Abstract
Aim:
Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan found in traditional Chinese herbs, has been determined to exhibit a variety of pharmacological activities, including anti-tumor, anti-inflammation, neuroprotection, and endurance enhancement. In the present study, we investigated the antioxidation and anti-fatigue effects of arctigenin in rats.
Methods:
Rat L6 skeletal muscle cell line was exposed to H2O2 (700 μmol/L), and ROS level was assayed using DCFH-DA as a probe. Male SD rats were injected with arctigenin (15 mg·kg−1·d−1, ip) for 6 weeks, and then the weight-loaded forced swimming test (WFST) was performed to evaluate their endurance. The levels of antioxidant-related genes in L6 cells and the skeletal muscles of rats were analyzed using real-time RT-PCR and Western blotting.
Results:
Incubation of L6 cells with arctigenin (1, 5, 20 μmol/L) dose-dependently decreased the H2O2-induced ROS production. WFST results demonstrated that chronic administration of arctigenin significantly enhanced the endurance of rats. Furthermore, molecular biology studies on L6 cells and skeletal muscles of the rats showed that arctigenin effectively increased the expression of the antioxidant-related genes, including superoxide dismutase (SOD), glutathione reductase (Gsr), glutathione peroxidase (GPX1), thioredoxin (Txn) and uncoupling protein 2 (UCP2), through regulation of two potential antioxidant pathways: AMPK/PGC-1α/PPARα in mitochondria and AMPK/p53/Nrf2 in the cell nucleus.
Conclusion:
Arctigenin efficiently enhances rat swimming endurance by elevation of the antioxidant capacity of the skeletal muscles, which has thereby highlighted the potential of this natural product as an antioxidant in the treatment of fatigue and related diseases.
Keywords: arctigenin, skeletal muscle, weight-loaded forced swimming test, fatigue, physical endurance, ROS, antioxidant, AMPK, PPARα, Nrf2
Introduction
Normal muscle contraction relies on the redox state of cells in the muscle1,2, and reactive oxygen species (ROS) generated by strenuous exercise can influence the contractile function of skeletal muscles and accelerate fatigue3. In addition, muscle fatigue also decreases mobility and quality of life for people with heart failure, cancer cachexia, sarcopenia and catabolic diseases4. Moreover, excessive ROS production and accumulation can even damage biomembranes5, proteins6, and DNA7, thereby initiating asthma, chronic obstructive pulmonary disease (COPD)8, atherosclerosis, inflammatory diseases9, and Parkinson's disease10. Therefore, increasing the antioxidant capacity of skeletal muscles can be beneficial for muscle performance, disease prevention and life-quality improvement.
AMP-activated protein kinase (AMPK) is an axis of energy metabolism. It is highly involved in the regulation of glucose uptake, fatty acid oxidation and mitochondrial biogenesis. AMPK activation is helpful to enhance endurance11. It has been reported that AMPK activation facilitates ROS reduction12. When exercising, the ROS that is produced by the mitochondrial respiratory chain accumulates in skeletal muscles, causing fatigue and increasing the oxidative damage to cells13. AMPK activation can prevent cells from overproducing and accumulating ROS in mitochondria14, which has been recently confirmed by a study on Cordyceps sinensis that elevated the exercise endurance capacity of rats via an increase in the expression of AMPK and its downstream antioxidant genes15.
Arctigenin (Figure 1A), a phenylpropanoid dibenzylbutyrolactone lignan, was isolated from the traditional Chinese herbs Arctium lappa L (burdock), Saussurea medusa (snow lotus) and Forsythia intermedia (border forsythia)12. Arctigenin was reported to possess important pharmacological properties, including anti-tumor, anti-inflammation, immunomodulation, neuroprotection, anti-virus, ER stress regulation, and endurance enhancement12,16,17,18,19. For example, arctigenin exhibits anti-inflammatory effects by inhibiting the exudation and recruitment of leukocytes into inflamed tissues via reducing the release/production of inflammatory mediators and also exhibits neuroprotective activity through reducing surplus ROS production and downregulating the mitochondrial membrane potential20,21. Additionally, arctigenin has therapeutic efficacy in experimental models of influenza virus and Japanese encephalitis (JE)12. Chronic oral administration of arctigenin can lower blood glucose and improve lipid metabolism in ob/ob mice22. Moreover, a previous study in our lab also proved that arctigenin can promote AMPK phosphorylation through CaMKK and LKB1-dependent pathways and improve mouse endurance by enhancing mitochondrial biogenesis and fatty acid synthesis and oxidation23.
Figure 1.
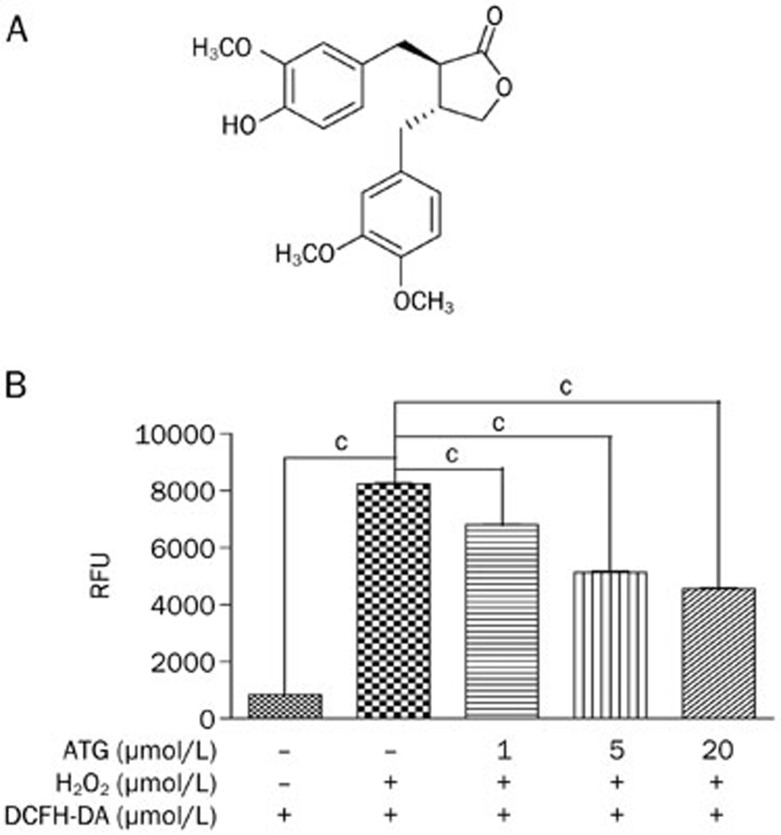
Arctigenin decreased reactive oxygen species (ROS) in L6 cells. (A) Chemical structure of arctigenin. (B) L6 cells were incubated with arctigenin (1, 5, and 20 μmol/L) or DMSO for 24 h. Cells were pretreated with 1 mmol/L DCFH-DA for 60 min at 37 °C, followed by treatment with 700 μmol/L H2O2 for 20 min. Fluorescence was determined with a fluorescence plate reader at 480 nm excitation/530 nm emission. The results shown are representative of three independent experiments. Values are expressed as the mean±SEM. cP<0.01; one-way ANOVA.
In the present study, we reported that arctigenin could also upregulate antioxidant-related genes to reduce ROS accumulation and production through phosphorylating AMPK, thereby enhancing rat swimming endurance.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine and horse serums were purchased from Invitrogen (Carlsbad, CA, USA). Compound C {6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine, AMPK inhibitor24} was obtained from Sigma (St Louis, MO, USA). The protease inhibitor cocktail (AEBSF, 104 mmol/L; aprotinin, 80 μmol/L; bestatin, 4 mmol/L; E-64, 1.4 mmol/L; leupeptin, 2 mmol/L; pepstatin A, 1.5 mmol/L) was obtained from Sigma-Aldrich (St Louis, MO, USA). The BCA protein assay kit was obtained from Pierce (Rockford, IL, USA). The Reactive Oxygen Species Assay Kit, pifithrin-α, ATP Assay Kit, Immunol Fluorescence Staining Kit and Nuclear and Cytoplasmic Protein Extraction Kit were obtained from Beyotime (Haimen, China). RNAiso, RT reagent Kit and SYBR Premix Ex Taq were purchased from TaKaRa (Dalian, China). Anti-AMPK, anti-phospho-AMPK (Thr172), anti-p53, anti-Lamin A, and anti-phospho-p53 (Ser15) were from Cell Signaling Technology, Inc (Beverly, MA, USA). Nuclear transcription factor erythroid-2-related factor 2 (Nrf2), peroxisome proliferator-activated receptor γ coactivator 1α (PGC 1α), and Cu-Zn superoxide dismutase (Cu,Zn-SOD) antibodies were purchased from Abcam (Cambridge, MA, USA). Secondary antibodies conjugated with horseradish peroxidase IgG (rabbit and mouse) were from Jackson-Immuno Research, and L6 cells (rat skeletal muscle myoblasts) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Arctigenin was isolated from the roots of Arctium lappa L, and the purity of arctigenin was determined to be 99%25.
Cell culture and differentiation
Rat L6 skeletal muscle cell line was cultured in DMEM supplemented with fetal bovine serum (FBS, 10%) with penicillin (100 IU/mL) and streptomycin (100 μg/mL), and grown at 37 °C in an environment of 5% CO2. To induce myotube differentiation, the cells were cultured in DMEM with 2% horse serum after 100% confluence in a 6-well plate. Differentiation medium was exchanged every 2 d for 6 d before experiments.
Intracellular ROS assay
ROS level in L6 cells was detected with a Reactive Oxygen Species Assay Kit by employing the cell-permeable fluorogenic probe 2',7'-dichlorodihydrofluorescin diacetate (DCFH-DA). In brief, DCFH-DA was diffused into the cells and deacetylated by cellular esterases to the non-fluorescent compound 2',7'-dichlorodihydrofluorescin (DCFH), which was rapidly oxidized to the highly fluorescent compound 2',7'-dichlorodihydrofluorescein (DCF) by ROS. The fluorescence intensity is proportional to the ROS level within the cell cytosol. In the assays, differentiated L6 cells were incubated with arctigenin (1, 5, and 20 μmol/L) or DMSO for 24 h in 6-well plate. Then, cells were treated with 1 mmol/L DCFH-DA for 60 min at 37 °C, followed by incubation with 700 μmol/L H2O2 for 20 min. Fluorescence was measured with a fluorescence plate reader (Molecular Devices, SpectraMax M5) at 480 nm excitation/530 nm emission.
Intracellular ATP assay
ATP level in L6 cells was detected with an ATP Assay Kit by employing firefly luciferase-catalyzed oxidation of D-luciferin to produce light in the presence of ATP. The light intensity is a direct indicator of the intracellular ATP concentration. Differentiated L6 cells were incubated with arctigenin (1, 5, and 20 μmol/L) or DMSO for 24 h in a 6-well plate, followed by the addition of 200 μL lysis buffer into each well and centrifuged at 12 000×g for 5 min; then, the supernatants were collected. For muscle tissue samples, after a 20 mg sample was homogenized in 200 μL lysis buffer and centrifuged at 12 000×g for 5 min, the supernatants were collected. Then, 100 μL of supernatant was transferred to each well of a white opaque 96-well plate, and 1 μL firefly luciferase, 1 μL D-luciferin and 98 μL dilution buffer were added. Finally, the luminescence was read on a luminometer (Molecular Devices, SpectraMax M5).
Tissue collection
Animals were euthanized 72 h after the last bout of the fatigue test. Gastrocnemius and quadriceps were then isolated, frozen, and stored at −80 °C until further analysis.
Western blot analysis
To denature the proteins in L6 cells and muscle tissues, 2× loading buffer (4% SDS, 62.5 mmol/L Tris-HCl, pH 6.8, 25% glycerol, and 0.1% bromophenol blue) was used. The separation of nuclear and cytoplasmic protein was conducted according to the protocol of the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime). Muscle tissues were homogenized in lysis buffer (25 mmol/L Tris-HCl, pH=7.5, 150 mmol/L NaCl, 1 mmol/L Na3VO4, 1% Triton X-100 and a protease inhibitor cocktail) by an ultrasonic processor. After centrifugation, the supernatants were collected, and protein concentrations were determined with a BCA protein assay kit. Equal amounts (2 mg/mL protein) of the total protein in tissue lysates and cells mixed with 2×loading buffer were boiled for 15 min at 99 °C, then separated by 10% SDS-polyacrylamide gel electrophoresis. After being transferred to nitrocellulose membranes, the proteins were blocked for one hour at room temperature with 5% skim milk and then incubated overnight at 4 °C with the primary antibodies. Membranes were washed for 15 min three times with wash buffer (TBST) at room temperature and incubated in TBST buffer (5% milk) for 2 h with horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit IgG or anti-mouse IgG). Finally, the membranes were washed three times by TBST for 15 min each, and a SuperSignal West Dura chemiluminescence kit (Pierce Biotechnology) was used to reveal the band. The image was captured by an Image Quant LAS 4000 mini (GE, healthcare).
RT-PCR and quantitative real-time PCR
Total mRNA was isolated from cells and rat skeletal muscles using RNAiso reagent in accordance with the kit instructions. The purity and quantity of the total RNA was measured by OD260/280. A 1 μg sample of total RNA was reverse-transcribed with a PrimeScript® RT Master Mix Perfect Real Time kit (TaKaRa). Quantitative real-time PCR was performed in 96-well plates using SYBR Premix Ex Taq on a DNA Engene Opticon TM2 system (MJ Research, Waltham, MA, USA). The primers are listed in Table 1.
Table 1. The list of PCR primers.
| Genes | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| UCP2 | ACTTTCCCTCTGGATACCGC | ACGGAGGCAAAGCTCATCTG |
| SOD | AATGTGTCCATTGAAGATCGTGTGA | GCTTCCAGCATTTCCAGTCTTTGTA |
| Gsr | GGGCAAAGAAGATTCCAGGTT | GGACGGCTTCATCTTCAGTGA |
| GPX1 | CAGTTCGGACATCAGGAGAAT | AGAGCGGGTGAGCCTTCT |
| Txn | TTCCCTCTGTGACAAGTATTCCAA | GGTCGGCATGCATTTGACT |
| P21 | CTGGATGCTAGAGGTCTGC | AGAGTTGTCAGTGTAGATGC |
| PPARα | CGACAAGTGTGATCGAAGCTGCAAG | GTTGAAGTTCTTCAGGTAGGCTTC |
| PGC-1α | GCCCGGTACAGTGAGTGTTC | CTGGGCCGTTTAGTCTTCCT |
| GAPDH | ACAGCAACAGGGTGGTGGAC | TTTGAGGGTGCAGCGAACTT |
| ERRα | CTCAGCTCTCTACCCAAACGC | CCGCTTTGGTGATCTCACACTC |
| Cytochrome c | CAGCTTCCATTGCGGACAC | GGCACTCACGGCAGAATGAA |
Immunohistochemistry (IHC-FR)
Gastrocnemius and quadriceps were collected and embedded in OCT cryostat sectioning medium and stored at −80 °C until ready for sectioning. The frozen tissue block was transferred to a cryotome cryostat (-20 °C) prior to sectioning and then cut to the desired thickness (5 μm) using a cryotome. The tissue sections were placed onto glass slides suitable for immunohistochemistry and dried overnight at room temperature. The slides were fixed with pre-cooled acetone for 10 min at room temperature and rinsed 3 times in PBS. The immunofluorescence staining was conducted following the instructions of the appropriate kit. The color of the antibody staining in the tissue sections was observed using a light microscope (OLYMPUS ix71). Fluorescence staining burdens were counted on every five fields throughout the entire gastrocnemius and quadriceps (8 rats per group) by Image-Pro Plus 6.0 software. Total number of fluorescence-stained plaques in the gastrocnemius and quadriceps (8 rats per group) was calculated for statistical analysis.
Animal experiments
All animal experiments were carried out in accordance with the Regulation of Experiments Animal Administration issued by the State Committee of Science and Technology of People's Republic of China. Permit numbers: SCXK(HU) 2008-0017 and SYXK(HU) 2008–0049.
Sprague-Dawley (SD) male rats at 7 weeks of age were purchased from the Shanghai Experimental Animal Center, Chinese Academy of Sciences, and acclimated to the IVC housing conditions for 2 d before any experimental intervention. Rats were accommodated under standard conditions (strict 12 h light/dark cycle, 22 °C, 60% humidity) and provided with water and food ad libitum.
A weight-loaded forced swimming test (WFST) was used to evaluate the rats' endurance and performed as described previously26 with minor modifications. There were 40 SD male rats in the first fatigue test at week 0. In the fatigue test, rats swam in water loaded with 3% of their body weight. The temperature and depth of the water was 30 °C and 70 cm. Exhaustion time was determined from the beginning of swimming to the point at which rats failed to return to the water surface within 10 s. After the first fatigue test, 30 rats with moderate endurance capacity were selected and randomly divided into arctigenin and vehicle treatment groups (n=10/group). Before the final fatigue assay, sedentary rats were treated with arctigenin (10 and 15 mg/kg) or vehicle (sterilized 0.9% sodium chloride containing 5% Tween-80) daily via an intraperitoneal injection for 6 weeks. After 6-week arctigenin administration, the final fatigue test was performed similar to the first fatigue test.
Statistical analysis
All data are reported as the mean±standard error of the mean (SEM). Data were analyzed using either a one-way ANOVA with an appropriate post hoc test for comparison of multiple groups or an unpaired Student's t-test for comparison of two groups, as described in the figure legends (GraphPad Prism 5.0 software). A P value of <0.05 was considered to be statistically significant.
Results
Arctigenin decreased ROS in L6 cells
Given that ROS accumulation is tightly linked with the function of skeletal muscles and that arctigenin is effective in enhancing mitochondrial biogenesis and fatty acid oxidation13,23, the potential effect of arctigenin on ROS accumulation was examined. In the assay, L6 cells, which have been widely used in the skeletal muscle endurance or fatigue studies27,28, were differentiated in DMEM with 2% horse serum, and then the ROS level in the cell line induced by H2O2 treatment was detected with the cell-permeable fluorogenic probe DCFH-DA. As shown in Figure 1B, arctigenin dose-dependently reduced ROS compared with the DMSO-treated (negative control) group in L6 cells.
Arctigenin increased AMPK phosphorylation and regulated its downstream antioxidant-related pathways in L6 cells
Arctigenin increased AMPK phosphorylation in L6 cells
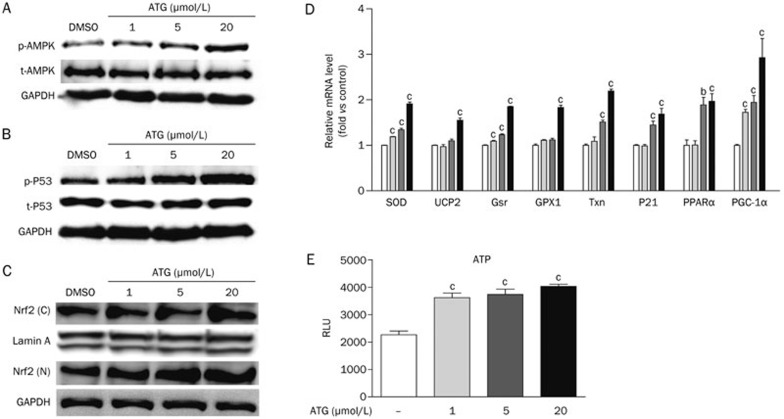
AMPK is an important checkpoint to maintain energy balance in both cells and organisms29 and is implicated in ROS reduction12. In our previous study, arctigenin was discovered to promote AMPK phosphorylation in mouse myoblast cells (C2C12 cells)23. Here, the effect of arctigenin on AMPK phosphorylation in L6 cells was also detected. As shown in Figure 2A, arctigenin dose-dependently increased AMPK phosphorylation in L6 cells similar to its effect on C2C12 cells.
Figure 2.
Arctigenin increased AMPK phosphorylation and regulated its downstream antioxidant-related pathways. (A–C) Differentiated L6 cells were incubated with arctigenin (1, 5, and 20 μmol/L) or DMSO for 2 h. After harvesting, the total- and phospho-AMPK (A), the total- and phospho-p53 (B), and the Nrf2 in the cell nucleus and cytoplasm (C) were determined by Western blotting. (D) Differentiated L6 cells were incubated with arctigenin (1, 5, and 20 μmol/L) or DMSO for 24 h. The expression levels of Cu,Zn-SOD, Txn, Gsr, GPX1, UCP2, p21, PPARα, and PGC-1α were measured by qRT-PCR. GAPDH RNA was used as an internal control for calculating mRNA fold changes. (E) Differentiated L6 cells were incubated with arctigenin (1, 5, and 20 μmol/L) or DMSO for 24 h. The levels of ATP were determined. The results shown were validated by three independent experiments. Values are expressed as the mean±SEM. bP<0.05, cP<0.01. one-way ANOVA.
Arctigenin upregulated antioxidant-related gene transcription involving AMPK/p53/Nrf2 pathway
Given that AMPK activation promoted p53 activity through phosphorylating Ser-15, followed by the regulation of glutathione peroxidase (GPX1)30 and p2131 expression levels, we next detected the potential regulation of arctigenin against the phosphorylation of p53 and mRNA level of p21 in L6 cells. Compared with the DMSO-treated group, arctigenin dose-dependently increased p53 phosphorylation and p21 expression in the cells (Figure 2B and 2D).
As reported, Nrf2 upregulates the gene expression of antioxidant enzymes or proteins, including Cu,Zn-SOD, thioredoxin (Txn) and glutathione reductase (Gsr) through binding to the antioxidant-responsive element (ARE)32,33,34,35. p21 can stabilize Nrf2 from degradation and increase the translocation of Nrf2 to the nucleus36. With these facts, we next examined the levels of Nrf2 in the nucleus (N) and cytoplasm (C). As indicated in Figure 2C, arctigenin dose-dependently increased Nrf2 in both the nucleus (N) and cytoplasm (C). Additionally, the mRNA levels of antioxidant genes regulated by Nrf2 and p53, such as Cu,Zn-SOD, GPX1, Txn and Gsr, were also investigated after treatment with arctigenin. As shown in Figure 2D, arctigenin obviously elevated the expression of these genes.
Arctigenin was involved in the regulation of AMPK/PGC1a/peroxisome proliferator activated receptor α (PPARα) pathway
As a downstream factor of AMPK, PGC-1α activation promotes the expression of antioxidant-related genes (eg, Cu,Zn-SOD and GPX-1) and nuclear receptor PPARα37, which can regulate uncoupling protein2 (UCP2) production in mitochondria37,38,39,40. Given that arctigenin could induce AMPK activation and upregulate PGC-1α gene expression in skeletal muscles23, the activity of arctigenin with respect to PGC-1α, PPARα and related antioxidant genes of UCP2, Cu,Zn-SOD and GPX-1 was investigated next. As expected, arctigenin dose-dependently enhanced the expression of these genes (Figure 2D). In addition, by considering that UCP2 uncouples ATP synthesis from the oxidative phosphorylation pathway41, the level of ATP was also examined after arctigenin incubation. As shown in Figure 2E, arctigenin could dose-dependently increase ATP levels in L6 cells.
Taken together, our results suggested that arctigenin can increase AMPK phosphorylation and upregulate its downstream antioxidant-related enzymes and proteins involving the regulation of AMPK/PGC1α/PPARα and AMPK/p53/Nrf2 pathways in L6 cells.
Regulation of arctigenin against antioxidant-related pathways was dependent on AMPK activation
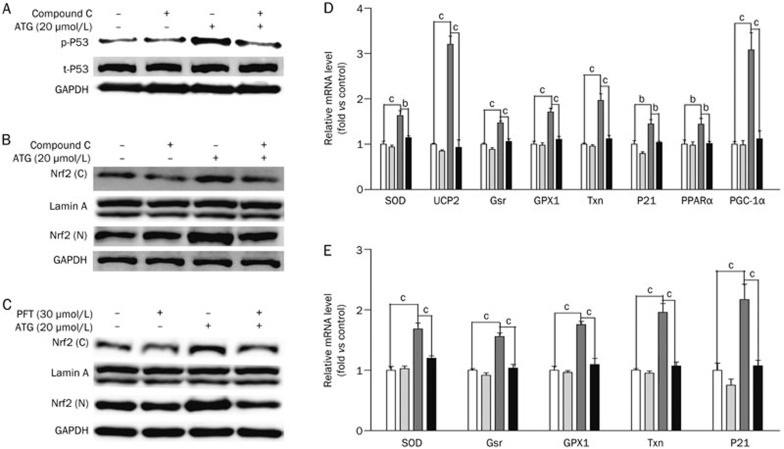
To further investigate whether arctigenin increased p53 phosphorylation, stabilized Nrf2 and upregulated antioxidant-related genes through AMPK activation, we next evaluated the activity of arctigenin against these genes and proteins together with an AMPK inhibitor (compound C) or p53 inhibitor (pifithrin-α, PFT) in L6 cells. As indicated in Figure 3A-B and D, treatment with compound C inhibited the arctigenin-induced p53 phosphorylation and Nrf2 accumulation in the nucleus (N) and cytoplasm (C), as well as the related gene expression in L6 cells. In Figure 3C and E, treatment of PFT inhibited Nrf2 accumulation in the nucleus (N) and cytoplasm (C) and decreased the expression of the p53 target gene p21 and related genes in L6 cells.
Figure 3.
Arctigenin regulated antioxidant-related pathways depending on AMPK activation. (A, B) Differentiated L6 cells were incubated with or without 10 μmol/L compound C (AMPK inhibitor) for 1 h before and during the incubation with arctigenin (20 μmol/L) for 2 h. After harvesting, total- and phospho-p53 protein levels (A), and Nrf2 in the cell nucleus and cytoplasm (B) were analyzed by Western blotting. (C) Differentiated L6 cells were incubated with 30 μmol/L PFT and arctigenin (20 μmol/L) for 2 h. After harvested, the amount of Nrf2 in the cell nucleus and cytoplasm were analyzed by Western blotting. (D) Differentiated L6 cells were treated with or without 10 μmol/L compound C (AMPK inhibitor) for 1 h before and during the incubation with arctigenin (20 μmol/L) for 24 h. The expression levels of Cu,Zn-SOD, Txn, Gsr, GPX1, UCP2, p21, PPARα and PGC-1α were measured by qRT-PCR. GAPDH RNA was used as an internal control for calculating mRNA fold changes. (E) Differentiated L6 cells were incubated with 30 μmol/L PFT and arctigenin (20 μmol/L) for 24 h. The expression levels of Cu,Zn-SOD, Txn, Gsr, GPX1, and p21 were measured by qRT-PCR. GAPDH RNA was used as an internal control for calculating mRNA fold changes. The results shown were validated by three independent experiments. Values are expressed as the mean±SEM. bP<0.05, cP<0.01. one-way ANOVA.
Therefore, the above-mentioned results imply that the regulation of arctigenin against p53 phosphorylation, Nrf2 and antioxidant-related genes are dependent on AMPK activation.
Arctigenin efficiently enhanced the swimming endurance of sedentary SD rats
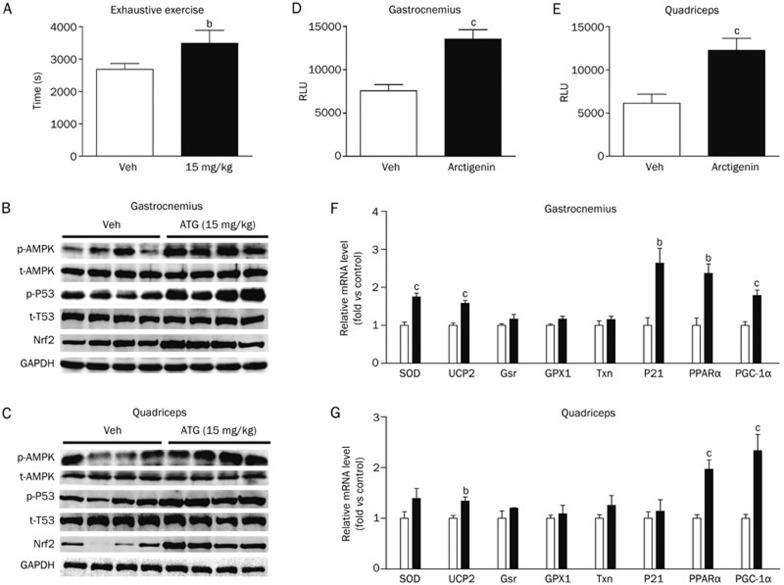
Given that improvement of skeletal muscle antioxidant capacity is beneficial for muscle performance4 and that arctigenin has been determined to be effective in reducing ROS levels, we next investigated the potential ability of this natural product to enhance rat endurance as measured by a weight-loaded forced swimming test (WFST)12. In the assay, before arctigenin administration, the swimming performance of all SD rats loaded with 3% of their body weight was recorded, and the rats with a swimming exhaustive time beyond the average were eliminated. After 6-week administration (10 and 15 mg/kg) of arctigenin via intraperitoneal injection, the swimming time until fatigue under a similar load was estimated as the maximal endurance capacity. As shown in Figure S1, arctigenin administration at 10 mg/kg increased the fatigue time by 24.97% (P=0.11) compared with vehicle, but without significance. However, arctigenin administration at 15 mg/kg increased fatigue time by 30.08% (P=0.04) compared with vehicle (Figure 4A), indicating that arctigenin efficiently enhanced the swimming endurance of sedentary rats.
Figure 4A–4G.
(A-G) Arctigenin efficiently enhanced swimming endurance of sedentary SD rats via increasing AMPK phosphorylation and regulating its downstream antioxidant-related pathways in vivo. (A) SD rats (n=10/group) were supplemented with arctigenin (15 mg/kg) or vehicle via intraperitoneal injection for 6 weeks, and the endurance performance was then measured by a weight-loaded forced swimming test. Exhaustion time was recorded. (B, C) Protein levels of phospho-AMPK, total-AMPK, phospho-p53, total-p53, and Nrf2 were determined by Western blotting in gastrocnemius (B) and quadriceps (C) (n=4/group). (D, E) The levels of ATP were determined in gastrocnemius (D) and quadriceps (E) (n=5/group). (F, G) The expression levels of Cu,Zn-SOD, Txn, Gsr, GPX1, UCP2, p21, PPARα, and PGC-1α from gastrocnemius (F) and quadriceps (G) were measured by qRT-PCR (n=5/group). GAPDH RNA was used as an internal control for calculating mRNA fold changes.
Arctigenin regulated downstream antioxidant-related pathways of AMPK in vivo
To further investigate the underlying mechanism of arctigenin in the improvement of the swimming endurance of sedentary rats, the relevant tissue-based assays were carried out. As expected, arctigenin administration obviously enhanced the phosphorylation of AMPK and p53 and the protein level of Nrf2 in both the gastrocnemius (Figure 4B) and quadriceps (Figure 4C) as measured by Western blot assays. A qRT-PCR assay demonstrated that arctigenin also upregulated the mRNA levels of PGC-1α, PPARα and UCP2 in the gastrocnemius and quadriceps (Figure 4F and 4G).
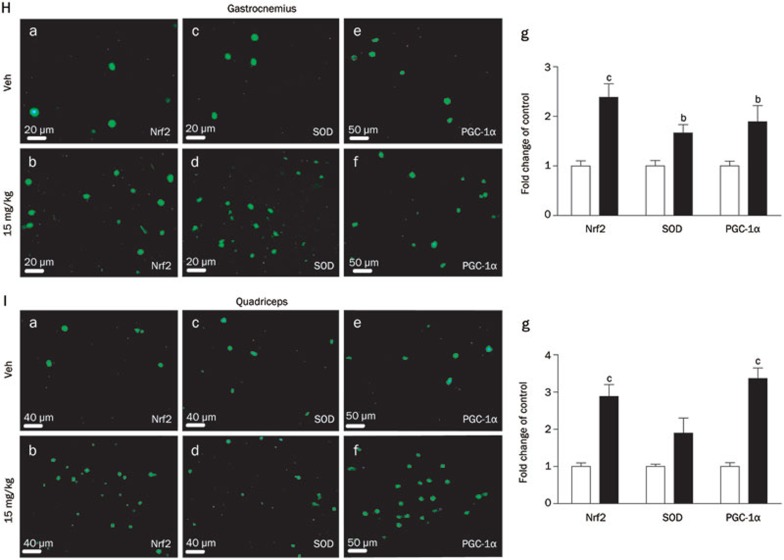
As shown in Figure 4F and 4G regarding the arctigenin group, the mRNA levels of Cu,Zn-SOD and p21 were elevated in the gastrocnemius, and there was a tendency towards increases in GPX1, Gsr, and Txn mRNA levels in the gastrocnemius and quadriceps, but without significance, and in the quadriceps, there was also a tendency towards increases in Cu,Zn-SOD and p21 mRNA levels although without significance. Similarly, the immunohistochemical results in both the gastrocnemius (Figure 4H) and quadriceps (Figure 4I) also suggested that arctigenin enhanced the levels of Nrf2, Cu,Zn-SOD and PGC-1α. Therefore, all in vivo results further support the potential of arctigenin in ROS elimination. Moreover, the effect of arctigenin on ATP levels was also examined, and the levels of ATP in both gastrocnemius (Figure 4D) and quadriceps (Figure 4E) were determined to be elevated in the arctigenin treatment group.
Figure 4H–4I.
(H, I) Immunofluorescence staining of Nrf2, Cu,Zn-SOD and PGC-1α with FITC in gastrocnemius (H) and quadriceps (I). The fluorescence was quantified using Image-Pro Plus6.0 software (n=8/group). Values are expressed as the mean±SEM. bP<0.05, cP<0.01. one-way ANOVA.
Discussion
ROS is a class of endogenous signaling molecule with specific functions depending on its subcellular localization, local concentration and duration of production42. A moderate level of ROS transiently oxidizes the cysteine sulphydryl that contributes to the active sites of most proteins43 and regulates a list of proteins, including tyrosine and serine/threonine phosphatases44,45, transcription factors46,47 and protease inhibitors48,49. When ROS accumulates to a certain level, its active effects might convert to inhibitory effects that damage cellular functions50. Oxidative stress will occur when the production of ROS exceeds its catabolism. Under oxidative stress, ROS may initiate the production of toxic compounds, thereby causing apoptosis42. Therefore, discovery of an efficient reagent that is able to reduce ROS is valuable in the treatment of related diseases.
AMPK as an axis of energy metabolism is highly involved in the regulation of the overproduction and accumulation of ROS in mitochondria14. AMPK activation upregulates the expression of PGC-1α or directly phosphorylates PGC-1α in skeletal muscles23, which subsequently increases the expression of the antioxidant enzymes responsible for ROS reduction, such as Cu,Zn-SOD and GPX151,52. UCP2 is one target gene of PPARα and is also regulated by PGC-1α activation38,39. UCP2 has been determined to play a critical role in the control of mitochondrial ROS production53. As a mitochondrial anion transporter, UCP2 uncouples ATP synthesis from the oxidative phosphorylation pathway by causing proton leakage across the mitochondrial inner membrane, finally reducing mitochondrial ROS production53,54. In addition, AMPK-induced ROS reduction also involves a series of pathways. For example, AMPK activates p53 through phosphorylating Ser-15 and subsequently affects the expressions of GPX130 and p21 (p53 target gene)31,55. Given that p21 stabilizes Nrf2 by interacting with Kelch-like ECH-associated protein 1 (Keap1) to block Nrf2 degradation in cytosol36, p21 stimulation may regulate the expression of the antioxidant enzymes or proteins, such as Cu,Zn-SOD, Txn and Gsr, through enhancing Nrf2 levels in the nucleus to bind the antioxidant-responsive element (ARE)32,33,34,35,36. Therefore, all results have addressed the functions of AMPK activation in ROS inhibition and anti-fatigue performance.
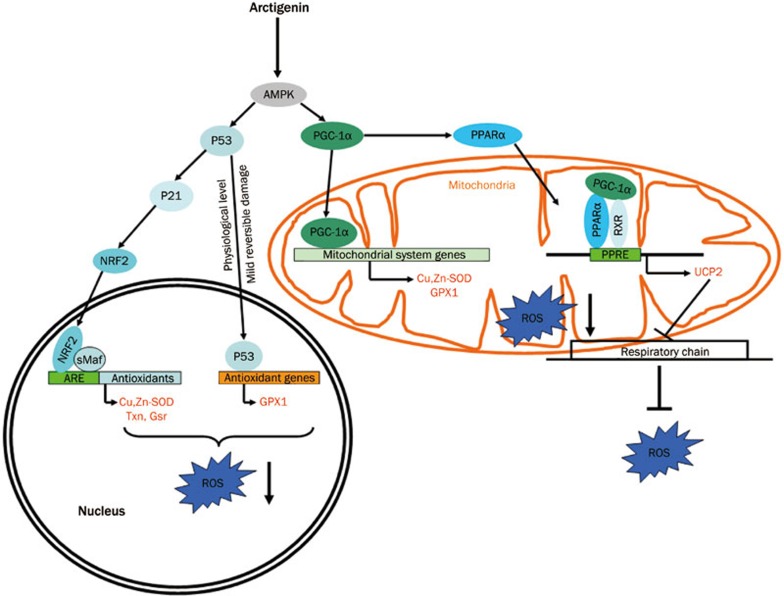
In the current work, we reported that arctigenin efficiently the enhanced swimming endurance of sedentary SD rats by improving the antioxidant capacity of the skeletal muscles through two antioxidant pathways: AMPK/PGC1a/PPARa in mitochondria and AMPK/p53/Nrf2 in the cell nucleus. In the regulation of the AMPK/PGC1a/PPARa pathway, arctigenin-stimulated AMPK activated PGC-1α and increased the expression of its downstream antioxidant-related genes (eg, Cu,Zn-SOD, GPX1). Additionally, PGC-1α activation also regulated PPARα expression, resulting in the elevation of its target gene UCP2. In AMPK/p53/Nrf2 signaling regulation, arctigenin-induced AMPK activation stimulated p53, thus directly promoting the expression of the antioxidant-related gene GPX1. Moreover, it was found that p53 activation also promoted the stabilizing factors Nrf2 and p21, leading to Nrf2 accumulation in both the cell nucleus and cytoplasm and the upregulation of the antioxidant-related genes Cu,Zn-SOD, Txn, and Gsr. Given that the localization of Nrf2 in the nucleus is essential for its function in the regulation of antioxidant gene expression56,57,58 and arctigenin has been determined to accelerate Nrf2 accumulation both in cell nucleus and cytoplasm, the capability of arctigenin to increase the Nrf2 in the nucleus is suggested to be attributed to upregulation of the antioxidant-related genes. Therefore, all these results have highlighted the potential of arctigenin to inhibit ROS production and accelerate ROS catabolism. Recently, arctigenin was reported to be effective in neuron protection via increasing SOD expression to degrade ROS12 and exert its anti-inflammatory effect by inhibiting the production of ROS in RAW264.7 cells59. Here, we discovered that arctigenin not only stimulated the expression of the key antioxidant-related genes of Cu,Zn-SOD, Txn, Gsr, and GPX1 to degrade ROS but also induced UCP2 expression to inhibit ROS production in skeletal muscles. Our results have thereby expanded the pharmacological functions of arctigenin. Notably, AMPK activator, like AICAR, can mimic exercise without additional training to enhance endurance in sedentary mice, and so does arctigenin in sedentary mice23,60. In our current work, we investigated whether arctigenin could mimic exercise to efficiently enhance the swimming endurance of sedentary rats by the upregulation of antioxidant related pathway, although further investigation is needed to underst and the effect of arctigenin in combination with exercise training on endurance and antioxidant capacity.
Figure 5.
A proposed model demonstrating the antioxidant mechanism of arctigenin.
A previous study in our lab reported that arctigenin could enhance mouse endurance by promoting AMPK phosphorylation and increasing mitochondrial biogenesis23. However, there are seemingly contradictory results in that arctigenin could inhibit mitochondrial respiratory chain complex I and reduce the ratio of AMP/ATP in L6 cells22,61. Actually, the effect of arctigenin on the ratio of AMP/ATP was only tested after 2 h incubation, while the arctigenin-enhanced mouse or rat endurance was a long-term effect with 24 h incubation in cells and a 6-week administration in a mouse or rat model. Therefore, to clarify the relationship between the arctigenin-mediated endurance enhancement and mitochondria function, the levels of ATP in L6 cells with long-term incubation and in skeletal muscle, including gastrocnemius and quadriceps, were examined. As shown in Figures 2E and 4D–4E, arctigenin obviously increased ATP levels in the related cells and tissues. Furthermore, the levels of ERRα and cytochrome c responsible for mitochondrial biogenesis in L6 cells, gastrocnemius and quadriceps (Figure S2) were also significantly elevated with arctigenin treatment, similar to the reported results23. These results therefore indicated that arctigenin upregulated ATP in rat skeletal muscle via increasing mitochondrial biogenesis to enhance the endurance. Therefore, the effect of arctigenin on endurance enhancement might be due to its functions in mitochondrial biogenesis and antioxidant capacity.
Additionally, fatigue has also been reported to be linked to disturbed cerebral dopaminergic neurotransmission, in that an increase in serotonin can inhibit cerebral dopamine neurotransmission to induce fatigue62, while improvement of dopamine transmission in the brain can increase the motivation and ability to exercise63. It was recently found that arctigenin could improve the movement behaviors and upregulate dopamine levels in mouse brains20, which might also contribute to the effect of this natural product on fatigue, although further investigation is needed.
In summary, we demonstrated that arctigenin efficiently enhanced the swimming endurance of sedentary SD rats by elevating the antioxidant capacity of the skeletal muscles through regulation of two antioxidant pathways: AMPK/PGC1α/PPARα in mitochondria and AMPK/p53/Nrf2 in the cell nucleus. Our results have revealed the potential of arctigenin in antioxidant and anti-fatigue research to treat related diseases.
Author contribution
Xu SHEN, Li-hong HU, and Jing CHEN designed the research. Ruo-ming WU, Jing-jing ZHUANG, and Xuan TANG performed the WFST. Ruo-ming WU also performed related assays to reveal the potential molecular mechanisms. Ruo-ming WU, Yan-yan SUN, Zhi-yuan ZHU, and Ting-ting ZHOU contributed to analysis and interpretation of data. Xu SHEN and Jing CHEN supervised the project. Xu SHEN, Jing CHEN, and Ruo-ming WU contributed to the manuscript writing. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81220108025 and 81373461) and the Science Foundation of Shanghai (12431900300).
Footnotes
(Supplementary Figures are available in the Acta Pharmacologica Sinica's website.
Supplementary Information
Arctigenin enhanced swimming endurance of sedentary SD rats
Arctigenin obviously increased the levels of ERRα and cytochrome c in L6 cells and muscle tissues
References
- Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely RA, et al. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur J Appl Physiol. 2002;87:272–7. doi: 10.1007/s00421-002-0631-3. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dynamic Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–31. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, et al. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005;32:633–8. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- Juranek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species — cause or consequence of tissue injury. Gen Physiol Biophys. 2005;24:263–78. [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino acids. 2003;25:207–18. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Melidou M, Riganakos K, Galaris D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. Free Radical Biol Med. 2005;39:1591–600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Zuo L, Otenbaker NP, Rose BA, Salisbury KS. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol. 2013;56:57–63. doi: 10.1016/j.molimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842:1282–94. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Ghosh J, Mishra MK, Basu A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J Antimicrobial Chemother. 2008;61:679–88. doi: 10.1093/jac/dkm503. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SM, Kim SG. Inhibition of arachidonic acid and iron-induced mitochondrial dysfunction and apoptosis by oltipraz and novel 1,2-dithiole-3-thione congeners. Mol Pharmacol. 2009;75:242–53. doi: 10.1124/mol.108.051128. [DOI] [PubMed] [Google Scholar]
- Kumar R, Negi PS, Singh B, Ilavazhagan G, Bhargava K, Sethy NK. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J Ethnopharmacol. 2011;136:260–6. doi: 10.1016/j.jep.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cho BJ, Park TW, Park BE, Kim SJ, Sim SS, et al. Dibenzylbutyrolactone lignans from Forsythia koreana fruits attenuate lipopolysaccharide-induced inducible nitric oxide synthetase and cyclooxygenase-2 expressions through activation of nuclear factor-kappab and mitogen-activated protein kinase in RAW264.7 cells. Biol Pharm Bull. 2010;33:1847–53. doi: 10.1248/bpb.33.1847. [DOI] [PubMed] [Google Scholar]
- Tsai WJ, Chang CT, Wang GJ, Lee TH, Chang SF, Lu SC, et al. Arctigenin from Arctium lappa inhibits interleukin-2 and interferon gene expression in primary human T lymphocytes. Chin Med. 2011;6:12. doi: 10.1186/1749-8546-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wen Q, Ren L, Liang W, Xia Y, Zhang X, et al. Neuroprotective effect of arctigenin via upregulation of P-CREB in mouse primary neurons and human SH-SY5Y neuroblastoma cells. Int J Mol Sci. 2013;14:18657–69. doi: 10.3390/ijms140918657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Sun XX, Ye JM, He L, Yan SS, Zhang HH, et al. Arctigenin alleviates ER stress via activating AMPK. Acta Pharmacol Sin. 2012;33:941–52. doi: 10.1038/aps.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HS, Lee JY, Kim CJ. Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol. 2008;116:305–12. doi: 10.1016/j.jep.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Li D, Liu Q, Jia D, Dou D, Wang X, Kang T. Protective effect of arctigenin against MPP+ and MPTP-induced neurotoxicity. Planta Med. 2014;80:48–55. doi: 10.1055/s-0033-1360171. [DOI] [PubMed] [Google Scholar]
- Huang SL, Yu RT, Gong J, Feng Y, Dai YL, Hu F, et al. Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia. 2012;55:1469–81. doi: 10.1007/s00125-011-2366-3. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhuang J, Chen J, Yu L, Hu L, Jiang H, et al. Arctigenin efficiently enhanced sedentary mice treadmill endurance. PloS One. 2011;6:e 24224. doi: 10.1371/journal.pone.0024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, et al. Arctigenin effectively ameliorates memory impairment in Alzheimer's disease model mice targeting both beta-amyloid production and clearance. J Neurosci. 2013;33:13138–49. doi: 10.1523/JNEUROSCI.4790-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang P, Wang C, Shan Y, Wang D, Qian F, et al. Effect of ginsenoside Rg3 on tyrosine hydroxylase and related mechanisms in the forced swimming-induced fatigue rats. J Ethnopharmacol. 2013;150:138–47. doi: 10.1016/j.jep.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Jung HY, Lee AN, Song TJ, An HS, Kim YH, Kim KD, et al. Korean mistletoe (Viscum album coloratum) extract improves endurance capacity in mice by stimulating mitochondrial activity. J Med Food. 2012;15:621–8. doi: 10.1089/jmf.2010.1469. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003;17:675–81. doi: 10.1096/fj.02-0951com. [DOI] [PubMed] [Google Scholar]
- Russo GL, Russo M, Ungaro P. AMP-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol. 2013;86:339–50. doi: 10.1016/j.bcp.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–6. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–73. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano MB. The guardian recruits cops: the p53-p21 axis delegates prosurvival duties to the Keap1-Nrf2 stress pathway. Mol Cell. 2009;34:637–9. doi: 10.1016/j.molcel.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–6. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Du X, Xu H, Jiang H, Xie J. Akt/Nrf2 activated upregulation of heme oxygenase-1 involves in the role of Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells. Neurotoxicity Res. 2013;24:71–9. doi: 10.1007/s12640-012-9362-3. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radical Biol Med. 2012;52:1054–66. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Kodde IF, van der Stok J, Smolenski RT, de Jong JW. Metabolic and genetic regulation of cardiac energy substrate preference. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:26–39. doi: 10.1016/j.cbpa.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J Mol Med. 2007;85:697–706. doi: 10.1007/s00109-007-0170-9. [DOI] [PubMed] [Google Scholar]
- Grabacka M, Reiss K. Anticancer properties of PPARalpha-effects on cellular metabolism and inflammation. PPAR Res. 2008;2008:930705. doi: 10.1155/2008/930705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Wu CA, Wu KL, Ho YH, Chang AY, Chan JY. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ Res. 2009;105:886–96. doi: 10.1161/CIRCRESAHA.109.199018. [DOI] [PubMed] [Google Scholar]
- Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18:52–8. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–61. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karisch R, Fernandez M, Taylor P, Virtanen C, St-Germain JR, Jin LL, et al. Global proteomic assessment of the classical protein-tyrosine phosphatome and "Redoxome". Cell. 2011;146:826–40. doi: 10.1016/j.cell.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JW, Sun C, Magalhaes MA, Glogauer M. Rac regulates PtdInsP(3) signaling and the chemotactic compass through a redox-mediated feedback loop. Blood. 2011;118:6164–71. doi: 10.1182/blood-2010-09-310383. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE, et al. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol. 2012;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keizer PLJ, Burgering BMT, Dansen TB. Forkhead box O as a sensor, mediator, and regulator of redox signaling. Antioxid Redox Sign. 2011;14:1093–106. doi: 10.1089/ars.2010.3403. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang J, Yi J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid Redox Signal. 2012;16:649–57. doi: 10.1089/ars.2011.4313. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Desrochers PE, Pizzo SV, Gonias SL, Sahakian JA, Levine RL, et al. Oxidative dissociation of human alpha(2)-macroglobulin tetramers into dysfunctional dimers. J Biol Chem. 1994;269:4683–91. [PubMed] [Google Scholar]
- Carp H, Janoff A. Inactivation of bronchial mucous proteinase inhibitor by cigarette smoke and phagocyte-derived oxidants. Exp Lung Res. 1980;1:225–37. doi: 10.3109/01902148009065462. [DOI] [PubMed] [Google Scholar]
- Castrogiovanni P, Imbesi R. Oxidative stress and skeletal muscle in exercise. Ital J Anat Embryol. 2012;117:107–17. [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Radak Z, Koltai E, Taylor AW, Higuchi M, Kumagai S, Ohno H, et al. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radial Biol Med. 2013;58:87–97. doi: 10.1016/j.freeradbiomed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–9. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Porter RK. Mitochondrial proton leak: a role for uncoupling proteins 2 and 3. Biochim Biophys Acta. 2001;1504:120–7. doi: 10.1016/s0005-2728(00)00246-2. [DOI] [PubMed] [Google Scholar]
- Leker RR, Aharonowiz M, Greig NH, Ovadia H. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol. 2004;187:478–86. doi: 10.1016/j.expneurol.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–29. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobon-Velasco JC, Vazquez-Victorio G, Macias-Silva M, Cuevas E, Ali SF, Maldonado PD, et al. S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radical Biol Med. 2012;53:1024–40. doi: 10.1016/j.freeradbiomed.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y, et al. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2-ARE signaling pathway as a potential mechanism. Free Radic Biol Med. 2014;73:1–11. doi: 10.1016/j.freeradbiomed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Kou XJ, Qi SM, Dai WX, Luo L, Yin ZM. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int Immunopharmacol. 2011;11:1095–102. doi: 10.1016/j.intimp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Selinger DW, Solomon JM, Wu H, Schmitt E, Serluca FC, et al. Integrated compound profiling screens identify the mitochondrial electron transport chain as the molecular target of the natural products manassantin, sesquicillin, and arctigenin. Acs Chem Biol. 2013;8:257–67. doi: 10.1021/cb300495e. [DOI] [PubMed] [Google Scholar]
- Blier P, Briley M. The noradrenergic symptom cluster: clinical expression and neuropharmacology. Neuropsychiatr Dis Treat. 2011;7 (Suppl 1):15–20. doi: 10.2147/NDT.S19613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman E, Lilja A. Increased monoaminergic neurotransmission improves compliance with physical activity recommendations in depressed patients with fatigue. Med Hypothese. 2013;80:47–9. doi: 10.1016/j.mehy.2012.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arctigenin enhanced swimming endurance of sedentary SD rats
Arctigenin obviously increased the levels of ERRα and cytochrome c in L6 cells and muscle tissues