Abstract
The polarized nature of epithelial cells allows for different responses to luminal or serosal stimuli. In kidney tubules, ATP is produced luminally in response to changes in luminal flow. Luminal increases in ATP have been previously shown to inhibit the renal epithelial Na+ channel (ENaC). On the other hand, ATP is increased basolaterally in renal epithelia in response to aldosterone. We tested the hypothesis that basolateral ATP can stimulate ENaC function through activation of the P2X4 receptor/channel. Using single channel cell-attached patch-clamp techniques, we demonstrated the existence of a basolaterally expressed channel stimulated by the P2X4 agonist 2-methylthio-ATP (meSATP) in Xenopus A6 cells, a renal collecting duct principal cell line. This channel had a similar reversal potential and conductance to that of P2X4 channels. Cell surface biotinylation of the basolateral side of these cells confirmed the basolateral presence of the P2X4 receptor. Basolateral addition of meSATP enhanced the activity of ENaC in single channel patch-clamp experiments, an effect that was absent in cells transfected with a dominant negative P2X4 receptor construct, indicating that activation of P2X4 channels stimulates ENaC activity in these cells. The effect of meSATP on ENaC activity was reduced after chelation of basolateral Ca2+ with EGTA or inhibition of phosphatidylinositol 3-kinase with LY-294002. Overall, our results show that ENaC is stimulated by P2X4 receptor activation and that the stimulation is dependent on increases in intracellular Ca2+ and phosphatidylinositol 3-kinase activation.
Keywords: purinergic receptor, phosphatidylinositol 3-kinase, collecting duct, principal cell, intracellular calcium
a hallmark of all epithelial tissues is that they are polarized with quite different proteins and lipids in the apical versus basolateral membranes of the tissues. In the aldosterone-sensitive distal nephron, there is definite polarization of transporters and channels, the clearest example of which is seen during transcellular Na+ transport, where Na+ moves apically through the epithelial Na+ channel (ENaC) and is actively pumped basolaterally via Na+-K+-ATPase. In epithelia, ATP can be secreted into the luminal or serosal side of the tissue, and it makes sense that the cell would want to be able to generate polarized responses to the ligand depending on which side of the cell the ATP is presented. ATP acts primarily through P2 purinergic receptors to regulate ion transport in the kidney, lung, salivary and pancreatic glands, gastrointestinal tract, and inner ear (7, 9, 33). These receptors are further divided into P2Y receptors, G protein-coupled receptors (22, 31), and P2X, ligand-activated, nonselective cation channels (10, 23). Both P2X and P2Y receptors are expressed in the collecting duct and distal tubule of the nephron (3). P2Y receptors are present in the apical and basolateral membranes (46). Basolateral P2Y receptors influence Ca2+-activated Cl− channels (11) and the vasopressin V2 receptor (14). P2X4 and P2X6 receptors are reportedly expressed both apically and basolaterally in the rat distal tubule (46). Besides polarization of receptors, transporters are also polarized. The distal tubule and cortical collecting ducts of the kidney express aldosterone-regulated ENaC only in the apical membrane.
Apical ATP inhibits ENaC through hydrolysis of phosphatidylinositol 4,5-bisphosphate (43). This effect occurs through stimulation of P2Y2 receptors and is consistent with P2Y2 receptor knockout mice having salt-resistant hypertension (37). Stimulation of apical P2X receptors inhibits ENaC as well, an effect thought to result from depolarization and Ca2+ movement mediated by P2X channels (48). The regulation of ENaC by basolateral P2X receptors has only recently been examined. Early work identified ATP production after an increase in aldosterone (13). This ATP caused cell contraction and cytoskeletal reorganization in a Xenopus collecting duct cell line (A6 cells) (18). The ATP-mediated effects on the cytoskeleton increase amiloride-sensitive current (50). Through an extensive pharmacological characterization, Zhang et al. (50) found that the purinergic receptor responsible for the initiation of this current is P2X4 like, meaning that the channel was pharmacologically similar to the P2X4 channel but with a slightly different agonist preference.
P2X4 receptor stimulation increases the amiloride-sensitive conductance of A6 cells, but ENaC itself has not been identified as the direct target (since the concentrations of amiloride used in the studies were relatively high). Furthermore, the mechanism whereby P2X4 receptors enhance amiloride-sensitive conductance remains unclear. We sought to expand the work of Zhang et al. to determine the effect of basolateral P2X4 receptor stimulation on ENaC and to characterize the signaling pathways involved. We tested the hypothesis that basolateral P2X4 receptors stimulate ENaC in the collecting duct through increases in the intracellular Ca2+ concentration ([Ca2+]i) and stimulation of phosphotidylinositol 3-kinase (PI3K).
METHODS
Cells.
All experiments used the 2F3 clone of A6 Xenopus distal tubule cells. Cells were grown on permeable supports to confluence, at which time cells were polarized and tight junctions were fully developed. Cell media were supplemented with aldosterone (1.5 μM) to increase ENaC activity. Cells were used between passages 97 and 104.
Transfection with dominant negative P2X4 DNA.
A plasmid containing a green fluorescent protein (GFP)-tagged dominant negative plasmid was obtained as a kind gift from Dr. Ruth Murrell-Lagnado (University of Cambridge) (6). A6 cells were transfected using the Xfect system (Clontech).
Single channel patch clamp.
Single channel patch clamp was performed as previously described (19, 49). Cell-attached recordings of ENaC single channel current from A6 distal nephron cells were carried out using an Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA). A6 cells were thoroughly washed with standard saline. Glass micropipettes with a pipette resistance of 7–10 MΩ were filled with standard saline solution (96 mM NaCl, 0.8 mM CaCl, 0.8 mM MgCl, and 20 mM HEPES, pH 7.4) and lowered to touch the apical membrane of a single cell. Suction was gently applied so that the membrane remained intact and a >1-GΩ seal was formed. Some electrodes were filled at the tip with physiological saline solution and then backfilled with physiological saline solution containing 2-methylthio-ATP (meSATP) to allow an initial recording period without meSATP and then a period with me-S-ATP present after meSATP diffusion to the tip of the pipette. Standard saline solution was used for both the luminal and basolateral baths. Unless otherwise stated, single channel currents were obtained with no applied pipette potential, filtered at 1 kHz, and sampled every 50 μs with pCLAMP 10 software. Experiments were conducted at room temperature. The total number of functional channels in the patch was estimated by observing the number of peaks detected on the current-amplitude histogram during at least a 10-min recording period. The open probability (Po) of ENaC was estimated using pCLAMP 10. ENaC was identified by its characteristic channel kinetics and current-voltage relationship. Empty apical patches (those with no apparent activity) comprised 30–50% of all patches and were excluded from study.
Basolateral single channel patch clamp.
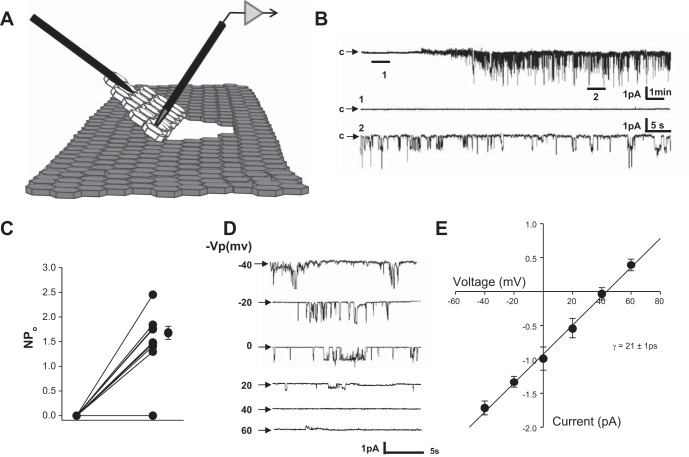
Cells were grown to confluency before a three-cornered cut was made in a small section of cells and the cell layer was flipped over to access the basolateral side (see Fig. 2A). Single channel measurements were performed as described above with pipettes backfilled with meSATP.
Fig. 2.
Effect of addition of 2-methylthio-ATP (meSATP) in the patch pipette. A: representative recording of current from a single patch (total time: ∼25 min) before (expanded section 1: ∼80 s) and after (expanded section 2: ∼80 s) the addition of meSATP. B: recording from an exposed basolateral membrane. Pipette tips were filled with saline solution and backfilled with saline solution plus meSATP. Therefore, initially, there was no meSATP present (expand section 1: ∼60 s), but after a diffusion period of 5–10 min, meSATP activated a channel (expanded section 2: ∼60 s) in the traces below the long-term record (∼17 min). C: individual channel open probability (NPo; where N is the number of channels and Po is opening probability). D: representative current-voltage relationship of the meSATP-inducible current shown in B. Vp, voltage potential. E: current-voltage curve of the currents shown in C showing a mean conductance (γ) of 21 ± 0.67 pS from three separate cells (r = 0.988).
Cell surface biotinylation.
Confluent cells were washed two times with PBS before the addition of 0.5 mg/ml biotin to the apical or basolateral surface. Biotin was quenched with 18.3 mg/ml l-lysine, and cells were lysed by scraping in RIPA buffer. The cell lysate was incubated on streptavidin beads. After incubation, the cytosolic fraction was removed and ran as protein on a SDS-PAGE gel. The biotinylated fraction was eluted from the beads with a solution containing Laemmli;s buffer and DTT and run on a SDS-PAGE gel.
Chemicals.
meSATP, LY-294002, and EGTA were purchased from Sigma-Aldrich. All reagents were dissolved in the physiological buffer described above.
Antibodies.
Antibodies to Na+-K+-ATPase and P2X4 receptor were obtained from Santa Cruz Biotechnology (H-300 and H-40, respectively). γ-ENaC antibody was generated in house to the same epitope as that used by Loffing et al. (28) and sold by StressMarq Biosciences (SPC405D, Victoria, BC, Canada). All antibodies were used at 1:1,000 dilution.
Statistics.
To compare ENaC Po, a paired t-test was performed. P values of <0.05 were considered significant.
RESULTS
P2X4 receptors are expressed in the basolateral membrane of A6 cells.
P2X4 receptors have been shown to be expressed basolaterally along the nephron (46). For our study, we chose to use a distal nephron principal cell culture model to allow for the addition of pharmacological agents selectively to either the apical or basolateral membrane and to be able to perform single channel patch clamp. To determine the localization of P2X4 receptors in Xenopus distal tubule A6 cells, we biotinylated the apical or basolateral surface of confluent monolayers of cells (Fig. 1). Using an anti-P2X4 receptor antibody, we detected biotinylated receptor only on the basolateral membrane with none detectable in the apical membrane. Higher-molecular-weight bands are typically present in P2X4 blots and are speculated to be P2X multimers that are difficult to dissociate (38). As a control, we showed that a known basolateral protein, Na+-K+-ATPase, was distributed just like the P2X4 receptor and that a known apical protein, γ-ENaC, could be detected after apical biotinylation.
Fig. 1.
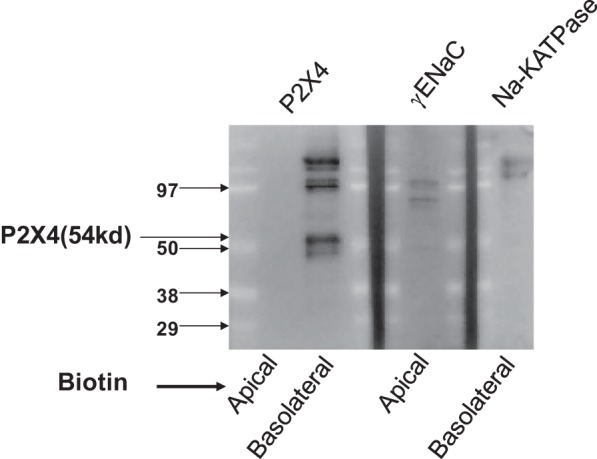
Localization of P2X4 receptors in A6 cells. Cell surface biotinylation of either the apical or basolateral side of A6 cells showed selective localization of P2X4 channels to the basolateral but not apical membrane (left). The blots on the right show that an apical protein [γ-epithelial Na+ channel (ENaC)] can be detected after apical biotinylation and that a basolateral protein (Na+-K+-ATPase) can be detected after basolateral biotinylation.
meSATP activates P2X4 channels on the basolateral surface of A6 cells.
P2X4 receptors are known to function as ATP-gated, nonselective cation channels. They have a reversal potential near 0 mV and a single channel conductance of 21 pS (39, 40, 45). In a physiological environment, however, they generally transport Na+ and Ca2+ down their electrochemical gradient into cells (32). To determine whether P2X4 channels exist in the basolateral membrane in A6 cells, we formed cell-attached patches on the basolateral surface of the cells (Fig. 2). Rather than applying the patch electrode apically as we typically do, we used a sharp microelectrode to make a three-cornered cut in the confluent cell layer and then folded over the cell layer to access the basolateral side. We filled the tip of the patch pipette with normal saline solution, and the rest of the pipette was then back filled with saline solution that contained 50 μM of the P2X4 agonist meSATP in the patch electrode. Thus, when we first formed the patch, there was no agonist, and after 5–10 min, the agonist diffused to the surface of the cell and activated P2X4 receptors (Fig. 2B). Sixty-nine percent (9 of 13) of the patches had no measurable current. If a patch had current before activation with meSATP, we did not continue. Of the nine patches that had no measurable current, eight patches (89%) responded strongly to meSATP (Fig. 2C). All responding patches had two or more channels, suggesting that meSATP-activated channels might be clustered. Since the channels were inside the patch pipette, they could not be responding to material from the apical surface of the cells. The current through the channels reversed at +40 mV, which is consistent with an apical membrane potential of approximately −40 mV and a nonselective channel with a reversal potential near 0 mV, as would be expected for P2X4 receptors (Fig. 2D). When we plotted current versus voltage, we found that the meSATP-activated channel had a conductance similar to that reported for P2X4 receptors in other tissues (Fig. 2E). We conclude that there is a P2X4-like channel in A6 cells that responds to meSATP.
Unlike the basolateral membrane, formation of seals with meSATP-containing pipettes on the apical surface produced no inducible currents. Application of meSATP outside the patch pipette inhibited ENaC activity within the patch pipette, as might be expected for activation of P2Y receptors (24, 25, 30, 34–36, 41).
Basolateral P2X4 receptor stimulation increases ENaC activity.
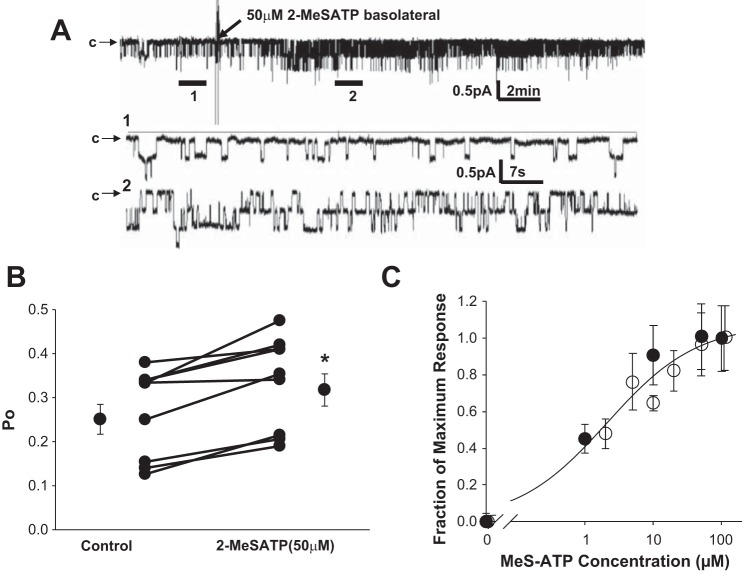
P2X4 receptor agonists can induce amiloride-sensitive conductance in A6 cells (50). To test whether this effect is through stimulation of ENaC, we tested the effects of basolateral meSATP on Po of apical ENaC in A6 cells using cell-attached patch clamp on the apical membrane (Fig. 3). Ten minutes after the basolateral addition of 50 μM meSATP, we saw a modest but significant increase in ENaC Po (Fig. 3B). To determine the dose response of ENaC to meSATP, we applied varying doses of meSATP to the basolateral side of cells undergoing either single channel patch clamp or transepithelial current measurements. Figure 3C shows both patch-clamp and transepithelial current responses to meSATP normalized to the maximal effect (maximum current or Po seen after 30 min of treatment with meSATP). Data were fit to the following Hill equation: relative current = Imax/[(Kslope)]/[meSATP]slope) + 1], where maximal current (Imax) = 1.06 ± 0.089, K = 2.4 ± 0.74, and slope = 0.756 ± 0.208 (r = 0.984, n ≥ 4 at each concentration).
Fig. 3.
Stimulation of ENaC with basolateral meSATP. meSATP (50 μM) was added basolaterally to A6 cells during single channel patch clamp on the apical side of the cell. A: representative recording of current from a single patch before (expanded section 1) and after (expanded section 2) the addition of meSATP. B: changes in ENaC Po in 8 patches treated with meSATP. *P < 0.05. C: dose response of ENaC to meSATP. Combined data from transepithelial current measurements (○) and patch-clamp measurements (●) were normalized to maximum effect (current or Po induced by the highest concentration of meSATP tested).
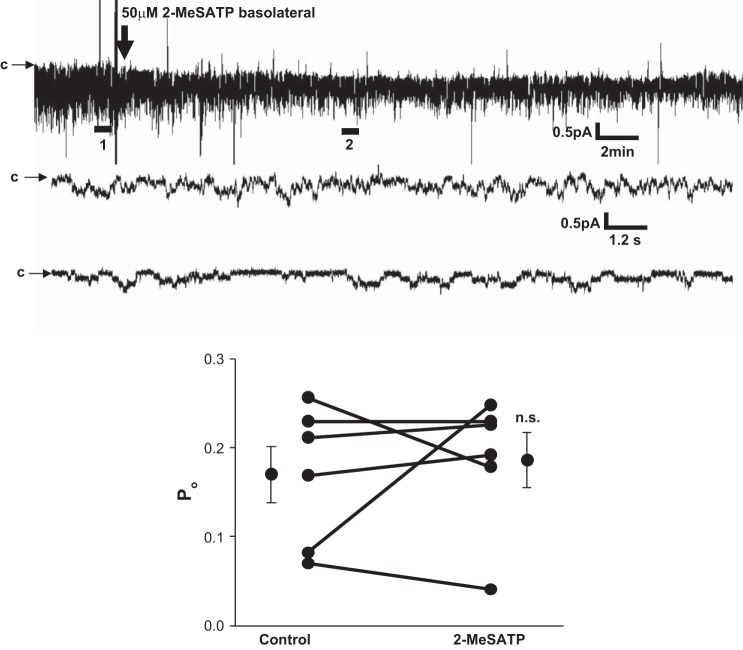
Pharmacological identification of purinergic receptors at present is difficult since no agonist or antagonist is 100% specific for any receptor subtype. Therefore, to confirm that the effects of meSATP on ENaC activity were due to P2X4 channel stimulation, we obtained a GFP-tagged dominant negative P2X4 construct from Dr. Ruth Murrell-Lagnado (University of Cambridge) (6). In A6 cells expressing dominant negative P2X4 channels, no effect of meSATP on ENaC activity was observed (Fig. 4). We therefore conclude that the stimulatory effects of meSATP on ENaC activity are due to stimulation of P2X4 channels.
Fig. 4.
Effect of meSATP on cells transfected with dominant negative P2X4. A6 cells were transfected with dominant negative P2X4 receptor, and patch clamp was performed as described in Fig. 3 with 50 μM meSATP added basolaterally after the formation of a seal. A: representative current recordings before and after 2- meSATP (top trace: ∼30 min; bottom traces corresponding to expanded sections 1 and 2: ∼50 s). B: ENaC Po in 5 patches before and after treatment with meSATP (P > 0.8). n.s., not significant.
ENaC stimulation by P2X4 receptors is Ca2+ dependent.
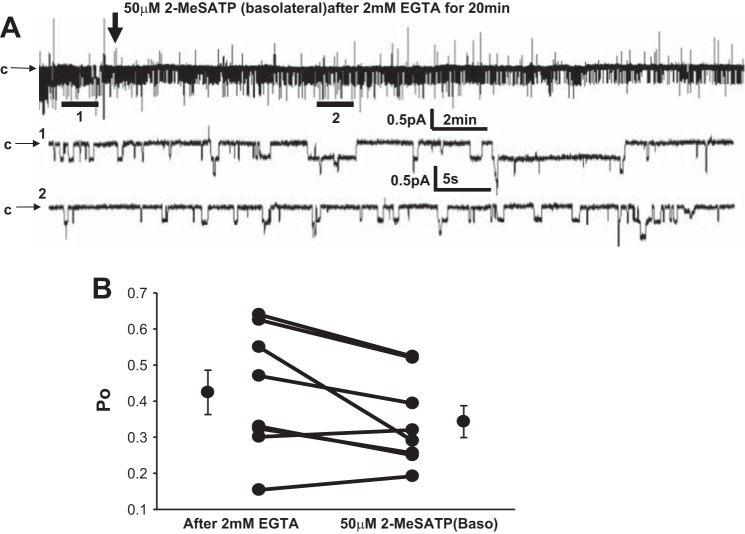
As previously described, P2X channels are typically nonselective cation channels (32). Given the ionic gradients across the the basolateral membranes of most epithelial cells, including A6 cells, Na+ and Ca2+ fluxes tend to be the major ions transported by these channels. Since changes in [Ca2+]I are known to have effects on ENaC (either stimulatory or inhibitory), we asked whether the effects of meSATP on ENaC were dependent on Ca2+ entry to the cell. To test this, we added the Ca2+ chelator EGTA basolaterally to A6 cells for 20 min before the basolateral addition of meSATP (Fig. 5). We found no significant effects of meSATP on ENaC Po in the presence of EGTA, suggesting that stimulation of ENaC requires P2X4 receptor-mediated Ca2+ entry and an increase in [Ca2+]i.
Fig. 5.
Effect of Ca2+ chelation with EGTA on P2X4 receptor-induced stimulation of ENaC. EGTA (2 mM) was applied apically 20 min before the addition of meSATP basolaterally. A: representative recording of ENaC current (∼25 min) after treatment with 2 mM EGTA (expanded section 1: ∼60 s) and after the addition of meSATP (expanded section 2: ∼60 s). B: effect of EGTA on ENaC Po before and after meSATP in 7 patches.
PI3K is required for P2X4 receptor-mediated ENaC stimulation.
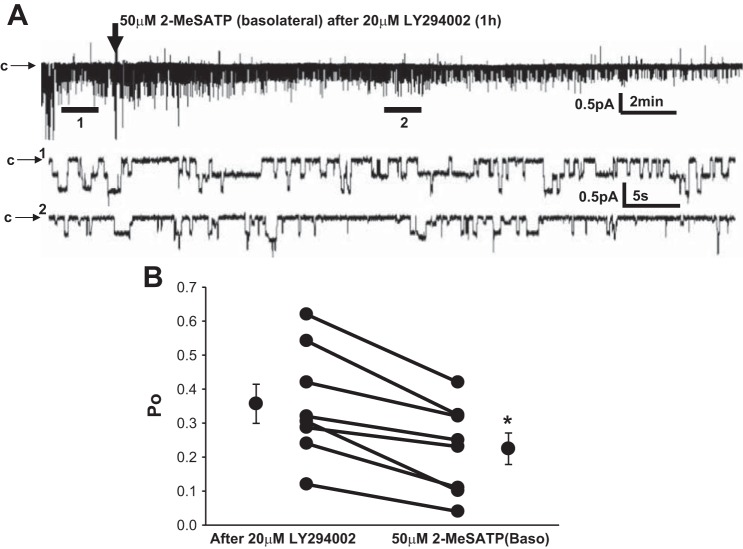
P2X4 receptors may be responsible for the cellular volume decrease after aldosterone exposure and these effects may be dependent on the stimulation of PI3K (18). PI3K activation is also known to activate ENaC (4, 5, 12, 44, 51). Therefore, to test whether stimulation of ENaC by P2X4 receptors in A6 cells is dependent on PI3K, we applied the selective PI3K antagonist LY-294002 to A6 cells 1 h before the basolateral addition of meSATP. While a significant increase in ENaC activity was observed in the presence of meSATP under normal conditions, in the absence of active PI3K, meSATP produced the opposite effect, resulting in significant inhibition of ENaC Po (Fig. 6). From these data, we conclude that the stimulatory effect of basolateral meSATP on ENaC is dependent on Ca2+-dependent activation of PI3K.
Fig. 6.
Effect of phosphatidylinositol 3-kinase inhibition with LY-294002 on meSATP-induced ENaC stimulation in A6 cells. LY-294002 was applied apically 1 h before the addition of meSATP basolaterally. A: representative recording of ENaC current (∼25 min) after treatment with 20 μM LY-294002 before (expanded section 1: ∼60 s) and after the addition of meSATP (expanded section 2: ∼60 s). B: effect of LY-294002 on ENaC Po before and after meSATP in 7 patches. *P < 0.05.
DISCUSSION
The polarization of epithelial tissues has evolved as a means to separate and generate unique responses to the environments inside and outside the body. In the aldosterone-sensitive nephron, where ionic transport occurs transcellulary, it is important to have polarized expression of transporters and channels. In addition, epithelial cells in this region of the nephron must be able to respond appropriately to signals from the tubular versus interstitial fluid. ATP, for example, is known to be secreted into the tubular lumen in response to changes in luminar flow to inhibit tubular transport in general (26). On the other hand, ATP release from cells may occur basolaterally in response to the action of aldosterone in the same cells (18). While a large body of work exists detailing the inhibitory effects of luminal (apical) ATP on ENaC function, little work is available detailing the effects of basolateral ATP on ENaC despite the suggestion of a requirement of ATP production in mediating aldosterone's effects on ENaC as early as 1963 (13).
Our experiments, in which we biotinylated both surfaces of the cells, showed that P2X4 receptors are in the basolateral membrane, with few, if any, receptors in the apical membrane. Our data are the first to show direct physiological evidence for the existence of a basolaterally expressed ATP-stimulated ion channel in a renal principal cell line. Using single channel cell-attached patch clamp, we identified a basolaterally expressed channel in A6 cells with a conductance and reversal potential similar to that which has been published for P2X4 receptors in other tissues (Fig. 2) (8, 39, 40). Experiments on cells labeled with cell surface biotin confirmed the basolateral presence of P2X4 channels in these cells (Fig. 1). These data support results showing immunohistochemical expression of P2X4 basolaterally along the tubule (46) and pharmacological data showing the effects of P2X4 agonists on amiloride-sensitive current in A6 cells (50).
Previous data have shown that, in A6 cells, basolateral ATP evoked an amiloride-sensitive increase in conductance and that this effect was dependent on increases in intracellular Ca2+ and PI3K activation (50). In the present study, we expanded on this research and used apical cell-attached single channel patch clamp to show that basolateral P2X4 receptor stimulation increases ENaC activity (Fig. 3). Furthermore, we demonstrated dependence of the stimulatory effect on increases in intracellular Ca2+, since the addition of EGTA to the bath diminisheds the effect (Fig. 5), and dependence on PI3K activation, since the addition of the PI3K inhibitor LY-294002 eliminated P2X4 receptor stimulation of ENaC (Fig. 6). Since PI3K is known to be important in ENaC trafficking, it is possible that PI3K is involved in P2X4 receptor stimulation only by assisting in ENaC trafficking and not through direct phosphorylation of PI3K downstream of P2X4 receptors. Furthermore, it is important to note that, while LY-294002 and EGTA are both known to have inhibitory effects on ENaC activity, it is impossible to tell whether that was the case in our experiments since we did not measure Po before the addition of these pharmacological agents. While our data and that of others suggest that Ca2+ and PI3K activate ENaC downstream of P2X4 channel activation (50), the exact underlying mechanisms remain a mystery. Since it is known that P2X4 receptors typically function as cation channels under physiological conditions (10, 23) and our data showed the dependence of ENaC stimulation by meSATP on intracellular Ca2+ changes (Fig. 5), it is likely that stimulation of P2X4 receptors by meSATP is causing an increase in intracellular Ca2+ before ENaC activation. Our data also showed the involvement of PI3K in mediating the ENaC response to meSATP (Fig. 6). Indeed, PI3K is known to stimulate ENaC by increasing the abundance of phosphatidylinositol (3,4,5)-trisphosphate at the plasma membrane and therefore increasing the membrane recruitment of ENaC (20, 29). How the initial increase in cytosolic Ca2+ from P2X4 channels activates PI3K, however, remains a mystery. PI3K is known to be downstream of receptor tyrosine kinases such as the EGF receptor and IGF receptor (17), both of which have been shown to activate ENaC (21, 27). Increases in cytosolic Ca2+ have been shown to enhance receptor tyrosine kinase transactivation (15, 16). It is possible that a local increase in Ca2+ activates these receptors. An alternate, and possibly more likely, scenario, however, involves the cell swelling that occurs after activation of P2X4 receptors in A6 cells (18, 50). This arrangement likely occurs subsequent to increases in cytosolic Ca2+ and subsequent phosphorylation of myosin light chain kinase (42). It is possible that this cell swelling results in changes in the interactions of the cell with the extracellular matrix, resulting in the activation of integrin proteins on the apical membrane that are known to activate PI3K (1). Investigation of these complex signaling pathways is beyond the scope of this this research, however, which was aimed only at elucidating the effects of basolateral ATP on ENaC activation. Future work should be aimed at identifying the various signaling molecules involved.
An interesting aspect to the present work is the stark contrast between the effects of apical and basolateral ATP on ENaC activity. Apical ATP application decreases both the number of ENaC channels in the membrane as well as Po of a single channel (43). This is done through stimulation of the ubiquitin ligase Nedd4-2, which tags ENaC for proteasomal degradation (47), and through stimulation of myristoylated alanine-rich C kinase substrate, which scavenges phosphatidylinositol 4,5-bisphosphate from the membrane, resulting in the removal of ENaC from the membrane (2). Interestingly, these effects occur after an increase in intracellular Ca2+ initiated by P2Y2 receptor activation of phospholipase C and the subsequent release of Ca2+ from endoplasmic reticulum stores (43). Oddly enough, we found that basolateral ATP application also increased intracellular Ca2+, but produced a stimulatory rather than inhibitory effect on ENaC (Figs. 3 and 4). It therefore follows that some mechanism must exist within the cell to result in a separation of the intracellular Ca2+ signals. Further work should be done to identify these Ca2+ signaling domains.
Overall, our data suggest that basolateral ATP enhances ENaC function through a pathway involving an increase in cytosolic Ca2+ and subsequent activation of PI3K. It is important to note that our experiments were performed in a cell culture line to easily be able to separate the apical and basolateral addition of pharmacological agents and for ease of patch clamping. Future experiments should be aimed at determining the relevance of this signaling pathway to whole animal physiology and ENaC regulation in the distal nephron in vivo.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37-DK-37960 and T32-DK-07656 and by American Heart Association Grant POST16820072.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.L.T., L.Y., D.C.E., B.J.D., and H.-F.B. conception and design of research; T.L.T., L.Y., D.C.E., B.J.D., O.K.A.-K., H.Y.C.L., H.M., and H.-F.B. performed experiments; T.L.T., D.C.E., H.M., and H.-F.B. analyzed data; T.L.T., D.C.E., and H.-F.B. interpreted results of experiments; T.L.T., D.C.E., and H.-F.B. prepared figures; T.L.T. and D.C.E. drafted manuscript; T.L.T., D.C.E., H.M., and H.-F.B. edited and revised manuscript; T.L.T., L.Y., D.C.E., B.J.D., O.K.A.-K., H.Y.C.L., H.M., and H.-F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Ruth Murrell-Lagnado for the kind gift of the dominant negative GFP-P2X4 plasmid.
REFERENCES
- 1.Alahari SK, Reddig PJ, Juliano RL. Biological aspects of signal transduction by cell adhesion receptors. Int Rev Cytol 220: 145–184, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Alli AA, Bao HF, Alli AA, Aldrugh Y, Song JZ, Ma HP, Yu L, Al-Khalili O, Eaton DC. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am J Physiol Renal Physiol 303: F800–F811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Baquero AF, Gilbertson TA. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am J Physiol Cell Physiol 300: C860–C871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazer-Yost BL, Paunescu TG, Helman SI, Lee KD, Vlahos CJ. Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am J Physiol Cell Physiol 277: C531–C536, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci 22: 4814–4824, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: renal pathophysiology and therapeutic potential. Clin Nephrol 78: 154–163, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Boucard D, Toulme JJ, Di Primo C. Bimodal loop-loop interactions increase the affinity of RNA aptamers for HIV-1 RNA structures. Biochemistry 45: 1518–1524, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. Bioessays 34: 218–225, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63: 641–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524: 77–90, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton DC, Malik B, Saxena NC, Al-Khalili OK, Yue G. Mechanisms of aldosterone's action on epithelial Na+ transport. J Membr Biol 184: 313–319, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Edelman IS, Bogoroch R, Porter GA. On the mechanism of action of aldosterone on sodium transport: the role of protein synthesis. Proc Natl Acad Sci USA 50: 1169–1177, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards RM. Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol 438: 179–181, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem 273: 8890–8896, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Fearn JC, King AC. EGF receptor affinity is regulated by intracellular calcium and protein kinase C. Cell 40: 991–1000, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 67: 481–507, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gorelik J, Zhang Y, Sanchez D, Shevchuk A, Frolenkov G, Lab M, Klenerman D, Edwards C, Korchev Y. Aldosterone acts via an ATP autocrine/paracrine system: the Edelman ATP hypothesis revisited. Proc Natl Acad Sci USA 102: 15000–15005, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol 290: L710–L722, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with γ-ENaC. J Biol Chem 280: 40885–40891, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Holzman JL, Liu L, Duke BJ, Kemendy AE, Eaton DC. Transactivation of the IGF-1R by aldosterone. Am J Physiol Renal Physiol 292: F1219–F1228, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal 8: 419–436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors–recent progress and persisting challenges. Purinergic Signal 8: 375–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142–143, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kunzelmann K, Schreiber R, Boucherot A. Mechanisms of the inhibition of epithelial Na+ channels by CFTR and purinergic stimulation. Kidney Int 60: 455–461, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Leipziger J. Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr Opin Nephrol Hypertens 20: 518–522, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Okada T, Assmann A, Soto J, Liew CW, Bugger H, Shirihai OS, Abel ED, Kulkarni RN. Insulin signaling regulates mitochondrial function in pancreatic β-cells. PLOS ONE 4: e7983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loffing J, Loffing-Cueni D, Macher A, Hebert SC, Olson B, Knepper MA, Rossier BC, Kaissling B. Localization of epithelial sodium channel and aquaporin-2 in rabbit kidney cortex. Am J Physiol Renal Physiol 278: F530–F539, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem 286: 32444–32453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol 282: F501–F505, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Nakata H, Suzuki T, Namba K, Oyanagi K. Dimerization of G protein-coupled purinergic receptors: increasing the diversity of purinergic receptor signal responses and receptor functions. J Recept Signal Transduct Res 30: 337–346, 2010 [DOI] [PubMed] [Google Scholar]
- 32.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Novak I. Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol (Oxf) 202: 501–522, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Palmer LG, Patel A, Frindt G. Regulation and dysregulation of epithelial Na+ channels. Clin Exp Nephrol 16: 35–43, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Poulsen AN, Klausen TL, Pedersen PS, Willumsen NJ, Frederiksen O. Regulation of ion transport via apical purinergic receptors in intact rabbit airway epithelium. Pflügers Arch 450: 227–235, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Rokic MB, Stojilkovic SS, Vavra V, Kuzyk P, Tvrdonova V, Zemkova H. Multiple roles of the extracellular vestibule amino acid residues in the function of the rat P2X4 receptor. PLOS ONE 8: e59411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen JB, Pappano AJ, Liang BT. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. FASEB J 20: 277–284, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA 93: 3684–3688, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903–1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokuda S, Niisato N, Morisaki S, Marunaka Y. Calmodulin-dependent regulation of hypotonicity-induced translocation of ENaC in renal epithelial A6 cells. Biochem Biophys Res Commun 298: 619–623, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Toney GM, Vallon V, Stockand JD. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na+ channel. Curr Opin Nephrol Hypertens 21: 52–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong Q, Booth RE, Worrell RT, Stockand JD. Regulation of Na+ transport by aldosterone: signaling convergence and cross talk between the PI3-K and MAPK1/2 cascades. Am J Physiol Renal Physiol 286: F1232–F1238, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Toulme E, Soto F, Garret M, Boue-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol 69: 576–587, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Peng Q, Lin Q, Childress C, Carey D, Yang W. Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J Biol Chem 285: 12279–12288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wildman SS, Kang ES, King BF. ENaC, renal sodium excretion and extracellular ATP. Purinergic Signal 5: 481–489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue G, Edinger RS, Bao HF, Johnson JP, Eaton DC. The effect of rapamycin on single ENaC channel activity and phosphorylation in A6 cells. Am J Physiol Cell Physiol 279: C81–C88, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, Korchev Y. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Renal Physiol 292: F1734–F1740, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zhu T, Zhang W, Wang DX. Insulin up-regulates epithelial sodium channel in LPS-induced acute lung injury model in rats by SGK1 activation. Injury 43: 1277–1283, 2012 [DOI] [PubMed] [Google Scholar]