Abstract
Several attempts have been made to directly date phytoliths, but most 14C results are not consistent with other independent chronologies. Due to the limited dataset, there is not a clear explanation for these discrepancies. Herein, we report the 14C ages of phytolith-occluded carbon (PhytOC) from contemporary rice and millet crops that were combusted at different temperatures to investigate the relationship between the combustion temperature and resulting 14C age. Our results show that the 14C age of PhytOC increases directly with combustion temperature (up to 1100°C) and results in age overestimations of hundreds of years. Considerably older ages are observed at higher temperatures, suggesting that it may be possible to distinguish between two fractions of organic carbon in phytoliths: labile and recalcitrant carbon. These findings challenge the assumption that PhytOC is homogeneous, an assumption made by those who have previously attempted to directly date phytoliths using 14C.
Rice and millet are two staple food crops that have been cultivated in the Yellow and Yangtze River basins of China since the early Neolithic period1,2. There is some uncertainty regarding the origin and spread of these important crops across East Asia, particularly China, due to the lack of adequate evidence from crop remains in archaeological sites3,4,5. In these archaeological sites, with climates ranging from moist and warm to semi-arid, typically only charred plant remains survive for several millennia6,7. As an alternative to these scarce, charred plant remains, phytoliths (biogenetic opals formed in plants when roots absorb soluble silica) represent a potentially useful geochronometer8,9. When plants die and decay, phytoliths are released into the soil and sediment10,11. Phytoliths are extremely durable and can be preserved in large amounts in most archaeological sites, and in some situations, they can form well-defined strata12,13.
Organic carbon (PhytOC) is occluded during the formation of a phytolith and sometimes represents up to 2% of the dry weight of a plant14,15,16,17. The PhytOC contents of millet and rice have been estimated to be 1.36 mg g−1 and 2.8 mg g−1, respectively18,19. The small sample size required by accelerator mass spectrometry (AMS) allows for the analysis of < 500 μg of carbon; therefore, only a handful of soil can provide a sufficient amount of phytoliths for radiocarbon analysis.
Over the past decades, there have been a few attempts to measure the 14C age of fossil phytoliths8,10,13,20,21,22. However, most 14C ages of phytoliths are not consistent with independent chronologies. These discrepancies have been attributed to preferential oxidation8, stratigraphic disturbances20, or sample extraction methods23,24,25. Moreover, some new 14C analyses of PhytOC from phytolith concentrates extracted from living grass or bamboo reported ages of up to several thousand years older26,27. A clear explanation for these surprisingly older dates is still lacking due to the limited dataset and exploration of extraction protocols27,28.
One hypothesis suggests that there may be two possible components of photosynthetic and recalcitrant organic matter in phytoliths27. The exact influence of these two components of PhytOC on 14C dating needs to be examined in detail because previous attempts to date 14C PhytOC have focused on the total organic matter within the phytolith, and these attempts have clearly failed. In some cases, the combustion temperature can be used to separate heterogeneous mixtures of labile and refractory carbonaceous components, each of which may have a different apparent radiocarbon age29,30,31. Recently, a more robust phytolith extraction protocol suitable for carbon isotopic analysis was reported32, and we use this protocol to further investigate the potential source of PhytOC. In this study, phytoliths were isolated from two different species of modern crops (rice and millet) using a modified version of the recently published protocol32 and were then combusted with CuO powder at different temperatures ranging from 160°C to greater than 1400°C. We analyse the 14C and δ13C ratios produced by the range of combustion temperatures and compare them with the current atmospheric ratio to investigate the influence of combustion temperature on 14C age. We explore these results in an attempt to delineate between the two hypothesised components of occluded organic carbon (PhytOC).
Results
Morphology of phytoliths
The extracted rice straw and millet phytoliths were examined using a scanning electron microscope (SEM) to ensure a lack of visible cellulose adhering to the outside of the phytoliths and to ensure the presence of intact samples with diameters of at least 20 μm (Supplementary Figure S1). When the phytoliths were combusted with CuO to form CO2 gas, a manometer was used to monitor the production of CO2 as the temperature increased from 160°C to 900°C. The combustion profiles of the phytolith samples show that the majority of CO2 is generated in two distinct temperature ranges: 500–600°C and 800–900°C (Supplementary Figure S2). This release pattern can be interpreted in terms of carbon being derived from a mixture of at least two components. As shown in Supplementary Figure S1, when the temperature reaches 900°C, most phytoliths lose their distinct morphological characteristics, and when the samples are heated to 1100°C, the phytoliths disappear.
14C concentrations in the modern atmosphere
The AMS 14C concentrations for modern crops and their phytolith samples, along with the δ13C values and carbon yield rates, are listed in Supplementary Table S1. The 14C concentration in the atmosphere can be estimated from the three living crops. The modern fraction (Fm, the deviation of the 14C/12C ratio of a sample from the 14C/12C ratio of “modern carbon”) values from the direct AMS dating of the plant material for AD 2011 rice straw, AD 2012 rice straw and AD 2012 millet are 1.0312 ± 0.0028, 1.0288 ± 0.0027, and 1.0223 ± 0.0027, respectively. These values are consistent with the atmospheric radiocarbon derived from the bomb radiocarbon curve in the Northern Hemisphere atmosphere33,34,35, suggesting that the 14C in the plant is in equilibrium with the 14C in the atmosphere.
14C concentrations in phytoliths with increasing combustion temperature
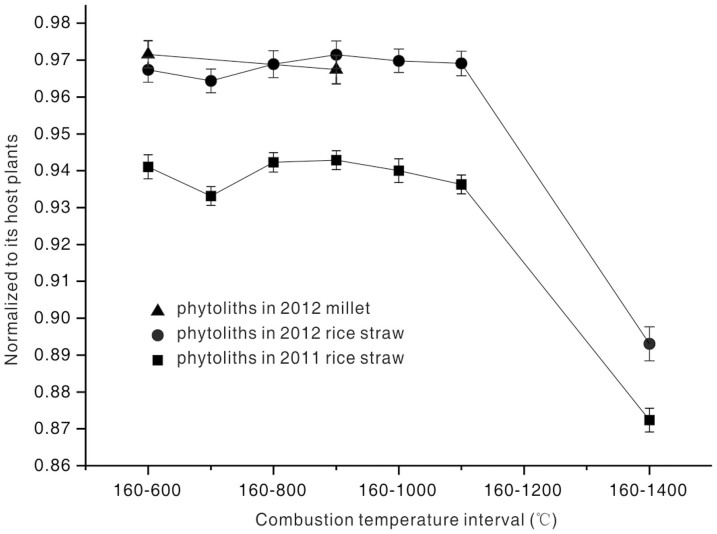
The 14C concentration in an individual phytolith sample was normalised to that in the host crop to more readily identify the deviation between the measured 14C concentration in phytoliths and that measured for the atmosphere. We observe that the AMS 14C concentrations in all 16 phytoliths are lower than those in the atmosphere (Figure 1).
Figure 1. Variations of the AMS 14C concentrations of phytoliths isolated from modern crop with increasing combustion temperature.
Error bars represent the 68% (±1σ) range for overall analytical confidence.
We can confirm a 14C depletion of several percent in PhytOC at combustion temperatures below 1100°C (Supplementary Table S1). For 14C of phytoliths in AD 2011 rice straw, a chi-square test was applied to compare the Fm values of phytoliths combusted at temperatures below 1100°C. The chi-square value of this dataset is 7.04, which is less than 11.07, the critical value for 95% confidence and 5 degrees of freedom, implying that the values are indistinguishable. The weighted mean for the 6 Fm values of phytoliths in AD 2011 rice straw is 0.9393±0.0013. This 14C level is 6% lower than the atmospheric concentration and corresponds to an apparent radiocarbon age of 480 yrs. Similarly, the Fm values of PhytOC from AD 2012 rice straw (C3 plant) and AD 2012 millet (C4 plant) also passed the chi-square test. We obtain weighted means of 0.9684±0.0019 and 0.9577±0.0013, respectively. These results indicate that the deficiency in the 14C content of the two samples is 3% to 4% lower when combusted at temperatures below 1100°C, equivalent to ages of 250 yrs and 320 yrs older than the true ages of the plants, respectively.
In a further experiment, 0.5 g and 0.3 g of phytolith samples were sent to Beta Analytic Inc. to investigate PhytOC during higher-temperature combustions. The phytolith samples were combusted at temperatures greater than 1400°C via the exothermic reaction between tin and oxygen21. The two PhytOC samples combusted at temperatures above 1400°C produced Fm values of 11% and 13% lower than those of the atmosphere, respectively, resulting in age estimates that were ~1000 yrs older than the true ages.
Similarly, the average δ13C values of PhytOC in AD 2011 rice straw and in AD 2012 rice straw combusted at temperatures below 1100°C are −33.66‰ and −32.67‰, respectively (Supplementary Table S1). In contrast, when combusted at temperatures over 1400°C, the δ13C values were 1.17‰ and 2.53‰ heavier, respectively, than those of PhytOC combusted at the lower temperature.
Background check
We also performed one 14C determination on a phytolith assemblage isolated from L1-3 loess to evaluate the background value for this extraction procedure. The OSL age of L1-3 loess is approximately 71 ka BP, which is beyond the limit of the radiocarbon dating method36. The Fm value of phytoliths from L1-3 loess is 0.0051±0.0001, corresponding to 42,380±180 yr BP, which is statistically indistinguishable from the background of our AMS line background, which is equivalent to 42,750±190 yr BP.
Discussion
If phytoliths are to become a new alternative candidate for 14C dating for establishing a reliable age control for archaeological sites, the 14C content in PhytOC must be in equilibrium with the 14C content in the atmosphere when the organism dies. However, in this study, the most striking feature is that the 14C contents in all 16 aliquots of phytoliths are lower than that in the atmosphere. These phytoliths were isolated from modern crops using a reliable, published protocol with only slight modifications. PhytOC accounted for 0.06–0.10% and 0.07% of the dry weight of rice and millet phytoliths, respectively (Supplementary Table S1), which is at least 20 times lower than the range of 14–34 mg g−1 for rice and 25.1 mg g−1 for millet18,19. These results indicate that the protocols used to extract phytoliths in this study are very harsh and can effectively eliminate possible sources of contamination during processing. We require 1200–2200 mg of phytoliths to produce graphite samples with ~0.8 mg of carbon for AMS 14C analysis. The precision and reproducibility of our experimental procedure, better than 0.3% for modern samples, could be evaluated by the mean Fm value of the standard material oxalic acid II during AMS 14C analysis. The background test of the phytolith extraction from L1-3 loess indicates that the protocols in our lab minimise the possibility of introducing contaminant carbon, specifically modern carbon, to the phytolith sample during extraction, graphitisation, and measurement. Moreover, the 6 14C overestimated ages (by hundreds of years) of phytolith samples with different weights from each rice crop are consistent when the samples are combusted at temperatures below 1100°C. The average δ13C values of PhytOC also implies that a new carbonaceous compound with a heavier carbon isotope is liberated from phytoliths when combusted at temperatures greater than 1400°C. Regardless, the change in the isotopic composition of PhytOC in rice straw cannot explain the systematic age offset. Therefore, this 14C depletion in the phytoliths most likely originates from the temperature interval at which the sample itself was combusted. A similar trend has been described in other studies that used the same laboratory protocols27. In addition, this problem is not unique to our laboratory protocols because the AMS 14C dates of phytoliths isolated from mature and recently senesced bamboo leaves using another separation method (microwave digestion) were inexplicably 3.5 ka and 1.9 ka too high, respectively28. These results clearly show a consistent shift in the 14C levels when comparing phytoliths to modern plants regardless of their locations, species, and extraction methods. As mentioned above, for fossil phytoliths, most 14C ages were inconsistent with expected or independent chronologies8,20,23,24,37. This problem could reveal that, even though the 14C of the plant is in equilibrium with the atmosphere, the 14C content of PhytOC is not.
Phytoliths can be changed by heating. Dry ashing has been widely used to recover phytoliths from plants since the beginning of phytolith analysis38. This method involves the incineration of plant tissue in muffled furnaces at temperatures of at least 500°C. This technique has been suggested to cause shrinkage, warping and changes to the refractive index of phytoliths39,40. For rice crop phytoliths, it has been found that the original physical characteristics are significantly altered when the extraction temperature exceeds 900°C41. Previous studies indicated that the relationship between the 14C age and combustion temperature can be used to quantify the relative contributions of different pools of organic carbon29,30,31. For phytoliths in AD 2012 rice straw, the Fm of PhytOC was depleted by approximately 4% when the sample was combusted below 1100°C and was even more depleted (to approximately 12%) when combusted over 1400°C. We observe a similar trend between the combustion temperature and Fm depletion of PhytOC in AD 2011 rice straw, suggesting that a mechanism may govern the 14C variation with temperature. Our results indicate multiple carbon species, each of which may potentially have a unique 14C concentration in phytoliths. One possible explanation is that there are two components of organic matter in phytoliths. The low-temperature pool (combusted below 900°C) is associated with relatively labile carbon, whereas the high pool (combusted above 900°C) is commonly associated with recalcitrant carbon.
We still have an incomplete understanding of PhytOC in phytoliths25,42,43,44,45. An early hypothesis suggested that recalcitrant PhytOC (biasing the 14C results towards age overestimates) could be brought from the soil to the plant by root uptake27, but this hypothesis is still under debate28,46. We entertain another hypothesis to explain the discrepancies in the 14C contents of our measured samples: two possible pools of 14C exist in the phytoliths, each of which is attributed to a different type of phytolith development. Phytoliths are biogenetic opals that form in the cell walls, cell lumina and intercellular spaces of plants14,47,48. If biosilicification primarily occurs in the cell walls, hollow forms of phytoliths are formed and often contain cellular organelles such as mitochondria and plastids25,49. However, if biosilicification occurs in cell lumina or between cells, solid forms of phytoliths develop and contain mostly lipids and nucleic acids25,49. For sugar cane, the amount of PhytOC retained within hollow rather than solid phytoliths has been estimated to be 10.12% and 0.15%, respectively50. These results suggest that, on average, the PhytOC from hollow phytolith cavities is 50 times more abundant than the PhytOC from solid phytoliths. Similar trends have also been observed in sorghum50. We also observe that organic materials are liberated from phytoliths in two distinct stages. For example, for rice straw from AD 2011, the carbon yield rate was 0.07%, and the Fm value was 0.9363±0.0038 when combusted below 1100°C (Supplementary Table S1). When combusted above 1400°C, although the carbon yield rates did not change, the Fm value was 0.8724 ± 0.0046. This Fm value, attributed to the recalcitrant fraction of PhytOC, increased the apparent age of phytoliths even though the amount of recalcitrant carbon is minor compared to the total amount of carbon in the samples. The ratio of carbon released below 1100°C to that released above 1100°C during combustion can be estimated using a simple, two end-member mixing model. If one end-member is the 14C released below 1100°C (Fm = 0.9363 ± 0.0038 for rice straw from AD 2011), then the other end-member is the 14C released above 1100°C (Fm = 0, dead carbon, no 14C); we calculate the ratio as 13. Similarly, for AD 2012 rice straw, we calculate the ratio as 12. Therefore, it is possible that the low-temperature carbon pool originates from hollow phytoliths formed in porous cell walls during photosynthesis. This carbon pool is more readily available for oxidation and lost from phytoliths at lower temperatures. Conversely, the high-temperature carbon pool may represent solid phytoliths formed in cell lumina. Further investigation of the mechanisms responsible for 14C differentiation in the two fractions of PhytOC is necessary.
Therefore, we are working toward a new protocol that can successfully remove the recalcitrant carbon from phytoliths such that we can establish a reliable 14C dating method for phytoliths. Among the many potential uses, a new protocol will allow us to better constrain the timing and origins of agriculture in East Asia.
Methods
Materials
Phytoliths were extracted from modern plants: two rice (Oryza sativa) stems (with leaves) and one millet (Panicum miliaceum). The dried rice straws were harvested from the Hubei province near the Yangtze River (115° E, 30° N) in two successive years: AD 2011 and AD 2012. The millet was obtained from the Nihewan basin (114° E, 40° N) in northwest Beijing in AD 2012. A fossil loess sample was collected from the Weinan section on the southeastern Chinese Loess Plateau and was dated by optically stimulated luminescence (OSL) to ca. 71 ka BP36.
Phytoliths isolated from modern crops and loess
Phytoliths were extracted from modern plants using a protocol involving both a sink-float specific gravity and a wet-digestion method detailed below (modified from a recently published protocol32). Approximately 700 g of each dried crop was used to obtain contemporary phytoliths. The rice straw samples were initially cut into cm-sized pieces and immersed in a distilled H2O ultrasonic bath to remove any matter adhering to their surfaces. The sample was then immersed in 1 N hydrochloric acid (HCl) for 4 h to eliminate carbonate material and stirred every half hour. The samples were washed three times in distilled H2O after the acid was decanted. The treated samples were dried in an oven at 70°C for three days. A 650 g aliquot of treated dry rice straw was weighed into a 4000 ml beaker and digested with ~5 ml of concentrated H2SO4 per gram of dry plant material under a fume hood. The digestion lasted for 2 h at 70°C, and the sample then sat unheated overnight. The following day, the hot plate was reheated to 70°C, and hydrogen peroxide (H2O2, 30%) was gradually added to destroy organic material until the liquid was clear and colourless. The supernatant liquid was poured off, and then the remains were rinsed 3 times with distilled water. The remaining solid material was then transferred into 100 ml beakers and dried at 70°C. After drying, we floated phytoliths using a heavy liquid (KI + HI + Zn) with a specific gravity (sp. gr.) of 2.351. The phytoliths were concentrated by centrifuging at 3000 rpm for 30 min. A few tiny dark minerals remained on the bottom of the cup after centrifugation. The liquid was then diluted with distilled water to a sp. gr. of 1.5. The supernatant liquid (with minimal organic matter) was poured off. This process was repeated three times. The recovered phytoliths were dried and retained.
After the phytoliths were recovered, they were washed and reheated at 70°C for 2 h in concentrated HNO3 and NaClO2 to ensure that any organic material clinging to the outer surfaces was removed. The sample sat unheated overnight, and the liquid was decanted. This step was repeated 4 times to maximise the oxidation of organic matter. The phytoliths were rinsed 3 times with distilled water and dried. Subsequently, they were immersed in a 0.001 M NaOH solution and heated at 70°C for 15 min to remove any alkali-soluble forms of organic matter. Afterward, the isolated phytoliths were washed again with concentrated HNO3 and rinsed 3 times with distilled water. Finally, phytolith concentrates were dried at 70°C, weighed and observed with an optical microscope.
The background extraction method for phytoliths in loess is similar to the aforementioned method for modern plants. Sand grains larger than phytoliths were first removed by wet-sieving through an 80-mesh screen and then added to 1 N HCl to eliminate carbonates. Subsequently, two 50-litre settling containers were used to separate the clays and organic colloids. The sedimentation-decanting procedure was repeated several times until the water was clear. The sample then was dried at 70°C. The subsequent steps were the same as for the modern plants, but the extraction is usually repeated twice to increase phytolith recovery.
Cellulose extracted from modern crops
The radiocarbon content of cellulose separated from modern crops was used to represent the atmospheric 14CO2 concentration. The crops were treated with sodium hydroxide at 70°C overnight. The alkali solution was refreshed until it remained clear, indicating that all soluble carbon had been removed. To yield alpha-cellulose, the sample was placed in a beaker and bleached with hot NaClO2/HCl. This solution was heated to 70°C. This bleaching procedure was repeated every 2 to 3 h until the sample appeared white. If the sample was not yet white, the bleaching procedure was repeated. After the straw had been satisfactorily bleached, it was rinsed with distilled water repeatedly and then dried in an oven at 70°C.
Measurements
Before isotope analyses, the phytolith morphologies were observed via SEM (a JSM-5800 equipped with an EDS system at the Beijing University of Aeronautics and Astronautics).
Approximately 1200 to 2200 mg of phytolith samples was transferred into clean 9-mm quartz tubes and combusted in a muffled furnace at 160°C overnight to release the carbon absorbed at the surface of phytoliths26. After the quartz tubes were cool, a measured amount of CuO powder and silver wire were added to the quartz tubes, and then they were connected to a graphite line and flame-sealed. PhytOC was combusted at temperature intervals ranging from 600°C to 1100°C to release CO2. Beyond 1000°C, the phytolith sample was placed in a 6-mm quartz tube and then enveloped in a 9-mm tube to avoid the rupture of the quartz tube during combustion. The CO2 was purified, and the volume of CO2 was measured. CO2 was then split, and a small portion of the sample was used to make a δ13C at the State Key Laboratory of Loess and Quaternary Geology, CAS, with a conventional isotope ratio mass spectrometer (except for cases in which an insufficient amount of CO2 was produced). Isotopic ratios in samples are expressed as per mil deviation relative to a VPDB standard with precision better than 0.2‰52. The remaining CO2 was converted into graphite over iron at 550°C in the presence of H253. AMS 14C dates were measured at PKU in a 0.5 MV AMS and reported in Fm values. The 14C errors of each date include statistical uncertainties based on 14C counts, standard and 14C background targets, and machine random error54. The AMS 14C dates are listed in Supplementary Table S1.
A series of standards run at the same time as the samples yielded the following values in agreement with the consensus values. The Fm values of 6 targets from the first wheel were 1.3406 ± 0.0042, 1.3357 ± 0.0024, 1.3342 ± 0.0028, 1.3448 ± 0.0044, 1.3447 ± 0.0032, and 1.3442 ± 0.0029, with a weighted average Fm of 1.3401 ± 0.0007. In addition, the Fm values of 6 targets from the second wheel were 1.3427 ± 0.0022, 1.3388 ± 0.0021, 1.3406 ± 0.0021, 1.3439 ± 0.0028, 1.3424 ± 0.0023, and 1.3356 ± 0.0022, and the weighted mean Fm can also be estimated to 1.3404 ± 0.0006. These results agree well with the consensus value of 1.3407 and demonstrate that the precision and reproducibility of our system are better than 0.3% for modern samples.
Author Contributions
J. Y. conceived the study, phytolith extraction, data interpretation, and wrote the manuscript. X. Y. performed the 14C graphite and discussion of the results. Y. Z. assigned sample collection and sample pre-clean.
Supplementary Material
Supplementary Information
Acknowledgments
This work was jointly supported by State Key Laboratory of Earthquake Dynamics (Project No. LED2013A08) and the fundamental scientific research special project of Institute of Geology, China Earthquake Administration (No: IGCEA-1116), and State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, CAS(SKLLQG0707). We are especially indebted to the technical support of the staff in PKUAMS center for their assistance and patient accommodation of our many AMS measurement requests.
References
- Lu H. et al. Millet noodles in late Neolithic China. Nature 437, 967–968 (2005). [DOI] [PubMed] [Google Scholar]
- Zong Y. et al. Fire and flood management of coastal swamp enabled first rice paddy cultivation in east China. Nature 449, 459–462 (2007). [DOI] [PubMed] [Google Scholar]
- Fuller D. Q., Harvey E. & Qin L. Presumed domestication? Evidence for wild rice cultivation and domestication in the fifth millennium BC of the Lower Yangtze region. Antiquity 81, 316–331 (2007). [Google Scholar]
- Crawford G. W. & Shen C. The origins of rice agriculture: recent progress in East Asia. Antiquity 72, 858–866 (1998). [Google Scholar]
- Zhang J. et al. Early Mixed Farming of Millet and Rice 7800 Years Ago in the Middle Yellow River Region, China. PLoS One 7, e52146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. New archaeobotanic data for the study of the origins of agriculture in China. Curr. Anthropol. 52, 295–306 (2011). [Google Scholar]
- Atahan P. et al. Holocene-aged sedimentary records of environmental changes and early agriculture in the lower Yangtze, China. Quaternary Sci. Rev. 27, 556–570 (2008). [Google Scholar]
- Wilding L. P. Radiocarbon dating of biogenetic opal. Science 156, 66–67 (1967). [DOI] [PubMed] [Google Scholar]
- Prasad V., Stromberg C. A. E., Alimohammadian H. & Sahni A. Dinosaur coprolites and the early evolution of grasses and grazers. Science 310, 1177–1180 (2005). [DOI] [PubMed] [Google Scholar]
- Mulholland S. C. & Prior C. [AMS radiocarbon dating of phytoliths] Current Research in Phytolith Analysis: Applications in Archaeology and Paleoecology, MASCA Research Papers in Science and Archaeology [Pearsall, D. M. & Piperno, D. R. (eds.)] [21–23] (The University Museum of Archaeology and Anthropology, University of Pennsylvania, Philadelphia, 1993).
- Parr J. F. & Sullivan L. A. Soil carbon sequestration in phytoliths. Soil Biol. Biochem. 37, 117–124 (2005). [Google Scholar]
- Albert R. M. et al. Phytolith-rich layers from the Late Bronze and Iron Ages at Tel Dor (Israel): mode of formation and archaeological significance. J. Archaeol. Sci. 35, 57–75 (2008). [Google Scholar]
- Piperno D. R. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. (AltaMira Press, Lanham MD, 2006). [Google Scholar]
- Prychid C. J., Rudall P. J. & Gregory M. Systematics and biology of silica bodies in monocotyledons. Bot. Rev. 69, 377–440 (2003). [Google Scholar]
- Jones L. H. P., Milne A. A. & Wadham S. M. Studies of silica in the oat plant. Plant Soil 18, 358–371 (1963). [Google Scholar]
- Huang Z.-T. et al. Long-term intensive management increased carbon occluded in phytolith (PhytOC) in bamboo forest soils. Sci. Rep. 4, 3602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Song Z. & Li B. The production and accumulation of phytolith-occluded carbon in Baiyangdian reed wetland of China. Appl. Geochem. 37, 117–124 (2013). [Google Scholar]
- Zuo X. & Lü H. Carbon sequestration within millet phytoliths from dry-farming of crops in China. Chinese Sci. Bull. 56, 3451–3456 (2011). [Google Scholar]
- Li Z., Song Z., Parr J. F. & Wang H. Occluded C in rice phytoliths: implications to biogeochemical carbon sequestration. Plant soil 370, 615–623 (2013). [Google Scholar]
- Kelly E. F., Amundson R. G., Marino B. D. & Deniro M. J. Stable isotope ratios of carbon in phytoliths as a quantitative method of monitoring vegetation and climate change. Quaternary Res. 35, 222–233 (1991). [Google Scholar]
- Piperno D. R. & Stothert K. E. Phytolith evidence for early Holocene Cucurbita domestication in southwest Ecuador. Science 299, 1054–1057 (2003). [DOI] [PubMed] [Google Scholar]
- McMichael C. H. et al. Spatial and temporal scales of pre-Columbian disturbance associated with western Amazonian lakes. Holocene 22, 131–141 (2012). [Google Scholar]
- Boaretto E. Dating materials in good archaeological contexts: the next challenge for radiocarbon analysis. Radiocarbon 51, 275–281 (2009). [Google Scholar]
- Prior C. A., Carter J. & Rieser U. Are phytolith radiocarbon dates reliable? the 10th International Conference on Accelerator Mass Spectrometry, Berkeley, USA. (http://llnl.confex.com/llnl/ams10/techprogram/P1592.HTM) (2005, September 5–10).
- Carter J. A. Atmospheric carbon isotope signatures in phytolith-occluded carbon. Quatern. Int. 193, 20–29 (2009). [Google Scholar]
- Santos G. M. et al. The phytolith 14C puzzle: a tale of background determinations and accuracy tests. Radiocarbon 52, 113–128 (2010). [Google Scholar]
- Santos G. M. et al. Possible source of ancient carbon in phytolith concentrates from harvested grasses. Biogeosciences 9, 1873–1884 (2012). [Google Scholar]
- Sullivan L. A. & Parr J. F. Comment on:” Possible source of ancient carbon in phytolith concentrates from harvested grasses” by G. M. Santos et al.(2012). Biogeosciences 9, 13773–13782, 10.5194/bgd-9-13773-2012 (2012). [Google Scholar]
- McGeehin J. et al. Stepped-combustion 14C dating of sediment: A comparison with established techniques. Radiocarbon 43, 255–261 (2001). [Google Scholar]
- Kolic E. D. Direct radiocarbon dating of pottery: Selective heat treatment to retrieve smoke-derived carbon. Radiocarbon 37, 275–284 (1995). [Google Scholar]
- Hatté C., Hodgins G., Holliday V. T. & Jull A. J. T. Dating Human Occupation on Diatom-Phytolith-Rich Sediment: Case Studies of Mustang Spring and Lubbock Lake, Texas, USA. Radiocarbon 52, 13–24 (2010). [Google Scholar]
- Corbineau R., Reyerson P. E., Alexandre A. & Santos G. M. Towards producing pure phytolith concentrates from plants that are suitable for carbon isotopic analysis. Rev. Palaeobot. Palyno. 197, 179–185 (2013). [Google Scholar]
- Levin I. et al. Observations and modelling of the global distribution and long–term trend of atmospheric 14CO2. Tellus B 62B, 26–46 (2010). [Google Scholar]
- Graven H. D., Guilderson T. P. & Keeling R. F. Observations of radiocarbon in CO2 at La Jolla, California, USA 1992–2007: Analysis of the long–term trend. J. Geophys. Res. 117 (2012). [Google Scholar]
- Hua Q., Barbetti M. & Rakowski A. Z. Atmospheric Radiocarbon for the Period 1950–2010. Radiocarbon 55, 2059–2072 (2013). [Google Scholar]
- Kang S., Lu Y. & Wang X. Closely-spaced recuperated OSL dating of the last interglacial paleosol in the southeastern margin of the Chinese Loess Plateau. Quat. Geochronol. 6, 480–490 (2011). [Google Scholar]
- McClaran M. P. & Umlauf M. Desert grassland dynamics estimated from carbon isotopes in grass phytoliths and soil organic matter. J. Veg. Sci. 11, 71–76 (2000). [Google Scholar]
- Rovner I. [Plant opal phytolith analysis: major advances in archaeobotanical research]. Advances in archaeological method and theory [Schiffer, M. B. (ed.)] [225–266] (Academic Press, New York, 1983). [Google Scholar]
- Elbaum R., Weiner S., Albert R. M. & Elbaum M. Detection of burning of plant materials in the archaeological record by changes in the refractive indices of siliceous phytoliths. J. Archaeol. Sci. 30, 217–226 (2003). [Google Scholar]
- Parr J. F., Lentfer C. J. & Boyd W. E. A comparative analysis of wet and dry ashing techniques for the extraction of phytoliths from plant material. J. Archaeol. Sci. 28, 875–886 (2001). [Google Scholar]
- Wu Y., Wang C. & Hill D. V. The transformation of phytolith morphology as the result of their exposure to high temperature. Microsc. Res. Techniq. 75, 852–855 (2012). [DOI] [PubMed] [Google Scholar]
- Pironon J. et al. [Individual characterization of phytoliths: experimental approach and consequences on paleoenvironment understanding]. Phytoliths:Applications in Earth Science and Human History [Meunier, J. D. & Colin, F. (eds.)] [329–341] (A.A. Balkema Publishers, Lisse, 2001). [Google Scholar]
- Smith F. A. & Anderson K. B. [Characterization of organic compounds in phytoliths: Improving the resolving power of phytolith δ13C as a tool for paleoecological reconstruction of C3 and C4 grasses]. Phytoliths: applications in earth sciences and human history [Meunier, J. D. & Colin, F. (eds.)] [317–327] (A.A. Balkema Publishers, Lisse, 2001). [Google Scholar]
- Perry C. C., Williams R. J. P. & Fry S. C. Cell wall biosynthesis during silicification of grass hairs. J. Plant Physiol. 126, 437–448 (1987). [Google Scholar]
- Elbaum R., Melamed-Bessudo C., Tuross N., Levy A. A. & Weiner S. New methods to isolate organic materials from silicified phytoliths reveal fragmented glycoproteins but no DNA. Quatern. Int. 193, 11–19 (2009). [Google Scholar]
- Evett R. Interactive comment on “Comment on: “Possible source of ancient carbon in phytolith concentrates from harvested grasses” by G. M. Santos et al.(2012)” by L. A. Sullivan and J. F. Parr. http://www.biogeosciences-discuss.net/9/C6286/2012/ (2012) Date of access: 5 December 2012.
- Song Z., Wang H., Strong P. J., Li Z. & Jiang P. Plant impact on the coupled terrestrial biogeochemical cycles of silicon and carbon: Implications for biogeochemical carbon sequestration. Earth-Sci. Rev. 115, 319–331 (2012). [Google Scholar]
- Piperno D. R. Phytolith analysis: an archaeological and geological perspective. (Academic Press, San Diego, 1988). [DOI] [PubMed] [Google Scholar]
- Hodson M. Interactive comment on “Comment on: “Possible source of ancient carbon in phytolith concentrates from harvested grasses” by G. M. Santos et al. (2012)” by L. A. Sullivan and J. F. Parr. http://www.biogeosciences-discuss.net/9/C5612/2012/ (2012) Date of access: 12 November 2012.
- Parr J. F. & Sullivan L. A. Comparison of two methods for the isolation of phytolith occluded carbon from plant material. Plant Soil 374, 45–53 (2014). [Google Scholar]
- Wang Y. & Lü H. The study of phytolith and its application (in Chinese). (China Ocean Press, Beijing, 1993). [Google Scholar]
- Liu W., An Z., Zhou W., M J., H. & Cai D. Carbon isotope and C/N ratios of suspended matter in rivers: an indicator of seasonal change in C4/C3 vegetation. Appl. Geochem. 18, 1241–1249 (2003). [Google Scholar]
- Vogel J. S., Southon J. R., Nelson D. E. & Brown T. A. Performance of catalytically condensed carbon for use in accelerator mass spectrometry. Nucl. Instrum. Meth. B 5, 289–293 (1984). [Google Scholar]
- Liu K. et al. A new compact AMS system at Peking University. Nucl. Instrum. Meth. B 259, 23–26 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information