Abstract
Protein SUMOylation is a critically important posttranslational protein modification that participates in nearly all aspects of cellular physiology. In the nearly 20 years since its discovery, SUMOylation has emerged as a major regulator of nuclear function, and more recently, it has become clear that SUMOylation has key roles in the regulation of protein trafficking and function outside of the nucleus. In neurons, SUMOylation participates in cellular processes ranging from neuronal differentiation and control of synapse formation to regulation of synaptic transmission and cell survival. It is a highly dynamic and usually transient modification that enhances or hinders interactions between proteins, and its consequences are extremely diverse. Hundreds of different proteins are SUMO substrates, and dysfunction of protein SUMOylation is implicated in a many different diseases. Here we briefly outline core aspects of the SUMO system and provide a detailed overview of the current understanding of the roles of SUMOylation in healthy and diseased neurons.
I. INTRODUCTION
The ability of cells to relay and respond to extracellular signals is a fundamental aspect of physiology. While in the long term, cells may respond to stimuli through alterations in gene expression, initially cells must transmit and react to signals through modification of the behavior and function of preexisting proteins. This is particularly relevant in the nervous system, where communication between cells typically takes place on a timescale on the order of milliseconds. To rapidly respond in such a manner, cells utilize a diverse toolbox of tags that, when conjugated to target proteins, alter the properties of the substrate. These tags can take many forms, and include chemical modifications, such as phosphate or acetyl groups, lipids, or even the covalent attachment of other polypeptides. Alone, or in combination, these posttranslational modifications (PTMs) rapidly coordinate changes in the behavior of their target proteins, setting in motion the complex cellular response to the stimulus.
The prototypical protein-based modifier is ubiquitin, a 76-amino acid protein covalently conjugated to lysine residues in target proteins. Initially reported in 1975, ubiquitin is now considered to be a central regulator of cellular function and is best understood for its role in targeting substrates for proteasomal degradation (97). Subsequently, a whole family of ubiquitin-like proteins (UBLs) have been discovered which, while not necessarily sharing high sequence homology with ubiquitin, share a similar three-dimensional structure, and also function as protein-based modifiers (130, 282). UBLs share analogous conjugation pathways to ubiquitin and are ultimately attached to substrates through a COOH-terminal glycine residue. One member of this family, small ubiquitin-like modifier (SUMO), was initially characterized for its role in regulating nuclear function. However, SUMO has subsequently been demonstrated to modify proteins throughout the cell and, in particular, has emerged as central regulator of neuronal and synaptic function. Moreover, defective protein SUMOylation is implicated in a growing number of disorders of the nervous system.
Here, we briefly review the basic cell biology and regulation of the SUMO pathway and discuss in detail the emerging roles of SUMOylation in neuronal function and dysfunction.
II. DISCOVERY AND RELEVANCE OF PROTEIN SUMOYLATION
SUMOylation is a lysine-targeted PTM in which members of the ∼11-kDa SUMO family of proteins are covalently conjugated to target proteins to alter their function. SUMO1 was originally identified by several different groups in the mid 1990s as a novel UBL that bound the DNA repair enzymes RAD51/52 (246), interacted with the PML protein (16), and protected cells from Fas-induced cell death (194). Subsequently, SUMO1 was confirmed as a genuine UBL modifier that regulated the partitioning of the nuclear pore protein RanGAP1 (166, 177). Through homology screening, these studies also predicted the existence of other SUMO paralogs, designated SUMO2 and SUMO3, which were subsequently also shown to be functional UBL proteins (124, 125).
Concerted effort by several groups identified the machinery required for SUMO conjugation. As for other UBL modifiers, SUMO is conjugated to proteins via a three-step enzymatic pathway involving an ATP-dependent activation step, performed by an E1 enzyme, transfer of the activated UBL to an E2 enzyme and, finally, E3-mediated conjugation of the UBL to the substrate (282). For SUMO, there is a single E1 enzyme, a heterodimer of SAE1 and SAE2 in humans (79), and in stark contrast to the ubiquitin system, a sole E2, Ubc9 (51, 79, 146, 237). A number of proteins with E3 or E3-like activity that catalyze the transfer of SUMO from the E2 to the substrate have also been identified as well as a growing number of enzymes which remove SUMO proteins from substrates (for reviews, see Refs. 57, 69, 94, 99, 119, 299).
Early studies indicated that SUMOylation is a predominantly nuclear modification, targeting many proteins involved in nuclear organization and function (126). Subsequent proteomic studies have identified several hundred targets of SUMOylation, highlighting it as a crucial regulator of nuclear function (15, 78, 104, 176, 235, 286, 287). This is underlined by the fact that knockout of the sole SUMO conjugating enzyme Ubc9, which prevents all protein SUMOylation, causes many major nuclear defects resulting in embryonic lethality in mice (187). Importantly, however, SUMOylation also targets many proteins in cellular compartments outside the nucleus, including plasma membrane proteins (for reviews, see Refs. 73, 299). Taken together, these findings have led to the realization that SUMOylation plays a key role in a wide variety of essential extranuclear cellular processes.
A. SUMO Proteins
There are four reported SUMO paralogs in humans, designated SUMO1–4. SUMO1 shares ∼50% identity to SUMO2, while SUMO2 and 3 differ in only three NH2-terminal residues and are thus generally collectively referred to as SUMO2/3 (119). SUMO4 possesses 86% homology with SUMO2 (18), but its role is unclear because a COOH-terminal proline residue unique to SUMO4 appears to prevent the generation of conjugatable SUMO4 from its precursor pro-SUMO4 (199). However, one study has suggested that under basal conditions SUMO4 is rapidly degraded but can be matured by a stress-induced endogenous hydrolase and conjugated to substrate proteins to regulate intracellular stress pathways (295). Notably, this study only examined the ability of recombinant or overexpressed SUMO4 to be matured. Thus, despite high mRNA expression in kidney (18), the functional relevance of SUMO4 as a genuine UBL modifier remains controversial.
SUMO1 and SUMO2/3 target overlapping sets of substrate proteins (286) but differ in their abundance and properties. At rest, mammalian cells contain very little unconjugated SUMO1 but a large pool of free SUMO2/3, which is also severalfold more abundant than SUMO1 (232). Furthermore, SUMO1 and SUMO2/3 differ in their conjugation dynamics throughout the cell cycle and in response to cellular stressors such as heat shock or hypoxia (6, 232). These findings suggest that, despite utilizing the same conjugation machinery, the SUMO proteins are differentially regulated in vivo, strongly supporting a functional distinction between the paralogs (6, 232). Most notably, SUMO2/3, but not SUMO1, are able to form SUMO chains on substrate proteins as a result of internal lysine residues that can be SUMOylated (268). Thus, similar to ubiquitination, substrates can undergo mono- or poly-SUMOylation to promote distinct functional consequences.
B. SUMO Knockout Mice
There have been mixed results from different studies that have generated SUMO1 knockout mice. Alkuraya et al. (2) generated transgenic mice in which the SUMO1 gene was interrupted by a β-galatosidase insertion. These mice exhibited cleft palate defects and increased rates of late embryonic and early postnatal death, supporting a role for SUMO1 in development (2). In apparent contrast, SUMO1 knockout mice generated in two other studies were initially reported to show normal phenotypes, consistent with SUMO2/3 compensating for loss of SUMO1 during mouse development (64, 317). However, more detailed analysis of these mice has revealed differences in body weight and adipogenesis (181), heart function (290), and inflammatory responses (285), suggesting that, while SUMO2/3 may compensate for many of the developmental roles of SUMO1, there are more subtle paralog-specific functions in which SUMO2/3 cannot compensate for SUMO1.
III. SUMOYLATION AND DESUMOYLATION
SUMO proteins are initially synthesized as nonconjugatable precursors that are processed by SUMO-specific proteases to expose a COOH-terminal diglycine motif that is required for for covalent conjugation. The mature SUMO proteins are then attached to target proteins through the actions of enzymes termed E1, E2, and E3 (Figure 1).
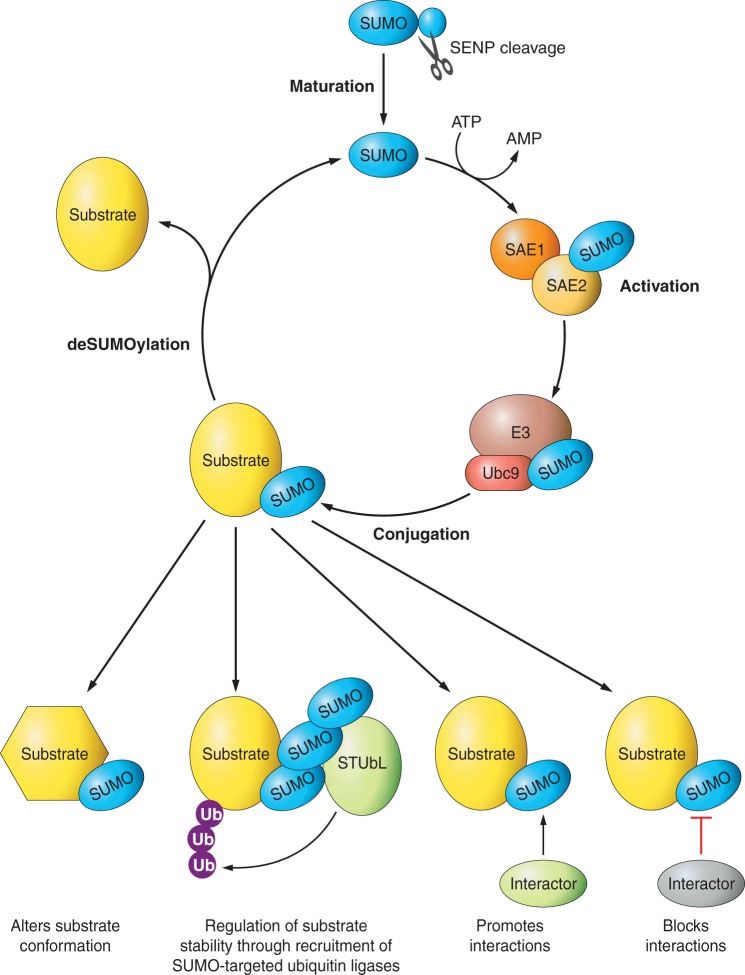
FIGURE 1.
The SUMO pathway. All SUMO proteins are first synthesized as inactive precursors that are cleaved by SUMO proteases (SENPs) to reveal mature (conjugatable) SUMO. SUMO is then activated in an ATP-dependent step by the E1 enzyme, a heterodimer of the subunits SAE1 and SAE2. SUMO is then passed to the active site cysteine of the sole SUMO conjugating enzyme, Ubc9, which, in combination with one of a growing number of described E3 enzymes, mediates target recognition and conjugation of SUMO to a lysine residue in the substrate protein. Broadly speaking, SUMOylation can have 4 nonmutually exclusive consequences: the attached SUMO can change the interaction profile of the substrate, either by blocking binding of an interacting protein, or by the attached SUMO acting as a recruitment factor for new proteins. Alternatively, SUMOylation can control substrate stability, through SUMO chain-mediated recruitment of SUMO-targeted ubiquitin ligases (STUbLs), or regulate substrate activity, through inducing a change in protein conformation.
A. The E1
Mature SUMO is activated by the E1 complex, which in humans is a heterodimer of SAE1 and SAE2 (80). This enzymatic complex stimulates a two-step reaction in which the E1 binds ATP-Mg2+ and SUMO to catalyze the adenylation of the SUMO COOH terminus. The second step of the reaction proceeds through a dramatic reorganization of the E1 active site, resulting in the attack of the SUMO adenylate by a catalytic cysteine residue in SAE2, forming a high-energy thioester bond between the catalytic cysteine and the COOH terminus of SUMO (195).
B. The E2, Ubc9
Once activated, SUMO is passed from E1 to the active site cysteine of the SUMO-specific conjugating enzyme Ubc9. Ubc9 binds the thioester-loaded E1 complex through a ubiquitin fold domain in SAE2 (275). Once loaded with SUMO, Ubc9, either directly or in conjunction with an E3 enzyme, catalyzes conjugation of SUMO to the ϵ-amino group of a lysine residue in the substrate via an isopeptide bond. Ubc9 plays a role in target specificity by recognizing and binding to a SUMOylation consensus site in substrate proteins, defined as ψ-K-x-D/E, where ψ represents a large hydrophobic residue (usually isoleucine, leucine or valine), K represents the target lysine, x is any residue, and D/E are acidic residues (227, 233).
C. E3 Ligases
E3 enzymes catalyze the transfer of UBL modifiers from thioester loaded E2s to the substrate. Since Ubc9 is sufficient for SUMO transfer to occur, at least in vitro, it was initially unclear whether E3 enzymes were required for SUMOylation. However, Ubc9 binding to the consensus motif is relatively weak, and SUMOylation of most substrates proceeds inefficiently in in vitro reactions lacking E3s.
1. PIAS proteins and RanBP2
The first E3s identified for the SUMO system were the Siz proteins, which mediate SUMO transfer to septins in the yeast S. cerevisiae, (120). The mammalian counterparts of Siz proteins are the PIAS (protein inhibitor of activated STAT) family that have E3 activity for a wide range of SUMO targets (for a comprehensive review of the PIAS proteins, see Ref. 230). There are four PIAS proteins expressed in humans, PIAS1, PIASx (which is alternatively spliced to give PIASxα and xβ), PIAS3, and PIASy.
The E3 activity of the Siz and PIAS proteins is mediated by a Siz/PIAS-RING (Really Interesting New Gene) motif that forms a zinc finger structural domain of 30–40 amino acid residues. In addition to the SP-RING, the PIAS E3s also contain a number of other motifs, including a SAP domain, a PINIT motif, a characteristic COOH-terminal domain (SP-CTD), and a SUMO interacting motif (SIM; see below). Together, these domains mediate protein-protein interactions and subcellular localization of the PIAS family. Mechanistically, the E3 ligase activity of the PIAS family is restricted to the SP-RING and SP-CTD domains, which bind Ubc9 and SUMO, respectively, and together are proposed to position thioester-loaded Ubc9 in a conformation conducive to SUMO transfer (316).
Interestingly, a number of proteins that lack the SP-RING motif have also been identified as SUMO E3s. For example, the SP-RING-lacking nuclear pore protein RanBP2 binds SUMO and Ubc9, and possesses SUMO E3 activity (205). As for the PIAS proteins, the minimal region required for E3 activity contains SUMO and Ubc9 binding sites, but does not appear to bind the substrate, suggesting a similar model whereby RanBP2 positions thioester-loaded Ubc9 in a position optimal for SUMO ligation to the substrate (205, 207, 223). However, in vivo, RanBP2 forms part of a much larger complex that also contains SUMO-modified RanGAP1, and two copies of Ubc9 (296). Together, this complex has been proposed to function as a novel multisubunit E3 ligase that couples substrate binding to facilitation of SUMO transfer, similar to those found in the ubiquitin system (296).
2. Other SUMO ligases
The PIAS family and RanBP2 are generally considered bona fide E3 enzymes because they are capable of enhancing SUMO transfer to a wide variety of substrates at substochiometric concentrations. In addition, a growing number of proteins are being identified that can enhance the SUMOylation of specific substrates, and thus function either as E3 ligases or cofactors of SUMOylation. For example, the histone deacetylases (HDACs) 4 and 7 have been reported to enhance SUMOylation of the androgen receptor and PML, respectively (71, 313). Furthermore, members of the tripartite motif (TRIM) family of proteins that contain a RING finger domain, B-box zinc finger domain(s), and a coiled coil region can stimulate SUMOylation in vitro (39, 71, 313). The RING domain-containing protein TOPORs, which acts as an E3 ligase for ubiquitin (219), also enhances SUMOylation of a number of targets, including p53 (294), topoisomerase I (90), and the NFκB regulator IKKϵ (221), suggesting it may function as a dual-specificity E3 for both the ubiquitin and SUMO systems. Furthermore, the protein RSUME has been reported to potentiate the SUMOylation of IκB, HIF1α, and glucocorticoid receptor (28, 58), although this effect appears to be due to a general ability to enhance SUMO loading of Ubc9, suggesting RSUME may function as a generalized cofactor of SUMOylation rather than as an E3 (28).
Since all SUMOylation events are mediated by only one E1 complex and one E2, the diversity of E3s may provide a mechanism for subtle, substrate-specific regulation of SUMOylation. Therefore, the regional and subcellular distributions of SUMO E3 ligases are likely to define which substrates are SUMOylated in those areas and compartments. For example, the protein MAPL (mitochondrial-associated protein ligase) has been proposed to be a SUMO E3 exclusively localized at mitochondria and has been reported to enhance SUMOylation of the GTPase dynamin-related protein 1 (Drp1) at the mitochondrial membrane (21). On a wider scale, tissue-specific expression patterns of SUMO ligases/cofactors may underlie the differences in substrate SUMOylation between tissues. The protein Rhes is enriched in the striatum and has been reported to specifically enhance SUMOylation of mutant Huntingtin, leading to the observed striatal-specific degeneration observed in patients with Huntington's disease (262).
D. DeSUMOylation: SUMO Proteases
SUMO proteases perform two core functions in the SUMOylation pathway: 1) they are responsible for the initial COOH-terminal cleavage of pro-SUMO to mature, conjugatable SUMO, via their endopeptidase activity; and 2) they deconjugate substrate-bound SUMO via their isopeptidase activity (for focused reviews, see Refs. 99, 186, 314).
1. SENP proteins
The SUMOylation status of a specific substrate is a delicate balance between E1/Ubc9/E3-mediated conjugation and SUMO protease-mediated deconjugation (Figure 2). Regulation of the distribution and activities of SUMO proteases therefore provides a mechanism to tightly control the location and levels of protein SUMOylation. In yeast, two SUMO proteases have been identified, Ulp1 and Ulp2 (151, 152). Their mammalian counterparts, the SENPs (sentrin-proteases), were subsequently isolated through homology screening. There are six SENPs in humans, designated SENP1, 2, 3, 5, 6, and 7. Individual SENPs differ in their subcellular localization, SUMO paralog specificity, the efficiency of their endopeptidase versus isopeptidase activities, and their ability to cleave monomeric SUMO versus SUMO chains (Table 1; for reviews, see Refs. 99, 186, 314). All SENPs contain a conserved COOH-terminal catalytic domain and an NH2-terminal domain that likely dictates their localization and protein interactions. SENP1 and SENP2 appear to preferentially cleave proSUMO to mature SUMO1 and SUMO2, respectively, while SENP5 appears most efficient at maturation of SUMO3 (52, 82, 222, 306).
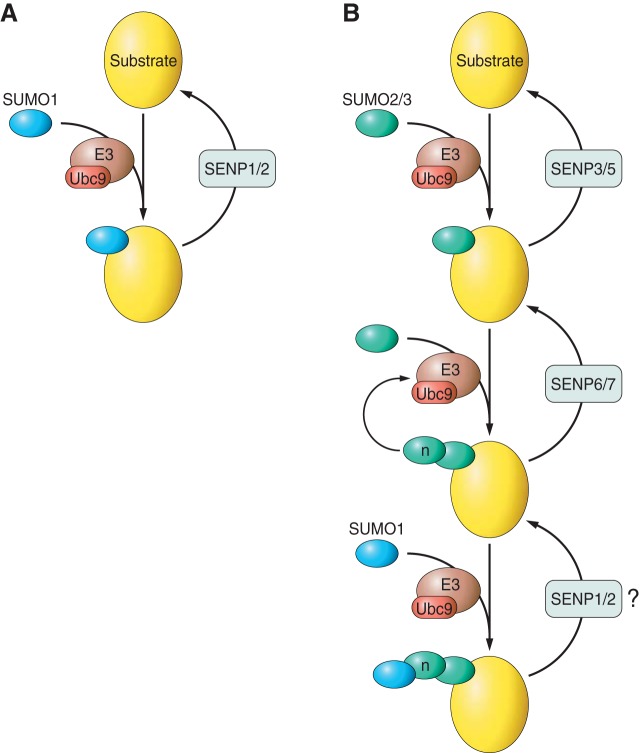
FIGURE 2.
Properties of SUMO1 and SUMO2/3 conjugation. Schematic highlighting the main differences between SUMO1 and SUMO2/3 modification. A: SUMO1 is conjugated to substrates via Ubc9 and cleaved mostly by SENP1/2. SUMO1 does not contain an internal SUMOylation site and therefore cannot form polySUMO chains. B: SUMO2/3 is conjugated by Ubc9 and cleaved by SENP3 or SENP5. Since SUMO2/3 contains SUMOylation sites, SUMO2/3 can form polySUMO chains of n length. SUMO chains are shortened by cleavage by SENP6/7. Conjugation of SUMO1 to SUMO2/3 can cap SUMO2/3 chains to terminate further elongation.
Table 1.
Localizations and actions of SUMO proteases
| SUMO Protease | Main Cellular Location | SUMO Selectivity | Cleavage of ProSUMO | Removal of SUMO From Target Substrates |
|---|---|---|---|---|
| SENP1 | Mainly nucleus and subnuclear structures but also present in cytoplasm and other extranuclear compartments | SUMO1 & SUMO2/3 | Yes SUMO1 & SUMO2 | Yes |
| SENP2 | Mainly nucleus and subnuclear structures but also present in cytoplasm and other extranuclear compartments | SUMO2/3 > SUMO1 | Yes SUMO1 & SUMO2 | Yes |
| SENP3 | Nucleolus as well as extranuclear compartments including mitochondria | SUMO2/3 | ND | Yes |
| SENP5 | Nucleolus as well as extranuclear compartments including mitochondria | SUMO2/3 | Yes SUMO3 | Yes |
| SENP6 | Nucleoplasm | SUMO2/3 chains | No | Yes, preferentially SUMO2/3 chains |
| SENP7 | Nucleoplasm | SUMO2/3 chains | No | Yes, preferentially SUMO2/3 chains |
| DeSI1 | Nucleus and cytoplasm | SUMO1 & SUMO2/3 | Possibly | Yes |
| DeSI2 | Nucleus and cytoplasm | ND | No | ND |
| USPL1 | Cajal bodies in nucleus | SUMO2/3 > SUMO1 | Possibly | Yes |
See text for details and references. ND, not determined.
In terms of deconjugation, SENP1 removes both SUMO1 and SUMO2/3 from substrate proteins in vivo, whereas SENPs 2, 3, and 5 show a preference towards SUMO2/3 (52, 82, 136, 222). SENP6 and 7 show preferential isopeptidase activity against SUMO2/3 chains (154, 185, 244). As for SUMO, the subcellular localization of SENPs is predominantly nuclear, and the different SENPs exhibit differing subnuclear localizations. SENP1 and 2 localize to the nuclear pore and discrete nuclear dots (8, 81, 91), SENP3 and 5 have a mainly nucleolar localization (82, 191), and SENP6 and SENP7 are found throughout the nucleoplasm (185, 244). Importantly, however, although highly enriched, SENPs are certainly not exclusively restricted to the nucleus. SENP3 and SENP5 are present at mitochondria (89, 328), and in neurons, SENP1, 3, and 6 have a widespread distribution throughout neuronal processes and at synapses, albeit at much lower levels than present in the nucleus (89, 159, 160, 174).
2. Other SUMO proteases
In addition to the SENP enzymes, two novel SUMO-specific proteases, DeSI1 and USPL1, have recently been reported (236, 248). DeSI1 was initially identified as an interactor of the transcription factor BZEL (248). When heterologously expressed, DeSI1 forms homodimers that distribute throughout both the cytosol and nucleus. DeSI1 can cleave SUMO1 and SUMO2/3 from BZEL, both in vivo and in vitro, and also deconjugates SUMO2/3 chains in vitro (248). However, DeSI1 appears to lack endopeptidase activity, since it does not mature SUMO1. Furthermore, in contrast to the SENPs, DeSI1 does not deSUMOylate the well-characterized SUMO substrates PML or ΔNp63, and knockdown of DeSI1 does not overtly alter the pattern of cellular SUMOylation. Thus these results suggest that DeSI1 has a much more restricted selection of substrates than the SENPs. From sequence alignment analysis, a similar protein, named DeSI2, has also been identified (248); however, at the time of writing, whether this protein also shows deSUMOylating activity has not yet been reported.
The other novel SUMO-specific protease, USPL1, was identified in a screen for proteins with deSUMOylating activity (236). It is primarily localized at Cajal bodies within the nucleus, binds SUMO, and deconjugates SUMO from the prototypic substrates RanGAP1 and Sp100, favoring deconjugation of SUMO2/3 over SUMO1 (236). However, the in vivo functions and targets of USPL1 remain to be elucidated.
IV. SUMOYLATION CONSENSUS MOTIFS
Early analysis of the modified lysine residues in SUMO substrates led to identification of a SUMOylation consensus site, ψ-K-x-D/E, to which Ubc9 binds directly, albeit with relatively low affinity (227, 233). Subsequent studies have built on these findings to define more extended SUMOylation target motifs, which appear to promote substrate SUMOylation by enhancing binding to Ubc9. For example, the negative charge-dependent SUMOylation motif (NDSM) contains an acidic patch located downstream of the modified consensus lysine, that binds to a cognate basic patch on Ubc9 (310). Similarly, the phosphorylation-dependent SUMOylation motif (PDSM) or phosphorylated SUMOylation motif (pSuM) contain phosphorylatable serine residues COOH-terminal to the modified lysine, or in place of the acidic residue of the consensus motif, respectively (100, 204). When phosphorylated, these residues provide the negative charge required to enhance substrate recognition by Ubc9 and provide an elegant mechanism to explain how substrate phosphorylation can regulate SUMOylation (100).
In addition to these “extended consensus motifs,” a number of other SUMO target sites have been defined by proteomic studies. For example, in some cases, methionine, phenylalanine, and cysteine can be tolerated in place of the large hydrophobic residue of the consensus motif. Furthermore, an inverted consensus motif (E/D-x-K-ψ) also functions for some substrates (176).
An important note of caution, however, is that while the presence of a consensus sequence in a protein of interest indicates the potential for that protein to undergo SUMO modification, the context of the consensus sequence is critical. Ubc9 binding and SUMOylation can only occur at consensus sequences located in unstructured regions of the target protein (206), and many, perhaps most, apparently consensus lysines are not modified. Equally importantly, many SUMO substrate proteins can be modified at nonconsensus lysines. How the SUMO machinery recognizes nonconsensus sites remains to be fully resolved and is an active area of investigation, but it is likely that E3 ligases may play a crucial role in recognition of these sites (69).
V. MOLECULAR CONSEQUENCES OF PROTEIN SUMOYLATION
The majority of reported SUMO substrates are located within the nucleus, and there are clear roles for SUMO modification in DNA repair, transcription, and nuclear organization. However, increasing numbers of SUMO substrates are being identified in compartments outside the nucleus and have been implicated in almost every aspect of cell biology. This diversity of substrate proteins means that it is difficult to predict the overall effects of global SUMOylation. Nonetheless, for specific substrates where the effects of SUMO conjugation have been defined, SUMOylation can be broadly considered to have four nonmutually exclusive effects on the target protein. Briefly, SUMOylation can do the following:
1)reduce interactions at the substrate protein by blocking protein-protein interaction sites;
2)enhance interactions at the substrate protein by providing a platform to recruit proteins that noncovalently bind the attached SUMO;
3)control target protein stability and turnover, especially via the recruitment of members of the SUMO-targeted ubiquitin ligase (STUbL) family of proteins; and
4)cause conformational changes in the target protein, which in turn regulate its function and/or activity (see Figure 1).
VI. SIMS AND RELATED SUMO BINDING MOTIFS
Many ubiquitin-binding motifs have been defined that mediate the downstream consequences of ubiquitin modification of the substrate. For SUMOylation, at least three distinct SUMO interacting motifs (SIMs) have been described in proteins that bind SUMO noncovalently. The first to be defined was the “classical” SIM, which consists of a hydrophobic core, often flanked COOH- or NH2-terminally by acidic residues (95, 182, 252, 253). Proteins containing this motif can bind to a hydrophobic groove present in all SUMO paralogs (95) and can bind SUMO in either orientation (95, 253). Although most classical SIMs do not show paralog preference, there are reported examples of paralog specificity (180, 198, 321).
Additional “nonclassical” SIMs have been discovered in Ubc9 (27, 53, 135) and in dipeptidyl peptidase 9 (DDP9), an enzyme which cleaves NH2-terminal dipeptides from substrates that have a proline residue at the second position (Xaa-Pro) (208). The SIMs in these proteins do not recognize the classical SIM binding groove in SUMO; rather, they bind to a surface surrounding Glu67 in SUMO1, which has been called the E67 interacting loop (EIL). Ubc9 binds equally to this EIL surface in all SUMO paralogs, whereas DDP9 binding is highly specific to SUMO1 (208). In addition, a zinc finger motif in the ubiquitin ligase HERC2 has recently been identified that binds SUMO1 and, to a lesser extent SUMO2, and this binding requires its ability to chelate zinc (48). Thus, similar to ubiquitin, we anticipate that beyond these three already identified SUMO interacting motifs, multiple other motifs capable of recognizing SUMO paralogs await discovery, and this emerging family of interacting domains will provide a mechanism for the versatile control of SUMOylation and its downstream pathways.
A. Functions of SIMs
SIMs can dictate the downstream effects of substrate SUMOylation by recruiting SIM-containing effector proteins to SUMOylated target proteins. For example, recruitment of the SIM-containing transcriptional repressor Daxx to many SUMOylated transcription factors mediates the repressive effects of SUMOylation on transcription (33, 142, 155). Furthermore, SUMO-SIM interactions can act as molecular “glue”, driving protein complex assembly, such as the formation of PML nuclear bodies (155, 245) or the recruitment of the DNA repair machinery to double strand breaks (217).
Beyond a role in the engagement of effector proteins, a number of identified SUMO substrates contain SIMs, including the deubiqutinating enzyme ubiquitin-specific peptidase 25 (USP25) (180) and the RecQ DNA helicase mutated in Bloom syndrome (BLM) (321). In these cases, the SIM recruits SUMO-loaded Ubc9 to the substrate, enhancing substrate SUMOylation. Alternatively, the SIM within the substrate can bind to the SUMO after it has been conjugated, leading to a change in conformation, as is the case for the DNA repair enzyme thymine d-glycosylase (TDG) (7, 92).
A recent advance in our understanding of how SIMs mediate the downsteam effects of SUMOylation has come from the discovery of SUMO-targeted ubiquitin ligases (STUbLs; for more detailed reviews, see Refs. 74, 202, 255). These proteins, of which RNF4 is the prototype, are ubiquitin ligases that harbor several tandem SIMs, allowing them to recognize SUMO chains. Recruitment of STUbLs to substrates via SUMO chains results in ubiquitination and degradation of the substrate. These findings highlight how mono-SUMOylation and poly-SUMOylation can have different effects and they provide an elegant example of the cooperation between the SUMO and ubiquitin pathways.
B. Regulation of SUMO-SIM Interactions
A number of studies have investigated the posttranslational modifications of SIMs and the SIM binding groove on SUMO that regulate their interaction properties (Figure 3). The SIM of the E3 enzyme PIAS1 can be phosphorylated by casein kinase 2 (CK2), which enhances its interaction with SUMO1 both in vitro and in vivo, and alters the ability of PIAS1 to function as a transcriptional coregulator (258). CK2-regulated SIMs have also been identified in PML, the exosome component PMSCL1, and the transcriptional repressor Daxx, suggesting that SUMO-SIM interactions may be generally regulated by phosphorylation (258). More recent studies have extended these findings to show that SUMO-SIM interactions can also be regulated through modification of the SIM-binding groove on SUMO (278). Acetylation of K37 in SUMO1, or K33 in SUMO2, blocked SUMO binding to various SIM-containing interactors. Furthermore, acetylation of SUMO dramatically reduced its capacity to repress transcription and the assembly of PML nuclear bodies, functions both dependent on SUMO-SIM interactions (278). We anticipate that other kinases and sites of phosphorylation, as well as other posttranslational modifications, regulate SUMO-SIM interactions and that, taken together, these processes provide the mechanisms to determine the extent and outcome of substrate protein SUMOylation under different conditions and circumstances.
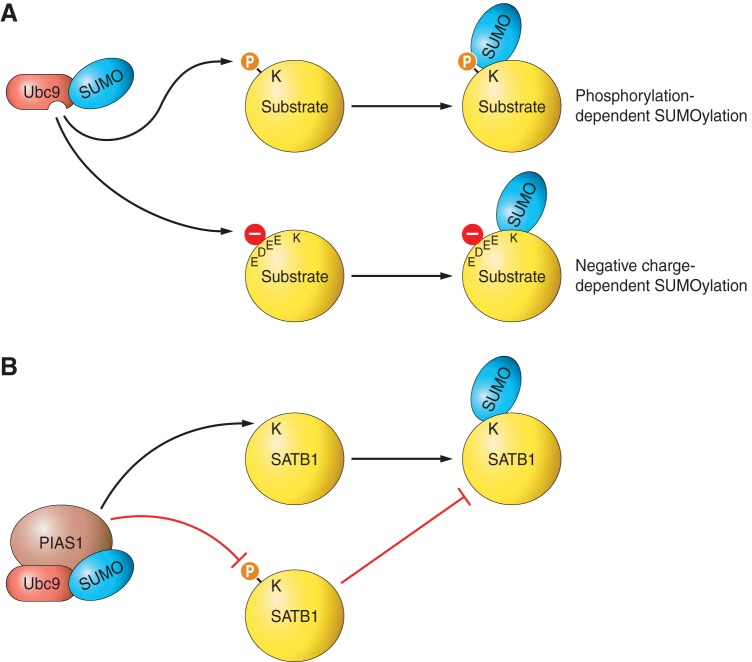
FIGURE 3.
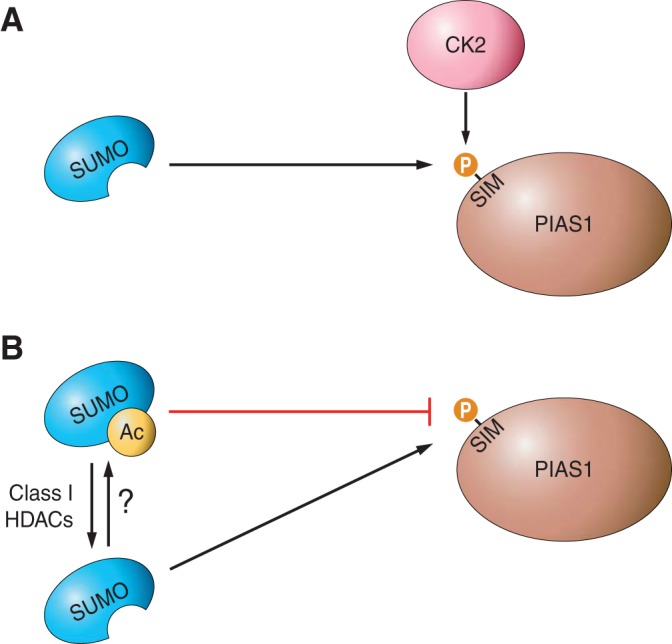
Regulation of SUMO-SIM interactions. SIM-containing proteins mediate the downstream effects of SUMOylation through binding to the SUMOylated substrate. Furthermore, SIMs are present in several components of the SUMOylation machinery, where they act to coordinate substrate SUMOylation. Intriguingly, SUMO-SIM interactions can be reciprocally regulated. A: phosphorylation of the SIM in the E3 ligase PIAS1 by casein kinase 2 (CK2) enhances its capacity to bind SUMO. B: acetylation of SUMO within the SIM-binding groove, which is reversed by class I histone deacetylases (HDACs), blocks SUMO-SIM interactions.
VII. REGULATION OF PROTEIN SUMOYLATION
As described above, SUMOylation proceeds with a highly restricted number of enzymes compared with the analogous ubiquitin system. Therefore, a key question in the field is how is substrate-specific regulation of SUMOylation achieved? As discussed below, recent advances revealing the roles of other PTMs of both substrate proteins and of specific components of the SUMO conjugation machinery are starting to provide partial answers. One particularly interesting emergent concept is that, in addition to regulation at the level of individual substrates, SUMOylation can also be regulated on a global level, as exemplified by the observation that various cellular stresses, including heat shock, osmotic shock, and ischemic challenge, lead to a global increase in SUMOylation, and dramatically alter the profile of cellular SUMOylation (88).
A. Interplay Between SUMOylation and Other PTMs of Substrate Proteins
A common theme in the regulation of substrate SUMOylation lies with crosstalk between SUMOylation and other PTMs. The best studied example is phosphorylation of the substrate protein, which can either enhance or reduce SUMOylation at a nearby lysine, depending on the circumstances and the specific substrate (Figure 4). As outlined above, the SUMO target lysine in a number of SUMO substrates lies within a PDSM motif which, when phosphorylated, enhances substrate recognition by Ubc9 (100). Furthermore, phosphorylation can also enhance substrate SUMOylation when not part of a PDSM motif, as is the case for the kainate receptor subunit GluK2, for which PKC-mediated phosphorylation of a serine 18 residues upstream of the SUMO target lysine promotes SUMOylation (32, 137). Conversely, phosphorylation of the transcription factor Elk-1 (311) and the DNA binding protein SATB1 (265) reduce SUMOylation, the latter through reducing the binding of the E3 PIAS1 to the substrate.
FIGURE 4.
Interplay between substrate SUMOylation and phosphorylation. A: phosphorylation of some substrates, such as MEF2A and GATA-1, promotes SUMOylation via enhaced recruitment of Ubc9. This is due to the negative charge of the phosphate group interacting with a cognate basic patch on Ubc9. Other substrates, such as Elk-1, are efficiently modified by SUMO due to the presence of a negatively charged region immediately COOH-terminal to the modified lysine, mimicking the effect of phosphorylation, a motif known as an NDSM (negatively charged SUMOylation motif). B: conversely, phosphorylation can also reduce SUMOylation of some substrates. For example, phosphorylation of SATB1 reduces its SUMOylation through blocking binding to the E3, PIAS1.
Another emerging regulatory mechanism is the interrelationship between substrate SUMOylation and other lysine-targeted PTMs, most notably by ubiquitin or acetylation. While these PTMs were originally suspected to compete with SUMOylation for the target lysine, it is now clear that the interactions can be far more nuanced, and that the interplay between different PTMs at the same lysine allows complex regulation of substrate properties and functions (for review, see Ref. 279). These different PTMs may be elicited by distinct stimuli or in different cellular locations and can be interchangeable and/or occur sequentially to regulate the substrate without necessarily being directly competitive. One example of this lies with the transcription factor MEF2A. MEF2A contains a PDSM-type SUMOylation site that, when phosphorylated, drives MEF2A SUMOylation (100, 240). Neuronal activation, however, leads to MEF2A dephosphorylation, deSUMOylation, and acetylation of the target lysine to regulate MEF2A transcriptional activity (240). Thus, while not in direct competition, SUMOylation and acetylation target the same lysine to tightly control the transcriptional response of MEF2A to neuronal activity. Analogous examples have emerged from the interplay between ubiquitin and SUMO. For example, the NFκB regulator NEMO is sequentially modified by SUMO and ubiquitin at the same lysine residue to control its nuclear localization (109). Furthermore, perhaps the most complex and elegant examples of the cross-regulation of substrate proteins through the interplay of PTMs are the STUbLs mentioned above (74, 202, 255).
B. Regulation Through Targeting of the SUMO Machinery
SUMOylation of all substrates uses the same core pathway and a very limited repertoire of enzymes. One consequence is that regulation of these SUMO machinery enzymes offers the potential to influence SUMOylation on a global level, or at least to spatiotemporally alter the SUMOylation of a subset of targets within a particular area or cellular compartment. Indeed, regulation of the SUMOylation machinery, either posttranslationally or through regulating enzyme turnover, is an important emerging concept in the field.
1. Regulation of the E1
High levels of H2O2 induce oxidative stress and increase SUMO conjugation (232), whereas low H2O2 levels rapidly decrease in SUMO conjugation (19). This dramatic biphasic effect is due to the direct and reversible inhibition of SUMO conjugating enzymes by low levels of reactive oxygen species. Inhibition occurs via the formation of a disulfide bond between the catalytic cysteines of the E1 subunit SAE2 and Ubc9 (19), providing an elegant mechanism to prevent SUMO conjugation without affecting deconjugation, and thereby reduce SUMOylation.
The SUMO E1 itself is also a SUMOylation substrate (261, 275). SUMOylation of the E1 subunit SAE2 occurs at multiple sites within both the Cys domain and near the COOH terminus of the protein (275). Cys domain SUMOylation appears to inhibit the transfer of activated SUMO from the E1 to the catalytic cysteine of Ubc9, while SUMOyation at the COOH terminus is required for nuclear retention of the E1 complex (275). Notably, heat shock, which increases SUMO2/3 modification of many substrate proteins, reduces SUMO modification of the E1 Cys domain (275). An attractive possibility is that SUMOylated E1 represents an inactive cellular reserve of E1 that, under stress conditions, can be activated by deSUMOylation to mediate the global increase in SUMO conjugation (275).
2. Regulation of Ubc9
Because it is the only E2, Ubc9 represents a prime target for global regulation of protein SUMOylation, and several PTMs of Ubc9 have been reported to modulate its target specificity (Figure 5). Mammalian Ubc9 is SUMOylated at a single lysine (K14), which, while not affecting its ability to modify the substrates HDAC4, PML, or E2–25K, impairs its ability to modify RanGAP1 and potentiates its activity against Sp100, in a manner dependent on the SIM in Sp100 (134). SUMOylation of Ubc9 thus acts to regulate its target specificity, skewing its activity to favor particular subsets of proteins.
FIGURE 5.
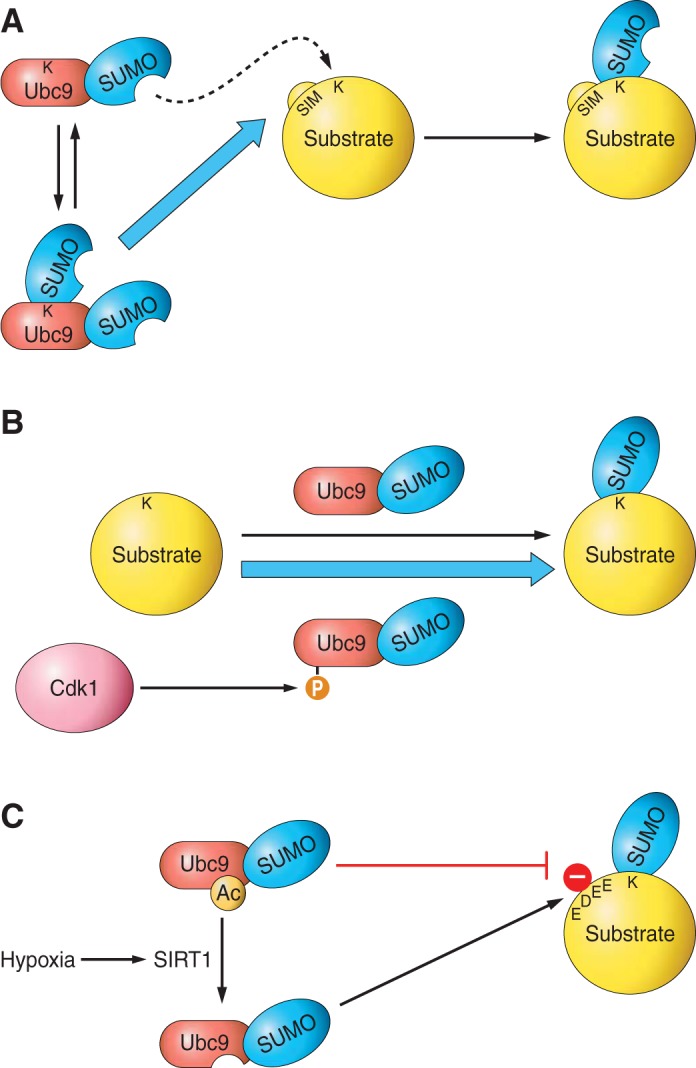
Posttranslational regulation of Ubc9 can alter global SUMOylation, or modification of particular subsets of proteins. A: SUMOylation of some target proteins, such as USP25 and BLM, is enhanced by the presence of a SIM in the substrate, which recruits SUMO-loaded Ubc9 through noncovalent binding to SUMO. However, Ubc9 can also be covalently modified by SUMO at lysine-14, and SUMOylation of Ubc9 acts to enhance its activity towards a subset of SIM-containing proteins, presumably due the covalently attached SUMO promoting recruitment to the substrate. B: phosphorylation of Ubc9 at serine-71 by the kinase Cdk1 enhances its activity in vitro. C: acetylation of Ubc9 at lysine-65 specifically reduces its activity towards NDSM-containing substrates. However, hypoxia results in SIRT1-mediated deacetylation of this residue, enhancing SUMOylation.
Phosphorylation of Ubc9 by CDK1/cyclin B also regulates SUMOylation in in vitro assays by enhancing thioester loading (260). As yet, however, the role of Ubc9 phosphorylation in regulating SUMO modification in vivo remains undetermined.
In contrast, acetylation of Ubc9 reduces its ability to modify substrates that contain an NDSM. Interestingly, enhanced SUMOylation during hypoxia correlates with SIRT1-mediated deacetylation of Ubc9, which relieves the inhibition to facilitate enhanced SUMOylation of NDSM containing substrates (105).
These are still relatively early days in this field, but it is already clear that, depending on the precise stimuli and pathways involved, PTMs can initiate global changes in E1 and Ubc9 activity, and it seems PTMs may also be capable of mediating more subtle changes to alter SUMOylation at certain sets of substrates.
3. Regulation of deSUMOylation
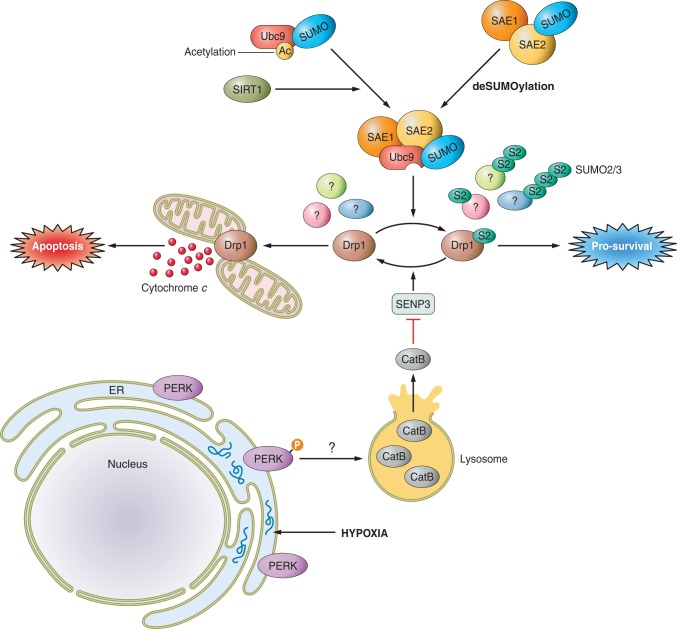
As outlined above, nine SUMO proteases have been reported that vary in their ability to process and/or deconjugate SUMO, as well as in their paralog and substrate specificity. Together with E3s, deSUMOylating enzymes provide the most diverse aspects of the SUMO pathway, and modulation of individual SUMO proteases offers the opportunity to regulate the paralog-specific SUMOylation of distinct subsets of substrate proteins. This has been most extensively examined in the field of ischemic and oxidative stress. In particular, regulation of the SUMO2/3-specific protease SENP3 has emerged as a central player in cellular stress responses. Under basal conditions, SENP3 is constitutively degraded through the ubiquitin-proteasome system. However, low doses of H2O2 that induce mild oxidative stress rapidly stabilize SENP3 protein levels (107). One important component of the cellular response to oxidative stress is the transcription factor hypoxia inducible factor 1α (HIF1α) which, along with its coactivator p300, drives expression of stress response genes (242). SENP3 stabilization following mild oxidative stress promotes deSUMOylation of p300, enhancing its interaction with HIF1α and promoting transcription (107). The ubiquitin ligase CHIP [COOH terminus of heat shock cognate protein (Hsc70) interacting protein] has been identified as the mediator of SENP3 ubiquitination and turnover (309). Interestingly, mild oxidative stress also causes oxidative modification of cysteines C243 and C274 in SENP3, indicating that SENP3 itself functions as a redox sensor. Redox modification of these cysteines recruits Hsp90, which prevents CHIP-mediated ubiquitination of SENP3 and stabilizes SENP3 levels (309). Thus direct oxidative modification of SENP3 can lead to differential interactions with chaperone proteins to ultimately stabilize SENP3 and drive the cellular response to oxidative stress.
Another study has also identified SENP3 as a central regulator of the cellular response to oxygen and glucose deprivation (OGD), a widely used model of ischemic insult (88, 89). This is described in more detail in the ischemia section below but, briefly, during OGD SENP3 is rapidly degraded by the lysosomal protease cathepsin B, and this loss correlates with increased SUMO2/3 conjugation (89). Thus cellular SUMOylation of subsets of proteins can be regulated by targeted degradation of deSUMOylating enzymes.
VIII. PARALOG SPECIFICITY OF SUMOYLATION
Ubc9 is capable of conjugating both SUMO1 and SUMO2/3 with similar efficiency (268). This raises the question as to the differences between SUMO1 versus SUMO2/3 conjugation, and how the paralog-specific modification observed for some substrates is achieved. While much remains to be discovered, a number of mechanisms are beginning to emerge. As outlined above, while many SIMs appear unable to distinguish between SUMO paralogs, several SIMs that can differentiate between SUMO1 and SUMO2/3 have been defined. For example, USP25 (180) and BLM (321) both contain SIMs that selectively recruit Ubc9 thioester-loaded with SUMO2/3 and promote consequent SUMO2/3-ylation.
The prototypic SUMO substrate RanGAP1, which is primarily modified by SUMO1, provides another example of paralog specificity. Intriguingly, this occurs via a separate regulatory mechanism since the conjugation machinery does not appear to distinguish between the covalent attachment of SUMO1 versus SUMO2/3 to RanGAP1. Instead, SUMO1 modification of RanGAP1 recruits it into a complex with the multifunctional protein RanBP2 that protects it from SENP-mediated deconjugation. In contrast, SUMO2/3-modified RanGAP1 is rapidly deconjugated, thereby generating effective SUMO1-specificity at equilibrium (323).
RanBP2 also provides a different mechanism for SUMO paralog specificity. One role of RanBP2 is as an E3 that conjugates both SUMO1 and SUMO2 to the substrates PML and Sp100, but conjugation is more efficient for SUMO2 (269). RanBP2 binds noncovalently to both Ubc9 and SUMO1, but not to SUMO2 and, like many E3s in the ubiquitin system, is also capable of catalyzing nonproductive automodification (autoSUMOylation). Essentially, autoSUMOylation versus substrate modification can be viewed as a competitive process. Because RanBP2 binds SUMO1, it preferentially autoSUMOylates with this paralog; however, because it does not directly bind to SUMO2, it preferentially conjugates SUMO2 to the substrate (269). Thus, while the E1 and E2 of the SUMO system seem to show little preference in paralog conjugation (268, 270), it is becoming increasingly clear that, for some targets, E3s can elegantly regulate paralog specificity.
IX. SUMOYLATION IN NEURONS
A. Distribution and Developmental Expression of the SUMO Machinery
Despite a growing appreciation of the importance of SUMOylation in neuronal function, the distribution and developmental profiles of the SUMOylation machinery have not yet been extensively characterized. However, this is an active area of investigation and a number of studies have begun to address this issue.
Investigation of the developmental profile of Ubc9 at the mRNA and protein levels in rat brain revealed highest expression between E13–18; thereafter, Ubc9 expression decreased following birth and remained low in adults. Total levels of substrate SUMOylation showed a corresponding developmental profile, consistent with an important role for SUMOylation in late embryonic development (292). The Ubc9 transcript was detected embryonically throughout the brain by in situ hybridization but was particularly concentrated in areas containing proliferating neural stem cells, with moderate expression elsewhere. After birth, Ubc9 expression was more restricted, being highest in dentate granular neurons and pyramidal neurons in the hippocampus, as well as large pyramidal neurons of the cerebral cortex (292). These results were interpreted to suggest key roles for Ubc9/SUMOylation in neuronal differentiation in the developing brain and, given the high expression levels in the adult hippocampus, synaptic plasticity in the adult (292).
A more recent study has also examined the developmental regulation and spatiotemporal distribution of the SUMOylation machinery with broadly similar results (160). Total protein conjugation by SUMO1 and SUMO2/3 peaked at E12, while levels of the deconjugating enzymes SENP1 and SENP6 were highest early in development and decreased thereafter. The highest levels of Ubc9 were detected in the developing brain (E15–18), with a decrease after birth. Interestingly, this study also investigated levels of SUMOylated Ubc9, and observed that this displayed a different profile to non-SUMOylated Ubc9, being low before birth, peaking at P14, and remaining high in the adult (160). The physiological consequences of this developmental switch from non-SUMOylated to predominantly SUMOylated Ubc9 are currently unclear. However, since Ubc9 SUMOylation is known to regulate target discrimination (134), an attractive possibility is that SUMOylation allows Ubc9 to target distinct sets of substrates during development.
B. Subcellular Localization of the SUMO Machinery
Because of the abundance of both SUMO and the conjugation machinery in nuclear compartments, historically SUMOylation has been regarded as a nuclear modification. Indeed, in cultured neurons, SUMO is predominantly localized in the nucleus, but significant SUMO immunoreactivity is also present in axons and dendrites, and at synapses (36, 67, 76, 113, 159, 160, 174, 210). In rat brain, SUMO1 and SUMO2/3 conjugation show similar profiles during development, with a peak at E12 in both nuclear and cytosolic fractions (160). Furthermore, SUMO1, SUMO2/3, and the conjugation machinery are readily detectable in synaptosome fractions, indicating a synaptic role for SUMOylation (67, 160, 174). Consistent with this, immunostaining of cultured neurons shows that SUMO proteins, the E1 complex, Ubc9, SENPs, and the PIAS proteins are localized in dendrites and axons as well as at synapses (113, 159, 160, 174). Interestingly, these proteins show differential localization patterns at synapses during neuronal development. In cultured hippocampal neurons, presynaptic levels of the E1 subunit SAE1 decreased between DIV10 and 20, whereas Ubc9 remained unchanged and SENP1 and SENP6 decreased. In contrast, postsynaptic levels of SAE1, Ubc9, and SENP6 all increased between DIV10 and DIV20, while SENP1 decreased (160). These findings illustrate that the SUMOylation machinery at synapses is dynamically regulated in a complex manner throughout neuronal development.
Targeting E3s or SENPs to a particular location at a particular time can locally alter SUMOylation to regulate complex cellular processes, such as cell cycle progression (120, 327) or DNA repair (217). However, exactly how SUMOylation and deSUMOylation enzymes are targeted to the right place at the right time in neurons is unknown. SUMO enzymes have been reported to undergo developmental and KCl depolarization-dependent redistribution (159) but, as yet, no experiments investigating the mechanisms and consequences of enzyme translocation under physiological and pathophysiological conditions have been reported.
An interesting concept that has emerged recently is that in yeast, anchoring of the E3 Siz2 catalyzes a local “SUMO spray” on nearby proteins to potentiate physical interactions and accelerate DNA repair (118). Neurons are far more morphologically complex cells, and spatiotemporally regulated events require sophisticated coordinatation of protein trafficking, retention, and turnover. Nonetheless, the concept that enzymes might be trafficked to cellular compartments to mediate global SUMOylation of substrate proteins present in a specific location at a particular time is an intriguing avenue for future study.
C. Activity-Dependent Regulation of Synaptic SUMOylation
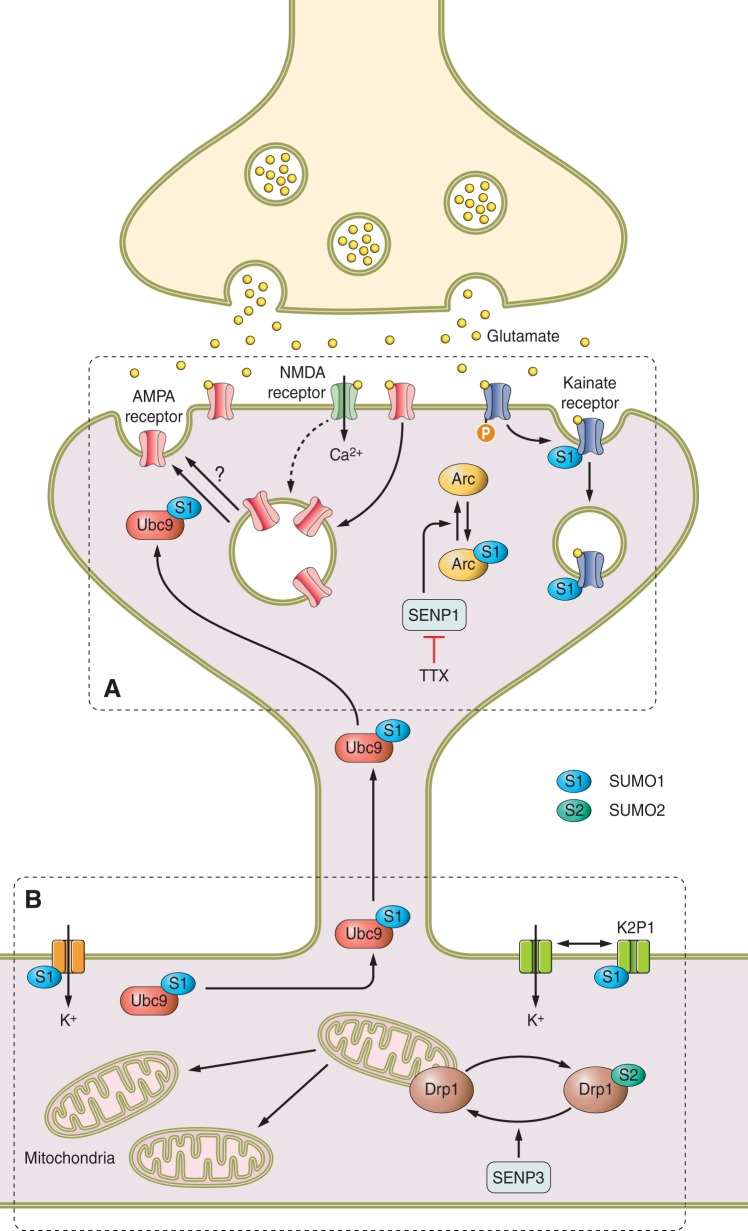
As well as being developmentally regulated, it is also becoming clear that the synaptic localization of SUMO paralogs and the SUMOylation machinery is under tight activity-dependent control. The pre- and postsynaptic distribution of the SUMO proteins and SUMO machinery in cultured neurons following depolarization with KCl has been investigated. KCl stimulation caused a sustained (at least 30 min) increase in presynaptic, but no change in postsynaptic, protein conjugation by SUMO1. In contrast, KCl stimulation caused a transient decrease in postsynaptic levels of SUMO2/3 conjugation but left presynaptic levels unchanged (159). KCl stimulation also led to complex and differential changes in the pre- and postsynaptic distribution of the E1 subunits SAE1, Ubc9, SENP1, and SENP6 (159).
Similarly, treatment of synaptosomes with AMPA has been shown to recruit Ubc9 to the presynaptic fraction (67), and stimulation of neurons with glycine, a coagonist of NMDA receptors, resulting in chemically induced LTP (Chem-LTP), also leads to an increase in colocalization of Ubc9 and SUMO1 with the postsynaptic marker PSD95 (113). Taken together, these results indicate that the dynamic recruitment and removal of the SUMO machinery provides a mechanism for the sophisticated control of substrate-specific SUMOylation at synapses in response to neuronal activity.
In addition to the localization of SUMO proteins and the SUMO machinery at synapses, an important role for SUMOylation in synaptic physiology has been inferred from the growing number of SUMO substrates identified in dendrites, axons, and synapses and the observation that perturbing SUMOylation has major consequences for synaptic function and plasticity (32, 36, 43, 113, 164, 174, 239, 301). In rather stark contrast, however, a report examining the distribution of SUMO reactivity in a His-HA-tagged SUMO1 knock-in mouse failed to detect any synaptic SUMOylation (274). Consistent with previous studies, the majority of SUMO in brains from these mice was present in the nuclear fraction but, in contrast to previously published immunostaining data, no colocalization was observed between His-HA-SUMO and the synaptic markers synapsin (all synapses), VIAAT (inhibitory synapses), or vGluT1 (excitatory synapses) (274). The precise reasons for these differences are unclear; however, it is notable that SUMO1 conjugation is decreased in the transgenic animals compared with wild-type mice, perhaps suggesting that any synaptic SUMO may be below the detection sensitivity of the techniques used (274). Notwithstanding the differences with transgenic mouse data, overall, a very substantial body of data suggests that, in addition to its roles in nuclear function, SUMOylation occurs throughout neurons, can be readily detected at synapses, and is integral to proper synaptic function.
X. SUMOYLATION IN NEURONAL DEVELOPMENT AND DIFFERENTIATION
The terminal differentiation of neurons requires complex transcriptional remodeling to control the activation of neuronal-specific genes in cells destined to become neurons. Soon after its discovery it was realized that SUMOylation is ideally placed to control the kind of transcriptional programming that drives neuronal differentiation. Indeed, a number of transcription factors involved in the expression of neuronal-specific genes, or in the repression of neuronal genes in nonneuronal cells, have been shown to be subject to control by SUMOylation. This is a research field of intense activity that is rapidly advancing. Therefore, to illustrate some of the pathways involved, we have selected several examples in which SUMOylation plays a key role in neuronal differentiation and outgrowth.
A. Pax Proteins
Pax proteins are a family of nine transcription factors that control embryonic neural development, several of which are regulated by SUMOylation (273). For example, Pax-6 performs an evolutionarily conserved role in eye and brain development (26). There are multiple isoforms of Pax-6 generated mainly by alternative splicing. Interestingly, however, the isoform p43 Pax-6 is a SUMOylated form of the smaller p32 isoform rather than a distinct alternative splice isoform (308). More specifically, Pax-6 SUMOylation at K91 enables p32 Pax-6 to bind DNA and become transcriptionally active. Furthermore, in developing mouse eyes, Pax-6 colocalizes with SUMO1 in a manner dependent on the presence of K91 (308). Thus, while the exact role of Pax-6 SUMOylation in regulating eye development is unclear, these data suggest that through modification of Pax-6, SUMOylation may play an important role in this process.
Pax-7 is required for neural crest development in mice and chicks and, in the adult, for muscle homeostasis and regeneration (24). Pax-7 is SUMOylated at a single nonconsensus lysine, K85, and while SUMOylation is not essential for DNA binding activity, it is required for normal neural crest development (163). Knockdown of Pax-7 in developing chick embryos leads to defective expression of neural crest markers that can be rescued by reexpression of wild-type Pax-7, but not a non-SUMOylatable Pax-7 mutant (163). Thus, although the exact mechanism of action remains unclear, Pax-7 SUMOylation is required for normal neural crest development.
B. BRAF35
LSD1-CoREST is a histone demethylase complex involved in the repression of neuronal genes in nonneuronal cells via recruitment to target promoters by the repression factor REST (5, 9). SUMOylation of one member of this complex, BRAF35, inhibits neuronal differentiation (31). BRAF35 can be SUMOylated at four lysine residues (K31, 125, 156, and 157), and overexpression of a mutant in which these residues have been mutated to non-SUMOylatable arginines (BRAF35–4KR) impaired the ability of the LDS1-CoREST complex to repress the expression of neuronal-specific sodium channel α-subunit genes in HeLa cells (31), suggesting that SUMO modification of BRAF35 is required for the repressive activity of the LSD1-CoREST complex. With the use of chromatin immunoprecipitation assays, overexpression of non-SUMOylatable BRAF35 was shown to decrease chromatin binding by the LSD1-CoREST, suggesting this modification is required to either recruit or stabilize the complex on chromatin (31). The P19 embryonic carcinoma stem cell line can be differentiated into neurons through overexpression of the transcription factor NeuroD2 and its dimerization partner E12 (65). Neuronal differentiation was strongly inhibited by coexpression of BRAF35, but not non-SUMOylatable BRAF35, demonstrating the requirement for BRAF35 SUMOylation on the repression of neuronal genes. Interestingly, mature neurons express an inhibitor of BRAF35, iBRAF, which heterodimerizes with BRAF35, sequestering it from the LSD1-CoREST complex and reducing BRAF35 SUMOylation (31). Thus SUMOylation of BRAF35 mediates the repression of neuronal genes in nonneuronal cells and, through targeting BRAF35 SUMOylation, iBRAF can effectively inhibit BRAF35 function to drive neuronal differentiation.
C. Verloren
Olfactory projection neurons extend dendrites that synapse onto a specific class of olfactory receptor neurons, and project axons to higher brain centers in a manner that has been extensively mapped in Drosophila (115–117, 171, 305). The protein Verloren (Velo) was originally identified in a genetic screen for proteins that regulated the specificity of dendrite targeting from olfactory projection neurons. Velo-deficient flies exhibit numerous axonal targeting defects (13) and, interestingly, sequence analysis suggested Velo encodes a SUMO protease, most similar to the human protease SENP7. In contrast to effective rescue with wild-type Velo, expression of a mutant Velo lacking the predicted active site cysteine did not rescue the effects of Velo perturbation, suggesting that its catalytic activity is essential for dendrite and axon targeting of olfactory projection neurons (13). The effects of Velo perturbation on dendritic targeting could be partially rescued by other Drosophila SUMO proteases, but the axonal defects could not, suggesting that Velo is specifically required for axonal targeting. Further underscoring a crucial role for SUMOylation, loss-of-function mutants of the Drosophila equivalents of both SUMO and Ubc9 also exhibited targeting defects (13). These results are particularly interesting because they demonstrate that targeted deSUMOylation is essential for the regulation of neuronal outgrowth, and future work will focus on identifying the specific substrate(s) involved.
Overall, these examples illustrate how SUMO regulation of transcription is an indispensible regulator of neuronal differentiation. The sophistication of this system is only just beginning to emerge, and the regulatory pathways are subtle and complex. SUMOylation of some transcription factors drives neuronal differentiation, whereas SUMOylation of others prevents transcription of neuronal specific genes. Nonetheless, it is already evident that differential substrate-specific modification participates in the control of highly complex cellular processes to ensure coordinated cellular reprogramming to a neuronal fate.
XI. SUMOYLATION IN ROD AND CONE DEVELOPMENT
The vertebrate retina is a widely used model system for investigating neuronal differentiation. The retina contains two distinct types of photoreceptor, rods and cones, which arise from a common progenitor. For progenitor cells to become rods, they must express rod-specific genes, and repress the expression of cone-specific genes. These cell fate decisions are regulated by expression of relatively few transcription factors such as Nrl and Nr2e3, which are expressed primarily in rod precursors, drive the expression of rod-specific genes and, in the same cells, repress cone-specific genes. Interestingly, SUMO-mediated regulation of Nrl and Nr2e3 is an important factor in rod differentiation in the retina (196, 228).
A. PIAS3 and Nr2e3
Expression of the SUMO E3 PIAS3 in the developing retina is almost exclusively restricted to Nr2e3-positive rod precursors (196). Furthermore, overexpression or knockdown of PIAS3 in the developing mouse retina led to an increase or decrease, respectively, in the number of cells showing mature rod phenotype. However, further examination of retinas in which PIAS3 had been knocked down revealed that over 60% of cells expressing the rod marker rhodopsin also expressed cone markers. The effect of PIAS3 knockdown was therefore attributed to defective repression of cone-specific genes in developing rods, a phenotype also observed upon mutation of Nrl or Nr2e3 (196). Following this line of investigation, PIAS3 was shown to bind to and occupy the same promoters as Nr2e3, and to catalyze the SUMO1 modification of Nr2e3 at three lysines: K178, K315, and K322 (196). Mice with a spontaneous null mutation in Nr2e3 exhibit a phenotype characterized by excessive numbers of cells expressing cone markers, and this can be partially rescued by expression of wild-type Nr2e3 but not by expression of an Nr2e3 mutant lacking the major SUMO acceptor site, K178, suggesting that SUMOylation of Nr2e3 is required for the effective repression of cone-specific genes. Taken together, these data have been interpreted to show that PIAS3-mediated SUMOylation of Nr2e3 on cone-specific promoters turns it from an activator of transcription into a potent repressor. Interestingly, expression of the viral protein Gam1, which specifically inhibits SUMOylation through targeting the E1 complex for degradation (17), caused defective repression of cone-specific genes in rods and defective expression of the rod marker rhodopsin. Thus, while PIAS3-mediated SUMOylation of Nr2e3 is required for the repression of cone markers, SUMOylation per se is also required to drive the expression of rhodopsin, suggesting both negative and positive regulation of gene expression by SUMOylation is required to drive rod differentiation (196).
B. Nrl
The rod-specific transcription factor Nrl is also SUMOylated, primarily at K20 (228). Like Nr2e3, Nrl represses cone-specific genes but, in addition, it also drives expression of many rod-specific genes, including rhodopsin and Nr2e3 itself. NonSUMOylatable Nrl is less effective than wild-type Nrl at activating rhodopsin and Nr2e3 promoters (228). Furthermore, Nrl knockout mice show reduced numbers of rhodopsin expressing cells and a loss of Nr2e3 expression. While expression of wild-type Nrl could almost completely rescue this phenotype, expression of non-SUMOylatable Nrl was much less effective (228). Thus SUMOylation of Nrl controls its ability to drive expression of rod-specific genes and is required for proper retinal development. Overall, these reports illustrate how SUMOylation of distinct targets on specific promoters can lead to the complex alterations in gene expression patterns required to determine cell fate and differentiation in the mammalian retina.
XII. SUMOYLATION IN SYNAPSE FORMATION AND SPINE MORPHOGENESIS
A. MEF2A SUMOylation and Synapse Formation
Activity-dependent synapse formation and remodeling is critical for neuronal development and circuit formation. In cerebellar granule neurons, specialized structures called dendritic claws act as postsynaptic targets for mossy fiber and Golgi neuron axons to form synapses onto. Dendritic claw formation is activity-dependently regulated by the transcription factor MEF2A. When activated, MEF2A promotes transcription of a set of genes, including Arc and synGAP, that suppress synapse formation (68). As discussed above, MEF2 transcriptional activity is controlled by the interplay between several posttranslational modifications including phosphorylation, acetylation, and SUMOylation (86, 127, 226, 240, 319). In brief, constitutive phosphorylation of S408 under basal conditions promotes MEF2A SUMOylation, negatively regulating its transcriptional activity. Calcium influx following neuronal activation causes calcineurin-mediated dephosphorylation of S408, which promotes a switch from SUMOylation to acetylation, leading to activation of MEF2A and the consequent transcription of genes that repress postsynaptic differentiation (240).
The role of SUMOylation in MEF2A transcriptional activity is further supported by the identification of the critical role of the SUMO E3 PIASx in dendritic claw formation (239). Knockdown of PIASx in cultured cerebellar granule neurons increases transcription of a MEF2A reporter gene and reduced the number of dendritic claws in cerebellar slices. Correspondingly, overexpression of PIASx increased the number of dendritic claws, and this effect was completely suppressed upon knockdown of MEF2A (239), indicating that MEF2A is the target of PIASx E3 activity that underlies dendritic claw formation.
As well as controlling postsynaptic specialization, MEF2A SUMOylation also plays a role in presynaptic differentiation (307). During development, immature neurotransmitter release sites assemble independently of postsynaptic contact. As networks mature, these “orphan” presynaptic sites are eliminated and their vesicles and proteins are redistributed to presynaptic terminals that have formed mature synapses with postsynaptic neurons (325). In cultured cerebellar granule neurons, knockdown of MEF2A caused an increase in these orphan presynaptic sites without affecting the number of mature synapses (307). The MEF2A knockdown phenotype was rescued by expression of wild-type MEF2A, but not by a non-SUMOylatable MEF2A mutant, indicating that the SUMOylated transcriptional repressor form of MEF2A mediates the suppression of orphan presynaptic sites. By microarray analysis, the authors identified synaptotagmin-1 as a target of MEF2A transcriptional activity and concluded that SUMOylation of MEF2A suppresses orphan presynapse number through repression of synaptotagmin-1 expression (307).
B. MeCP2 and Synapse Formation
Methyl CpG binding protein 2 (MeCP2) is an X-linked DNA binding protein that functions as a transcriptional repressor through the recruitment of HDACs (188). Mutations in MeCP2 are the primary cause of Rett Syndrome (3), a neurodevelopmental disorder that almost exclusively affects females and is characterized by developmental regression, loss of hand skills, impaired mobility and speech, and other symptoms commonly associated with autism (70). SUMOylation of MeCP2, primarily at K223, has been reported to be involved in the regulation of synapse development (37). Interestingly, non-SUMOylatable MeCP2 did not bind HDAC1 and 2, suggesting a role for MeCP2 SUMOylation in transcriptional repression. Knockdown of MeCP2 in neurons caused upregulation of multiple genes in a whole genome cDNA microarray assay. More detailed analysis of five of these genes revealed that their upregulation caused by MeCP2 knockdown was returned to normal levels by exogenous expression of an RNAi-insensitive wild-type MeCP2. However, expression of an RNAi-resistant non-SUMOylatable MeCP2 did not reverse the upregulation, suggesting that SUMOylation of MeCP2 is required for its repressive capability (37). Furthermore, knockdown of MeCP2 reduced synaptic density both in cultured hippocampal neurons and in the hippocampus in vivo after in utero electroporation with an shRNA targeting MeCP2. Reexpression of wild-type MeCP2 rescued this phenotype, but expression of non-SUMOylatable MeCP2 did not (37). These data highlight how SUMOylation of MeCP2 acts as a regulator of its transcriptional activity and synapse formation and open new possibilities for defining mechanistically how defects in SUMOylation and/or MeCP2 are involved in the cellular pathology of Rett Syndrome.
C. SUMOylation of CASK Regulates Dendritic Spine Morphogenesis
Calcium/calmodulin-dependent serine protein kinase (CASK) is a member of the membrane-associate guanylate kinase (MAGUK) family of proteins that includes the postsynaptic density protein PSD95. CASK binds to cell adhension molecules including syndecan-2 and synaptic cell adhesion molecule (SynCAM) and plays a key role in the formation of dendritic spines by connecting transmembrane adhesion molecules to the actin cytoskeleton via interaction with the scaffold protein 4.1 (106). Knockdown of CASK in cultured hippocampal neurons reduces spine size and number, suggesting that CASK stabilizes or maintains spine morphology. The knockdown phenotype can be rescued by expression of wild-type CASK but not CASK lacking the protein 4.1 binding site (36). Interestingly, CASK has been shown to undergo activity-dependent SUMOylation at K679, and this modification regulates binding to protein 4.1, since a CASK-SUMO1 fusion construct or SUMO1 overexpression reduced the CASK-protein 4.1 interaction (36). CASK overexpression increases spine size and density, whereas overexpression of the CASK-SUMO1 fusion protein has opposite effects, leading to a decrease in both spine size and number. These results suggest that SUMOylation of CASK downregulates spine formation via uncoupling membrane adhesion molecules from protein 4.1 and the cytoskeleton (36).
XIII. REGULATION OF ION CHANNELS AND CELL EXCITABILITY
Modulation of neuronal excitability can profoundly affect synaptic properties and neuronal networking. SUMOylation plays a fundamental role in controlling membrane potential and cell excitability via regulation of ion channels in neurons, as well as cells isolated from different organs including heart and pancreas. SUMOylation has been proposed to regulate a wide range of channels but, as outlined below, in many cases the evidence is not yet conclusive.
A. SUMO Regulation of K2P1 (TWIK1) Activity
The K2P family of potassium channels helps maintain negative resting membrane potentials below the firing threshold of excitable cells by contributing to the leak K+ conductance. Most K2P channels are actively modulated by neurotransmitters, and within the wider family, channel subunits mediate time- and voltage-independent potassium leak currents whereas other subtypes, including TASK channels, increase channel open probability with depolarization (211).
The K2P1 channel that regulates the background potassium current in many cells has been reported to be a target for SUMOylation. When wild-type K2P1 channel is heterologously expressed at the plasma membrane of Xenopus oocytes, its deSUMOylation is necessary to generate an outward potassium current. This led to the suggestion that SUMOylation of K274 maintains the channel in an inactive state (218). Notably, mutation of K274 to glutamic acid or overexpression of SENP1 allows the channel to operate, exhibiting K+ selectivity and sensitivity to extracellular pH (218). However, the role of K274 SUMOylation has been challenged by another group who reported that the mutation K274E caused an increase in K2P1 current density, while the mutation K274R, which would also prevent SUMOylation, did not (66). These data suggested a charge effect of amino acid substitution rather than an effect of SUMOylation was responsible for the observed alteration in K2P1 activity. Furthermore, they also suggest that endocytosis rather than SUMO-mediated inactivation accounts for K2P1 silencing (66). However, the authors of the original Rajan et al. (218) report have disputed these findings in a subsequent publication in which they demonstrate that silent K2P1 channels in excised patches of plasma membrane can be activated by application of recombinant SENP1 and resilenced by addition of SUMO1 (209). In these experiments, mutation of K274 to glutamine, arginine, glutamic acid, aspartic acid, cysteine, or alanine residues all resulted in constitutively active K2P1 channels, which were insensitive to SUMO1 and SENP1 (209). Thus, as things stand, whether the K2P1-mediated current is controlled by SUMOylation remains a matter of contention.
Interestingly, K2P1 can form heteromeric assemblies with two P domain, acid-sensitive K+ channel (TASK) subunits K2P3 or K2P9, and the resultant channels mediate the noninactivating standing outward K+ current (IKso). In addition to controlling the resting membrane potential, IKso defines cerebellar granule neuron responses to, among other things, membrane stretching, intracellular acidosis, and volatile anesthetics such as halothane (161). Inclusion of picomolar concentrations of recombinant SENP1 in the recording patch pipette revealed rapidly increased IKso in rat cerebellar granule neurons, suggesting that the endogenous SUMO pathway suppresses a fraction of IKso (212). However, channels formed exclusively by K2P3 or K2P9 were shown to be insensitive to SENP1. These results suggest that SUMOylation in cerebellar granule neurons silences IKso channels containing K2P1 and that deSUMOylation by infusion of SENP1 releases these channels from suppression to reduce their excitability (212). Extraplolation of these data suggests that SUMOylation likely impacts on the many homeostatic mechanisms mediated via TASK channels containing K2P1.
B. Voltage-Gated Potassium Channels and SUMOylation
The voltage-gated potassium channel Kv2.1 is widely expressed in many tissues including brain, heart, muscle, and pancreas where it controls membrane excitability by mediating the majority of the delayed inwardly rectifying current (IDR). During periods of increased activity, the voltage-dependent activation of Kv2.1 is shifted to more negative membrane potentials, resulting in homeostatic suppression of excitability (59, 60). Inclusion of SUMO1 in the recording electrode in current clamp experiments on hippocampal neurons increased action potential firing rates, whereas SENP1 decreased action potentials (210). The increased excitability mediated by SUMO1 was attributed to decreased voltage sensitivity of the delayed inwardly-rectifying current and shown to be due to SUMOylation of K470 of Kv2.1 (210). SUMO conjugation makes the channels more sensitive to depolarization by shifting the half-maximal activation voltage of the channels. Kv2.1 channels comprise four subunits, but only two nonadjacent subunits can be simultaneously SUMOylated. SUMOylation of one subunit shifts voltage for half-activation (V1/2) by 15 mV, whereas SUMOylation of two subunits shifts the V1/2 by 35 mV. Thus direct SUMOylation of Kv2.1 provides a graded regulatory system for controlling the excitability of hippocampal neurons, and most likely other excitable cells (210).
Kv2.1 also regulates pancreatic β-cell excitability and insulin secretion (165). In HEK 293 cells, infusion of recombinant SUMO1 inhibited current through exogenously expressed Kv2.1 by 80%, and expression of the YFP-SUMO1 in human β-cells or insulinoma cells significantly inhibited native voltage-gated potassium currents (47). In HEK 293 cells, SUMOylation of Kv2.1 accelerated inactivation and inhibited recovery from inactivation of the Kv2.1-mediated current. These properties result in the widening of action potentials and a decreased firing frequency, suggesting SUMOylation has a strong inhibitory action on Kv2.1 to regulate cellular excitability in native β-cells and, by extrapolation, other cell types (47).
Another voltage-gated potassium channel, Kv1.5, is present in brain (284) but is best characterized for mediating the K+ current (IKur) central to action potential repolarization in atrial myocytes (190). Kv1.5 is directly SUMOylated at two sites located close to plasma membrane spanning domains (12). SUMOylation alters the biophysical properties of the channel to accelerate inactivation and inhibit recovery from inactivation, resulting in decreased firing frequency (12). In Cos-7 cells, deSUMOylation of the Kv1.5 channel, either by disruption of the SUMOylation sites or by overexpression of SENP2, causes a hyperpolarizing shift in the steady-state inactivation of the related IKur current. This may represent a significant mechanism to fine tune electrical activity. Although no direct experimental evidence has yet been reported, multiple other Kv channel α-subunits contain consensus SUMOylation motifs in similar positions, suggesting that SUMOylation may represent a general mechanism of channel regulation. In this respect it may also be significant that KChAP, a protein originally described as a potassium channel chaperone that increases expression (298), was subsequently identified as the SUMO E3 ligase PIAS3 (297).
C. TRPM4
Transient receptor potential melastatin (TRPM) ion channel type 4 (TRPM4) is a nonselective cation channel that is activated by, but is not permeable to, intracellular Ca2+. It is widely expressed in neurons as well as in heart, pancreas, and kidney and is particularly abundant in cardiac Purkinje fibers (141). TRPM4 is a SUMO substrate, although the specific lysine residue(s) involved have not yet been defined (289). However, a glutamate to lysine mutation at residue 7 in the NH2 terminus of TRPM4, which enhances channel SUMOylation and increases plasma membrane expression and consequent channel activity, has been linked to the autosomal dominant disease progressive familial heart block type I (141).
Further genetic linkage analyses of families with autosomal dominant isolated cardiac conduction block revealed other missense mutations in the TRPM4 gene. Each of these mutations resulted in an increased current density gain of function, which was suggested to be attributable to impaired endocytosis caused by misregulation of SUMOylation (156). However, given that the first reported mutation adds a lysine residue to the NH2 terminus and the other three do not involve lysines (R164W, A432T, and G844D), how this would occur is unclear.
XIV. SUMOYLATION IN POLARIZED MRNA AND TRAFFICKING
Many neuronal processes, including synapse formation and maintenance, and synaptic plasticity, require local protein synthesis (288) which, in turn, requires the transport of mRNA to distal processes by mRNA binding proteins. SUMOylation of one such transport protein, La (283), regulates its interaction with the motor proteins kinesin and dynein, which are required for retrograde and anterograde transport, respectively (101). In a seemingly simple and elegant “switch” system, SUMOylated La binds only dynein, whereas non-SUMOylated La binds only kinesin, thus determining the direction of the axonal transport of La and its associated mRNAs (283). It remains unclear how the SUMOylation of La is regulated, and it is possible that other mRNA binding proteins could also be subject to such directional regulation. It is therefore likely that SUMOylation plays important roles in the delivery of mRNAs throughout the neuron.
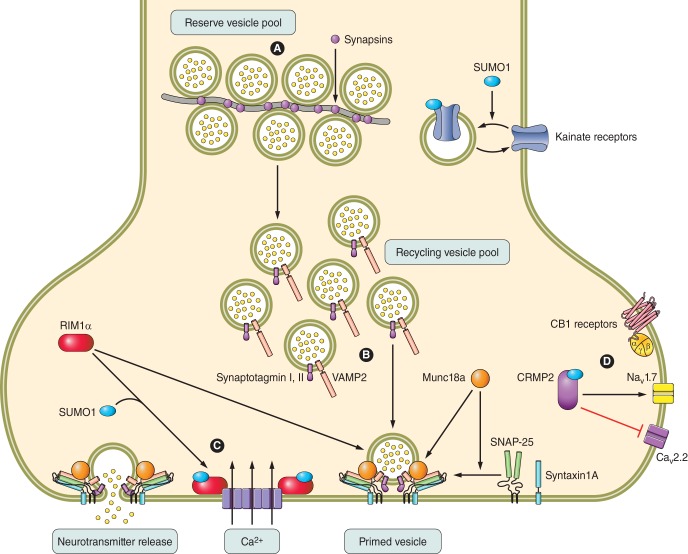
XV. SYNAPTIC SUMOYLATION AND NEUROTRANSMISSION
A. Presynaptic SUMOylation and Neurotransmitter Release
Timely and appropriate neurotransmitter release is fundamental to synaptic function. Moreover, activity-dependent changes in release properties underlie some forms of synaptic plasticity (30). The fusion of synaptic vesicles with the presynaptic membrane is very precisely controlled by the interplay between active zone proteins. While many of these proteins have been identified, it is unclear how the complex web of interactions is regulated, particularly since several of these proteins are multifunctional. Intriguingly, there are multiple, mainly unidentified, SUMO substrate proteins present in synaptosomes and purified presynaptic membrane fractions (67). Furthermore, presynaptic SUMOylation appears to be activity-dependently regulated since application of AMPA to isolated synaptosomes increases the extent of protein SUMOylation, while kainate application decreases synaptosomal protein SUMOylation and NMDA stimulation has no discernible effect (67).
During the preparation of isolated synaptosomes, presynaptic boutons are sheared from axons and then reseal. It is therefore possible to entrap compounds, peptides, or even recombinant proteins inside the synaptosomes during the resealing process. Synaptosomes loaded with SENP1 displayed increased KCl-evoked glutamate release, whereas synaptosomes loaded with SUMO1 exhibited decreased KCl-evoked release. Conversely, presynaptic release evoked by stimulation with kainate was enhanced by SUMO1 (but not SUMO1ΔGG, which lacks the two COOH-terminal glycines required for conjugation), and reduced by SENP1 (67). Thus SUMOylation of presynaptic proteins can either up- or downregulate neurotransmitter release depending on the precise stimulus. In synaptosomes containing SENP1, KCl- or AMPA-induced Ca2+ influx was enhanced, whereas in synaptosomes containing SUMO1, KCl- or AMPA-induced Ca2+ influx was reduced. Consistent with the release assays, the opposite effects were observed following kainate stimulation (67).
These results illustrate the complexity of the roles of SUMOylation in neurotransmitter release. Overall, the data suggest that SUMO conjugation to distinct subsets of presynaptic proteins acts to tune presynaptic release in an activity-dependent and stimulus-specific manner.
Exactly which proteins mediate these effects is the subject of a concerted research effort, and several presynaptic proteins known to regulate release have been identified as SUMO substrates (Figure 6). For example, the syntaxin 1A interacting protein tomosyn is a SUMO2/3 substrate at K730 (303). Mutation of K730 enhances tomosyn inhibition of presynaptic exocytosis in PC12 cells, but does not alter the interaction with syntaxin1A, suggesting that SUMOylation of this protein could downregulate release (303).
FIGURE 6.
The roles of protein SUMOylation at the presynapse. Schematic highlighting proteins involved in key stages of presynaptic vesicle exocytosis. A: the reserve pool of synaptic vesicles is tethered to the actin cytoskeleton by synapsin proteins. On depolarization, calcium influx activates protein kinases, e.g., CaMKII and PKA, which phosphorylate synapsins allowing migration of vesicles from the reserve to the recycling pool. B: vesicles in the recycling pool are docked and primed for release. This process involves many proteins including the SNARE complex (VAMP2, SNAP-25, and syntaxin-1A), munc18a, synaptotagmins, munc13–1, and RIM1α. C: calcium influx through voltage-gated CaV channels (clustered by RIM1α) triggers exocytosis of neurotransmitter by binding to synaptotagmin. D: collapsin response mediator protein 2 (CRMP2) is reported to be SUMOylated in neurons. SUMOylation of CRMP2 has been implicated in inhibition of Ca2+ entry through CaV2.2 channels, and increasing surface expression of NaV1.7 channels. Named proteins are potential or confirmed SUMO substrates. For confirmed SUMO substrates, the role of SUMOylation is shown, i.e., SUMOylation of RIM1α is required to cluster CaV channels and allow coordinated Ca2+ entry, and GluK2 SUMOylation triggers kainate receptor endocytosis.
Some neurotransmitter receptors that are partly or exclusively localized at the presynaptic terminal have also been proposed as SUMOylation targets. These include and cannabinoid receptor type 1 (CB1) (85) and group III mGluRs (62, 266, 302), but as yet no compelling functional evidence has been reported to support a physiological role for SUMOylation of these receptor proteins.
1. Rab3 interacting molecule 1α
To date, the only active zone protein that has been extensively characterized as a SUMO substrate is Rab3 interacting molecule (RIM1α). RIM1α is a large, multifunctional protein which coordinates many of the critical stages of neurotransmitter release (25), including docking and priming (50) of synaptic vesicles, presynaptic LTP (29), and clustering of Ca2+ channels (123) to allow coordindated Ca2+ entry in response to an action potential. Despite its critical role in neurotransmitter release, little is known about how the many functions of RIM1α are coordinated. In a recent study, Girach et al. (76) demonstrated that RIM1α is SUMOylated in neurons at a single lysine, K502, and that SUMOylation of this site is required for normal presynaptic exocytosis. Specifically, knockdown of RIM1α and replacement with a non-SUMOylatable mutant eliminates the fast component of synaptic vesicle exocytosis, due to a defect in depolarization-induced calcium entry. SUMOylation of RIM1α makes its PDZ domain available for interaction with CaV2.1 and is required for the Ca2+-channel clustering function of RIM1α, although docking of synaptic vesicles appears to be unimpaired by a lack of SUMOylation. Therefore, RIM1α SUMOylation acts as a molecular switch, coordinating the diverse functions of this protein. This study found no activity dependence of RIM1α SUMOylation, implying instead that there may be two distinct populations of SUMOylated and non-SUMOylated RIM1α, which carry out distinct functions at the presynapse.
As outlined above, the effects of altering SUMOylation levels on presynaptic glutamate release are diverse and stimulus dependent (67). This kind of SUMO-mediated molecular switch may represent a general mechanism for the coordination of multifunctional presynaptic proteins, possibly explaining how many proteins (e.g., syntaxin1A, synaptotagmin, synapsins) participate in different stages of the synaptic vesicle cycle. Interestingly, syntaxin1A has been suggested to be a SUMO substrate in pancreatic β-cells (170) where it acts as a “brake” on exocytosis. If this were the case in neurons, it would mean that SUMOylation has different effects on synaptic vesicle exocytosis depending on the substrate protein, consistent with a highly sophisticated level of regulation of presynaptic function by SUMOylation. Clearly, this is an underdeveloped field with many intriguing questions remaining to be answered.
2. Collapsin response mediator 2
Collapsin response mediator 2 (CRMP2) is a microtubule-binding protein involved in axon guidance and the establishment of neuronal polarity (112). CRMP2 can regulate presynaptic release through interactions with the calcium channel subunit CaV2.2 (304). In hippocampal neurons, CRMP2 promotes neurotransmitter release through enhanced surface expression of CaV2.2. Interestingly, CRMP2 has been identified as a SUMO substrate, which is modified at a single lysine residue, K374, in the CAD cell line (121). In adult dorsal root ganglion (DRG) neurons, overexpression of CRMP2, SUMO, and Ubc9 reduced KCl depolarization-induced calcium influx. Conversely, overexpression of a non-SUMOylatable CRMP2 mutant along with SUMO and Ubc9 increased KCl-induced calcium influx, suggesting CRMP2 SUMOylation negatively regulates calcium influx (121). However, exactly how SUMOylation of CRMP2 affects CaV2.2 trafficking and calcium influx in DRG neurons has not yet been investigated.
In addition to CaV2.2, CRMP2 has also been reported to regulate surface expression of the voltage-gated sodium channel subunit NaV1.7 in DRG neurons (61). The ability of CRMP2 to regulate NaV1.7 trafficking is also regulated by SUMOylation. CRMP2 mutants lacking the SUMOylation site impair sodium currents in CAD cells, and decrease surface trafficking of NaV1.7 (61). This effect was specific to NaV1.7, since NaV1.1 and NaV1.3 currents were unaffected. Furthermore, in DRG neurons, overexpression of non-SUMOylatable CRMP2 reduced sodium current density (61). Together, these data suggest that SUMOylation of CRMP2 is required for correct surface trafficking of NaV1.7 sodium channels. However, whether CRMP2 interacts directly with NaV1.7, and the mechanism underlying how SUMOylaton of CRMP2 regulates NaV1.7 channel trafficking, is currently unclear. Furthermore, in addition to these examples, CRMP2 has also been reported to regulate the trafficking and signaling of NMDA and kainate-type glutamate receptors (22, 23, 172). Further work will be necessary to test if SUMOylation of CRMP2 also controls glutamate receptor function.
B. Postsynaptic SUMOylation
As for the presynaptic terminal, there has been rapid development in the identification and understanding of the roles of SUMOylation of a growing number of predominantly postsynaptic proteins (Figure 7). As outlined below, the best characterized of these is the GluK2 subunit of kainate receptors.
FIGURE 7.
SUMOylation at the postsynapse. SUMOylation regulates postsynaptic structure and function through modification of multiple substrates. A: agonist activation of the kainate receptor (KAR) leads to PKC-mediated phosphorylation and SUMOylation of the GluK2 receptor subunit, ultimately resulting in KAR endocytosis. Although not direct targets themselves, SUMOylation also regulates the trafficking of AMPA receptors. Induction of LTP with the NMDAR coagonist glycine recruits Ubc9 and SUMO to the postsynapse, and SUMOylation is essential for AMPAR insertion into the postsynaptic membrane. Suppression of synaptic activity by TTX blockade of voltage-gated sodium channels causes increased AMPAR insertion and synaptic upscaling. This treatment also causes the loss of the SUMO protease SENP1, promoting SUMOylation of the synaptic protein Arc, which in turn reduces AMPAR endocytosis, resulting in increased AMPAR surface expression. B: SUMOylation modulates neuronal excitability through modification of the potassium channels K2P1 and Kv1.5, and potentially regulates the ability of synapses to meet energy demands through modification of the mitochondrial GTPase Drp1, which controls mitochondrial number.
1. Kainate receptors
Kainate receptors (KARs) are tetrameric glutamate-gated cation channels composed of the subunits GluK1–5 (formerly GluR5–7, KA1, KA2) (83). They are present at pre- and postsynaptic membranes at many synapses throughout the brain. Presynaptic KARs control neurotransmitter release, extrasynaptic KARs regulate neuronal excitability, and postsynaptic KARs contribute to postsynaptic depolarization (42, 83). Furthermore, KARs are highly expressed in many neuronal circuits during development where they regulate synapse formation and stabilization, after which their expression is reduced (131, 143, 144, 267).
The KAR subunit GluK2 is strongly linked with autism (133, 203, 250) and intellectual disability (183). GluK2 is SUMOylated at a single lysine residue (K886) in its intracellular COOH terminus in response to agonist activation, and this modification is required for agonist-induced receptor internalization (175). Furthermore, postsynaptic KAR responses at mossy fiber-CA3 synapses are decreased by infusion from the recording electrode of SUMO1, or increased by infusion of the catalytic domain of the SUMO-specific deconjugating enzyme SENP1 (175).
Protein kinase C (PKC)-mediated phosphorylation of KARs can enhance or depress KAR surface expression in cultured hippocampal neurons (173). Subsequent work demonstrated that PKC phosphorylation of GluK2 at S868 precedes and regulates receptor SUMOylation. The PKC activator PMA enhances GluK2 SUMOylation and consequent internalization, whereas mutation of S868 to a nonphosphorylatable alanine prevents kainate-induced GluK2 SUMOylation at K886 and endocytosis (137, 300). Further analysis of the functional interrelationship between phosphorylation and SUMOylation of GluK2 was performed using patch-clamp electrophysiology of HEK 293 cells expressing recombinant wild-type or mutated GluK2. Infusion of conjugatable SUMO1 from the electrode caused a decrease in the KAR current amplitude in HEK 293 cells expressing wild-type GluK2. However, SUMO1 infusion did not decrease the KAR current amplitude evoked from HEK 293 cells expressing K886R GluK2 or in cells expressing a nonphosphorylatable alanine at serine-868 (32).
The physiological importance of GluK2 SUMOylation was highlighted by the observation that it is required for long-term depression (LTD) of postsynaptic KARs (32). At mossy fiber-CA3 synapses, inclusion of nonconjugatable SUMO1-ΔGG had no effect on electrophysiologically induced LTD compared with control conditions. However, infusion of conjugatable SUMO1 decreased KAR EPSCs and completely occluded LTD. Furthermore, infusion of SUMO1 into CA3 neurons virally expressing wild-type GluK2 depressed KAR EPSCs, and induction of KAR LTD was indistinguishable between wild-type GluK2-transduced and nontransduced neurons. In contrast, infusion of SUMO1 into neurons virally expressing either non-SUMOylatable K886R GluK2 or nonphosphorylatable S868A GluK2 did not depress KAR EPSCs, and KAR LTD was completely blocked (32). Taken together, these results indicate that SUMOylation of GluK2 at K886 and phosphorylation at S868 are required for KAR LTD.
These studies on the GluK2 subunit provide a clear example of how SUMOylation and phosphorylation can combine to modulate synaptic function by orchestrating the temporal and spatial regulation of KARs. While the underlying mechanics of how phosphorylation and SUMOylation modulate KAR surface expression is an area of active investigation, these findings illustrate the complexity and refinement of receptor trafficking pathways and provide insight into the mechanisms that control synaptic transmission, and consequently network activity and brain function. More generally, these results underscore the importance of the interplay between posttranslational modifications in controlling substrate protein behavior.
XVI. SUMOYLATION AND SYNAPTIC PLASTICITY
A. AMPARs
AMPA receptors (AMPARs) mediate most fast excitatory neurotransmission in the CNS and are key determinants of neuronal function and dysfunction (96, 110). While there have been investigations looking for AMPAR SUMOylation in recombinant systems (302) and investigating the effects of modulating synaptic SUMOylation on basal AMPAR responses (174), to date, there are no reports of AMPARs themselves being directly SUMOylated. Nonetheless, as discussed below, it is becoming increasingly apparent that SUMOylation may play import roles in activity-dependent AMPAR trafficking through SUMOylation of AMPAR interacting proteins.
B. Long-Term Potentiation
Synaptic plasticity generally refers to alterations in the number, subunit composition, and/or properties of synaptic AMPARs that potentiate (long-term potentiation; LTP) or depress (LTD) the efficiency of synaptic transmission. This AMPAR-mediated synaptic plasticity occurs at synapses throughout the CNS and can last minutes, hours, or years and underlies many neuronal, network, and systems level functions including learning, memory, and intellectual processing (96, 110, 167). The most extensively studied form of LTP is the NMDAR-dependent increase in the functional activity and number of postsynaptic AMPARs (129). NMDARs require both agonist activation and membrane depolarization to remove an intracellular channel block by Mg2+ to allow Ca2+ gating (200). The increased intracellular Ca2+ due to NMDAR opening activates calcium/calmodulin-dependent protein kinase II (CaMKII), which triggers the phosphorylation events necessary for AMPAR insertion into the postsynaptic membrane (158, 168, 169).
The possible roles of SUMOylation in plasticity have been investigated in several experimental models. In cultured rat hippocampal neurons, increased AMPAR surface expression mediated by application of the NMDAR coagonist glycine (ChemLTP) (138, 162) recruits SUMO1 to synapses and increases colocalization of SUMO1 with Ubc9 (113). Interestingly, SUMO1 and Ubc9 mRNAs were also increased and trafficked to dendrites following ChemLTP, highlighting SUMO1 and Ubc9 as activity-dependent plasticity-related genes (113). Preventing SUMOylation by expression of dominant negative Ubc9 or the catalytic domain of SENP1 prevented the ChemLTP-induced increase in AMPAR surface expression, indicating that SUMOylation is required for this form of plasticity (113). However, the SUMO substrates responsible for AMPAR trafficking during LTP are currently unclear.
C. Synaptic Scaling
Another form of plasticity is homeostatic, or synaptic, scaling in which neurons modulate the sensitivity of their synaptic inputs in response to prolonged changes in network activity. This process is important because it allows neurons to remain excitable despite large changes in background network activity (215, 276). Chronic blockade of neuronal activity with tetrodotoxin (TTX) or NMDAR and AMPAR antagonists, or increasing neuronal network activity by blocking inhibitory transmission with the GABAA receptor antagonist bicuculline, results in compensatory increases or decreases in AMPAR surface expression and synaptic strength, respectively.
The signaling mechanisms underpinning synaptic scaling are the focus of intense investigation, and a number of secreted signaling molecules have been implicated as key mediators including brain-derived neurotrophic factor (BDNF) (229) and tumor necrosis factor (TNF)-α (259). Furthermore, scaling has been reported to be dependent on various CaMK isoforms (111, 272). Perhaps the best-characterized protein involved in synaptic scaling is the activity-regulated immediate early gene product Arc/Arg3.1 (Arc) (247), which has been proposed to mediate AMPAR endocytosis. Levels of Arc protein are decreased during TTX-mediated upscaling, and this decrease reduces AMPAR internalization resulting in increased surface expression (38, 224). Interestingly, in parallel with TTX-induced increases in AMPAR surface expression, levels of the deSUMOylating enzyme SENP1 are reduced, and there is a consequent increase in protein SUMOylation. Furthermore, overexpression of the catalytic domain of SENP1 prevents the TTX-induced increase in surface AMPARs (45), suggesting that SUMOylation is required for AMPAR upscaling. Mutation of two consensus SUMOylation sites in Arc (Arc-ΔKK) disrupts Arc localization to dendrites, which has been interpreted to suggest that Arc SUMOylation plays a role in structural changes required for some forms of LTP consolidation (20). In cultured neurons, viral overexpression of Arc-ΔKK, but not wild-type Arc, prevents TTX-induced synaptic scaling, indicating that Arc SUMOylation is required for scaling and that non-SUMOylatable Arc-ΔKK acts as a dominant negative (45). These initial data are particularly interesting because Arc-dependent synaptic scaling has been implicated in intellectual disability (251) and neurodegenerative diseases (216).
XVII. SUMOYLATION AND SYNAPTIC SIGNALING CASCADES
A. Kinases and Phosphatases as SUMO Substrates
As outlined above, phosphorylation can regulate substrate SUMOylation. However, these two systems also participate in intricate reciprocal regulation, since important components of the phosphorylation machinery that play direct roles in synaptic regulation are themselves subject to SUMO modification.
1. GSK3β
The multifunctional proline-directed serine/threonine kinase glycogen synthase kinase 3β (GSK3β) plays an important role in the phosphorylation cascade that initiates NMDAR-dependent LTD (132, 201). Interestingly, inhibition of GSK-3β has no effect on NMDAR-dependent LTP, but GSK-3β overexpression blocks LTP, and LTP induction inhibits the activity of GSK-3β (102, 322). These data have been interpreted to suggest that GSK-3β may act as a molecular bridge between LTP and LTD pathways (102, 201). GSK3β is SUMOylated at a single lysine residue (K292), and mutating this site to a non-SUMOylatable arginine decreased its kinase activity and protein stability, and reduced its nuclear localization (145). The conditions that promote GSK3β SUMOylation and the physiological relevance to synaptic plasticity have not yet been established. However, given that SUMOylation decreases GSK3β activity, it is tempting to speculate that SUMOylation will act to modify the control of plasticity exerted by GSK3β. We expect that defining exactly how SUMOylation regulates GSK3β activity is likely to be an active and potentially fruitful area of future research.
2. PTEN
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a lipid phosphatase that dephosphorylates phosphatidylinositol 3,4,5-trisphosphate (PIP3) to generate phosphatidylinositol 4,5-bisphosphate (PIP2). This downregulates the PI3K-Akt pathway, a major cell regulatory system, and acts as a brake on cell growth and division (254). Thus PTEN is a tumor suppressor, and its mutation is implicated in a large number of cancers (256).
Interestingly, PTEN is highly expressed in adult brain, and defects in PTEN have been proposed to be a potential cause of autism spectrum disorders (189). Downregulating PTEN negatively regulates mTOR and promotes axon branching (56) and neuronal regeneration (157). Furthermore, activation of NMDA receptors enhances PDZ interactions between PTEN and PSD95 leading to the recruitment of PTEN to spines where its phosphatase activity decreases AMPAR-mediated synaptic responses (122). Importantly, inhibition of PTEN activity prevents NMDAR-dependent LTD, but not mGluR-dependent LTD or LTP. Thus PTEN appears to play a key role as a synaptic signaling molecule required for NMDAR-dependent LTD (122).
Separate groups have independently reported that PTEN can be SUMOylated at K254 and K266, and that SUMOylation increases PTEN membrane association and activity (10, 84, 108). Because PTEN is a negative regulator, its SUMOylation acts to downregulate the Akt pathway. In mitotic cells, this has been proposed to suppress cell proliferation and tumor growth in vivo (108). As yet, there have been no reports on the effects of PTEN SUMOylation in neurons, but we believe that this too represents an exciting avenue for future investigation.
XVIII. POSSIBLE FUTURE DIRECTIONS FOR THE ANALYSIS OF PROTEIN SUMOYLATION IN NEURONS
Although the techniques used so far have advanced understanding of neuronal and synaptic SUMOylation, and provided some insights into the SUMO-regulation of synaptic transmission, there are limitations in the approaches currently available. Inclusion of either SUMO1 or SENP1 in recording electrodes when studying ion channels or synaptic plasticity (e.g., Refs. 32, 210) has been fairly widely used. This nongenetic approach to manipulating SUMOylation has the advantage that, where an acute effect is seen, it is highly likely to be due to changes in SUMOylation of extranuclear proteins, rather than an alteration in gene expression. However, the disadvantage of this approach is a lack of specificity and fine control, in that the SUMOylation of many different substrate proteins will be either up- or downregulated simultaneously. To draw conclusions relating to specific substrates, therefore, it is essential to show the insensitivity of a non-SUMOylatable mutant to these manipulations. Furthermore, it is not possible to empirically determine the extent of up- or downregulation of SUMOylation of particular proteins using this approach. As a result, considerable care needs to be taken in interpreting such data, and safe conclusions can only be drawn when this approach is used in combination with other techniques. Similarly, when SUMOylation levels in neurons are manipulated by overexpression of either SENPs or SUMO (e.g., using transfection or viral infection), many different SUMO substrates will be affected, and thus it is not possible to ascribe a functional effect to the modification of a particular protein without adopting a combinatorial approach (e.g., Refs. 44, 113). An advantage of this overexpression approach over inclusion of SUMO or SENP proteins in recording electrodes is that it allows direct biochemical analysis of the changes in SUMOylation levels of individual proteins, and the SUMO state of the cell as a whole. However, a disadvantage is that, due to the time scale of transfection/viral infection, effects of SUMOylation at a transcriptional level cannot be discounted. It also needs to be recognized that proteins are substrates for different SUMO paralogs and that the SUMOylated forms are preferentially deSUMOylated by different SENPs. Clearly, these parameters should ideally be established before such studies are performed.
Increasingly elegant techniques are being utilized to offer a more precise analysis of the effect of SUMOylation of individual proteins on neuronal function. One of these is the application of an acute molecular replacement (knockdown-rescue) strategy (e.g., Refs. 76, 89). This approach involves the replacement of endogenous protein with a nonSUMOylatable mutant, allowing much more precise analysis. However, there are caveats: the level of knockdown is rarely 100%, diluting the observed effect by the continuing presence of the wild-type protein. Additionally, rigorous controls must be conducted to ensure that the rescue protein is expressed to the same level as the wild-type. Since the substrate cannot be SUMOylated at any stage, this approach offers limited insights into the temporal and spatial regulation of SUMOylation. A powerful parallel approach will be to generate knock-in transgenic mice in which the endogenous protein is replaced with a non-SUMOylatable mutant. To our knowledge, this has not yet been attempted, but it has the potential to yield valuable insight into the role of individual SUMO substrates, not just at a cellular level, but also on whole animal behavior.
One major drawback to the genetically encoded approaches listed above is that it is currently only possible to reduce or eliminate SUMOylation. As yet, there is no SUMO equivalent of the phosphomimetic mutations, which have provided key insights in the study of synaptic regulation by phosphorylation. While directly fusing SUMO to the NH2 or COOH terminus of the target protein has been utilized for a number of substrates (e.g., Refs. 36, 226, 257), this approach is highly substrate dependent, since the fused SUMO does not necessarily replicate the functional effects of SUMO conjugation to an internal lysine residue. One potential answer is to substitute the SUMOylation site of a neuronal protein with that from a protein that is an intrinsically good SUMO substrate. However, this technique has not yet been tested and is likely to have many potential problems and caveats to overcome. Another potential solution is to use an inducible Ubc9/substrate dimerization system in which SUMOylation of a specific substrate can be induced by addition of rapamycin (324). Again, this system has yet to be validated, and extensive and rigorous controls will be needed.
In summary, there are many potential emergent techniques for the analysis of the role of protein SUMOylation on neuronal function, but all have limitations and drawbacks. However, when used in combination, we anticipate that these approaches will continue to drive rapid progress towards understanding the roles and consequences of protein SUMOylation in neurons.
XIX. SUMOYLATION AND NEURONAL DYSFUNCTION
In addition to being a critical regulator of neuronal and synaptic function under normal conditions, an increasing body of evidence is accumulating demonstrating that protein SUMOylation is an important factor in a very wide range of diseases. As well as aberrant SUMOylation being implicated in disease etiology, an emergent theme is that SUMOylation can be a powerful cytoprotective mechanism in cells subjected to severe stress. This is a very active and rapidly advancing field of investigation, especially in elucidating roles of SUMOylation in transcriptional regulation and cancer biology (14, 46, 69, 118) and in cardiac dysfunction (289, 326). In addition, the potential roles of SUMOylation in neurological and neurodegenerative diseases are receiving increased attention, and genetic linkage studies in particular have implicated the SUMO pathway in multiple hereditary brain disorders (4, 140). Here we summarize some of the latest developments linking SUMOylation with different forms of brain disease (Table 2).
Table 2.
SUMOylation and disease
| Disease | SUMOylated Proteins | Effect | Reference Nos. |
|---|---|---|---|
| Huntington's disease | Htt (mediated by Rhes) | Toxic | 178, 256, 261 |
| Spinal and bulbar muscular atrophy (SBMA) | Androgen receptor | Uncertain, possibly protective? Attenuates AR aggregation and transcriptional activity | 183, 191, 212 |
| Dentatorubral-pallidoluysian atrophy (DRPLA) | Intranuclear inclusions are SUMO1 positive, no substrate yet identified | SUMOylation possibly increases nuclear inclusions and apoptosis | 270 |
| Spinocerebellar ataxias (SCAs) | Ataxin-1 | Unclear, possibly increases aggregation and cytotoxicity | 224, 230 |
| Ataxin-3 | Possibly increases cytotoxicity of polyQ protein | 319 | |
| Ataxin-7 (by SUMO1 and SUMO2) | Reduces protein aggregation and cytotoxicity | 113 | |
| Alzheimer's disease | APP | Uncertain. Increasing cellular SUMOylation increases Aβ production, but SUMOylation of APP reduces Aβ production. Further studies show increasing cellular SUMOylation rescues cytotoxicity | 54, 102, 152, 314, 317 |
| Tau | Controversial. Tau aggregates in AD model mice stain for SUMO1, but this has not been shown in human brain. Tau can be SUMOylated at K340, but there is no in vivo evidence | 53, 213, 263 | |
| Parkinson's disease | α-Synuclein | Uncertain. Has been reported to reduce aggregation and toxicity. Another study has shown it to increase its aggregation. | 138, 192 |
| Parkin | Noncovalent interaction with SUMO1 increases ubiquitin E3 ligase activity | 279 | |
| DJ-1 | L166P mutation leads to aberrant SUMOylation and loss of oxidative stress response | 248 | |
| Ischemia | General increase in SUMO2/3-ylation | Likely to be neuroprotective | 41, 147, 149, 310, 311 |
| Drp1 | SUMO2/3-ylation is anti-apoptotic during reperfusion | 88 |
See text for details.
A. Polyglutamine Disorders
Polyglutamine (polyQ) disorders are gain-of-function disorders caused by expansion of a CAG repeat in disease-specific genes (for review, see Ref. 72). These disorders are generally dominantly inherited, with age of onset dependent on the length of the CAG expansion. The polyQ expansion results in neuronal dysfunction and degeneration, as well as the formation of neuronal inclusions of the disease protein in question, although there is some doubt as to whether these inclusions are directly pathogenic or represent a cellular response to the pathogenic protein. Examples of polyQ disorders include Huntington's disease, spinal and bulbar muscular atrophy (SBMA), dentatorubral-pallidoluysian atrophy (DRPLA), and spinocerebellar ataxias (SCA). All of these disorders show enhanced neuronal staining for SUMO1, implicating SUMOylation in the pathology of these diseases (277).
1. Huntington's disease
Probably the most studied of the polyQ disorders, Huntington's disease, is caused by a polyQ expansion in the NH2-terminal domain of the Huntingtin protein (Htt). It is an incurable genetic neurodegenerative disorder that causes cognitive impairment and psychiatric problems as well as affecting muscle coordination (75). Typically, this disease manifests in mid-adulthood, and early damage is predominantly in the striatum. However, in some cases mutant proteins containing 60 or more CAG repeats can manifest earlier (before 20 years of age) (75). Huntingtin is a substrate for both ubiquitination and SUMOylation on the same lysine residue (257), and the effect of these two modifications appears to be antagonistic; in a Drosophila model, ubiquitination of Httex1p (a pathogenic fragment of mutant Htt) reduces neurodegeneration, whereas SUMOylation exacerbates it (257). In cultured cells, SUMOylation stabilizes Httex1p, reducing its ability to form aggregates and promoting its capacity to repress transcription. SUMOylation of Htt has also been postulated as an explanation for the specific degeneration of the corpus striatum observed in Huntington's disease; the small GTP-binding protein Rhes is specifically expressed in the corpus striatum and acts as a SUMO E3 ligase for mutant, but not wild-type Htt, enhancing its SUMOylation and cytotoxicity (262). This study showed that, in both HEK 293T cells and striatal neurons, mutant Htt expression was not cytotoxic in the absence of Rhes. Therefore, it seems likely that SUMOylation of Htt is required for its cytotoxic effects, and the expression pattern of Rhes seemingly explains how the ubiquitously expressed mutant Htt leads to the characteristically specific pathology of Huntington's disease. This hypothesis is further supported by a recent study showing that genetic ablation of Rhes is neuroprotective in the striatal-specific 3-nitropropionic acid (3-NP) mouse model of HD (179). However, as this model elicits striatal lesion via 3-NP toxicity rather than mutant Htt, it remains unclear whether the neuroprotective effect of the Rhes knockout is due to its interaction with Htt and SUMO E3 ligase activity. Furthermore, the mechanism of SUMO-Htt toxicity is unlikely to be a simple antagonism of ubiquitination, as removal of the target lysines for both results in a reduced mutant Htt toxicity (257).
2. Spinal and bulbar muscular atrophy
Spinal and bulbar muscular atrophy (SBMA) is a disease that causes degeneration of the motor neurons in the brain stem and spinal cord resulting in muscle cramp and progressive weakness (128). Unlike the other polyQ disorders, which are all autosomal, SBMA is an X-linked disorder caused by a polyQ expansion in the androgen receptor (AR). The AR is a transcription factor, which is responsive to androgenic hormones, e.g., testosterone (11). Like Htt, the AR can be SUMOylated and ubiquitinated at the same lysine residue (213), and this SUMOylation represses the transcriptional activity of the AR (192). Additionally, SUMOylation of polyQ AR inhibits its aggregation (184), whereas overexpression of a nondeconjugatable SUMO3 in HeLa cells attenuates polyQ AR aggregation, and this effect is dependent on the SUMOylation sites in AR. This effect was not seen for an NH2-terminal fusion of SUMO3 with polyQ AR, indicating it is the branched peptide nature of SUMOylation that disrupts aggregation (184). These data have led to suggestions that enhancing SUMOylation might be a therapeutic strategy for SBMA. However, it is still unclear whether the aggregation of polyQ AR is cytotoxic in itself, or whether it represents a cellular response to a toxic protein. Indeed, in several SBMA models, no correlation has been found between large intracellular inclusions and neurotoxicity (11).
3. Dentatorubral-pallidoluysian atrophy
Dentatorubral-pallidoluysian atrophy (DRPLA) is an autosomal dominant progressive neurological disorder caused by a polyQ expansion in the atrophin-1 gene. The disease usually manifests in midlife and has a similar pathology to Huntington's disease, including ataxia, tremors, and dementia (234). The function of atrophin-1 is not currently understood, but the polyQ expansion associated with DRPLA leads to characteristic intranuclear inclusions, which are cytotoxic and stain positively for SUMO1 (271). Coexpression of SUMO1 with polyQ atrophin-1 in PC12 cells leads to an increase in nuclear inclusions and subsequent apoptosis (271). Expression of a nonconjugatable mutant of SUMO1 had the opposite effect, thus implicating SUMOylation in the toxicity of polyQ atrophin-1. However, whether this is by direct modification of the disease protein or another cellular substrate remains unclear.
4. Spinocerebellar ataxias
Spinocerebellar ataxias (SCAs) are progressive neurodegenerative disorders characterized by atrophy of cerebellar Purkinje cells (197). There are six polyQ spinocerebellar ataxias: SCA types 1, 2, 3, 7 are caused by polyQ expansions in the proteins ataxin 1, 2, 3 and 7, respectively; SCA type 6 is caused by a polyQ expansion in the calcium channel subunit CACNA1A, and the polyQ expansion in SCA17 is in the TBP gene (197). Interestingly, there is mounting evidence that SUMOylation can play a role in the toxicity of the polyQ mutant protein in SCAs 1, 3, and 7.
SUMOylation of ataxin-1 is inhibited by the polyQ expansion (225), and overexpression of either Ubc9 or SUMO1 increases aggregation of polyQ ataxin-1. Since the protein aggregates seen in SCAs are presumed to be cytotoxic, protein SUMOylation is implicated in the pathology of SCA type 1. Additionally, hydrogen peroxide-induced oxidative stress increases SUMOylation of ataxin-1, resulting in higher levels of aggregation of the polyQ but not the wild-type protein (231). This oxidative stress-induced aggregation seems to involve the JNK pathway, although the relationship between this and SUMOylation is unclear. Indeed, this proposed pathway is unusual because SUMOylation is generally perceived to reduce aggregation and enhance protein solubility. Interestingly, however, a phosphorylation site mutant of ataxin-1, S776A, prevents oxidative stress-induced aggregation in a similar manner to JNK inhibition (225).
Ataxin-3 interacts with SUMO1 (243), and a recent study has also implicated the SUMOylation of polyQ ataxin-3 in the pathology of SCA type 3 (320). In HEK 293 cells, SUMOylation of ataxin-3 at K166 did not affect its cellular localization or ubiquitination; however, the non-SUMOylatable mutant polyQ ataxin-3 showed reduced stability. Additionally, cells expressing non-SUMOylatable polyQ ataxin-3 showed reduced rates of early apoptosis compared with cells expressing SUMOylatable polyQ ataxin-3 (320). Thus SUMOylation may have a role in SCA type 3 pathology by increasing the toxicity of polyQ ataxin-3.
In contrast, SUMOylation of ataxin-7 may play the opposite role in SCA type 7. Ataxin-7 is modified by both SUMO1 and SUMO2, and ataxin-7 inclusions stain positively for both of these proteins (114). The polyQ expansion in mutant ataxin-7 does not alter the levels of SUMOylation, but expression of a SUMO-deficient polyQ ataxin-7 in Cos-7 cells resulted in greater ataxin-7 aggregation and cell death. Thus, in contrast to ataxin-1 and -3, SUMOylation of ataxin-7 appears to ameliorate the cytotoxic effects of the polyQ expansion, a pathway more in line with the known roles of SUMOylation in other models of protein aggregation.
B. Alzheimer's Disease
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder of the elderly. It is characterized clinically by progressive dementia and histologically by amyloid plaques and neurofibrillary tangles. Amyloid plaques are composed of aggregated amyloid-beta protein (Aβ), a cleavage product of the amyloid precursor protein (APP), and neurofibrillary tangles consist of a hyperphosphorylated form of the microtubule-associated protein tau. As with other progressive dementias, some doubt still remains as to whether these plaques and tangles are the direct cause of neurodegeneration, or represent a cellular response to the disease. Intriguingly, several genome-wide association studies have identified polymorphisms in genes involved in SUMOylation as risk factors in sporadic AD, including SAE2 (87), SENP3 (293), and Ubc9 (1). Furthermore, there is growing evidence of a general involvement of SUMOylation in AD pathology (147), and both APP (318) and tau (54, 77) have been identified as potential SUMO substrates.
1. SUMO and Aβ
The role of SUMOylation in the generation of cytotoxic Aβ from APP is somewhat controversial, with several different studies reaching opposing conclusions. Li et al. (153) noted in 2003 that SUMO3 overexpression reduced Aβ production, whereas overexpression of the K11R mutant, which cannot form polySUMO chains, had the opposite effect. In contrast, Dorval et al. (55) showed that overexpression of SUMO3 increased Aβ secretion, but that this was independent of SUMO conjugation. That study also found that SUMO3 overexpression increased levels of BACE1 (β-secretase, the Aβ cleaving enzyme). Interestingly, a recent study has reported similar findings, that SUMO1 modification increases BACE1 levels and consequently increases Aβ generation (315). Conversely, a 2008 study showed that APP itself can be SUMOylated in vivo at two sites, K587 and K595, which are directly adjacent to the sites of β-secretase cleavage (318) and that this SUMOylation reduced levels of Aβ aggregates. This study further reported that overexpression of SUMO1 and Ubc9 reduced levels of Aβ aggregates in cells expressing the AD-associated V642F APP mutant (318). Another recent study demonstrated that increasing levels of SUMO1 conjugation in astrocytes decreased Aβ-induced astrocyte reactivity (103), which has been implicated in neuronal loss in AD. Clearly further incisive work is required to resolve these apparently contradictory lines of evidence pointing to SUMOylation either reducing or exacerbating the neurotoxic effects of Aβ. Although the reasons for the current discrepancies are unclear, it almost certainly reflects the complexity of AD and the multiple and varied roles of SUMOylation in cellular systems.
2. SUMO and tau
The role of SUMOylation in tau aggregation has been less extensively investigated than its roles in Aβ generation and toxicity. Tau can be SUMOylated in heterologous cells, primarily at K340, located in the fourth microtubule binding site (54). Interestingly, evidence suggests that K340 is also a ubiquitination site and that SUMOylation may antagonize ubiquitination of tau. Tau SUMOylation is also regulated by phosphorylation, as levels are increased by phosphatase inhibition (54). Phospho-tau aggregates in the Tg2576 APP mouse model of AD also stain positively for SUMO1 (264), implicating tau-SUMOylation in the pathology of AD. However, these results have not been recapitulated by studies of tangles in the post-mortem human brain (214). Thus it remains unclear what role, if any, SUMOylation of tau may have in the pathology of AD.
3. SUMO regulation of synaptic protein trafficking in AD
Away from the two major disease proteins, there are other indications that SUMOylation has a role in AD pathology. A study profiling the levels of SUMOylated and ubiquitinated proteins in the brains of Tg2576 AD model mice found changes in the abundance of specific SUMOylated proteins compared with wild-type mice (178). Although the identity of many of these proteins is unclear, these results indicate that, in addition to SUMOylation of APP and tau, dysfunction of SUMOylation of multiple substrates may be involved in AD progression.
Importantly, synaptic dysfunction and loss is the primary cause of dementia (35, 238, 241). However, the precise signaling cascades underlying synapse collapse and the resulting cognitive loss remain elusive. As outlined above, SUMOylation of synaptic proteins is a key modulator of synaptic kainate receptor trafficking and function (32, 113, 174), and is also necessary for AMPAR trafficking in synaptic scaling (45) and LTP (113). The fact that AMPAR trafficking pathways are disrupted in AD, combined with published reports of a strong association between SUMOylation and AD (55, 153, 178, 315), raise the possibility that misregulation of SUMO-dependent AMPAR trafficking may be a causative factor in the synaptic dysfunction observed during AD, and we expect that this is likely to be an active area of future investigation.
C. Parkinson's Disease
Parkinson's disease (PD) is a progressive neuropathy resulting from the specific loss of dopaminergic neurons projecting from the substantia nigra compacta to the striatum. These neurons are associated with the initiation of voluntary movements, and as a result the symptoms of the disease include muscular rigidity, resting tremor, postural instability, and difficulty initiating movements. The vast majority of PD cases are idiopathic, although there are known genetic risk factors, and SUMOylation has been implicated in PD disease pathology (63).
One of the characteristics of PD is the presence of inclusions called Lewy bodies in the cytosol of affected neurons. These inclusions stain strongly for α-synuclein, a natively unfolded protein of undetermined function, which is a known SUMO substrate (54). In particular, SUMOylation of α-synuclein has been shown in the brains of transgenic mice expressing His6-tagged SUMO2 (139), mainly at K96 and K102. SUMOylation reduces aggregation and toxicity of α-synuclein, both in heterologous cells and in the dopaminergic neurons of the substantia nigra in a rat model of PD. However, another study has suggested that SUMOylation of α-synuclein increases its aggregation (193). Thus the exact function of α-synuclein SUMOylation in PD remains uncertain.
About 1–2% of early-onset PD cases are caused by mutations in the transcriptional regulator DJ-1 (63). DJ-1 is a multifunctional protein, but one of its major roles is in the cellular response to oxidative stress (263). SUMOylation of DJ-1 at K130 is enhanced by UV irradiation and is required for full activity of the protein (249). Interestingly, the most severe DJ-1 mutation associated with PD is L166P, which leads to aberrant DJ-1 SUMOylation and a decrease in protein solubility (249). L166P DJ-1 expressing cells showed a similar sensitivity to UV-induced apoptosis as DJ-1 knockdown cells, implying that the PD mutation and consequent aberrant SUMOylation causes a loss of the oxidative stress response.
The most common autosomal recessive cause of early-onset PD is mutation of the PARK2 gene, encoding parkin, a ubiquitin E3 ligase (63). Parkin is present in the majority of Lewy bodies in both familial and sporadic PD, implicating this protein in the pathology of the disease. Although it has not been shown to be SUMOylated, parkin interacts noncovalently with SUMO1, and this interaction increases its E3 ligase activity, including autoubiquitination (280). In addition, interaction of SUMO1 with parkin increases its nuclear transport. Therefore, noncovalent interaction of SUMO-1 with parkin clearly plays an important regulatory role, although whether this has any relation to the pathology of PD is unknown. It is also of note that one of the targets of parkin ubiquitin ligase activity is RanBP2 (281), which is itself a SUMO E3 ligase, thus demonstrating the often complex interplay between these two modifications.
D. SUMOylation in Ischemia
Rapid global increases in neuronal protein SUMOylation occur in response to excessive neuronal excitation, or other forms of cellular stress such as heat shock, oxidative stress and ischemia (147, 301), leading to the hypothesis that this modification is cytoprotective during extreme cell stress. Indeed, it is well established that protein SUMOylation levels are greatly increased in the brain, liver, and kidney during hibernation torpor in squirrels (149), when blood flow is severely reduced, and that this SUMOylation represents a critical cytoprotective mechanism. Another study has shown that transient focal cerebral ischemia causes a massive increase in SUMO2/3 conjugation (312), with the largest increase seen in the parietal cortex (∼20-fold). Similar results were obtained using two different animal models of stroke (41). In the rat model used in this study, a dramatic increase in conjugation of SUMO1 and SUMO2/3 was observed in the infarct region at 6 and 24 h. Interestingly, there was also an increase in SUMO2/3 conjugation in the hippocampus, which was not subject to ischemia, and in the mouse model this was the only site of increased SUMO2/3 conjugation, whereas increases in SUMO1 conjugation were found in the infarct region (41). Significantly, kainate receptor levels were decreased in the infarct region, in line with the observation that SUMOylation of GluK2 causes decreased KAR EPSCs (174).
Furthermore, OGD increases SUMO1 and SUMO2/3 conjugation to substrate proteins in cultured hippocampal neurons (40). Surprisingly, levels of SENP1 were also increased by OGD, suggesting that the neuronal response to OGD involves a sophisticated interrelationship between SUMOylation and deSUMOylation (40). Broadly in line with these results, blockade of neuronal SUMO2/3 translation in primary cortical neurons increased neuronal vulnerability following OGD, consistent with SUMO2/3 conjugation being an endogenous neuroprotective stress response (49).
Taken together, these different studies support the concept that increases in protein SUMOylation represent a cytoprotective neuronal response to ischemic insult. SUMOylation also has a role in ischemic preconditioning, an intrinsic process in which repeated short subtoxic episodes of ischemia protect against a subsequent major ischemic insult, and overexpression of SUMO1 or SUMO2 in either cortical neurons or SHSY5Y cells leads to increased survival following OGD (148). Consistent with this, RNAi depletion of SUMO1 has the opposite effect and attenuated the effect of preconditioning. Furthermore, transgenic mice overexpressing Ubc9 have elevated global SUMOylation levels, and also exhibit increased protection against focal cerebral ischemic damage (150). Therefore, the evidence points strongly to a cytoprotective role of neuronal protein SUMOylation during ischemic insult and preconditioning. However, until very recently no SUMO targets were identified as critical to this protective response.
1. Drp1
The GTPase Drp1 plays a major role in regulating mitochondrial morphology and integrity (220, 326). Under conditions of extreme cell stress, Drp1 is recruited to mitochondria and mediates, among other things, fragmentation and cytochrome c release, which are potent signals for cell death (34). Drp1 is SUMOylated by both SUMO1 and SUMO2/3, and this modification appears to regulate its mitochondrial partitioning (88, 89, 93, 291). Interestingly, SUMO2/3 modification of Drp1 has been shown to mediate its partitioning away from mitochondria, promoting cell survival during OGD (89) (Figure 8). The deSUMOylating enzyme SENP3 mediates SUMO2/3 removal from Drp1, and SENP3 is rapidly degraded during ischemia (89). The OGD-induced loss of SENP3 is triggered by activation of the endoplasmic reticulum (ER) unfolded protein response (UPR), a cell stress pathway that is activated by the accumulation of unfolded proteins in the ER (98). The UPR seeks to restore cellular homeostasis but, when overwhelmed, can initiate cell death. More specifically, OGD activates the ER-resident UPR kinase PERK, resulting in cathepsin B-mediated degradation of SENP3 (89). Downregulation of SENP3 during the insult stabilizes global SUMO2/3 conjugation and prolongs SUMO2/3 modification of Drp1, sequestering it away from mitochondria, and reducing Drp1-mediated cytochrome c release and consequent cell death (88, 89).
FIGURE 8.
SUMOylation and ischemia. Hypoxia induces the accumulation of unfolded proteins in the ER, resulting in ER stress, which is sensed by the ER-resident kinase PERK. Activation of PERK, through an unknown mechanism, results in lysosomal rupture and release of the protease cathepsin B (CatB) into the cytosol, which mediates degradation of the SUMO2/3-specific SUMO protease SENP3. Loss of SENP3 leads to increased SUMOylation of multiple SUMO2/3 substrates, including the mitochondrial GTPase Drp1. Drp1 is a crucial mediator of mitochondrial fission and pro-apoptotic cytochrome c release. Enhanced SUMOylation of Drp1 during ischemia favors its distribution to the cytosol, where it is unable to stimulate cytochrome c release, promoting cell survival. In addition to stimulating degradation of SENP3, hypoxia also promotes SUMOylation by inducing SIRT1-mediated deacetylation of Ubc9, which enhances its activity against NDSM-containing substrates, and deSUMOylation of the E1 subunit SAE2, which promotes its activity.
A notable feature of ischemic damage is that very few cells die during the ischemic episode; rather, they undergo delayed cell death following the reintroduction of oxygen, a phenomena termed “reperfusion injury.” Correlating with this, SENP3 levels rapidly recover during reoxygenation, thereby reducing SUMO2/3 modification of Drp1, and promoting cytochrome c release and cell death. A key observation is that knockdown of SENP3 blocks the cell death associated with reoxygenation, suggesting that SENP3, acting as a novel arm of the UPR, mediates the delayed cell death observed upon ischemic insult (88, 89).
This represents a novel adaptive pathway to extreme cell stress in which dynamic changes in SENP3 stability and regulation of Drp1 SUMOylation by SUMO2/3 are crucial determinants of cell fate (89). Furthermore, these data highlight SENP3 as a potentially useful therapeutic target to prevent reperfusion injury. It is highly likely that other ischemia-induced SUMO substrates will be identified in the coming years, and will ultimately lead to novel therapies for ischemic insults such as stroke.
XX. SUMMARY
The field of posttranslational modification is undergoing a renaissance with increased understanding of the multifaceted roles of ubiquitination and the emergence of multiple UBL proteins. SUMO is currently the best characterized of the UBL family, but there many other, as yet much less studied, family members, and more are continually being discovered (282). Overall, the effects of SUMOylation in different neurological and neurodegenerative diseases are complex, and it is clear a great deal of further work is required. As highlighted throughout this review, despite the many advances achieved, many questions remain. For example, we still do not yet know in any detail which steps in synaptic transmission, plasticity, and neuroprotection pathways are regulated by SUMOylation. Nor do we know how the process of SUMOylation itself is regulated to control synaptic function and cell survival in neurons. However, we expect that progress in this field will continue to advance rapidly and further define the molecular triggers, targets, and functional outcomes of protein SUMOylation in neurons. On a larger scale, while much evidence supports a role for SUMOylation in neuronal and synaptic function, no studies have yet investigated the impact of perturbed SUMOylation on learning and memory in vivo, or examined how SUMOylation of specific substrates regulates brain function. Together with the progress already made, these studies will reveal exciting and potentially clinically important data on the targets, mechanisms, and consequences of SUMOylation, and we anticipate that components and substrates of the SUMO pathway will eventually provide attractive and tractable therapeutic targets for a wide range of neurological and neurodegenerative diseases.
GRANTS
We are grateful to the European Research Council, the Medical Research Council, and the Biotechnology and Biological Sciences Research Council for financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
Address for reprint requests and other correspondence: J. M. Henley, School of Biochemistry, Medical Sciences Building, Univ. of Bristol, Bristol BS8 1TD, UK (e-mail: j.m.henley@bristol.ac.uk).
REFERENCES
- 1.Ahn K, Song JH, Kim DK, Park MH, Jo SA, Koh YH. Ubc9 gene polymorphisms and late-onset Alzheimer's disease in the Korean population: a genetic association study. Neurosci Lett 465: 272–275, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313: 1751, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Anderson DB, Wilkinson KA, Henley JM. Protein SUMOylation in neuropathological conditions. Drug News Perspect 22: 255–265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres ME, Burger C, Peral-Rubio MJ, Battaglioli E, Anderson ME, Grimes J, Dallman J, Ballas N, Mandel G. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci USA 96: 9873–9878, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayaydin F, Dasso M. Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol Biol Cell 15: 5208–5218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba D, Maita N, Jee JG, Uchimura Y, Saitoh H, Sugasawa K, Hanaoka F, Tochio H, Hiroaki H, Shirakawa M. Crystal structure of thymine DNA glycosylase conjugated to SUMO-1. Nature 435: 979–982, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bailey D, O'Hare P. Herpes simplex virus 1 ICP0 co-localizes with a SUMO-specific protease. J Gen Virol 83: 2951–2964, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron 31: 353–365, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Stambolic V. PTEN, here, there, everywhere. Cell Death Differ 20: 1595–1596, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beitel LK, Alvarado C, Mokhtar S, Paliouras M, Trifiro M. Mechanisms mediating spinal and bulbar muscular atrophy: investigations into polyglutamine-expanded androgen receptor function and dysfunction. Front Neurol 4: 53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iniguez-Lluhi JA, Martens JR. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5 Proc Natl Acad Sci USA 104: 1805–1810, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berdnik D, Favaloro V, Luo L. The SUMO protease Verloren regulates dendrite and axon targeting in olfactory projection neurons. J Neurosci 32: 8331–8340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettermann K, Benesch M, Weis S, Haybaeck J. SUMOylation in carcinogenesis. Cancer Lett. In press [DOI] [PubMed] [Google Scholar]
- 15.Blomster HA, Imanishi SY, Siimes J, Kastu J, Morrice NA, Eriksson JE, Sistonen L. In vivo identification of sumoylation sites by a signature tag and cysteine-targeted affinity purification. J Biol Chem 285: 19324–19329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boddy MN, Howe K, Etkin LD, Solomon E, Freemont PS. PIC 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene 13: 971–982, 1996 [PubMed] [Google Scholar]
- 17.Boggio R, Colombo R, Hay RT, Draetta GF, Chiocca S. A mechanism for inhibiting the SUMO pathway. Mol Cell 16: 549–561, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem 279: 27233–27238, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21: 349–357, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res 200: 125–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10: 748–754, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bretin S, Rogemond V, Marin P, Maus M, Torrens Y, Honnorat J, Glowinski J, Premont J, Gauchy C. Calpain product of WT-CRMP2 reduces the amount of surface NR2B NMDA receptor subunit. J Neurochem 98: 1252–1265, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Brustovetsky T, Pellman JJ, Yang XF, Khanna R, Brustovetsky N. Collapsin response mediator protein 2 (CRMP2) interacts with N-methyl-d-aspartate (NMDA) receptor and Na+/Ca2+ exchanger and regulates their functional activity. J Biol Chem 289: 7470–7482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol 23: 645–673, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron 42: 889–896, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Callaerts P, Halder G, Gehring WJ. PAX-6 in development and evolution. Annu Rev Neurosci 20: 483–532, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Capili AD, Lima CD. Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. J Mol Biol 369: 608–618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell 131: 309–323, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature 415: 327–330, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron 76: 70–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceballos-Chavez M, Rivero S, Garcia-Gutierrez P, Rodriguez-Paredes M, Garcia-Dominguez M, Bhattacharya S, Reyes JC. Control of neuronal differentiation by sumoylation of BRAF35, a subunit of the LSD1-CoREST histone demethylase complex. Proc Natl Acad Sci USA 109: 8085–8090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain SE, Gonzalez-Gonzalez IM, Wilkinson KA, Konopacki FA, Kantamneni S, Henley JM, Mellor JR. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat Neurosci 15: 845–852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CC, Lin DY, Fang HI, Chen RH, Shih HM. Daxx mediates the small ubiquitin-like modifier-dependent transcriptional repression of Smad4. J Biol Chem 280: 10164–10173, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann NY Acad Sci 1201: 34–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease: advantages, caveats, and future outlook. Eur J Neurosci 35: 1908–1916, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Chao HW, Hong CJ, Huang TN, Lin YL, Hsueh YP. SUMOylation of the MAGUK protein CASK regulates dendritic spinogenesis. J Cell Biol 182: 141–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Huang M, Zhu Y, Xin YJ, Zhao YK, Huang J, Yu JX, Zhou WH, Qiu Z. SUMOylation of MeCP2 is essential for transcriptional repression and hippocampal synapse development. J Neurochem 128: 798–806, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52: 445–459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu Y, Yang X. SUMO E3 ligase activity of TRIM proteins. Oncogene 30: 1108–1116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cimarosti H, Ashikaga E, Jaafari N, Dearden L, Rubin P, Wilkinson KA, Henley JM. Enhanced SUMOylation and SENP-1 protein levels following oxygen and glucose deprivation in neurones. J Cereb Blood Flow Metab 32: 17–22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology 54: 280–289, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci 34: 154–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craig TJ, Henley JM. Protein SUMOylation in spine structure and function. Curr Opin Neurobiol 22: 480–487, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craig TJ, Henley JM. SUMOylation, Arc and the regulation homeostatic synaptic scaling: implications in health and disease. Commun Integr Biol 5: 634–636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig TJ, Jaafari N, Petrovic MM, Jacobs SC, Rubin PP, Mellor JR, Henley JM. Homeostatic synaptic scaling is regulated by protein SUMOylation. J Biol Chem 287: 22781–22788, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cubenas-Potts C, Matunis MJ. SUMO: a multifaceted modifier of chromatin structure and function. Dev Cell 24: 1–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci 122: 775–779, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Danielsen JR, Povlsen LK, Villumsen BH, Streicher W, Nilsson J, Wikstrom M, Bekker-Jensen S, Mailand N. DNA damage-inducible SUMOylation of HERC2 promotes RNF8 binding via a novel SUMO-binding Zinc finger. J Cell Biol 197: 179–187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab 31: 2152–2159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron 69: 317–331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desterro JM, Thomson J, Hay RT. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett 417: 297–300, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Di Bacco A, Ouyang J, Lee HY, Catic A, Ploegh H, Gill G. The SUMO-specific protease SENP5 is required for cell division. Mol Cell Biol 26: 4489–4498, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding H, Xu Y, Chen Q, Dai H, Tang Y, Wu J, Shi Y. Solution structure of human SUMO-3 C47S and its binding surface for Ubc9. Biochemistry 44: 2790–2799, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem 281: 9919–9924, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Dorval V, Mazzella MJ, Mathews PM, Hay RT, Fraser PE. Modulation of Abeta generation by small ubiquitin-like modifiers does not require conjugation to target proteins. Biochem J 404: 309–316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 65: 341–357, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Droescher M, Chaugule VK, Pichler A. SUMO rules: regulatory concepts and their implication in neurologic functions. Neuromol Med 15: 639–660, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Druker J, Liberman AC, Antunica-Noguerol M, Gerez J, Paez-Pereda M, Rein T, Iniguez-Lluhi JA, Holsboer F, Arzt E. RSUME enhances glucocorticoid receptor SUMOylation and transcriptional activity. Mol Cell Biol 33: 2116–2127, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol 522: 19–31, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience 84: 37–48, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Dustrude ET, Wilson SM, Ju W, Xiao Y, Khanna R. CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. J Biol Chem 288: 24316–24331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dutting E, Schroder-Kress N, Sticht H, Enz R. SUMO E3 ligases are expressed in the retina and regulate SUMOylation of the metabotropic glutamate receptor 8b. Biochem J 435: 365–371, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Eckermann K. SUMO and Parkinson's disease. Neuromol Med 15: 737–759, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Evdokimov E, Sharma P, Lockett SJ, Lualdi M, Kuehn MR. Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J Cell Sci 121: 4106–4113, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127: 693–702, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Feliciangeli S, Bendahhou S, Sandoz G, Gounon P, Reichold M, Warth R, Lazdunski M, Barhanin J, Lesage F. Does sumoylation control K2P1/TWIK1 background K+ channels? Cell 130: 563–569, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Feligioni M, Nishimune A, Henley JM. Protein SUMOylation modulates calcium influx and glutamate release from presynaptic terminals. Eur J Neurosci 29: 1348–1356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311: 1008–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem 82: 357–385, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Gadalla KK, Bailey ME, Cobb SR. MeCP2 and Rett syndrome: reversibility and potential avenues for therapy. Biochem J 439: 1–14, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Gao C, Ho CC, Reineke E, Lam M, Cheng X, Stanya KJ, Liu Y, Chakraborty S, Shih HM, Kao HY. Histone deacetylase 7 promotes PML sumoylation and is essential for PML nuclear body formation. Mol Cell Biol 28: 5658–5667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet 6: 743–755, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8: 947–956, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Geoffroy MC, Hay RT. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol 10: 564–568, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Gil JM, Rego AC. Mechanisms of neurodegeneration in Huntington's disease. Eur J Neurosci 27: 2803–2820, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Girach F, Craig TJ, Rocca DL, Henley JM. RIM1alpha SUMOylation is required for fast synaptic vesicle exocytosis. Cell Rep 5: 1294–1301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gocke CB, Yu H, Kang J. Systematic identification and analysis of mammalian small ubiquitin-like modifier substrates. J Biol Chem 280: 5004–5012, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Golebiowski F, Matic I, Tatham MH, Cole C, Yin Y, Nakamura A, Cox J, Barton GJ, Mann M, Hay RT. System-wide changes to SUMO modifications in response to heat shock. Sci Signal 2: ra24, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J Biol Chem 272: 28198–28201, 1997 [DOI] [PubMed] [Google Scholar]
- 80.Gong L, Li B, Millas S, Yeh ET. Molecular cloning and characterization of human AOS1 and UBA2, components of the sentrin-activating enzyme complex. FEBS Lett 448: 185–189, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Gong L, Millas S, Maul GG, Yeh ET. Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J Biol Chem 275: 3355–3359, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Gong L, Yeh ET. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J Biol Chem 281: 15869–15877, 2006 [DOI] [PubMed] [Google Scholar]
- 83.González-González IM, Konopaki F, Rocca DL, Doherty AJ, Jaafari N, Wilkinson KA, Henley JM. Kainate receptor trafficking. WIRES Membr Transp Signal 1: 31–44, 2012 [Google Scholar]
- 84.Gonzalez-Santamaria J, Campagna M, Ortega-Molina A, Marcos-Villar L, de la Cruz-Herrera CF, Gonzalez D, Gallego P, Lopitz-Otsoa F, Esteban M, Rodriguez MS, Serrano M, Rivas C. Regulation of the tumor suppressor PTEN by SUMO. Cell Death Dis 3: e393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gowran A, Murphy CE, Campbell VA. Delta(9)-tetrahydrocannabinol regulates the p53 post-translational modifiers Murine double minute 2 and the Small Ubiquitin MOdifier protein in the rat brain. FEBS Lett 583: 3412–3418, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Gregoire S, Yang XJ. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol Cell Biol 25: 2273–2287, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grupe A, Abraham R, Li Y, Rowland C, Hollingworth P, Morgan A, Jehu L, Segurado R, Stone D, Schadt E, Karnoub M, Nowotny P, Tacey K, Catanese J, Sninsky J, Brayne C, Rubinsztein D, Gill M, Lawlor B, Lovestone S, Holmans P, O'Donovan M, Morris JC, Thal L, Goate A, Owen MJ, Williams J. Evidence for novel susceptibility genes for late-onset Alzheimer's disease from a genome-wide association study of putative functional variants. Hum Mol Genet 16: 865–873, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Guo C, Henley J. Wrestling with stress: roles of protein SUMOylation and deSUMOylation in cell stress response. IUBMB Life. In press [DOI] [PubMed] [Google Scholar]
- 89.Guo C, Hildick KL, Luo J, Dearden L, Wilkinson KA, Henley JM. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J 32: 1514–1528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammer E, Heilbronn R, Weger S. The E3 ligase Topors induces the accumulation of polysumoylated forms of DNA topoisomerase I in vitro and in vivo. FEBS Lett 581: 5418–5424, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Hang J, Dasso M. Association of the human SUMO-1 protease SENP2 with the nuclear pore. J Biol Chem 277: 19961–19966, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Hardeland U, Steinacher R, Jiricny J, Schar P. Modification of the human thymine-DNA glycosylase by ubiquitin-like proteins facilitates enzymatic turnover. EMBO J 21: 1456–1464, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14: 340–345, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Hay RT. SUMO: a history of modification. Mol Cell 18: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I. Specification of SUMO1- SUMO2-interacting motifs. J Biol Chem 281: 16117–16127, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci 34: 258–268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 67: 425–479, 1998 [DOI] [PubMed] [Google Scholar]
- 98.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 13: 755–766, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA 103: 45–50, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirokawa N. mRNA transport in dendrites: RNA granules, motors, and tracks. J Neurosci 26: 7139–7142, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci 25: 81–86, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Hoppe JB, Rattray M, Tu H, Salbego CG, Cimarosti H. SUMO-1 conjugation blocks beta-amyloid-induced astrocyte reactivity. Neurosci Lett 546: 51–56, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Hsiao HH, Meulmeester E, Frank BT, Melchior F, Urlaub H. “ChopNSpice,” a mass spectrometric approach that allows identification of endogenous small ubiquitin-like modifier-conjugated peptides. Mol Cell Proteomics 8: 2664–2675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsieh YL, Kuo HY, Chang CC, Naik MT, Liao PH, Ho CC, Huang TC, Jeng JC, Hsu PH, Tsai MD, Huang TH, Shih HM. Ubc9 acetylation modulates distinct SUMO target modification and hypoxia response. EMBO J 32: 791–804, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsueh YP. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem 13: 1915–1927, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, Chen Y, Cang H, Li H, Shi G, Cheng J, Tang X, Yi J. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J 28: 2748–2762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, Mustelin T, Feng GS, Chen G, Yu J. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun 3: 911, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell 115: 565–576, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron 80: 704–717, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Ip JP, Fu AK, Ip NY. CRMP2: functional roles in neural development and therapeutic potential in neurological diseases. Neuroscientist. In press [DOI] [PubMed] [Google Scholar]
- 113.Jaafari N, Konopacki FA, Owen TF, Kantamneni S, Rubin P, Craig TJ, Wilkinson KA, Henley JM. SUMOylation is required for glycine-induced increases in AMPA receptor surface expression (ChemLTP) in hippocampal neurons. PLoS One 8: e52345, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janer A, Werner A, Takahashi-Fujigasaki J, Daret A, Fujigasaki H, Takada K, Duyckaerts C, Brice A, Dejean A, Sittler A. SUMOylation attenuates the aggregation propensity and cellular toxicity of the polyglutamine expanded ataxin-7. Hum Mol Genet 19: 181–195, 2010 [DOI] [PubMed] [Google Scholar]
- 115.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature 414: 204–208, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128: 1187–1203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, Luo L. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development 131: 117–130, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Jentsch S, Psakhye I. Control of nuclear activities by substrate-selective and protein-group SUMOylation. Annu Rev Genet 47: 167–186, 2013 [DOI] [PubMed] [Google Scholar]
- 119.Johnson ES. Protein modification by SUMO. Annu Rev Biochem 73: 355–382, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106: 735–744, 2001 [DOI] [PubMed] [Google Scholar]
- 121.Ju W, Li Q, Wilson SM, Brittain JM, Meroueh L, Khanna R. SUMOylation alters CRMP2 regulation of calcium influx in sensory neurons. Channels 7: 153–159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jurado S, Benoist M, Lario A, Knafo S, Petrok CN, Esteban JA. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J 29: 2827–2840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144: 282–295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamitani T, Kito K, Nguyen HP, Fukuda-Kamitani T, Yeh ET. Characterization of a second member of the sentrin family of ubiquitin-like proteins. J Biol Chem 273: 11349–11353, 1998 [DOI] [PubMed] [Google Scholar]
- 125.Kamitani T, Nguyen HP, Kito K, Fukuda-Kamitani T, Yeh ET. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J Biol Chem 273: 3117–3120, 1998 [DOI] [PubMed] [Google Scholar]
- 126.Kamitani T, Nguyen HP, Yeh ET. Preferential modification of nuclear proteins by a novel ubiquitin-like molecule. J Biol Chem 272: 14001–14004, 1997 [DOI] [PubMed] [Google Scholar]
- 127.Kang J, Gocke CB, Yu H. Phosphorylation-facilitated sumoylation of MEF2C negatively regulates its transcriptional activity. BMC Biochem 7: 5, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katsuno M, Adachi H, Waza M, Banno H, Suzuki K, Tanaka F, Doyu M, Sobue G. Pathogenesis, animal models and therapeutics in spinal and bulbar muscular atrophy (SBMA). Exp Neurol 200: 8–18, 2006 [DOI] [PubMed] [Google Scholar]
- 129.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22: 159–180, 2006 [DOI] [PubMed] [Google Scholar]
- 131.Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400: 569–573, 1999 [DOI] [PubMed] [Google Scholar]
- 132.Kim JI, Lee HR, Sim SE, Baek J, Yu NK, Choi JH, Ko HG, Lee YS, Park SW, Kwak C, Ahn SJ, Choi SY, Kim H, Kim KH, Backx PH, Bradley CA, Kim E, Jang DJ, Lee K, Kim SJ, Zhuo M, Collingridge GL, Kaang BK. PI3Kgamma is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat Neurosci 14: 1447–1454, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Kim SA, Kim JH, Park M, Cho IH, Yoo HJ. Family-based association study between GRIK2 polymorphisms and autism spectrum disorders in the Korean trios. Neurosci Res 58: 332–335, 2007 [DOI] [PubMed] [Google Scholar]
- 134.Knipscheer P, Flotho A, Klug H, Olsen JV, van Dijk WJ, Fish A, Johnson ES, Mann M, Sixma TK, Pichler A. Ubc9 sumoylation regulates SUMO target discrimination. Mol Cell 31: 371–382, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J 26: 2797–2807, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kolli N, Mikolajczyk J, Drag M, Mukhopadhyay D, Moffatt N, Dasso M, Salvesen G, Wilkinson KD. Distribution and paralogue specificity of mammalian deSUMOylating enzymes. Biochem J 430: 335–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Konopacki FA, Jaafari N, Rocca DL, Wilkinson KA, Chamberlain S, Rubin P, Kantamneni S, Mellor JR, Henley JM. Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proc Natl Acad Sci USA 108: 19772–19777, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci 26: 2000–2009, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krumova P, Meulmeester E, Garrido M, Tirard M, Hsiao HH, Bossis G, Urlaub H, Zweckstetter M, Kugler S, Melchior F, Bahr M, Weishaupt JH. Sumoylation inhibits α-synuclein aggregation and toxicity. J Cell Biol 194: 49–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krumova P, Weishaupt JH. Sumoylation in neurodegenerative diseases. Cell Mol Life Sci 70: 2123–2138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119: 2737–2744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuo HY, Chang CC, Jeng JC, Hu HM, Lin DY, Maul GG, Kwok RP, Shih HM. SUMO modification negatively modulates the transcriptional activity of CREB-binding protein via the recruitment of Daxx. Proc Natl Acad Sci USA 102: 16973–16978, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lanore F, Labrousse VF, Szabo Z, Normand E, Blanchet C, Mulle C. Deficits in morphofunctional maturation of hippocampal mossy fiber synapses in a mouse model of intellectual disability. J Neurosci 32: 17882–17893, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JT, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron 50: 415–429, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Lee EJ, Hyun SH, Chun J, Shin SH, Yeon KH, Kwak MK, Park TY, Kang SS. Regulation of glycogen synthase kinase 3β functions by modification of the small ubiquitin-like modifier. Open Biochem J 2: 67–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee GW, Melchior F, Matunis MJ, Mahajan R, Tian Q, Anderson P. Modification of Ran GTPase-activating protein by the small ubiquitin-related modifier SUMO-1 requires Ubc9, an E2-type ubiquitin-conjugating enzyme homologue. J Biol Chem 273: 6503–6507, 1998 [DOI] [PubMed] [Google Scholar]
- 147.Lee YJ, Hallenbeck JM. SUMO and ischemic tolerance. Neuromolecular Med 15: 771–781, 2013 [DOI] [PubMed] [Google Scholar]
- 148.Lee YJ, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM. SUMOylation participates in induction of ischemic tolerance. J Neurochem 109: 257–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab 27: 950–962, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee YJ, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS One 6: e25852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li SJ, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature 398: 246–251, 1999 [DOI] [PubMed] [Google Scholar]
- 152.Li SJ, Hochstrasser M. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol Cell Biol 20: 2367–2377, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Y, Wang H, Wang S, Quon D, Liu YW, Cordell B. Positive and negative regulation of APP amyloidogenesis by sumoylation. Proc Natl Acad Sci USA 100: 259–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lima CD, Reverter D. Structure of the human SENP7 catalytic domain and poly-SUMO deconjugation activities for SENP6 and SENP7. J Biol Chem 283: 32045–32055, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin DY, Huang YS, Jeng JC, Kuo HY, Chang CC, Chao TT, Ho CC, Chen YC, Lin TP, Fang HI, Hung CC, Suen CS, Hwang MJ, Chang KS, Maul GG, Shih HM. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol Cell 24: 341–354, 2006 [DOI] [PubMed] [Google Scholar]
- 156.Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3: 374–385, 2010 [DOI] [PubMed] [Google Scholar]
- 157.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13: 1075–1081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci USA 92: 11175–11179, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loriol C, Khayachi A, Poupon G, Gwizdek C, Martin S. Activity-dependent regulation of the sumoylation machinery in rat hippocampal neurons. Biol Cell 105: 30–45, 2013 [DOI] [PubMed] [Google Scholar]
- 160.Loriol C, Parisot J, Poupon G, Gwizdek C, Martin S. Developmental regulation and spatiotemporal redistribution of the sumoylation machinery in the rat central nervous system. PLoS One 7: e33757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lotshaw DP. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys 47: 209–256, 2007 [DOI] [PubMed] [Google Scholar]
- 162.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Luan Z, Liu Y, Stuhlmiller TJ, Marquez J, Garcia-Castro MI. SUMOylation of Pax7 is essential for neural crest and muscle development. Cell Mol Life Sci 70: 1793–1806, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luo J, Ashikaga E, Rubin PP, Heimann MJ, Hildick KL, Bishop P, Girach F, Josa-Prado F, Tang LT, Carmichael RE, Henley JM, Wilkinson KA. Receptor trafficking and the regulation of synaptic plasticity by SUMO. Neuromolecular Med 15: 692–706, 2013 [DOI] [PubMed] [Google Scholar]
- 165.MacDonald PE, Ha XF, Wang J, Smukler SR, Sun AM, Gaisano HY, Salapatek AM, Backx PH, Wheeler MB. Members of the Kv1 and Kv2 voltage-dependent K+ channel families regulate insulin secretion. Mol Endocrinol 15: 1423–1435, 2001 [DOI] [PubMed] [Google Scholar]
- 166.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88: 97–107, 1997 [DOI] [PubMed] [Google Scholar]
- 167.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004 [DOI] [PubMed] [Google Scholar]
- 168.Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340: 554–557, 1989 [DOI] [PubMed] [Google Scholar]
- 169.Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245: 862–866, 1989 [DOI] [PubMed] [Google Scholar]
- 170.Manning Fox JE, Hajmrle C, Macdonald PE. Novel roles of SUMO in pancreatic beta-cells: thinking outside the nucleus. Can J Physiol Pharmacol 90: 765–770, 2013 [DOI] [PubMed] [Google Scholar]
- 171.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell 109: 243–255, 2002 [DOI] [PubMed] [Google Scholar]
- 172.Marques JM, Rodrigues RJ, Valbuena S, Rozas JL, Selak S, Marin P, Aller MI, Lerma J. CRMP2 tethers kainate receptor activity to cytoskeleton dynamics during neuronal maturation. J Neurosci 33: 18298–18310, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martin S, Bouschet T, Jenkins EL, Nishimune A, Henley JM. Bidirectional regulation of kainate receptor surface expression in hippocampal neurons. J Biol Chem 283: 36435–36440, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 447: 321–325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci 8: 948–959, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matic I, Schimmel J, Hendriks IA, van Santen MA, van de Rijke F, van Dam H, Gnad F, Mann M, Vertegaal AC. Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol Cell 39: 641–652, 2010 [DOI] [PubMed] [Google Scholar]
- 177.Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol 135: 1457–1470, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McMillan LE, Brown JT, Henley JM, Cimarosti H. Profiles of SUMO and ubiquitin conjugation in an Alzheimer's disease model. Neurosci Lett 502: 201–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mealer RG, Subramaniam S, Snyder SH. Rhes deletion is neuroprotective in the 3-nitropropionic acid model of Huntington's disease. J Neurosci 33: 4206–4210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meulmeester E, Kunze M, Hsiao HH, Urlaub H, Melchior F. Mechanism and consequences for paralog-specific sumoylation of ubiquitin-specific protease 25. Mol Cell 30: 610–619, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Mikkonen L, Hirvonen J, Janne OA. SUMO-1 regulates body weight and adipogenesis via PPARgamma in male and female mice. Endocrinology 154: 698–708, 2013 [DOI] [PubMed] [Google Scholar]
- 182.Minty A, Dumont X, Kaghad M, Caput D. Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J Biol Chem 275: 36316–36323, 2000 [DOI] [PubMed] [Google Scholar]
- 183.Motazacker MM, Rost BR, Hucho T, Garshasbi M, Kahrizi K, Ullmann R, Abedini SS, Nieh SE, Amini SH, Goswami C, Tzschach A, Jensen LR, Schmitz D, Ropers HH, Najmabadi H, Kuss AW. A defect in the ionotropic glutamate receptor 6 gene (GRIK2) is associated with autosomal recessive mental retardation. Am J Hum Genet 81: 792–798, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mukherjee S, Thomas M, Dadgar N, Lieberman AP, Iniguez-Lluhi JA. Small ubiquitin-like modifier (SUMO) modification of the androgen receptor attenuates polyglutamine-mediated aggregation. J Biol Chem 284: 21296–21306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mukhopadhyay D, Ayaydin F, Kolli N, Tan SH, Anan T, Kametaka A, Azuma Y, Wilkinson KD, Dasso M. SUSP1 antagonizes formation of highly SUMO2/3-conjugated species. J Cell Biol 174: 939–949, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci 32: 286–295, 2007 [DOI] [PubMed] [Google Scholar]
- 187.Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell 9: 769–779, 2005 [DOI] [PubMed] [Google Scholar]
- 188.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386–389, 1998 [DOI] [PubMed] [Google Scholar]
- 189.Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D, Angelastro J, Omanska-Klusek A, Schoenfeld R, Giulivi C. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS One 7: e42504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nattel S, Yue L, Wang Z. Cardiac ultrarapid delayed rectifiers: a novel potassium current family of functional similarity and molecular diversity. Cell Physiol Biochem 9: 217–226, 1999 [DOI] [PubMed] [Google Scholar]
- 191.Nishida T, Tanaka H, Yasuda H. A novel mammalian Smt3-specific isopeptidase 1 (SMT3IP1) localized in the nucleolus at interphase. Eur J Biochem 267: 6423–6427, 2000 [DOI] [PubMed] [Google Scholar]
- 192.Nishida T, Yasuda H. PIAS1 and PIASxalpha function as SUMO-E3 ligases toward androgen receptor and repress androgen receptor-dependent transcription. J Biol Chem 277: 41311–41317, 2002 [DOI] [PubMed] [Google Scholar]
- 193.Oh Y, Kim YM, Mouradian MM, Chung KC. Human polycomb protein 2 promotes alpha-synuclein aggregate formation through covalent SUMOylation. Brain Res 1381: 78–89, 2011 [DOI] [PubMed] [Google Scholar]
- 194.Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei CF, Chang HM, Yeh ET. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol 157: 4277–4281, 1996 [PubMed] [Google Scholar]
- 195.Olsen SK, Capili AD, Lu X, Tan DS, Lima CD. Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature 463: 906–912, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Onishi A, Peng GH, Hsu C, Alexis U, Chen S, Blackshaw S. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron 61: 234–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Orr HT. Cell biology of spinocerebellar ataxia. J Cell Biol 197: 167–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ouyang J, Shi Y, Valin A, Xuan Y, Gill G. Direct binding of CoREST1 to SUMO-2/3 contributes to gene-specific repression by the LSD1/CoREST1/HDAC complex. Mol Cell 34: 145–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Owerbach D, McKay EM, Yeh ET, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun 337: 517–520, 2005 [DOI] [PubMed] [Google Scholar]
- 200.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14: 383–400, 2013 [DOI] [PubMed] [Google Scholar]
- 201.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53: 703–717, 2007 [DOI] [PubMed] [Google Scholar]
- 202.Perry JJ, Tainer JA, Boddy MN. A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem Sci 33: 201–208, 2008 [DOI] [PubMed] [Google Scholar]
- 203.Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, Van Maldergem L, Penet C, Feingold J, Brice A, Leboyer M. Genome-wide scan for autism susceptibility genes. Paris Autism Research International Sibpair Study. Hum Mol Genet 8: 805–812, 1999 [DOI] [PubMed] [Google Scholar]
- 204.Picard N, Caron V, Bilodeau S, Sanchez M, Mascle X, Aubry M, Tremblay A. Identification of estrogen receptor beta as a SUMO-1 target reveals a novel phosphorylated sumoylation motif and regulation by glycogen synthase kinase 3beta. Mol Cell Biol 32: 2709–2721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108: 109–120, 2002 [DOI] [PubMed] [Google Scholar]
- 206.Pichler A, Knipscheer P, Oberhofer E, van Dijk WJ, Korner R, Olsen JV, Jentsch S, Melchior F, Sixma TK. SUMO modification of the ubiquitin-conjugating enzyme E2–25K. Nat Struct Mol Biol 12: 264–269, 2005 [DOI] [PubMed] [Google Scholar]
- 207.Pichler A, Knipscheer P, Saitoh H, Sixma TK, Melchior F. The RanBP2 SUMO E3 ligase is neither HECT- nor RING-type. Nat Struct Mol Biol 11: 984–991, 2004 [DOI] [PubMed] [Google Scholar]
- 208.Pilla E, Moller U, Sauer G, Mattiroli F, Melchior F, Geiss-Friedlander R. A novel SUMO1-specific interacting motif in dipeptidyl peptidase 9 (DPP9) that is important for enzymatic regulation. J Biol Chem 287: 44320–44329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Plant LD, Dementieva IS, Kollewe A, Olikara S, Marks JD, Goldstein SA. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci USA 107: 10743–10748, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Plant LD, Dowdell EJ, Dementieva IS, Marks JD, Goldstein SA. SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J Gen Physiol 137: 441–454, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Plant LD, Rajan S, Goldstein SA. K2P channels and their protein partners. Curr Opin Neurobiol 15: 326–333, 2005 [DOI] [PubMed] [Google Scholar]
- 212.Plant LD, Zuniga L, Araki D, Marks JD, Goldstein SA. SUMOylation silences heterodimeric TASK potassium channels containing K2P1 subunits in cerebellar granule neurons. Sci Signal 5: ra84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Poukka H, Karvonen U, Janne OA, Palvimo JJ. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1). Proc Natl Acad Sci USA 97: 14145–14150, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pountney DL, Huang Y, Burns RJ, Haan E, Thompson PD, Blumbergs PC, Gai WP. SUMO-1 marks the nuclear inclusions in familial neuronal intranuclear inclusion disease. Exp Neurol 184: 436–446, 2003 [DOI] [PubMed] [Google Scholar]
- 215.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66: 337–351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci 14: 1112–1114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151: 807–820, 2012 [DOI] [PubMed] [Google Scholar]
- 218.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 121: 37–47, 2005 [DOI] [PubMed] [Google Scholar]
- 219.Rajendra R, Malegaonkar D, Pungaliya P, Marshall H, Rasheed Z, Brownell J, Liu LF, Lutzker S, Saleem A, Rubin EH. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J Biol Chem 279: 36440–36444, 2004 [DOI] [PubMed] [Google Scholar]
- 220.Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67: 103–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Renner F, Moreno R, Schmitz ML. SUMOylation-dependent localization of IKKepsilon in PML nuclear bodies is essential for protection against DNA-damage-triggered cell death. Mol Cell 37: 503–515, 2010 [DOI] [PubMed] [Google Scholar]
- 222.Reverter D, Lima CD. A basis for SUMO protease specificity provided by analysis of human Senp2 and a Senp2-SUMO complex. Structure 12: 1519–1531, 2004 [DOI] [PubMed] [Google Scholar]
- 223.Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature 435: 687–692, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52: 461–474, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Riley BE, Zoghbi HY, Orr HT. SUMOylation of the polyglutamine repeat protein, ataxin-1, is dependent on a functional nuclear localization signal. J Biol Chem 280: 21942–21948, 2005 [DOI] [PubMed] [Google Scholar]
- 226.Riquelme C, Barthel KK, Liu X. SUMO-1 modification of MEF2A regulates its transcriptional activity. J Cell Mol Med 10: 132–144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rodriguez MS, Dargemont C, Hay RT. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem 276: 12654–12659, 2001 [DOI] [PubMed] [Google Scholar]
- 228.Roger JE, Nellissery J, Kim DS, Swaroop A. Sumoylation of bZIP transcription factor NRL modulates target gene expression during photoreceptor differentiation. J Biol Chem 285: 25637–25644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21: 521–530, 1998 [DOI] [PubMed] [Google Scholar]
- 230.Rytinki MM, Kaikkonen S, Pehkonen P, Jaaskelainen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci 66: 3029–3041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ryu J, Cho S, Park BC, Lee do H. Oxidative stress-enhanced SUMOylation and aggregation of ataxin-1: implication of JNK pathway. Biochem Biophys Res Commun 393: 280–285, 2010 [DOI] [PubMed] [Google Scholar]
- 232.Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem 275: 6252–6258, 2000 [DOI] [PubMed] [Google Scholar]
- 233.Sampson DA, Wang M, Matunis MJ. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276: 21664–21669, 2001 [DOI] [PubMed] [Google Scholar]
- 234.Schilling G, Wood JD, Duan K, Slunt HH, Gonzales V, Yamada M, Cooper JK, Margolis RL, Jenkins NA, Copeland NG, Takahashi H, Tsuji S, Price DL, Borchelt DR, Ross CA. Nuclear accumulation of truncated atrophin-1 fragments in a transgenic mouse model of DRPLA. Neuron 24: 275–286, 1999 [DOI] [PubMed] [Google Scholar]
- 235.Schimmel J, Larsen KM, Matic I, van Hagen M, Cox J, Mann M, Andersen JS, Vertegaal AC. The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Mol Cell Proteomics 7: 2107–2122, 2008 [DOI] [PubMed] [Google Scholar]
- 236.Schulz S, Chachami G, Kozaczkiewicz L, Winter U, Stankovic-Valentin N, Haas P, Hofmann K, Urlaub H, Ovaa H, Wittbrodt J, Meulmeester E, Melchior F. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep 13: 930–938, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schwarz SE, Matuschewski K, Liakopoulos D, Scheffner M, Jentsch S. The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc Natl Acad Sci USA 95: 560–564, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science 298: 789–791, 2002 [DOI] [PubMed] [Google Scholar]
- 239.Shalizi A, Bilimoria PM, Stegmuller J, Gaudilliere B, Yang Y, Shuai K, Bonni A. PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J Neurosci 27: 10037–10046, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 311: 1012–1017, 2006 [DOI] [PubMed] [Google Scholar]
- 241.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 14: 837–842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5: 437–448, 2004 [DOI] [PubMed] [Google Scholar]
- 243.Shen L, Tang JG, Tang BS, Jiang H, Zhao GH, Xia K, Zhang YH, Cai F, Tan LM, Pan Q. Research on screening and identification of proteins interacting with ataxin-3. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 22: 242–247, 2005 [PubMed] [Google Scholar]
- 244.Shen LN, Geoffroy MC, Jaffray EG, Hay RT. Characterization of SENP7, a SUMO-2/3-specific isopeptidase. Biochem J 421: 223–230, 2009 [DOI] [PubMed] [Google Scholar]
- 245.Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP. The mechanisms of PML-nuclear body formation. Mol Cell 24: 331–339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shen Z, Pardington-Purtymun PE, Comeaux JC, Moyzis RK, Chen DJ. UBL1, a human ubiquitin-like protein associating with human RAD51/RAD52 proteins. Genomics 36: 271–279, 1996 [DOI] [PubMed] [Google Scholar]
- 247.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52: 475–484, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shin EJ, Shin HM, Nam E, Kim WS, Kim JH, Oh BH, Yun Y. DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO Rep 13: 339–346, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shinbo Y, Niki T, Taira T, Ooe H, Takahashi-Niki K, Maita C, Seino C, Iguchi-Ariga SM, Ariga H. Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ 13: 96–108, 2006 [DOI] [PubMed] [Google Scholar]
- 250.Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH, Ling YS, Ruan Y, Yang XL, Zhang D. Family-based association study between autism and glutamate receptor 6 gene in Chinese Han trios. Am J Med Genet B Neuropsychiatr Genet 131B: 48–50, 2004 [DOI] [PubMed] [Google Scholar]
- 251.Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci 30: 16910–16921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci USA 101: 14373–14378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Song J, Zhang Z, Hu W, Chen Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: a reversal of the bound orientation. J Biol Chem 280: 40122–40129, 2005 [DOI] [PubMed] [Google Scholar]
- 254.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13: 283–296, 2012 [DOI] [PubMed] [Google Scholar]
- 255.Sriramachandran AM, Dohmen RJ. SUMO-targeted ubiquitin ligases. Biochim Biophys Acta 1843: 75–85, 2014 [DOI] [PubMed] [Google Scholar]
- 256.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15: 356–362, 1997 [DOI] [PubMed] [Google Scholar]
- 257.Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington's disease pathology. Science 304: 100–104, 2004 [DOI] [PubMed] [Google Scholar]
- 258.Stehmeier P, Muller S. Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Mol Cell 33: 400–409, 2009 [DOI] [PubMed] [Google Scholar]
- 259.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature 440: 1054–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 260.Su YF, Yang T, Huang H, Liu LF, Hwang J. Phosphorylation of Ubc9 by Cdk1 enhances SUMOylation activity. PLoS One 7: e34250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Subramaniam S, Mealer RG, Sixt KM, Barrow RK, Usiello A, Snyder SH. Rhes, a physiologic regulator of sumoylation, enhances cross-sumoylation between the basic sumoylation enzymes E1 and Ubc9. J Biol Chem 285: 20428–20432, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science 324: 1327–1330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 5: 213–218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Takahashi K, Ishida M, Komano H, Takahashi H. SUMO-1 immunoreactivity co-localizes with phospho-Tau in APP transgenic mice but not in mutant Tau transgenic mice. Neurosci Lett 441: 90–93, 2008 [DOI] [PubMed] [Google Scholar]
- 265.Tan JA, Song J, Chen Y, Durrin LK. Phosphorylation-dependent interaction of SATB1 and PIAS1 directs SUMO-regulated caspase cleavage of SATB1. Mol Cell Biol 30: 2823–2836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tang Z, El Far O, Betz H, Scheschonka A. Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. J Biol Chem 280: 38153–38159, 2005 [DOI] [PubMed] [Google Scholar]
- 267.Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors. A two-step model of synaptogenesis. Neuron 38: 773–784, 2003 [DOI] [PubMed] [Google Scholar]
- 268.Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem 276: 35368–35374, 2001 [DOI] [PubMed] [Google Scholar]
- 269.Tatham MH, Kim S, Jaffray E, Song J, Chen Y, Hay RT. Unique binding interactions among Ubc9, SUMO and RanBP2 reveal a mechanism for SUMO paralog selection. Nat Struct Mol Biol 12: 67–74, 2005 [DOI] [PubMed] [Google Scholar]
- 270.Tatham MH, Kim S, Yu B, Jaffray E, Song J, Zheng J, Rodriguez MS, Hay RT, Chen Y. Role of an N-terminal site of Ubc9 in SUMO-1, -2, and -3 binding and conjugation. Biochemistry 42: 9959–9969, 2003 [DOI] [PubMed] [Google Scholar]
- 271.Terashima T, Kawai H, Fujitani M, Maeda K, Yasuda H. SUMO-1 co-localized with mutant atrophin-1 with expanded polyglutamines accelerates intranuclear aggregation and cell death. Neuroreport 13: 2359–2364, 2002 [DOI] [PubMed] [Google Scholar]
- 272.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. Alpha-betaCaMKII: inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron 36: 1103–1114, 2002 [DOI] [PubMed] [Google Scholar]
- 273.Thompson JA, Ziman M. Pax genes during neural development and their potential role in neuroregeneration. Prog Neurobiol 95: 334–351, 2011 [DOI] [PubMed] [Google Scholar]
- 274.Tirard M, Hsiao HH, Nikolov M, Urlaub H, Melchior F, Brose N. In vivo localization and identification of SUMOylated proteins in the brain of His6-HA-SUMO1 knock-in mice. Proc Natl Acad Sci USA 109: 21122–21127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Truong K, Lee TD, Chen Y. Small ubiquitin-like modifier (SUMO) modification of E1 Cys domain inhibits E1 Cys domain enzymatic activity. J Biol Chem 287: 15154–15163, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135: 422–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ueda H, Goto J, Hashida H, Lin X, Oyanagi K, Kawano H, Zoghbi HY, Kanazawa I, Okazawa H. Enhanced SUMOylation in polyglutamine diseases. Biochem Biophys Res Commun 293: 307–313, 2002 [DOI] [PubMed] [Google Scholar]
- 278.Ullmann R, Chien CD, Avantaggiati ML, Muller S. An acetylation switch regulates SUMO-dependent protein interaction networks. Mol Cell 46: 759–770, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ulrich HD. Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends Cell Biol 15: 525–532, 2005 [DOI] [PubMed] [Google Scholar]
- 280.Um JW, Chung KC. Functional modulation of parkin through physical interaction with SUMO-1. J Neurosci Res 84: 1543–1554, 2006 [DOI] [PubMed] [Google Scholar]
- 281.Um JW, Min DS, Rhim H, Kim J, Paik SR, Chung KC. Parkin ubiquitinates and promotes the degradation of RanBP2. J Biol Chem 281: 3595–3603, 2006 [DOI] [PubMed] [Google Scholar]
- 282.Van der Veen AG, Ploegh HL. Ubiquitin-like proteins. Annu Rev Biochem 81: 323–357, 2012 [DOI] [PubMed] [Google Scholar]
- 283.Van Niekerk EA, Willis DE, Chang JH, Reumann K, Heise T, Twiss JL. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc Natl Acad Sci USA 104: 12913–12918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci 7: 2189–2205, 1995 [DOI] [PubMed] [Google Scholar]
- 285.Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, Nilsson LM, Parini P, Janne OA, Gustafsson JA, Steffensen KR, Treuter E. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev 24: 381–395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Vertegaal AC, Andersen JS, Ogg SC, Hay RT, Mann M, Lamond AI. Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol Cell Proteomics 5: 2298–2310, 2006 [DOI] [PubMed] [Google Scholar]
- 287.Vertegaal AC, Ogg SC, Jaffray E, Rodriguez MS, Hay RT, Andersen JS, Mann M, Lamond AI. A proteomic study of SUMO-2 target proteins. J Biol Chem 279: 33791–33798, 2004 [DOI] [PubMed] [Google Scholar]
- 288.Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci 33: 173–182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang J. Cardiac function and disease: emerging role of small ubiquitin-related modifier. Wiley Interdiscip Rev Syst Biol Med 3: 446–457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang J, Chen L, Wen S, Zhu H, Yu W, Moskowitz IP, Shaw GM, Finnell RH, Schwartz RJ. Defective sumoylation pathway directs congenital heart disease. Birth Defects Res A Clin Mol Teratol 91: 468–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177: 439–450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Watanabe M, Takahashi K, Tomizawa K, Mizusawa H, Takahashi H. Developmental regulation of Ubc9 in the rat nervous system. Acta Biochim Pol 55: 681–686, 2008 [PubMed] [Google Scholar]
- 293.Weeraratna AT, Kalehua A, Deleon I, Bertak D, Maher G, Wade MS, Lustig A, Becker KG, Wood W, 3rd, Walker DG, Beach TG, Taub DD. Alterations in immunological and neurological gene expression patterns in Alzheimer's disease tissues. Exp Cell Res 313: 450–461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett 579: 5007–5012, 2005 [DOI] [PubMed] [Google Scholar]
- 295.Wei W, Yang P, Pang J, Zhang S, Wang Y, Wang MH, Dong Z, She JX, Wang CY. A stress-dependent SUMO4 sumoylation of its substrate proteins. Biochem Biophys Res Commun 375: 454–459, 2008 [DOI] [PubMed] [Google Scholar]
- 296.Werner A, Flotho A, Melchior F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol Cell 46: 287–298, 2012 [DOI] [PubMed] [Google Scholar]
- 297.Wible BA, Wang L, Kuryshev YA, Basu A, Haldar S, Brown AM. Increased K+ efflux and apoptosis induced by the potassium channel modulatory protein KChAP/PIAS3beta in prostate cancer cells. J Biol Chem 277: 17852–17862, 2002 [DOI] [PubMed] [Google Scholar]
- 298.Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. Cloning and expression of a novel K+ channel regulatory protein, KChAP. J Biol Chem 273: 11745–11751, 1998 [DOI] [PubMed] [Google Scholar]
- 299.Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 428: 133–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wilkinson KA, Konopacki F, Henley JM. Modification and movement: phosphorylation and SUMOylation regulate endocytosis of GluK2-containing kainate receptors. Commun Integr Biol 5: 223–226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev 64: 195–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wilkinson KA, Nishimune A, Henley JM. Analysis of SUMO-1 modification of neuronal proteins containing consensus SUMOylation motifs. Neurosci Lett 436: 239–244, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Williams AL, Bielopolski N, Meroz D, Lam AD, Passmore DR, Ben-Tal N, Ernst SA, Ashery U, Stuenkel EL. Structural and functional analysis of tomosyn identifies domains important in exocytotic regulation. J Biol Chem 286: 14542–14553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wilson SM, Brittain JM, Piekarz AD, Ballard CJ, Ripsch MS, Cummins TR, Hurley JH, Khanna M, Hammes NM, Samuels BC, White FA, Khanna R. Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels 5: 449–456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109: 229–241, 2002 [DOI] [PubMed] [Google Scholar]
- 306.Xu Z, Au SW. Mapping residues of SUMO precursors essential in differential maturation by SUMO-specific protease, SENP1. Biochem J 386: 325–330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yamada T, Yang Y, Huang J, Coppola G, Geschwind DH, Bonni A. Sumoylated MEF2A coordinately eliminates orphan presynaptic sites and promotes maturation of presynaptic boutons. J Neurosci 33: 4726–4740, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yan Q, Gong L, Deng M, Zhang L, Sun S, Liu J, Ma H, Yuan D, Chen PC, Hu X, Liu J, Qin J, Xiao L, Huang XQ, Zhang J, Li DW. Sumoylation activates the transcriptional activity of Pax-6, an important transcription factor for eye and brain development. Proc Natl Acad Sci USA 107: 21034–21039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yan S, Sun X, Xiang B, Cang H, Kang X, Chen Y, Li H, Shi G, Yeh ET, Wang B, Wang X, Yi J. Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J 29: 3773–3786, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yang SH, Galanis A, Witty J, Sharrocks AD. An extended consensus motif enhances the specificity of substrate modification by SUMO. EMBO J 25: 5083–5093, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang SH, Jaffray E, Hay RT, Sharrocks AD. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol Cell 12: 63–74, 2003 [DOI] [PubMed] [Google Scholar]
- 312.Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab 28: 269–279, 2008 [DOI] [PubMed] [Google Scholar]
- 313.Yang Y, Tse AK, Li P, Ma Q, Xiang S, Nicosia SV, Seto E, Zhang X, Bai W. Inhibition of androgen receptor activity by histone deacetylase 4 through receptor SUMOylation. Oncogene 30: 2207–2218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yeh ET. SUMOylation and De-SUMOylation: wrestling with life's processes. J Biol Chem 284: 8223–8227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yun SM, Cho SJ, Song JC, Song SY, Jo SA, Jo C, Yoon K, Tanzi RE, Choi EJ, Koh YH. SUMO1 modulates Abeta generation via BACE1 accumulation. Neurobiol Aging 34: 650–662, 2013 [DOI] [PubMed] [Google Scholar]
- 316.Yunus AA, Lima CD. Structure of the Siz/PIAS SUMO E3 ligase Siz1 and determinants required for SUMO modification of PCNA. Mol Cell 35: 669–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhang FP, Mikkonen L, Toppari J, Palvimo JJ, Thesleff I, Janne OA. Sumo-1 function is dispensable in normal mouse development. Mol Cell Biol 28: 5381–5390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zhang YQ, Sarge KD. Sumoylation of amyloid precursor protein negatively regulates Abeta aggregate levels. Biochem Biophys Res Commun 374: 673–678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol Cell Biol 25: 8456–8464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhou YF, Liao SS, Luo YY, Tang JG, Wang JL, Lei LF, Chi JW, Du J, Jiang H, Xia K, Tang BS, Shen L. SUMO-1 modification on K166 of polyQ-expanded ataxin-3 strengthens its stability and increases its cytotoxicity. PLoS One 8: e54214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhu J, Zhu S, Guzzo CM, Ellis NA, Sung KS, Choi CY, Matunis MJ. Small ubiquitin-related modifier (SUMO) binding determines substrate recognition and paralog-selective SUMO modification. J Biol Chem 283: 29405–29415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci 27: 12211–12220, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhu S, Goeres J, Sixt KM, Bekes M, Zhang XD, Salvesen GS, Matunis MJ. Protection from isopeptidase-mediated deconjugation regulates paralog-selective sumoylation of RanGAP1. Mol Cell 33: 570–580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zimnik S, Gaestel M, Niedenthal R. Mutually exclusive STAT1 modifications identified by Ubc9/substrate dimerization-dependent SUMOylation. Nucleic Acids Res 37: e30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci 5: 385–399, 2004 [DOI] [PubMed] [Google Scholar]
- 326.Zungu M, Schisler J, Willis MS. All the little pieces. Regulation of mitochondrial fusion and fission by ubiquitin and small ubiquitin-like modifer and their potential relevance in the heart. Circ J 75: 2513–2521, 2011 [DOI] [PubMed] [Google Scholar]
- 327.Zunino R, Braschi E, Xu L, McBride HM. Translocation of SenP5 from the nucleoli to the mitochondria modulates DRP1-dependent fission during mitosis. J Biol Chem 284: 17783–17795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci 120: 1178–1188, 2007 [DOI] [PubMed] [Google Scholar]