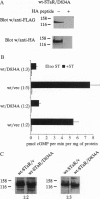
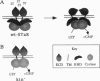
Abstract
Infection with enterotoxigenic Escherichia coli is a leading cause of traveler's diarrhea. Many enterotoxigenic E. coli strains produce heat-stable enterotoxin (ST), a peptide that binds to the intestinal receptor guanylyl cyclase C known as STaR. The toxin-receptor interaction elevates intracellular cGMP, which then activates apical chloride secretion, resulting in secretory diarrhea. In this report, we examine how the intracellular domains of STaR participate in the propagation and regulation of signaling. We show that STaR exists as an oligomer in both the presence and the absence of toxin. We also demonstrate that deletion of the intracellular kinase-homology domain produces a constitutively active mutant, suggesting that this domain subserves an autoinhibitory function. Finally, we constructed a point mutant within a highly conserved region of the cyclase domain that completely inactivates the catalytic activity of guanylyl cyclase. Cotransfection of this point mutant with wild-type receptor causes a dominant-negative effect on receptor activation. This suggests that interaction of receptor subunits is required for toxin-induced activation and that the cyclase domain is involved in this essential interaction. We propose that the binding of ST to STaR promotes a conformational change across the cell membrane. This removes the inhibitory effects of the kinase-homology domain and promotes an interaction between cyclase domains that leads to receptor activation. The data suggest a paradigm of signal transduction that may also be relevant to other members of the guanylyl cyclase receptor family.
Full text
PDF

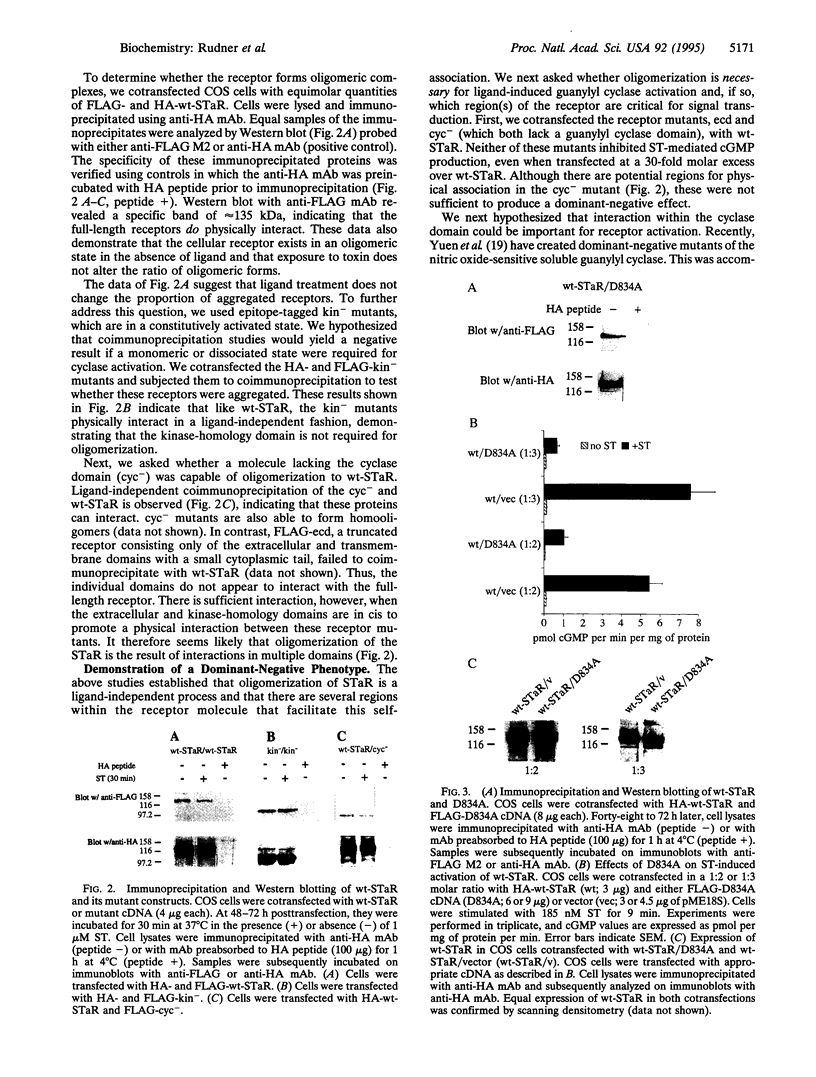


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J. S., Williams S. I., Scheving L. A., Judd A. K., Schoolnik G. K. Ligand-based histochemical localization and capture of cells expressing heat-stable enterotoxin receptors. Mol Microbiol. 1993 May;8(5):865–873. doi: 10.1111/j.1365-2958.1993.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Chao A. C., de Sauvage F. J., Dong Y. J., Wagner J. A., Goeddel D. V., Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994 Mar 1;13(5):1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989 Sep 22;245(4924):1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Wilson E. M. Ligand-independent oligomerization of natriuretic peptide receptors. Identification of heteromeric receptors and a dominant negative mutant. J Biol Chem. 1992 Sep 15;267(26):18589–18597. [PubMed] [Google Scholar]
- Cohen M. B., Mann E. A., Lau C., Henning S. J., Giannella R. A. A gradient in expression of the Escherichia coli heat-stable enterotoxin receptor exists along the villus-to-crypt axis of rat small intestine. Biochem Biophys Res Commun. 1992 Jul 15;186(1):483–490. doi: 10.1016/s0006-291x(05)80833-2. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewett J. G., Garbers D. L. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 1994 Apr;15(2):135–162. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Ericsson C. D. Prevention and treatment of traveler's diarrhea. N Engl J Med. 1993 Jun 24;328(25):1821–1827. doi: 10.1056/NEJM199306243282507. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Eber S. L., Turner J. T., Freeman R. H., Fok K. F., Currie M. G. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest. 1993 Jun;91(6):2423–2428. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Wada A., Hidaka Y., Fujisawa J., Takeda Y., Shimonishi Y. Expression of a truncated guanylate cyclase (GC-C), a receptor for heat-stable enterotoxin of enterotoxigenic Escherichia coli, and its dimer formation in COS-7 cells. Microb Pathog. 1993 Oct;15(4):283–291. doi: 10.1006/mpat.1993.1078. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Jewett J. R., Koller K. J., Goeddel D. V., Lowe D. G. Hormonal induction of low affinity receptor guanylyl cyclase. EMBO J. 1993 Feb;12(2):769–777. doi: 10.1002/j.1460-2075.1993.tb05711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K. J., de Sauvage F. J., Lowe D. G., Goeddel D. V. Conservation of the kinaselike regulatory domain is essential for activation of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biol. 1992 Jun;12(6):2581–2590. doi: 10.1128/mcb.12.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Lowe D. G. Human natriuretic peptide receptor-A guanylyl cyclase is self-associated prior to hormone binding. Biochemistry. 1992 Nov 3;31(43):10421–10425. doi: 10.1021/bi00158a001. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Evans P. R. Structural basis of the allosteric behaviour of phosphofructokinase. Nature. 1990 Jan 11;343(6254):140–145. doi: 10.1038/343140a0. [DOI] [PubMed] [Google Scholar]
- Schulz S., Chrisman T. D., Garbers D. L. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992 Aug 15;267(23):16019–16021. [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Thompson D. K., Garbers D. L. Dominant negative mutations of the guanylyl cyclase-A receptor. Extracellular domain deletion and catalytic domain point mutations. J Biol Chem. 1995 Jan 6;270(1):425–430. doi: 10.1074/jbc.270.1.425. [DOI] [PubMed] [Google Scholar]
- Tien X. Y., Brasitus T. A., Kaetzel M. A., Dedman J. R., Nelson D. J. Activation of the cystic fibrosis transmembrane conductance regulator by cGMP in the human colonic cancer cell line, Caco-2. J Biol Chem. 1994 Jan 7;269(1):51–54. [PubMed] [Google Scholar]
- Vaandrager A. B., van der Wiel E., Hom M. L., Luthjens L. H., de Jonge H. R. Heat-stable enterotoxin receptor/guanylyl cyclase C is an oligomer consisting of functionally distinct subunits, which are non-covalently linked in the intestine. J Biol Chem. 1994 Jun 10;269(23):16409–16415. [PubMed] [Google Scholar]
- Yuen P. S., Doolittle L. K., Garbers D. L. Dominant negative mutants of nitric oxide-sensitive guanylyl cyclase. J Biol Chem. 1994 Jan 14;269(2):791–793. [PubMed] [Google Scholar]
- de Sauvage F. J., Camerato T. R., Goeddel D. V. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991 Sep 25;266(27):17912–17918. [PubMed] [Google Scholar]
- de Sauvage F. J., Horuk R., Bennett G., Quan C., Burnier J. P., Goeddel D. V. Characterization of the recombinant human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1992 Apr 5;267(10):6479–6482. [PubMed] [Google Scholar]
- de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]