °C. The eluted proteins were separated by SDS–PAGE and transferred to nitrocellulose for immunoblotting. The membranes were blocked with 5% milk in TBS (10 mM Tris–HCl, pH 7.6, 150 mM NaCl) and incubated with the indicated antibody. Bound antibody was revealed with the appropriate secondary antibody, and protein was visualized by enhanced chemiluminescence (ECL, Amersham).
Results and discussion
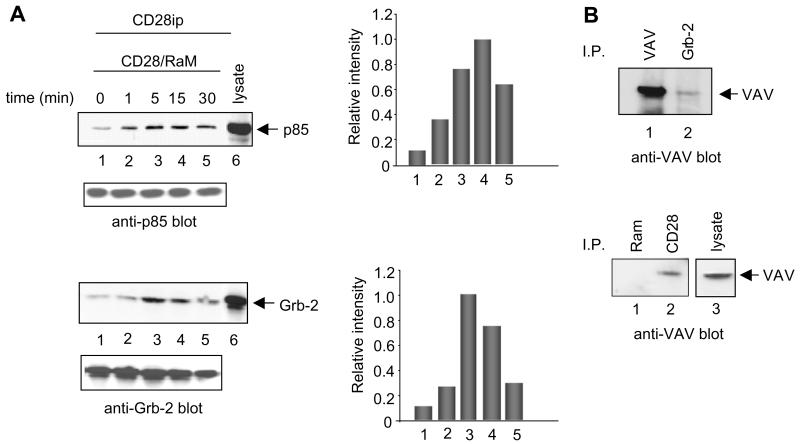
CD28 has been shown to bind to several intracellular proteins including PI3 kinase, Grb-2, Gads and ITK [4]. As shown in Fig. 1A, ligation of the co-receptor with anti-CD28 leads to the recruitment of PI3 kinase (upper panel) and Grb-2 (lower panel) with maximum binding at 5 and 15 min (histograms, right panels). Small amounts of constitutive p85 and Grb-2 binding could also be observed in the Jurkat T cell line (lane 1). Equal loading was confirmed by blotting of cell lysates with anti-p85 (upper panel) and anti-Grb-2 (lower panel) antibodies. Next, Grb-2 has been reported to bind to the guanine nucleotide exchange factor Vav1 through dimerization of the SH3 domains in both molecules (Fig. 1B, upper panel) [33]. To assess whether CD28 associates with Vav1, anti-CD28 was used to precipitate antigen followed by blotting with anti-Vav1. As shown in Fig. 1B (lower panel), in Jur-kat T cells binding of Vav1 to CD28 was observed, albeit at low amounts. This was observed in three experiments and indicates that CD28 can engage Vav1.
Fig. 1.
CD28 binding to PI3 K, Grb-2 and Vav1. (A) Jurkat T-cells were either left untreated (lane 1) or stimulated with anti-CD28 (4 μg/ml) and rabbit anti-hamster (2 μg/ml) antibodies (lanes 2–5). Cells were washed and solubilized in Triton X-100 lysis buffer containing protease and phosphatase inhibitors. Anti-CD28 immunoprecipitates and lysates were separated by SDS–PAGE gel, transferred to nitrocellulose and immunoblotted with anit-p85 (upper panel) or anti-Grb-2 (lower panel) antibodies. Lane 6 shows the position of p85 (upper panel) and Grb-2 (lower panel) in cell lysates. Cell lysates blotted for p85 (upper panel) and Grb-2 (lower panel) served as loading controls. (B, upper panel): Jurkat cells were lysed, immunoprecipitated with anti-Vav1 (lane 1) or anti-Grb-2 (lane 2) antibodies and blotted with anti-Vav1. Lower panel: Jurkat cells were lysed, immunoprecipitated with rabbit anti-mouse (lane 1) or anti-CD28 (lane 2) antibodies and blotted for associated Vav1 with anti-Vav1 mAb. Lane 3 shows the position of Vav1 in cell lysates.
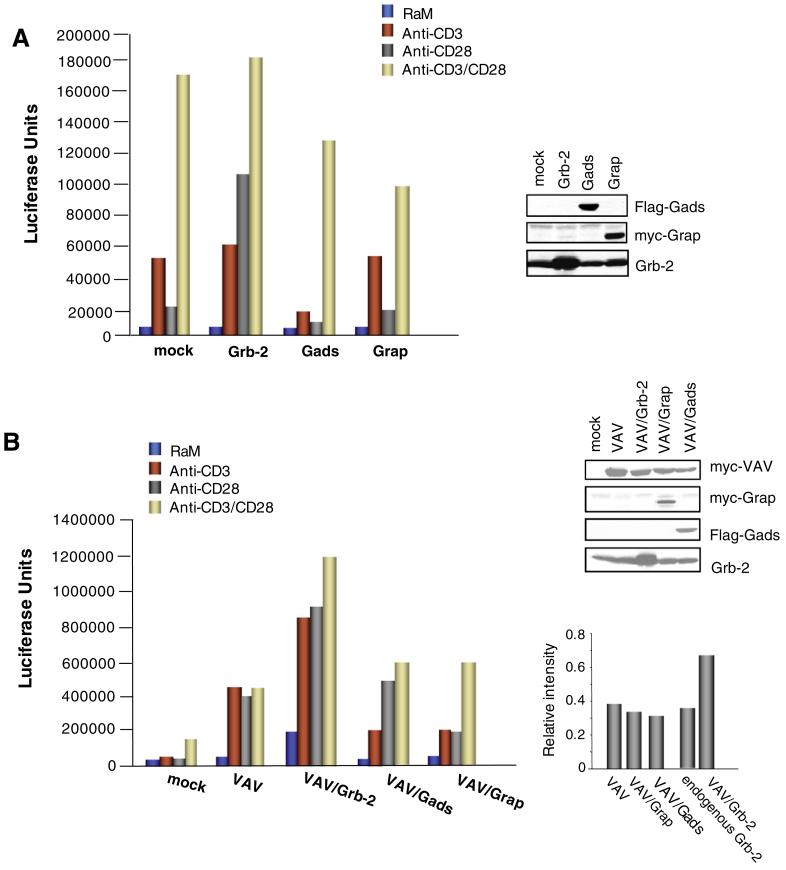
To further assess whether Grb-2 could co-operate in a specific manner with CD28 ligation, Grb-2 and related proteins Gads and Grap were individually expressed followed by CD3, CD28 and CD3/CD28 ligation and an assessment of NFAT/AP-1 transcription using the NFAT/AP-1 luciferase assay. As seen in Fig. 2A, the expression of Grb-2 led to a 3-to 4-fold increase in CD28 mediated NFAT/AP-1 gene activity. By contrast, neither Grap nor Gads co-operated with anti-CD28 to induce NFAT/AP-1 activation. In fact, a partial reduction was often observed, although the proteins were expressed at similar levels (upper right panel). This observation indicates that Grb-2 has a specific ability amongst the Grb-2-like proteins to co-operate with CD28 in the regulation of NFAT/AP-1 activity.
Fig. 2.
Grb-2 specifically co-operates with CD28 in the up-regulation of NFAT/AP-1 transcription, (A) Jurkat cells were transfected with mock, Grb-2, Gads and Grap together with a reporter plasmid containing the luciferase gene linked to 3XNFAT/AP-1. NFAT/AP-1 activation was determined as a measure of luciferase activity after anti-CD3, anti-CD28 and anti-CD3/CD28 stimulation for 6 h. Expression of the individual proteins are shown in the upper right panel. (B) Grb-2 co-operates with Vav1 in the up-regulation of NFAT/AP-1 activity. Jurkat cells were transfected with mock, Vav1, Vav1/Grb-2, Vav1/Gads and Vav1/Grap together with a reporter plasmid containing the luciferase gene linked to 3XNFAT/AP-1 and stimulated with anti-CD3, anti-CD28 and anti-CD3/CD28 antibodies. Six hours later, luciferase activity was determined. Expression of the individual proteins are shown in the upper right panel. Lower right histogram shows the relative intensity of the expressed proteins.
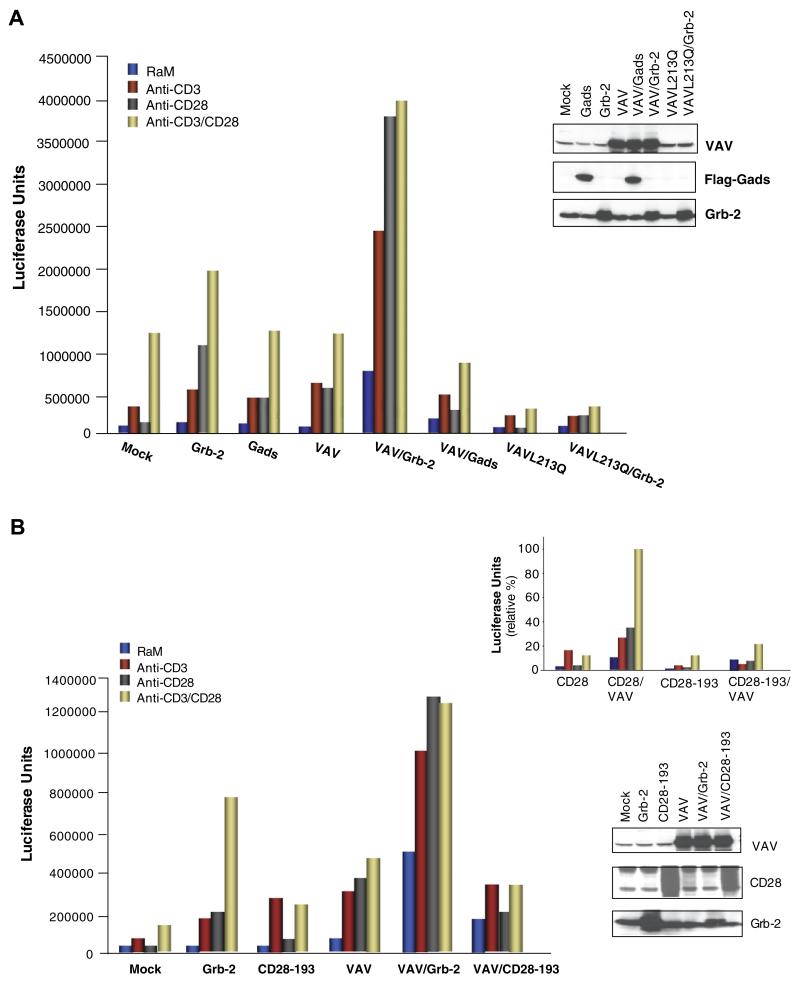
Previous studies have shown that CD28 can synergize with Vav1 (and Vav1/SLP-76) in the generation of signals leading to IL-2 transcription [34-36]. Further, we previously reported that the loss of the Grb-2 binding site resulted in a loss of Vav1 phosphorylation upon anti-CD3/CD28 co-ligation [16]. The CD28-Vav1 pathway can also operate independently of the CD28-PI3K pathway [26]. These observations are consistent with the notion that CD28-Grb-2 binding may act as a bridge to Vav1. To test whether CD28-Grb-2 amplification of NFAT/AP-1 activation could be linked to Vav1, Grb-2, Gads or Grap were co-expressed with Vav1 and assessed for effects on NFAT/AP-1 transcription. While co-expression of Grb-2 and Vav1 enhanced the activation, Gads or Grap either failed or only weakly co-operated with CD28 and Vav1 (Fig. 2B). In fact, the combination of the latter one was generally inhibitory when compared to the Vav1 mediated effects on NFAT/AP-1 activation. Further, the ability of Grb-2 to collaborate with Vav1 depended on the presence of an intact guanine nucleotide exchange factor (GEF) domain. The L213Q mutant, defective in Vav GEF activity markedly inhibited co-operativity (Fig. 3A). These data indicate that Grb-2 is unique amongst the Grb-2-like adaptors in being able to collaborate with Vav1 in the generation of CD28 signals.
Fig. 3.
Defective Vav GEF activity (VavL213Q) markedly inhibited co-operativity in CD28 induced NFAT/AP-1 activity, (A) Jurkat cells were transfected with either mock, Grb-2, Gads and Vav1 alone or together with Vav1 or with VavL213Q and VavL213Q and Grb-2 in addition with the reporter plasmid containing the luciferase gene linked to 3XNFAT/AP-1. Luciferase activity was measured after anti-CD3, anti-CD28 and anti-CD3/CD28 stimulation for 6 h. Expression of the individual plasmids are shown in the upper right panel. (B) Loss of Grb-2 binding to CD28 abrogates the co-operativity with Vav1 in the up-regulation of NFAT/AP-1 activity. Jurkat cells were transfected with either mock, Grb-2, CD28-193 and Vav1 alone or together with Vav1 and CD28 or CD28-193 in addition with the reporter plasmid containing the luciferase gene linked to 3XNFAT/AP-1. Luciferase activity was measured after anti-CD3, anti-CD28 and anti-CD3/CD28 stimulation for 6 h. Expression of the individual proteins are shown in the lower right panel. Upper right panel: Jurkat cells were transfected with either mock, CD28 wild type, CD28-193 mutant alone or together with Vav1 in addition with the reporter plasmid containing the luciferase gene linked to 3XNFAT/AP-1. Luciferase activity was measured as described above.
Similarly, the loss of Grb-2 binding to CD28 abrogated the ability of Grb-2 to co-operate with Vav1 in the up-regulation of NFAT/AP-1 transcription (Fig. 3B). This indicates that the mere co-expression of Grb-2/Vav1 is insufficient to co-operate with CD28. Instead, there is a requirement for the presence of an asparagine in the pYMNM motif needed for Grb-2 binding. Over-expression of CD28 wild type and CD28-193 mutant with and without Vav1 clearly shows that the ability to collaborate with Vav1 in the up-regulation of NFAT/AP-1 activation is dependent on the asparagine (Fig. 3B, upper right panel).
Vav1 has been connected upstream in the regulation of the activation cassette leading to activation of JNK [23]. Given the fact that Grb-2 and Vav1 co-operate with CD28 in the up-regulation of NFAT/AP-1 activation, it would be expected that there also exists co-operativity in the activation of the JNK pathway. Overall, our study indicates that CD28 can co-operate with Grb-2 in a specific manner with Vav1 in the activation of NFAT/AP-1. This extends our previous findings that the loss of Grb-2 binding to CD28 blocks the CD28 dependent IL-2 production, Vav1 phosphorylation and JNK activation [16]. Consistent with this, we also provided evidence of a physical interaction between CD28 and Vav1. The weak level of co-precipitation may be indicative of either a low stoichiometry of binding or an inefficient co-precipitation due to indirect binding via Grb-2. Alternatively, it may be indicative of a close connection to Vav1 at a functional level that in part involves physical binding but is not dependent on a protein–protein interaction. In either case, the specificity of co-operativity between CD28 and Grb-2 was clear and involved the ability of CD28 to generate a co-operative signal in the absence of TcR ligation, as previously described [34]. In addition, the Vav1 mutant L213Q could block the process. An intriguing question is whether the CD28-Grb-2-Vav1 connection could substitute for the LAT-Grb-2 interaction, given the fact that the major activator of the p21ras-Erk pathway is due to Ras guanyl nucleotide releasing protein-1 (RasGRP1) [37,38]. Our findings complement a recent study showing that Gads, but not Grb-2, is essential for CD28-mediated NFkB activation [18]. Further, CD28-Grb-2–Vav1 could up-regulate NFAT/AP-1 activation, while Gads could modulate the NFkB pathway. JNK is upstream of NFAT/AP-1, while the combination of CD28-Vav1-SLP-76 has been shown to alter NFAT translocation [34]. Further downstream, Vav1 may also intersect with NFkB [39]. Further studies will be needed to dissect the relative contributions of different pathways in regulating other aspects of T-cell immunity.
Acknowledgments
C.E. Rudd was supported by a Programme Grant and is a Principal Research Fellow (PRF) of the Wellcome Trust. H. Schneider is supported by a grant from the BBSRC (UK).
References
- [1].June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol. Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- [2].Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- [3].Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- [4].Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat. Rev. Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- [5].Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- [7].Linsley PS, Golstein P. Lymphocyte activation: T-cell regulation by CTLA-4. Curr. Biol. 1996;6:398–400. doi: 10.1016/s0960-9822(02)00506-7. [DOI] [PubMed] [Google Scholar]
- [8].Linsley PS. New look at an old costimulator. Nat. Immunol. 2005;6:231–232. doi: 10.1038/ni0305-231. [DOI] [PubMed] [Google Scholar]
- [9].Prasad KV, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc. Natl. Acad. Sci. USA. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Truitt KE, Hicks CM, Imboden JB. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat T cells. J. Exp. Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].August A, Dupont B. CD28 of T lymphocytes associates with phosphatidylinositol 3-kinase. Int. Immunol. 1994;6:769–774. doi: 10.1093/intimm/6.5.769. [DOI] [PubMed] [Google Scholar]
- [12].Klasen S, Pages F, Peyron JF, Cantrell DA, Olive D. Two distinct regions of the CD28 intracytoplasmic domain are involved in the tyrosine phosphorylation of Vav and GTPase activating protein-associated p62 protein. Int. Immunol. 1998;10:481–489. doi: 10.1093/intimm/10.4.481. [DOI] [PubMed] [Google Scholar]
- [13].Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- [14].Harada Y, Tanabe E, Watanabe R, Weiss BD, Matsumoto A, Ariga H, Koiwai O, Fukui Y, Kubo M, June CH, Abe R. Novel role of phosphatidylinositol 3-kinase in CD28-mediated costimulation. J. Biol. Chem. 2001;276:9003–9008. doi: 10.1074/jbc.M005051200. [DOI] [PubMed] [Google Scholar]
- [15].Schneider H, Cai YC, Prasad KV, Shoelson SE, Rudd CE. T cell antigen CD28 binds to the Grb-2/SOS complex, regulators of p21ras. Eur. J. Immunol. 1995;25:1044–1050. doi: 10.1002/eji.1830250428. [DOI] [PubMed] [Google Scholar]
- [16].Kim HH, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J. Biol. Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- [17].Okkenhaug K, Rottapel R. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J. Biol. Chem. 1998;273:21194–21202. doi: 10.1074/jbc.273.33.21194. [DOI] [PubMed] [Google Scholar]
- [18].Watanabe R, Harada Y, Takeda K, Takahashi J, Ohnuki K, Ogawa S, Ohgai D, Kaibara N, Koiwai O, Tanabe K, Toma H, Sugamura K, Abe R. Grb2 and Gads exhibit different interactions with CD28 and play distinct roles in CD28-mediated costimulation. J. Immunol. 2006;177:1085–1091. doi: 10.4049/jimmunol.177.2.1085. [DOI] [PubMed] [Google Scholar]
- [19].Carey KD, Dillon TJ, Schmitt JM, Baird AM, Holdorf AD, Straus DB, Shaw AS, Stork PJ. CD28 and the tyrosine kinase lck stimulate mitogen-activated protein kinase activity in T cells via inhibition of the small G protein Rap1. Mol. Cell. Biol. 2000;20:8409–8419. doi: 10.1128/mcb.20.22.8409-8419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holdorf AD, Green JM, Levin SD, Denny MF, Straus DB, Link V, Changelian PS, Allen PM, Shaw AS. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J. Exp. Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bustelo XR. Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kuhne MR, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J. Biol. Chem. 2000;275:2185–2190. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- [24].Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- [25].Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- [26].Wood JE, Schneider H, Rudd CE. TcR and TcR-CD28 engagement of protein kinase B (PKB/AKT) and glycogen synthase kinase-3 (GSK-3) operates independently of guanine nucleotide exchange factor VAV1. J. Biol. Chem. 2006;281:32385–32394. doi: 10.1074/jbc.M604878200. [DOI] [PubMed] [Google Scholar]
- [27].Charvet C, Canonigo AJ, Becart S, Maurer U, Miletic AV, Swat W, Deckert M, Altman A. Vav1 promotes T cell cycle progression by linking TCR/CD28 costimulation to FOXO1 and p27kip1 expression. J. Immunol. 2006;177:5024–5031. doi: 10.4049/jimmunol.177.8.5024. [DOI] [PubMed] [Google Scholar]
- [28].Skolnik EY, Batzer A, Li N, Lee CH, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- [29].Pages F, Ragueneau M, Klasen S, Battifora M, Couez D, Sweet R, Truneh A, Ward SG, Olive D. Two distinct intracytoplasmic regions of the T-cell adhesion molecule CD28 participate in phosphatidylinositol 3-kinase association. J. Biol. Chem. 1996;271:9403–9409. doi: 10.1074/jbc.271.16.9403. [DOI] [PubMed] [Google Scholar]
- [30].Law CL, Ewings MK, Chaudhary PM, Solow SA, Yun TJ, Marshall AJ, Hood L, Clark EA. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J. Exp. Med. 1999;189:1243–1253. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yankee TM, Yun TJ, Draves KE, Ganesh K, Bevan MJ, Murali-Krishna K, Clark EA. The Gads (GrpL) adaptor protein regulates T cell homeostasis. J. Immunol. 2004;173:1711–1720. doi: 10.4049/jimmunol.173.3.1711. [DOI] [PubMed] [Google Scholar]
- [32].Shen R, Ouyang YB, Qu CK, Alonso A, Sperzel L, Mustelin T, Kaplan MH, Feng GS. Grap negatively regulates T-cell receptor-elicited lymphocyte proliferation and interleukin-2 induction. Mol. Cell. Biol. 2002;22:3230–3236. doi: 10.1128/MCB.22.10.3230-3236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramos-Morales F, Romero F, Schweighoffer F, Bismuth G, Camonis J, Tortolero M, Fischer S. The proline-rich region of Vav binds to Grb2 and Grb3-3. Oncogene. 1995;11:1665–1669. [PubMed] [Google Scholar]
- [34].Raab M, Pfister S, Rudd CE. CD28 signaling via VAV/SLP-76 adaptors: regulation of cytokine transcription independent of TCR ligation. Immunity. 2001;15:921–933. doi: 10.1016/s1074-7613(01)00248-5. [DOI] [PubMed] [Google Scholar]
- [35].Michel F, Mangino G, Attal-Bonnefoy G, Tuosto L, Alcover A, Roumier A, Olive D, Acuto O. CD28 utilizes Vav1 to enhance TCR-proximal signaling and NF-AT activation. J. Immunol. 2000;165:3820–3829. doi: 10.4049/jimmunol.165.7.3820. [DOI] [PubMed] [Google Scholar]
- [36].Michel F, Acuto O. CD28 costimulation: a source of Vav1 for TCR signaling with the help of SLP-76? Sci. STKE. 2002:PE35. doi: 10.1126/stke.2002.144.pe35. [DOI] [PubMed] [Google Scholar]
- [37].Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL, 2nd, Cox AD, Philips MR. Ras signalling on the endoplasmic reticulum and the golgi. Nat. Cell Biol. 2002;4:343–350. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- [38].Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 2004;5:363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- [39].Marinari B, Costanzo A, Viola A, Michel F, Mangino G, Acuto O, Levrero M, Piccolella E, Tuosto L. Vav cooperates with CD28 to induce NF-kappaB activation via a pathway involving Rac-1 and mitogen-activated kinase kinase 1. Eur. J. Immunol. 2002;32:447–456. doi: 10.1002/1521-4141(200202)32:2<447::AID-IMMU447>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]