Abstract
The kidney adjusts K+ excretion to match intake in part by regulation of the activity of apical K+ secretory channels, including renal outer medullary K+ (ROMK)-like K+ channels, in the cortical collecting duct (CCD). ANG II inhibits ROMK channels via the ANG II type 1 receptor (AT1R) during dietary K+ restriction. Because AT1Rs and ANG II type 2 receptors (AT2Rs) generally function in an antagonistic manner, we sought to characterize the regulation of ROMK channels by the AT2R. Patch-clamp experiments revealed that ANG II increased ROMK channel activity in CCDs isolated from high-K+ (HK)-fed but not normal K+ (NK)-fed rats. This response was blocked by PD-123319, an AT2R antagonist, but not by losartan, an AT1R antagonist, and was mimicked by the AT2R agonist CGP-42112. Nitric oxide (NO) synthase is present in CCD cells that express ROMK channels. Blockade of NO synthase with N-nitro-l-arginine methyl ester and free NO with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt completely abolished ANG II-stimulated ROMK channel activity. NO enhances the synthesis of cGMP, which inhibits phosphodiesterases (PDEs) that normally degrade cAMP; cAMP increases ROMK channel activity. Pretreatment of CCDs with IBMX, a broad-spectrum PDE inhibitor, or cilostamide, a PDE3 inhibitor, abolished the stimulatory effect of ANG II on ROMK channels. Furthermore, PKA inhibitor peptide, but not an activator of the exchange protein directly activated by cAMP (Epac), also prevented the stimulatory effect of ANG II. We conclude that ANG II acts at the AT2R to stimulate ROMK channel activity in CCDs from HK-fed rats, a response opposite to that mediated by the AT1R in dietary K+-restricted animals, via a NO/cGMP pathway linked to a cAMP-PKA pathway.
Keywords: angiotensin II, angiotensin II type 2 receptor, cortical collecting duct, renal outer medullary potassium channel, protein kinase A
the kidney plays a pivotal role in maintaining K+ homeostasis in response to dietary K+ loading or restriction. This is accomplished, in large part, by modifications in urinary K+ excretion. Alterations in the activity of K+ secretory channels in the apical membrane of distal nephron segments, including the connecting tubule and cortical collecting duct (CCD), are critical in this adaptive response. Two types of conducting K+ channels have been identified in the apical membrane of these segments: a K+ secretory channel encoded by the renal outer medullary K+ (ROMK) channel, considered to mediate baseline K+ secretion (11, 54), and the Ca2+-activated maxi-K+ channel, proposed to mediate flow-stimulated K+ secretion (31, 62). The expression and activity of both channels have been shown to respond to changes in dietary K+ intake (28, 31, 49). However, the molecular mechanisms underlying the regulation of channel activity remain incompletely understood.
The renal adaptation to a high-K+ (HK) diet is associated with an increase in the density but not open probability of the well-characterized ROMK channel in the mammalian distal nephron (11, 32, 54). Patch-clamp studies of the apical membrane of CCD principal cells have revealed that ROMK channel density, measured as channel number per µm2 patch membrane area, increased more than threefold after only 6 h on a HK diet and more than fivefold after 48 h on the same HK diet compared with animals fed a normal-K+ (NK) diet (33). This aldosterone-insensitive response (33) was proposed not to be due to an increase in ROMK mRNA in single CCDs or channel protein abundance, as determined by in situ hybridization and immunoblot analysis (12), respectively. The rapid and stable increase in the density of the channel was attributed by the authors to the activation of a silent pool of channels in the cell membrane or translocation of existing ROMK proteins from the cytoplasm to the cell membrane (12). In support of the latter mechanism, emerging evidence suggests that ROMK channel density in the plasma membrane depends on the balance of phosphorylation and dephosphorylation of a specific tyrosine residue in the channel protein (27). Protein tyrosine kinase responds to changes in the extracellular K+ balance (51). Dietary K+ loading and the consequent increased ROMK channel activity are associated with reduced tyrosine phosphorylation of the channel protein and attenuated endocytotic removal of channels from the apical cell membrane (19), whereas dietary K+ restriction induces an opposite response (51).
The renal intratubular renin-angiotensin system (RAS), which is independently regulated from the RAS in the plasma circulation (for a review, see Ref. 48), contributes to the control of salt and water transport within the kidney (60). ANG II mediates its biological effects by interacting with two major subtypes of receptors: ANG II type 1 and 2 receptors (AT1Rs and AT2Rs, respectively). Each of the receptors has an unique pattern of expression, signal transduction mechanisms, and effects (6). Both AT1R and AT2R expression have been identified in the adult kidney (26). We (60) have recently reported that ANG II via AT1Rs inhibits ROMK channel activity through a protein tyrosine kinase-dependent pathway under conditions of dietary K+ restriction. Given that AT1Rs and AT2Rs generally function in an antagonistic manner (6), we speculated that AT2R activation would stimulate ROMK channel activity. This hypothesis was confirmed, and the signaling pathway was explored in the present study. Specifically, our results indicate that ANG II, via the AT2R, stimulates ROMK channel activity in CCDs from HK-fed rats above and beyond the well-described dietary K+-induced increase in channel activity (33) via nitric oxide (NO)/cGMP/phosphodiesterase (PDE) linked to a cAMP/PKA pathway.
METHODS
Preparation of CCDs.
Pathogen-free Sprague-Dawley rats of either sex (5–6 wk old, Taconic Farms, Germantown, NY) were fed either NK diet (1.1% K+ and 0.4% Na+ lab rodent diet 5001, LabDiet, St. Louis, MO, for the data shown in Fig. 1; and 1% K+ and 0.32% Na+ rodent control diet TD.88238 from Harlan Teklad Laboratory, Madison, WI, for the remainder of the control experiments) or 5% (2.6% K+ and 0.4% Na+, TD.10214) or 10% (5.2% K+ and 0.4% Na+, TD.76448) (9) HK diets (both from Harlan Teklad) for 4–7 days. Animals were allowed free access to tap water. Animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of New York Medical College and Icahn School of Medicine at Mount Sinai.
Fig. 1.
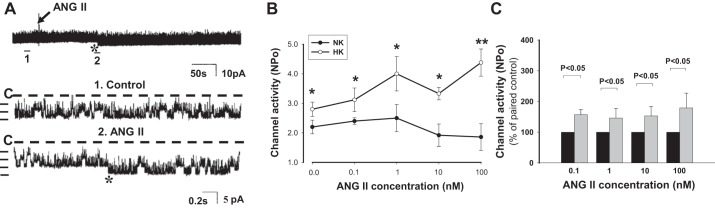
Effect of ANG II on renal outer medullary K+ (ROMK) channel activity in cortical collecting ducts (CCDs) isolated from rats fed a normal-K+ (NK) diet or high-K+ (HK) diet. A: representative traces of the stimulatory effect of ANG II (100 nM) on the apical ROMK channel in a CCD from a HK-fed rat. The top trace shows the time course of the entire experiment. The expanded traces (traces 1 and 2) show detailed channel activity at a faster time resolution. The channel closed state (C) is indicated by a dotted line. Current levels are indicated by the short horizontal bars on the left of each trace. The experiment was performed in a cell-attached patch at a holding potential of 0 mV. B: ANG II in concentrations ranging from 0.1 to 100 nM increased the activity (NPo; where Po is open probability and N is channel number) of ROMK channels in CCDs from HK- but not NK-fed rats. *P < 0.05 and **P < 0.01 vs. the same ANG II concentration in the NK-fed group via an unpaired t-test. C: ANG II increased NPo in HK-fed (gray bars) vs. NK-fed (black bars) rats without a clear dose dependence.
Rats were euthanized by cervical dislocation. Kidneys were immediately removed, and several thin coronal slices were cut with a razor blade and placed in ice-cold Ringer solution for microdissection of CCDs, as previously described (60). Ringer solution contained (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 10 HEPES (pH 7.4). To immobilize tubules for patch clamp or immunolabeling, CCDs were affixed to 5 × 5-mm coverglasses coated with poly-d-lysine.
Patch-clamp recordings.
The basic patch-clamp methods have been previously described (58, 60). In brief, each isolated CCD affixed to a coverglass was transferred to a chamber (total volume: 1,000 μl) mounted on the stage of a Nikon inverted microscope. The chamber was filled with Ringer solution. The CCD was cut open with a sharpened micropipette to expose the apical membrane. Patch-clamp pipettes were pulled using a Flaming/Brown micropipette puller (model P-87, Sutter Instrument, Novato, CA) in two stages with borosilicate glass capillaries (Dagan, Minneapolis, MN). Pipettes were fire polished using a microforge (model MF-830, Narishige Scientific Instrument Laboratory, Tokyo, Japan) and had resistances of 2–5 MΩ when filled with a solution containing (in mM) 140 KCl, 1.8 MgCl2, and 5 HEPES (pH 7.4). Experiments were performed at room temperature. Agonists and inhibitors were added to the chamber for at least 5 min, but not longer than 30 min, before recordings were obtained. Currents originating in each cell-attached patch were recorded using an Axon200B patch-clamp amplifier (Axon Instruments, Burlingame, CA). Currents were low-pass filtered at 1 kHz. Data were digitized using an Axon interface (Digidata 1200) and stored on the computer hard drive.
Immunohistochemical staining and confocal microscopy.
Isolated CCDs affixed to a coverglass were fixed with 2.5% paraformaldehyde prepared in 1× PBS plus 0.05% picric acid (pH 7.4) for 2–3 min and then rinsed with base buffer (1× PBS containing 1% BSA and 0.1% glycine). Thereafter, tubules were permeabilized with permeabilization solution (0.1% Triton dissolved in the base buffer) for 1 h at room temperature and then incubated with rabbit polyclonal IgG anti-AT2R antibody (1:200 dilution in permeabilization solution) overnight at 4°C; this antibody, directed against a predicted sequence in the amino terminus of the protein, was selected as it has been well validated and characterized (30, 56). Tubules were rinsed three times with the base buffer and then incubated for 1 h with a goat anti-rabbit IgG secondary antibody conjugated with Alexa 488 at a 1:1,000 dilution in 1× PBS containing 1% BSA at room temperature. After being rinsed with 3 volumes of 1× PBS, tubules were colabeled with the principal cell (apical membrane) marker, Dolichos biflorus agglutinin (DBA; conjugated to rhodamine, 1:200 dilution in 1 × PBS), by incubation for 30 min at room temperature. After a final rinse, a single drop of antifade reagent was deposited on each tubule, and the coverglass was sealed onto the slide with clear nail polish. Fluorescence imaging was performed with an inverted Zeiss laser scanning microscope (510-META) using a ×40 oil-immersion objective.
Relative quantitation of AT1R and AT2R mRNA in single CCDs.
CCDs were microdissected in 1× PBS containing 10 mM vanadyl ribonucleoside complexes for no longer than 90 min after the death of the animal. A total length of ∼10 mm of CCDs was pooled for each sample. RNA was extracted from microdissected CCDs, and cDNA was synthesized using random primers as previously described (60).
AT1R and AT2R transcript expression was quantitated via real-time PCR using the TaqMan assay and inventoried AT1R and AT2R Gene Expression Assays (Applied Biosystems, Foster City, CA). The Gene Expression Assay kit for rodent 18S was chosen as the internal positive reference control (Applied Biosystems). Real-time PCR was performed as previously described (60). The following was added to each 2-μl cDNA sample: 8 μl of a cocktail mix containing 0.05 μl Platinum Taq DNA polymerase, 0.1 unit AmpErase uracil N-glycosylase (UNG), 0.2 μl Gene Amp dNTPs with dUTP, 0.2 μl passive reference ROX dye, 0.2 μl (20 pM) forward and reverse primers, and 0.04 μl Taqman probe for 18S or 0.5 μl for 20× inventoried specific Taqman Gene Expression Assay. Taq DNA polymerase and ROX were purchased from Invitrogen (Carslbad, CA), and AmpErase uracil N-glycosylase and dNTPs with dUTP were purchased from Applied Biosystems (Foster City, CA). Nuclease-free water was added for a total volume of 10 μl. Each plate was then covered with optical adhesive film and, after the initial steps of 50°C for 2 min and 95°C for 10 min and 40 cycles of 95°C for 15 s (melt) and 60°C for 1 min (anneal/extend), detection was performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Measurement of cAMP content in single CCDs.
Multiple individual CCDs were microdissected from a single kidney in cold Ringer solution within 1 h of euthanization of the rat, and total lengths of ∼10 mm were transferred to a 1.5-ml microcentrifuge tube to generate a single sample. Thereafter, fresh Ringer solution (50 μl) was added to each tube, and samples were allowed to equilibrate to room temperature for 5 min. Next, samples were centrifuged, the supernatant was removed, and Ringer solution (50 μl) with IBMX (100 μM) or vehicle (DMSO) alone was added. After incubation at room temperature for 20 min, samples were centrifuged, and the supernatant was stored at −80°C. For measurements of extracellular cAMP, samples of the supernatant were boiled 5 min at 95°C and then centrifuged, and 45 μl of the supernatant were mixed with 5 μl of the internal standard solution and then subjected to direct analysis. To extract intracellular cAMP from CCDs, 0.5 ml of ice-cold 1-propanol was added to the tissue pellet, and samples were placed in the cold room at 4°C on a shaker for 2 h; 1-propanol extracts were stored at −80°C. For analysis of intracellular cAMP, 1-propanol samples were taken to dryness, reconstituted in 100 μl pure water, and then centrifuged at 8,000 rpm for 10 min. Samples (90 μl) were mixed with 10 μl of internal standard solution, and this was subjected to direct analysis. cAMP measurements were performed by HPLC-tandem mass spectrometry using a triple quadrupole mass spectrometery (TSQ Quantum-Ultra, Thermo Fisher Scientific, Waltham, MA) as previously described in detail (37, 38). For each sample, total CCD cAMP content was calculated as the sum of the cAMP content measured in the extracts of single tubules plus that detected in the supernatant normalized to tubule length (in mm).
Immunoblot analysis.
Western blot analysis was performed as previously described (42). Renal cortexes isolated from rats fed the NK diet or one of the two HK diets (5% and 10% KCl, Harland) for 4–7 days were homogenized in buffer containing 50 mM HEPES, 150 mM NaCl, 1% Triton X-100 and 5 mM EDTA (pH 7.4) supplemented with a protease inhibitor cocktail (Roche Applied Science). Aliquots of the total protein lysate (∼50 μg) were resolved by 5–15% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% nonfat dried milk in PBS-0.05% Tween 20 for 1 h at room temperature and washed three times for 5 min each with PBS-0.05% Tween 20. Blots were then probed with an AT2R polyclonal primary antibody (1:500, Alomone Labs) at 4°C overnight, washed, and then incubated with a horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:10,000, Sigma, St. Louis, MO) for 1 h at room temperature. Bound antibody was visualized using the West Pico enhanced chemiluminescence kit (Pierce, Rockford, IL). Membranes were then stripped, blocked, probed with an anti-tubulin antibody (1:1,000, Sigma), and visualized using the same methods as described above for the primary antibody. The intensity of the identified bands was quantified with ImageJ (National Institutes of Health). Relative expression of the AT2R in HK-fed rats was compared with that detected in NK-fed rats and presented as the fold increase.
Chemicals.
N-nitro-l-arginine methyl ester (l-NAME), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (carboxy-PTIO), 8-bromo-cGMP (8-Br-cGMP), cilostamide, KT-5823, and myristoylated PKA inhibitor 14–22 amide (PKI) were obtained from Calbiochem (La Jolla, CA). IBMX was purchased from BioMol (Plymouth Meeting, PA). ANG II, PD-123319, CGP-42112, 8-pCPT-2′-O-Me-cAMP (8-pCPT), and other chemicals, including vanadyl ribonucleoside complexes, were purchased from Sigma. Losartan and the affinity-purified AT2R antibody were generous gifts from Dr. Charles T. Stier (New York Medical College) and Dr. Robert M. Carey (University of Virginia School of Medicine) (30, 56), respectively. AT2R antibody was purchased from Santa Cruz Biotechnology and Alomone Labs. Alexa 488-conjugated goat anti-rabbit IgG secondary antibody was purchased from Invitrogen, and rhodamine-conjugated DBA was purchased from Vector Laboratories (Burlingame, CA). The antifade reagent (Prolong Gold) was obtained from Molecular Probes (Eugene, OR). For those reagents prepared as stocks in DMSO, the concentration of this vehicle in the final solutions to which CCDs were exposed was always <0.1%.
Data analysis and statistics.
Data are expressed as means ± SE; n is the number of animals studied for patch-clamp, Western blot, real-time PCR, or cAMP analyses. Patch-clamp data were analyzed using pCLAMP software system 6.04 (Axon Instruments). Channel activity was defined as NPo, the product of open probability (Po) and channel number (N), which was calculated from data samples of 60-s duration in the steady state as follows: NPo = Σ(t1 + 2t2 +…iti) (1), where ti is the fractional open time spent at each of the observed current levels. Statistical analysis was performed using SigmaPlot (SPSS). Paired or unpaired Student's t-tests were performed to determine statistical significance as appropriate. Comparisons of mRNA abundance among the three dietary groups were performed by one-way ANOVA followed by a Bonferroni posttest. cAMP data were analyzed using the Aspin-Welch unequal-variance test. Significance was asserted at P values of <0.05.
Real-time PCR data were analyzed using SDS 2.1 software for the calculation of threshold cycle (CT) values of each transcript. Using the 2−ΔΔCT method (21), data are represented as fold changes in mRNA expression, normalized to 18S, relative to the control. The SD of the difference (s) was calculated from the SDs (S) of the AT1R, AT2R, and 18S values using the following formula: s = √S12 + S22 (21).
RESULTS
As we (54) and others (32, 33) have previously reported, dietary K+ loading (4–7 days) leads to an increase in ROMK channel activity, defined as NPo, in principal cells of the rat CCD (Fig. 1, B and C; ANG II concentration: 0 nM). Because ROMK channels are clustered in the cell membrane (8) and patches with no apparent ROMK channel activity in NK-fed rats were not included in the analysis of the effect of ANG II on channel activity, the magnitude of the increase in NPo in HK rats (as shown in Fig. 1B; ANG II concentration = 0 nM) was not as great as that reported by others (33). Our patch-clamp recordings showed that the addition of ANG II to the bathing solution further increased NPo in cell-attached patches in HK-fed rats without a clear dose dependence (Fig. 1, A-C); specifically, ANG II in concentrations of 0.1 nM (n = 8, P < 0.05), 1 nM (n = 6, P < 0.05), 10 nM (n = 6, P < 0.05), and 100 nM (n = 10, P < 0.01) increased NPo (Fig. 1, B and C). However, ANG II was without a consistent effect in CCDs isolated from rats fed a NK diet; only 3 of 19 patches responded to 1 or 10 nM ANG II with an increase in ROMK channel activity (Fig. 1B).
The AT1R acts to inhibit ROMK channel activity in dietary K+-restricted rats (60). Given that AT1Rs and AT2Rs generally mediate antagonistic effects (6), we speculated that the ANG II-induced stimulation of ROMK channels in HK-fed rats was mediated by AT2Rs. To test this, CCDs isolated from HK-fed rats were pretreated with the AT2R antagonist PD-123319 (10 μM) before exposure to ANG II. PD-123319 completely abolished the stimulatory effect of ANG II (100 nM) on the channel [n = 7, P = not significant (NS); Fig. 2]. In contrast, in CCDs isolated from HK-fed rats and pretreated with losartan (20 μM), an AT1R antagonist, ANG II (100 nM) elicited a 37 ± 14% (n = 9, P < 0.05) increase in ROMK channel activity, a degree of stimulation identical to that observed in CCDs not treated with any receptor antagonist (Fig. 2). The critical role of the AT2R in mediating the ANG II-induced stimulation of ROMK channels was confirmed by exposing CCDs from HK-fed rats to the AT2R agonist CGP-42112. Patch-clamp recordings revealed that CGP-42112 (10 nM) increased channel activity by 52 ± 10% in these tubules (n = 5, P < 0.05; Fig. 2).
Fig. 2.
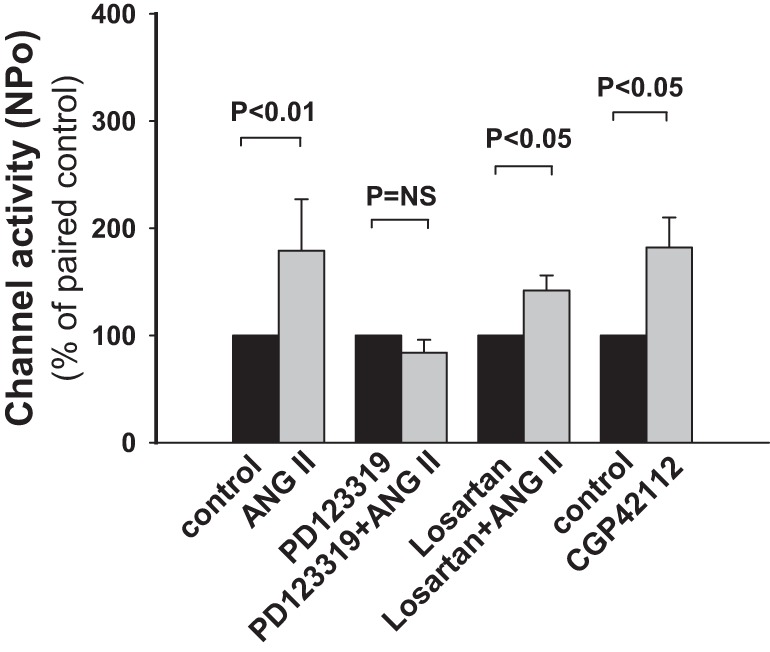
Role of ANG II type 1 and 2 receptors (AT1Rs and AT2Rs, respectively) in the ANG II-induced increase in ROMK channel activity in CCDs isolated from HK-fed rats. An antagonist of the AT2R (PD123319) but not AT1R (losartan) inhibited the ANG II-induced stimulation of ROMK channel activity. The AT2R agonist CGP-42112 stimulated ROMK channel activity to a similar extent to that induced by ANG II.
The AT2R is expressed in the adult kidney, including the CCD (26). Immunoblot analysis confirmed the presence of AT2R protein in the renal cortex and revealed an increase in steady-state expression in response to dietary K+ loading relative to that measured in NK-fed rats (Fig. 3). AT2R expression increased approximately threefold (n = 3, P < 0.01) in animals fed the 5% HK diet and approximately sixfold (n = 3, P < 0.001) in rats fed the 10% HK diet.
Fig. 3.
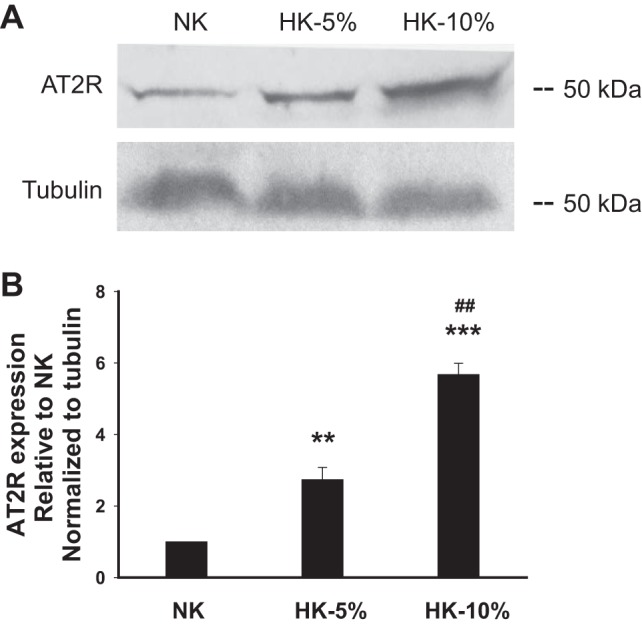
Dietary K+ induces expression of AT2R in the rat renal cortex. A: representative Western blots of lysates of the kidney cortex harvested from rats fed NK, 5% HK, or 10% HK diets. B: densitometric analyses of Western blots (n = 3 in each group) demonstrating an increase in AT2R expression with increasing dietary K+ intake. Data are shown as means ± SE. **P < 0.01 and ***P < 0.001 vs. expression in NK-fed group; ##P < 0.01 vs. the 5% HK-fed group via an unpaired t-test.
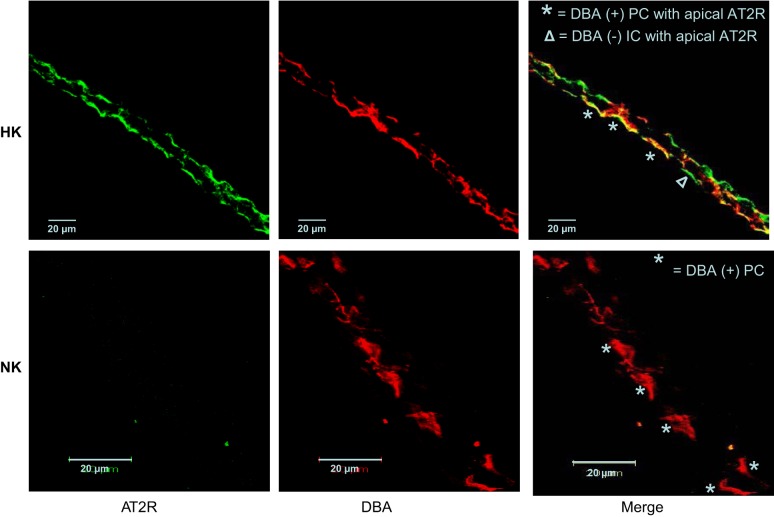
To confirm AT2R expression in the CCD in HK-fed rats and determine its localization, we performed immunofluorescence labeling of isolated CCDs using antibodies directed against the AT2R, visualized with an Alexa 488-conjugated secondary antibody. Antibody binding colocalized with apical DBA, a principal cell-specific marker (Fig. 4), but was also present in the apical membrane of DBA-negative cells. We observed abundant apical expression of immunodetectable AT2R in tubules isolated from HK-fed rats (Fig. 4). However, only 1 of 20 CCDs from NK-fed rats labeled with the anti-AT2R antibody, confirming a report by others (23) showing that expression of the AT2R is low in adult rats fed a NK diet. As the focus of this study was on the AT2R and steady-state mRNA expression for the AT1R was not affected by the HK diet (see below and Fig. 5), and as we have already published that the AT1R is critical for the renal response to a low-K+ diet (60), we elected not to perform an immunocharacterization of the AT1R in the CCD.
Fig. 4.
Immunolocalization of AT2Rs in CCDs from HK or NK-fed rats. Tubules were colabeled with an anti-AT2R antibody and visualized with an Alexa 488-conjugated secondary antibody (green) and rhodamine-conjugated Dolichos biflorus agglutinin (DBA; red), a marker of apical membrane of principal cells, in the CCD. Abundant apical membrane expression of AT2R was present in the CCD isolated from HK, but not NK-fed rats.
Fig. 5.

Relative expression of mRNA encoding the AT1R and AT2R in CCDs from rats fed NK or HK diets. Administration of the HK diet for 48 h, but not 12 h, upregulated AT2R mRNA in single CCDs compared with that detected in NK-fed rats. AT1R message was not affected by the HK diet. *P < 0.05 vs. the NK-fed group.
To determine whether dietary K+ loading and the consequent increase of ROMK channel activity are associated with an upregulation of the message encoding the AT2R, the relative abundance of receptor-specific mRNA was examined by real-time PCR analysis of single CCDs isolated from rats 12 or 48 h after initiation of HK or NK diets. These time intervals were selected based on 1) a report (33) showing that an increase in the density of conducting ROMK channels was detected as early as 6 h after a K+-rich meal and that the density of channels continued to increase for 48 h while on the same diet and 2) a report (29) showing that once adaptation to a dietary manipulation has occurred, changes in the steady-state expression of genes regulated in the process may be missed if they occur early in adaptation. AT2R mRNA expression in CCDs isolated from rats fed a HK diet for 48 h (n = 4) but not 12 h was significantly greater than that detected in NK-fed rats (n = 4; Fig. 5). AT1R message abundance was similar in HK and NK-fed rats at both time points studied (Fig. 5). These data suggest that the increase in ROMK channel activity after only 6 h on a HK diet (8) may be mediated by existing AT2Rs and/or an alternate mechanism, whereas that observed after 48 h on a HK diet may be due, at least in part, to a dietary K+-dependent increase in the steady-state abundance of AT2R (Fig. 5).
One signaling pathway known to be coupled to the AT2R is the NO/cGMP pathway (6). The CCD expresses three NO synthase (NOS) isoforms, including endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS) (1, 44, 55). To test whether the ANG II-induced stimulation of ROMK channel activity in the CCD was mediated by activation of the NO pathway, we first examined the effect of ANG II on ROMK channel activity in CCDs isolated from HK-fed rats pretreated with l-NAME (200 nM), an inhibitor of NOS. Pretreatment of CCDs with this inhibitor prevented the ANG II-induced stimulation of channel activity (n = 9, P = NS; Fig. 6). It should be noted, however, that in 3 of 13 total patches on l-NAME-treated CCDs, ANG II still increased channel activity. We consider that the latter responses may reflect incomplete inhibition of NOS by l-NAME at the low concentration used. Carboxy-PTIO is a free NO scavenger. Pretreatment of CCDs with carboxy-PTIO (300 nM) alone or with l-NAME completely abolished the stimulatory effect of ANG II on the channel (n = 4, P = NS; Fig. 6), consistent with a role for NO in the ANG II-mediated response. Neither l-NAME nor carboxy-PTIO had any significant effect on basal channel activity (Fig. 6).
Fig. 6.
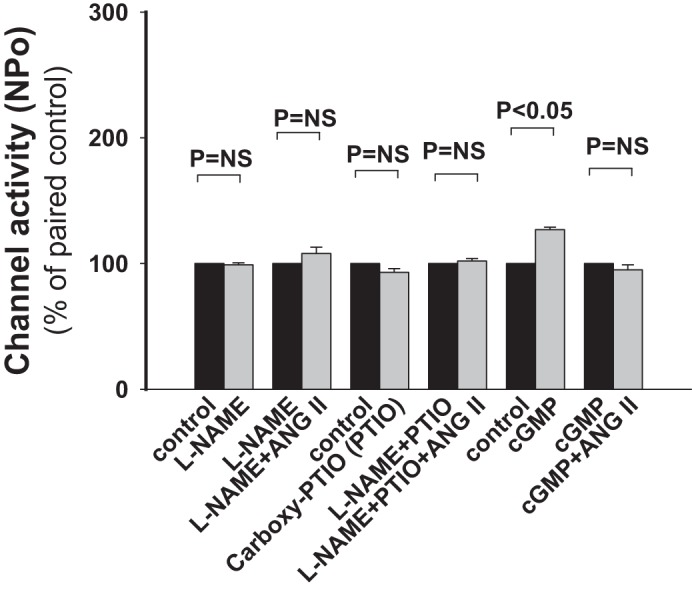
The stimulatory effect of ANG II on ROMK channel activity in CCDs of HK-fed rats is mediated through a nitric oxide (NO)/cGMP pathway. In CCDs from HK-fed rats, N-nitro-l-arginine methyl ester (l-NAME; 200 nM) and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (carboxy-PTIO; 300 nM), an inhibitor of NO synthase and a scavenger of free NO, respectively, abolished the stimulatory effect of ANG II on ROMK channel activity. Application of cGMP (200 nM) increased NPo. However, cGMP prevented a further ANG II-induced increase in NPo. These data suggest that a NO/cGMP pathway is involved in the ANG II response. NS, not significant.
NO enhances cGMP synthesis via the activation of soluble guanylyl cyclases (35). In contrast to the inhibitory effect of cGMP on ROMK channels in NK-fed animals (22), the addition of cell-permeable 8-Br-cGMP to the solution bathing CCDs from HK-fed rats led to an increase in channel activity (n = 4, P < 0.05) and prevented a further increase in channel activity by ANG II (Fig. 6). cGMP mediates most of its intracellular effects through PKG. To explore whether PKG participates in the response of ROMK channels to ANG II, we examined the effect of KT-5823 (1 μM), an inhibitor of PKG, on ROMK channel activity in CCDs from HK-adapted rats. Patch-clamp recordings confirmed that KT-5823 increased ROMK channel activity (n = 5, P < 0.05; Fig. 7), as has been previously described (22); PKG stimulates protein phosphatase 2A (66), which, in turn, participates in the regulation of ROMK channels (17). Moreover, ANG II still stimulated channel activity (n = 7, P < 0.05; Fig. 7) in another group of KT-5823-pretreated CCDs to a degree similar to that detected in untreated CCDs. These data suggest that the effect of ANG II on the ROMK channel is not mediated via cGMP/PKG.
Fig. 7.
The stimulatory effect of ANG II on ROMK channel activity in HK-fed rats is not mediated by cGMP-associated downstream PKG. A: representative traces of the stimulatory effect of KT-5823 (1 μM), a PKG inhibitor, and ANG II (100 nM) on apical ROMK channels in a CCD from a HK-fed rat. See Fig. 1 for details describing the tracings. B: summary of the effect of KT-5823 on ROMK channel activity. ANG II increased ROMK channel activity in KT-5823-pretreated CCDs.
A recent study (64) has suggested that there is cross-talk between cGMP and cAMP signaling pathways. Intracellular cyclic nucleotide concentrations and effects are determined, in large part, by the activity of PDEs. The >60 distinct isoforms comprising the large PDE superfamily differ in tissue distribution, sensitivity to specific inhibitors, and mechanisms of regulation (7). Whereas some isoforms preferentially hydrolyze cAMP or cGMP, others hydrolyze both cyclic nucleotides and can thus regulate the intracellular ratio of cAMP to cGMP. cAMP stimulates ROMK channel activity in a PKA-dependent manner (22, 50).
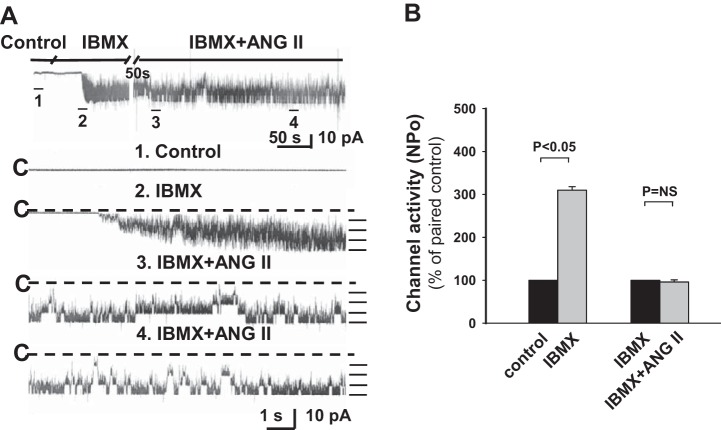
We next sought to test whether the ANG II-induced stimulation of ROMK channel activity was due to an effect of the hormone on cGMP-dependent PDE. To test this, CCDs from HK-fed rats were pretreated with IBMX, a broad-spectrum PDE inhibitor, and the effect of ANG II or cGMP on channel activity was examined. We found that IBMX (100 μM) activated ROMK channels (n = 6, P < 0.05; Fig. 8) and prevented further channel activation by ANG II (n = 6, P = NS; Fig. 8) or cGMP (data not shown). Of note was that pretreatment of CCDs isolated from HK-fed rats with IBMX (100 μM) for 20 min led to a significant increase in tubular cAMP content to 1.43 ± 0.35 pg/mm tubule length (n = 14) compared with that measured in vehicle-treated controls (0.61 ± 0.13 pg/mm tubule length, n = 12, P < 0.05; Fig. 9). These data suggest that ANG II-induced changes in cAMP or cGMP content mediate the hormone-induced stimulation of channel activity.
Fig. 8.
The stimulatory effect of ANG II on ROMK channel activity in HK-fed rats is mediated by phosphodiesterases (PDEs). A: representative traces of the stimulatory effect of IBMX (100 μM), a broad-spectrum inhibitor of PDEs, and ANG II (100 nM) on apical ROMK channel activity in a CCD from a HK-fed rat. See Fig. 1 for details describing the tracings. The experiment was performed in a cell-attached patch at a holding potential of 0 mV. B: IBMX increased NPo but prevented further stimulation of NPo by ANG II.
Fig. 9.
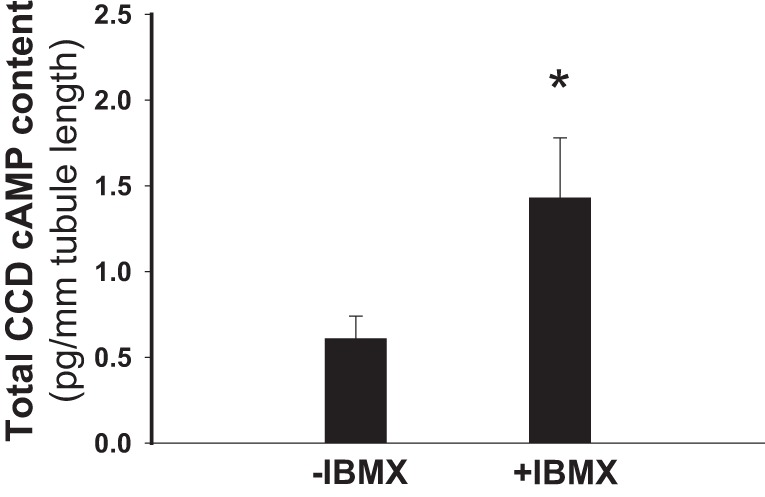
Total cAMP content in single CCDs of HK-fed rats is increased after IBMX treatment. The cAMP content of single CCDs exposed to IBMX (+IBMX; 100 μM), a broad-spectrum inhibitor of PDEs, was greater than that detected in vehicle-treated CCDs (−IBMX). *P < 0.05.
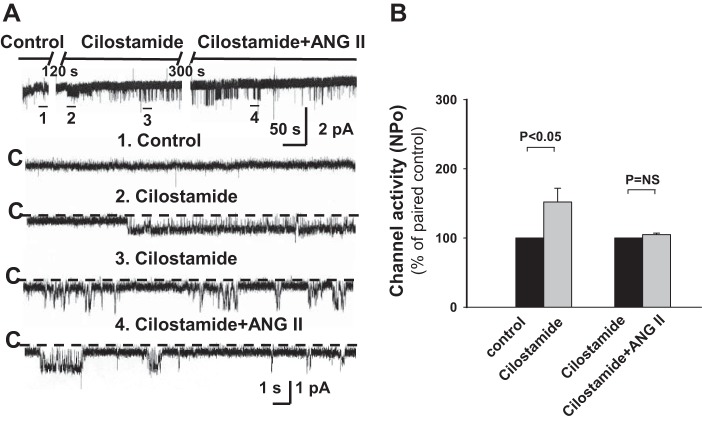
PDE3 is expressed in the mammalian CCD (36) and has a high affinity for cGMP; cAMP and cGMP are competitive substrates for PDE3 (7). Biological effects of cGMP have been proposed to be mediated by inhibition of PDE3, leading to a reduction in the degradation of cAMP and subsequent activation of PKA (24). Indeed, addition of the PDE3 inhibitor cilostamide (10 μM) to the bathing solution led to a significant increase in ROMK channel activity (n = 5, P < 0.05; Fig. 10), consistent with an accumulation of cAMP. ROMK channel activity in cilostamide-treated CCDs was not further stimulated by ANG II (n = 8, P = NS; Fig. 10) or cGMP (data not shown). These observations provide support for the notion that PDE3 participates in the ANG II-mediated stimulation of ROMK channel activity in HK-fed rats and suggest that the effect of ANG II on ROMK channels is mediated via cAMP.
Fig. 10.
The stimulatory effect of ANG II on ROMK channel activity in HK-fed rats is mediated by cGMP-related PDEs. A: representative traces of the stimulatory effect of cilostamide (10 μM), a specific inhibitor of PDE3, and ANG II (100 nM) on apical ROMK channel activity in a CCD from a HK-fed rat. See Fig. 1 for details describing the tracings. The experiment was performed in a cell-attached patch at a holding potential of 0 mV. B: cilostamide increased channel NPo but prevented further stimulation of NPo by ANG II.
cAMP is an important intracellular second messenger that activates PKA and exchange protein activated by cAMP (Epac)1/Epac2, two intracellular cAMP receptors (3). Both Epac1 and Epac2 have been detected in CCDs, and Epac2 is expressed in principal cells of CCDs (18). To confirm a role of PKA in the ANG II-induced response, the effect of ANG II on CCDs pretreated with PKI, a reagent that inhibits PKA only in the presence of cAMP (5), was examined. Cell-permeable myristoylated PKI (10 μM) inhibited ROMK channel activity (n = 6, P < 0.05), as has been previously described for this channel, which is activated by PKA (22), and prevented the stimulatory effect of ANG II on the channel (n = 12, P = NS; Fig. 11), implicating the cAMP-PKA pathway in the stimulatory effect of ANG II on ROMK channels. Meanwhile, a role for Epac1/2 participation in the ANG II-induced activation of ROMK channel activity was not supported by our finding that 8-pCPT-cAMP (10 μM), a specific Epac1/2 activator (3), did not affect either basal channel activity (n = 6, P = NS) or the effect of ANG II on ROMK channels (n = 10, P < 0.05; Fig. 12).
Fig. 11.

The stimulatory effect of ANG II on ROMK channel activity in HK-fed rats is mediated by cAMP-dependent PKA. Myristoylated PKI (10 μM), a cell-permeable PKA inhibitor peptide, inhibited channel activity and blocked the stimulatory effect of ANG II on the channel.
Fig. 12.
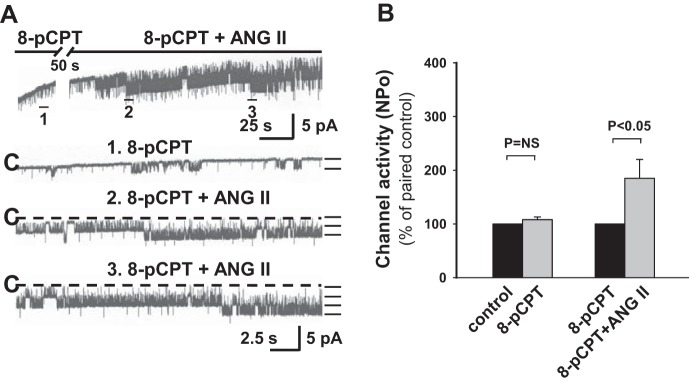
The stimulatory effect of ANG II on ROMK channel activity in HK-fed rats is not mediated by cAMP-dependent exchange protein activated by cAMP (Epac)1/2. A: representative traces of the effect of 8-pCPT-cAMP (10 μM) on apical ROMK channel activity in a CCD from a HK-fed rat. See Fig. 1 for details describing the tracings. The experiment was performed in a cell-attached patch at a holding potential of 0 mV. B: activation of Epac1/2 by 8-pCPT-cAMP did not significantly increase or prevent the stimulatory effect of ANG II on ROMK channel activity. Data are normalized to paired controls and are presented as means ± SE. Please see results for n values.
DISCUSSION
ANG II plays a major role in regulating total body salt and fluid balance. Although almost all biological actions of ANG II have been assigned to the AT1R, emerging evidence suggests that the AT2R plays a physiological role in the regulation of blood pressure and urinary Na+ excretion by opposing the vasoconstrictor and antinatriuretic actions of ANG II (4). We now provide experimental evidence that ANG II occupation of the AT2R in the rat CCD functions in a counterregulatory mode to the AT1R, stimulating ROMK channel activity in rats adapted to a HK diet. The results of this present study are, to our knowledge, the first to attribute a physiological role of the AT2R in the regulation of K+ homeostasis, specifically by regulating renal K+ secretion.
AT2R mRNA and protein in the kidney is abundant in fetal and early postnatal life (26). Within the adult rat kidney, immunodetectable AT2Rs have been identified in the proximal and collecting tubules (26). Here, we extend these previously published studies to show that dietary K+ loading induces AT2R expression in the cortex of adult rats (Fig. 3). Note that van der Lubbe et al. (47) has reported that rats fed a HK diet identical to that used in the present study for 8 days showed no difference in kidney ANG II levels compared with controls; however, it is important to recognize that whole kidney ANG II may differ from renal tubular ANG II (45).
Little is known about the function of the AT2R. The present study demonstrates that the steady-state abundance of AT2R mRNA increases in response to dietary K+ loading (Fig. 5), a finding consistent with our detection of abundant immunodetectable AT2Rs in the apical membrane of CCDs isolated from HK-fed animals (Fig. 4). AT2Rs in the latter nephron segments are functional, specifically in HK-fed rats, as demonstrated by our observations that 1) ANG II and CGP-42112, an AT2R agonist, stimulate the ROMK channel activity (Figs. 1, 2) the ANG II-induced increase in ROMK channel activity is blocked by pretreatment with an AT2R but not AT1R antagonist (Fig. 2). We propose that our ability to detect functional AT2Rs in HK-fed rats is due, at least in part, to an increase in AT2R abundance.
Of note is that the ANG II effect in NK-fed rats is minimal (Fig. 1B). Our study suggests that ANG II-mediated regulation of ROMK channel activity and thus presumably renal K+ secretion becomes apparent only in response to dietary K+ manipulations. Although much progress has been made during the past several decades in elucidating the role of the RAS in physiology (13), the specific effects of the local intrarenal versus circulating RAS and the contributions of AT1Rs and AT2Rs in the regulation of renal epithelial cell function remain to be more fully explored. Also uncertain is how the activity and responses of AT1Rs and AT2Rs, both present in the same nephron segment (CCD) and mediating opposite effects under HK and low-K+ conditions, are coordinated.
Signaling via the AT2R is, with few exceptions, distinct from that coupled to the AT1R (6). Like the AT1R, the AT2R is a G protein-coupled receptor, but it has not been associated with any classical G protein-related signaling pathway (6). An unconventional pathway coupled to the AT2R is activation of NO release with a subsequent increase in intracellular cGMP levels (6); this pathway may be partially shared by the AT1R, if expressed by the cell (46). An AT2R-coupled increase in NO generation has been observed in many tissue/cell types, including vascular tissue (61) and neuronal cells (65). In the kidney, in vivo infusion of ANG II into conscious rats resulted in a twofold increase in renal cortical interstitial fluid cGMP content; this effect was significantly attenuated by coadministration of either the AT2R antagonist PD-123319 or the NOS inhibitor l-NAME, implicating the AT2R and NO, respectively, in this response (39). Consistent with the notion that the AT2R in the kidney is coupled to a NO/cGMP pathway is the finding that AT2R-null mice exhibit markedly reduced basal and ANG II-induced cGMP and bradykinin levels in renal interstitial fluid (40). The fact that ANG II-induced NO production in these mice was partially but not completely blocked by the nNOS-specific inhibitor 7-nitroindazole implicated nNOS in NO/cGMP production. As indicated above, nNOS is expressed in the CCD principal cell, the same cell that expresses ROMK channels (55).
One aim of the present study was to test whether AT2R signal transduction in the CCD involves activation of NO/cGMP, a pathway that regulates the basolateral K+ channel in this segment (52). NO enhances cGMP synthesis via activation of soluble guanylyl cyclases (35). cGMP inhibits PDE3 activity, which, in turn, inhibits cAMP degradation (7). The net effect of this series of events, in which PDE3 appears to act as a “cross-linker” between the cGMP and cAMP signaling pathways, is an increase in cAMP accumulation and subsequent activation of cAMP-dependent PKA. Our observations that pretreatment of CCDs with l-NAME and carboxy-PTIO completely abolished the stimulatory effect of ANG II on channel activity (Fig. 6) demonstrate that NO is involved in the ANG II-induced stimulation of ROMK channels in HK-fed animals. Furthermore, our finding that 8-Br-cGMP increased ROMK channel activity and prevented a further stimulatory effect of ANG II (Fig. 6) in HK-fed rats supports the conclusion that NO/cGMP is involved in this ANG II effect. It is well established that cAMP (22) and cAMP-dependent PKA (53) stimulate ROMK channel activity by phosphorylation of the channel protein (25), thereby increasing the sensitivity of the channel to phosphatidylinositol 4,5-bisphosphate, an essential “gating molecule” of the channel (20). ROMK mutants in which potential PKA phosphorylation sites are abolished exhibit low channel activity (20, 63). A role for cAMP-PKA in the ANG II-induced response was confirmed by our detection of inhibition of the expected ANG II-induced increase in ROMK channel activity in CCDs pretreated with myristoylated PKI, a specific cell-permeant PKA inhibitor that is effective only in the presence of cAMP (5). We thus propose that ANG II stimulates NO release, which, in turn, increases cellular cGMP, which inhibits PDE3, leading to an increase in cellular cAMP. Notably, in this pathway, PDE3 cross-links cGMP to the cAMP signaling pathway to activate ROMK channels via PKA (Figs. 8–11).
The finding of a greater increase in channel activity in CCDs treated with IBMX (Fig. 8) compared with those treated with ANG II or PDE3 inhibitor (Figs. 1 and 10) implies that PDEs other than PDE3 contribute to the regulation of ROMK channel activity. PDE4 is the predominant PDE in the renal vasculature (16), and preclinical evidence suggests that inhibitors of PDE4 may be useful in treating/preventing sepsis-induced acute kidney injury (14) The results of the present study raise the possibility that PDE (and perhaps PDE4) inhibition, via stimulation of ROMK channel activity, could attenuate acute kidney injury-associated hyperkalemia.
Another signaling pathway reported to be coupled to the AT2R is that associated with protein phosphatases (6), including tyrosine phosphatase-related MAPK phosphatase (15) and SH2 domain-containing protein-tyrosine phosphatase 1 (2). The balance of activity of protein tyrosine kinase and protein tyrosine phosphatase within the principal cell regulates ROMK channel density via phosphorylation or dephosphorylation of a key tyrosine residue in the ROMK channel (27). Of relevance to the present study are the observations that inhibition of protein tyrosine phosphatase reduces ROMK channel activity in the apical membrane of the CCD in HK-fed rats (57) and that HK feeding reduces tyrosine phosphorylation and attenuates endocytotic removal of ROMK channels from the apical membrane, which directly increases channel density (19). The effect of dietary K+ on renal expression of tyrosine phosphatase-related MAPK phosphatase and SH2 domain-containing protein-tyrosine phosphatase 1 has not been explored. Nor is it known whether the ANG II-induced increase in ROMK channel activity in HK-fed rats is due to alterations in AT1R-activated tyrosine phosphorylation and thus trafficking of channel proteins to the apical membrane versus AT2R-mediated activation of protein tyrosine phosphatase activity, as has been suggested by others (2, 6, 15). Future efforts will be directed at exploring these possibilities.
While not a focus of this study, the AT1R, which is also expressed in the aldosterone-sensitive distal nephron, may indirectly participate in CCD K+ secretion in NK but not HK-fed rats. Previous studies (23, 34, 43) have demonstrated that ANG II stimulates the epithelial Na+ channel (ENaC) in the CCD via the AT1R; an increase in ENaC activity would be expected to enhance K+ secretion. However, in HK-fed rats, an increased fraction of K+ secretion is independent of ENaC activity (10). A HK intake also increases the production of epoxyeicosatrienoic acid, which inhibits ENaC and is thus expected to reduce the driving force for K+ secretion through ROMK channels (41, 59). However, we speculate that epoxyeicosatrienoic acid inhibits ENaC predominantly in the late distal convoluted tubule, with a lesser effect in the CCD during HK feeding, based on two unpublished observations: 1) HK intake significantly increases epoxygenase expression in the distal convoluted tubule and 2) ROMK channel activity in the late distal convoluted tubule is lower and less responsive to HK stimulation than in the CCD. The net effect of these adaptive responses on transepithelial K+ secretion in the aldosterone-sensitive distal nephron of HK-fed animals must await measurements of transepithelial transport in perfused tubules.
In summary, our data build on our previous work (60) to provide further evidence that ANG II plays an important role in the regulation of ROMK channel activity in the distal nephron in response to alterations in dietary K+ intake. This mechanism of regulation is distinct from the indirect effect of dietary-induced changes in circulating levels of aldosterone on the channel. Specifically, the results of the present study reveal that AT1Rs and AT2Rs play opposing roles in channel regulation (Fig. 13). Whereas we have previously reported that AT1Rs activation inhibits ROMK channels in rats fed a low-K+ diet (60) (Fig. 13, right), we now show that the AT2R in HK-fed animals stimulates channel activity via a NO/cGMP/PDE3/cAMP/PKA pathway. We consider it likely that the coordinated regulation and functional balance of AT1Rs and AT2Rs in the CCD, regulated by dietary K+ intake and shown in Fig. 13, determines, at least in part, total body K+ balance.
Fig. 13.
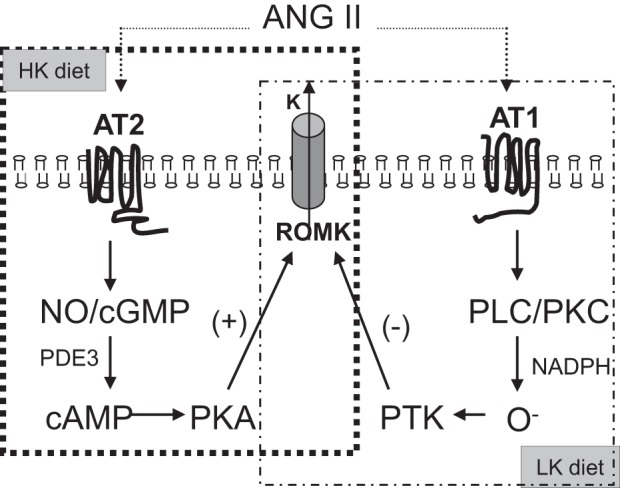
Proposed signaling pathway by which the stimulatory effect of ANG II on ROMK channel activity in HK-fed rats is mediated via AT2Rs. AT2Rs and AT1Rs cooperate in the renal regulation of K+ excretion. The pathway by which ANG II inhibits ROMK channels in low-K+ (LK)-fed rats was previously reported (58). PTK, protein tyrosine kinase; PLC, phospholipase C.
GRANTS
This work was supported by American Heart Association Scientist Development Grant 0530092N (to Y. Wei). W. H. Wang was supported by NIH Grant DK-47402, and L. M. Satlin was supported by NIH Grants DK-38470 and DK-51391. L. M. Satlin and E. K. Jackson were further supported by NIH Grant P30-DK-079307 (Pittsburgh Center for Kidney Research). R. Rohatgi was supported by Department of Veterans Affairs Merit Review 1I01BX000388.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.W., W.-H.W., and L.M.S. conception and design of research; Y.W., Y.L., B.Z., J.R., W.L., P.C., R.R., and G.E. performed experiments; Y.W., G.E., E.K.J., and L.M.S. analyzed data; Y.W. and L.M.S. interpreted results of experiments; Y.W. prepared figures; Y.W. drafted manuscript; Y.W. and L.M.S. edited and revised manuscript; Y.W., Y.L., B.Z., J.R., W.L., P.C., R.R., G.E., E.K.J., W.-H.W., and L.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Tung-Tien Sun for the stimulating discussion and support in the Western blot experiment. The authors acknowledge the support of the MSSM Microscopy Shared Resource Facility, which was supported by National Institutes of Health (NIH) Shared Resources Grant 5-R24-CA-095823-04, National Science Foundation Major Research Instrumentation Grant DBI-9724504, and NIH Shared Instrumentation Grant 1-S10-RRO-9145-01.
REFERENCES
- 1.Ahn KY, Mohaupt MG, Madsen KM, Kone BC. In situ hybridization localization of mRNA encoding inducible nitric oxide synthase in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F748–F757, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bedecs K, Elbaz N, Sutren M, Masson M, Susini C, Strosberg AD, Nahmias C. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J 325: 449–454, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Carey RM, Jin XH, Siragy HM. Role of the angiotensin AT2 receptor in blood pressure regulation and therapeutic implications. Am J Hypertens 14: 98S-102S, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Dalton GD, Dewey WL. Protein kinase inhibitor peptide (PKI): a family of endogenous neuropeptides that modulate neuronal cAMP-dependent protein kinase function. Neuropeptides 40: 23–34, 2006 [DOI] [PubMed] [Google Scholar]
- 6.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 7.Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem 272: 6823–6826, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Frindt G, Zhou H, Sackin H, Palmer LG. Dissociation of K channel density and ROMK mRNA in rat cortical collecting tubule during K adaptation. Am J Physiol Renal Physiol 274: F525–F531, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med 264: 224–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holthoff JH, Wang Z, Patil NK, Gokden N, Mayeux PR. Rolipram improves renal perfusion and function during sepsis in the mouse. J Pharmacol Exp Ther 347: 357–364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem 272: 19022–19026, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Jackson EK, Mi Z, Carcillo JA, Gillespie DG, Dubey RK. Phosphodiesterases in the rat renal vasculature. J Cardiovasc Pharmacol 30: 798–801, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Kubokawa M, McNicholas CM, Higgins MA, Wang W, Giebisch G. Regulation of ATP-sensitive K+ channel by membrane-bound protein phosphatases in rat principal tubule cell. Am J Physiol Renal Fluid Electrolyte Physiol 269: F355–F362, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Konings IB, Zhao J, Price LS, de Heer E, Deen PM. Renal expression of exchange protein directly activated by cAMP (Epac) 1 and 2. Am J Physiol Renal Physiol 295: F525–F533, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA 96: 5820–5825, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lu M, MacGregor GG, Wang W, Giebisch G. Extracellular ATP inhibits the small-conductance K channel on the apical membrane of the cortical collecting duct from mouse kidney. J Gen Physiol 116: 299–310, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62: 1111–1122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys 322: 1–13, 1995 [DOI] [PubMed] [Google Scholar]
- 25.McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proc Natl Acad Sci USA 91: 8077–8081, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol 277: F437–F446, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Moral Z, Dong K, Wei Y, Sterling H, Deng H, Ali S, Gu R, Huang XY, Hebert SC, Giebisch G, Wang WH. Regulation of ROMK1 channels by protein-tyrosine kinase and -tyrosine phosphatase. J Biol Chem 276: 7156–7163, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Nowik M, Lecca MR, Velic A, Rehrauer H, Brandli AW, Wagner CA. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 30: 1238–1246, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pyriochou A, Papapetropoulos A. Soluble guanylyl cyclase: more secrets revealed. Cell Signal 17: 407–413, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Reinhardt RR, Chin E, Zhou J, Taira M, Murata T, Manganiello VC, Bondy CA. Distinctive anatomical patterns of gene expression for cGMP-inhibited cyclic nucleotide phosphodiesterases. J Clin Invest 95: 1528–1538, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J, Mi Z, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther 325: 920–926, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328: 855–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA 96: 6506–6510, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun P, Lin DH, Yue P, Jiang H, Gotlinger KH, Schwartzman ML, Falck JR, Goli M, Wang WH. High potassium intake enhances the inhibitory effect of 11,12-EET on ENaC. J Am Soc Nephrol 21: 1667–1677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct. J Am Soc Nephrol 20: 513–523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terada Y, Tomita K, Nonoguchi H, Marumo F. Polymerase chain reaction localization of constitutive nitric oxide synthase and soluble guanylate cyclase messenger RNAs in microdissected rat nephron segments. J Clin Invest 90: 659–665, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J Clin Invest 116: 1110–1116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toda N, Ayajiki K, Okamura T. Interaction of endothelial nitric oxide and angiotensin in the circulation. Pharmacol Rev 59: 54–87, 2007 [DOI] [PubMed] [Google Scholar]
- 47.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol 5: 89–100, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol 66: 547–569, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Wang W. Regulation of the ROMK channel: interaction of the ROMK with associate proteins. Am J Physiol Renal Physiol 277: F826–F831, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channels. Am J Physiol Renal Physiol 278: F165–F171, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Wang WH. The cGMP-dependent protein kinase stimulates the basolateral 18-pS K channel of the rat CCD. Am J Physiol Cell Physiol 278: C1212–C1217, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Wang WH, Giebisch G. Dual modulation of renal ATP-sensitive K+ channel by protein kinases A and C. Proc Natl Acad Sci USA 88: 9722–9725, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Lu M, Gao Y, Papapetropoulos A, Sessa WC, Wang W. Neuronal nitric oxide synthase is expressed in principal cell of collecting duct. Am J Physiol Renal Physiol 275: F395–F399, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Wang ZQ, Moore AF, Ozono R, Siragy HM, Carey RM. Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension 32: 78–83, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Wei Y, Bloom P, Gu R, Wang W. Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct. J Biol Chem 275: 20502–20507, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol 140: 809–824, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J Biol Chem 271: 9313–9319, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res 100: 1569–1578, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Biermann T, Luther C, Unger T, Culman J, Gohlke P. Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells. J Neurochem 85: 759–767, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Zhou XB, Ruth P, Schlossmann J, Hofmann F, Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J Biol Chem 271: 19760–19767, 1996 [DOI] [PubMed] [Google Scholar]