Abstract
α-Klotho exerts pleiotropic biological actions. Heterozygous α-Klotho haplo-insufficient mice (kl/+) appear normal at baseline except for age-related changes in the lung, suggesting heightened pulmonary susceptibility to α-Klotho deficiency. We used in vivo and in vitro models to test whether α-Klotho protects lung epithelia against injury. Normally, α-Klotho is not expressed in the lung, but circulating α-Klotho levels are reduced ∼40% in kl/+ mice and undetectable in homozygous α-Klotho-deficient mice (kl/kl). kl/+ mice show distal air space enlargement at a given airway pressure, with elevated lung oxidative damage marker (8-hydroxydeoxyguanosine; 8-OHdG); these abnormalities are exacerbated in kl/kl mice. Studies were performed in A549 lung epithelial cells and/or primary culture of alveolar epithelial cells. Hyperoxia (95% O2) and high inorganic phosphate concentrations (Pi, 3–5 mM) additively caused cell injury (lactate dehydrogenase release), oxidative DNA damage (8-OHdG), lipid oxidation (8-isoprostane), protein oxidation (carbonyl), and apoptosis (caspase-8 activity and TUNEL stain). Transfection of transmembrane or soluble α-Klotho, or addition of soluble α-Klotho-containing conditioned media, increased cellular antioxidant capacity (Cu- and Fe-based assays) via increased nuclear factor erythroid-derived 2-related factors 1 and 2 (Nrf1/2) transcriptional activity and ameliorated hyperoxic and phosphotoxic injury. To validate the findings in vivo, we injected α-Klotho-containing conditioned media into rat peritoneum before and during hyperoxia exposure and found reduced alveolar interstitial edema and oxidative damage. We conclude that circulating α-Klotho protects the lung against oxidative damage and apoptosis partly via increasing endogenous antioxidative capacity in pulmonary epithelia. Cytoprotection by α-Klotho may play an important role in degenerative diseases of the lung.
Keywords: lung injury, hyperoxia, phosphate toxicity, Klotho cytoprotection, alveolar epithelial cells, antioxidant capacity
klotho was originally identified as an antiaging gene based on the premature multiorgan failure as a consequence of its disruption (27). Three members of the Klotho family have been described, with the original prototypic paralog termed α-Klotho and the other two members termed β-Klotho and γ-Klotho (21, 22). α-Klotho encodes a single-pass transmembrane protein that is predominantly expressed in the kidney and the choroid plexus of the brain (27); a secreted soluble form of the protein is found in the blood, cerebrospinal fluid, and urine and exerts pleiomorphic endocrine as well as paracrine actions in distant organs (19, 30). Transmembrane α-Klotho functions as a coreceptor for fibroblast growth factor-23 (FGF23) to suppress renal inorganic phosphate (Pi) reabsorption and active vitamin D biosynthesis (17), thus promoting negative external Pi balance. α-Klotho also suppresses cellular Pi uptake, thereby contributing to negative intracellular Pi balance (13). In addition, soluble α-Klotho can protect cells from oxidative stress, partly via inducing the expression of antioxidant proteins including catalase and mitochondrial manganese superoxide dismutase (SOD) and reduction of reactive oxygen species (ROS) (12, 25, 40). Part of the α-Klotho cytoprotective effect may be via inhibition of cellular Pi uptake to mitigate cellular phosphotoxicity (14, 28).
Genetically homozygous hypomorphic α-Klotho-deficient (kl/kl) mice exhibit premature universal organ degeneration and early death, suggesting that α-Klotho sustains fundamental “housekeeping” functions for many cell types (27). Heterozygous hypomorphic α-Klotho mice (kl/+) are normal at baseline in all organs except the lung. The lung is the only organ reported to show age-exacerbated degenerative changes, such as air space enlargement and increased compliance in an unperturbed state in heterozygous animals, suggesting higher sensitivity of the lung to α-Klotho action (38). Because α-Klotho is not expressed in the lung, the pulmonary defect in α-Klotho deficiency reflects either a direct effect of low circulating α-Klotho on lung cells or a secondary effect via the action of α-Klotho on some other intermediate(s). Up to now, no study has attempted to delineate these mechanisms in the lung.
In contrast to genetic deletion of α-Klotho, transgenic α-Klotho overexpression extends life span (29), but the protective action of increased α-Klotho on the lung against imposed insults has never been examined. Conditions of heightened systemic oxidative stress, e.g., the metabolic syndrome (obesity and type 2 diabetes mellitus), are associated with low renal and circulating α-Klotho levels independent of renal insufficiency (1, 7, 41); the low α-Klotho level could contribute to oxidative stress-induced accelerated end-organ degeneration. Systemic delivery of exogenous α-Klotho has been shown to protect against renal ischemia-reperfusion injury, an effect that is partially mediated via relief of oxidative stress (12, 15).
Given the apparent importance of α-Klotho in lung health, and the paucity of systematic investigation of α-Klotho action on the lung, our objective was to determine the potential cytoprotective role of α-Klotho in the lung. Using both in vitro cell culture and in vivo animal models, we tested the hypothesis that α-Klotho expression protects the lung against oxidative damage by increasing endogenous antioxidant capacity.
MATERIALS AND METHODS
Cell Culture
Human lung epithelial (A549) cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco's Modified Eagle Medium (DMEM)-nutrient mixture F12 (Life Technologies, Grand Island, NY) with 1% L-glutamine. Human alveolar type 1 (AT1) epithelial cells (Applied Biological Materials, Richmond, BC, Canada) were grown in Prigrow III medium (Applied Biological Materials). Rat vascular smooth muscle cells (A10) derived from the embryonic thoracic aorta (A10 cells, American Type Culture Collection) were grown in DMEM. All culture media were also supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin.
Approximately 50,000 cells were seeded in six-well plates, and each experiment was performed in triplicate. AT1 cells were grown in extracellular matrix-coated flasks and incubated for 12 h at 37°C in 5% CO2 and 95% air. Pi concentration in regular medium (1 mM) was increased to 3 or 5 mM using a sodium phosphate buffer concentrate. Cells were either placed in the incubator directly or in a modulator incubator chamber (Billups-Rothenberg, Del Mar, CA) flushed with 95% O2-5% CO2 mixture for 15 min. The sealed humidified chamber was placed in an incubator at 37°C for 24 h.
α-Klotho-conditioned medium (CM) was prepared from cultured Chinese hamster ovary cells stably transfected with α-Klotho or vector. Serum-free media was added 16 h posttransfection. The subsequent conditioned medium was harvested after 16 h as the α-Klotho CM or control CM, respectively.
α-Klotho Plasmids and Transfection
Full-length (pEFmKLcFT) and secreted (pEFmKLΔTMcFT) α-Klotho cDNAs (Makoto Kuro-o, UT Southwestern Medical Center, Dallas, TX) were cloned into the vector pEF1/myc-His[A] (Life Technologies). A FLAG tag was inserted before the stop codon. In the case of the secreted α-Klotho cDNA, the amino acids (LLVFISFLVFTFIISLALIFH) in the COOH terminus corresponding to the transmembrane domain were deleted. Transfection was performed with 2 μg/well of pEFmKLcFT or pEFmKLΔTMcFT cDNA using OPTI-MEM I and Lipofectamine 2000, followed by 24 h of incubation in DMEM at 37°C and 5% CO2-95% air. Empty pEF1/myc-His[A] plasmids were used as controls in all transfection experiments.
Quantitation of α-Klotho
Soluble α-Klotho in rodent plasma was determined by immunoprecipitation-immunoblot assay using recombinant α-Klotho (R&D Systems, Minneapolis, MN) as calibration standard curve. α-Klotho from 100 μl of rat serum plasma was incubated with 2 μg/ml of synthetic sb106 Fabs to α-Klotho. The immune complex was precipitated with anti-Flag-Beads (murine IgG1 mono-Ab, Sigma-Aldrich) overnight at 4°C, released with SDS-buffer, and resolved on 7.5% SDS-PAGE. The equivalent of 35 μl of serum was loaded per lane. Immunoblot was performed with primary rat anti-α-Klotho antibody (KM2076, 1:5,000) and horseradish peroxidase-coupled goat anti-rat secondary antibody (1:5,000). Signals were visualized with enhanced chemiluminescence and quantified by densitometry.
Assays
Cell injury.
Lactate dehydrogenase (LDH) cytotoxicity detection kit (Clontech Laboratories, Mountain View, CA) was used to measure release of the cytoplasmic enzyme into the culture supernatant when the plasma membrane is damaged. LDH generates NADH from lactate, which reacts with diaphorase to generate formazan dye products for colorimetric absorbance (490 nm).
Cell proliferation.
Bromodeoxyuridine (BrdU) (Calbiochem, Billerica, MA) incorporation into newly synthesized DNA of actively proliferating cells was measured from the conversion of the chromogenic substrate tetramethylbenzidine at a spectrophotometric absorbance at dual wavelengths of 450–540 nm.
Apoptosis.
Caspase-8 activity and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) were performed. Caspase-8 activity was measured using a colorimetric assay with spectrophotometric detection (wavelength 405 nm) of the chromophore p-nitroaniline after its cleavage by caspase from the labeled caspase-specific substrates (ApoAlert; Clontech Laboratories). For TUNEL, alveolar type I epithelial cells on coverslips in 12-well plates were rinsed with ice-cold PBS, fixed (4% paraformaldehyde; 60 min; 25°C), and incubated in permeabilization solution (2 min; 4°C). Apoptotic cells were detected in situ using Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions, costained with DAPI, and visualized with a Zeiss Axioplan-2 Fluorescent Microscope (Carl Zeiss, Jena, Germany).
Total antioxidant capacity.
Both copper- and iron-based assays were performed. Copper-reducing equivalents were measured using a calorimetric assay (OxiSelect total antioxidant capacity, Cell BioLabs, San Diego, CA) based on the reduction of copper (II) to copper (I) by uric acid and further reaction with a coupling chromogenic reagent (maximum absorbance 490 nm). The net absorbance of antioxidants, proportional to the total reductive capacity of the sample, is compared with a known uric acid standard curve. The Trolox antioxidant assay (Sigma-Aldrich) is based on formation of a ferryl myoglobin radical from metmyoglobin and hydrogen peroxide, which oxidizes to 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), a soluble chromogen measured spectrophotometrically (λAb 405 nm). Trolox, a water-soluble vitamin E analog, serves as a standard control antioxidant.
Antioxidant reporter.
A dual-luciferase assay (Cignal Antioxidant Reporter Kit; SA Biosciences, Valencia, CA) was used to measure the activity of nuclear factor erythroid-derived 2-related factors 2 and 1 (Nrf2 and Nrf1) transcription factor activation of tandem antioxidant response elements (AREs) regulating genes protecting cells from oxidative damage. The ARE reporter contains inducible antioxidant-responsive firefly luciferase construct and constitutively expressed Renilla luciferase construct. Upon transfection with the ARE reporter, the activities of the firefly and Renilla luciferases quantify transcriptional activities of the antioxidant response pathway.
Heme oxygenase-1 protein.
Heme oxygenase-1 (HOX-1) levels were measured in primary epithelial cell lysate by immunoblot of SDS-PAGE using a mouse anti-HOX-1 monoclonal antibody (Abcam Cambridge, MA).
Oxidative Damage
DNA damage.
The formation of 8-hydroxydeoxyguanosine (8-OHdG) is a ubiquitous marker of oxidative DNA injury. DNA was extracted using DNAzol (Life Technologies), precipitated in 100% ethanol, washed with 70% ethanol, and suspended in 8 mM NaOH, and the 8-OHdG concentration was determined by ELISA (OxiSelect Oxidative DNA damage, Cell BioLabs) compared with that of a 8-OHdG standard curve.
Protein oxidation.
OxiSelect Protein Carbonyl ELISA Kit (Cell BioLabs) was used to measure protein oxidation against a known reduced/oxidized BSA standard curve at 450 nm.
Lipid oxidation.
The lipid-based marker of oxidative stress, 8-isoprostane, was measured using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) based on competitive binding between 8-isoprostane and 8-isoprostane-acetylcholinesterase (AChE) conjugate (8-Isoprostane Tracer) for limited specific binding sites compared against a standard curve at 412 nm.
Animal Studies
All animal protocols were conducted following the Guide for the Care and Use of Laboratory Animals by The National Institutes of Health and approved by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center.
Mice.
Homozygous (kl/kl, n = 9) and heterozygous (kl/+, n = 4) α-Klotho hypomorphic mice and wild-type control (+/+, n = 5) mice were bred on 129 sv genetic background. The mice were euthanized by an overdose intraperitoneal injection of pentobarbital and phenytoin. The left lung was perfused and snap-frozen in liquid nitrogen for the assays described above. The right lung was fixed by intratracheal instillation of 2.5% buffered glutaraldehyde at a constant airway pressure (25 cm H2O) for separate morphometric analysis. The kidneys, spleen, liver, serum, and urine were also collected and frozen.
Rats.
Male Sprague-Dawley rats (body weight ∼300 g; Charles River, Wilmington, MA) were exposed to normoxia (room air, 21% O2) or hyperoxia (90% inspired O2 in an environmental chamber; Biospherix, Lacona, NY) continuously for 3 days. At 12 h before and again 24 h and 48 h after the start of O2 exposure, the animals received intraperitoneal injections of one of three preparations: DMEM (100 μl n = 6), control CM (100 μl, n = 6), or α-Klotho CM (100 μl, ∼60 pmol, n = 8). The animals were taken out of the chamber briefly once daily for body weight measurements. At the end of exposure, the rats were euthanized by an overdose intraperitoneal injection of Euthasol. The left lung was perfused and snap-frozen in liquid nitrogen for the assays described above. The right lung was fixed by intratracheal instillation of 4% paraformaldehyde at a constant airway pressure (25 cm H2O). Tissue blocks from the right caudal lobe were sampled, embedded in paraffin, sectioned (4-μm thickness), and stained with trichrome for histological evaluation. The kidneys, spleen, liver, serum, and urine were collected and frozen.
Edema estimation.
Lung tissue (∼100 mg) was weighed and transferred to a platinum ashing crucible and placed on a hot plate set at 100°C under a heat lamp for 2 h. Then, the dry sample-containing crucible was weighed to determine the sample dry weight. The crucible was placed overnight in the ash oven set at 600°C, removed, and weighed to determine the ash weight. The ash was dissolved in 2 ml of HCl, and the sodium content was measured by flame photometry. Sodium-to-dry weight ratio serves as a surrogate marker for the relative amount of interstitial fluid (extracellular fluid sodium concentration 135–140 mM; intracellular sodium concentration 10–15 mM).
Data Analysis
All experiments were done in triplicate. Results were expressed as means ± SD and compared across treatment conditions by two-way ANOVA. Results across Klotho genotypes were compared by one-way ANOVA with post hoc test by Fisher's protected least-significant-difference method. A P value of <0.05 was considered significant.
RESULTS
Klotho-Deficient Mice
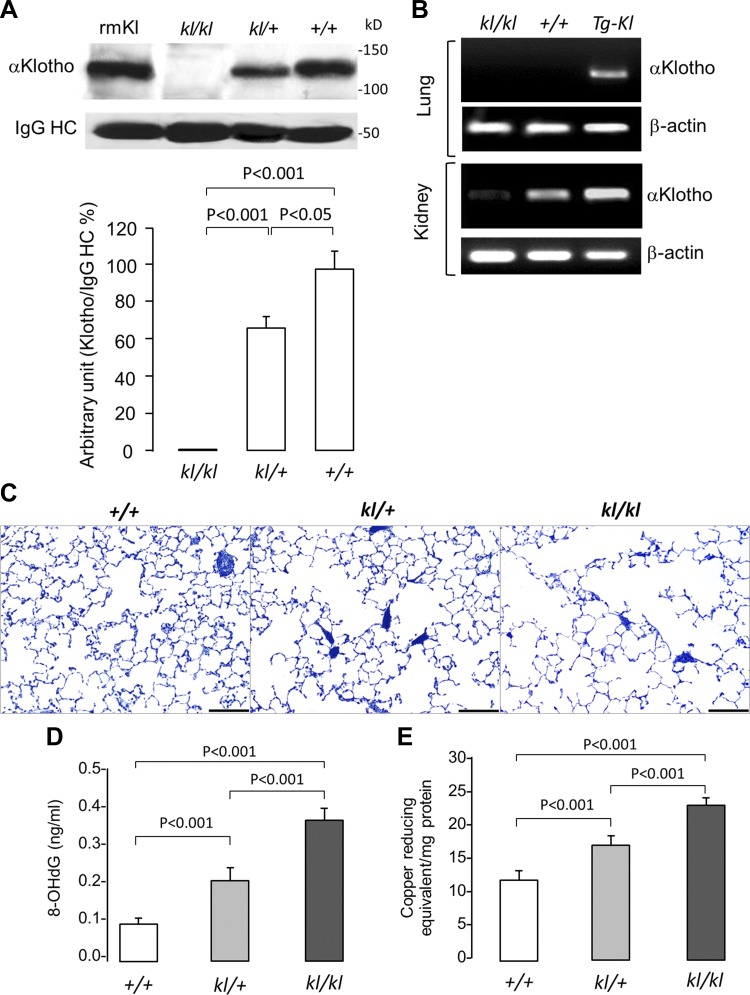
Using an immunoprecipitation-immunoblot assay, we found that circulating α-Klotho levels in the serum were almost undetectable in kl/kl mice and reduced by about 30–40% in heterozygous kl/+ compared with +/+ mice (Fig. 1A). We also confirmed the absence of native α-Klotho expression in the lung using nonquantitative RT-PCR (Fig. 1B) (27). When driven by a ubiquitous promoter (elongation factor) in the transgenic α-Klotho-overexpressing mice (Tg-Kl) (29), α-Klotho transcript is detectable in the lung, assuring the ability of this assay to detect α-Klotho mRNA when present in the lung. The renal expression is as expected in previous reports (14, 15, 29). The lungs from kl/kl mice exhibited enlarged distal air spaces at a given airway pressure compared with kl/+ and +/+ mice (Fig. 1C). The kl/kl lungs also exhibited higher 8-OHdG levels than kl/+ lungs, which in turn was higher than +/+ lungs, indicating greater oxidative damage in the lung with more severe circulating α-Klotho deficiency (Fig. 1C). Interestingly, the more pronounced oxidative damage in α-Klotho-insufficient states was accompanied by higher copper-reducing equivalents, suggesting the recruitment of α-Klotho-independent compensatory pathways in response to the oxidative damage (Fig. 1D). These observations are compatible with the model of circulating α-Klotho exerting an effect on the lung. However, the complexity of the whole organism does not permit a definitive conclusion that α-Klotho acts directly on the lung or the dissection of potential protective mechanisms. To show direct effects, we next examined cultured lung cells.
Fig. 1.
Alterations in the lungs of α-Klotho-deficient mice. kl/kl homozygous and kl/+ heterozygous disruption of the murine α-Klotho gene promoter results in hypomorphism. +/+ denotes wild-type. A: serum α-Klotho concentration was measured by immunoprecipitation-immunoblot. rmKl, recombinant mouse α-Klotho standard; IgG-HC, immunoglobulin G heavy chain. B: RT-PCR of murine α-Klotho and β-actin mRNA in lung and kidney in kl/kl and +/+ mice along with transgenic Klotho-overexpressing mice (Tg-Kl) as controls. C: representative histological sections from the distal lung of kl/kl and kl/+ α-Klotho mice compared with +/+ mice, stained with toluidine blue. Bar = 200 μm. D: oxidative damage measured by 8-hydroxydeoxyguanosine (8-OHdG) concentration. E: total antioxidant capacity measured as copper-reducing equivalents. Means ± SD. Statistical significance was determined by ANOVA.
Cell Culture
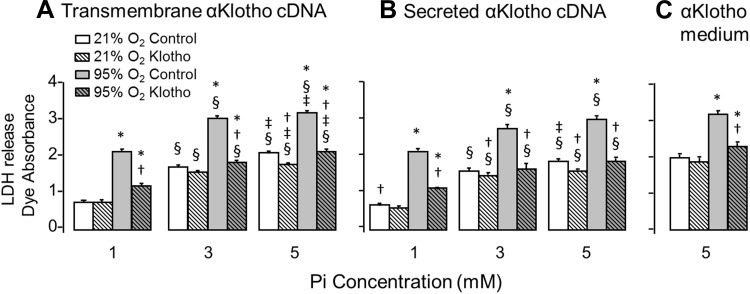
Cytoprotection by α-Klotho has been shown in other cell types to be mediated at least partly via inhibition of cellular Pi uptake (14). In addition to antioxidation, we tested the interaction between hyperoxic and phosphotoxic insults on cultured A549 lung epithelial cells. Increasing either O2 or Pi concentration increased the amount of LDH release, indicating cell injury, and the deleterious effects of Pi and hyperoxia were additive (Figs. 2–5). Because transmembrane α-Klotho and soluble α-Klotho have differential functions (16), and some have even proposed intracellular and intranuclear actions of α-Klotho (11), we separately examined three maneuvers to increase α-Klotho exposure: 1) transfecting cDNA of full-length transmembrane α-Klotho, 2) transfecting cDNA of secreted α-Klotho, and 3) exogenously added soluble α-Klotho. The control for cDNA transfection was empty plasmid, and the control for conditioned medium was either plain cell culture medium or cell culture medium from cells not subjected to α-Klotho plasmid transfection. All three maneuvers reduced LDH release from cultured cells compared with unperturbed controls during exposure to hyperoxia and Pi (Fig. 2). The protection conferred by α-Klotho against hyperoxia was more pronounced than that against phosphotoxicity for all three forms of α-Klotho augmentation in these cells (Fig. 2). Cell proliferation measured by BrdU incorporation was not significantly changed during O2 or Pi exposure (data not shown).
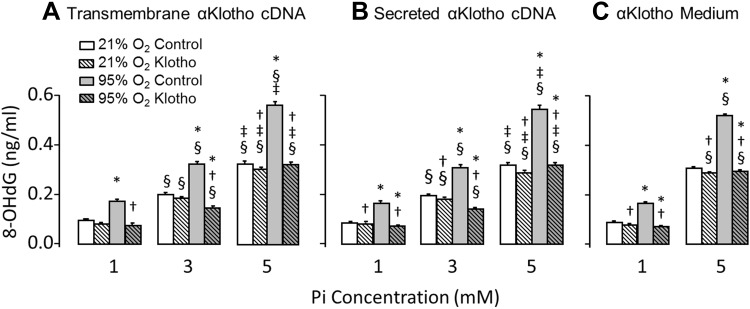
Fig. 2.
Effect of α-Klotho on lung epithelial cell viability during hyperoxic and phosphotoxic insults. Lactate dehydrogenase (LDH) release was measured in A549 cells transfected with transmembrane (A) or secreted (B) α-Klotho cDNA, or incubated with α-Klotho conditioned medium (Klotho CM, C) and exposed to 21% or 95% O2 at inorganic phosphate (Pi) concentration = 1, 3, or 5 mM. Control cells were transfected with empty vector or incubated with regular unconditioned medium. Means ± SD. P ≤ 0.05: *95% vs. 21% O2 at the same Pi and cDNA; † vs. control at the same Pi concentration and O2 exposure; § vs. Pi concentration of 1 mM and ‡ vs. 3 mM at the same cDNA and O2 exposure, by ANOVA.
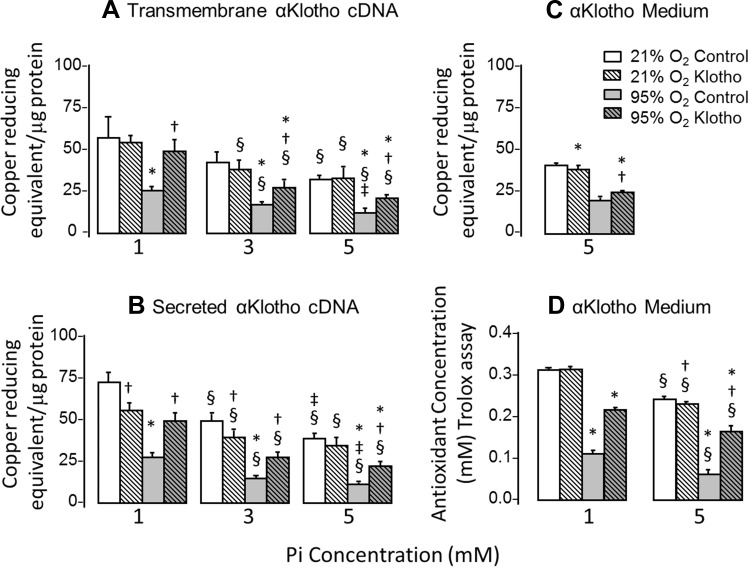
Fig. 5.
Effect of α-Klotho on total antioxidant capacity during hyperoxic and phosphotoxic insults. A–C: copper-reducing equivalent (per microgram of protein) was measured in A549 cells transfected with transmembrane (A) or secreted (B) α-Klotho cDNA or incubated with α-Klotho CM (C), and exposed to 21% or 95% O2 at [Pi] = 1, 3, or 5 mM. Control cells were transfected with empty vector or incubated with regular unconditioned medium. D: A549 cells were treated as in C, and the iron-based Trolox assay was performed to assess antioxidant capacity. Means ± SD. P ≤ 0.05: *95% vs. 21% O2 at the same Pi and cDNA; † vs. control at the same Pi concentration and O2 exposure; § vs. 1 mM and ‡ vs. 3 mM at the same cDNA and O2 exposure, by ANOVA.
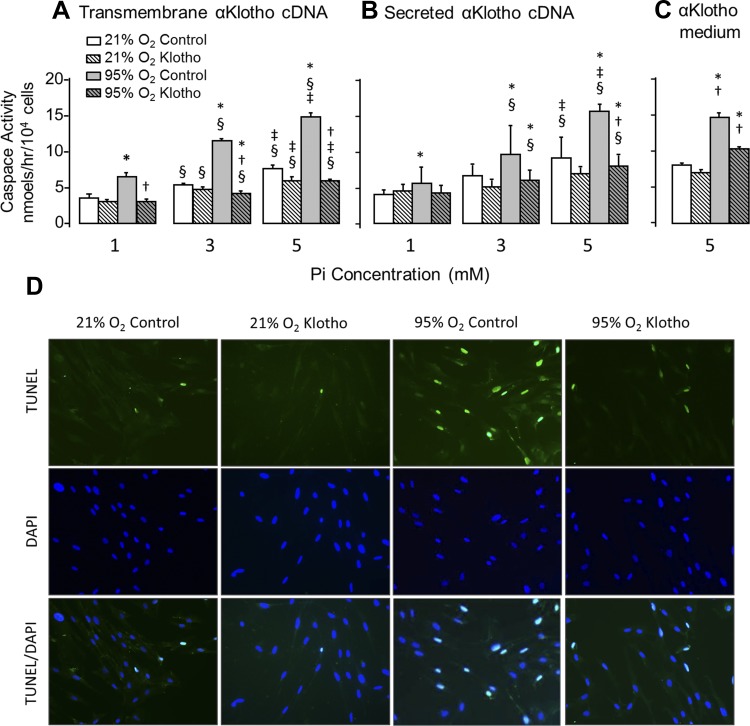
We next examined whether the cell injury was mediated by apoptosis using caspase activity and TUNEL staining as readouts (Fig. 3). Hyperoxia and Pi independently and additively induced apoptosis. Similar to cell viability, all three forms of α-Klotho expression ameliorated the apoptotic markers (caspase-8 and TUNEL labeling) with more pronounced effect on hyperoxia than phosphotoxicity (Fig. 3). Because both hyperoxia and phosphotoxicity can elicit oxidative stress, we measured 8-OHdG as a marker of oxidative damage to DNA. Similar to the scenario with cell viability and apoptosis, hyperoxia and Pi induced oxidative DNA damage in an additive fashion, and all three forms of α-Klotho lessened the damage (Fig. 4). Finally, we examined whether α-Klotho restores the antioxidative capacity of cells using both a copper-based (Fig. 5, A–C) and an iron-based (Trolox, Fig. 5D) read out. Whereas Pi modestly lowered antioxidant activity, hyperoxia caused a drastic reduction of this parameter (Fig. 5, A–D). Transfected transmembrane and secreted α-Klotho increased antioxidant activity with both assays (Fig. 5, A and B), whereas exogenously added α-Klotho induced a smaller though still statistically significant increase (Fig. 5C). The experiment in Fig. 5C was repeated using an Fe-based measure of total antioxidant capacity, and similar results were obtained (Fig. 5D).
Fig. 3.
Effect of α-Klotho on lung epithelial cell apoptosis during hyperoxic and phosphotoxic insults. A–C: caspase activity was measured in A549 cells transfected with transmembrane (A) or secreted (B) α-Klotho cDNA or incubated with α-Klotho CM (C) and exposed to 21% or 95% O2 at Pi concentration = 1, 3, or 5 mM. Control cells were transfected with empty vector or incubated with regular unconditioned medium. Means ± SD. P ≤ 0.05: *95% vs. 21% O2 at the same Pi and cDNA; † vs. control at the same Pi concentration and O2 exposure; § vs. 1 mM and ‡ vs. 3 mM at the same cDNA and O2 exposure, by ANOVA. D: TUNEL and DAPI labeling of type I epithelial cells under different treatments: incubated with regular medium (control) or α-Klotho CM and exposed to 21% or 95% O2 as described above.
Fig. 4.
Effect of α-Klotho on oxidative DNA damage during hyperoxic and phosphotoxic insults. 8-OHdG concentration (ng/ml) was measured in A549 cells transfected with transmembrane (A) or secreted (B) α-Klotho cDNA or incubated with α-Klotho CM (C), and exposed to 21% or 95% O2 at [Pi] = 1, 3, or 5 mM. Control cells were transfected with empty vector or incubated with regular medium. Means ± SD. P ≤ 0.05. *95% vs. 21% O2 at the same Pi and cDNA; † vs. control at the same Pi concentration and O2 exposure; § vs. 1 mM and ‡ vs. 3 mM at the same cDNA and O2 exposure, by ANOVA.
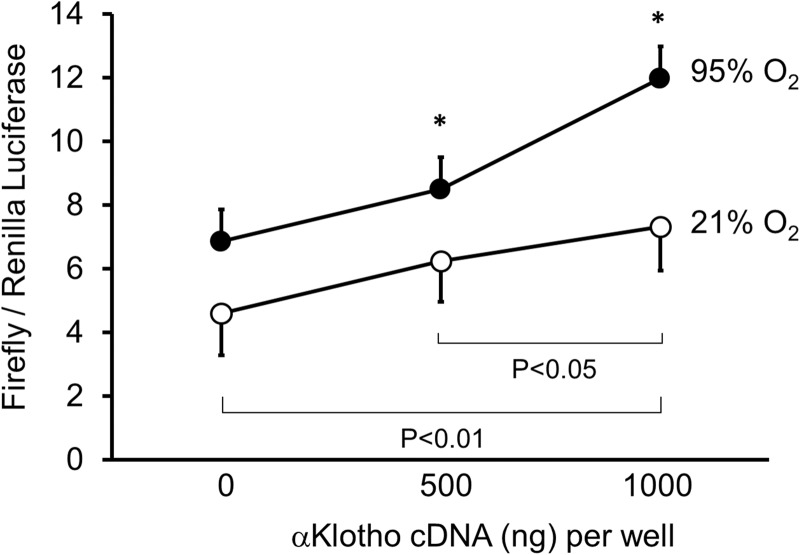
Expression of genes encoding antioxidative enzymes is regulated at the transcriptional level through cis-active sequences known as ARE. Nrf1 and Nrf2 play central roles in mediating defense against oxidant insults through the ARE (23). We next examined the activation of antioxidative pathways using an ARE reporter for the Nrf1 and Nrf2 pathway that transactivates a number of genes involved in antioxidative responses (33). Hyperoxia alone increased the ARE reporter activity, and the expression of α-Klotho further enhanced the response (Fig. 6), consistent with the biochemical studies of total antioxidant capacity shown above.
Fig. 6.
Effect of α-Klotho and hyperoxia on activation of antioxidant response element. A549 cells were cotransfected with the luciferase reporter plasmid along with either α-Klotho-expression plasmid or empty vector, then exposed to 21% or 95% O2. The cell lysate was subjected to luciferase assay (normalized to Renilla). Means ± SD. *P ≤ 0.05 between 95% and 21% O2 for the same amount of transfected α-Klotho cDNA.
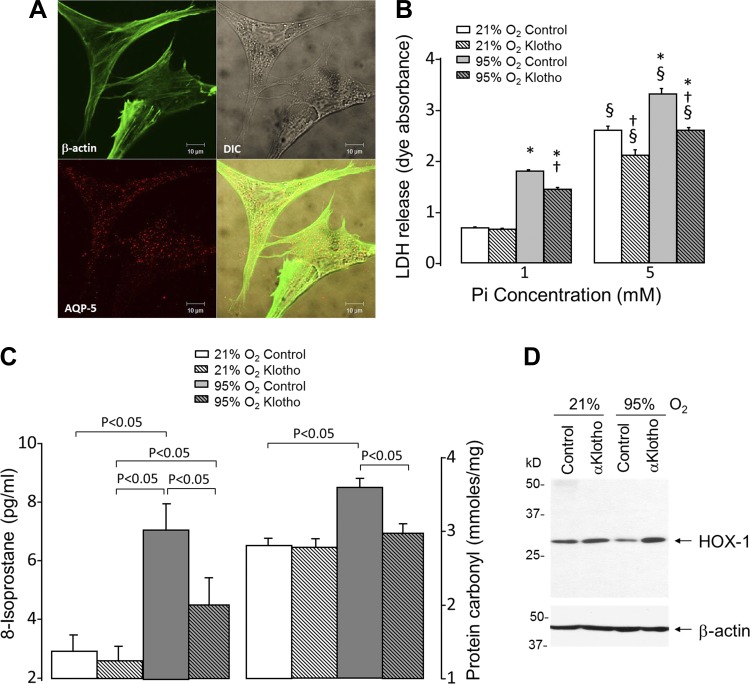
Because A549 cell is a transformed cell line, we tested whether the same protective effect is also present in primary culture of human alveolar type I epithelial cells. We first confirmed that these cells maintain adequate differentiation by their expression of aquaporin-5 (Fig. 7A). In these primary cells, addition of α-Klotho-containing CM reduced hyperoxia-induced cell damage similar to that seen in A549 cells (Fig. 7B). In addition to oxidative DNA damage, we assessed lipid and protein oxidation. Both of these parameters were increased by hyperoxia, and addition of α-Klotho ameliorated the damage (Fig. 7C). In keeping with the increased total antioxidant capacity and ARE reporter activity, we examined one downstream target antioxidant HOX-1, and α-Klotho addition increased HOX-1 under hyperoxic stress (Fig. 7D). These in vitro studies confirmed that α-Klotho directly protects lung epithelial cells against oxidative damage and apoptosis induced by hyperoxia and phosphotoxicity.
Fig. 7.
Effect of α-Klotho on primary human alveolar type I (AT1) epithelial cells during hyperoxic and phosphotoxic insults. A: photomicrographs of aquaporin-5 (AQP-5) and β-actin expression, shown in differential interference contrast (DIC) and composite image (bottom, right). B: LDH release in AT1 cells incubated with or without α-Klotho CM and exposed to 21% or 95% O2 at Pi = 1 or 5 mM. Control cells were incubated with CM without α-Klotho. Means ± SD. P ≤ 0.05: *95% vs. 21% O2 at the same Pi and cDNA; † vs. control at the same Pi concentration and O2 exposure; § vs. 1 mM at the same cDNA and O2 exposure. C: cells were exposed to normoxia or hyperoxia in the presence or absence of α-Klotho as in B, and cell lysates were assayed for lipid (8-isoprostane; left) and protein (protein carbonyl; right) oxidation. D: immunoblot for heme oxygenase 1 (HOX-1) protein under the same experimental conditions as C.
Animal Studies
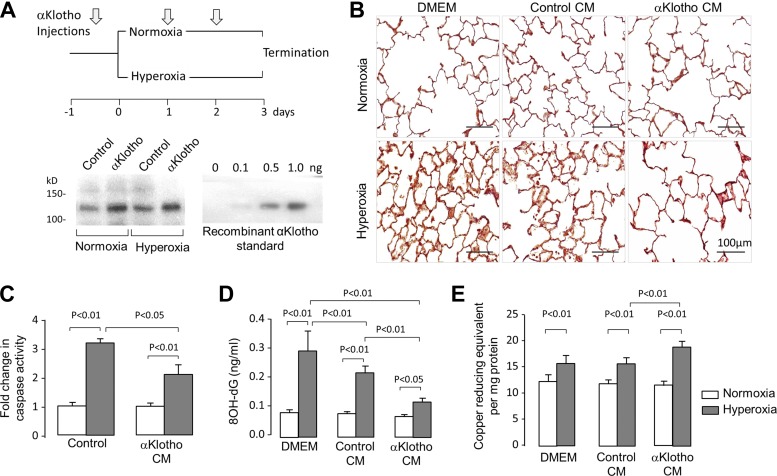
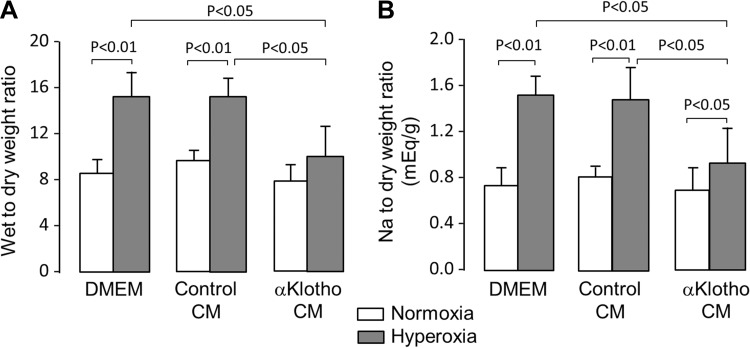
The above studies demonstrated direct cytoprotection by α-Klotho in cultured cells. As further proof of principle, we tested whether α-Klotho-mediated cellular protection in the lung can be observed in vivo using an acute hyperoxia model. Rats exposed to acute hyperoxia received exogenous α-Klotho-containing CM or control medium (either plain cell culture medium or medium conditioned by mock-transfected cells) (Fig. 8A). The administration of α-Klotho-containing CM effectively increased circulating α-Klotho concentrations although there were large variations from animal to animal because of the long duration that elapsed between the last injection and the serum α-Klotho measurement (Fig. 8A, mean ± SD in pg/ml: normoxia control 1.35 ± 0.38, normoxia Klotho 4.91 ± 3.26, hyperoxia control 1.91 ± 0.13, hyperoxia Klotho 2.43 ± 0.48; n = 3 per group). Histological sections in α-Klotho-treated animals exposed to hyperoxia showed significant attenuation of the expected perivascular and interstitial edema, patchy atelectasis, and alveolar exudation compared with treatment with DMEM and control CM (Fig. 8B). The hyperoxia-induced increase in lung caspase activity was also attenuated by α-Klotho CM (Fig. 8C). Interestingly, both the mock-transfected (control) CM and α-Klotho-containing CM reduced 8-OHdG in lung tissue compared with plain cell culture medium (DMEM), but the α-Klotho-containing CM had an effect above and beyond that of mock-transfected CM (Fig. 8D). The data suggest that the contents released from mock-transfected cells offered some protection independent of α-Klotho, but α-Klotho clearly exerted additional protective effects against oxidative DNA damage. Furthermore, exogenous α-Klotho treatment escalated the hyperoxia-stimulated upregulation of total antioxidant capacity in the lung (Fig. 8E). In addition to ameliorating cellular damage, α-Klotho also attenuated lung fluid accumulation measured by the dry-to-wet weight ratio (Fig. 9A) and the sodium (predominant extracellular ion)-to-wet weight ratio (Fig. 9B). These effects of systemically injected CM indicate that the lung is an endocrine target of α-Klotho action.
Fig. 8.
Effect of exogenous α-Klotho on the lung during acute hyperoxic insult in vivo. Sprague-Dawley rats received intraperitoneal injections of plain DMEM, mock-transfected CM (control CM), or α-Klotho-transfected CM before and during 3 days of exposure to 21% or 90% O2. A, top: experimental scheme. Bottom: plasma Klotho levels by immunoprecipitation-immunoblot at the end of exposure in the four groups of animals. B: representative histological sections of the distal lung stained with trichrome. Bar = 100 μm. C: caspase-8 concentration in lung tissue was expressed as a ratio with respect to that in the corresponding normoxia-exposed animal. D: 8-OHdG concentration (ng/ml) in lung tissue. E: total antioxidant capacity (copper-reducing equivalent per microgram of protein) in lung tissue.
Fig. 9.
Effect of exogenous α-Klotho on lung fluid during acute hyperoxic insult in vivo. Sprague-Dawley rats received intraperitoneal injections of plain DMEM, mock-transfected CM (control CM), or α-Klotho-transfected CM before and during 3 days of exposure to 21% or 90% O2. Wet-to-dry weight ratio (A) and sodium-to-dry weight ratio (B) in the lungs of animals. Means ± SD. Statistical significance was determined by ANOVA.
DISCUSSION
Summary of Findings
This is the first study to systemically examine cytoprotection by α-Klotho on the lung. 1) Despite a lack of endogenous α-Klotho expression in the lung, kl/kl mice with near absence of circulating Klotho exhibit heightened oxidative DNA damage in the lung, whereas kl/+ mice with ∼40% reduction in circulating α-Klotho exhibit intermediate oxidative damage in the lung. 2) In cultured lung epithelial cells, transfection of transmembrane or soluble α-Klotho or addition of α-Klotho-containing CM containing increased soluble α-Klotho protected the cells from oxidative and, to some extent, phosphotoxic damage by increasing the antioxidative capacity of the cells via the Nrf1/2 pathways. 3) In an acute hyperoxic lung injury animal model, systemic elevation of α-Klotho CM alleviated oxidative damage and interstitial edema and stimulated an increase in total antioxidant capacity in the lung possibly via Nrf pathways. In combination, these findings strongly support a model of lung protection by α-Klotho as an endocrine substance that activates endogenous antioxidative pathways.
The morphological hallmark of kl/kl mice suffering from premature senescence is age-accelerated air space enlargement (20), reminiscent of that found in the aging human lung (10). Airway cell apoptosis and oxidative stress are accentuated in these animals (20, 25). Our observation of increased oxidative DNA, lipid, and protein damage supports the interpretation that heightened oxidative tissue damage contributes to premature alveolar degeneration, whereas the associated increase in total antioxidant capacity likely represents a compensatory response via α-Klotho-independent pathways. In heterozygous kl/+ lungs, both DNA damage and antioxidative capacity are elevated to an intermediate degree, suggesting that α-Klotho-independent pathways, although present, are inadequate to fully alleviate DNA damage, thereby attesting to a crucial role for α-Klotho-mediated antioxidative mechanisms. Most of the organs that undergo premature senescence in the kl/kl mice in fact do not express α-Klotho (27), suggesting that organ degeneration is likely related to deficient circulating α-Klotho. α-Klotho is primarily expressed in the kidney and choroid plexus (27), but low levels of transcript can be detected in various organs other than the lung using highly sensitive RT-PCR. α-Klotho is cleaved by β-secretases (2, 6) and released into the circulation with the kidneys being a major source (34). Although the kl/+ mice show lower circulating α-Klotho levels, it is impossible to conclude from the genetically altered mice whether α-Klotho insufficiency is directly responsible for the age-related changes in the lung. We addressed this question by directly increasing α-Klotho level in cultured pulmonary epithelial cells to prove that α-Klotho protects lung epithelia. It is important to note that the presence of a direct effect on the lung does not rule out a simultaneous secondary effect of α-Klotho acting through other intermediate mediators on the lung.
Our in vitro studies employ a combination of ambient hyperoxia and phosphate loading (26) as cytotoxic insults. Oxygen and phosphate toxicity can act independently in parallel or converge upon common cytotoxic pathways. Human vascular endothelial cells exposed to high phosphate medium produce higher levels of ROS and undergo apoptosis (8). Renal phosphate retention caused by α-Klotho deficiency in mice leads to oxidative damage and apoptosis in hippocampus neurons, resulting in cognitive impairment, an effect that can be rescued by administration of antioxidant (32). Mitochondrial membrane potential, NADH concentration, and oxygen consumption in isolated mitochondria escalate dramatically as extramitochondrial phosphate concentration increases (3); the membrane potential is positively correlated with ROS production and can contribute to apoptosis (36). High phosphate concentration enhances delivery of reducing equivalent to cytochrome c in Complex III in the electron transport chain, which increases ROS production (3) and induces nonselective inner membrane permeabilization in the mitochondria (24). The cytotoxicity induced by hyperoxia and high phosphate was additive in cell culture, and α-Klotho effectively ameliorated the damage imparted by both insults (Figs. 2, 3, 4, and 7B). α-Klotho is known to inhibit cellular phosphate uptake via inhibition of the Pit family of Na+-coupled phosphate transporters (14, 17), so part of its cytoprotective effect in pulmonary epithelia may be mediated via reduction of cellular phosphate uptake.
Transgenic overexpression of α-Klotho in mice and addition of soluble α-Klotho to cells lead to increased manganese SOD2 expression and decreased Forkhead box O phosphorylation (regulator of SOD2) and lower 8-OHdG (18, 40). Interestingly, SOD2 is a downstream effector of the Nrf1/2 pathway (37). Thus the effect of α-Klotho on pulmonary epithelial cells is probably mediated by dual actions of inhibition of phosphate uptake as well as enhanced antioxidation.
Although we elucidated the cellular mechanism of α-Klotho-mediated cytoprotection, the molecular mechanisms underlying α-Klotho cytoprotection remain enigmatic. Transmembrane α-Klotho functions as a coreceptor for FGF23 in extrapulmonary organs (25). However, transmembrane α-Klotho is not present in the lung, and there is no added FGF23 in our cell culture system. Therefore, it is more likely that the transmembrane α-Klotho is cleaved by β-secretases endogenous to A549 cells releasing the extracellular domain α-Klotho, and the cytoprotective effect is actually mediated by soluble α-Klotho. The fact that transfected transmembrane α-Klotho, secreted α-Klotho, and α-Klotho-containing cell culture medium exerted the same effect strongly suggests that α-Klotho is acting in the extracellular space. Again, this mechanism of action is compatible with the increased DNA damage observed in kl/- mice with low circulating levels of α-Klotho. As a glycan-modifying enzyme, soluble α-Klotho can modify ion channels and transporters by cleaving sialic acid (4, 5) and glucuronate residues (13, 35) through changing surface protein as well as direct gating in renal tubular epithelial cells (4, 5, 13). Similar mechanisms may operate in lung epithelial cells. It is unclear whether these enzymatic actions mediate the α-Klotho-induced increases in cellular antioxidant capacity.
Implications and Significance
These studies clearly establish the lung as an endocrine target of α-Klotho cytoprotective action in which antioxidation and antiapoptosis play important roles. α-Klotho deficiency or insufficiency has been implicated in normal aging, acute kidney injury, chronic kidney disease, and acute systemic inflammation (14, 15, 31). Each of these conditions is also associated with elevated risks of various pulmonary complications (9, 10, 39). It is plausible that α-Klotho deficiency may hasten age-related pulmonary decline, predispose to or exacerbate exogenous insult causing lung injury, impair repair, and increase the likelihood of secondary pulmonary complications in extrapulmonary conditions. Our findings lay the essential foundation for further investigation to elucidate the mechanisms of α-Klotho action in the lung to determine its pathophysiological role in pulmonary diseases and, eventually, consideration as therapy.
GRANTS
The research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK091392 and DK092461 (O. Moe, M. Kuro-o, M. Hu), National Heart, Lung, and Blood Institute grants R01 HL40070 and U01 HL111146 (C. Hsia), National Institute of Aging grant R01 AG019712 (M. Kuro-o), the Ruth L. Kirschstein National Research Service Award F32 HL103043 (P. Ravikumar), Division of Nephrology Training Grant T32-DK007257-32 (P. Ravikumar), the O'Brien Kidney Research Center (NIH P30DK-07938), the Simmons Family Foundation (O. Moe), and the Charles and Jane Pak Foundation (O. Moe, M. Kuro-o, M. Hu). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.R., M.C.H., O.W.M., and C.C.H. conception and design of research; P.R., J.Y., J.Z., S.N.P., and M.C.H. performed experiments; P.R., M.C.H., and C.C.H. analyzed data; P.R., M.C.H., M.K.-o., C.C.H, and O.W.M. interpreted results of experiments; P.R., C.C.H., and O.W.M. prepared figures; P.R. and C.C.H. drafted manuscript; P.R., M.C.H., C.C.H., and O.W.M. edited and revised manuscript; P.R., C.C.H., and O.W.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Roshni Iyer and Blaine Smith for technical assistance.
REFERENCES
- 1.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81: 539–547, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem 278: 39155–39165, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76: 38–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105: 9805–9810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng MF, Chen LJ, Cheng JT. Decrease of Klotho in the kidney of streptozotocin-induced diabetic rats. J Biomed Biotechnol 2010: 513853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstadt H. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol 294: F1381–F1387, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Doi K, Ishizu T, Fujita T, Noiri E. Lung injury following acute kidney injury: kidney-lung crosstalk. Clin Exp Nephrol 15: 464–470, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc 6: 570–572, 2009 [DOI] [PubMed] [Google Scholar]
- 11.German DC, Khobahy I, Pastor J, Kuro OM, Liu X. Nuclear localization of Klotho in brain: an anti-aging protein. Neurobiol Aging 33: 1483 e1425–1430, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu MC, Shi M, Cho HJ, Zhang J, Pavlenco A, Sidhu SS, Huang LS, Moe OW. Erythropoietin receptor- A downstream effector of aKlotho-induced cytoprotection. Kidney Int 84: 468–481, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Phys 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Shiizaki K, Kuro OM, Moe OW. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 339: 827–832, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ishii M, Yamaguchi Y, Yamamoto H, Hanaoka Y, Ouchi Y. Airspace enlargement with airway cell apoptosis in klotho mice: a model of aging lung. J Gerontol A Biol Sci Med Sci 63: 1289–1298, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576: 341–345, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima Y. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98: 115–119, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med 47: 1304–1309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett 495: 12–15, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem 389: 233–241, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kuro-o M. A potential link between phosphate and aging—lessons from Klotho-deficient mice. Mech Ageing Dev 131: 270–275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Kuro OM. A potential link between phosphate and aging-Lessons from Klotho-deficient mice. Mech Ageing Dev 131: 270–275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol 22: 1315–1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J 17: 50–52, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem 283: 33554–33562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 23: 1641–1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panesso MC, Shi M, Cho HJ, Paek J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85: 855–870, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem 174: 305–319, 1997 [PubMed] [Google Scholar]
- 37.Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp 287: 60–63; discussion 63–69, 2007 [PubMed] [Google Scholar]
- 38.Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro-o M, Nabeshima Y, Nagai R. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol 22: 26–33, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Turcios NL. Pulmonary complications of renal disorders. Paediatr Respir Rev 13: 44–49, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Ko M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280: 38029–38034, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes 60: 1907–1916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]