Abstract
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide. Discovered 30 years ago, it is produced as a consequence of alternative RNA processing of the calcitonin gene. CGRP has two major forms (α and β). It belongs to a group of peptides that all act on an unusual receptor family. These receptors consist of calcitonin receptor-like receptor (CLR) linked to an essential receptor activity modifying protein (RAMP) that is necessary for full functionality. CGRP is a highly potent vasodilator and, partly as a consequence, possesses protective mechanisms that are important for physiological and pathological conditions involving the cardiovascular system and wound healing. CGRP is primarily released from sensory nerves and thus is implicated in pain pathways. The proven ability of CGRP antagonists to alleviate migraine has been of most interest in terms of drug development, and knowledge to date concerning this potential therapeutic area is discussed. Other areas covered, where there is less information known on CGRP, include arthritis, skin conditions, diabetes, and obesity. It is concluded that CGRP is an important peptide in mammalian biology, but it is too early at present to know if new medicines for disease treatment will emerge from our knowledge concerning this molecule.
I. INTRODUCTION
Calcitonin gene-related peptide (CGRP) is a 37-amino acid peptide, which is primarily localized to C and Aδ sensory fibers. These fibers display a wide innervation throughout the body, with extensive perivascular localization, and have a dual role in sensory (nociceptive) and efferent (effector) function (261, 339). CGRP is also localized in nonneuronal tissues, of which less is known at present. The role of CGRP remains unclear, despite excellent and previous reviews including (28, 41, 86, 430, 435).
Originally, CGRP was shown to mediate sympathetic outflow from the brain (123). However, it was soon established that the major cardiovascular activity of CGRP is its potent vasodilator activity that is obvious when exogenous CGRP is administered at femtomolar doses to the skin of human and animal species (45), and supported by evidence that CGRP has a vascular protective role through studies mainly carried out in rodent models. It has been suggested that CGRP may have potential as a therapy for treating cardiovascular diseases, but progress here has been limited. However, the sensory fibers that CGRP is contained in are also associated with pain processes, and the development of CGRP antagonists has revealed the pivotal role that CGRP plays in migraine, and with it the therapeutic potential of CGRP receptor antagonists, which has led to a vibrant drug discovery program (302, 311). The aim of this review is to summarize the current understanding of the role of CGRP in physiology and pathophysiology, with special reference to the cardiovascular system.
CGRP was discovered when it was realized that alternative processing (tissue-specific splicing) of the mRNA for calcitonin in the thyroid of the ageing rat leads to CGRP production, and CGRP was found to be widely expressed in neuronal tissue (11, 338, 339). It was then isolated from the thyroid of patients with medullary thyroid carcinoma (294). The gene family is comprised of adrenomedullin, adrenomedullin 2 (intermedin), and amylin, in addition to the calcitonin gene. There are two major CGRP isoforms, which have similar structures and biological activities but are formed by separate genes (10).
The realization that CGRP was present in sensory nerves led to studies with the chili extract capsaicin, which is now known to activate transient receptor potential vanilloid 1 (TRPV1) receptors, commonly found on sensory C and Aδ-fibers. Capsaicin has long been known to cause pain and redness on acute application; thus its ability to release CGRP and the colocalized neuropeptide substance P (SP), characterized a few decades earlier, was not surprising (139, 255, 448). There were also two key indicators of future importance. First, CGRP was found to be released and active in the cerebral circulation (162). Second, CGRP was not only a potent vasodilator, but also had a close reciprocal interaction with the sympathetic nervous system in the periphery (212, 394). Other aspects, such as the role of CGRP in inflammation, have been debated with evidence for both a pro- and anti-inflammatory role, depending on situation (see Ref. 39).
II. SYNTHESIS
A. The Two Isoforms of CGRP: αCGRP and βCGRP
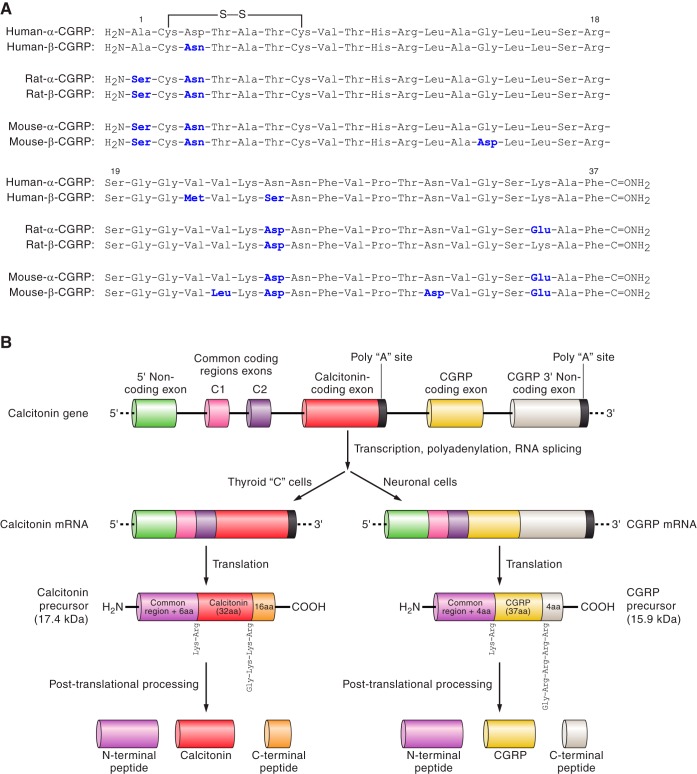
The two forms of CGRP, α and βCGRP, otherwise known as CGRPI and II, are synthesized from two distinct genes at different sites on chromosome 11 in the human (436). The CALC I gene can undergo alternative splicing to produce either calcitonin or αCGRP (Figure 1), whereas βCGRP is known to be transcribed from its own distinct CALC II gene (7, 371). αCGRP and βCGRP share >90% homology (Figure 1A) and differ by only three amino acids in the human; thus it is perhaps not surprising that they share similar biological activities (10, 294, 371). Traditionally, it has been considered that αCGRP is the principal form found in the central and peripheral nervous system, whereas βCGRP is found mainly in the enteric nervous system (41, 297).
Fig. 1.
A: amino acid residues of human, rat, and mouse α- and β-CGRPs. The secondary structure regions and disulfide bonds are indicated. The residues in bold are nonidentical homologs to the human-α-CGRP. In italics are the residues that are nonidentical homologs between the α- and β-CGRP of the same species. B: processing of the calcitonin CALC I gene leading to either primarily calcitonin in the thyroid or α-CGRP in sensory neurons.
To produce calcitonin from the CALC I gene, it is necessary for exon 4 within the gene to be expressed in the mature protein. This is the situation that occurs predominantly in the thyroid where calcitonin is the major gene product. Conversely, expression of exons 5 and 6 results in production of αCGRP mRNA, which is first translated into a 121-amino acid pro-hormone, and then subsequently cleaved to create the mature 37-amino acid peptide (Figure 1B). αCGRP is predominantly expressed throughout the central and peripheral nervous system. However, the mechanism that determines this alternate splicing remains unclear (249).
B. Structure of CGRP
The structure of human αCGRP has been revealed to contain four clear domains, similar to that of βCGRP (75). The first seven residues of the NH2 terminus make up the first domain and form a ringlike structure, held together by a disulfide bridge. The peptide CGRP antagonist, CGRP8–37, is formed from the removal of this first domain. Residues 8–18 make up domain 2, which consists of an α helix, deletion of which causes a 50- to 100-fold decrease in affinity (341). In particular, residues 11 and 18 within the hydrophilic face of the α-helix play a crucial role in promoting high-affinity binding (190). Residues 19–27 make up domain 3 of CGRP and form either a β- or γ-twist (75). The final fourth domain contains the COOH terminus and the remaining residues from 28–37, and has two turn regions that are thought to form a binding epitope (46, 59). The species differences and structure-activity relationships for CGRP have been extensively investigated, and key amino acids playing pivotal roles in receptor binding and activation have been identified (429). Figure 1A shows the amino acid sequences of human, rat, and mouse α and βCGRP.
C. Regulation of Synthesis and Release of CGRP
The regulation of CGRP synthesis is still poorly understood. CGRP synthesis is known to be upregulated in models of nerve damage, such as peripheral axotomy, and it is thought that synthesis of the peptide is enhanced in tissues that are undergoing an inflammatory response (98). This may be linked to local release of nerve growth factor (NGF) from cells such as macrophages and keratinocytes. NGF is vitally important for the growth of sensory nerves and for the maintenance of function of mature nerves (393). After the depletion of sensory neuropeptides from nerve terminals following treatment with the TRPV1 agonist capsaicin, NGF is required for the synthesis of new peptide (97). Moreover, NGF has been linked to the upregulation of CGRP production within the dorsal root ganglia (DRG) and promotion of CGRP expression in the genetically bred spontaneously hypertensive rat (SHR) (384) and is now considered to be involved in influencing both the sensory and sympathetic nervous systems in a complex manner during cardiovascular dysfunction (167). Increased levels of both NGF and CGRP are also observed in the plasma and saliva from migraine patients, a condition which CGRP is heavily implicated in, as will be discussed later in this review (199). There is also the potential for other factors, e.g., brain-derived neurotrophic factor (BDNF) to influence CGRP release and activity (346).
After synthesis, CGRP is stored in large, dense-core vesicles within the sensory nerve terminal (273). Following neuronal depolarization, CGRP is released from the terminal via calcium-dependent exocytosis mediated by classical exocytotic pathways that involve members of the SNARE (soluble N-ethymalemide-sensitive factor attachment protein receptor) family of proteins (284). The release of CGRP from sensory neurons was first demonstrated using capsaicin. The TRPV1 receptor is an ion channel that is also activated by noxious heat (>43°C) and sensitized by low pH as well as various endogenous lipids and other soluble mediators acting through a range of receptors on the nerve terminal (60, 415). However, the actual importance of specific endogenous agonists in releasing CGRP is still being defined. Substantial work has been carried out with the compound rutaecarpine isolated from the plant, Evodia rutaecarpa, which is used to treat hypertension in traditional Chinese medicine (236). Rutaecarpine is a reported TRPV1 agonist that releases CGRP and acts in a hypotensive, cardiovascular protective manner in humans (236). It is likely that endogenous agonists/stimuli might be more relevant in terms of TRPV1 activation and subsequent CGRP release in vivo, especially in areas of inflammation that are likely to have raised local temperature and increased proton concentrations (9). One possible endogenous TRPV1 agonist is anandamide, an endocannabinoid. Anandamide has been shown to release CGRP to produce vasodilatation in tissues that include the mesentery (320, 462). Peroni et al. (320) have suggested that this pathway is under the regulatory control of estrogen, with isoflavones acting to promote mesenteric CGRP levels as estrogen levels are lost in ageing females. In general support of this concept, it has been shown that plasma levels of CGRP are reduced in postmenopausal women, but restored by hormone replacement therapy (409). Aside from TRPV1, other members of the TRP family have been shown to release CGRP following stimulation, such as transient receptor potential ankyrin 1 (TRPA1). TRPA1 is often coexpressed alongside TRPV1 in certain populations of sensory neurons. Indeed, it is estimated that 60–75% of TRPV1-expressing neurons also express TRPA1 (412). In our hands, the topical application of TRPA1 agonists (including a lipid peroxidation product, 4-ONE) leads to an increase in cutaneous blood flow due to CGRP release. However, TRPA1 agonists do not appear to modulate blood pressure regulation, in the mouse at least (151, 326). The gas hydrogen sulfide is also involved in TRPA1-mediated CGRP release (327, 433).
The release of CGRP also occurs in response to known pressor agents [e.g., angiotensin II (ANG II) and norepinephrine], possibly as a direct consequence of receptors activated at the level of the perivascular sensory nerve, and of potential relevance to the pathophysiology of hypertension as discussed later (213). There is evidence that norepinephrine acts via the α2-adrenoceptors to inhibit activation of sensory nerves as NGF-induced release of CGRP was restored by the α2-adrenoceptor antagonist yohimbine in DRG (86, 210, 380). The relevance of this to hypertension will be discussed in due course.
Angeli's salt generates nitroxyl anion (HNO), which acts via CGRP to mediate positive ionotrophic effects on the heart, and also vasodilatation (53, 114, 317). The endogenous relevance of this release mechanism is not yet known. However, a new HNO donor (1-nitrosocyclohexyl acetate, NCA), which has a longer half-life and predominantly releases HNO, has been suggested as a potential therapeutic agent due to its ability to counteract contractile effects and block platelet aggregation (99).
D. CGRP Metabolism
Several aspects concerning the regulation of CGRP activity are well understood, but it is still unclear as to how the peptide is metabolized following release from the cell. Initial studies investigating the capacity of the neuropeptide SP to elicit neurogenic inflammation showed that low concentrations of CGRP were able to potentiate plasma extravasation induced by SP in rat abdominal skin (129). Undoubtedly, this result may have been as a result of many different pharmacological effects, but it was later suggested that CGRP was capable of inhibiting SP degradation and therefore augmenting its bioactivity by competing for degradation by the same endopeptidase, although the identity of this molecule was not determined (231, 232). The lack of an identified candidate for this effect may be attributed to a false interpretation of data from these groups. Indeed, it is perhaps more likely that the potentiation of edema formation in this model was due to the potent vasodilator activity of CGRP working in synergy with the potent endothelial junctional destabilization activity of SP (43). To determine mechanisms involved in the breakdown of CGRP, experiments were performed in skin. CGRP was hydrolyzed following mast cell activation, implicating tryptase in this system (44). Subsequent to this study, the identity of a shared removal enzyme common to both SP and CGRP has been identified as neutral endopeptidase, more commonly known in the field of neurodegenerative disease as neprilysin (208). More recently, a proteomic approach has been used to identify novel CGRP fragments in mouse tissue and to reveal the endogenous cleavage sites within the primary sequence of the peptide. Mass spectrometry revealed the presence of 10 endogenously produced peptide fragments in murine spinal cord homogenates that highlighted two primary cleavage sites in vivo: Ser17-Arg18 and Asn26-Phe27. Subsequent biochemical assays identified a role for a metalloprotease in the regulation of CGRP proteolysis, of which insulin-degrading enzyme (IDE) was found to be particularly important in processing of the peptide at the aforementioned cleavage sites (221). Endothelin-converting enzyme-1 (ECE-1) also degrades CGRP, and this has been suggested to be of functional importance in murine pulmonary fibrosis (164).
An alternative mechanism has been proposed to exist to regulate CGRP removal. In the mouse vas deferens, application of the TRPV1 agonist capsaicin was shown to attenuate electrically induced twitch responses, an effect that was attributable to the release of CGRP from the sensory nerve terminal. However, following a second exposure to capsaicin, its ability to blunt muscle twitch was markedly reduced, suggesting sensory afferents had depleted their stores of CGRP following the first stimulation. This effect was rescued by preincubating the preparation for a short time with exogenous CGRP. The authors suggest that this restoration of function is attributable to reuptake of CGRP into the neuron by an active transport system (350). Indeed, this aspect of reuptake is similar to the reuptake process in sympathetic nerves (264). Similar results for CGRP have been shown in the guinea pig basilar artery and in rat dura mater encephali, where reuptake may prove to be an important mechanism by which CGRPergic signaling is regulated in conditions such as migraine (158, 350). In summary, a wide range of removal mechanisms have been proposed to be important in the removal or breakdown of CGRP following its release from nerves or vascular cells. Specific targeting of each of these mechanisms may yield a novel means by which to augment or suppress CGRP bioactivity for therapeutic gain.
III. CENTRAL AND PERIPHERAL DISTRIBUTION OF CGRP
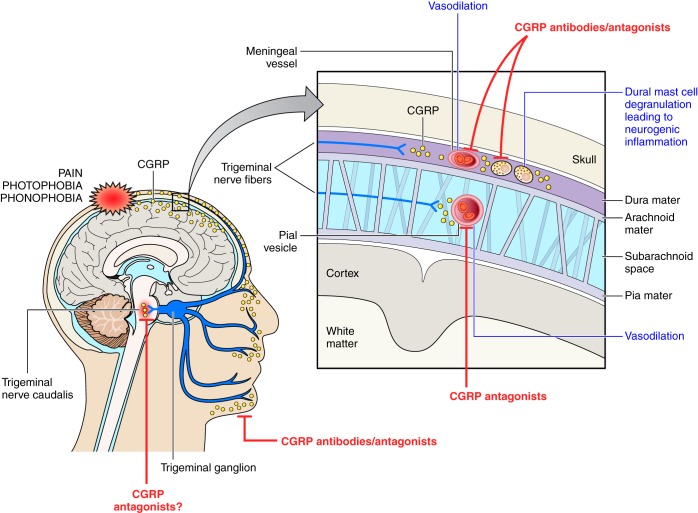
CGRP is widely distributed in discrete areas of the central and peripheral nervous system (339) and was shown at an early stage to be released from trigeminal ganglion cells (270) and DRG, both major sites of neuropeptide synthesis (140). The primary association of CGRP with sensory neurons, especially the unmyelinated C fibers and thinly myelinated Aδ fibers, is clear. It is commonly colocalized with SP (139). CGRP is also coexpressed with ACh in motor neurons and may be involved in acetylcholine receptor synthesis (305).
Of importance for the vasodilator activity of CGRP is the finding that CGRP is contained in perivascular nerves, providing a major link with the cardiovascular system (449). Immunohistochemical localization of CGRP indicates a perivascular innervation that can extend from the adventitia to the medial muscle layers of blood vessels. The localization of CGRP in perivascular neurons is common to all vascular beds, with perhaps a higher localization in arterial than venous tissues, and some have suggested that levels may be lower in human than laboratory species (28, 41, 186, 297, 405). Perivascular nerves have been suggested to be a major source of plasma CGRP, that has been found to become deficient in certain types of hypertension (86, 367, 435). However, the understanding of when and how CGRP is released in hypertension remains poor. There is a dense perivascular innervation of CGRP, for example associated with the resistance vessels of the mesentery (212) and the kidney (414), and these sites may be key to release. This again links the sites of CGRP neuronal innervation with cardiovascular regulation. Indeed, CGRP can be readily released into the plasma under certain circumstances, including pregnancy, some flushing syndromes, kidney dialysis, certain cardiovascular states (where it also may become depleted) and migraine, all of which will be discussed in later sections (41, 168, 373). Generally, however, CGRP plasma levels are low, and it is likely that CGRP can mediate its biological effects, without the need to circulate in plasma. Moreover, it is considered that plasma CGRP is the result of an “overspill” from perivascular sensory neurons, and the major effects of CGRP are exerted locally, in the vessel wall, close to its site of release. Of importance, there is a dense perivascular innervation in the trigeminal system that is related to CGRP release during migraine and subarachnoid hemorrhage (104, 312), although again mechanisms involved in its release remain unclear. A deficiency of CGRP release has also been suggested to be involved in the lack of reflex vasodilatation observed in Raynaud's disease and, indeed, here CGRP administration was shown to have a beneficial effect (55).
The α-CGRP form is considered to be the primary neuronal form, both centrally as well as peripherally. It has been suggested that αCGRP immunoreactivity is severalfold higher than that for βCGRP, and the αCGRP form is primarily subject to capsaicin depletion (298, 359). Historically, βCGRP was suggested to be mainly found in the intestine, with up to seven times more expression detected than αCGRP (298). However, more recently βCGRP has been observed in the adventitia of mesenteric branch arteries in rat, in the myenteric plexus and mucosa (369). In addition, βCGRP has been shown to be released alongside αCGRP in the vascular system (235). Thus it is now becoming clear that both isoforms can be expressed in the nervous system, depending on situation.
CGRP was discovered as a consequence of its nonneuronal upregulation in the thyroid of ageing rats and in medullary thyroid carcinoma. Whilst the nerves are its primary source, it is now clear that CGRP is located in nonneuronal cells, such as endothelial cells and adipocytes (57, 159, 315). There is also some evidence that CGRP is produced by several types of immune cells, including activated B-lymphocytes, peripheral blood mononuclear cells, and macrophages (38, 248, 421). Perhaps surprisingly, the release of CGRP from nonneuronal cells has also been linked to the activation of TRPV1 [e.g., in endothelial cells (257)]. Both α and βCGRP have also been found in endothelial progenitor cells that repair damaged endothelium and influence vascular remodeling (453). It has been suggested that CGRP expression is more abundant in early rather than late endothelial progenitor cells (113).
Finally, there is increasing evidence that CGRP is localized to keratinocytes. There are several papers that suggest low levels of CGRP are found in keratinocytes from immunohistochemical studies. Recently, it has been shown that βCGRP mRNA and to a lesser extent αCGRP are expressed in keratinocyte cultures derived from human and rodents (189).
IV. RECEPTORS
A. Discovery of CLR
Early radioligand binding studies mapped out tissue preparations that had the capacity to bind CGRP and therefore expressed a CGRP receptor. Early studies revealed the existence of two “receptors”: CGRP1 and CGRP2. The linearized CGRP analog [Cys(ACM)2,7]hCGRP was found to be a potent agonist in the rat vas deferens bioassay, but not guinea pig atrial preparations. Furthermore, the truncated peptide fragments hCGRP12–37 and CGRP8–37 had antagonist properties in atrial preparations but not in the vas deferens (69). Generally speaking, receptors that are antagonized by CGRP8–37 with a pKb of ∼7.0 are considered to be CGRP1 receptors, while those that are blocked with a pKb of 6.0 or less are considered to be CGRP2 receptors. While the existence of CGRP1 and CGRP2 receptors was debated for many years, it is now accepted that only one true CGRP receptor exists, and the apparent existence of additional receptors for CGRP may be explained by the peptide having some affinity (albeit low) for other related receptors of distinct molecular composition, such as receptors for adrenomedullin and intermedin (124, 169).
The molecular nature of the CGRP receptor took some years to understand as it is composed of two subunits. In addition, some supposed CGRP receptor structures were suggested that are now known to be incorrect (see Ref. 171). Studies by Chang et al. (67) and Fluhmann et al. (124) in the early 1990s hinted at the discovery of a “calcitonin receptor-like receptor” (CLR) in both rat and human that were unresponsive to CGRP stimulation when expressed in cell lines. The human protein was found to be 461 amino acids in length and had 7 transmembrane domains as part of its structure (Figure 2). The receptor was found to have 96% sequence homology with the rat protein and around 56% homology with human calcitonin receptor (124). In 1996, Aiyar et al. (5) discovered that in human embryonic kidney (HEK293) cells, the same cloned receptor as Chang and Fluhmann surprisingly produced a 60-fold increase in cAMP generation following CGRP stimulation, and this could be blocked by CGRP8–37. This response was attributed to the HEK293 cells endogenously expressing a hitherto unknown protein that, when coexpressed with CLR, responded to CGRP. This protein was christened a “receptor activity modifying protein,” or RAMP. Three RAMPs are now known: RAMP1, RAMP2, and RAMP3 (280). The heterodimerization of both CLR and one of the single transmembrane RAMP peptides is required if the mature protein is to be exported from the endoplasmic reticulum and inserted into the plasma membrane, a mechanism dependent on the glycosylation status of the receptor (280). When RAMPs are expressed in isolation, they are retained by the Golgi apparatus in the form of a disulfide-linked homodimer (179). However, when the RAMP translocates to the cell membrane, it is stabilized in a heterodimeric complex with the CLR facilitated by noncovalent interactions. The terminology was quickly agreed upon that coexpression of CLR and RAMP1 creates a CGRP receptor with a high affinity for CGRP, while dimerization of the CLR and RAMP2 creates a receptor that is highly responsive to the related peptide adrenomedullin (AM1 receptor). The RAMP3 receptor confers a second adrenomedullin receptor (AM2 receptor) that has some selectivity for CGRP (70, 296).
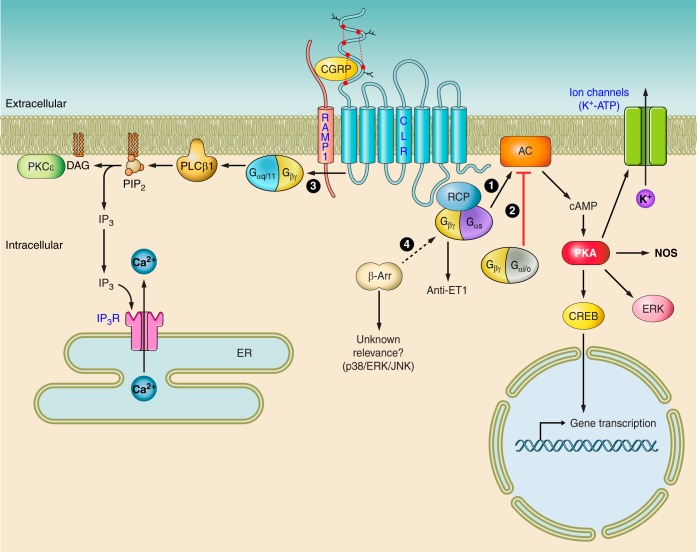
Fig. 2.
CGRP receptor-mediated intracellular signaling. Binding of CGRP ligand to the CLR/RAMP1 receptor can cause activation of multiple signaling pathways and subsequent recruitment of many more downstream effectors. Perhaps the best known is 1) where the activation of adenylate cyclase (AC) by Gαs provokes the elevation of intracellular cAMP, thereby activating protein kinase A (PKA), resulting in the phosphorylation of multiple downstream targets. These targets may include potassium-sensitive ATP channels (KATP channels), extracellular signal-related kinases (ERKs), or transcription factors, such as cAMP response element-binding protein (CREB). Nitric oxide generation following CGRP receptor activation may be secondary to phosphorylation of nitric oxide synthase (NOS), although this has not been directly shown. Alternatively, the CGRP receptor may couple to Gαi/o, thus attenuating AC activity and decreasing intracellular cAMP, resulting in a loss of PKA activity (2). Reports in osteoblasts have also shown evidence of Gαq/11-mediated signaling (3), involving activation of PLC-β1, cleaving phosphatidylinositol 4,5-bisphosphate (PIP2) to form inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3R) on the endoplasmic reticulum (ER), causing calcium release and thus raising cytoplasmic concentrations. DAG may activate PKC-ϵ, which in turn phosphorylates proteins further downstream. Finally, there is evidence to suggest G protein-independent signaling pathways (4) that require the translocation of scaffolding proteins such as β-arrestins (β-Arr) to the activated receptor. Additionally, βγ G protein subunits may signal in a unique way to specifically terminate endothelin (ET)-mediated effects. Solid arrows represent known pathways, and broken arrows represent potential new pathways. CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like receptor; RAMP1, receptor activity-modifying protein 1; RCP, receptor component protein.
B. CLR and RAMPs
The CLR belongs to the class B “secretin-like” family of G protein-coupled receptors (GPCRs), which also includes receptors for calcitonin, vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase activating polypeptide (PACAP), and parathyroid hormone (PTH). The RAMP family of proteins is comprised of three members: RAMP1, RAMP2, and RAMP3, each with <30% sequence homology but sharing a similar structure. They are small membrane proteins that possess a large extracellular NH2 terminus of ∼100 amino acids, a single transmembrane domain, and a short intracellular domain of 10 amino acids. Heterodimerization of RAMP1 and CLR produces a receptor for CGRP (Figure 2) that can be antagonized by the truncated peptide CGRP8–37 and nonpeptide CGRP antagonists (e.g., BIBN4096BS). Association of CLR and RAMP2 leads to the formation of a receptor for adrenomedullin (AM1 receptor), which is blocked by AM22–52, and association with RAMP3 produces the second adrenomedullin receptor (AM2 receptor). As the CLR is widely expressed, it has been proposed that the tissue-specific expression of the RAMPs regulates the site-specific bioactivity of the CGRP-related peptides (160). It is still unclear how the dimerization of RAMPs with the CLR is regulated, especially in cell types that coexpress more than one RAMP isoform. It is proposed that RAMPs compete with each other to interact with CLR, and studies in rabbit aortic endothelial cells have shown that cotransfection of different RAMPs can change the potency of CGRP at the receptor (296). It is suggested that in cells that coexpress both RAMP2 and RAMP1, a preference for RAMP1 to colocalize with the CLR is established, to form a receptor for CGRP (52).
While the true receptor for CGRP is considered to be formed by the CLR/RAMP1 complex, it has been shown in vitro that CGRP has some affinity for the CGRP/RAMP3 complex (the AM2 receptor). Hay et al. (170) showed that, as expected, αCGRP was ∼15 times less potent than AM at the CLR/RAMP3 complex present in COS-7 cells, whilst βCGRP was only ∼2.5 times less potent than AM (170). It is unclear whether this finding reflects the action of βCGRP in vivo, but it certainly presents an interesting alternative mechanism by which CGRP might mediate its effects.
C. Discovery of Receptor Component Protein
An additional third protein, receptor component protein (RCP), is required to form an optimally functional CGRP receptor (Figure 2). RCP is a small (∼17 kDa), hydrophilic, membrane-associated protein that has little homology to other known protein sequences. RCP was first identified in the guinea pig organ of Corti, where an isolated cDNA was shown to encode for a protein that conferred specific pharmacological sensitivity to CGRP, and not other related ligands (254). Indeed, when antisense RNA was constructed against RCP and transfected into NIH3T3 cells, cAMP generation subsequent to CGRP receptor stimulation was lost. Disruption of RCP synthesis had no effect on the ability of CGRP to bind to its receptor but significantly attenuated cAMP generation. The RCP was shown to coimmunoprecipitate with the CLR protein, confirming that association of RCP with the CGRP receptor is important for optimal signal transduction (111). In addition, this unique protein has since been shown to be important in the regulation of AM receptor-driven signaling (328). A potentially important role for RCP in vivo has recently been uncovered in the setting of a hypertension model. Rats rendered hypertensive via subtotal nephrectomy surgery and saline consumption were found to have an augmented depressor response to intravenous CGRP compared with sham-operated animals, and isolated resistance vessels from these animals were found to be more sensitive to exogenous CGRP. This was an effect that was suggested to be directly attributable to an increased expression of RCP protein within the resistance vasculature of the hypertensive rats, whereas expression of other CGRP receptor proteins was found to be unchanged (381). It is therefore conceivable that the fine-tuning of RCP expression in disease conditions may drastically change CGRP-mediated responses.
D. Tachyphylaxis of Receptors
An important feature of GPCR signaling is that of tachyphylaxis, or desensitization. Whilst receptor desensitization has been extensively studied in typical class A GPCRs, such as the β2-adrenergic receptor, the kinetics of class B receptor tachyphylaxis are now being investigated. Early studies investigating signal desensitization showed that cAMP responses in a variety of cell types were indeed attenuated after a second exposure to CGRP and that this decrease in signal was dependent on the activity of PKA (101, 102). Other studies have highlighted an additional role for PKC in this process (323). After agonist stimulation, the CLR component of the receptor is rapidly phosphorylated and subsequently internalized via the recruitment of β-arrestins and movement of the receptor complex to clathrin-coated pits for endocytosis (178). Therefore, in spite of its atypical heterodimeric composition, the CGRP receptor appears to behave similarly to other seven transmembrane receptors in terms of the mechanisms by which it becomes desensitized. Duration of stimulus can also affect receptor fate: transient stimulation of the receptor with CGRP induces internalization of the receptor to endosomes, which allows rapid recycling back to the plasma membrane for another round of agonist challenge. Chronic exposure of the agonist initiates a distinct desensitization pathway whereby the internalized receptor is trafficked from the endosome to the lysosome for degradation (80). Recovery from this desensitized state is therefore slow, as it requires the de novo synthesis of receptor protein. An additional layer of complexity has been added to CGRP receptor cycling, whereby it has been discovered that isoforms of ECE-1 cointernalize with the CGRP receptor to the early endosome where they degrade CGRP. Degradation of CGRP promotes receptor cycling, and if ECE-1 activity is inhibited, receptor complexes remain trapped within the endosomal compartment (281, 316). Therefore, regulation of ECE isoforms may prove an attractive drug target for the treatment of migraine through maintenance of the acute vasoregulatory effects of CGRP while abrogating the nociceptive effects of sustained CGRP receptor stimulation.
E. CGRP Receptor Antagonists
The removal of the first seven amino acid residues of CGRP yields the peptide antagonist CGRP8–37, used since 1989 until today, as a valuable experimental tool to interrogate CGRP-derived responses (69). Other peptide antagonists have also been investigated [e.g., CGRP27–37, which is the minimal fragment required for binding (441)]. While these peptide fragments have been useful for probing the calcitonin family of receptors, there are issues related to selectivity. Furthermore, the nature of peptides mean they do not readily lend themselves to complex in vivo assays. Therefore, there was a great need to develop small molecule antagonists of the CGRP receptor to fully explore its biological and translational relevance. Development of selective nonpeptide antagonists of the CGRP receptor (and, indeed, other class B GPCRs) has met with difficulty owing to the fact that the endogenous ligand of the receptor is a peptide. Therefore, a low-molecular-weight antagonist working at the orthosteric site would have to prevent the binding of a much larger molecule that is likely to have a complex interaction with its receptor. Furthermore, the heterogeneity of the CGRP family of receptors begets further complexity; namely, the design of novel antagonists must overcome structural differences between CLR/RAMP heteromers. However, much progress has been made in drug discovery programs.
The first reported small molecule CGRP receptor antagonist was SB-273779, developed by SmithKline Beecham. This molecule was found to inhibit CGRP binding with an IC50 of 0.31 μM and could inhibit 50% of CGRP-induced cyclase activity at a similar concentration (4). Following this, evidence for the first potent CGRP receptor antagonist was published in 2000 by Boehringer Ingelheim. They patented the new chemical entity BIBN4096BS, or, olcegepant: 1-piperidinecarboxamide,N-[2-[[5-amino-1-[[4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)-methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-[R-(R*,S*)] (100). This novel noncompetitive antagonist of the CGRP receptor blocked binding of the endogenous ligand to receptor in both cells and tissue preparations and also produced a rightward shift in cAMP accumulation curves. Indeed, it has an affinity of 14.4 ± 6.3 pM for human CGRP receptors in SK-N-MC cells and 3.4 ± 0.5 nM for CGRP receptors found in spleen (109). This is a striking difference compared with the 1.3 nM affinity of CGRP8–37 for CGRP receptors in SK-N-MC cells (109). In vivo preparations demonstrated that the antagonist, although not orally active, was further capable of inhibiting increases in dermal blood flow following trigeminal ganglion stimulation in the marmoset (69, 100). While this antagonist has been shown to have increased potency over the peptide antagonist, it is considered to have 200-fold increased potency in humans and non-human primates than in rodent tissue (100).
Based on the promising pharmacology of olcegepant, an additional member of the “gepant” family was identified by Merck via high-throughput screening. Originally termed “compound 2,” the benzodiazepine-like lead structure was shown to possess low affinity for the human CGRP receptor, with a Ki of 4.8 μM. Optimization of this structure led to the development of telcagepant, which was considerably more potent at the same receptor. This compound was found to have good oral bioavailability (319). Early characterization studies showed that telcagepant had a Ki of 1.1 nM at CGRP receptors expressed in HEK293 cells and could inhibit capsaicin-induced increases in blood flow in the rhesus forearm (347). Merck continued with the success of telcagepant with the development of a more potent antagonist, MK-3207. This compound displayed a Ki of 0.024 nM at human and rhesus CGRP receptors but again this high potency was not conserved in species other than humans and primates (349).
Other less well-characterized antagonists have also been developed to block CGRP activity. A second compound was filed under the Boehringer patent WO98/11128, (4-(2-oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidine-1-carboxylic acid [1–3,4-dibromo-4-hydroxyl-benzyl)-2-oxo-2-(4-phenyl-piperazin-1-yl)-ethyl]-amide) or “compound 1.” Compound 1 was shown to have pKi values of 7.8 in SK-N-MC cells (compared with that of around 8.9 for CGRP8–37). It was shown to be a weak antagonist of CGRP responses in human cerebral and guinea pig basilar arteries (109), but not in porcine coronary arteries (166). Interestingly, compound 1 also antagonizes the effects of CGRP in the coronary vasculature, albeit from humans (165), demonstrating the species difference for CGRP in receptor pharmacology. There has also been a range of cyclic nonpeptide antagonists based on CGRP27–37 with potencies in the nanomolar range (441).
More recently, Bristol-Myers Squibb (BMS) have published data on a novel set of non-peptide antagonists at the CGRP receptor. With accumulating evidence for many small molecule CGRP receptor antagonists unfortunately being potent inhibitors of hepatic CYP3A4 (leading to the withdrawal of telcagepant from clinical trials; see below), BMS have developed compounds that possess modified benzthiophene side chains to circumvent these issues, including BMS-694153, a compound with high affinity for the human CGRP receptor (Ki = 13 pM). Significantly, this compound was shown to have excellent intranasal bioavailability in the rabbit, a physicochemical characteristic that may suit the treatment of an acute migraine attack (85). For an excellent and thorough review of all major CGRP receptor antagonists, including their pharmacological characterization, see Reference 293.
A feature common to the small molecule CGRP receptor antagonists appears to be their increased selectivity for receptors derived from humans and non-human primates versus rodents. Clearly, this points to differences in the molecular composition of CGRP receptors between species that may confer functional changes in pharmacology. Definitive work in 2002 by Mallee et al. (263) utilizing receptor chimeras described how the RAMP molecule of the receptor was most likely to confer species selectivity, rather than the CLR. A series of elegant experiments showed that olcegepant had similar potencies on receptors comprised of rat CLR and human RAMP1 compared with those comprised of human CLR and human RAMP1. This equivalence in potency was lost when rat RAMP1 was introduced. Following from this finding, Mallee et al. (263) were able to find a region within the primary amino acid sequence of RAMP1 that was required for species selectivity, specifically residues 66–112 (263). Strikingly, they found that mutagenesis of rat RAMP1 residue 74 from lysine to tryptophan recapitulated the human phenotype and restored potency of olcegepant to that similar to its pharmacology at the human receptor. This residue is critical for small molecule antagonist binding. Kusano et al. (226) made progress in resolving the crystal structure of the extracellular domains of human RAMP1 in 2008, which uncovered four additional residues that potentially could be important for antagonist binding: Arg67, Asp71, Glu78, and Trp84. Validation of these key residues in conferring antagonist affinity uncovered for the first time a key role for Trp84 in the regulation, of not only affinity for small molecule antagonists, but also affinity for peptide fragment antagonists and peptide agonists alike (292). This residue exists as part of the binding interface between RAMP1 and the CLR, indicating that the ligand-binding domain of the CGRP receptor is formed of a hydrophobic pocket located between both structures. Interestingly, mutation of Trp84 to alanine results in a lowered membrane expression of the receptor, bolstering the role for RAMP1 as a molecular chaperone for CLR to reach the plasma membrane and supporting the role of Trp84 in mediating RAMP1:CLR interactions.
Antagonist binding is not solely dictated by critical residues present within the RAMPs. Certain aspects of the CLR structure also appear to be important in regulating affinity. The rationale for investigating the role of the CLR in antagonist binding came from the observation that CLR:RAMP1 and calcitonin receptor CTR:RAMP1 heterodimers possessed different affinities for CGRP antagonists, indicating a role for the CLR. Indeed, formation of CTR/CLR chimeras implicated a role for amino acids 37–63 of the CLR in the high-affinity binding of gepant-like antagonist structures, whilst aspects of transmembrane domain 7 appeared to be important for a second class of RAMP-independent antagonists (e.g., compound 4; Ref. 348). Having an antagonist that is dependent on binding to a site that is so far removed from the CLR:RAMP1 interface may be indicative of a site of allosteric modulation. Collectively, however, identification of two distinct regions of the extracellular domain of CLR critical for antagonist binding further highlights the complexity of this receptor family and the challenges that are faced by pharmacologists who wish to design drugs targeted against them.
The mechanism by which CGRP binds to its receptor is commonly described as part of the “two-domain model,” proposed by Hoare (183). In this model, the COOH terminus of the peptide ligand interacts with high affinity with the NH2 terminus of the extracellular domain of the receptor to form an “affinity trap.” This initial high-affinity binding greatly increases the local concentration of the ligand at the receptor complex, which facilitates the delivery of the NH2 terminus of the ligand to its typically low-affinity binding site as part of the juxtamembrane region of the receptor. This secondary binding event allows for receptor activation (183). This model is strengthened by the observation that truncated CGRP peptides (CGRP8–37, for example) are capable of binding the receptor but not activating it, and therefore act as competitive antagonists. It is thought that the small molecule antagonists work in a different manner by blocking access to the peptide-binding cleft at the interface between RAMP1 and CLR, preventing the initial ligand capture event, thereby explaining their higher antagonistic potency compared with the peptide class of antagonists (392).
While molecular determinants of both agonist and antagonist binding to the CGRP receptor have been identified, it has been difficult to determine the crystal structure of this family of receptors. Early ab initio modeling of RAMP1 predicted a structure composed of three α-helices (362). Kusano et al. (226) developed this prediction by scrutinizing the soluble extracellular domains of the RAMP1 molecule and confirmed that the structure was trihelical. As previously mentioned, Trp74 is an important residue as part of this structure and forms part of a hydrophobic cleft that exists between the CLR and RAMP1 that is essential for forming a ligand-binding domain. Alongside Arg67, Asp71, and Glu78, Trp74 was found to exist within the structure of the second α-helix of RAMP1 whilst the aforementioned Trp84 was found to exist on the loop adjoining helix two and three, with its side-chain oriented in the same direction as Trp74, towards the solvent-exposed side of the structure.
However, these isolated resolutions of the RAMP1 extracellular domain shed no light on how CLR and RAMP1 complex together, and this is clearly critical for function. To address this issue, Koth et al. (223) created a construct consisting of both extracellular regions of RAMP1 and CLR to study drug interactions with the shared ligand-binding domain between both molecules. This construct was validated by its ability to compete with wild-type CGRP receptors for CGRP in SK-N-MC cells. However, the construct displayed a lower affinity for CGRP than the full-length receptor (IC50 = 12 μM) but was shown to retain high-affinity binding for each of the small molecule antagonists (223). Resolution of the crystal structure of the extracellular domain in the liganded and unliganded state may pave the way for rational drug design. Whilst difficulties do exist in resolving complex GPCR crystal structures, only with the full structure will we be able to gain a full understanding of how CGRP receptors interact with their endogenous ligands and exogenous drug molecules.
F. Intracellular Signaling Pathways
RAMP1 binding 1:1 to CLR causes the relocation of CLR to the plasma membrane, but the RAMP also functions to modulate glycosylation state and influence ligand binding of CGRP and antagonist, as discussed above. In addition, CLR:RAMP interactions are essential for conformational changes following ligand binding and coupling to intracellular signaling pathways. The synthesis of the common marker of CGRP receptor activation, cAMP, is increased following Gαs-dependent stimulation of adenylate cyclase. This is directly linked with CGRP's effect on the vasculature (see Ref. 41), especially in vascular smooth muscle (224) and in neuronal cells (e.g., DRG; Ref. 13), as well as in a wide range of other cells including lymphocytes (278). Evidence soon became available that CGRP-induced increases in cAMP led to activation of protein kinase A and in some cases the opening of ATP-sensitive K+ channels, which is now considered an important pathway leading to vasodilatation (303). Other K+ channels have also been suggested to be involved, such as large-conductance Ca2+-activated K+ channels in pial arteries (188), with little evidence of other calcium-dependent pathways. CGRP has also been shown to mediate endothelial-dependent vasodilatation, and this also involves cAMP (41). Indeed, CGRP may have a positive influence on the nitric oxide pathway as it protects against its loss in a model of hypertension (368).
CGRP did not affect intracellular calcium concentrations in the DRG, but increased cAMP that led to phosphorylation of the cAMP response element binding (CREB) protein, via a PKA-dependent pathway, indicating that CGRP is able to affect gene transcription (13). In lymphocyte cell lines, McGillis et al. (279) have shown that inhibition of PKA blocked c-fos induction by CGRP, via a mechanism that involved nuclear AP-1 binding. This again demonstrates that CGRP can influence gene expression via this pathway (279). This has implications for pain sensitization, as discussed later.
CGRP has been shown to activate the mitogen-activated protein kinases (MAPKs), which are phosphorylated in some tissues following CGRP activation. This can lead to the proliferation of gingival fibroblasts (215), via PKA-dependent and -independent pathways (214). CGRP can protect cultured vascular smooth muscle cells from oxidative stress-induced apoptosis by a signaling pathway involving activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 MAPKs (354). This protective effect of CGRP was also observed in response to the effect of CGRP on ANG II-induced proliferation of rat vascular smooth muscle cells (VSMC) (331). In neuronal cells morphine tolerance involves CGRP, which acts via ERK and p38 signaling. This occurs via CGRP regulating the activity of neuronal calcium/calmodulin-dependent protein kinase II (CaMKII)-CREB, microglial p38-NFκB and astroglial ERK-STAT1/3 pathways (427). Additionally, CGRP increases the transcription of a large range of MAPK reporter genes in trigeminal ganglion cells, enriched with glia, as determined by microarray analysis (411).
CGRP intracellular signaling is a complex area, for which more work is required. While there is a common intracellular signaling pathway involving Gαs cAMP, PKA and CREB, as cited throughout this review, a range of downstream signaling factors and also other pathways (involving Gαi/a, Gαq/11, and β-arrestin signaling) have been reported (Figure 2). These have been implicated, usually in a cell-specific manner, and have been comprehensively reviewed elsewhere (416).
V. PHYSIOLOGICAL ACTIONS OF CGRP
A. Vasodilator Activities of CGRP
CGRP as a microvascular vasodilator has a potency that is ∼10-fold higher than the most potent prostaglandins and 10–100 times greater than other vasodilators such as ACh and SP. This makes CGRP the most potent microvascular vasodilator currently known (45). Injections of femtomole amounts of CGRP cause reddening due to increased blood flow in the cutaneous microcirculation (45). As well as having high potency, the vasodilatory effects of CGRP appear to be more enduring than other vasodilators. When injected into human skin, picomole amounts of CGRP result in an erythema lasting for 5–6 h (42). The action of CGRP as a selective potent dilator is seen in cerebral, coronary, and kidney vascular beds (41); this vasodilator activity is substantially inhibited by selective CGRP receptor antagonists (e.g., Ref. 388). The cardiovascular activity of CGRP has previously been reviewed extensively (28, 41, 86, 267, 329, 367, 430, 435).
Due to its high potency, the vasodilator effect of CGRP has been studied intensely in vivo since its discovery. When CGRP was administered systemically, it reduced the BP in both normotensive and hypertensive rats (132, 198, 435). The mechanisms behind this are thought to involve both NO-dependent and -independent dilatatory pathways in peripheral arterial vessels (198). CGRP has also been given systemically to humans, both healthy volunteers and cardiovascular patients, and is seen to act as a vasodilator (135, 329). Positive inotropic and chronotropic responses in the heart, in addition to vasodilator effects, are observed after intravenous CGRP administration (14, 134). This is thought to be an attempt to combat the hypotension and is believed to be mediated via both direct actions on the cardiac muscle and reflex sympathetic nervous activity (14). However, when CGRP is injected directly into the ventricular system of the brain, it actually causes a hypertensive response. This was shown to be through selective activation of noradrenergic sympathetic nerves (123). Yet it appears that despite these actions, CGRP does not play a crucial role in the physiological control of systemic BP in normal individuals. Overwhelming data from CGRP antagonists appears to confirm that antagonism of CGRP receptors does not particularly affect BP. CGRP receptor antagonists have been tested in a number of species including rats (19, 451) and humans (311, 321) and have not affected resting heart rate or BP. This is favorable for drug discovery programs as these antagonists have been found to be effective as acute treatments for migraine (discussed in a later section).
Several pathways are thought to be involved in CGRP-dependent vasorelaxation, including endothelium-dependent and -independent pathways (41). The endothelium- and nitric oxide (NO)-independent pathway is the most commonly observed pathway. A rise in cAMP is seen after administration of CGRP, and relaxation can occur in the absence of the endothelium, suggesting that CGRP directly stimulates adenylate cyclase (AC) in smooth muscle cells to trigger cAMP production (Figure 3 and as discussed in the cell signaling section). CGRP increases cAMP in human endothelial and VSMC, but is unable to stimulate cGMP directly in either, even at high CGRP doses (81). CGRP is also active in endothelium-denuded arteries in cat brain vessels (107) and in human intracranial arteries (108). The subsequent activation of protein kinase A by cAMP can lead to the phosphorylation and opening of ATP-sensitive potassium (K+) channels, resulting in relaxation (Figure 3). Glibenclamide, the ATP-sensitive K+ channel blocker, can inhibit this CGRP-induced relaxation via inhibition of arterial smooth muscle hyperpolarization (303).
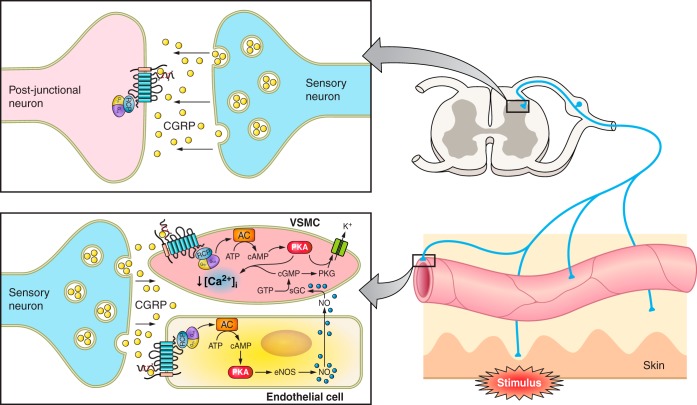
Fig. 3.
A diagram representing the interactions between the sensory nerves, skin, and arterioles. Antidromic sensory nerve stimulation results in an electrical impulse firing towards the spinal cord and axon reflex to the peripheral vasculature, where CGRP is released from the nerve terminals acting on the arterioles, causing vasodilatation. CGRP can mediate this response by: 1) directly activating its receptors on the vascular smooth muscles and mediating relaxation via the Gαs pathway; and 2) activating receptors on endothelial cells to enhance NO production, which can diffuse into the vascular smooth muscles to mediate vasorelaxation via GC activation. CGRP is also released from the central projections of DRG neurons where it may play a role in central sensitization. AC, adenylyl cyclase; eNOS, endothelial nitric oxide synthase; GC, guanylyl cyclase; NO, nitric oxide; PKA, protein kinase A; PKG, protein kinase G; VSMC, vascular smooth muscle cell.
The endothelium-dependent relaxation pathway can also be stimulated by CGRP. CGRP acts via an endothelial receptor, resulting in a rise in cAMP and production of NO. Diffusion of NO into the smooth muscle cell causes activation of guanylate cyclase, leading to the production of cGMP (Figure 3), and thus, ultimately relaxation (155).
The bicyclic peptide endothelin-1 (ET-1) is involved in pulmonary hypertension and a strong link has been discovered between this contractile peptide and CGRP. Coexpression has been observed of the ET receptors with CGRP in perivascular sensory motor nerves (41, 282). ET-1-induced arterial contractions are sensitive to relaxation by CGRP, and this effect is independent of NO, cyclic nucleotides, and K+ channels (282). A recent ligand binding and inhibitor study in isolated rat mesenteric resistance arteries provided evidence for reversal of the effect of ET-1 to be mediated via the G protein βγ subunits (283).
B. CGRP: Measurement in Plasma and Potential Significance for Cardiovascular Regulation
The perivascular location of neuronal CGRP makes it perfectly situated to mediate potent vascular effects at a local level, with little reaching plasma. However, there are physiological/pathophysiological situations where circulating CGRP levels are elevated, of which pregnancy is a notable physiological situation (373). It is thought that inappropriate CGRP release is key to the facial reddening in blushing syndromes; postmenopausal women with facial flushing have elevated CGRP plasma levels compared with premenopausal women (157, 168, 438). There are also flushing episodes associated with medullary thyroid carcinoma, and this is possibly related to the raised CGRP plasma concentrations found in these patients (42). Pathological situations where plasma CGRP levels are raised include kidney dialysis (307), where CGRP is possibly acting as a defense mechanism (306), and myocardial infarction (262), where a protective role is also assumed.
In humans, CGRP has been shown to possess a circadian rhythm, with higher amounts released into the plasma at night. This rhythm was retained in hypertensives, although the levels measured were lower (324). The question of whether CGRP is released in response to BP increases was answered at an early stage. It was discovered that infusion of the pressor agent ANG II to normotensive humans caused dose-dependent increases in plasma CGRP levels, in parallel with an increase in BP induced by ANG II (325). This provides evidence that CGRP is released as a response to the acute increase in BP in humans. This study also revealed that plasma CGRP levels are altered as part of the response to postural changes, providing further evidence for the release of CGRP in cardiovascular regulation, although not whether the release is functionally important (325). It has also been seen that circulating levels of CGRP progressively rise during exercise in both normotensive and hypertensive patients, but no significant differences were seen between patient groups. (247). Plasma CGRP concentrations have also been found to be nonsignificantly raised in a small study where elevated arterial BP was observed in patients with secondary hypertension (272). Interestingly, these levels were reduced after treatment emphasizing the possible compensatory nature of the release of CGRP in response to high BP.
It may appear from the above studies that the concentration of CGRP in the plasma is modified as a normal physiological response to vasomotor changes, and CGRP may potentially play a role in regulating peripheral vascular tone and BP in humans. However, many studies show reduced or unchanged levels of CGRP in the plasma in patients with essential hypertension compared with normotensive controls (106, 235, 324, 356, 390, 428). The reasons behind these findings are of interest: it is presumed that CGRP release is enhanced early in the pathogenesis of hypertension, in order for it to act in either a compensatory or protective manner. The differences seen in plasma CGRP levels in these studies may be due in part to variations in treatment and sampling methods, the metabolism, or the nature of the hypertension in the patients studied (86, 367). On the other hand, knowing that a wide range of receptors exist on sensory nerve terminals, it is possible that neuronal activation and neuropeptide release becomes attenuated through such mechanisms. Examples involving NGF, anandamide, and α2 adrenoceptors are discussed in the following sections.
Studies with CGRP antagonists have revealed that injections of either the peptide receptor antagonist CGRP8–37, or the non-peptide antagonists BIBN4096BS and telcagepant, had no effect on baseline heart rate or systemic BP in humans, as mentioned above (19, 90, 311). It may be that the fundamental vasodilator properties of CGRP at the microvascular level have to be diminished or antagonized for some time, before any functional consequences are observed. Alternatively, it is possible that a number of classic vasodilator mediators (e.g., NO and PGs) can potentially operate at the resistance vessel level, leading to redundancy of CGRP. This includes the coreleased SP, which has been shown to mediate vasodilator properties in the microvascular bed of the mouse following capsaicin application (154). In this study both CGRP and SP had to be blocked before a substantial loss of peripheral neurogenic vasodilatation was seen. However, in human skin it has been suggested that CGRP acts alone following capsaicin application (410).
There have been some key studies of the cardiovascular safety of CGRP antagonists performed. As will be discussed later, one of the main advantages of the CGRP antagonists is their inability to affect coronary arteries and BP compared with the currently used 5-HT1B/1D receptor agonist class of triptans for acute migraine treatment. The Merck CGRP antagonist telcagepant does not contract or relax human coronary vessels, unlike zolmitriptan, and does not cause BP increases in migraine patients, unlike sumatriptan (66, 90). However, overall studies in humans have been limited in terms of defining the influence and role of CGRP in patients with ongoing cardiovascular disease. To date, to our knowledge, CGRP antagonists have not been examined in hypertensive patients.
Interestingly, one genetic study has shown that the CALC I gene that encodes CGRP and calcitonin is associated with a polymorphism that is linked to essential hypertension susceptibility. In a study of more than 400 hypertensive and normotensive individuals, it was found that those with at least one C allele at CALC I T-692C possessed an increased hypertensive risk (259). However, this was a small study, and the functional significance of this polymorphism is not yet known.
C. The CGRP Knockout Mice and Baseline Hemodynamics
Genetically modified mice are a powerful tool to examine the effect of life-long αCGRP deletion. Several different αCGRP knockout (KO) strains of mice have been generated, and differing results have been obtained dependent on the KO studied. The original αCGRP KO mouse generated by Lu et al. (250) exhibited no difference in BP at baseline, after exercise, or post phenylephrine infusion compared with wild-type (WT) (250). In contrast, Gangula et al. (133) and Li et al. (244) saw an increase in basal BP and renin-angiotensin activity in αCGRP KO hypertensive mice that also had a combined deletion of calcitonin. Thus it seems likely taking the evidence from the CGRP antagonist and human studies that it is this additional deletion of calcitonin that led to the change in baseline cardiovascular parameters and not the lack of CGRP. However, a different group using an αCGRP KO mouse raised on a 129/Sv × C57BL/6 hybrid genetic background presented data showing the KO mice to have increased mean arterial pressure and heart rate (225, 309). They suggested this was due to increased sympathetic nervous activity when lacking αCGRP, as the increased mean arterial pressure in the KOs could be inhibited by the α-adrenergic antagonist, prazosin (309). The extent of the differing results with the αCGRP KO mouse strains is surprising. As CGRP does not appear to play a role in the regulation of healthy BP in humans, it would appear that the most suitable CGRP KO mouse is one that does not show baseline cardiovascular differences and involves deletion of CGRP only, without affecting the calcitonin gene (250, 367). Overall though, as now discussed, evidence from rodent studies suggests that the functional consequences of a reduction or blockade of CGRP on the cardiovascular system only become pathologically important in the compromised vasculature.
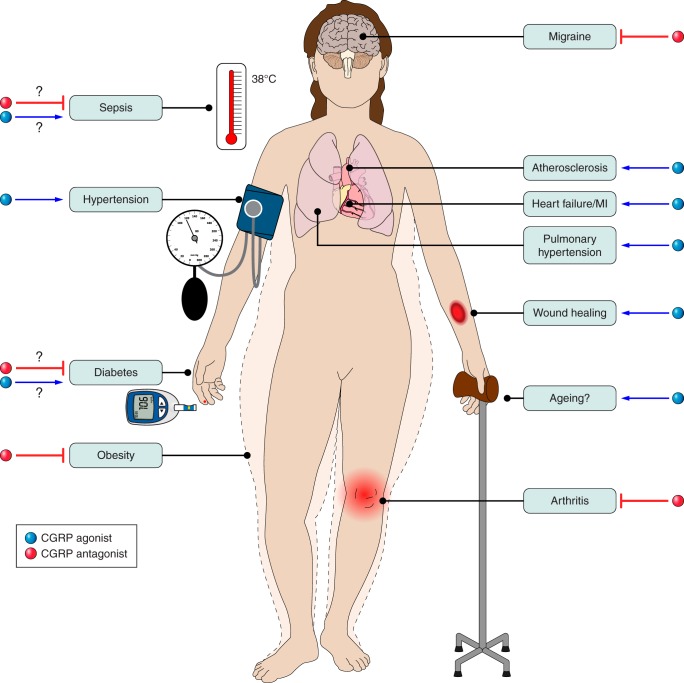
VI. INVOLVEMENT IN CARDIOVASCULAR DISEASE
A. CGRP and Hypertension
Experimental animal models of hypertension are being utilized to examine the role that CGRP plays under pathophysiological conditions. Increasing evidence suggests that CGRP activity is important in resisting the onset of hypertension and vascular dysfunction (see also reviews in Refs. 41, 86, 367 and Figure 4). Importantly, there is evidence that the role of CGRP depends on the experimental model chosen, as discussed in reviews on the function of CGRP in the regulation of blood pressure and hypertension (86, 367). Specifically, Deng and Li (86) discussed how CGRP responds with increased synthesis and release, considered as a compensatory and/or protective mechanism, in some models involving the kidney (e.g., the DOCA, the two-kidney, one-clip, and in a phenol model involving intrarenal injection). They compared this with the reduced CGRP activity that correlated with the worsened cardiovascular phenotype that is observed in the SHR and in the CGRP KO mice (86).
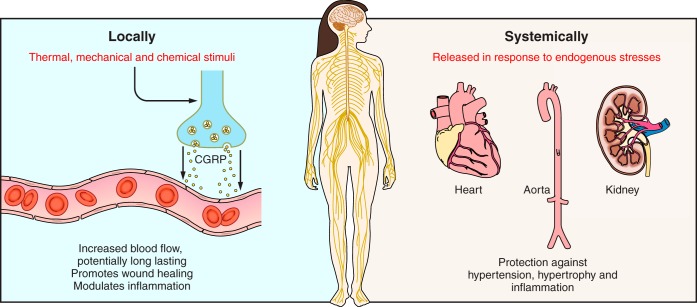
Fig. 4.
Local and systemic mechanisms involving CGRP in cardiovascular regulation. Locally (e.g., in skin), CGRP is released from the peripheral sensory nerve endings (left). CGRP acts to increase blood flow, in a long-acting manner, which can lead to involvement in neurogenic inflammation and as a regulatory factor in inflammation. These effects can contribute to enhanced wound healing. Systemically, CGRP is not considered to have a major role in the normal individual. However, animal studies imply that CGRP may delay or protect against cardiovascular disease (right). This leads to protection against hypertension, hypertrophy, and inflammation and may be via direct mechanisms, or indirectly as a consequence of vasodilator activity.
1. Models involving the primary induction of hypertension
CGRP activity and function has been antagonized in experimental models, through the use of CGRP8–37. This results in an exaggerated cardiovascular response during the onset of hypertension. These models include salt-induced hypertension (37, 385, 425) and l-NAME-induced hypertension (131). In a model of subtotal nephrectomy hypertension, hypertensive Sprague-Dawley rats showed an additional elevation in mean arterial pressure when given CGRP8–37 (384). However, the mechanisms involved remain unclear. CGRP dose-dependently lowered mean arterial pressure and increased the heart rate in Wistar rats and was able to significantly attenuate an ANG II-induced pressor response. This is in keeping with the concept that ANG II and CGRP work in tandem, to influence vascular tone and thus blood pressure, and support the results from human studies discussed above (198). A later study involving ANG II showed that ANG II infusion over 10 days in Wistar rats caused increased CLR and RAMP1 mRNA expression in the mesenteric vessels, which was pressure-dependent (239), although this group has also shown that ANG II given over 2 wk by osmotic mini-pump does not influence DRG CGRP mRNA (379). These studies provide evidence for the potential pressure-dependent regulation of the receptor components, in addition to stimulatory effects on peptide release alone. Thus collectively it may be said that there is a “compensatory” stimulation of the vasodilator activity of the CGRP system in hypertension.
2. The influence of CGRP on important vascular beds
The microvasculature and in particular the resistance arteries play an important role in regulating blood pressure and tissue blood flow (299), and an increase in total peripheral vascular resistance is a factor contributing to hypertension (285). Generally, the signaling systems involved in the release of CGRP in the microvasculature remain unclear. Early studies proposed that CGRP released from sensory nerves played an important role in the rat mesentery via an interaction with sympathetic nerves (212). Interestingly, under conditions of both raised angiotensin levels and noradrenergic stimulation, mesenteric CGRP release and activity was reduced (210, 213). Thus there appears to be an intimate relationship between known pressor agents and release of CGRP in the mesentery (167). Indeed, it is established that a wide range of receptors are localized to sensory nerves where they have the potential to influence CGRP release, that extend beyond those discussed here, and of which the best known are opioid receptors (40).
A recent study by Smillie et al. (368), involving the CGRP knockouts in the ANG II model has shown that CGRP deletion not only leads to increased hypertension, but enhances loss of eNOS. In addition, there is evidence of oxidative stress in the aorta, whilst NO-dependent vasodilatation within the mesenteric vessels is not affected (368).
3. CGRP and the sympathetic system
A subpressor dose of CGRP can counteract the effect of norepinephrine as well as ANG II, in short-term 6-day studies, leading to the suggestion that CGRP acts via an effect on peripheral resistance (127). Kawasaki et al. (210) showed that the sympathetic nervous system signals to the sensory CGRP system via α2 adrenergic receptors. The α2 receptor has been suggested to mediate the inhibition of NGF-stimulated CGRP release in the DRG (380). This has been further investigated in a phenol model of hypertension, where intrarenal injection of phenol is associated with hypertension, decreased sensory nerve activation, and, in turn, reduced CGRP. While an α1 receptor antagonist was found to reduce the hypertension, only an α2 receptor antagonist was able to restore CGRP levels (via a mechanism involving NGF), but the α2 antagonist was not able to reduce BP (86). This suggests that even in the hypertensive state when CGRP levels are raised sufficiently to reach the plasma, CGRP does not influence BP regulation in this hypertensive model. The authors suggested that this may be due to α2 receptors acting also to influence sympathetic tone, increasing the pressor component. The loss of CGRP following phenol administration has been suggested to mimic a remodeling process that may occur during hypertension, which can be rescued by NGF (167).
4. Role of CGRP in hypertension models where the kidney plays a central role
The role of CGRP has also been investigated in the mineralcorticoid (DOCA)-salt induced hypertension model. Here CGRP mRNA and protein levels were shown to be increased for 3–4 wk following hypertension induction and suggested to be raised to compensate for the DOCA-salt administration (379). CGRP8–37 improved the hypertensive state, leading the authors to suggest that CGRP plays a compensatory vasodilator role in this model, as mentioned above (385).
A study by Supowit et al. (383) in mice attempted to identify the role of αCGRP in hypertension by uninephrectomy, DOCA-salt administration, and 0.9% saline drinking water in WT and calcitonin/αCGRP KO mice. This treatment produced a significant 35% mean arterial pressure increase in both WT and KO mice. Heart and kidney analysis from the hypertensive WT mice showed no pathological changes compared with their normotensive controls. However, thickening and inflammation of the vessel walls was observed in the hearts of hypertensive KO mice. In addition to this, the kidneys of these KO mice exhibited glomerular changes. Thus deletion of calcitonin/αCGRP makes the heart and kidneys more vulnerable to hypertension-induced end organ damage, indicating a protective role for CGRP in these organs (383). In a later study by the same group, the role of αCGRP was investigated in DOCA-salt hypertension-induced renal damage with the use of the calcitonin/αCGRP KO mouse. Although these mice had an increased BP at basal levels, there were no morphological or functional changes in the kidney under normal conditions. Vascular cell adhesion molecule (VCAM)-1, monocyte chemoattractant protein (MCP)-1, and intracellular adhesion molecule (ICAM)-1 expression in the kidney was measured by immunohistochemistry in WT and calcitonin/αCGRP KO mice after either 14 or 21 days DOCA-salt treatment. At baseline there was no difference in expression of these markers between the genotypes. These markers were elevated at the onset of hypertension at day 14 and were progressively more elevated at day 21. The CGRP KOs demonstrated increased levels of these inflammatory markers compared with the WTs (37). However, it was concluded that it was not possible based on these findings to elucidate whether the changes observed were dependent on an increase in BP, or were intrinsically αCGRP dependent (37). More recently, this group has again tried to elucidate the mechanistic role of αCGRP in the DOCA-salt hypertension model using the same calcitonin/αCGRP KO mice, which display a higher basal mean arterial pressure compared with matched WTs. In this study they added an additional group of KOs that were pretreated with 0.025% hydralazine in drinking water, which reduced their mean arterial pressure to a value comparable to the WTs. Consequently, when treated with DOCA-salt, the final mean arterial pressure of the KOs did not differ from the matched hypertensive WTs. However, urinary markers of oxidative stress and renal inflammatory markers were higher in the KOs than WTs, although there were no renal histological differences. It was concluded that the renal protective effect of αCGRP in DOCA-salt hypertension has a BP-independent protective component (237). These studies provided evidence that the long-term lack of CGRP when calcitonin is also deleted can affect the cardiovascular balance, leading to enhanced hypertension and an increased severity in terms of heart and kidney damage.
In the two-kidney, one-clip renovascular hypertension model in the rat, BP was significantly elevated 10 days post operation, and treatment with capsaicin at a dose chosen to deplete all the neurotransmitters within the sensory nerves, caused the BP to elevate further. Mesenteric mRNA CGRP expression was significantly increased in control rats, but this was prevented with capsaicin pretreatment (88). Thus it was suggested that in this model the increased production of CGRP acts as a compensatory dilatory mechanism to counteract the increase in BP. The mechanisms involved are still relatively unclear; however, ANG II is considered to play a key role in this model and thus may be the link (88).
5. Role of CGRP in hypertension in a model involving pregnancy
Alternatively, a hormone-related mechanism has been proposed for the role of CGRP in hypertension. Gangula et al. (132) reported that CGRP reversed hypertension induced by NO inhibition in pregnant rats through a possible progesterone-dependent mechanism. In a later study, they showed that CGRP induced a dose-dependent decrease in mean arterial pressure. This response was significantly greater in pregnant rats compared with nonpregnant rats, indicating that the decrease in vascular tone observed during pregnancy may be mediated in part by a sex steroid hormone-induced increase in the vascular sensitivity to CGRP (132).
6. Role of CGRP in the SHR model
The SHR model is a commonly used model of essential hypertension, usually involving the Wistar-Kyoto rat strain, inbred to be hypertensive. The comparative effect of CGRP has been investigated in a range of vascular beds in both normal and SHR Wistar-Kyoto rats using a microsphere technique. Results showed that CGRP similarly reduced BP in both normotensive and hypertensive rats, and this was due to a change in total peripheral vascular resistance and apparently not influenced by the higher sympathetic tone in the SHR group (14). These authors noted the ability of CGRP to specifically dilate the cutaneous and gastric beds and linked this to its pathophysiological potential. On the other hand, a reduction in blood flow was observed in the cerebral circulation (14).
NGF has also been suggested to influence CGRP release and hypotensive potential at some time points in the SHR (384). Supowit et al. (382) have shown that neuronal CGRP gene expression is decreased at the DRG level in the SHR. Moreover, the sensory neuronal contribution of CGRP in the mesentery is substantially reduced in SHR compared with WT and suggested to be lacking and linked to ageing in the SHR rat (210). This was reversed by prolonged (7 wk) treatment with captopril, an angiotensin-converting enzyme inhibitor (211). Kawasaki et al. (213) showed that ANG II, generated at the mesenteric vascular wall, causes an inhibition of sensory neurogenic CGRP induced vasodilatation and suggested that ANG II receptors are found on the sensory neuron in the mesentery, as mentioned earlier. Importantly, this response was only observed in the SHR model, suggesting that a form of sensory nerve remodeling may be occurring (213). Interestingly, the long-term treatment of SHR for 7 wk with calcium antagonists (amlodipine and nicardipine) has led to the suggestion that these antagonists have the ability to reduce noradrenergic constrictor tone, whilst not affecting the sensory CGRP function, thus leading to a total beneficial effect (301).
7. Modulation of the CGRP receptor to study CGRP in hypertension
An alternate approach to antagonists to examine the function of CGRP is to carry out experiments on mice with genetically modified receptor components. The G protein-coupled CLR component is a common receptor component for all the CGRP family peptides and, as a consequence, is not ideal for the specific study of the action of CGRP. On the other hand, the single transmembrane RAMP1 component constitutes the major receptor for CGRP. RAMP1-deficient mice have been found to exhibit increased BP compared with WT mice. αCGRP administration caused potent relaxation of the arteries in WT mice and suppressed the production of proinflammatory cytokines in dendritic cells stimulated by lipopolysaccharide (LPS) (402). These suppressor mechanisms were absent in RAMP1-deficient mice, indicating that CGRP via the CLR/RAMP1 receptor plays a vital role in BP regulation and suppression of inflammatory mediator generation (402). RAMP1 upregulation has also been shown to enhance the antihypertensive actions of endogenous agonists (considered to be primarily CGRP) in RAMP1 transgenic mice chronically infused with ANG II (345). Interestingly, although the current evidence supports the belief that the antihypertensive actions of CGRP are due to its role as a vasodilator, Sabharwal et al. (345) investigated other mechanisms. They provided evidence for a role of CGRP in the modulation of baroreflex sensitivity (345). The baroreflex acts to buffer BP fluctuations by responding to reflex changes in heart rate and vascular resistance. High baroreflex sensitivity leads to a lower BP, whereas low baroreflex sensitivity results in an increase in mean arterial pressure and thus, consequently, increases the cardiovascular risk (391). Sabharwal et al. (345) observed that RAMP1 transgenic mice had increased baroreflex sensitivity; therefore, CGRP may be protecting against the onset of hypertension through its involvement in this baroreflex response.
8. Mechanisms involving the release of CGRP
It is perhaps not surprising that studies have also involved a search for a role for TRPV1 in hypertension due to its CGRP-releasing ability (reviewed in Ref. 419). A study using electrophysiological and immunohistochemical techniques provided evidence for a distinct population of CGRP-positive sensory nerves in the kidney that are thought to play a role in renal pathophysiology, as they were found to be more sensitive to TRPV1-mediated acid stimulation (94). Also, in a rat model of hypertension, the cannabinoid TRPV1 agonist anandamide regulated the generation and activity of CGRP with the limiting factor being the anandamide transporter (235). The TRPV1 agonist rutaecarpine has been shown to possess an antihypertensive effect due to increased neuronal CGRP release into the circulation in SHRs, two-kidney one-clip (2K1C), and renal phenol injury models of hypertension (87, 236, 332). CGRP released by rutaecarpine treatment has also been shown to reduce platelet aggregation through inhibition of platelet-derived tissue factor (236); in addition, rutaecarpine may also have NO inhibitory effects (418). The consumption of dietary capsaicin has been suggested to be beneficial in lowering BP and improving endothelial function due to increased vasodilatation by CGRP (163, 442). Therefore, TRPV1 agonists, such as capsaicin and rutaecarpine, provide evidence that CGRP released via the TRPV1 pathway may be protective and important for the treatment of hypertension (163, 442). However, it should be remembered that TRPV1 also mediates pain; thus the difference between activation of sensory nerves to cause pain, compared with CGRP-dependent vasodilatation, is of interest.
Alternatively, it has been suggested that the anandamide transporter may regulate the production of CGRP in hypertension, and this is deficient in hypertensive conditions where CGRP is lacking (235). Anandamide is a TRPV1 agonist, and lack of anandamide transportation reduces the activation of TRPV1, leading to a loss of CGRP release (235). A later publication has shown that CGRP levels recover following treatment of the SHR with an ANG II type 1 receptor antagonist. ANG II mediates a decrease in the anandamide transporter activity which in turn decreases CGRP via a reactive oxygen species (ROS)-dependent mechanism (361). However, rodent data also show that TRPV1 KO mice do not have the predicted hypertensive phenotype that would be expected if this were so (268).
B. CGRP and Pulmonary Hypertension
The investigation of CGRP in pulmonary hypertension is important as the current pharmacological agents used to treat pulmonary hypertension have unwanted side effects and limited efficacy (82, 217). αCGRP is abundant in the lung (298), with CGRP-like immunoreactivity localized in the nerve fibers of airway mucosa (56) and the airway epithelium (398). CGRP dilates precontracted pulmonary arteries in vitro (274), and CGRP protects against hypoxia-induced tissue remodeling in human pulmonary hypertension (398). Circulating plasma levels of CGRP are reduced in rats with pulmonary hypertension (216), and effects of αCGRP infusion into these pulmonary hypertensive rats can be prevented/partially reversed in a time-dependent manner and exacerbated by CGRP8-37 (216, 217, 398). Adenovirus-mediated transfer of CGRP into the lungs of mice prior to exposure of chronic hypoxia protected against pulmonary resistance via cAMP induced vasodilatation, in addition to the suppression of ET-1 and ANG II release. This in turn resulted in reduced pulmonary vascular resistance and subsequent remodeling compared with controls (64). It has also been suggested that degeneration of capsaicin-sensitive nerves enhances pulmonary hypertension due to the loss of CGRP being released via TRPV1 activation, as reviewed (408). More recently, Li et al. (242) induced pulmonary hypertension in rats by hypoxia and then depleted endogenous CGRP via capsaicin. This resulted in exacerbated hypoxia-induced pulmonary hypertension characterized by raised systolic pressure, vascular hypertrophy, and increased ERK1/2 expression. Exogenous application of CGRP reversed this pulmonary arterial remodeling by inhibiting the ERK1/2 pathway; therefore, this study suggests CGRP is protective via inhibition of the ERK1/2 pathway (242). Injection of endothelial progenitor cells (EPC), genetically altered to express CGRP, attenuated established pulmonary hypertension and subsequent pulmonary vascular remodeling in pulmonary hypertensive rats (457). This study suggests that therapy with CGRP-overexpressing EPCs may be successful in the treatment of pulmonary hypertension diseases in the future. Prior to this, Deng et al. (89) also proposed CGRP supplementation to be a novel therapy for conditions such as pulmonary hypertension, as genetically engineered marrow stromal cells that secrete CGRP inhibit VSMC proliferation in vitro.
C. CGRP and Heart Failure
CGRP-immunoreactive fibers have been reported to innervate the heart vasculature, in a diffuse, but comprehensive manner throughout the myocardium and coronary vessels (197), thus are positioned to act in a sensory-efferent manner. A cardioprotective role for CGRP has been suggested, but as for hypertension, knowledge of the exact mechanisms involved is limited. Although the best-characterized effects of CGRP have been studied within the context of the vasculature, there is undoubtedly a role for CGRP in maintaining cardiac homeostasis. Prior to the functional characterization of CGRP in cardiovascular tissues, it was known that CGRP-containing nerve fibers were present in the heart, with particularly dense innervation around the coronary arteries, papillary muscle, and also excitatory regions, such as the sinoatrial and atrioventricular nodes (297). Other experiments investigating the effect of capsaicin on the spontaneously beating guinea pig auricle showed that application of the TRPV1 agonist resulted in a positive chronotropic and inotropic response from the atrial tissue (256), and the transmitter responsible was identified as CGRP (255).
CGRP has been suggested to have a role in protection against heart failure (see Ref. 41). This has been confirmed in an isoprenaline-induced model of cardiac failure, where CGRP release (by the TRPV1 agonist rutaecarpine) was found to protect against hypertrophy (240). Increased levels of CGRP after heart failure are observed in genetically predisposed individuals (191), and infusion of CGRP improves the circulation in heart disease (103, 136, 374). The mechanisms involved have been suggested, as for hypertension, to include vasodilatation and generation of protective mediators. A recent study has used a transverse aortic constriction model in the calcitonin/αCGRP KO mice to probe mechanisms. The WT mice had raised levels of CGRP, and the KO mice had lower survival rates. KOs exhibited a worsened phenotype in terms of cardiac remodeling, left ventricular mass, and fibrosis. It was suggested that CGRP protects against inflammation, measured in terms of macrophage accumulation, TNF-α generation, cell death, and interstitial and perivascular fibrosis (237). CGRP is a powerful proangiogenic growth factor in human umbilical vein endothelial cells (HUVECs) (403), and endogenous CGRP has been shown to play a role in promoting tumor-associated angiogenesis and tumor growth (400). Of note, CGRP appeared to protect against cell death, with the WT hearts favoring angiogenesis whilst an increased loss of cardiac myocytes at sites of collagen staining was observed in the KOs, leading to the suggestion that CGRP is an important angiogenic factor for microvascular growth (238). However, there was evidence by 21 days after initiation of the transverse aortic constriction model that the heart content of CGRP was not being sustained (238). This is in keeping with lower CGRP levels found in the later stages of human heart failure (103).
CGRP is able to activate p38 and ERK1/2 kinase, both of which are involved in protective pathways against apoptosis (354). Pretreatment with CGRP markedly inhibits apoptosis and intracellular ROS production of isoprenaline-treated cultured rat cardiomyocytes (240). This protective effect of CGRP is reversed by the addition of a CGRP receptor antagonist (239). Of note, CGRP has recently been shown to inhibit norepinephrine-mediated apoptosis via Bcl-2/Bax in cultured rat cardiomyocytes (260). Also, Al-Rubaiee et al. (6) have attempted to define the mechanisms by which CGRP has positive inotropic effects on the heart; blocking CGRP using CGRP8-37 increased the level of sarcomere shortening in isolated adult rat cardiomyocytes (6).
D. Ischemia
Since the identification of CGRP as a cardiac signaling molecule, it has been shown to be capable of acting as a very potent endogenous mediator of preconditioning; the phenomenon whereby preexposure of the heart to a preconditioning agent can attenuate subsequent damage incurred by an ischemic episode (245). In the Langendorff-perfused rat heart, exogenous CGRP mimics preconditioning induced by transient ischemia via ligation of the left anterior descending coronary artery. Furthermore, application of the small molecule CGRP antagonist BIBN 4096BS inhibited the preconditioning response from either exogenous CGRP or transient ischemia, indicating that CGRP plays an important role in the physiological response to myocardial ischemia (62). CGRP is also protective against ischemia in a range of other organs including gut, kidney, and brain (41). It is considered that ischemic episodes in central organs, such as heart and liver, may not only involve CGRP acting locally, but also protects tissues, such as the gastric mucosa, through the release of CGRP, following axon reflexes, for instance (48).
In humans following acute myocardial infarction, immunoreactive CGRP is raised in plasma, as well as in nerves, implicating its release in response to either the stressed metabolic environment that occurs after ischemia, or to the decreased vasodilatation (262, 329, 340). There is a wealth of evidence that sensory nerves innervate and respond to the ischemic, constricted environment, but some of this is obtained from studies involving capsaicin depletion, where there is a loss of the total sensory neuronal component, rather than just CGRP. Calcitonin/αCGRP KO are reported to have increased susceptibility to ischemia/reperfusion injury. This coincides with increased vascular cell damage and an increase in the measurement of malonaldehyde, indicative of ROS generation, in the KOs (194). Recovery following this ischemia/reperfusion injury is also blunted in the KO mice, a response that may be directly linked to the loss of the vasodilator mechanisms (194). In cultured rodent cardiomyocytes, CGRP inhibits apoptosis of the cells after stimulation by noradrenaline (456), suggesting a protective role for CGRP. The CGRP receptors and their cardioprotective effects of ischemic pre/postconditioning and infarct reduction have recently been reviewed by Maslov et al. (269).
CGRP is also implicated in coronary artery disease (CAD) patients where serum levels were reduced compared with normal healthy individuals (422). Endogenous CGRP is protective in the hearts of pigs in a model of myocardial infarction, although this is not the case with exogenously administered CGRP (205, 206). Of importance, a recent study has investigated the effect of the CGRP antagonist telecagepant on exercise-induced (stable) angina and ischemic ST-segment depression. The antagonist did not significantly affect either the time to onset of angina, or ischemic ST-segment depression. Moreover, the profile for BP and heart rate changes were similar in both placebo and antagonist-treated patients. (63). However, exogenous CGRP can prolong exercise time (407).
The overall clinical weight of CGRP's role in mediating the preconditioning response has still to be evaluated. However, it may be especially relevant in myocardial ischemia suffered by diabetic patients. In rodent experimental models of diabetes, the ischemic preconditioning response is markedly attenuated (252), an effect that can be reversed via adenoviral transfection of the CGRP gene to myocardial tissue (458). In addition, deletion of the TRPV1 gene produces a similar loss of preconditioning in the heart, and thus it was suggested that the beneficial effects of TRPV1 stimulation is through the release of CGRP, and also SP (459). The ability of CGRP to produce a preconditioning effect also appears to be attenuated with the advancement of age (251). As both diabetes and ageing are associated with sensory nerve degeneration, it is conceivable that a loss of myocardial sensory innervation is a key process in increasing susceptibility to myocardial damage following ischemia.
There are further data to support the role of CGRP as a protective factor in ischemia. Diabetic mice are less able to generate cardioprotection via ischemic preconditioning, and this is thought to be due to a loss of CGRP activity. Administration of CGRP into these rodents was shown to improve their cardioprotective ability (458). In addition, sensory CGRP depletion by chronic capsaicin treatment has been reported to induce exacerbated hypoxia-induced hypertension and vascular remodeling in rats (399).
E. CGRP, Atherosclerosis, and Vessel Remodeling
Hypertension and cardiovascular diseases such as atherosclerosis display both increased BP and vascular remodeling and inflammation. During the remodeling, there is an increase in the rate of hypertrophy and proliferation of the VSMC within the vessel wall. Endothelial dysfunction also occurs, and subsequently, NO production is impaired (355). CGRP has been shown to be protective in the onset of cardiovascular disease in a variety of studies that include vessel injury and remodeling, as discussed in previous sections. CGRP and its role in atherosclerotic models are not as widely documented. However, despite this, CGRP has been shown to be protective in several reports involving the inhibition of growth factors, which influence cell proliferation in response to vessel injury, with earlier studies being coherently reviewed by DeFeudis (84). Vessel remodeling/stiffness and plaque formation is associated with age and nerve deterioration. CGRP levels have been reported to decrease with age (see below). In an in vitro atherosclerotic model with ageing rabbits, the effect of CGRP was reduced in a time-dependent manner in the mesentery as the rabbits aged and atherosclerosis progressed (375). More recently, Yang et al. (443) have elucidated a protective role for endogenous CGRP in neointimal hyperplasia following wire-induced vascular injury. This study showed that neointimal formation was significantly enhanced in CGRP KOs compared with their matched WTs, thus suggesting a vasoprotective role for CGRP, making it a potential therapeutic target in cardiovascular disease and vessel remodeling.
Overexpression of RAMP1 in RAMP1 transgenic mice protects against ANG II-induced endothelial dysfunction through the increase of CGRP-induced vasodilatation, shown previously by Chrissobolis et al. (71) when studying vascular responses to CGRP in carotid and basilar arteries in vitro. CGRP stimulation has also been shown to reduce the numbers of migrating neutrophils and monocytes through the endothelium into the LPS-stimulated mouse peritoneal cavity (150). A decrease in chemokine expression was found through the inhibition of the NF-κB pathway by this CGRP stimulation in cultured endothelial cells (193).
EPCs play a critical role in vascular endothelial repair, and a decline in EPC content is thought to predispose subjects to future cardiovascular complications. CGRP synthesis has recently been discovered in EPCs, as mentioned before, and this EPC derived CGRP has been shown to be protective against EPC senescence induced by ANG II (461). To further emphasize the protective importance of EPC derived CGRP, VSMC hypertrophy was attenuated in ANG II-induced hypertension (113). This inhibitory effect of CGRP on VSMC proliferation has also been demonstrated in in vitro studies using the ANG II stimulus (331). Furthermore, when administering CGRP containing EPCs to rats with pulmonary hypertension, a lowered vascular resistance results together with inhibited VSMC proliferation and thickening of the vessel wall (457). In addition to this, in cultured human dermal microvascular endothelial cells, application of CGRP significantly reduces chemokine production, thus reducing the recruitment of monocytes and neutrophils to the vessel wall (193). With regards to the role of CGRP and inflammatory markers, MCP-1 expression was inhibited by CGRP transfection into rabbit isolated jugular vein grafts with chronic inflammatory vein graft disease (454). In addition to this, gene transfer of CGRP into Lewis rats suppressed VCAM-1 mRNA expression during the development of allograft vasculopathy (455). These studies suggest an anti-inflammatory role for CGRP.
Research has found that endogenous CGRP is involved in the depressor effect and regression of vascular remodeling in reno-hypertensive rats (332). Previous findings have also shown that CGRP inhibited the proliferation of VSMCs from rabbit and rat aorta, induced by fetal bovine serum, through an increase in cAMP production (243). Intramuscular gene transfer of CGRP was also shown to inhibit neointimal hyperplasia after balloon injury in the abdominal aorta of the rat through inhibition of VSMC and CGRP-mediated apoptosis (423).
It is well documented that ERK1/2, the important protein kinases in cell proliferation, are activated by many growth factors via different upstream signal proteins. It has been reported that ANG II activates ERK1/2 via PKC-ζ by cross-talk with the tyrosine kinase receptor pathway (246). CGRP was recently shown to inhibit both ANG II-induced and hypoxia-induced proliferation of rat aortic and pulmonary smooth muscle cells via inactivation of ERK1/2, therefore suggesting that the inhibitory effect of CGRP on the proliferation of VSMCs involves the MAPK signaling pathway (242, 331).
The increasing literature therefore provides evidence for a protective role of the CGRP signaling pathway in the onset of vascular inflammation. Manipulation of this specific signaling pathway could prove to be a therapeutic target in the prevention of hypertension induced vascular inflammation; however, the mechanisms in which it plays this role are not well understood. Despite this, it has been shown that statins reduce the expression of CGRP in rat cultured DRG (49), an effect described by the authors to more closely relate CGRP to its inflammatory rather than cardio-protective role.
F. Sepsis
CGRP plasma levels are raised in patients with sepsis (203) and here is suggested to mediate hypotension (17, 195). On the other hand, CGRP may reduce production of some proinflammatory cytokines [e.g., keratinocyte chemoattractant (KC) and macrophage inflammatory protein-2 (MIP-2)] and protect mice from fatal endotoxic shock (150, 424). RAMP-1-deficient mice were shown to exhibit a loss of immunosuppression (204). Moreover, the deletion of TRPV1 is associated with enhanced sepsis in mice (72). A recent study suggests that this worsened inflammatory response is linked to a loss of TRPV1-dependent CGRP release, as plasma levels were reduced in the TRPV1 KO mice (426). Conversely, Fernandes et al. (117) did not find support for this concept in a similar model. Holzmann (187) has recently reviewed this area and suggests that CGRP is released at an early stage following infection. CGRP is then able to act on macrophages and other cells in an anti-inflammatory manner to upregulate IL-10, among other mechanisms. However, it is suggested that in mixed-bacterial infections, CGRP may over compensate resulting in adverse events of immunosuppression and compromised host defense (187).
VII. CGRP AND OTHER PATHOPHYSIOLOGICAL CONDITIONS
A. CGRP in Neurogenic Inflammation and Pain
Sensory nerve fibers are branched in a collateral manner; it is thought that when a peripheral terminal of a sensory nerve is activated, action potentials are not only transmitted to the dorsal horn, but are also transmitted antidromically down the branches. This results in neuropeptide release from the peripheral branches (Figure 3). It is this axon reflex (a reflex in a single neuron) that is thought to account for the flare seen after intradermal injection of histamine into human skin which can extend for several centimeters away from the injection site (234). Neurogenic inflammation is the term given to the microvascular effects caused by the release of neuropeptides from the nerve terminals (Figure 3). The two main proinflammatory neuropeptides are CGRP and substance P (SP). SP acting on NK1 receptors is a potent mediator of increased microvascular permeability (58) and, as extensively discussed, CGRP is an extremely potent vasodilator, thus release of these peptides results in edema formation, increased blood flow, and recruitment of inflammatory cells to the local area.
The role of CGRP in inflammation has been reviewed (39, 118). In general, CGRP has been suggested to mediate a host of immune regulatory responses, relevant to host defense and inflammation, but few have been fully supported by strong subsequent in vivo studies. However, without doubt, depending on situation, CGRP is able to promote inflammation, secondary to vasodilatation, and inhibit inflammation/mediator release through its ability to increase cAMP. Thus, to a certain extent, it depends on when and how CGRP is released as to its net effect (39). There is a strong indication that CGRP has the ability to inhibit lymphocyte differentiation and proliferation (278, 406, 420). CGRP has also been suggested to influence the activity of other inflammatory cells, including Langerhans cells (20, 172) and macrophages (196). CGRP can inhibit IL-1β-induced reactive oxygen species generation in alveolar epithelial cells (241). Overall, it is considered that CGRP can inhibit allergic conditions such as irritant dermatitis (73, 286). CGRP, via increases in cAMP, was able to inhibit ovalbumin-induced airway inflammation in the mouse (287). In keeping with this, CGRP deletion is associated with an inhibition of airway hyperresponsiveness (16).
CGRP has been proposed to influence neutrophil-endothelial cell interactions in a positive manner (142), and also a negative manner (150). The positive effect has been suggested to be secondary to microvascular dilation (51). Monneret et al. (291) have shown that CGRP can inhibit cytokine production from LPS- and fMLP-stimulated blood cells, secondary to increasing cAMP (291). An inhibition of chemokines (CXCL1, CXCL8, and CCL2) from LPS-stimulated cultured human microvascular endothelial cells in vitro was observed (193), and this correlated with the ability of local and systemic CGRP to inhibit monocytes and neutrophils in response to LPS in the mouse peritoneal cavity (150). This inhibition of recruitment may be secondary to systemic hypotension in this model. In vivo, deletion of RAMP1 is associated with hypertension and proinflammatory cytokine production (402), providing further evidence for an inhibitory role of CGRP. In the kidney, CGRP has been shown to be proinflammatory in a model of obstructive nephritis, and this may in part be related to the potentiating vasodilator activity, as inflammatory markers, as well as resulting fibrosis were obvious (220). Thus it would seem sensible to assume that CGRP can act in a pro- or anti- inflammatory role depending on situation.
It has long been known that capsaicin-sensitive neurons are important in neurogenic inflammation, since the hypersensitivity and flare produced from intradermal injection of capsaicin can be prevented by denervation or by prolonged exposure to capsaicin that produces sensory nerve desensitization (334). Furthermore, evidence has shown that intraplantar injection of capsaicin in rats leads to the secretion of proinflammatory cytokines, such as IL-1β, TNF-α, and NGF (344). The functional importance of CGRP in this process is seen by the upregulation of these proinflammatory mediators being prevented by pretreatment with CGRP antagonists (271). In humans, the role of CGRP has been examined in a model of increased dermal blood flow induced by topical application of capsaicin (364). Pretreatment with the CGRP antagonist telcagepant inhibited this increased blood flow after capsaicin, demonstrating a key role for CGRP in neurogenic vasodilatation in humans (364).
Although the potent vasodilatory effects of CGRP cannot be denied, its role in pain is continually debated. Hyperalgesia is the heightened sensitivity to painful stimuli and occurs under conditions that lead to sensitization of peripheral nerve terminals (peripheral sensitization), or sensitization of the spinal cord dorsal horn (central sensitization). Perhaps more focus has been given to the tachykinin neuropeptide SP, acting on the NK1 receptor on the dorsal horn after high-intensity stimuli and contributing to central sensitization (437). Controversies exist as to the importance of CGRP in the generation of hyperalgesia as differing results have been observed between different laboratories and models. Small effects of peripheral CGRP injection have been observed. Injection of CGRP alone induced hyperalgesia in the rat hindpaw, although with a far less potent effect than SP (300), and only injections of high nonphysiologically relevant doses of CGRP caused any changes in the thermal nociceptive threshold in the mouse hindpaw (352). However, the injection of CGRP into human skin is not associated with axon-reflex flare initiation or increased nociception (42, 45, 431). It is possible that CGRP plays more of an important role centrally than peripherally. Mechanical hyperalgesia caused by intrathecal CGRP has been observed, and electrophysiological data show CGRP-induced sensitization of spinal dorsal horn neurons (310, 376). To contradict this view, other groups have provided evidence for intrathecal injection of CGRP resulting in no detectable pain behaviors in mice (129), and no change in response in the tail immersion test or thermal or mechanical thresholds in rats (202, 446).
It may be that the role of CGRP in pain becomes more important under conditions of abnormal pain processing such that occurs during inflammatory or neuropathic pain states. CGRP antagonists inhibited hyperalgesia induced by intraplantar injection of capsaicin and carrageenan in rats and reduced pain in neuropathic models (29, 271, 444, 445). CGRP KO mice have also been subjected to kaolin- and carrageenan-induced inflammation, and these mice did not develop hyperalgesia (452). Mice lacking the essential CGRP receptor component RAMP1 were protected from a complex regional pain syndrome associated with distal limb fracture. Chiefly, allodynia was attenuated, and inflammatory cytokines (except for IL-6) were reduced (156). In addition, electrophysiological studies in rats have shown the importance of CGRP in the hyperexcitability in spinal cord neurons during joint inflammation (304).
Conversely, in our hands, we have seen that CGRP was not important in the development of bilateral hyperalgesia in a model of TNF-α-induced inflammation in the mouse paw (343). Additionally, expression of CGRP mRNA was upregulated bilaterally in trigeminal neurons in a chronic inflammatory rodent model of periodontitis induced by LPS (1). Reduced pain thresholds are not observed in this model, or in patients with periodontitis; thus the authors proposed that the upregulation of CGRP is involved with the inflammatory process and not nociception (1).
The discrepancy in results may be due to the different models, experimental conditions, and the many different ways of measuring pain. However, an intriguing report by Mogil et al. (290) provides evidence that different mouse strains differ widely in their expression of the CGRP gene and this correlates with their variable thermal sensitivity. In mouse strains that expressed low CGRP levels of CGRP, peripheral injection of CGRP into the hindpaw caused a decrease in thermal nociceptive threshold. In comparison, CGRP-rich strains, which include C57/BL6 mice, were insensitive to CGRP injection (290). Thus the authors suggest that unknowingly using these CGRP-rich strains would lead to the failure to demonstrate CGRP effects. Perhaps as a matter of course all CGRP studies should look at gene expression and levels of CGRP release to be aware of this factor.
B. CGRP and Migraine
Migraine is a complex neurovascular disorder; for a full review of the neurobiology, see Reference 144. It is a common, disabling condition affecting 8% of men and 18% of women with 13% of people affected experiencing more than one attack per week (372), making it an economically costly disease. Symptoms are wide-ranging and can include painful debilitating headache, increased sensitivity to light and sound, nausea, vomiting, fatigue, and dizziness. While the etiology of migraine is unknown, it has long been recognized that CGRP is involved in its pathophysiology. It was thought that the pain sensitivity arises from nociceptive sensory afferent fibers from the trigeminal nerve that provide dense innervation to the meningeal blood vessels within the skull (Figure 5). These afferent fibers contain vasoactive peptides, and activation of the trigeminal ganglion causes release of these peptides, particularly CGRP, in both animal and human studies (147, 447). Furthermore, intravenous infusion of CGRP can induce migraine-like attacks in migraine patients (229, 230). A significant elevation of plasma levels of CGRP in humans during migraine has been observed, which can be normalized with the anti-migraine agents, the triptans (5-HT1B/1D agonists), with a concurrent resolution of the headache component (128, 146, 148, 351). However, even this condition is not without its CGRP controversy as in 2005 a study was published using intrapatient comparison that showed no increase in CGRP levels in the jugular blood in some migraine patients (404); for a full analysis of these discrepancies, see Reference 395. Thus CGRP may not be increased for all migraine sufferers, or it may be that, as for rodents, it depends on the particular individual's gene expression for how susceptible they are to the effects of CGRP.
Fig. 5.
CGRP is involved in the pathophysiology of migraine. CGRP is released from trigeminal afferent nerve fibers during a migraine and causes vasodilatation and neurogenic inflammation. Raised levels of CGRP are observed both peripherally and centrally in migraine patients. CGRP antibodies and antagonists are thought to reduce migraine by reducing these CGRP levels or through blocking the actions of CGRP. CGRP antibodies are peripherally restricted, whereas CGRP antagonists may have central actions.
Yet, as much evidence had shown an important role for CGRP in migraine, work has been under way for many years to develop CGRP antagonists specifically to treat this debilitating condition (see Table 1 for a summary of studied CGRP antagonists). The disease-specific currently available drugs on the market for migraine, the triptans, are limited by cardiovascular problems due to their vasoconstrictive properties, complications with medication overuse headache, and inefficacy for a proportion of patients (302). In 2000, Boehringer Ingelheim published the pharmacological profile of BIBN4096BS (Olcegepant), the first selective nonpeptide CGRP antagonist, which could block vasodilatation caused by stimulation of the trigeminal nerve in the marmoset (100). Trials showed that BIBN409BS had no effect on baseline regional or systemic hemodynamics in animal and human studies (19, 321, 322), suggesting that unlike the triptan class of drugs, the CGRP antagonists may not lead to problems with cardiovascular side effects, as discussed in earlier sections. In a phase II proof of concept study, BIBN4096BS was tested in an international multicenter double-blind randomized clinical trial in 126 patients with migraine. The BIBN treated group had a response rate of 60% compared with 27% for placebo, and this response became apparent from 30 min after treatment (311). Importantly, this response was comparable to that published for the available triptans (121). However, although BIBN4096BS had high affinity for the human CGRP receptor, is selective, and is a pure antagonist with good tolerability, its high molecular weight meant it could only be administered by intravenous injection (342). As migraine attacks occur at infrequent episodic intervals and these antagonists need to be administered as soon as the attack manifests itself, intravenous drug administration is not a particularly commercially viable option. Therefore, much research went to develop an orally available CGRP antagonist, and in 2007 Merck published details of their compound MK-0974 (or telcagepant) which was potent and showed good oral bioavailability (319, 360). As with BIBN4096BS, the initial phase II proof of concept study showed good efficacy and tolerability for telcagepant (181). It was then tested in a larger phase III trial of 1,380 patients, and telcagepant was shown to be more effective than placebo in relieving pain and reducing other symptoms such as nausea and photophobia (181). This was comparable to the triptan zolmitriptan, but the CGRP antagonist had fewer associated adverse side effects (181). A second randomized, controlled study confirmed these results showing efficacy and tolerability of telcagepant at, and this time, also 150 mg (77). A randomized, double-blind, placebo-controlled trial was also carried out with telcagepant administered together with either ibuprofen or acetaminophen and looked at pain freedom at 2 h. Combination therapy and monotherapy were significantly better than placebo, although no statistical differences were seen between treatment groups. However, numerically greater treatment effects were seen in the combination groups, suggesting there could be some benefit to this (177).
Table 1.
Current CGRP antagonists/antibodies under investigation
| Name | Type | Affinity* (Ki, nM) | Indication | Route of Administration | Efficacious Doses, mg | Current Stage in Clinical Trials | Reference Nos. |
|---|---|---|---|---|---|---|---|
| Olcegepant (BIBN4096BS) | Nonpeptide receptor antagonist | 0.01 | Acute migraine treatment | Intravenous | 2.5 | Phase II | 100, 293, 311, 349 |
| Telcegepant (MK-0974) | Nonpeptide receptor antagonist | 0.8 | Acute migraine treatment/prophylactic for episodic migraine | Oral | 150, 300 | Discontinued after phase III due to liver toxicity | 76, 77, 180 |
| MK-3207 | Nonpeptide receptor antagonist | 0.02 | Acute migraine treatment | Oral | 10, 100, 200 | Discontinued after phase II due to liver toxicity | 176, 349 |
| BI 44370 TA | Nonpeptide receptor antagonist | Not reported | Acute migraine treatment | Oral | 400 | Phase II | 92 |
| BMS-927711 | Nonpeptide receptor antagonist | 0.027 | Acute migraine treatment | Oral | 75, 150, 300 | Phase II | 258, 266 |
| BMS-742413 | Nonpeptide receptor antagonist | 0.023 | Acute migraine treatment | Intranasal | Preclinical | 68 | |
| MK-2918 | Nonpeptide receptor antagonist | 0.05 | Acute migraine treatment | Oral | Preclinical | 318 | |
| LY2951742 | Humanized monoclonal antibody against CGRP | Prophylactic for episodic migraine/also in preclinical studies for osteoarthritic pain | Subcutaneous | 150 | Phase II results reported at 2014 AAN meeting; clinical trial NCT01625988 | 31, 95 | |
| ALD403 | Humanized monoclonal antibody against CGRP | Prophylactic for episodic migraine | Intravenous | 1,000 | Phase I results reported at 2014 AAN meeting; clinical trial NCT01772524 | 145 | |
| AMG 334 | Fully humanized monoclonal antibody against CGRP receptor | Prophylactic for episodic and chronic migraine/also in phase I for hot flashes associated with menopause | Subcutaneous | Not reported | Phase II ongoing; clinical trial NCT01952574 | ||
| LBR-101 | Fully humanized antibody against CGRP | Prophylactic for episodic and chronic migraine | Subcutaneous | Dose-ranging | Phase I -published Phase II ongoing | 32 |
Affinity is given for human calcitonin gene-related peptide (CGRP) receptor.
A further large phase III trial then looked at how effective telcagepant was at treating migraine symptoms across four attacks within the same patient (180). Both 140 and 280 mg telcagepant were found to be more consistently effective for the treatment of migraine than control. Few patients experienced adverse effects, and these were mainly limited to drowsiness and vomiting. No abnormal changes were seen in liver transaminase levels of patients; however, these were only assessed at least 7 days after treatment (180). In contrast, in a clinical trial (NCT00797667) where 140 or 280 mg telcagepant was given twice daily for up to 3 mo as a migraine prophylactic treatment, two patients were seen to have elevated symptomatic transaminase levels. This led to the early termination of the trial.
A further long-term study was carried out over 18 mo to assess the safety of telcagapant for the intermittent acute treatment of migraine. During this large phase III trial, a total of 19,820 migraine attacks were treated with 300 mg telcagepant and 10,981 attacks with the triptan rizatriptan (76). Patients were allowed to treat up to eight attacks per month, and telcagepant was seen to lead to fewer adverse effects than rizatriptan. Both treatments showed consistent efficacy without any suggestion of tolerance developing. Interestingly, there were some observations to suggest that telcagepant may provide more of a sustained response than the triptan, thus potentially may reduce both migraine duration and frequency over time (76). Despite the concerns over liver transaminase levels when telcagepant was given daily, in this trial with repeated but intermittent drug administration only three patients saw an increase in enzyme levels, and these were discovered to be transient and temporally unrelated to the dosage of medication (76).
As mentioned above, one of the major problems of the triptan class of drugs is that they are contraindicated for people with cardiovascular disease. Thus a major advantage for the CGRP antagonists would be if they were safe for this patient population. Multiple doses of telcagepant were initially evaluated to be safe in patients with coronary artery disease in a small study (27). Further investigation was then undertaken treating patients with telcagepant that were comorbid for stable coronary artery disease and migraine (182). Unfortunately, due to recruitment difficulties, the study was underpowered so it was not possible to fully evaluate the efficacy of telcagepant over placebo. However, telcagepant was generally well tolerated with no serious drug-related cardiovascular adverse effects. Importantly, as problems had been seen in the prophylactic trial, no elevation in liver transaminase levels was seen (182).
However, in July 2011, Merck stopped the development of telcagepant announcing in a press release this statement: “Merck is discontinuing the clinical development program for telcagepant, the company's investigational calcitonin gene-related peptide receptor antagonist for the treatment of acute migraine. The decision is based on an assessment of data across the clinical program, including findings from a recently completed six-month Phase III study.” In their review, Negro et al. (302) state that this was due to the liver toxicity concerns with the drug.
Merck had also developed a second orally bioavailable CGRP receptor antagonist that was structurally distinct from telcagepant, MK-3207. MK-3207 was shown to be 50- to 100-fold more potent than telcagepant both in vitro and in vivo (349) and was examined in a phase II clinical trial for the use as an acute treatment for migraine at doses ranging from 2.5–200 mg (176). Significant efficacy was observed for 10, 100, and 200 mg MK-3207 compared with placebo, when measuring pain freedom at 2 h. Interestingly, the study investigators had calculated that the clinically efficacious dose would be between 2.5 and 100 mg, yet there was a trend towards a dose-response with greater effect at 200 mg; thus the authors suggested that doses higher than 200 mg may have even greater efficacy. This is important as though they did not compare to a triptan in this study, the 26% 2-h pain-free response for 200 mg MK-3207 is close to both the 29% seen with 100 mg sumatriptan in a meta-analysis (121) and the 27% with 300 mg telcagepant in the phase III trial (181). Thus higher doses of MK-3207 have the potential to be an improvement on the triptans and the original CGRP oral antagonist telcagepant. Higher drug plasma levels could result in central penetration; thus these results have led to the suggestion that there is a potential role for central CGRP receptors in migraine (105), although this argument is under debate, as discussed later. Unfortunately, there were also liver toxicity issues with MK-3207. During an extended phase 1 study, result abnormalities were observed in some subjects in the liver enzyme test causing Merck to discontinue development of this compound (176).
A phase II clinical trial for acute migraine treatment has been carried out on a third oral CGRP antagonist, developed by Boehringer Ingelheim, BI 44370 TA, at 50, 200, and 400 mg (92). The highest dose of 400 mg achieved significant pain freedom at 2 h in more patients than placebo with a response rate of 27%, similar to that seen previously with triptans and earlier CGRP antagonists (92, 121, 181). There were few adverse events, although one subject did show increased liver function tests; however, this subject was also taking other medications concurrently and had a history of alcohol consumption, so the authors stated it was hard to conclude whether BI 44370 TA caused the anomalous liver result. They stated that internal data showed no liver toxicity data with the compound (92). This study only included 341 subjects so we await further larger phase III studies to confirm the safety of BI 44370 TA.
To illustrate the excitement for CGRP antagonists, it is notable that Bristol-Myers Squibb have two CGRP antagonists in their pipeline. The first of these is an oral CGRP antagonist, termed BMS-927711 (258), and results from a phase II trial have recently been published (266). BMS-927711, at doses from 75 to 300 mg, led to significant increases in the number of patients reaching pain freedom at 2 h post dose compared with placebo. There was approximately a 30% response rate for all doses, making it comparable to the other CGRP antagonists and the triptans (266). No adverse events were reported, and no signs of liver toxicity were detected; however, this was a single dose study, so long-term safety and tolerability could not be assessed (266).
The second CGRP antagonist developed by Bristol-Myers Squibb is BMS-742413, (R)-N-3-(7-methyl-1H-indazol-5-yl)-1-(4–1-methylpiperidine-1-carboxamide) (68). To increase the speed of pain relief, ways are always sought to improve onset of action; thus it is of importance that this compound is suitable for intranasal delivery. BMS-742413 displayed good bioavailability following insufflation and was shown to dose-dependently inhibit facial blood flow in the marmoset. Of particular note, this compound showed no vasomotor activity in isolated human coronary arteries, contrasting with the clear vasoconstrictor effect of sumatriptan in the same preparation (68). It will be interesting to see how this compound fares in toxicological studies as part of a clinical trial, if carried forward.
Despite shutting down development of at least two of their oral CGRP antagonists, Merck published a paper in 2011 illustrating their efforts in improving upon the structure of telecagepant and developing CGRP antagonists with lower projected clinical doses (318). It remains to be seen whether their new imidazoazepane compound, MK-2918, has the same liver effects as the previous CGRP receptor antagonists.
An alternative approach to small molecule antagonists has been the use of antibodies to target CGRP, thus reducing its bioavailability. Zeller et al. (451) gave a systemic injection of an anti-αCGRP antibody to rats and showed it could inhibit neurogenic vasodilatation in two blood flow models but had no effect on heart rate or BP. The onset of action was apparent after 1–2 h and lasted for 7 days. This slow but prolonged inhibition makes this approach different from the CGRP receptor antagonists, allowing the use of antibodies as a prophylactic treatment as opposed to acute migraine medication. The obvious inability for monoclonal antibodies to cross the blood-brain barrier requires that the antibodies are an effective migraine treatment simply through a peripheral site of action (Figure 5).
There are several monoclonal antibodies in phase I or II trials for migraine (33, 96). Table 1 lists the characteristics and progress of four antibodies. LY2951742 is a humanized monoclonal antibody, developed by Arteaus Therapeutics, which potently and selectively binds to CGRP. The phase I trial showed that the drug was well-tolerated (www.arteaus.com). The phase II clinical trial (NCT01625988) examined the change in number of migraine headache days in a 28-day period after subcutaneous administration of drug or placebo once every other week for 12 wk. In January 2014, Eli Lilly issued a press release stating they had acquired all development rights for this drug based on positive phase II data (https://investor.lilly.com/). In May 2014, results from the phase II trial were announced at the American Academy of Neurology (AAN) meeting. LY2951742 significantly decreased the number of migraine days compared with placebo (95).
Another antibody showing promise is ALD403 by Alder Biopharmaceuticals that has been tested for efficacy and safety in a single dose study of 163 migraine patients (NCT01772524). Goadsby et al. (145) presented the results at the AAN 2014 meeting and showed that intravenous ALD403 significantly reduced the mean migraine days per month compared with placebo. Interestingly, a proportion of patients experienced a complete abolition of their migraines, suggesting that CGRP inhibition may be key for certain patients. Alder Biopharmaceuticals use a novel yeast expression system to produce functional antibodies quicker, and reportedly, cheaper than other traditionally used systems, which may speed up the drug discovery process.
AMG 334 is being developed by Amgen, and in contrast to the other antibodies, it is targeted against the CGRP receptor rather than the free peptide (33). As well as being in phase II studies for episodic and chronic migraine, AMG 334 is also being examined in a phase I study for women with menopausal hot flushes (35).
To date, very few details have been published with regards to the antibodies in development. However, Labrys Biologics have recently published all the phase I results for their CGRP antibody LBR-101 (32, 34). LBR-101 was well tolerated for single intravenous doses ranging from 0.2 to 2,000 mg and did not affect BP, temperature, or heart rate (32). It is of note that LBR-101 did not result in any liver abnormalities (32). An additional study examined LBR-101 in women over 40 yr of age. Despite this population having an increased age risk of cardiovascular events, LBR-101 did not result in any hemodynamic changes (34). However, this study only enrolled healthy volunteers, excluding those with cardiovascular disease or known cardiovascular risk factors such as diabetes or high cholesterol (34).
It will be of great interest to see if the CGRP antibodies are efficacious, as theories exist that the CGRP antagonists work partly through a central mode of action. Sixt et al. (366) showed that systemic olcegepant decreased spinal trigeminal activity in the rat. Expression of CGRP receptors have been observed on central primary afferent endings presynaptically in the rat and in trigeminal neurons in humans (110, 233); thus antagonists could block the release of neurotransmitters, but the antibodies would be unable to reach this site of action (Figure 5). An apparent central mode of action is thought to be validated by the much higher dose of CGRP antagonist required to treat a migraine attack compared with the calculated dose from in vitro potency. The high dose would allow sufficient blood-brain barrier penetration; however, one study has shown that the high dose required for the anti-migraine effect is equivalent to the dose required to inhibit capsaicin-induced dermal blood flow in humans (396). This does not support a central mode of action and suggests that high doses are required due to other reasons, for example, significant plasma protein binding (396). Other studies have examined the mechanisms of exogenous CGRP and triptans in healthy volunteers; intravenous CGRP was shown to dilate extracranial but not intracranial vessels, and this could be reversed with sumatriptan (21). In addition, an fMRI study showed that neither exogenous CGRP nor sumatriptan given systemically had any effect on brain activity (22). Therefore, it is far from clear whether it is necessary to have a central block of CGRP for efficacy of migraine treatment.
As of yet, no antagonist has reached the clinic due to the aforementioned problems with liver toxicity. The advantage with the antibodies is that they are structurally very different from the CGRP antagonists; thus it is unlikely that the same liver problems will be encountered. However, as with all antibody therapies, they have the problems of higher cost and injectable route of administration, not to mention that chronically depleting systemic levels of CGRP may have adverse side effects. Then again, it is perhaps relevant that a study, very recently published, has shown for the first time that patients with chronic migraine (more than 15 migraine days a month) have significantly elevated CGRP levels measured by ELISA in their peripheral blood outside their migraine attacks, compared with healthy controls (61). The authors suggest this CGRP measure could act as a biomarker for chronic migraine; however, it would also be imperative to determine whether reducing peripheral CGRP levels with long-term antibody administration would be sufficient to reduce number of migraine attacks.
It is still not exactly clear what causes the persistent pain during a migraine attack. Almost 80% of CGRP expressing trigeminal sensory nerves also coexpress the P2X3 receptor, which is activated by extracellular ATP, a major algogenic substance (112, 288). It has been shown that just 1-h exposure to CGRP stimulates both trafficking of P2X3 receptors to the cell-surface membrane of cultured trigeminal neurons, and synthesis of new receptors via various signaling pathways including PKA and PKC activation, cAMP-response element binding protein (CREB) phosphorylation, and nuclear translocation (112, 363). This culminates in a prolonged P2X3 receptor upregulation that persists for up to 10 h and may account for the sensitization of the trigeminal nociceptive neurons and enduring pain observed during a migraine attack. It is important to note that the neurotrophin growth factors, BDNF and NGF, are also known to sensitize trigeminal neurons and are thought to play a role in migraine. Although BDNF can potentiate the sensitizing effect of CGRP, it can also act on CGRP-insensitive neurons (363). In addition, NGF can upregulate P2X3 receptors via a different mechanism and in a quicker timeframe than CGRP (83, 143). Thus, for full therapeutic efficacy for migraine, it may be necessary to block all three of these migraine mediators, or choose to develop antagonists for the common downstream target, the P2X3 receptor, as discussed in the review by Ford (125).
C. CGRP and Arthritis
Arthritic conditions encompass a range of chronic inflammatory joint disorders that are thought to affect ∼20% of adults in the Western world (173). It has been known for a long time that arthritic patients have increased levels of CGRP in their plasma and synovial fluid (8, 18, 174, 185, 228), and CGRP may be a very early mediator in the disease process as mRNA levels of CGRP are increased from 30 min in an adjuvant-induced rodent model (54). Furthermore, modulating CGRP activity affects important disease components of arthritis. CGRP can cause cytokine production from whole blood cells and fibroblasts from rheumatoid arthritis (RA) and osteoarthritis (OA) patients (175, 333). The CGRP antagonist CGRP8–37 inhibits the proliferation of RA synovial cells and production of pro-inflammatory cytokines and MMPs (387). The use of capsaicin and surgical denervation on arthritic ankle joints in rats can reduce peripheral and DRG levels of CGRP and SP and reduce, although not completely prevent, the development of joint inflammation (2, 3). In a ligament injury model of the knee, which leads to joint instability and invariably OA, knee joint hyperemia was observed that was neurogenically mediated and thought to be via increased CGRP release (277). Also, as mentioned previously, CGRP8–37 reduces the hypersensitivity seen in joint neurons during joint inflammation, thus perhaps playing a role in the painful component of the disease (304). Increased sensory innervation is observed throughout arthritic joints, and most of these nerve fibers are seen to be CGRP immunoreactive (24, 138, 201). Concurrent with the nerve growth is neovascularization; thus the inflammatory state of the joint can be enhanced, as release of CGRP, together with other vasoactive neuropeptides, such as SP, from the nascent nerve terminals can promote further angiogenesis as well as other neurogenic inflammatory effects (23, 265). Interestingly, the gold standard nonbiologic treatment for RA, methotrexate, can reduce CGRP-positive nerve fibers in the thymus of rats, which may partially account for its therapeutic benefit (26).
Arthritic conditions can be broadly divided into two main types: degenerative, such as OA, and inflammatory conditions, such as RA. Due to its major role in neurogenic inflammation, it may be thought that CGRP would play more of a role in the inflammatory arthritides. Capsaicin-induced CGRP release did not increase over time in two models of OA (25), and some groups have observed greater CGRP expression or levels in RA compared with OA tissue (18, 295). However, it is now known that viewing OA purely as a “wear and tear” disease is a huge oversimplification, and there is no doubt that inflammation also plays a role in this condition (149), with increasing evidence for the prominence of CGRP. Groups have shown increased CGRP expressing DRG joint neurons in models of OA (116, 120, 122), and CGRP positive neurons were upregulated in patients with hip OA compared with those with painless failed total hip arthroplasties (353). Recently, in a model of OA in mice, using intra-articular sodium mono-iodoacetate (MIA) injection to degrade joint articular cartilage, enhanced levels of CGRP were observed in MIA dorsal horn neurons compared with control. Additionally, MIA joints exhibited mechanical allodynia, and this allodynia could be attenuated by intrathecal treatment with the CGRP antagonist CGRP8–37 (308). A TRPV1 antagonist has been shown to be analgesic in OA and was thought to work via reducing CGRP release in the spinal cord (330). Another compound with analgesic activity, eugenol, a component of clove oil, also reduced spinal CGRP content in the MIA model (115). However, it remains to be seen whether antagonism of CGRP could be a viable, safe, and efficacious therapeutic option to join the currently available arthritis treatments.
Interestingly, a recent paper examined the effect of LY2951742, the neutralizing CGRP antibody, in several OA rodent models. The antibody provided pain relief in these models via a different mechanism to NSAIDs (31). LY2951742 was recently acquired by Eli Lilly due to its promise as a migraine therapy, but this study indicates its potential as a treatment for other pain conditions.
D. Skin
There has been great interest in the role of CGRP in skin, since its vasodilator activity was discovered in this tissue (45). CGRP injected at femtomolar doses causes a sustained increase in blood flow in human, as well as other species. It is more potent than most vasodilators tested and has a sustained action. CGRP is released in skin from sensory afferents (Figure 4), and there is also the suggestion that keratinocytes, as well as immune cells, may be sources (337). Meanwhile, the CLR receptor has been found in human skin, on dermal smooth muscle and endothelial cells, by both studies of gene and protein expression (161, 193). The vasodilator activity of CGRP has been studied in humans as well as rodents. It has been suggested to be the mediator of local skin axon reflex flares, in addition to antidromic vasodilatation, and thus has possible relevance to facial flushing (41). Flushing is observed when CGRP is given by systemic infusion and is observed at subpressor doses (135). Others have found that CGRP levels in plasma and urine correlate with postmenopausal flushing (reviewed by Ref. 168). However, the neuronal mechanisms that lead to its release are currently unclear. It has also been suggested that CGRP may be involved in thermoregulation. On the other hand, perhaps surprisingly, CGRP plasma levels have not been found to be raised during sweating in young women (370).
CGRP has been shown, as a consequence of its vasodilator activity, to potentiate edema formation induced by mediators of increased microvascular permeability (such as histamine and bradykinin) and leukocyte accumulation (for example induced by IL-1) in skin (43, 50). It has also been suggested to directly influence leukocyte accumulation in a range of studies (see Refs. 39, 41, 193, 337), but these have not been strongly supported by cross confirmation by findings from different laboratories as discussed in Reference 39. On the other hand, two laboratories have provided evidence that CGRP is able to inhibit human dermal microvascular endothelial cell activities, including chemokine production, adhesion molecule expression, and neutrophil/monocyte adhesion (193, 358).
Scratching has been suggested to quickly (within 1–3 days) influence sensory nerve growth in skin, and as a consequence, upregulate levels of CGRP as well as other neuropeptides via NGF-associated mechanisms (440). Levels of immune-reactive peptides are influenced by the health of the skin. CGRP has been suggested to influence the immune response in several situations. In lymphocytes taken from atopic dermatitis, it has been suggested that CGRP increases IL-13 production in peripheral blood mononuclear cells and memory T cells, and high levels of IL-13 are associated with worsened dermatitis. Thus CGRP may influence the generation of Th2-type response from cutaneous T lymphocytes, thereby exacerbating the problem (15).
Of relevance, the CGRP antagonist CGRP8–37 blocks itch induced by trypsin (79). Indeed, recent studies have involved a knock-in mouse with a LoxP-stopped cell ablation construct (human diphtheria toxin receptor) to the CALCA locus (276). A GFP reporter was used to determine that 50% of calcitonin/αCGRP DRG neurons expressed functional TRPV1. These fibers responded to histamine and chloroquine, indicative of a role in itch and also to heat (276). This was confirmed in a later study that also provided evidence that loss of αCGRP-containing nerves is associated with cold hypersensitivity as linked with neuropathy (275). The findings with these mice also indicate a possible role for CGRP in temperature regulation and weight loss (275). On the other hand, the injection of CGRP at a picomolar dose induces sustained reddening but is not associated with itching in human skin (42).
E. Wound Healing
There is a range of research that indicates that the presence of CGRP facilitates tissue repair and wound healing. First, capsaicin has been used as a tool to deplete, through neonatal application, the sensory neurogenic component in rodents. This is associated with loss of skin health and cutaneous lesions (397). While CGRP is depleted in these studies, it is also clear that other sensory nerve components such as SP or alternative colocalized neuropeptides and the TRP channels are also depleted in these nerves (39). A study involving capsaicin depletion that focused on CGRP was performed in a rat skin-flap model. Capsaicin depletion led to decreased flap survival, as normally wound healing is associated with early growth of new CGRP-containing sensory nerves (207, 222). However, intravenous CGRP increased both blood flow and flap survival (222). CGRP has also been shown to improve wound healing in a blister model, where the healing was delayed in either capsaicin-depleted or aged mice (219).
There are also various studies in CGRP KO mice or with CGRP antagonists where the genetic deletion or blockade of CGRP receptors has been suggested to be detrimental to wound healing (39, 41, 400). Toda et al. (400) showed that CGRP KO mice exhibited deficient wound-induced angiogenesis and wound closure compared with WT mice (400). The role of CGRP in wound healing is likely to be due, in part, as a consequence of its vasodilator activity. Another possibility is that CGRP promotes wound healing by upregulating VEGF expression locally in the wound (400). It has also been demonstrated that CGRP enhances revascularization in a murine hindlimb ischemia model. Here, CGRP KO mice exhibited decreased blood flow and capillary formation (as assessed by CD31-positive cells). The ischemia was associated with an upregulation of CGRP in the DRG in WT mice, and a lack of CGRP (or treatment with CGRP8–37) was associated with decreased gene expression of VEGF, basic fibroblast growth factor, and transforming growth factor-β (TGF-β) in the ischemic tissue (219, 289), all factors involved in angiogenesis. CGRP may also have an alternative beneficial role. Transfection of CGRP attenuated expression of inflammatory mediators, such as MCP, tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), and matrix metallopeptidase-9 (MMP-9), as well as macrophage infiltration, and presumably as a consequence, neointimal hyperplasia in a model of vein graft disease in the rabbit jugular vein (454). This provides an example where CGRP not only promotes revascularization, but also limits concomitant adverse inflammatory events.
CGRP itself may also support the regeneration of skin directly as evidence exists that it directly promotes proliferation of keratinocytes (336). There is also evidence that CGRP stimulates fibroblasts (357), that, in the kidney at least, exacerbate obstructive nephritis (220). CGRP enhances wound healing of human bronchial epithelial cells via PKC and MAPK pathways through effects thought to involve migration, proliferation, and blockade of apoptosis (460). Epithelial cells express CGRP, and levels may be reduced in asthma (36). Indeed, it is also known that CGRP influences allergic airway inflammation by altering dendritic cell function (335) and that CGRP receptors on dendritic cells can be altered.
A role for CGRP and other neuropeptides has been suggested in burn models as levels are high in early human burns in hospitalized patients (314). This subject has been recently reviewed (137). Both the major sensory neuropeptides, SP and CGRP, are involved in the acute edema formation following dermal burns (365). This fits with the observation that there is a strong upregulation of CGRP containing nerves centrally following UV irradiation; this has been suggested to be linked to immunosuppression (141, 142). However, under related circumstances CGRP may be less beneficial. For instance, the CGRP antagonist BIBN4096BS attenuated the adverse effects of smoke exposure in sheep, which was associated with a reduction in tracheal blood flow (227).
F. Diabetes and Obesity
The CGRP-related peptide amylin was discovered soon after CGRP. It is structurally related to CGRP, but differs in amino acid region 20–29 region (the amyloid-like region). This peptide is found locally in the β cells of the pancreas and influences pancreatic events in association with insulin (432), and deposits are found in the diabetic pancreas (78). It has been investigated as a drug target for diabetes with some success. CGRP is also localized to the pancreas (in particular the β cells of the islets of Langerhans), and also to blood cells. The use of capsaicin to deplete the sensory component revealed that capsaicin-sensitive αCGRP and also capsaicin-independent βCGRP was found, demonstrating at an early stage the presence of two entities of CGRP in the pancreas (298).
CGRP modulates glucose-stimulated insulin release, most usually acting to reduce it, with some cases resulting in a hyperglycemia (for review, see Ref. 47). CGRP mediates insulin resistance in skeletal muscle and can possibly influence events in vivo, if released in sufficient amount from perivascular tissues. The normally-fed αCGRP KO mouse exhibits normal plasma glucose levels (S. Brain, personal communication). On the other hand, there has also been a thorough study of the role of CGRP in obesity, through investigation of αCGRP KO mice, and evidence obtained shows that these mice are protected from diet-induced obesity. The αCGRP KO mice showed improved glucose handling and insulin sensitivity. The effect of CGRP on lipid metabolism was investigated using a high-fat diet for several weeks. The CGRP KO mice developed a range of parameters in keeping with higher metabolic rate and, as a consequence, decreased body weight. These included raised body temperature and energy use. This led the authors to suggest that CGRP blockade may be useful in the treatment of obesity (417). There is some evidence that CGRP levels are raised in obese females humans (450), but the changes were quite small (although significant). Likewise, a similar increase was observed in preobese Zucker rats (153). In keeping with this concept, capsaicin-induced sensory nerve blockade/desensitization benefited glucose tolerance in these Zucker rats (153). These authors later showed that CGRP-containing sensory nerves in the pancreatic islets are capsaicin-sensitive. Indeed, capsaicin destroys them with a resulting beneficial effect on insulin secretion/glucose tolerance (152). In addition, a TRPV1 antagonist has been shown to normalize fasting glucose levels in the ob/ob mouse, with an effect suggested to be similar to that of the type II diabetic treatment pioglitazone (389). Thus these data support the concept that increased CGRP/sensory nerve activity occurs at an early stage in the metabolic syndrome.
Diabetes mellitus is associated with a sensory neuropathy, especially in the periphery. This is related to downregulation of NGF, and as a consequence, a loss of CGRP-containing sensory neurons; accordingly, it can be reversed with insulin and NGF (see Refs. 30, 401). It presents as a loss of nociceptive sensation but is also directly linked to the poor wound healing and ulcers that these patients suffer, as the diabetes worsens. A painful neuropathy can also be, and it is suggested that this may be due to abnormal stimulation to sensory nerves and altered responses, although the precise role of CGRP is unclear (126, 200). There is an increase in circulating insulin levels in a fructose drinking rat model of type II diabetes, which is accompanied by loss of CGRP-containing fibers, following a decrease in NGF (167).
Diabetic patients are linked to a range of adverse circumstances in terms of cardiovascular disease. It is possible that the loss of neuronal CGRP exacerbates this, as also discussed earlier. It has been more recently shown that circulating CGRP levels are decreased in diabetes mellitus patients that also suffer heart disease (422). This study complements the finding that CGRP gene transfer could protect against ischemic-reperfusion injury in both diabetic and nondiabetic mice (458). Interestingly, Oltman et al. (313) tried to reverse vascular dysfunction with treatment in Zucker diabetic fatty rats. They found the loss of endothelium-dependent vasodilatation to acetylcholine was easier to reverse than the loss of CGRP vasodilatation (313). It has been suggested recently that insulin resistance-induced hypertension is associated with a downregulation of sensory CGRP nerves and function, but an increase in sympathetic nerves, with a corresponding increase in tone (386).
Finally, CGRP gene transfer selectively suppresses T-cell proliferation and the synthesis of Th1 cytokines but enhances Th2 subsets, which are more normally associated with anti-inflammatory effects. This protected against the insulin-dependent diabetes onset in the mouse, exhibiting an autoimmune effect of CGRP (378). However, overall, there needs to be further study in this complex area to fully understand the mechanisms and the apparent beneficial versus harmful effects of CGRP in diabetes.
G. CGRP and Ageing
Peripheral neurons in general are negatively affected by the ageing process in terms of their ability to conduct action potentials and to sprout collaterals in regenerating fibers (412). One of the first studies investigating the developmental changes that occur to peptidergic sensory neurons was performed in the ageing guinea pig, where it was discovered that density of peptidergic neurons surrounding the mesenteric and carotid arteries increased with fetal development, with a peak at birth. CGRPergic nerve plexuses then declined with age, waning to about half-maximal density once the animal had reached ∼2 yr of age (91). Interestingly, a study investigating peptidergic innervation of the ageing rat aorta showed that CGRP-positive neuronal fibers were present in animals younger than 6 mo, but gradually disappeared to complete absence when animals reached 1 yr of age (74). Conversely, a separate study has indicated no change in DRG αCGRP mRNA expression in rats aged 6 versus 24 mo (253). Therefore, it is still unclear what happens to peptidergic perivascular innervation as time progresses. Additionally, however, some aspects of neuronal physiology can be compromised as part of ageing. As CGRP is a peptide that is synthesized directly by the nucleus (unlike small molecules that require complex metabolic pathways for synthesis) but has the majority of its effects when released from the nerve terminal, it requires axoplasmic transport from the cell body to the axon terminal. The rate of active transport from nucleus to terminal is something that is significantly diminished with age, and this may represent a reduction in CGRP bioactivity as age advances (119).
In addition to stored CGRP, circulating levels of released CGRP have been noted to both increase and decrease with advancing age. One possible reason why serum levels of CGRP are seen to be increased as age increases may be related to the change in CALC I gene splicing in favor of CGRP rather than calcitonin (note that CGRP was first discovered in the thyroid tissue of ageing rats and isolated in humans from thyroid carcinoma cells). Increased production of CGRP by an endocrine gland such as the thyroid would presumably explain increases in plasma concentrations (218, 434). However, as circulating levels of CGRP are so low, it is unlikely that this source of peptide has any significant biological effect. It seems more likely that controlled localized release of CGRP is more important than this suggested “spillover,” and as the local sources are more often seen to be reduced than increased as one ages, it is possible that a very valuable store of CGRP is being lost as part of the ageing process.
Some functional experiments investigating changes in CGRP bioactivity with age have been discussed earlier as they also involve the induction of cardiovascular disease or diabetes. As such, results obtained cannot be directly attributed to ageing alone. Nonetheless, some interesting results have been uncovered. In aged female rats, circulating levels of CGRP have been shown to decline slightly compared with younger animals, and the content of bioavailable CGRP found within the mesenteric vascular bed showed an even more profound drop. This reduction in CGRP availability could be reversed with supplementation of female sex steroid hormones (130). Several longitudinal studies have also been performed utilizing the SHR as discussed in earlier sections. αCGRP mRNA expression within DRG neurons was found to decrease in the aged rat, while no change in expression was found in normotensive rats (439). This decline in CGRP mRNA expression in hypertensive animals appears to be accompanied by a measurable decrease in CGRP-positive sensory nerves that innervate the mesenteric resistance arteries (209, 210). This reduction in neuronal density was found to result in an overall decrease of releasable CGRP following peripheral nerve electrical stimulation (211) and also following capsaicin stimulation (resulting in the activation of TRPV1 channels and subsequent exocytosis of CGRP-loaded vesicles) (377). There has been proposed a strong interaction between the angiotensin signaling system and CGRP and its receptors within the SHR model. One longitudinal study has been performed to show that long-term inhibition of the angiotensin system using temocapril and losartan is capable of preventing the reduction in perivascular sensory nerve innervation in a BP-independent manner (184). Thus hypertension may have a negative effect as one ages on CGRP-containing nerves and the amount of CGRP available for action.
CGRP-containing sensory nerves also appear to be important in governing the cardiac preconditioning response to intermittent periods of ischemia, to prepare for an impending ischemic episode that may accompany myocardial infarction. It is true that older humans are more susceptible to myocardial infarction and isolated heart preparations from older laboratory animals appear to lose this preconditioning response (192, 251). This has been attributed to a reduction in release of endogenous stores of CGRP found within the heart and, curiously, a reduction in the ability of exogenous CGRP to also have a protective effect (251). A similar impairment in CGRP bioactivity with increasing age has been demonstrated in vascular preparations from ageing rats. The ability of CGRP to relax aortic and caudal artery rings was significantly impaired as the animal grew older and intravenous injections of CGRP to older animals resulted in an attenuated hypotensive BP response (65). Likewise, a similar reduction in sensitivity to CGRP has been shown in isolated perfused mesenteric beds in both normotensive and hypertensive ageing rats, although this effect was not exclusive to CGRP as vasodilatation to other relaxant agents was also impaired (12).
It is still relatively unclear how the expression, release, and bioactivity of CGRP changes with advancing age. This lack of clarity mainly arises from the use of tissue derived from different species of laboratory animal, differing lengths of longitudinal study employed, and a majority of information stemming from the use of the SHR model. That said, enough information has been uncovered to warrant future research into this area, particularly as the current consensus appears to be that local stores of CGRP are depleted as age advances and this results in a decrease in its bioactivity. If CGRP is indeed an important mediator of cardiovascular (and otherwise) health, then it is conceivable that we are losing a very important protective mediator, and CGRP replacement may be a viable future therapeutic strategy. It is important to note that as migraine prevalence declines with age (413), the benefits of CGRP agonist therapy in the elderly may outweigh the potential disadvantages.
VIII. CONCLUSIONS
Although it is now known that CGRP is involved in the pathophysiology of migraine, there is much that remains unclear with respect to the role of CGRP in biology and pathophysiology. Perhaps most excitingly, there is evidence that CGRP may have a role in pain and itch associated with arthritis and skin disease. Consequently, this information provides an additional incentive for those involved in the development of anti-CGRP therapies. However, CGRP's role in cardiovascular regulation is still speculative. Figure 6 summarizes the potential roles that CGRP may play in all these conditions. Without doubt, CGRP is one of the most potent peripheral microvascular vasodilators known, although it does not have a role in the physiological regulation of BP in humans. On the other hand, a range of studies in animal models indicate that deletion or blockade of CGRP worsens cardiovascular disease through effects that extend beyond the heart and kidney and probably its vasodilator action. There is a clear indication from the evidence provided here, that there is the potential for CGRP activity to be increased in a compensatory manner as a consequence of adverse cardiovascular events, and evidence that it can play a fundamental role associated with healthy living. However, the precise cardiovascular protective mechanisms of CGRP remain unclear, and as a consequence, its pathophysiological importance remains to be established in humans. At present, CGRP antibodies are being developed for possible long-term therapies in migraine and their investigation will allow insight into the long-term effect of depleting CGRP levels in humans. One important factor that needs clarifying is whether CGRP replacement therapy is beneficial, as this opens up a potential therapeutic option of using a CGRP agonist of which at least one form is known. Novo Nordisk have available a stabilized αCGRP agonist, conformationally constrained, acetylated CGRP α analog, (1/2 life >7 h, compared with CGRP, <30 min; patent number WO 2011/051312 A1). Other opportunities for CGRP involve the modulation of its synthesis and release either chemically or electrically. Of note, transcutaneous electrical stimulation (TENS) has been suggested to improve bladder dysfunction via CGRP and cAMP (93). On the other hand, the role of CGRP in the metabolic aspects that relate to diabetes are complex and require further work; this emerging evidence may provide additional support for the biomedical significance of this peptide. It is perhaps surprising that after more than 20 years of intense study fundamental questions remain over the functional relevance of CGRP and potential therapeutic options.
Fig. 6.
Summary of disease conditions where CGRP plays a role. CGRP can be targeted pharmacologically with either the use of a CGRP analog to increase CGRP levels or a CGRP antagonist to block the actions of CGRP. These approaches are shown against the conditions that may benefit. For some conditions, e.g., diabetes, it is not yet clear whether CGRP supplementation or CGRP inhibition would be the most beneficial approach.
GRANTS
We thank Arthritis Research UK (to F. A. Russell), the British Heart Foundation (to S.-J. Smillie and R. King), the British Pharmacological Society (to X. Kodji), and a Biotechnology and Biological Sciences Research Council-led mammalian biology capacity building award in integrative mammalian biology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
NOTE ADDED IN PROOF
Riera et al. (Cell 157: 1023-1026, 2014) have recently shown that inhibition of CGRP with aging may increase metabolic health and control longevity
acknowledgments
We are grateful to Pratish Thakore for help with illustrations.
Address for reprint requests and other correspondence: S. D. Brain, Cardiovascular Division, BHF Centre of Research Excellence & Centre of Integrative Biomedicine, King's College London, Franklin Wilkins Building, Waterloo Campus, London SE1 9NH, UK (e-mail: sue.brain@kcl.ac.uk).
REFERENCES
- 1.Abd El-Aleem SA, Morales-Aza BM, Donaldson LF. Sensory neuropeptide mRNA up-regulation is bilateral in periodontitis in the rat: a possible neurogenic component to symmetrical periodontal disease. Eur J Neurosci 19: 650–658, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Bjurholm A, Srinivasan GR, Lundeberg T, Theodorsson E, Schultzberg M, Kreicbergs A. Capsaicin effects on substance P and CGRP in rat adjuvant arthritis. Regul Pept 55: 85–102, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ahmed M, Srinivasan GR, Theodorsson E, Schultzberg M, Kreicbergs A. Effects of surgical denervation on substance P and calcitonin gene-related peptide in adjuvant arthritis. Peptides 16: 569–579, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Aiyar N, Daines RA, Disa J, Chambers PA, Sauermelch CF, Quiniou M, Khandoudi N, Gout B, Douglas SA, Willette RN. Pharmacology of SB-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J Pharmacol Exp Ther 296: 768–775, 2001 [PubMed] [Google Scholar]
- 5.Aiyar N, Rand K, Elshourbagy NA, Zeng Z, Adamou JE, Bergsma DJ, Li Y. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J Biol Chem 271: 11325–11329, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Al-Rubaiee M, Gangula PR, Millis RM, Walker RK, Umoh NA, Cousins VM, Jeffress MA, Haddad GE. Inotropic and lusitropic effects of calcitonin gene-related peptide in the heart. Am J Physiol Heart Circ Physiol 304: H1525–H1537, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alevizaki M, Shiraishi A, Rassool FV, Ferrier GJ, MacIntyre I, Legon S. The calcitonin-like sequence of the beta CGRP gene. FEBS Lett 206: 47–52, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Co-variation of neuropeptide Y, calcitonin gene-related peptide, substance P and neurokinin A in joint fluid from patients with temporomandibular joint arthritis. Arch Oral Biol 40: 127–135, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24: 4300–4312, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 229: 1094–1097, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298: 240–244, 1982 [DOI] [PubMed] [Google Scholar]
- 12.Amerini S, Mantelli L, Filippi S, Ledda F. Effects of aging and hypertension on vasorelaxant activity of calcitonin gene-related peptide: a comparison with other vasodilator agents. J Cardiovasc Pharmacol 23: 432–437, 1994 [PubMed] [Google Scholar]
- 13.Anderson LE, Seybold VS. Calcitonin gene-related peptide regulates gene transcription in primary afferent neurons. J Neurochem 91: 1417–1429, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Ando K, Pegram BL, Frohlich ED. Hemodynamic effects of calcitonin gene-related peptide in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 258: R425–R429, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Antunez C, Torres MJ, Lopez S, Rodriguez-Pena R, Blanca M, Mayorga C, Santamaria-Babi LF. Calcitonin gene-related peptide modulates interleukin-13 in circulating cutaneous lymphocyte-associated antigen-positive T cells in patients with atopic dermatitis. Br J Dermatol 161: 547–553, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Aoki-Nagase T, Nagase T, Oh-Hashi Y, Shindo T, Kurihara Y, Yamaguchi Y, Yamamoto H, Tomita T, Ohga E, Nagai R, Kurihara H, Ouchi Y. Attenuation of antigen-induced airway hyperresponsiveness in CGRP-deficient mice. Am J Physiol Lung Cell Mol Physiol 283: L963–L970, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Arden WA, Fiscus RR, Wang X, Yang L, Maley R, Nielsen M, Lanzo S, Gross DR. Elevations in circulating calcitonin gene-related peptide correlate with hemodynamic deterioration during endotoxic shock in pigs. Circ Shock 42: 147–153, 1994 [PubMed] [Google Scholar]
- 18.Arnalich F, de Miguel E, Perez-Ayala C, Martinez M, Vazquez JJ, Gijon-Banos J, Hernanz A. Neuropeptides and interleukin-6 in human joint inflammation relationship between intraarticular substance P and interleukin-6 concentrations. Neurosci Lett 170: 251–254, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Arulmani U, Schuijt MP, Heiligers JP, Willems EW, Villalon CM, Saxena PR. Effects of the calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS on alpha-CGRP-induced regional haemodynamic changes in anaesthetised rats. Basic Clin Pharmacol Toxicol 94: 291–297, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Asahina A, Hosoi J, Murphy GF, Granstein RD. Calcitonin gene-related peptide modulates Langerhans cell antigen-presenting function. Proc Assoc Am Physicians 107: 242–244, 1995 [PubMed] [Google Scholar]
- 21.Asghar MS, Hansen AE, Kapijimpanga T, van der Geest RJ, van der Koning P, Larsson HB, Olesen J, Ashina M. Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology 75: 1520–1526, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Asghar MS, Hansen AE, Larsson HB, Olesen J, Ashina M. Effect of CGRP and sumatriptan on the BOLD response in visual cortex. J Headache Pain 13: 159–166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol 20: 573–580, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis 70: 523–529, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Averbeck B, Rudolphi K, Michaelis M. Osteoarthritic mice exhibit enhanced prostaglandin E2 and unchanged calcitonin gene-related peptide release in a novel isolated knee joint model. J Rheumatol 31: 2013–2020, 2004 [PubMed] [Google Scholar]
- 26.Baig JA, Iqbal MP, Rehman R, Qureshi AA, Ahmed M. Anti-inflammatory role of methotrexate in adjuvant arthritis: effect on substance P and calcitonin gene-related peptide in thymus and spleen. J Coll Physicians Surg Pak 17: 490–494, 2007 [PubMed] [Google Scholar]
- 27.Behm MO, Blanchard RL, Murphy MG, Palcza JS, Harris DE, Butterfield KL, Smith WB, Preston RA, Chodakewitz JA, Krucoff MW. Effect of telcagepant on spontaneous ischemia in cardiovascular patients in a randomized study. Headache 51: 954–960, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Bell D, McDermott BJ. Calcitonin gene-related peptide in the cardiovascular system: characterization of receptor populations and their (patho)physiological significance. Pharmacol Rev 48: 253–288, 1996 [PubMed] [Google Scholar]
- 29.Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP(8–37) in a rodent model of chronic central pain. Pain 86: 163–175, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Bennett GS, Garrett NE, Diemel LT, Brain SD, Tomlinson DR. Neurogenic cutaneous vasodilatation and plasma extravasation in diabetic rats: effect of insulin and nerve growth factor. Br J Pharmacol 124: 1573–1579, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, Lynch RA, Li B, McCarty D, Oskins JL, Lin C, Johnson KW, Chambers MG. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage 22: 578–585, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Bigal ME, Escandon R, Bronson M, Walter S, Sudworth M, Huggins JP, Garzone P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: results of the Phase 1 program. Cephalalgia 34: 483–492, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Bigal ME, Walter S. Monoclonal antibodies for migraine: preventing calcitonin gene-related peptide activity. CNS Drugs 28: 389–399, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Bigal ME, Walter S, Bronson M, Alibhoy A, Escandon R. Cardiovascular and hemodynamic parameters in women following prolonged CGRP inhibition using LBR-101, a monoclonal antibody against CGRP. Cephalalgia. In press [DOI] [PubMed] [Google Scholar]
- 35.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 53: 1230–1244, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Bonner K, Kariyawasam HH, Ali FR, Clark P, Kay AB. Expression of functional receptor activity modifying protein 1 by airway epithelial cells with dysregulation in asthma. J Allergy Clin Immunol 126: 1277–1283, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Bowers MC, Katki KA, Rao A, Koehler M, Patel P, Spiekerman A, DiPette DJ, Supowit SC. Role of calcitonin gene-related peptide in hypertension-induced renal damage. Hypertension 46: 51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Bracci-Laudiero L, Aloe L, Buanne P, Finn A, Stenfors C, Vigneti E, Theodorsson E, Lundeberg T. NGF modulates CGRP synthesis in human B-lymphocytes: a possible anti-inflammatory action of NGF? J Neuroimmunol 123: 58–65, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology 37: 133–152, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol 147 Suppl 1: S202–S211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Brain SD, Tippins JR, Morris HR, MacIntyre I, Williams TJ. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Invest Dermatol 87: 533–536, 1986 [DOI] [PubMed] [Google Scholar]
- 43.Brain SD, Williams TJ. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol 86: 855–860, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature 335: 73–75, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature 313: 54–56, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Breeze AL, Harvey TS, Bazzo R, Campbell ID. Solution structure of human calcitonin gene-related peptide by 1H NMR and distance geometry with restrained molecular dynamics. Biochemistry 30: 575–582, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Bretherton-Watt D, Ghatei MA, Jamal H, Gilbey SG, Jones PM, Bloom SR. The physiology of calcitonin gene-related peptide in the islet compared with that of islet amyloid polypeptide (amylin). Ann NY Acad Sci 657: 299–312, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Kwiecien S, Pawlik M, Drozdowicz D, Sliwowski Z, Pawlik WW. Ischemic preconditioning of remote organs attenuates gastric ischemia-reperfusion injury through involvement of prostaglandins and sensory nerves. Eur J Pharmacol 499: 201–213, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Bucelli RC, Gonsiorek EA, Kim WY, Bruun D, Rabin RA, Higgins D, Lein PJ. Statins decrease expression of the proinflammatory neuropeptides calcitonin gene-related peptide and substance P in sensory neurons. J Pharmacol Exp Ther 324: 1172–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Buckley TL, Brain SD, Collins PD, Williams TJ. Inflammatory edema induced by interactions between IL-1 and the neuropeptide calcitonin gene-related peptide. J Immunol 146: 3424–3430, 1991 [PubMed] [Google Scholar]
- 51.Buckley TL, Brain SD, Rampart M, Williams TJ. Time-dependent synergistic interactions between the vasodilator neuropeptide, calcitonin gene-related peptide (CGRP) and mediators of inflammation. Br J Pharmacol 103: 1515–1519, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buhlmann N, Leuthauser K, Muff R, Fischer JA, Born W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology 140: 2883–2890, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Bullen ML, Miller AA, Andrews KL, Irvine JC, Ritchie RH, Sobey CG, Kemp-Harper BK. Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid Redox Signal 14: 1675–1686, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Bulling DG, Kelly D, Bond S, McQueen DS, Seckl JR. Adjuvant-induced joint inflammation causes very rapid transcription of beta-preprotachykinin and alpha-CGRP genes in innervating sensory ganglia. J Neurochem 77: 372–382, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Bunker CB, Reavley C, O'Shaughnessy DJ, Dowd PM. Calcitonin gene-related peptide in treatment of severe peripheral vascular insufficiency in Raynaud's phenomenon. Lancet 342: 80–83, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Cadieux A, Springall DR, Mulderry PK, Rodrigo J, Ghatei MA, Terenghi G, Bloom SR, Polak JM. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: effect of capsaicin treatment and surgical denervations. Neuroscience 19: 605–627, 1986 [DOI] [PubMed] [Google Scholar]
- 57.Cai WQ, Bodin P, Loesch A, Sexton A, Burnstock G. Endothelium of human umbilical blood vessels: ultrastructural immunolocalization of neuropeptides. J Vasc Res 30: 348–355, 1993 [DOI] [PubMed] [Google Scholar]
- 58.Cao T, Pinter E, Al Rashed S, Gerard N, Hoult JR, Brain SD. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J Immunol 164: 5424–5429, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Carpenter KA, Schmidt R, von Mentzer B, Haglund U, Roberts E, Walpole C. Turn structures in CGRP C-terminal analogues promote stable arrangements of key residue side chains. Biochemistry 40: 8317–8325, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 81: 1191–1196, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Chai W, Mehrotra S, Jan Danser AH, Schoemaker RG. The role of calcitonin gene-related peptide (CGRP) in ischemic preconditioning in isolated rat hearts. Eur J Pharmacol 531: 246–253, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Chaitman BR, Ho AP, Behm MO, Rowe JF, Palcza JS, Laethem T, Heirman I, Panebianco DL, Kobalava Z, Martsevich SY, Free AL, Bittar N, Chrysant SG, Ho TW, Chodakewitz JA, Murphy MG, Blanchard RL. A randomized, placebo-controlled study of the effects of telcagepant on exercise time in patients with stable angina. Clin Pharmacol Ther 91: 459–466, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Champion HC, Bivalacqua TJ, Lambert DG, McNamara DB, Kadowitz PJ. The influence of candesartan and PD123319 on responses to angiotensin II in the hindquarters vascular bed of the rat. J Am Soc Nephrol 10 Suppl 11: S95–97, 1999 [PubMed] [Google Scholar]
- 65.Chan GH, Fiscus RR. Severe impairment of CGRP-induced hypotension in vivo and vasorelaxation in vitro in elderly rats. Eur J Pharmacol 434: 133–139, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Chan KY, Edvinsson L, Eftekhari S, Kimblad PO, Kane SA, Lynch J, Hargreaves RJ, de Vries R, Garrelds IM, van den Bogaerdt AJ, Danser AH, Maassenvandenbrink A. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther 334: 746–752, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Chang CP, Pearse RV, 2nd, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 11: 1187–1195, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Chaturvedula PV, Mercer SE, Pin SS, Thalody G, Xu C, Conway CM, Keavy D, Signor L, Cantor GH, Mathias N, Moench P, Denton R, Macci R, Schartman R, Whiterock V, Davis C, Macor JE, Dubowchik GM. Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-y l)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett 23: 3157–3161, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8–37). Am J Physiol Endocrinol Metab 256: E331–E335, 1989 [DOI] [PubMed] [Google Scholar]
- 70.Choksi T, Hay DL, Legon S, Poyner DR, Hagner S, Bloom SR, Smith DM. Comparison of the expression of calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) with CGRP and adrenomedullin binding in cell lines. Br J Pharmacol 136: 784–792, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chrissobolis S, Zhang Z, Kinzenbaw DA, Lynch CM, Russo AF, Faraci FM. Receptor activity-modifying protein-1 augments cerebrovascular responses to calcitonin gene-related peptide and inhibits angiotensin II-induced vascular dysfunction. Stroke 41: 2329–2334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 21: 3747–3755, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Clementi G, Caruso A, Prato A, De Bernardis E, Fiore CE, Amico-Roxas M. A role for nitric oxide in the anti-ulcer activity of calcitonin gene-related peptide. Eur J Pharmacol 256: R7–8, 1994 [DOI] [PubMed] [Google Scholar]
- 74.Connat JL, Busseuil D, Gambert S, Ody M, Tebaldini M, Gamboni S, Faivre B, Quiquerez AL, Millet M, Michaut P, Rochette L. Modification of the rat aortic wall during ageing: possible relation with decrease of peptidergic innervation. Anat Embryol 204: 455–468, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Conner AC, Hay DL, Howitt SG, Kilk K, Langel U, Wheatley M, Smith DM, Poyner DR. Interaction of calcitonin-gene-related peptide with its receptors. Biochem Soc Trans 30: 451–455, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Connor KM, Aurora SK, Loeys T, Ashina M, Jones C, Giezek H, Massaad R, Williams-Diaz A, Lines C, Ho TW. Long-term tolerability of telcagepant for acute treatment of migraine in a randomized trial. Headache 51: 73–84, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 73: 970–977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA 84: 8628–8632, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, Paszcuk AF, Quintao NL, Juliano L, Brain SD, Calixto JB. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol 154: 1094–1103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem 282: 12260–12271, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Crossman DC, Dashwood MR, Brain SD, McEwan J, Pearson JD. Action of calcitonin gene-related peptide upon bovine vascular endothelial and smooth muscle cells grown in isolation and co-culture. Br J Pharmacol 99: 71–76, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuiper LL, Price PV, Christman BW. Systemic and pulmonary hypertension after abrupt cessation of prostacyclin: role of thromboxane A2. J Appl Physiol 80: 191–197, 1996 [DOI] [PubMed] [Google Scholar]
- 83.D'Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, Fabbretti E. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci 27: 8190–8201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeFeudis FV. Coronary atherosclerosis: current therapeutic approaches and future trends. Life Sci 49: 689–705, 1991 [DOI] [PubMed] [Google Scholar]
- 85.Degnan AP, Chaturvedula PV, Conway CM, Cook DA, Davis CD, Denton R, Han X, Macci R, Mathias NR, Moench P, Pin SS, Ren SX, Schartman R, Signor LJ, Thalody G, Widmann KA, Xu C, Macor JE, Dubowchik GM. Discovery of (R)-4-(8-fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)-N-(3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl)piperidine-1-carboxami de (BMS-694153): a potent antagonist of the human calcitonin gene-related peptide receptor for migraine with rapid and efficient intranasal exposure. J Med Chem 51: 4858–4861, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Deng PY, Li YJ. Calcitonin gene-related peptide and hypertension. Peptides 26: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Deng PY, Ye F, Cai WJ, Tan GS, Hu CP, Deng HW, Li YJ. Stimulation of calcitonin gene-related peptide synthesis and release: mechanisms for a novel antihypertensive drug, rutaecarpine. J Hypertens 22: 1819–1829, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Deng PY, Ye F, Zhu HQ, Cai WJ, Deng HW, Li YJ. An increase in the synthesis and release of calcitonin gene-related peptide in two-kidney, one-clip hypertensive rats. Regul Pept 114: 175–182, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Deng W, St Hilaire RC, Chattergoon NN, Jeter JR, Jr, Kadowitz PJ. Inhibition of vascular smooth muscle cell proliferation in vitro by genetically engineered marrow stromal cells secreting calcitonin gene-related peptide. Life Sci 78: 1830–1838, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Depre M, Macleod C, Palcza J, Behm M, de Lepeleire I, Han T, Panebianco D, Smith W, Blanchard R, Chodakewitz J, Murphy M, de Hoon J. Lack of hemodynamic interaction between CGRP-receptor antagonist telcagepant (MK-0974) and sumatriptan: results from a randomized study in patients with migraine. Cephalalgia 33: 1292–1301, 2013 [DOI] [PubMed] [Google Scholar]
- 91.Dhall U, Cowen T, Haven AJ, Burnstock G. Perivascular noradrenergic and peptide-containing nerves show different patterns of changes during development and ageing in the guinea-pig. J Auton Nerv Syst 16: 109–126, 1986 [DOI] [PubMed] [Google Scholar]
- 92.Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia 31: 573–584, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Ding L, Song T, Yi C, Huang Y, Yu W, Ling L, Dai Y, Wei Z. Transcutaneous electrical nerve stimulation (TENS) improves the diabetic cytopathy (DCP) via up-regulation of CGRP and cAMP. PLoS One 8: e57477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ditting T, Tiegs G, Rodionova K, Reeh PW, Neuhuber W, Freisinger W, Veelken R. Do distinct populations of dorsal root ganglion neurons account for the sensory peptidergic innervation of the kidney? Am J Physiol Renal Physiol 297: F1427–F1434, 2009 [DOI] [PubMed] [Google Scholar]
- 95.Dodick DW, Goadsby PJ, Spierings E, Scherer J, Sweeney S, Grayzel D. American Academy of Neurology. 2014 https://http://www.aan.com/PressRoom/Home/GetDigitalAsset/11217. [Google Scholar]
- 96.Dolgin E. Antibody drugs set to revive flagging migraine target. Nat Rev Drug Discov 12: 249–250, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience 49: 693–698, 1992 [DOI] [PubMed] [Google Scholar]
- 98.Donnerer J, Stein C. Evidence for an increase in the release of CGRP from sensory nerves during inflammation. Ann NY Acad Sci 657: 505–506, 1992 [DOI] [PubMed] [Google Scholar]
- 99.Donzelli S, Fischer G, King BS, Niemann C, DuMond JF, Heeren J, Wieboldt H, Baldus S, Gerloff C, Eschenhagen T, Carrier L, Boger RH, Espey MG. Pharmacological characterization of 1-nitrosocyclohexyl acetate, a long-acting nitroxyl donor that shows vasorelaxant and antiaggregatory effects. J Pharmacol Exp Ther 344: 339–347, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol 129: 420–423, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drake WM, Ajayi A, Lowe SR, Mirtella A, Bartlett TJ, Clark AJ. Desensitization of CGRP and adrenomedullin receptors in SK-N-MC cells: implications for the RAMP hypothesis. Endocrinology 140: 533–537, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Drake WM, Lowe SR, Mirtella A, Bartlett TJ, Clark AJ. Desensitisation of calcitonin gene-related peptide responsiveness but not adrenomedullin responsiveness in vascular smooth muscle cells. J Endocrinol 165: 133–138, 2000 [DOI] [PubMed] [Google Scholar]
- 103.Dubois-Rande JL, Merlet P, Benvenuti C, Sediame S, Macquin-Mavier I, Chabrier E, Braquet P, Castaigne A, Adnot S. Effects of calcitonin gene-related peptide on cardiac contractility, coronary hemodynamics and myocardial energetics in idiopathic dilated cardiomyopathy. Am J Cardiol 70: 906–912, 1992 [DOI] [PubMed] [Google Scholar]
- 104.Edvinsson L. Innervation and effects of dilatory neuropeptides on cerebral vessels. New Aspects Blood Vessels 28: 35–45, 1991 [DOI] [PubMed] [Google Scholar]
- 105.Edvinsson L, Chan KY, Eftekhari S, Nilsson E, de Vries R, Saveland H, Dirven CM, Danser AH, MaassenVanDenBrink A. Effect of the calcitonin gene-related peptide (CGRP) receptor antagonist telcagepant in human cranial arteries. Cephalalgia 30: 1233–1240, 2010 [DOI] [PubMed] [Google Scholar]
- 106.Edvinsson L, Ekman R, Thulin T. Reduced levels of calcitonin gene-related peptide (CGRP) but not substance P during and after treatment of severe hypertension in man. J Hum Hypertens 3: 267–270, 1989 [PubMed] [Google Scholar]
- 107.Edvinsson L, Fredholm BB, Hamel E, Jansen I, Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett 58: 213–217, 1985 [DOI] [PubMed] [Google Scholar]
- 108.Edvinsson L, Gulbenkian S, Barroso CP, Cunha e Sa M, Polak JM, Mortensen A, Jorgensen L, Jansen-Olesen I. Innervation of the human middle meningeal artery: immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides 19: 1213–1225, 1998 [DOI] [PubMed] [Google Scholar]
- 109.Edvinsson L, Sams A, Jansen-Olesen I, Tajti J, Kane SA, Rutledge RZ, Koblan KS, Hill RG, Longmore J. Characterisation of the effects of a non-peptide CGRP receptor antagonist in SK-N-MC cells and isolated human cerebral arteries. Eur J Pharmacol 415: 39–44, 2001 [DOI] [PubMed] [Google Scholar]
- 110.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169: 683–696, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275: 31438–31443, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Fabbretti E, D'Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 26: 6163–6171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fang L, Chen MF, Xiao ZL, Liu Y, Yu GL, Chen XB, Xie XM. Calcitonin gene-related peptide released from endothelial progenitor cells inhibits the proliferation of rat vascular smooth muscle cells induced by angiotensin II. Mol Cell Biochem 355: 99–108, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Favaloro JL, Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res 73: 587–596, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Ferland CE, Beaudry F, Vachon P. Antinociceptive effects of eugenol evaluated in a monoiodoacetate-induced osteoarthritis rat model. Phytother Res 26: 1278–1285, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav 97: 603–610, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Fernandes ES, Liang L, Smillie SJ, Kaiser F, Purcell R, Rivett DW, Alam S, Howat S, Collins H, Thompson SJ, Keeble JE, Riffo-Vasquez Y, Bruce KD, Brain SD. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J Immunol 188: 5741–5751, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Fernandes ES, Schmidhuber SM, Brain SD. Sensory-nerve-derived neuropeptides: possible therapeutic targets. Handb Exp Pharmacol 393–416, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Fernandez HL, Hodges-Savola CA. Axoplasmic transport of calcitonin gene-related peptide in rat peripheral nerve as a function of age. Neurochem Res 19: 1369–1377, 1994 [DOI] [PubMed] [Google Scholar]
- 120.Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett 388: 75–80, 2005 [DOI] [PubMed] [Google Scholar]
- 121.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 22: 633–658, 2002 [DOI] [PubMed] [Google Scholar]
- 122.Ferreira-Gomes J, Adaes S, Sarkander J, Castro-Lopes JM. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum 62: 3677–3685, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Fisher LA, Kikkawa DO, Rivier JE, Amara SG, Evans RM, Rosenfeld MG, Vale WW, Brown MR. Stimulation of noradrenergic sympathetic outflow by calcitonin gene-related peptide. Nature 305: 534–536, 1983 [DOI] [PubMed] [Google Scholar]
- 124.Fluhmann B, Muff R, Hunziker W, Fischer JA, Born W. A human orphan calcitonin receptor-like structure. Biochem Biophys Res Commun 206: 341–347, 1995 [DOI] [PubMed] [Google Scholar]
- 125.Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal 8: 3–26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fuchs D, Birklein F, Reeh PW, Sauer SK. Sensitized peripheral nociception in experimental diabetes of the rat. Pain 151: 496–505, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Fujioka S, Sasakawa O, Kishimoto H, Tsumura K, Morii H. The antihypertensive effect of calcitonin gene-related peptide in rats with norepinephrine- and angiotensin II-induced hypertension. J Hypertens 9: 175–179, 1991 [DOI] [PubMed] [Google Scholar]
- 128.Gallai V, Sarchielli P, Floridi A, Franceschini M, Codini M, Glioti G, Trequattrini A, Palumbo R. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia 15: 384–390, 1995 [DOI] [PubMed] [Google Scholar]
- 129.Gamse R, Saria A. Nociceptive behavior after intrathecal injections of substance P, neurokinin A and calcitonin gene-related peptide in mice. Neurosci Lett 70: 143–147, 1986 [DOI] [PubMed] [Google Scholar]
- 130.Gangula PR, Chauhan M, Reed L, Yallampalli C. Age-related changes in dorsal root ganglia, circulating and vascular calcitonin gene-related peptide (CGRP) concentrations in female rats: effect of female sex steroid hormones. Neurosci Lett 454: 118–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gangula PR, Supowit SC, Wimalawansa SJ, Zhao H, Hallman DM, DiPette DJ, Yallampalli C. Calcitonin gene-related peptide is a depressor in NG-nitro-l-arginine methyl ester-induced hypertension during pregnancy. Hypertension 29: 248–253, 1997 [DOI] [PubMed] [Google Scholar]
- 132.Gangula PR, Zhao H, Supowit S, Wimalawansa S, DiPette D, Yallampalli C. Pregnancy and steroid hormones enhance the vasodilation responses to CGRP in rats. Am J Physiol Heart Circ Physiol 276: H284–H288, 1999 [DOI] [PubMed] [Google Scholar]
- 133.Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, Gagel RF, Yallampalli C. Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension 35: 470–475, 2000 [DOI] [PubMed] [Google Scholar]
- 134.Gardiner SM, Compton AM, Kemp PA, Bennett T, Bose C, Foulkes R, Hughes B. Antagonistic effect of human alpha-calcitonin gene-related peptide (8–37) on regional hemodynamic actions of rat islet amyloid polypeptide in conscious Long-Evans rats. Diabetes 40: 948–951, 1991 [DOI] [PubMed] [Google Scholar]
- 135.Gennari C, Fischer JA. Cardiovascular action of calcitonin gene-related peptide in humans. Calcif Tissue Int 37: 581–584, 1985 [DOI] [PubMed] [Google Scholar]
- 136.Gennari C, Nami R, Agnusdei D, Fischer JA. Improved cardiac performance with human calcitonin gene related peptide in patients with congestive heart failure. Cardiovasc Res 24: 239–241, 1990 [DOI] [PubMed] [Google Scholar]
- 137.Gherardini G, Curinga G, Colella G, Freda N, Rauso R. Calcitonin gene-related peptide and thermal injury: review of literature. Eplasty 9: e30, 2009 [PMC free article] [PubMed] [Google Scholar]
- 138.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Coughlin KA, Kaczmarska MJ, Castaneda-Corral G, Bloom AP, Kuskowski MA, Mantyh PW. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum 64: 2223–2232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett 57: 125–130, 1985 [DOI] [PubMed] [Google Scholar]
- 140.Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci 4: 3101–3111, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gillardon F, Moll I, Michel S, Benrath J, Weihe E, Zimmermann M. Calcitonin gene-related peptide and nitric oxide are involved in ultraviolet radiation-induced immunosuppression. Eur J Pharmacol 293: 395–400, 1995 [DOI] [PubMed] [Google Scholar]
- 142.Gillardon F, Schrock H, Morano I, Zimmermann M. Long-term increase in CGRP levels in rat spinal dorsal horn following skin ultraviolet irradiation. A mechanism of sunburn pain? Ann NY Acad Sci 657: 493–496, 1992 [DOI] [PubMed] [Google Scholar]
- 143.Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol 37: 83–90, 2008 [DOI] [PubMed] [Google Scholar]
- 144.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience 161: 327–341, 2009 [DOI] [PubMed] [Google Scholar]
- 145.Goadsby PJ, Dodick DW, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Woodinville J, Smith J. American Academy of Neurology. 2014 doi: 10.1016/S1474-4422(14)70209-1. https://http://www.aan.com/PressRoom/Home/GetDigitalAsset/11219. [DOI] [PubMed] [Google Scholar]
- 146.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33: 48–56, 1993 [DOI] [PubMed] [Google Scholar]
- 147.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 23: 193–196, 1988 [DOI] [PubMed] [Google Scholar]
- 148.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28: 183–187, 1990 [DOI] [PubMed] [Google Scholar]
- 149.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol 213: 626–634, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock 24: 590–594, 2005 [DOI] [PubMed] [Google Scholar]
- 151.Graepel R, Fernandes ES, Aubdool AA, Andersson DA, Bevan S, Brain SD. 4-Oxo-2-nonenal (4-ONE): evidence of transient receptor potential ankyrin 1-dependent and -independent nociceptive and vasoactive responses in vivo. J Pharmacol Exp Ther 337: 117–124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gram DX, Ahren B, Nagy I, Olsen UB, Brand CL, Sundler F, Tabanera R, Svendsen O, Carr RD, Santha P, Wierup N, Hansen AJ. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci 25: 213–223, 2007 [DOI] [PubMed] [Google Scholar]
- 153.Gram DX, Hansen AJ, Wilken M, Elm T, Svendsen O, Carr RD, Ahren B, Brand CL. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol 153: 963–969, 2005 [DOI] [PubMed] [Google Scholar]
- 154.Grant AD, Gerard NP, Brain SD. Evidence of a role for NK1 and CGRP receptors in mediating neurogenic vasodilatation in the mouse ear. Br J Pharmacol 135: 356–362, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gray DW, Marshall I. Human alpha-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol 107: 691–696, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain 8: 85, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupta P, Harte A, Sturdee DW, Sharma A, Barnett AH, Kumar S, McTernan PG. Effects of menopausal status on circulating calcitonin gene-related peptide and adipokines: implications for insulin resistance and cardiovascular risks. Climacteric 11: 364–372, 2008 [DOI] [PubMed] [Google Scholar]
- 158.Gupta S, Amrutkar DV, Mataji A, Salmasi H, Hay-Schmidt A, Sheykhzade M, Messlinger K, Olesen J, Jansen-Olesen I. Evidence for CGRP re-uptake in rat dura mater encephali. Br J Pharmacol 161: 1885–1898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gupta S, Mehrotra S, Villalon C, De Vries R, Garrelds I, Saxena P, Vandenbrink AM. Effects of female sex hormones on responses to CGRP, acetylcholine, and 5-HT in rat isolated arteries. Headache 47: 564–575, 2007 [DOI] [PubMed] [Google Scholar]
- 160.Hagner S, Haberberger RV, Overkamp D, Hoffmann R, Voigt KH, McGregor GP. Expression and distribution of calcitonin receptor-like receptor in human hairy skin. Peptides 23: 109–116, 2002 [DOI] [PubMed] [Google Scholar]
- 161.Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res 310: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 162.Hanko J, Hardebo JE, Kahrstrom J, Owman C, Sundler F. Calcitonin gene-related peptide is present in mammalian cerebrovascular nerve fibres and dilates pial and peripheral arteries. Neurosci Lett 57: 91–95, 1985 [DOI] [PubMed] [Google Scholar]
- 163.Hao X, Chen J, Luo Z, He H, Yu H, Ma L, Ma S, Zhu T, Liu D, Zhu Z. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflügers Arch 461: 345–353, 2011 [DOI] [PubMed] [Google Scholar]
- 164.Hartopo AB, Emoto N, Vignon-Zellweger N, Suzuki Y, Yagi K, Nakayama K, Hirata K. Endothelin-converting enzyme-1 gene ablation attenuates pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am J Respir Cell Mol Biol 48: 465–476, 2013 [DOI] [PubMed] [Google Scholar]
- 165.Hasbak P, Saetrum Opgaard O, Eskesen K, Schifter S, Arendrup H, Longmore J, Edvinsson L. Investigation of CGRP receptors and peptide pharmacology in human coronary arteries. Characterization with a nonpeptide antagonist. J Pharmacol Exp Ther 304: 326–333, 2003 [DOI] [PubMed] [Google Scholar]
- 166.Hasbak P, Sams A, Schifter S, Longmore J, Edvinsson L. CGRP receptors mediating CGRP-, adrenomedullin- and amylin-induced relaxation in porcine coronary arteries. Characterization with “Compound 1” (WO98/11128), a non-peptide antagonist. Br J Pharmacol 133: 1405–1413, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hashikawa-Hobara N, Hashikawa N, Zamami Y, Takatori S, Kawasaki H. The mechanism of calcitonin gene-related peptide-containing nerve innervation. J Pharmacol Sci 119: 117–121, 2012 [DOI] [PubMed] [Google Scholar]
- 168.Hay DL, Poyner DR. Calcitonin gene-related peptide, adrenomedullin and flushing. Maturitas 64: 104–108, 2009 [DOI] [PubMed] [Google Scholar]
- 169.Hay DL, Poyner DR, Quirion R. International Union of Pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev 60: 143–145, 2008 [DOI] [PubMed] [Google Scholar]
- 170.Hay DL, Poyner DR, Smith DM. Desensitisation of adrenomedullin and CGRP receptors. Regul Pept 112: 139–145, 2003 [DOI] [PubMed] [Google Scholar]
- 171.Hay DL, Walker CS, Poyner DR. Adrenomedullin and calcitonin gene-related peptide receptors in endocrine-related cancers: opportunities and challenges. Endocr Relat Cancer 18: C1–14, 2011 [DOI] [PubMed] [Google Scholar]
- 172.He Y, Ding G, Wang X, Zhu T, Fan S. Calcitonin gene-related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J 113: 747–751, 2000 [PubMed] [Google Scholar]
- 173.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 58: 15–25, 2008 [DOI] [PubMed] [Google Scholar]
- 174.Hernanz A, De Miguel E, Romera N, Perez-Ayala C, Gijon J, Arnalich F. Calcitonin gene-related peptide II, substance P and vasoactive intestinal peptide in plasma and synovial fluid from patients with inflammatory joint disease. Br J Rheumatol 32: 31–35, 1993 [DOI] [PubMed] [Google Scholar]
- 175.Hernanz A, Medina S, de Miguel E, Martin-Mola E. Effect of calcitonin gene-related peptide, neuropeptide Y, substance P, and vasoactive intestinal peptide on interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha production by peripheral whole blood cells from rheumatoid arthritis and osteoarthritis patients. Regul Pept 115: 19–24, 2003 [DOI] [PubMed] [Google Scholar]
- 176.Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia 31: 712–722, 2011 [DOI] [PubMed] [Google Scholar]
- 177.Hewitt DJ, Martin V, Lipton RB, Brandes J, Ceesay P, Gottwald R, Schaefer E, Lines C, Ho TW. Randomized controlled study of telcagepant plus Ibuprofen or acetaminophen in migraine. Headache 51: 533–543, 2011 [DOI] [PubMed] [Google Scholar]
- 178.Hilairet S, Belanger C, Bertrand J, Laperriere A, Foord SM, Bouvier M. Agonist-promoted internalization of a ternary complex between calcitonin receptor-like receptor, receptor activity-modifying protein 1 (RAMP1), and beta-arrestin. J Biol Chem 276: 42182–42190, 2001 [DOI] [PubMed] [Google Scholar]
- 179.Hilairet S, Foord SM, Marshall FH, Bouvier M. Protein-protein interaction and not glycosylation determines the binding selectivity of heterodimers between the calcitonin receptor-like receptor and the receptor activity-modifying proteins. J Biol Chem 276: 29575–29581, 2001 [DOI] [PubMed] [Google Scholar]
- 180.Ho AP, Dahlof CG, Silberstein SD, Saper JR, Ashina M, Kost JT, Froman S, Leibensperger H, Lines CR, Ho TW. Randomized, controlled trial of telcagepant over four migraine attacks. Cephalalgia 30: 1443–1457, 2010 [DOI] [PubMed] [Google Scholar]
- 181.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372: 2115–2123, 2008 [DOI] [PubMed] [Google Scholar]
- 182.Ho TW, Ho AP, Chaitman BR, Johnson C, Mathew NT, Kost J, Fan X, Aurora SK, Brandes JL, Fei K, Beebe L, Lines C, Krucoff MW. Randomized, controlled study of telcagepant in patients with migraine and coronary artery disease. Headache 52: 224–235, 2012 [DOI] [PubMed] [Google Scholar]
- 183.Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today 10: 417–427, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Hobara N, Gessei-Tsutsumi N, Goda M, Takayama F, Akiyama S, Kurosaki Y, Kawasaki H. Long-term inhibition of angiotensin prevents reduction of periarterial innervation of calcitonin gene-related peptide (CGRP)-containing nerves in spontaneously hypertensive rats. Hypertens Res 28: 465–474, 2005 [DOI] [PubMed] [Google Scholar]
- 185.Holmlund A, Ekblom A, Hansson P, Lind J, Lundeberg T, Theodorsson E. Concentrations of neuropeptides substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid of the human temporomandibular joint. A correlation with symptoms, signs and arthroscopic findings. Int J Oral Maxillofac Surg 20: 228–231, 1991 [DOI] [PubMed] [Google Scholar]
- 186.Holzer P. Peptidergic sensory neurons in the control of vascular functions: mechanisms and significance in the cutaneous and splanchnic vascular beds. Rev Physiol Biochem Pharmacol 121: 49–146, 1992 [DOI] [PubMed] [Google Scholar]
- 187.Holzmann B. Modulation of immune responses by the neuropeptide CGRP. Amino Acids 45: 1–7, 2013 [DOI] [PubMed] [Google Scholar]
- 188.Hong KW, Yoo SE, Yu SS, Lee JY, Rhim BY. Pharmacological coupling and functional role for CGRP receptors in the vasodilation of rat pial arterioles. Am J Physiol Heart Circ Physiol 270: H317–H323, 1996 [DOI] [PubMed] [Google Scholar]
- 189.Hou Q, Barr T, Gee L, Vickers J, Wymer J, Borsani E, Rodella L, Getsios S, Burdo T, Eisenberg E, Guha U, Lavker R, Kessler J, Chittur S, Fiorino D, Rice F, Albrecht P. Keratinocyte expression of calcitonin gene-related peptide beta: implications for neuropathic and inflammatory pain mechanisms. Pain 152: 2036–2051, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Howitt SG, Kilk K, Wang Y, Smith DM, Langel U, Poyner DR. The role of the 8–18 helix of CGRP8–37 in mediating high affinity binding to CGRP receptors; coulombic and steric interactions. Br J Pharmacol 138: 325–332, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hsu JH, Yeh JL, Dai ZK, Chen IJ, Wu JR. Increased circulating calcitonin gene-related peptide in congestive heart failure caused by congenital heart disease. Int Heart J 46: 867–875, 2005 [DOI] [PubMed] [Google Scholar]
- 192.Hu CP, Peng J, Xiao L, Ye F, Deng HW, Li YJ. Effect of age on alpha-calcitonin gene-related peptide-mediated delayed cardioprotection induced by intestinal preconditioning in rats. Regul Pept 107: 137–143, 2002 [DOI] [PubMed] [Google Scholar]
- 193.Huang J, Stohl LL, Zhou X, Ding W, Granstein RD. Calcitonin gene-related peptide inhibits chemokine production by human dermal microvascular endothelial cells. Brain Behav Immun 25: 787–799, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang R, Karve A, Shah I, Bowers MC, DiPette DJ, Supowit SC, Abela GS. Deletion of the mouse alpha-calcitonin gene-related peptide gene increases the vulnerability of the heart to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 294: H1291–H1297, 2008 [DOI] [PubMed] [Google Scholar]
- 195.Huttemeier PC, Ritter EF, Benveniste H. Calcitonin gene-related peptide mediates hypotension and tachycardia in endotoxic rats. Am J Physiol Heart Circ Physiol 265: H767–H769, 1993 [DOI] [PubMed] [Google Scholar]
- 196.Ichinose M, Sawada M. Enhancement of phagocytosis by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Peptides 17: 1405–1414, 1996 [DOI] [PubMed] [Google Scholar]
- 197.Ieda M, Kanazawa H, Ieda Y, Kimura K, Matsumura K, Tomita Y, Yagi T, Onizuka T, Shimoji K, Ogawa S, Makino S, Sano M, Fukuda K. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation 114: 2351–2363, 2006 [DOI] [PubMed] [Google Scholar]
- 198.Itabashi A, Kashiwabara H, Shibuya M, Tanaka K, Masaoka H, Katayama S, Ishii J. The interaction of calcitonin gene-related peptide with angiotensin II on blood pressure and renin release. J Hypertens Suppl 6: S418–420, 1988 [DOI] [PubMed] [Google Scholar]
- 199.Jang MU, Park JW, Kho HS, Chung SC, Chung JW. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis 17: 187–193, 2011 [DOI] [PubMed] [Google Scholar]
- 200.Jiang Y, Nyengaard JR, Zhang JS, Jakobsen J. Selective loss of calcitonin gene-related peptide-expressing primary sensory neurons of the a-cell phenotype in early experimental diabetes. Diabetes 53: 2669–2675, 2004 [DOI] [PubMed] [Google Scholar]
- 201.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 14: R101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jolicoeur FB, Menard D, Fournier A, St-Pierre S. Structure-activity analysis of CGRP's neurobehavioral effects. Ann NY Acad Sci 657: 155–163, 1992 [DOI] [PubMed] [Google Scholar]
- 203.Joyce CD, Fiscus RR, Wang X, Dries DJ, Morris RC, Prinz RA. Calcitonin gene-related peptide levels are elevated in patients with sepsis. Surgery 108: 1097–1101, 1990 [PubMed] [Google Scholar]
- 204.Jusek G, Reim D, Tsujikawa K, Holzmann B. Deficiency of the CGRP receptor component RAMP1 attenuates immunosuppression during the early phase of septic peritonitis. Immunobiology 217: 761–767, 2012 [DOI] [PubMed] [Google Scholar]
- 205.Kallner G. Release and effects of calcitonin gene-related peptide in myocardial ischaemia. Scand Cardiovasc J Suppl 49: 1–35, 1998 [PubMed] [Google Scholar]
- 206.Kallner G, Gonon A, Franco-Cereceda A. Calcitonin gene-related peptide in myocardial ischaemia and reperfusion in the pig. Cardiovasc Res 38: 493–499, 1998 [DOI] [PubMed] [Google Scholar]
- 207.Karanth SS, Dhital S, Springall DR, Polak JM. Reinnervation and neuropeptides in mouse skin flaps. J Auton Nerv Syst 31: 127–134, 1990 [DOI] [PubMed] [Google Scholar]
- 208.Katayama M, Nadel JA, Bunnett NW, Di Maria GU, Haxhiu M, Borson DB. Catabolism of calcitonin gene-related peptide and substance P by neutral endopeptidase. Peptides 12: 563–567, 1991 [DOI] [PubMed] [Google Scholar]
- 209.Kawasaki H, Saito A, Goto K, Takasaki K. Age-related changes in calcitonin gene-related peptide (CGRP)-mediated neurogenic vasodilation of the mesenteric resistance vessel in SHR. Clin Exp Hypertens A 13: 745–754, 1991 [DOI] [PubMed] [Google Scholar]
- 210.Kawasaki H, Saito A, Takasaki K. Age-related decrease of calcitonin gene-related peptide-containing vasodilator innervation in the mesenteric resistance vessel of the spontaneously hypertensive rat. Circ Res 67: 733–743, 1990 [DOI] [PubMed] [Google Scholar]
- 211.Kawasaki H, Takasaki K. Age-related decrease of neurogenic release of calcitonin gene-related peptide from perivascular nerves in spontaneously hypertensive rats. Clin Exp Hypertens A 14: 989–1001, 1992 [DOI] [PubMed] [Google Scholar]
- 212.Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 335: 164–167, 1988 [DOI] [PubMed] [Google Scholar]
- 213.Kawasaki H, Takenaga M, Araki H, Futagami K, Gomita Y. Angiotensin inhibits neurotransmission of calcitonin gene-related peptide-containing vasodilator nerves in mesenteric artery of spontaneously hypertensive rats. J Pharmacol Exp Ther 284: 508–515, 1998 [PubMed] [Google Scholar]
- 214.Kawase T, Okuda K, Burns DM. Immature osteoblastic MG63 cells possess two calcitonin gene-related peptide receptor subtypes that respond differently to [Cys(Acm)(2,7)] calcitonin gene-related peptide and CGRP(8–37). Am J Physiol Cell Physiol 289: C811–C818, 2005 [DOI] [PubMed] [Google Scholar]
- 215.Kawase T, Okuda K, Wu CH, Yoshie H, Hara K, Burns DM. Calcitonin gene-related peptide acts as a mitogen for human Gin-1 gingival fibroblasts by activating the MAP kinase signalling pathway. J Periodontal Res 34: 160–168, 1999 [DOI] [PubMed] [Google Scholar]
- 216.Keith IM, Ekman R. Dynamic aspects of regulatory lung peptides in chronic hypoxic pulmonary hypertension. Exp Lung Res 18: 205–224, 1992 [DOI] [PubMed] [Google Scholar]
- 217.Keith IM, Tjen ALS, Kraiczi H, Ekman R. Three-week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 279: H1571–H1578, 2000 [DOI] [PubMed] [Google Scholar]
- 218.Kendall CH, Homer CE, Bishop AE, Polak JM. Age-related peptide production by human thyroid C cells. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol 410: 97–101, 1986 [DOI] [PubMed] [Google Scholar]
- 219.Khalil Z, Helme R. Sensory peptides as neuromodulators of wound healing in aged rats. J Gerontol A Biol Sci Med Sci 51: B354–361, 1996 [DOI] [PubMed] [Google Scholar]
- 220.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol 24: 229–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim YG, Lone AM, Nolte WM, Saghatelian A. Peptidomics approach to elucidate the proteolytic regulation of bioactive peptides. Proc Natl Acad Sci USA 109: 8523–8527, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kjartansson J, Dalsgaard CJ. Calcitonin gene-related peptide increases survival of a musculocutaneous critical flap in the rat. Eur J Pharmacol 142: 355–358, 1987 [DOI] [PubMed] [Google Scholar]
- 223.Koth CM, Abdul-Manan N, Lepre CA, Connolly PJ, Yoo S, Mohanty AK, Lippke JA, Zwahlen J, Coll JT, Doran JD, Garcia-Guzman M, Moore JM. Refolding and characterization of a soluble ectodomain complex of the calcitonin gene-related peptide receptor. Biochemistry 49: 1862–1872, 2010 [DOI] [PubMed] [Google Scholar]
- 224.Kubota M, Moseley JM, Butera L, Dusting GJ, MacDonald PS, Martin TJ. Calcitonin gene-related peptide stimulates cyclic AMP formation in rat aortic smooth muscle cells. Biochem Biophys Res Commun 132: 88–94, 1985 [DOI] [PubMed] [Google Scholar]
- 225.Kurihara H, Shindo T, Oh-Hashi Y, Kurihar Y, Kuwaki T. Targeted disruption of adrenomedullin and alphaCGRP genes reveals their distinct biological roles. Hypertens Res 26 Suppl: S105–108, 2003 [DOI] [PubMed] [Google Scholar]
- 226.Kusano S, Kukimoto-Niino M, Akasaka R, Toyama M, Terada T, Shirouzu M, Shindo T, Yokoyama S. Crystal structure of the human receptor activity-modifying protein 1 extracellular domain. Protein Sci 17: 1907–1914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lange M, Enkhbaatar P, Traber DL, Cox RA, Jacob S, Mathew BP, Hamahata A, Traber LD, Herndon DN, Hawkins HK. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol 107: 176–184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Larsson J, Ekblom A, Henriksson K, Lundeberg T, Theodorsson E. Concentration of substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scand J Rheumatol 20: 326–335, 1991 [DOI] [PubMed] [Google Scholar]
- 229.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 22: 54–61, 2002 [DOI] [PubMed] [Google Scholar]
- 230.Lassen LH, Jacobsen VB, Haderslev PA, Sperling B, Iversen HK, Olesen J, Tfelt-Hansen P. Involvement of calcitonin gene-related peptide in migraine: regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain 9: 151–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Le Greves P, Nyberg F, Hokfelt T, Terenius L. Calcitonin gene-related peptide is metabolized by an endopeptidase hydrolyzing substance P. Regul Pept 25: 277–286, 1989 [DOI] [PubMed] [Google Scholar]
- 232.Le Greves P, Nyberg F, Terenius L, Hokfelt T. Calcitonin gene-related peptide is a potent inhibitor of substance P degradation. Eur J Pharmacol 115: 309–311, 1985 [DOI] [PubMed] [Google Scholar]
- 233.Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507: 1277–1299, 2008 [DOI] [PubMed] [Google Scholar]
- 234.Lewis T. Nocifensor system of nerves (II). Br Med J 1: 491–494, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li D, Chen BM, Peng J, Zhang YS, Li XH, Yuan Q, Hu CP, Deng HW, Li YJ. Role of anandamide transporter in regulating calcitonin gene-related peptide production and blood pressure in hypertension. J Hypertens 27: 1224–1232, 2009 [DOI] [PubMed] [Google Scholar]
- 236.Li D, Peng J, Xin HY, Luo D, Zhang YS, Zhou Z, Jiang DJ, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats. Peptides 29: 1781–1788, 2008 [DOI] [PubMed] [Google Scholar]
- 237.Li J, Carnevale KA, Dipette DJ, Supowit SC. Renal protective effects of alpha-calcitonin gene-related peptide in deoxycorticosterone-salt hypertension. Am J Physiol Renal Physiol 304: F1000–F1008, 2013 [DOI] [PubMed] [Google Scholar]
- 238.Li J, Levick SP, Dipette DJ, Janicki JS, Supowit SC. Alpha-calcitonin gene-related peptide is protective against pressure overload-induced heart failure. Regul Pept 185C: 20–28, 2013 [DOI] [PubMed] [Google Scholar]
- 239.Li J, Wang DH. Development of angiotensin II-induced hypertension: role of CGRP and its receptor. J Hypertens 23: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 240.Li JZ, Peng J, Xiao L, Zhang YS, Liao MC, Li XH, Hu CP, Deng HW, Li YJ. Reversal of isoprenaline-induced cardiac remodeling by rutaecarpine via stimulation of calcitonin gene-related peptide production. Can J Physiol Pharmacol 88: 949–959, 2010 [DOI] [PubMed] [Google Scholar]
- 241.Li W, Wang T, Ma C, Xiong T, Zhu Y, Wang X. Calcitonin gene-related peptide inhibits interleukin-1beta-induced endogenous monocyte chemoattractant protein-1 secretion in type II alveolar epithelial cells. Am J Physiol Cell Physiol 291: C456–C465, 2006 [DOI] [PubMed] [Google Scholar]
- 242.Li XW, Hu CP, Wu WH, Zhang WF, Zou XZ, Li YJ. Inhibitory effect of calcitonin gene-related peptide on hypoxia-induced rat pulmonary artery smooth muscle cells proliferation: role of ERK1/2 and p27. Eur J Pharmacol 679: 117–126, 2012 [DOI] [PubMed] [Google Scholar]
- 243.Li Y, Fiscus RR, Wu J, Yang L, Wang X. The antiproliferative effects of calcitonin gene-related peptide in different passages of cultured vascular smooth muscle cells. Neuropeptides 31: 503–509, 1997 [DOI] [PubMed] [Google Scholar]
- 244.Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci 24: 9623–9631, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Li YJ, Song QJ, Xiao J. Calcitonin gene-related peptide: an endogenous mediator of preconditioning. Acta Pharmacol Sin 21: 865–869, 2000 [PubMed] [Google Scholar]
- 246.Liao DF, Monia B, Dean N, Berk BC. Protein kinase C-zeta mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J Biol Chem 272: 6146–6150, 1997 [DOI] [PubMed] [Google Scholar]
- 247.Lind H, Brudin L, Lindholm L, Edvinsson L. Different levels of sensory neuropeptides (calcitonin gene-related peptide and substance P) during and after exercise in man. Clin Physiol 16: 73–82, 1996 [DOI] [PubMed] [Google Scholar]
- 248.Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med 32: 1715–1721, 2004 [DOI] [PubMed] [Google Scholar]
- 249.Lou H, Gagel RF. Alternative RNA processing–its role in regulating expression of calcitonin/calcitonin gene-related peptide. J Endocrinol 156: 401–405, 1998 [DOI] [PubMed] [Google Scholar]
- 250.Lu JT, Son YJ, Lee J, Jetton TL, Shiota M, Moscoso L, Niswender KD, Loewy AD, Magnuson MA, Sanes JR, Emeson RB. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol Cell Neurosci 14: 99–120, 1999 [DOI] [PubMed] [Google Scholar]
- 251.Lu R, Hu CP, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated ischemic preconditioning in the rat heart: influence of age. Regul Pept 99: 183–189, 2001 [DOI] [PubMed] [Google Scholar]
- 252.Lu R, Hu CP, Peng J, Deng HW, Li YJ. Role of calcitonin gene-related peptide in ischaemic preconditioning in diabetic rat hearts. Clin Exp Pharmacol Physiol 28: 392–396, 2001 [DOI] [PubMed] [Google Scholar]
- 253.Lu R, Zhu HQ, Peng J, Li NS, Li YJ. Endothelium-dependent vasorelaxation and the expression of calcitonin gene-related peptide in aged rats. Neuropeptides 36: 407–412, 2002 [DOI] [PubMed] [Google Scholar]
- 254.Luebke AE, Dahl GP, Roos BA, Dickerson IM. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc Natl Acad Sci USA 93: 3455–3460, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lundberg JM, Franco-Cereceda A, Hua X, Hokfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol 108: 315–319, 1985 [DOI] [PubMed] [Google Scholar]
- 256.Lundberg JM, Hua Y, Fredholm BB. Capsaicin-induced stimulation of the guinea-pig atrium. Involvement of a novel sensory transmitter or a direct action on myocytes? Naunyn-Schmiedebergs Arch Pharmacol 325: 176–182, 1984 [DOI] [PubMed] [Google Scholar]
- 257.Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ, Li D, Deng HW, Li YJ. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul Pept 150: 66–72, 2008 [DOI] [PubMed] [Google Scholar]
- 258.Luo G, Chen L, Conway CM, Denton R, Keavy D, Signor L, Kostich W, Lentz KA, Santone KS, Schartman R, Browning M, Tong G, Houston JG, Dubowchik GM, Macor JE. Discovery of (5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyri din-9-yl 4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxylate (BMS-927711): an oral calcitonin gene-related peptide (CGRP) antagonist in clinical trials for treating migraine. J Med Chem 55: 10644–10651, 2012 [DOI] [PubMed] [Google Scholar]
- 259.Luo XL, Yang TL, Chen XP, Li YJ. Association of CALCA genetic polymorphism with essential hypertension. Chin Med J 121: 1407–1410, 2008 [PubMed] [Google Scholar]
- 260.Ma YX, Guo Z, Sun T. CGRP inhibits norepinephrine induced apoptosis with restoration of Bcl-2/Bax in cultured cardiomyocytes of rat. Neurosci Lett 549: 130–134, 2013 [DOI] [PubMed] [Google Scholar]
- 261.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol 45: 1–98, 1995 [DOI] [PubMed] [Google Scholar]
- 262.Mair J, Lechleitner P, Langle T, Wiedermann C, Dienstl F, Saria A. Plasma CGRP in acute myocardial infarction. Lancet 335: 168, 1990 [DOI] [PubMed] [Google Scholar]
- 263.Mallee JJ, Salvatore CA, LeBourdelles B, Oliver KR, Longmore J, Koblan KS, Kane SA. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem 277: 14294–14298, 2002 [DOI] [PubMed] [Google Scholar]
- 264.Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J Neurochem 97: 310–333, 2006 [DOI] [PubMed] [Google Scholar]
- 265.Mapp PI, McWilliams DF, Turley MJ, Hargin E, Walsh DA. A role for the sensory neuropeptide calcitonin gene-related peptide in endothelial cell proliferation in vivo. Br J Pharmacol 166: 1261–1271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia 34: 114–125, 2014 [DOI] [PubMed] [Google Scholar]
- 267.Marquez-Rodas I, Longo F, Rothlin RP, Balfagon G. Pathophysiology and therapeutic possibilities of calcitonin gene-related peptide in hypertension. J Physiol Biochem 62: 45–56, 2006 [DOI] [PubMed] [Google Scholar]
- 268.Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie SJ, Lalgi K, Fernandes ES, Gnudi L, Brain SD. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 61: 246–252, 2013 [DOI] [PubMed] [Google Scholar]
- 269.Maslov LN, Gorbunov AS, Lishmanov YB. Cardioprotective effect of ischemic postconditioning on the model of isolated heart. Bull Exp Biol Med 153: 313–314, 2012 [DOI] [PubMed] [Google Scholar]
- 270.Mason RT, Peterfreund RA, Sawchenko PE, Corrigan AZ, Rivier JE, Vale WW. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature 308: 653–655, 1984 [DOI] [PubMed] [Google Scholar]
- 271.Massaad CA, Safieh-Garabedian B, Poole S, Atweh SF, Jabbur SJ, Saade NE. Involvement of substance P, CGRP and histamine in the hyperalgesia and cytokine upregulation induced by intraplantar injection of capsaicin in rats. J Neuroimmunol 153: 171–182, 2004 [DOI] [PubMed] [Google Scholar]
- 272.Masuda A, Shimamoto K, Mori Y, Nakagawa M, Ura N, Iimura O. Plasma calcitonin gene-related peptide levels in patients with various hypertensive diseases. J Hypertens 10: 1499–1504, 1992 [DOI] [PubMed] [Google Scholar]
- 273.Matteoli M, Haimann C, Torri-Tarelli F, Polak JM, Ceccarelli B, De Camilli P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci USA 85: 7366–7370, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.McCormack DG, Mak JC, Coupe MO, Barnes PJ. Calcitonin gene-related peptide vasodilation of human pulmonary vessels. J Appl Physiol 67: 1265–1270, 1989 [DOI] [PubMed] [Google Scholar]
- 275.McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 78: 138–151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.McCoy ES, Taylor-Blake B, Zylka MJ. CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PLoS One 7: e36355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.McDougall JJ, Ferrell WR, Bray RC. Neurogenic origin of articular hyperemia in early degenerative joint disease. Am J Physiol Regul Integr Comp Physiol 276: R745–R752, 1999 [DOI] [PubMed] [Google Scholar]
- 278.McGillis JP, Humphreys S, Rangnekar V, Ciallella J. Modulation of B lymphocyte differentiation by calcitonin gene-related peptide (CGRP). I. Characterization of high-affinity CGRP receptors on murine 70Z/3 cells. Cell Immunol 150: 391–404, 1993 [DOI] [PubMed] [Google Scholar]
- 279.McGillis JP, Miller CN, Schneider DB, Fernandez S, Knopf M. Calcitonin gene-related peptide induces AP-1 activity by a PKA and c-fos-dependent mechanism in pre-B cells. J Neuroimmunol 123: 83–90, 2002 [DOI] [PubMed] [Google Scholar]
- 280.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333–339, 1998 [DOI] [PubMed] [Google Scholar]
- 281.McNeish AJ, Roux BT, Aylett SB, Van Den Brink AM, Cottrell GS. Endosomal proteolysis regulates calcitonin gene-related peptide responses in mesenteric arteries. Br J Pharmacol 167: 1679–1690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Meens MJ, Fazzi GE, van Zandvoort MA, De Mey JG. Calcitonin gene-related peptide selectively relaxes contractile responses to endothelin-1 in rat mesenteric resistance arteries. J Pharmacol Exp Ther 331: 87–95, 2009 [DOI] [PubMed] [Google Scholar]
- 283.Meens MJ, Mattheij NJ, van Loenen PB, Spijkers LJ, Lemkens P, Nelissen J, Compeer MG, Alewijnse AE, De Mey JG. G-protein betagamma subunits in vasorelaxing and anti-endothelinergic effects of calcitonin gene-related peptide. Br J Pharmacol 166: 297–308, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci 120: 2864–2874, 2007 [DOI] [PubMed] [Google Scholar]
- 285.Michel D, Zimmermann W. [Treatment of arterial hypertension in the aged]. Fortschr Med 101: 2031–2038, 1983 [PubMed] [Google Scholar]
- 286.Mikami N, Matsushita H, Kato T, Kawasaki R, Sawazaki T, Kishimoto T, Ogitani Y, Watanabe K, Miyagi Y, Sueda K, Fukada S, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol 186: 6886–6893, 2011 [DOI] [PubMed] [Google Scholar]
- 287.Mikami N, Miyagi Y, Sueda K, Takatsuji M, Fukada S, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide and cyclic adenosine 5'-monophosphate/protein kinase A pathway promote IL-9 production in Th9 differentiation process. J Immunol 190: 4046–4055, 2013 [DOI] [PubMed] [Google Scholar]
- 288.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol 57: 1–164, 1999 [DOI] [PubMed] [Google Scholar]
- 289.Mishima T, Ito Y, Hosono K, Tamura Y, Uchida Y, Hirata M, Suzsuki T, Amano H, Kato S, Kurihara Y, Kurihara H, Hayashi I, Watanabe M, Majima M. Calcitonin gene-related peptide facilitates revascularization during hindlimb ischemia in mice. Am J Physiol Heart Circ Physiol 300: H431–H439, 2011 [DOI] [PubMed] [Google Scholar]
- 290.Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA 102: 12938–12943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Monneret G, Arpin M, Venet F, Maghni K, Debard AL, Pachot A, Lepape A, Bienvenu J. Calcitonin gene related peptide and N-procalcitonin modulate CD11b upregulation in lipopolysaccharide activated monocytes and neutrophils. Intensive Care Med 29: 923–928, 2003 [DOI] [PubMed] [Google Scholar]
- 292.Moore EL, Gingell JJ, Kane SA, Hay DL, Salvatore CA. Mapping the CGRP receptor ligand binding domain: tryptophan-84 of RAMP1 is critical for agonist and antagonist binding. Biochem Biophys Res Commun 394: 141–145, 2010 [DOI] [PubMed] [Google Scholar]
- 293.Moore EL, Salvatore CA. Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol 166: 66–78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Morris HR, Panico M, Etienne T, Tippins J, Girgis SI, MacIntyre I. Isolation and characterization of human calcitonin gene-related peptide. Nature 308: 746–748, 1984 [DOI] [PubMed] [Google Scholar]
- 295.Mousa SA, Straub RH, Schafer M, Stein C. Beta-endorphin, Met-enkephalin and corresponding opioid receptors within synovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann Rheum Dis 66: 871–879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Muff R, Leuthauser K, Buhlmann N, Foord SM, Fischer JA, Born W. Receptor activity modifying proteins regulate the activity of a calcitonin gene-related peptide receptor in rabbit aortic endothelial cells. FEBS Lett 441: 366–368, 1998 [DOI] [PubMed] [Google Scholar]
- 297.Mulderry PK, Ghatei MA, Bishop AE, Allen YS, Polak JM, Bloom SR. Distribution and chromatographic characterisation of CGRP-like immunoreactivity in the brain and gut of the rat. Regul Pept 12: 133–143, 1985 [DOI] [PubMed] [Google Scholar]
- 298.Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 25: 195–205, 1988 [DOI] [PubMed] [Google Scholar]
- 299.Mulvany MJ. Structure and function of small arteries in hypertension. J Hypertens Suppl 8: S225–232, 1990 [PubMed] [Google Scholar]
- 300.Nakamura-Craig M, Gill BK. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci Lett 124: 49–51, 1991 [DOI] [PubMed] [Google Scholar]
- 301.Nakatsuma A, Kawasaki H, Kurosaki Y, Futagami K, Araki H, Gomita Y. Effects of long-term treatment with calcium antagonists on periarterial nerve function in the mesenteric artery of spontaneously hypertensive rats. Jpn J Pharmacol 84: 156–162, 2000 [DOI] [PubMed] [Google Scholar]
- 302.Negro A, Lionetto L, Simmaco M, Martelletti P. CGRP receptor antagonists: an expanding drug class for acute migraine? Expert Opin Investig Drugs 21: 807–818, 2012 [DOI] [PubMed] [Google Scholar]
- 303.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 344: 770–773, 1990 [DOI] [PubMed] [Google Scholar]
- 304.Neugebauer V, Rumenapp P, Schaible HG. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat's knee joint and in the generation and maintenance of hyperexcitability of dorsal horn-neurons during development of acute inflammation. Neuroscience 71: 1095–1109, 1996 [DOI] [PubMed] [Google Scholar]
- 305.New HV, Mudge AW. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature 323: 809–811, 1986 [DOI] [PubMed] [Google Scholar]
- 306.Odar-Cederlof I, Theodorsson E, Ericsson F, Kjellstrand CM. Plasma concentrations of calcitonin gene-related peptide in fluid overload. Lancet 338: 411–412, 1991 [DOI] [PubMed] [Google Scholar]
- 307.Odar-Cederlof I, Theodorsson E, Eriksson CG, Kjellstrand CM. Plasma concentrations of calcitonin gene-related peptide increase during haemodialysis: relation to blood pressure. J Intern Med 226: 177–182, 1989 [DOI] [PubMed] [Google Scholar]
- 308.Ogbonna AC, Clark AK, Gentry C, Hobbs C, Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur J Pain 17: 514–526, 2013 [DOI] [PubMed] [Google Scholar]
- 309.Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, Hirata Y, Yazaki Y, Nagai R, Kuwaki T, Kurihara H. Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res 89: 983–990, 2001 [DOI] [PubMed] [Google Scholar]
- 310.Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res 403: 350–354, 1987 [DOI] [PubMed] [Google Scholar]
- 311.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350: 1104–1110, 2004 [DOI] [PubMed] [Google Scholar]
- 312.Olesen J, Edvinsson L. Migraine: a research field matured for the basic neurosciences. Trends Neurosci 14: 3–5, 1991 [DOI] [PubMed] [Google Scholar]
- 313.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Adebara ET, Yorek MA. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition to reverse. Diabetes Obes Metab 10: 64–74, 2008 [DOI] [PubMed] [Google Scholar]
- 314.Onuoha GN, Alpar EK. Levels of vasodilators (SP, CGRP) and vasoconstrictor (NPY) peptides in early human burns. Eur J Clin Invest 31: 253–257, 2001 [DOI] [PubMed] [Google Scholar]
- 315.Ozaka T, Doi Y, Kayashima K, Fujimoto S. Weibel-Palade bodies as a storage site of calcitonin gene-related peptide and endothelin-1 in blood vessels of the rat carotid body. Anat Rec 247: 388–394, 1997 [DOI] [PubMed] [Google Scholar]
- 316.Padilla BE, Cottrell GS, Roosterman D, Pikios S, Muller L, Steinhoff M, Bunnett NW. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J Cell Biol 179: 981–997, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci USA 98: 10463–10468, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Paone DV, Nguyen DN, Shaw AW, Burgey CS, Potteiger CM, Deng JZ, Mosser SD, Salvatore CA, Yu S, Roller S, Kane SA, Selnick HG, Vacca JP, Williams TM. Orally bioavailable imidazoazepanes as calcitonin gene-related peptide (CGRP) receptor antagonists: discovery of MK-2918. Bioorg Med Chem Lett 21: 2683–2686, 2011 [DOI] [PubMed] [Google Scholar]
- 319.Paone DV, Shaw AW, Nguyen DN, Burgey CS, Deng JZ, Kane SA, Koblan KS, Salvatore CA, Mosser SD, Johnston VK, Wong BK, Miller-Stein CM, Hershey JC, Graham SL, Vacca JP, Williams TM. Potent, orally bioavailable calcitonin gene-related peptide receptor antagonists for the treatment of migraine: discovery of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1- (2,2,2-trifluoroethyl)azepan-3-yl]-4- (2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin- 1-yl)piperidine-1-carboxamide (MK-0974). J Med Chem 50: 5564–5567, 2007 [DOI] [PubMed] [Google Scholar]
- 320.Peroni RN, Abramoff T, Neuman I, Podesta EJ, Adler-Graschinsky E. Phytoestrogens enhance the vascular actions of the endocannabinoid anandamide in mesenteric beds of female rats. Int J Hypertens 2012: 647856, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, Olesen J. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia 25: 139–147, 2005 [DOI] [PubMed] [Google Scholar]
- 322.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther 77: 202–213, 2005 [DOI] [PubMed] [Google Scholar]
- 323.Pin SS, Bahr BA. Protein kinase C is a common component of CGRP receptor desensitization induced by distinct agonists. Eur J Pharmacol 587: 8–15, 2008 [DOI] [PubMed] [Google Scholar]
- 324.Portaluppi F, Trasforini G, Margutti A, Vergnani L, Ambrosio MR, Rossi R, Bagni B, Pansini R, degli Uberti EC. Circadian rhythm of calcitonin gene-related peptide in uncomplicated essential hypertension. J Hypertens 10: 1227–1234, 1992 [DOI] [PubMed] [Google Scholar]
- 325.Portaluppi F, Vergnani L, Margutti A, Ambrosio MR, Bondanelli M, Trasforini G, Rossi R, Degli Uberti EC. Modulatory effect of the renin-angiotensin system on the plasma levels of calcitonin gene-related peptide in normal man. J Clin Endocrinol Metab 77: 816–820, 1993 [DOI] [PubMed] [Google Scholar]
- 326.Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res 87: 760–768, 2010 [DOI] [PubMed] [Google Scholar]
- 327.Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemeny A, Materazzi S, Nassini R, Helyes Z, Szolcsanyi J, Pinter E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol 689: 56–64, 2012 [DOI] [PubMed] [Google Scholar]
- 328.Prado MA, Evans-Bain B, Oliver KR, Dickerson IM. The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides 22: 1773–1781, 2001 [DOI] [PubMed] [Google Scholar]
- 329.Preibisz JJ. Calcitonin gene-related peptide and regulation of human cardiovascular homeostasis. Am J Hypertens 6: 434–450, 1993 [DOI] [PubMed] [Google Scholar]
- 330.Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, Lewis LG, Bianchi BR, Mikusa JP, Koenig JR, Perner RJ, Kort ME, Honore P, Faltynek CR, Kym PR, Reilly RM. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydr onaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain 150: 319–326, 2010 [DOI] [PubMed] [Google Scholar]
- 331.Qin XP, Ye F, Hu CP, Liao DF, Deng HW, Li YJ. Effect of calcitonin gene-related peptide on angiotensin II-induced proliferation of rat vascular smooth muscle cells. Eur J Pharmacol 488: 45–49, 2004 [DOI] [PubMed] [Google Scholar]
- 332.Qin XP, Zeng SY, Li D, Chen QQ, Luo D, Zhang Z, Hu GY, Deng HW, Li YJ. Calcitonin gene-related Peptide-mediated depressor effect and inhibiting vascular hypertrophy of rutaecarpine in renovascular hypertensive rats. J Cardiovasc Pharmacol 50: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 333.Raap T, Justen HP, Miller LE, Cutolo M, Scholmerich J, Straub RH. Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol 27: 2558–2565, 2000 [PubMed] [Google Scholar]
- 334.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 302: 839–845, 2002 [DOI] [PubMed] [Google Scholar]
- 335.Rochlitzer S, Veres TZ, Kuhne K, Prenzler F, Pilzner C, Knothe S, Winkler C, Lauenstein HD, Willart M, Hammad H, Muller M, Krug N, Lambrecht BN, Braun A. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin Exp Allergy 41: 1609–1621, 2011 [DOI] [PubMed] [Google Scholar]
- 336.Roggenkamp D, Kopnick S, Stab F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 133: 1620–1628, 2013 [DOI] [PubMed] [Google Scholar]
- 337.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86: 1309–1379, 2006 [DOI] [PubMed] [Google Scholar]
- 338.Rosenfeld MG, Amara SG, Evans RM. Alternative RNA processing: determining neuronal phenotype. Science 225: 1315–1320, 1984 [DOI] [PubMed] [Google Scholar]
- 339.Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304: 129–135, 1983 [DOI] [PubMed] [Google Scholar]
- 340.Roudenok V, Gutjar L, Antipova V, Rogov Y. Expression of vasoactive intestinal polypeptide and calcitonin gene-related peptide in human stellate ganglia after acute myocardial infarction. Ann Anat 183: 341–344, 2001 [DOI] [PubMed] [Google Scholar]
- 341.Rovero P, Giuliani S, Maggi CA. CGRP antagonist activity of short C-terminal fragments of human alpha CGRP, CGRP(23–37) and CGRP(19–37). Peptides 13: 1025–1027, 1992 [DOI] [PubMed] [Google Scholar]
- 342.Rudolf K, Eberlein W, Engel W, Pieper H, Entzeroth M, Hallermayer G, Doods H. Development of human calcitonin gene-related peptide (CGRP) receptor antagonists. 1. Potent and selective small molecule CGRP antagonists 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]car bonyl]-d-tyrosyl]-l-lysyl]-4-(4-pyridinyl)piperazine: the first CGRP antagonist for clinical trials in acute migraine. J Med Chem 48: 5921–5931, 2005 [DOI] [PubMed] [Google Scholar]
- 343.Russell FA, Fernandes ES, Courade JP, Keeble JE, Brain SD. Tumour necrosis factor alpha mediates transient receptor potential vanilloid 1-dependent bilateral thermal hyperalgesia with distinct peripheral roles of interleukin-1beta, protein kinase C and cyclooxygenase-2 signalling. Pain 142: 264–274, 2009 [DOI] [PubMed] [Google Scholar]
- 344.Saade NE, Massaad CA, Ochoa-Chaar CI, Jabbur SJ, Safieh-Garabedian B, Atweh SF. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol 545: 241–253, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sabharwal R, Zhang Z, Lu Y, Abboud FM, Russo AF, Chapleau MW. Receptor activity-modifying protein 1 increases baroreflex sensitivity and attenuates Angiotensin-induced hypertension. Hypertension 55: 627–635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Salio C, Averill S, Priestley JV, Merighi A. Costorage of BDNF and neuropeptides within individual dense-core vesicles in central and peripheral neurons. Dev Neurobiol 67: 326–338, 2007 [DOI] [PubMed] [Google Scholar]
- 347.Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, Mosser SD, Burgey CS, Paone DV, Shaw AW, Graham SL, Vacca JP, Williams TM, Koblan KS, Kane SA. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther 324: 416–421, 2008 [DOI] [PubMed] [Google Scholar]
- 348.Salvatore CA, Mallee JJ, Bell IM, Zartman CB, Williams TM, Koblan KS, Kane SA. Identification and pharmacological characterization of domains involved in binding of CGRP receptor antagonists to the calcitonin-like receptor. Biochemistry 45: 1881–1887, 2006 [DOI] [PubMed] [Google Scholar]
- 349.Salvatore CA, Moore EL, Calamari A, Cook JJ, Michener MS, O'Malley S, Miller PJ, Sur C, Williams DL, Jr, Zeng Z, Danziger A, Lynch JJ, Regan CP, Fay JF, Tang YS, Li CC, Pudvah NT, White RB, Bell IM, Gallicchio SN, Graham SL, Selnick HG, Vacca JP, Kane SA. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J Pharmacol Exp Ther 333: 152–160, 2010 [DOI] [PubMed] [Google Scholar]
- 350.Sams-Nielsen A, Orskov C, Jansen-Olesen I. Pharmacological evidence for CGRP uptake into perivascular capsaicin sensitive nerve terminals. Br J Pharmacol 132: 1145–1153, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20: 907–918, 2000 [DOI] [PubMed] [Google Scholar]
- 352.Saxen MA, Welch SP, Dewey WL. The mouse paw withdrawal assay: a method for determining the effect of calcitonin gene-related peptide on cutaneous heat nociceptive latency time. Life Sci 53: 397–405, 1993 [DOI] [PubMed] [Google Scholar]
- 353.Saxler G, Loer F, Skumavc M, Pfortner J, Hanesch U. Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain 11: 67–74, 2007 [DOI] [PubMed] [Google Scholar]
- 354.Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta 1643: 65–73, 2003 [DOI] [PubMed] [Google Scholar]
- 355.Schiffrin EL. Beyond blood pressure: the endothelium and atherosclerosis progression. Am J Hypertens 15: 115S–122S, 2002 [DOI] [PubMed] [Google Scholar]
- 356.Schifter S. Circulating concentrations of calcitonin gene-related peptide (CGRP) in normal man determined with a new, highly sensitive radioimmunoassay. Peptides 12: 365–369, 1991 [DOI] [PubMed] [Google Scholar]
- 357.Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol 7: 81–96, 1998 [DOI] [PubMed] [Google Scholar]
- 358.Scholzen TE, Kalden DH, Brzoska T, Fisbeck T, Fastrich M, Schiller M, Bohm M, Schwarz T, Armstrong CA, Ansel JC, Luger TA. Expression of proopiomelanocortin peptides in human dermal microvascular endothelial cells: evidence for a regulation by ultraviolet light and interleukin-1. J Invest Dermatol 115: 1021–1028, 2000 [DOI] [PubMed] [Google Scholar]
- 359.Schutz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J Comp Neurol 476: 32–43, 2004 [DOI] [PubMed] [Google Scholar]
- 360.Shaw AW, Paone DV, Nguyen DN, Stump CA, Burgey CS, Mosser SD, Salvatore CA, Rutledge RZ, Kane SA, Koblan KS, Graham SL, Vacca JP, Williams TM. Caprolactams as potent CGRP receptor antagonists for the treatment of migraine. Bioorg Med Chem Lett 17: 4795–4798, 2007 [DOI] [PubMed] [Google Scholar]
- 361.Shi RZ, Hu CP, Luo D, Li D, Pan W, Li SX, Yang TL, Li YJ, Zhang GG. Decreased anandamide transporter activity and calcitonin gene-related peptide production in spontaneously hypertensive rats: role of angiotensin II. Eur J Pharmacol 680: 81–87, 2012 [DOI] [PubMed] [Google Scholar]
- 362.Simms J, Hay DL, Wheatley M, Poyner DR. Characterization of the structure of RAMP1 by mutagenesis and molecular modeling. Biophys J 91: 662–669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem 283: 18743–18752, 2008 [DOI] [PubMed] [Google Scholar]
- 364.Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depre M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974). Br J Clin Pharmacol 69: 15–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Siney L, Brain SD. Involvement of sensory neuropeptides in the development of plasma extravasation in rat dorsal skin following thermal injury. Br J Pharmacol 117: 1065–1070, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sixt ML, Messlinger K, Fischer MJ. Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain 132: 3134–3141, 2009 [DOI] [PubMed] [Google Scholar]
- 367.Smillie SJ, Brain SD. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides 45: 93–104, 2011 [DOI] [PubMed] [Google Scholar]
- 368.Smillie SJ, King R, Kodji X, Outzen E, Pozsgai G, Fernandes E, Marshall N, de Winter P, Heads RJ, Dessapt-Baradez C, Gnudi L, Sams A, Shah AM, Siow RC, Brain SD. An ongoing role of alpha-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension 63: 1056–1062, 2014 [DOI] [PubMed] [Google Scholar]
- 369.Somasundaram C, Diz DI, Coleman T, Bukoski RD. Adventitial neuronal somata. J Vasc Res 43: 278–288, 2006 [DOI] [PubMed] [Google Scholar]
- 370.Spetz AC, Ellefsen K, Theodorsson E, Lassvik CT, Hammar ML. Calcitonin gene-related peptide during sweating in young healthy women. Gynecol Obstet Invest 60: 149–153, 2005 [DOI] [PubMed] [Google Scholar]
- 371.Steenbergh PH, Hoppener JW, Zandberg J, Visser A, Lips CJ, Jansz HS. Structure and expression of the human calcitonin/CGRP genes. FEBS Lett 209: 97–103, 1986 [DOI] [PubMed] [Google Scholar]
- 372.Steiner TJ, Scher AI, Stewart WF, Kolodner K, Liberman J, Lipton RB. The prevalence and disability burden of adult migraine in England and their relationships to age, gender and ethnicity. Cephalalgia 23: 519–527, 2003 [DOI] [PubMed] [Google Scholar]
- 373.Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J 293: 1329–1330, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Stevenson RN, Roberts RH, Timmis AD. Calcitonin gene-related peptide: a haemodynamic study of a novel vasodilator in patients with severe chronic heart failure. Int J Cardiol 37: 407–414, 1992 [DOI] [PubMed] [Google Scholar]
- 375.Stewart-Lee AL, Aberdeen J, Burnstock G. The effect of atherosclerosis on neuromodulation of sympathetic neurotransmission by neuropeptide Y and calcitonin gene-related peptide in the rabbit mesenteric artery. Eur J Pharmacol 216: 167–174, 1992 [DOI] [PubMed] [Google Scholar]
- 376.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 92: 2859–2866, 2004 [DOI] [PubMed] [Google Scholar]
- 377.Sun W, Guo J, Tang Y, Wang X. Alteration of capsaicin and endotoxin-induced calcitonin gene-related peptide release from mesenteric arterial bed and spinal cord slice in 18-month-old rats. J Neurosci Res 53: 385–392, 1998 [DOI] [PubMed] [Google Scholar]
- 378.Sun W, Wang L, Zhang Z, Chen M, Wang X. Intramuscular transfer of naked calcitonin gene-related peptide gene prevents autoimmune diabetes induced by multiple low-dose streptozotocin in C57BL mice. Eur J Immunol 33: 233–242, 2003 [DOI] [PubMed] [Google Scholar]
- 379.Supowit SC, Gururaj A, Ramana CV, Westlund KN, DiPette DJ. Enhanced neuronal expression of calcitonin gene-related peptide in mineralocorticoid-salt hypertension. Hypertension 25: 1333–1338, 1995 [DOI] [PubMed] [Google Scholar]
- 380.Supowit SC, Hallman DM, Zhao H, DiPette DJ. Alpha 2-adrenergic receptor activation inhibits calcitonin gene-related peptide expression in cultured dorsal root ganglia neurons. Brain Res 782: 184–193, 1998 [DOI] [PubMed] [Google Scholar]
- 381.Supowit SC, Katki KA, Hein TW, Gupta P, Kuo L, Dickerson IM, Dipette DJ. Vascular reactivity to calcitonin gene-related peptide is enhanced in subtotal nephrectomy-salt induced hypertension. Am J Physiol Heart Circ Physiol 301: H683–H688, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Supowit SC, Ramana CV, Westlund KN, DiPette DJ. Calcitonin gene-related peptide gene expression in the spontaneously hypertensive rat. Hypertension 21: 1010–1014, 1993 [DOI] [PubMed] [Google Scholar]
- 383.Supowit SC, Rao A, Bowers MC, Zhao H, Fink G, Steficek B, Patel P, Katki KA, Dipette DJ. Calcitonin gene-related peptide protects against hypertension-induced heart and kidney damage. Hypertension 45: 109–114, 2005 [DOI] [PubMed] [Google Scholar]
- 384.Supowit SC, Zhao H, DiPette DJ. Nerve growth factor enhances calcitonin gene-related peptide expression in the spontaneously hypertensive rat. Hypertension 37: 728–732, 2001 [DOI] [PubMed] [Google Scholar]
- 385.Supowit SC, Zhao H, Hallman DM, DiPette DJ. Calcitonin gene-related peptide is a depressor of deoxycorticosterone-salt hypertension in the rat. Hypertension 29: 945–950, 1997 [DOI] [PubMed] [Google Scholar]
- 386.Takatori S, Zamami Y, Hashikawa-Hobara N, Kawasaki H. Insulin resistance-induced hypertension and a role of perivascular CGRPergic nerves. Curr Protein Pept Sci 14: 275–281, 2013 [DOI] [PubMed] [Google Scholar]
- 387.Takeba Y, Suzuki N, Kaneko A, Asai T, Sakane T. Evidence for neural regulation of inflammatory synovial cell functions by secreting calcitonin gene-related peptide and vasoactive intestinal peptide in patients with rheumatoid arthritis. Arthritis Rheum 42: 2418–2429, 1999 [DOI] [PubMed] [Google Scholar]
- 388.Tam CW, Husmann K, Clark NC, Clark JE, Lazar Z, Ittner LM, Gotz J, Douglas G, Grant AD, Sugden D, Poston L, Poston R, McFadzean I, Marber MS, Fischer JA, Born W, Brain SD. Enhanced vascular responses to adrenomedullin in mice overexpressing receptor-activity-modifying protein 2. Circ Res 98: 262–270, 2006 [DOI] [PubMed] [Google Scholar]
- 389.Tanaka H, Shimaya A, Kiso T, Kuramochi T, Shimokawa T, Shibasaki M. Enhanced insulin secretion and sensitization in diabetic mice on chronic treatment with a transient receptor potential vanilloid 1 antagonist. Life Sci 88: 559–563, 2011 [DOI] [PubMed] [Google Scholar]
- 390.Tang JA, Xu D, Yuan QX, Meng ZH, Cheng FR, Chen MZ, Liu GZ, Liu LS, Zhang ZK. Calcitonin gene-related peptide in the pathogenesis and treatment of hypertension. Chin Med J 102: 897–901, 1989 [PubMed] [Google Scholar]
- 391.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 50: 325–332, 2007 [DOI] [PubMed] [Google Scholar]
- 392.Ter Haar E, Koth CM, Abdul-Manan N, Swenson L, Coll JT, Lippke JA, Lepre CA, Garcia-Guzman M, Moore JM. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure 18: 1083–1093, 2010 [DOI] [PubMed] [Google Scholar]
- 393.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat 194: 1–14, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Terenghi G, Polak JM, Rodrigo J, Mulderry PK, Bloom SR. Calcitonin gene-related peptide-immunoreactive nerves in the tongue, epiglottis and pharynx of the rat: occurrence, distribution and origin. Brain Res 365: 1–14, 1986 [DOI] [PubMed] [Google Scholar]
- 395.Tfelt-Hansen P, Le H. Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain 10: 137–143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Tfelt-Hansen P, Olesen J. Possible site of action of CGRP antagonists in migraine. Cephalalgia 31: 748–750, 2011 [DOI] [PubMed] [Google Scholar]
- 397.Thomas DA, Dubner R, Ruda MA. Neonatal capsaicin treatment in rats results in scratching behavior with skin damage: potential model of non-painful dysesthesia. Neurosci Lett 171: 101–104, 1994 [DOI] [PubMed] [Google Scholar]
- 398.Tjen ALS, Ekman R, Lippton H, Cary J, Keith I. CGRP and somatostatin modulate chronic hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 263: H681–H690, 1992 [DOI] [PubMed] [Google Scholar]
- 399.Tjen ALS, Kraiczi H, Ekman R, Keith IM. Sensory CGRP depletion by capsaicin exacerbates hypoxia-induced pulmonary hypertension in rats. Regul Pept 74: 1–10, 1998 [DOI] [PubMed] [Google Scholar]
- 400.Toda M, Suzuki T, Hosono K, Kurihara Y, Kurihara H, Hayashi I, Kitasato H, Hoka S, Majima M. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother 62: 352–359, 2008 [DOI] [PubMed] [Google Scholar]
- 401.Tomlinson DR, Fernyhough P, Diemel LT. Neurotrophins and peripheral neuropathy. Philos Trans R Soc Lond B Biol Sci 351: 455–462, 1996 [DOI] [PubMed] [Google Scholar]
- 402.Tsujikawa K, Yayama K, Hayashi T, Matsushita H, Yamaguchi T, Shigeno T, Ogitani Y, Hirayama M, Kato T, Fukada S, Takatori S, Kawasaki H, Okamoto H, Ikawa M, Okabe M, Yamamoto H. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci USA 104: 16702–16707, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tuo Y, Guo X, Zhang X, Wang Z, Zhou J, Xia L, Zhang Y, Wen J, Jin D. The biological effects and mechanisms of calcitonin gene-related peptide on human endothelial cell. J Recept Signal Transduct Res 33: 114–123, 2013 [DOI] [PubMed] [Google Scholar]
- 404.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 58: 561–568, 2005 [DOI] [PubMed] [Google Scholar]
- 405.Uddman R, Edvinsson L, Ekblad E, Hakanson R, Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept 15: 1–23, 1986 [DOI] [PubMed] [Google Scholar]
- 406.Umeda Y, Takamiya M, Yoshizaki H, Arisawa M. Inhibition of mitogen-stimulated T lymphocyte proliferation by calcitonin gene-related peptide. Biochem Biophys Res Commun 154: 227–235, 1988 [DOI] [PubMed] [Google Scholar]
- 407.Uren NG, Seydoux C, Davies GJ. Effect of intravenous calcitonin gene related peptide on ischaemia threshold and coronary stenosis severity in humans. Cardiovasc Res 27: 1477–1481, 1993 [DOI] [PubMed] [Google Scholar]
- 408.Vaishnava P, Wang DH. Capsaicin sensitive-sensory nerves and blood pressure regulation. Curr Med Chem Cardiovasc Hematol Agents 1: 177–188, 2003 [DOI] [PubMed] [Google Scholar]
- 409.Valentini A, Petraglia F, De Vita D, Nappi C, Margutti A, degli Uberti EC, Genazzani AR. Changes of plasma calcitonin gene-related peptide levels in postmenopausal women. Am J Obstet Gynecol 175: 638–642, 1996 [DOI] [PubMed] [Google Scholar]
- 410.Van der Schueren BJ, Rogiers A, Vanmolkot FH, Van Hecken A, Depre M, Kane SA, De Lepeleire I, Sinclair SR, de Hoon JN. Calcitonin gene-related peptide8–37 antagonizes capsaicin-induced vasodilation in the skin: evaluation of a human in vivo pharmacodynamic model. J Pharmacol Exp Ther 325: 248–255, 2008 [DOI] [PubMed] [Google Scholar]
- 411.Vause CV, Durham PL. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: findings from array analysis. Neurosci Lett 473: 163–167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst 5: 191–208, 2000 [DOI] [PubMed] [Google Scholar]
- 413.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 30: 1065–1072, 2010 [DOI] [PubMed] [Google Scholar]
- 414.Villarreal D, Reams G, Freeman R. Calcitonin gene-related peptide and the kidney. Curr Opin Nephrol Hypertens 3: 453–458, 1994 [DOI] [PubMed] [Google Scholar]
- 415.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 416.Walker CS, Conner AC, Poyner DR, Hay DL. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci 31: 476–483, 2010 [DOI] [PubMed] [Google Scholar]
- 417.Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, Sewell MA, Ruggiero K, Phillips AR, Kraegen EW, Hay DL, Cooper GJ, Loomes KM. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151: 4257–4269, 2010 [DOI] [PubMed] [Google Scholar]
- 418.Wang B, Chen B, Hu D. [The changes of calcitonin gene-related peptide (CGRP)-containing nerve fibers in adrenal gland of burned rats]. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 15: 99–101, 1999 [PubMed] [Google Scholar]
- 419.Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin 26: 286–294, 2005 [DOI] [PubMed] [Google Scholar]
- 420.Wang F, Millet I, Bottomly K, Vignery A. Calcitonin gene-related peptide inhibits interleukin 2 production by murine T lymphocytes. J Biol Chem 267: 21052–21057, 1992 [PubMed] [Google Scholar]
- 421.Wang H, Xing L, Li W, Hou L, Guo J, Wang X. Production and secretion of calcitonin gene-related peptide from human lymphocytes. J Neuroimmunol 130: 155–162, 2002 [DOI] [PubMed] [Google Scholar]
- 422.Wang LH, Zhou SX, Li RC, Zheng LR, Zhu JH, Hu SJ, Sun YL. Serum levels of calcitonin gene-related peptide and substance P are decreased in patients with diabetes mellitus and coronary artery disease. J Int Med Res 40: 134–140, 2012 [DOI] [PubMed] [Google Scholar]
- 423.Wang W, Sun W, Wang X. Intramuscular gene transfer of CGRP inhibits neointimal hyperplasia after balloon injury in the rat abdominal aorta. Am J Physiol Heart Circ Physiol 287: H1582–H1589, 2004 [DOI] [PubMed] [Google Scholar]
- 424.Wang X, Ebong SJ, Call DR, Newcomb DE, Bolgos GR, Remick DG. Calcitonin gene-related peptide partially reverses decreased production of chemokines KC and MIP-2 following murine sepsis. Inflammation 26: 167–174, 2002 [DOI] [PubMed] [Google Scholar]
- 425.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension 47: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 426.Wang Y, Wang DH. TRPV1 ablation aggravates inflammatory responses and organ damage during endotoxic shock. Clin Vaccine Immunol 20: 1008–1015, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wang Z, Ma W, Chabot JG, Quirion R. Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFkappaB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain 151: 194–205, 2010 [DOI] [PubMed] [Google Scholar]
- 428.Wang ZG, Liu JL, Liu Y, Wen SJ, Wen J, Liu YP, Chen XJ, Wu ZS. [Increased plasma vasoactive substances and antioxidant enzymes levels in prehypertensive patients]. Zhonghua Xin Xue Guan Bing Za Zhi 35: 719–722, 2007 [PubMed] [Google Scholar]
- 429.Watkins HA, Rathbone DL, Barwell J, Hay DL, Poyner DR. Structure-activity relationships for alpha calcitonin gene-related peptide. Br J Pharmacol 170: 1308–1322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Watson RE, Supowit SC, Zhao H, Katki KA, Dipette DJ. Role of sensory nervous system vasoactive peptides in hypertension. Braz J Med Biol Res 35: 1033–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 431.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin–a microdialysis study. J Invest Dermatol 115: 1015–1020, 2000 [DOI] [PubMed] [Google Scholar]
- 432.Westermark P, Wernstedt C, O'Brien TD, Hayden DW, Johnson KH. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol 127: 414–417, 1987 [PMC free article] [PubMed] [Google Scholar]
- 433.White BJ, Smith PA, Dunn WR. Hydrogen sulphide-mediated vasodilatation involves the release of neurotransmitters from sensory nerves in pressurized mesenteric small arteries isolated from rats. Br J Pharmacol 168: 785–793, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wimalawansa SJ. Age-related increase of calcitonin gene-related peptide in rat thyroid and circulation. Peptides 12: 1143–1147, 1991 [DOI] [PubMed] [Google Scholar]
- 435.Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17: 533–585, 1996 [DOI] [PubMed] [Google Scholar]
- 436.Wimalawansa SJ, Morris HR, Etienne A, Blench I, Panico M, MacIntyre I. Isolation, purification and characterization of beta-hCGRP from human spinal cord. Biochem Biophys Res Commun 167: 993–1000, 1990 [DOI] [PubMed] [Google Scholar]
- 437.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci USA 96: 7723–7730, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wyon Y, Frisk J, Lundeberg T, Theodorsson E, Hammar M. Postmenopausal women with vasomotor symptoms have increased urinary excretion of calcitonin gene-related peptide. Maturitas 30: 289–294, 1998 [DOI] [PubMed] [Google Scholar]
- 439.Yamaga N, Kawasaki H, Inaizumi K, Shimizu M, Nakamura A, Kurosaki Y. Age-Related decrease in calcitonin gene-related peptide mRNA in the dorsal root ganglia of spontaneously hypertensive rats. Jpn J Pharmacol 86: 448–450, 2001 [DOI] [PubMed] [Google Scholar]
- 440.Yamaoka J, Di ZH, Sun W, Kawana S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J Dermatol Sci 46: 41–51, 2007 [DOI] [PubMed] [Google Scholar]
- 441.Yan LZ, Johnson KW, Rothstein E, Flora D, Edwards P, Li B, Li J, Lynch R, Vaughn R, Clemens-Smith A, McCarty D, Chow C, McKnight KL, Lu J, Nisenbaum ES, Mayer JP. Discovery of potent, cyclic calcitonin gene-related peptide receptor antagonists. J Pept Sci 17: 383–386, 2011 [DOI] [PubMed] [Google Scholar]
- 442.Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 12: 130–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Yang L, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Kawate H, Yoshizawa T, Koyama T, Iesato Y, Uetake R, Yamauchi A, Tanaka M, Toriyama Y, Igarashi K, Shindo T. Endogenous CGRP protects against neointimal hyperplasia following wire-induced vascular injury. J Mol Cell Cardiol 59: 55–66, 2013 [DOI] [PubMed] [Google Scholar]
- 444.Yu LC, Hansson P, Brodda-Jansen G, Theodorsson E, Lundeberg T. Intrathecal CGRP8–37-induced bilateral increase in hindpaw withdrawal latency in rats with unilateral inflammation. Br J Pharmacol 117: 43–50, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8–37 increases the latency to withdrawal responses bilaterally in rats with unilateral experimental mononeuropathy, an effect reversed by naloxone. Neuroscience 71: 523–531, 1996 [DOI] [PubMed] [Google Scholar]
- 446.Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8–37 increases the latency to withdrawal responses in rats. Brain Res 653: 223–230, 1994 [DOI] [PubMed] [Google Scholar]
- 447.Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 16: 69–75, 1990 [DOI] [PubMed] [Google Scholar]
- 448.Zaidi M, Bevis PJ, Girgis SI, Lynch C, Stevenson JC, MacIntyre I. Circulating CGRP comes from the perivascular nerves. Eur J Pharmacol 117: 283–284, 1985 [DOI] [PubMed] [Google Scholar]
- 449.Zaidi M, Breimer LH, MacIntyre I. Biology of peptides from the calcitonin genes. Q J Exp Physiol 72: 371–408, 1987 [DOI] [PubMed] [Google Scholar]
- 450.Zelissen PM, Koppeschaar HP, Lips CJ, Hackeng WH. Calcitonin gene-related peptide in human obesity. Peptides 12: 861–863, 1991 [DOI] [PubMed] [Google Scholar]
- 451.Zeller J, Poulsen KT, Sutton JE, Abdiche YN, Collier S, Chopra R, Garcia CA, Pons J, Rosenthal A, Shelton DL. CGRP function-blocking antibodies inhibit neurogenic vasodilatation without affecting heart rate or arterial blood pressure in the rat. Br J Pharmacol 155: 1093–1103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain 89: 265–273, 2001 [DOI] [PubMed] [Google Scholar]
- 453.Zhang LL, Yan LD, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res 100: 1063–1070, 2007 [DOI] [PubMed] [Google Scholar]
- 454.Zhang X, Zhuang J, Wu H, Chen Z, Su J, Chen S, Chen J. Inhibitory effects of calcitonin gene-related peptides on experimental vein graft disease. Ann Thorac Surg 90: 117–123, 2010 [DOI] [PubMed] [Google Scholar]
- 455.Zhang XN, Chen DZ, Zheng YH, Liang JG, Yang HX, Lin XW, Zhuang J. Gene transfer of calcitonin gene-related peptide suppresses development of allograft vasculopathy. Transplant Proc 41: 1900–1905, 2009 [DOI] [PubMed] [Google Scholar]
- 456.Zhao FP, Guo Z, Wang PF. Calcitonin gene related peptide (CGRP) inhibits norepinephrine induced apoptosis in cultured rat cardiomyocytes not via PKA or PKC pathways. Neurosci Lett 482: 163–166, 2010 [DOI] [PubMed] [Google Scholar]
- 457.Zhao Q, Liu Z, Wang Z, Yang C, Liu J, Lu J. Effect of prepro-calcitonin gene-related peptide-expressing endothelial progenitor cells on pulmonary hypertension. Ann Thorac Surg 84: 544–552, 2007 [DOI] [PubMed] [Google Scholar]
- 458.Zheng LR, Han J, Yao L, Sun YL, Jiang DM, Hu SJ, Shao L, Sun ZH, Wang LH. Up-regulation of calcitonin gene-related peptide protects streptozotocin-induced diabetic hearts from ischemia/reperfusion injury. Int J Cardiol 156: 192–198, 2012 [DOI] [PubMed] [Google Scholar]
- 459.Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293: H1791–H1798, 2007 [DOI] [PubMed] [Google Scholar]
- 460.Zhou Y, Zhang M, Sun GY, Liu YP, Ran WZ, Peng L, Guan CX. Calcitonin gene-related peptide promotes the wound healing of human bronchial epithelial cells via PKC and MAPK pathways. Regul Pept 184C: 22–29, 2013 [DOI] [PubMed] [Google Scholar]
- 461.Zhou Z, Hu CP, Wang CJ, Li TT, Peng J, Li YJ. Calcitonin gene-related peptide inhibits angiotensin II-induced endothelial progenitor cells senescence through up-regulation of klotho expression. Atherosclerosis 213: 92–101, 2010 [DOI] [PubMed] [Google Scholar]
- 462.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di MV, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999 [DOI] [PubMed] [Google Scholar]