Abstract
Reduced spinal synaptic inputs to phrenic motor neurons elicit a unique form of spinal plasticity known as inactivity-induced phrenic motor facilitation (iPMF). iPMF requires tumor necrosis factor-α (TNF-α) and atypical protein kinase C (aPKC) activity within spinal segments containing the phrenic motor nucleus to stabilize early, transient increases in phrenic burst amplitude into long-lasting iPMF. Here we tested the hypothesis that spinal N-methyl-d-aspartate receptor (NMDAR) activation constrains long-lasting iPMF in some rat substrains. Phrenic motor output was recorded in anesthetized, ventilated Harlan (HSD) and Charles River (CRSD) Sprague-Dawley rats exposed to a 30-min central neural apnea. HSD rats expressed a robust, long-lasting (>60 min) increase in phrenic burst amplitude (i.e., long-lasting iPMF) when respiratory neural activity was restored. By contrast, CRSD rats expressed an attenuated, transient (∼15 min) iPMF. Spinal NMDAR inhibition with DL-2-amino-5-phosphonopentanoic acid (APV) before neural apnea or shortly (4 min) prior to the resumption of respiratory neural activity revealed long-lasting iPMF in CRSD rats that was phenotypically similar to that in HSD rats. By contrast, APV did not alter iPMF expression in HSD rats. Spinal TNF-α or aPKC inhibition impaired long-lasting iPMF enabled by NMDAR inhibition in CRSD rats, suggesting that similar mechanisms give rise to long-lasting iPMF in CRSD rats with NMDAR inhibition as those giving rise to long-lasting iPMF in HSD rats. These results suggest that NMDAR activation can impose constraints on TNF-α-induced aPKC activation after neural apnea, impairing stabilization of transient iPMF into long-lasting iPMF. These data may have important implications for understanding differential responses to reduced respiratory neural activity in a heterogeneous human population.
Keywords: control of breathing, inactivity, iPMF, phrenic, plasticity
reduced respiratory neural activity (in the absence of hypoxia and hypercapnia) elicits plasticity in multiple respiratory-related neurons, including the phrenic motor circuit (2, 5, 35, 47). For example, following a prolonged (30 min) central neural apnea, a rebound increase in phrenic burst amplitude is apparent when respiratory neural activity is restored (7, 46, 48), a form of plasticity called inactivity-induced phrenic motor facilitation (iPMF) (2, 35, 48). Mechanisms local to the phrenic motor pool give rise to iPMF because unilaterally blocking C2 axon conduction, while maintaining brainstem respiratory neural activity, is sufficient to elicit iPMF in the ipsilateral phrenic motor pool (46); thus spinal mechanisms sense and respond to reductions in phrenic synaptic inputs. Evidence suggests that iPMF consists of at least two phases; an early, transient phase that can be converted to a long-lasting, stable increase in phrenic burst amplitude (10, 57). Whereas mechanisms giving rise to transient iPMF are unknown, our working model of long-lasting iPMF suggests that reduced respiratory-related synaptic inputs to phrenic motor neurons induce the release of tumor necrosis factor-α (TNF-α) within the phrenic motor pool (7, 46), which activates TNF receptors on phrenic motor neurons and leads to formation of a signaling complex between atypical protein kinase C (aPKC) isoforms (PKCζ and/or PKCι) and the scaffolding molecule p62/ZIP to give rise to iPMF through unknown downstream mechanisms (7, 48). Interestingly, the necessary window for PKCζ/ι-p62/ZIP activity is during transient iPMF expression, but once long-lasting iPMF is established, PKCζ/ι-p62/ZIP is no longer required (57).
By happenstance, we discovered that iPMF differs among Sprague-Dawley rats supplied by Harlan (HSD) and Charles River (CRSD) Laboratories; specifically, CRSD rats appear to express an attenuated, transient iPMF (unpublished observations). We hypothesized that differential expression of iPMF in HSD and CRSD rats was due to differential N-methyl-d-aspartate receptor (NMDAR) activation. This hypothesis was based on observations in hippocampal neurons showing that mechanisms of homeostatic synaptic plasticity occur several times faster when NMDARs are inhibited at the same time as hippocampal activity deprivation. Indeed, in the hippocampus, a prolonged period (>12 h) of activity deprivation is generally required to elicit an increase in synaptic strength in response to action potential blockade with tetrodotoxin (TTX) (59); however, if hippocampal NMDARs are also blocked at the same time as action potential blockade, mechanisms of homeostatic plasticity may be activated as early as 4 h following activity deprivation (50, 51). Mechanisms that underlie NMDAR-mediated stabilization of synapses during activity deprivation are not entirely clear, but may involve tonic suppression of dendritic protein synthesis (50), suggesting that NMDAR activation by miniature excitatory postsynaptic currents (mEPSCs, or minis) that persist during inhibition of action potential–mediated synaptic events delays the expression of some forms of inactivity-induced plasticity.
Here we demonstrate that blockade of spinal NMDARs during activity deprivation or shortly (∼4 min) prior to the resumption of respiratory neural activity reveals a robust, long-lasting iPMF in a rat substrain in which iPMF is normally small and transient (∼15 min; CRSD), suggesting that genetic or epigenetic differences in NMDARs may underlie phenotypic differences in iPMF among genetically isolated rat substrains. Moreover, long-lasting iPMF in CRSD rats revealed by spinal NMDAR inhibition requires spinal TNF-α and aPKC activity, suggesting that NMDAR activation in CRSD rats prevents the stabilization of transient iPMF into a long-lasting iPMF by impairing TNF-α-induced activation of aPKC activity. Understanding mechanisms that may constrain plasticity in diverse populations may be crucial in the development of novel treatment and/or prevention strategies for devastating respiratory control disorders characterized by inadequate respiratory neural activity.
MATERIALS AND METHODS
Animals
Eighty-five adult male outbred Sprague-Dawley rats (2–4 mo old, 230–550 g) from two different suppliers, Harlan (n = 20, Colony 217; Indianapolis, IN) and Charles River Laboratories (n = 65, Colony P09; Portage, MI), were used for this study (referred to as HSD and CRSD, respectively). Rats were housed in pairs in a controlled environment (12 h light/dark cycles) with food and water ad libitum at the University of Wisconsin-Madison. A small but significant difference was found in the average age (HSD 96 ± 2 days; CRSD 83 ± 1 days; P < 0.05) and weight (HSD 341 ± 7 g; CRSD 373 ± 8 g; P < 0.05) of HSD and CRSD rats at the time of electrophysiological experiments; however, because the age difference was less than 2 wk and the weight difference was ∼30 g, it is unlikely that this difference gave rise to the observed differential response to neural apnea. Indeed, linear regression analysis indicated that phrenic burst amplitudes at 5 and 60 min were not significantly correlated with either age (P > 0.05) or weight (P > 0.05; data not shown). All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison.
Electrophysiology Surgery and Preparation
Anesthesia was induced with isoflurane in a closed chamber. Rats were transferred to a heated table where anesthesia was maintained with 3% isoflurane (in 50% O2:N2 balance) through a nose cone. Core body temperature was monitored with a rectal probe (700 1H; Physitemp) and kept between 36–38°C. A tail vein catheter was placed for intravenous delivery of urethane and fluids. The trachea was cannulated, rats were pump-ventilated (Rodent Ventilator 683; Harvard Apparatus), and a bilateral vagotomy was performed. End-tidal CO2 (ETCO2) was monitored (Capnogard; Respironics, Murrysville, PA), and inspired gases were adjusted to maintain spontaneous respiratory neural activity throughout the surgery (ETCO2 approximately 45–50 mmHg). A femoral arterial catheter was placed to monitor blood pressure and sample blood gases throughout the protocol (ABL-500; Radiometer, Copenhagen, Denmark). Using a dorsal approach, the left phrenic nerve was isolated, cut distally, and desheathed. The C2 spinous process was exposed and a C2 laminectomy was performed over the spinal midline leaving the lamina near the lateral edges intact. A small hole was cut in the dura of the exposed spinal segment and a silicone catheter (2 French; Access Technologies) connected to a 50-μl Hamilton syringe was inserted into the intrathecal space and carefully advanced caudally (∼5 mm) so that the tip of the catheter laid on the dorsal surface of the C4 spinal segment.
Following surgery, rats were converted (6 ml/h; Harvard Apparatus syringe pump) to urethane anesthesia (1.7–1.8 g/kg iv) while inspired isoflurane was slowly decreased. After rats were converted to urethane, the neuromuscular paralytic pancuronium bromide (2.5 mg/kg iv; Sicor Pharmaceuticals) was delivered, followed by a slow infusion of a 1:1:4 solution (6% hetastarch/8.4% sodium bicarbonate/lactated Ringers) to maintain blood pressure and fluid homeostasis. Adequate depth of anesthesia was monitored by blood pressure responses to toe-pinch; rats were supplemented with urethane as necessary. The left phrenic nerve was placed on a bipolar silver recording electrode and the cavity was filled with mineral oil. Compound action potentials were amplified (×10 K), band-pass filtered (300 Hz to 10 kHz), and integrated (time constant 50 ms). Raw and integrated signals were digitized and recorded with a PowerLab data acquisition system (Lab Chart 7.0; ADInstruments).
Intrathecal Compounds
The following compounds were dissolved in artificial cerebral spinal fluid (aCSF; in mM: 120 NaCl, 3 KCl, 2 CaCl, 2 MgCl, 23 NaHCO3, 10 glucose, bubbled with 95% O2-5% CO2; pH 7.4): the NMDAR antagonist DL-2-amino-5-phosphonopentanoic acid (APV; doses below; Sigma Aldrich); the aPKC inhibitory peptide, myristoylated ζ-pseudosubstrate inhibitory peptide (PKCζ-PS; 2 μg/μl; Tocris Bioscience); the scrambled version of the aPKC inhibitory peptide, myristoylated scrambled ζ-pseudosubstrate peptide (scrPKCζ-PS; 2 μg/μl; Tocris Bioscience); and the TNF-α signaling inhibitor, soluble TNF-α receptor 1 (sTNFR1; 0.1 μg/μl; R&D Systems). Doses for PKCζ-PS, scrPKCζ-PS, and sTNFR1 were determined in previous studies (7, 48). Preliminary experiments were performed to establish a dose-response curve for APV in CRSD rats to determine the smallest effective dose. Briefly, CRSD rats received 10 μl of APV at one of the following doses 20 min prior to neural apnea: 200 ng/μl (n = 3), 20 ng/μl (n = 4), and 2 ng/μl (n = 4); 20 ng/μl was chosen and used for all remaining experiments. Intrathecal compounds were slowly delivered over approximately 1–2 min, for a total volume of 10 μl. An additional 3 μl of sTNFR1 was delivered prior to the end of neural apnea in sTNFR1-treated rats. Vehicle-treated rats received 10 μl of aCSF.
Electrophysiology Protocols
One hour after isoflurane was withdrawn, baseline phrenic activity was established by setting baseline burst frequency at approximately 45–50 bursts/min because previous studies in HSD rats indicate that baseline burst frequency is expected at an ETCO2 of approximately 2–3 mmHg above the recruitment threshold (2, 7, 46), and we wished to avoid exposure to neural apnea prior to the protocol. In a small subset of initial experiments, CRSD rats received a preprotocol apneic/recruitment threshold test to ensure average phrenic burst frequency at an ETCO2 2–3 mmHg above the recruitment threshold was similar to that of HSD rats (5/8 CRSD rats + aCSF; 3/8 CRSD rats + APV). In these rats, central respiratory activity was briefly (∼1.5 min) decreased by lowering inspired CO2 below the threshold for breathing (apneic threshold); inspired CO2 was then increased until central respiratory neural activity was restored (recruitment threshold). ETCO2 was then set 2–3 mmHg above the recruitment threshold, and baseline burst frequency was noted (47 ± 3 bursts/min). In remaining rats, baseline burst frequency was set at approximately 45–50 bursts/min. Similar to a previous study (7), there were no differences in iPMF in CRSD rats that received a preprotocol apneic/recruitment threshold test vs. those that did not (P > 0.05), and as such, the data were combined.
Following 15–20 min of stable phrenic neural activity, a baseline arterial blood gas sample was taken. Rats were exposed to one of the following protocols: central neural apnea or time control (described below). In all rats, arterial blood gas samples were taken during baseline, central neural apnea, and 5 and/or 15, 30, and 60 min after respiratory neural activity resumed or an equivalent duration in time controls not receiving reduced respiratory neural activity. At the end of each protocol, rats were presented with a severe hypercapnic challenge (∼10% CO2) to assure our preparation did not deteriorate and to assess the dynamic range of phrenic motor output.
Neural apnea.
In subgroups of CRSD and HSD rats, a reversible central neural apnea was induced by slowly reducing inspired CO2 and/or increasing the ventilator rate until ETCO2 was ∼5 mmHg below the apneic threshold. After 30 min of neural apnea, baseline PaCO2 levels were restored, and phrenic neural activity was monitored for 60 min. CRSD rats exposed to neural apnea received intrathecal 1) vehicle 20 min prior to neural apnea (n = 8); 2) APV 20 min prior to neural apnea (n = 8); 3) APV 4 min prior to the end of neural apnea (referred to as APV-post; n = 8); 4) sTNFR1 20 min prior to neural apnea + APV-post (n = 7); 5) APV-post + scrPKCζ-PS 10 min after the resumption of phrenic neural activity (n = 7); or 6) APV-post + PKCζ-PS 10 min after the resumption of phrenic neural activity (n = 7). HSD rats exposed to a neural apnea received intrathecal 1) vehicle 20 min prior to neural apnea (n = 8) or 2) APV 20 min prior to neural apnea (n = 6).
Time controls.
To control for time-dependent variations in phrenic neural output inherent to our preparation or as a consequence of intrathecal compounds, separate groups of CRSD and HSD rats received the same surgery and electrophysiology protocol (described above), but were not exposed to neural apnea; we refer to these rats as time controls. Four groups of CRSD time controls receiving the following intrathecal compounds were included: 1) vehicle (n = 3); 2) APV (n = 4); 3) sTNFR1 and APV (n = 3); or 3) APV and PKCζ-PS (n = 3). Two groups of HSD time controls were also included and received the following intrathecal compounds: 1) vehicle (n = 3) or 2) APV (n = 3). Intrathecal APV, sTNFR1, or PKCζ-PS did not alter baseline burst amplitude (at 60 min, vehicle: 12.7 ± 11; APV: 1.7 ± 6; APV + sTNFR1: 11.5 ± 6; APV + PKCζ-PS: 13.9 ± 11% baseline; P > 0.05) or frequency (vehicle: 5 ± 2; APV: 4 ± 3; APV + sTNFR1: 2 ± 1; APV + PKCζ-PS: 2 ± 2 Δ baseline; P > 0.05). There were no significant differences in phrenic burst amplitude or frequency among time control groups (P > 0.05), so groups were combined for statistical analysis (n = 19). For comparison purposes, average amplitude and frequency of this combined time control group is shown in Figs. 1–4, A and B, respectively.
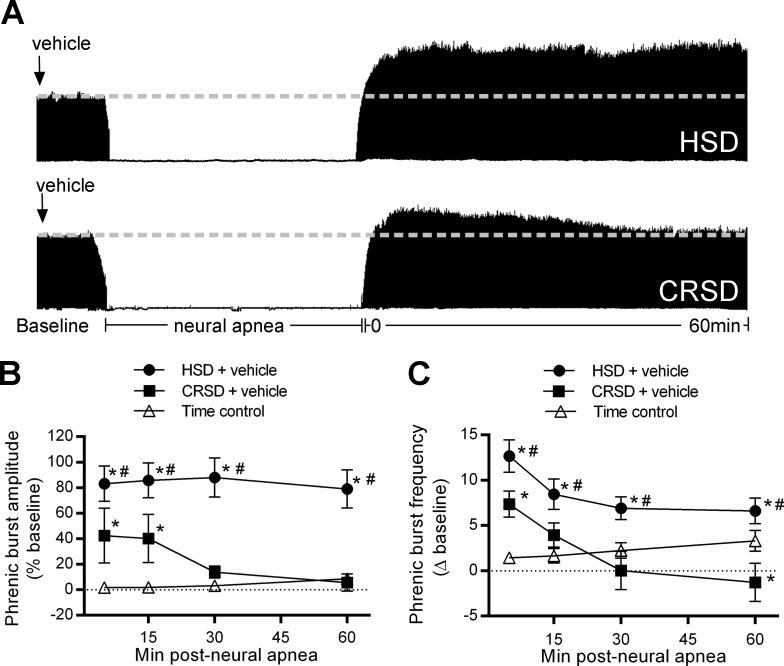
Fig. 1.
Inactivity-induced phrenic motor facilitation (iPMF) and burst frequency facilitation are differentially regulated in Harlan Sprague-Dawley (HSD) and Charles River Sprague-Dawley (CRSD) rat substrains. A: representative compressed phrenic neurograms depicting baseline, 30 min of neural apnea, and for 60 min after the resumption of phrenic neural activity in HSD and CRSD rats receiving intrathecal vehicle. Black arrows denote intrathecal delivery of vehicle. B: average phrenic burst amplitude (% baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in HSD (circles) and CRSD (squares) rats pretreated with vehicle. HSD rats exposed to neural apnea expressed a significant increase in phrenic burst amplitude relative to time controls at all time points, indicating long-lasting iPMF. By contrast, CRSD rats exposed to neural apnea expressed a smaller but significant increase in phrenic burst amplitude relative to time controls at 5 and 15 min, which returned to baseline 30 min post neural apnea, suggesting transient iPMF. C: average phrenic burst frequency (Δ baseline) in HSD (circles) and CRSD (squares) rats pretreated with vehicle for 60 min post neural apnea or an equivalent duration in time controls (triangles). HSD rats exposed to neural apnea expressed a significant increase in phrenic burst frequency relative to time controls at all time points. CRSD rats exposed to neural apnea expressed a smaller but significant increase relative to time controls only at 5 min post neural apnea. Values are means ± SE. *Significantly different than time controls; #significantly different than CRSD rats; P < 0.05.
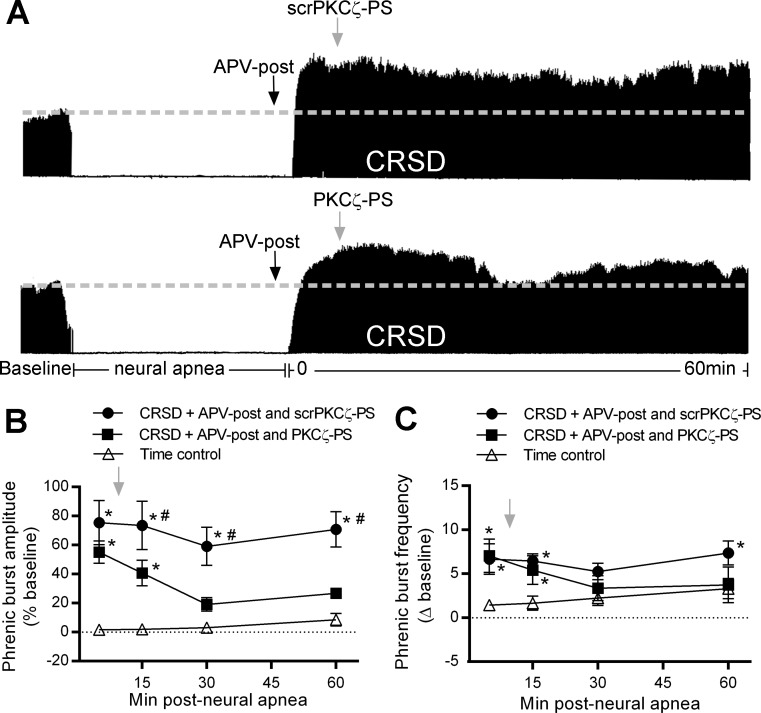
Fig. 4.
Long-lasting iPMF in CRSD rats receiving APV requires spinal atypical protein kinase C (aPKC) activity. A: representative compressed phrenic neurograms depicting baseline, 30 min of neural apnea, and for 60 min after the resumption of phrenic neural activity in CRSD rats receiving intrathecal APV-post and either the scrambled version of the atypical PKC inhibitory peptide, myristoylated scrambled ζ-pseudosubstrate peptide (scrPKCζ-PS) or the atypical PKC inhibitory peptide, myristoylated ζ-pseudosubstrate inhibitory peptide (PKCζ-PS) 10 min after the resumption of neural activity. Black arrows denote intrathecal delivery of APV-post; gray arrows indicate intrathecal delivery of scrPKCζ-PS or PKCζ-PS. B: average phrenic burst amplitude (% baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in rats receiving APV-post and scrPKCζ-PS (circles) or PKCζ-PS (squares). Gray arrow indicates intrathecal delivery of scrPKCζ-PS or PKCζ-PS. CRSD rats receiving APV-post and scrPKCζ-PS express a significant increase in phrenic burst amplitude relative to time controls at all time points post neural apnea, indicating that the scrambled peptide did not impair long-lasting iPMF enabled by NMDAR inhibition. By contrast, CRSD rats receiving APV-post and PKCζ-PS expressed a small but significant increase in phrenic burst amplitude compared with time controls only at 5 and 15 min post neural apnea; however, phrenic burst amplitude was not significantly increased from time controls at 30 or 60 min. Furthermore, phrenic burst amplitude in CRSD rats receiving APV-post and PKCζ-PS was significantly lower than in CRSD rats receiving APV-post and scrPKCζ-PS, indicating that long-lasting iPMF in CRSD rats enabled by NMDAR inhibition requires spinal aPKC activity. C: average phrenic burst frequency (Δ baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in rats receiving APV-post and scrPKCζ-PS (circles) or PKCζ-PS (squares). Gray arrow indicates intrathecal delivery of scrPKCζ-PS or PKCζ-PS. CRSD rats receiving intrathecal APV-post and scrPKCζ-PS expressed a significant increase in phrenic burst frequency from time controls at 5, 15, and 60 min post neural apnea. CRSD rats receiving APV-post and PKCζ-PS expressed a significant increase in phrenic burst frequency from time controls at 5 and 15 min post neural apnea. Values are means ± SE. *Significantly different than time controls; P < 0.05.
Statistical Analysis
Criteria for including data in the analysis were mean arterial blood pressure (MAP) >70 mmHg, PaO2 >140 mmHg, PaCO2 within 1.5 mmHg ± baseline, and hypercapnic response >25%. Integrated phrenic burst amplitude and frequency were averaged over 30- to 60-s periods (immediately preceding blood gas samples) during baseline and 5, 15, 30, and 60 min post neural apnea or an equivalent duration in time controls. Integrated phrenic amplitude was reported as a percent increase over baseline (normalized to zero), and phrenic burst frequency was expressed as an absolute change from baseline. A two-way ANOVA with repeated measures design was used for statistical comparisons of amplitude, frequency, and blood gas parameters (Prism 6; GraphPad Software). Individual time point comparisons were determined by Fisher least significant difference post hoc tests. A one-way ANOVA was used to detect significant differences in the maximum phrenic response to hypercapnia, and individual comparisons were made using a Fisher least significant difference post hoc test (Prism 6; GraphPad Software). For this analysis, CRSD rats receiving intrathecal APV prior to neural apnea or shortly before the end of neural apnea (APV-post) were combined for clarity. For all analyses, the significance level was set to 0.05. Results are shown as means ± SE.
RESULTS
Central respiratory neural activity was temporarily reduced in ventilated HSD and CRSD rats by reducing arterial CO2 below the threshold for breathing (apneic threshold). Following 30 min of central neural apnea, baseline arterial CO2 was restored and respiratory neural activity was monitored for 60 min. Table 1 lists average arterial Pco2, pH, Po2, and MAP during baseline and 60 min post neural apnea or an equivalent duration in time controls not receiving reduced respiratory activity. There were no significant differences in any variable from baseline to 60 min post neural apnea within any rat group (P > 0.05).
Table 1.
Average arterial partial pressures of CO2 and O2, pH, and MAP before and 60 min after central neural apnea or an equivalent duration in time control rats receiving a similar surgery, but no neural apnea
| PaCO2 | PaO2 | pH | MAP | ||
|---|---|---|---|---|---|
| Time control | Baseline | 46.2 ± 0.9 | 279 ± 8 | 7.4 ± 0.0 | 126 ± 4 |
| 60 min | 46.0 ± 1.0 | 280 ± 6 | 7.4 ± 0.0 | 111 ± 5 | |
| HSD + vehicle | Baseline | 47.5 ± 0.8 | 277 ± 9 | 7.4 ± 0.0 | 129 ± 5 |
| Neural apnea | 60 min | 47.3 ± 0.7 | 270 ± 8 | 7.4 ± 0.0 | 122 ± 7 |
| CRSD + vehicle | Baseline | 46.8 ± 1.8 | 274 ± 6 | 7.4 ± 0.0 | 107 ± 7*† |
| Neural apnea | 60 min | 46.9 ± 1.8 | 264 ± 5 | 7.4 ± 0.0 | 101 ± 8† |
| HSD + APV | Baseline | 46.4 ± 1.5 | 248 ± 9 | 7.3 ± 0.0 | 129 ± 5 |
| Neural apnea | 60 min | 46.3 ± 1.3 | 251 ± 9 | 7.4 ± 0.0 | 125 ± 6 |
| CRSD + APV | Baseline | 48.3 ± 1.4 | 264 ± 4 | 7.4 ± 0.0 | 114 ± 6 |
| Neural apnea | 60 min | 48.2 ± 1.4 | 259 ± 6 | 7.4 ± 0.0 | 102 ± 5§ |
| CRSD + APV-post | Baseline | 46.3 ± 1.0 | 258 ± 5 | 7.4 ± 0.0 | 116 ± 7 |
| Neural apnea | 60 min | 46.4 ± 0.8 | 253 ± 4 | 7.4 ± 0.0 | 100 ± 7 |
| CRSD + sTNFR1 and APV-post | Baseline | 51.1 ± 1.1*‡ | 264 ± 15 | 7.3 ± 0.0 | 107 ± 11* |
| Neural apnea | 60 min | 51.5 ± 1.2*‡ | 249 ± 17 | 7.3 ± 0.0*‡ | 95 ± 10 |
| CRSD + APV-post and PKCζ-PS | Baseline | 48.6 ± 1.1 | 266 ± 6 | 7.3 ± 0.0 | 113 ± 7 |
| Neural apnea | 60 min | 48.2 ± 1.3 | 264 ± 3 | 7.3 ± 0.0 | 89 ± 4* |
| CRSD + APV-post and scrPKCζ-PS | Baseline | 48.0 ± 1.1 | 265 ± 10 | 7.3 ± 0.0 | 120 ± 7 |
| Neural apnea | 60 min | 48.8 ± 1.1 | 259 ± 11 | 7.3 ± 0.0* | 101 ± 9 |
APV, DL-2-amino-5-phosphonopentanoic acid; APV-post, APV administered 4 min before the end of neural apnea; CRSD, Charles River Sprague-Dawley rats; HSD, Harlan Sprague-Dawley rats; MAP, mean arterial blood pressure; PaO2, arterial partial pressure of O2, PaCO2, arterial partial pressure of CO2; PKCζ-PS, myristoylated ζ-pseudosubstrate inhibitory peptide; scrPKCζ-PS, myristoylated scrambled ζ-pseudosubstrate peptide; sTNFR1, soluble tumor necrosis factor-α receptor. Values are means ± SE
Significantly different than time controls (P < 0.05);
significantly different than HSD + vehicle;
significantly different than CRSD + APV-post;
significantly different than HSD + APV.
Slight differences in PaCO2, pH, and MAP were detected across groups (Table 1). CRSD rats pretreated with intrathecal sTNFR1 prior to neural apnea and receiving APV-post had a significantly higher PaCO2 (at baseline and 60 min) and lower pH (60 min) relative to time controls and CRSD rats receiving APV only (P < 0.05). CRSD rats receiving APV-post and scrPKCζ-PS exhibited a significantly lower pH relative to time controls (P < 0.05). MAP was significantly lower in CRSD rats pretreated with vehicle prior to neural apnea than in time controls and HSD rats receiving vehicle (P < 0.05). MAP was also lower in CRSD rats receiving pretreatment with APV prior to neural apnea relative to HSD rats receiving APV (P < 0.05). CRSD rats pretreated with sTNFR1 prior to neural apnea and receiving APV-post had a significantly lower MAP during baseline compared with time controls (P < 0.05). Finally, CRSD rats receiving APV-post and PKCζ-PS had a significantly lower MAP at 60 min relative to time controls (P < 0.05). These changes are not considered to have affected our study because 1) all observed changes were small and within normal physiological limits, 2) no time-dependent changes were observed within any group, and 3) there was no relationship with the expression/blockade of iPMF.
iPMF Is Differentially Regulated Among Sprague-Dawley Rat Substrains
Representative compressed phrenic neurograms from vehicle-treated HSD and CRSD rats during baseline, 30 min of reduced respiratory neural activity, and 60 min following the resumption of respiratory neural activity are shown in Fig. 1A. Average change in phrenic burst amplitude (% baseline) for 60 min post neural apnea in HSD and CRSD rats receiving intrathecal vehicle or an equivalent duration in time controls is shown in Fig. 1B. Phrenic burst amplitude in time controls was not significantly different than baseline at any time point (all P > 0.05), suggesting that phrenic burst amplitude is stable throughout the protocol. Similar to previous findings (2, 48), ventilated HSD rats exposed to central neural apnea expressed a prolonged increase in phrenic burst amplitude that was significantly increased from baseline and time controls at all time points following neural apnea (all P < 0.05; Fig. 1B), indicating long-lasting iPMF. By contrast, CRSD rats expressed a small but significant increase in phrenic burst amplitude relative to baseline and time controls at 5 and 15 min (both <0.05); however, at 30 and 60 min, phrenic burst amplitude was no longer significantly increased from baseline or time controls (both P > 0.05), and phrenic burst amplitude at 30 and 60 min was significantly lower than the response at 5 and 15 min post neural apnea (P < 0.05), suggesting an attenuated, transient iPMF. Indeed, phrenic burst amplitude in CRSD rats was significantly lower than HSD rats at all time points post neural apnea (all P < 0.05; Fig. 1B).
Average phrenic burst frequency (Δ baseline) for 60 min post neural apnea in HSD and CRSD rats receiving intrathecal vehicle or an equivalent duration in time controls is shown in Fig. 1C. Similar to a previous study (35), time controls expressed a small but significant increase in phrenic burst frequency over the recording period (P < 0.05); as such, we only make inferences regarding changes from time controls. HSD rats exposed to central neural apnea expressed a significant increase in phrenic burst frequency relative to time controls at all time points post neural apnea (all P < 0.05), suggesting sustained phrenic burst frequency facilitation (Fig. 1C). By contrast, CRSD rats expressed a significant increase in phrenic burst frequency compared with time controls only at 5 min post neural apnea (P < 0.05). Further, phrenic burst frequency in CRSD rats was significantly lower than HSD rats at all time points (all P < 0.05), suggesting that CRSD rats express an attenuated, transient phrenic burst frequency facilitation. Together, these results demonstrate that HSD and CRSD rats express differential phrenic burst amplitude and frequency facilitation following central neural apnea.
Activation of Spinal NMDARs in CRSD Rats Impairs Long-Lasting iPMF
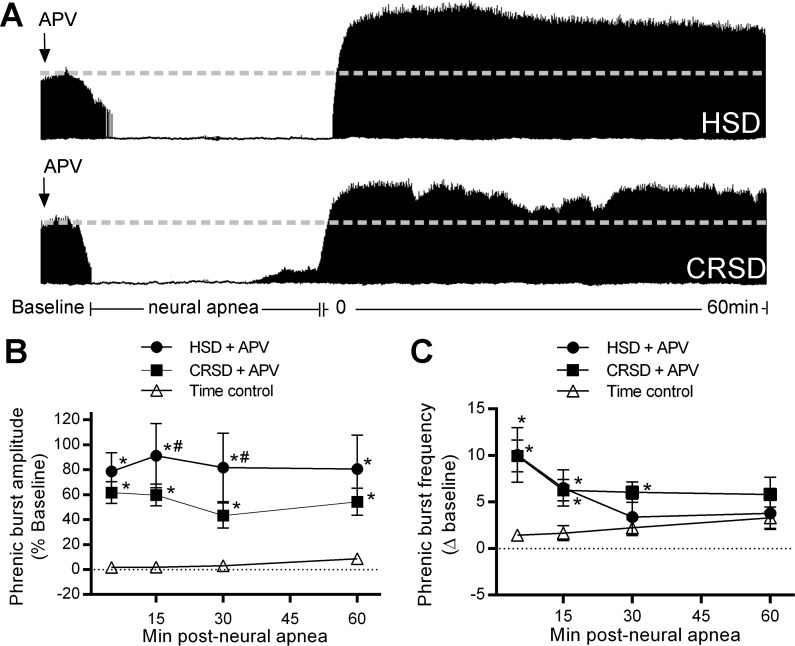
To test the hypothesis that activation of spinal NMDARs in or near the phrenic motor nucleus in CRSD rats impairs long-lasting iPMF following central neural apnea, the competitive NMDAR antagonist APV (20 ng/μl) was delivered intrathecally 20 min prior to central neural apnea. Intrathecal APV in time controls did not alter phrenic burst amplitude (at 60 min, 1.7 ± 6% baseline). Representative compressed phrenic neurograms during baseline, 30 min of reduced respiratory neural activity, and 60 min following the resumption of respiratory neural activity in CRSD and HSD rats pretreated with intrathecal APV are shown in Fig. 2A. Average change (% baseline) in phrenic burst amplitude for 60 min post neural apnea in HSD and CRSD rats pretreated with intrathecal APV or an equivalent duration in time controls is shown in Fig. 2B. HSD rats receiving APV 20 min prior to neural apnea expressed a significant increase in phrenic burst amplitude relative to baseline and time controls at all time points post neural apnea (all P < 0.05; Fig. 2B) that was phenotypically similar to that observed in HSD rats that did not receive APV (Fig. 1B), suggesting that APV did not alter the development of long-lasting iPMF. CRSD rats receiving APV 20 min prior to neural apnea expressed a significant increase in phrenic burst amplitude relative to baseline and time controls at all time points following neural apnea (all P < 0.05; Fig. 2B), indicating long-lasting iPMF. Phrenic burst amplitude in CRSD rats pretreated with APV was not significantly different than HSD rats pretreated with APV at 5 or 60 min following resumption of respiratory neural activity (both P < 0.05), although CRSD rats expressed a slightly lower phrenic burst amplitude compared with HSD rats at 15 and 30 min (both P < 0.05). Together, these results suggest that inhibition of spinal NMDARs reveals long-lasting iPMF in CRSD rats.
Fig. 2.
Spinal N-methyl-d-aspartate receptor (NMDAR) activation in CRSD rats, but not HSD rats constrains long-lasting iPMF. A: representative compressed phrenic neurograms depicting baseline, 30 min of neural apnea, and for 60 min after the resumption of phrenic neural activity in HSD and CRSD rats pretreated with intrathecal DL-2-amino-5-phosphonopentanoic acid (APV). Black arrows denote intrathecal delivery of APV. B: average phrenic burst amplitude (% baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in HSD (circles) and CRSD (squares) rats receiving APV prior to neural apnea. Both HSD and CRSD rats pretreated with APV and exposed to neural apnea expressed a significant increase compared with time controls at all time points, indicating long-lasting iPMF. This increase in CRSD rats was significantly lower than in HSD rats at 15 and 30 min post neural apnea. C: average phrenic burst frequency (Δ baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in HSD (circles) and CRSD (squares) rats pretreated with intrathecal APV prior to neural apnea. HSD rats receiving APV before neural apnea expressed a significant increase in phrenic burst frequency relative to time controls at 5 and 15 min. CRSD rats receiving intrathecal APV prior to neural apnea expressed a significant increase from time controls at 5, 15, and 30 min post neural apnea. Values are means ± SE. *Significantly different than time controls; #significantly different than HSD rats; P < 0.05.
Average phrenic burst frequency (Δ baseline) for 60 min post neural apnea in HSD and CRSD rats receiving APV 20 min prior to neural apnea or an equivalent duration in time controls is shown in Fig. 2C. HSD rats pretreated with APV prior to neural apnea expressed a significant increase in phrenic burst frequency relative to time controls at 5 and 15 min (both P < 0.05); however, at 30 and 60 min post neural apnea, phrenic burst frequency was no longer significantly increased from time controls (both P > 0.05; Fig. 2C). Similarly, CRSD rats receiving APV prior to neural apnea expressed a significant increase in phrenic burst frequency relative to time controls at 5, 15, and 30 min post neural apnea (all P < 0.05), but not at 60 min (P > 0.05; Fig. 2C). Thus CRSD and HSD rats with spinal NMDAR inhibition express transient burst frequency facilitation following neural apnea.
The Time Domain in which Spinal NMDAR Activation Impairs Long-Lasting iPMF in CRSD Rats
To determine whether spinal NMDAR activation during or following reduced respiratory neural activity is necessary to constrain iPMF, intrathecal APV (20 ng/μl) was delivered shortly (∼4 min) prior to the end of neural apnea and the resumption of respiratory neural activity in CRSD rats (designated as APV-post). A representative compressed phrenic neurogram during baseline, 30 min of reduced respiratory neural activity, and 60 min following the resumption of respiratory neural activity in CRSD rats receiving intrathecal APV-post is shown in Fig. 3A, whereas the average responses are shown in Fig. 3B. Similar to CRSD rats pretreated with APV (Fig. 2B), CRSD rats receiving APV-post expressed a significant increase in phrenic burst amplitude relative to baseline and time controls at all time points post neural apnea (P < 0.05), suggesting that activation of spinal NMDARs during transient iPMF expression (vs. during neural apnea) in CRSD rats constrains long-lasting iPMF.
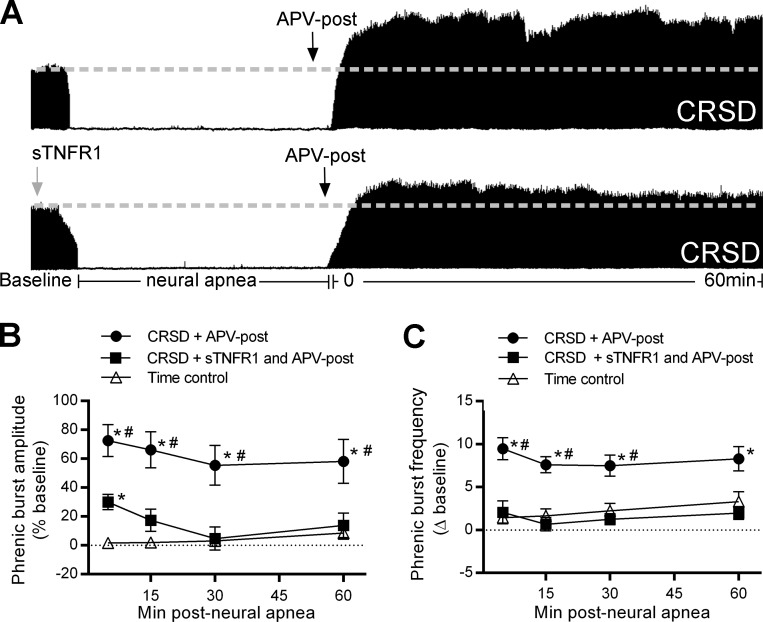
Fig. 3.
Long-lasting iPMF in CRSD rats receiving APV requires spinal tumor necrosis factor-α (TNF-α). A: representative compressed phrenic neurograms during baseline, 30 min of neural apnea, and for 60 min after the resumption of phrenic neural activity in CRSD rats receiving intrathecal APV shortly prior to the resumption of respiratory neural activity (APV-post) or TNF-α signaling inhibitor, soluble TNF-α receptor 1 (sTNFR1) and APV-post. Black arrows denote intrathecal delivery of APV-post; gray arrow indicates intrathecal delivery of sTNFR1. B: average phrenic burst amplitude (% baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in rats receiving APV-post (circles) or pretreatment of sTNFR1 and APV-post (squares). CRSD rats receiving APV-post expressed a significant increase in phrenic burst amplitude relative to time controls at all time points post neural apnea, indicating long-lasting iPMF. By contrast, CRSD rats receiving sTNFR1 and APV-post expressed a small but significant increase in phrenic burst amplitude compared with time controls at 5 min post neural apnea; however, phrenic burst amplitude was not significantly increased from time controls at subsequent time points. Furthermore, phrenic burst amplitude in CRSD rats receiving sTNFR1 and APV-post was significantly lower than in CRSD rats receiving APV-post at all time points post neural apnea, indicating that long-lasting iPMF in CRSD rats with NMDAR inhibition requires spinal TNF-α. C: average phrenic burst frequency (Δ baseline) for 60 min post neural apnea or an equivalent duration in time controls (triangles) in rats receiving APV-post (circles) or sTNFR1 and APV-post (squares). CRSD rats receiving intrathecal APV-post expressed a significant increase in phrenic burst frequency from time controls at all time points. By contrast, phrenic burst frequency in CRSD rats receiving sTNFR1 and APV-post was not significantly increased from time controls at any time point following neural apnea. Values are means ± SE. *Significantly different than time controls; #significantly different than sTNFR1 and APV-post; P < 0.05.
Long-Lasting iPMF in CRSD Rats with NMDAR Inhibition Requires Spinal TNF-α
Recently, we reported that spinal TNF-α is necessary for iPMF in HSD rats (7). In these studies, intrathecal delivery of recombinant soluble TNF receptor 1 (sTNFR1), which scavenges TNF-α and prevents binding to endogenous TNF receptors, prior to neural apnea impaired long-lasting iPMF, but not early iPMF. To determine whether spinal TNF-α is necessary for long-lasting iPMF in CRSD rats with NMDAR inhibition, intrathecal sTNFR1 was delivered 20 min prior to neural apnea in CRSD rats treated with APV-post. CRSD rats receiving intrathecal sTNFR1 and APV-post expressed a small but significant increase in phrenic burst amplitude relative to baseline and an equivalent time point in time controls at 5 min (both P < 0.05; Fig. 3B); however, subsequent time points were not significantly different than baseline or time controls (all P > 0.05). Furthermore, phrenic burst amplitude in CRSD rats receiving intrathecal sTNFR1 and APV-post was significantly lower than CRSD receiving APV-post alone at all time points following the resumption of respiratory neural activity (all P < 0.05; Fig. 3B). Taken together, our results suggest that the long-lasting iPMF revealed in CRSD rats with NMDAR inhibition requires spinal TNF-α, similar to iPMF in HSD (without NMDAR inhibition).
Average phrenic burst frequency (Δ baseline) for 60 min post neural apnea in CRSD rats receiving intrathecal APV-post or intrathecal sTNFR1 and APV-post, or an equivalent duration in time controls is shown in Fig. 3C. CRSD rats receiving intrathecal APV-post expressed a significant increase in phrenic burst frequency relative to time controls at all time points (all P < 0.05). Phrenic burst frequency in CRSD rats receiving intrathecal sTNFR1 and APV-post was not significantly increased relative to time controls at any time point (all P > 0.05). In CRSD rats receiving APV-post, phrenic burst frequency was significantly increased from that of CRSD rats receiving intrathecal sTNFR1 and APV-post at early time points (5, 15, 30 min; all P < 0.05); by 60 min post neural apnea, phrenic burst frequency was no longer significantly different between these two groups (P < 0.05).
Long-Lasting iPMF in CRSD Rats with NMDAR Inhibition Requires Spinal aPKC Activity
Because aPKC isoforms PKCζ, PKCι/λ, or both are necessary during early phases of iPMF to transition to a long-lasting iPMF (48), we tested the hypothesis that spinal aPKC activity is necessary for long-lasting iPMF in CRSD rats with NMDAR inhibition. CRSD rats received intrathecal APV-post (20 ng/μl) and either the cell-permeable pseudosubstrate inhibitory peptide that inhibits all aPKC isoforms (PKCζ-PS) or the scrambled version of the peptide (scrPKCζ-PS) 10 min after phrenic neural activity was restored. Representative compressed phrenic neurograms from CRSD rats during baseline, 30 min of reduced respiratory neural activity, and 60 min following the resumption of respiratory neural activity in rats receiving APV-post and either scrPKCζ-PS or PKCζ-PS are shown in Fig. 4A. Average change in phrenic burst amplitude (% baseline) for 60 min post neural apnea in CRSD rats receiving APV-post and either scrPKCζ-PS or PKCζ-PS, or an equivalent duration in time controls is shown in Fig. 4B. Phrenic burst amplitude was significantly increased compared with baseline and time controls before (5 min; P < 0.05) delivery of scrPKCζ-PS or PKCζ-PS, which was not significantly different between groups (P > 0.05), suggesting a similar magnitude of early iPMF in both groups. CRSD rats receiving APV-post and scrPKCζ-PS expressed a significant increase in phrenic burst amplitude relative to baseline and time controls at all time points post neural apnea (all P < 0.05; Fig. 1B), suggesting that scrPKCζ-PS had no effect on long-lasting iPMF enabled by NMDAR inhibition. By contrast, in CRSD rats treated with APV-post and PKCζ-PS, phrenic burst amplitude was significantly increased from baseline and time controls shortly after PKCζ-PS treatment (15 min; P < 0.05), but then slowly declined, such that at 30 and 60 min following resumption of respiratory neural activity, phrenic burst amplitude was no longer significantly different than that of time controls (both P > 0.05). A small but significant increase in phrenic burst amplitude relative to baseline was apparent at 60 min post neural apnea in CRSD rats treated with APV-post and PKCζ-PS (P < 0.05), but not at 30 min (P > 0.05); however, phrenic burst amplitude at both 30 and 60 min was significantly decreased relative to the 5-min time point post neural apnea (P < 0.05). Phrenic burst amplitude in rats receiving APV-post and PKCζ-PS was significantly lower than in rats treated with APV-post and scrPKCζ-PS at 15, 30, and 60 min post neural apnea (all P < 0.05; Fig. 4B). Collectively, these results suggest that long-lasting iPMF in CRSD rats with NMDAR inhibition requires spinal aPKC signaling.
Average phrenic burst frequency (Δ baseline) for 60 min post neural apnea in CRSD rats receiving APV-post and either scrPKCζ-PS or PKCζ-PS or an equivalent duration in time controls is shown in Fig. 4C. Rats receiving intrathecal APV-post and scrPKCζ-PS following the resumption of neural activity expressed a significant increase in phrenic burst frequency relative to time controls at 5, 15, and 60 min post neural apnea (all P < 0.05). Rats receiving intrathecal APV-post and PKCζ-PS expressed a significant increase from time controls at 5 and 15 min post neural apnea (both P < 0.05); however, phrenic burst frequency in rats receiving scrPKCζ-PS was not significantly different from rats receiving PKCζ-PS at any time point (all P > 0.05).
iPMF Is Associated with an Enhanced Hypercapnic Response
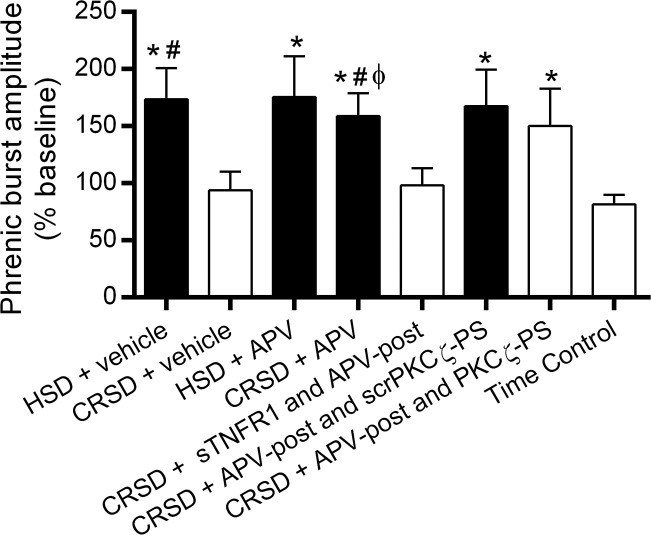
At the end of each experimental protocol, rats were exposed to a brief hypercapnic challenge (ETCO2 ∼98) to determine the peak phrenic response to a respiratory challenge. Average phrenic burst amplitude (% baseline) during hypercapnia is shown in Fig. 5. Shaded bars indicate rat groups in which a significant iPMF was expressed at 60 min. As expected, all rat groups expressed a significant increase in phrenic burst amplitude during hypercapnia (P < 0.05). HSD rats receiving intrathecal vehicle or APV prior to neural apnea expressed a significant increase in the phrenic burst amplitude response to hypercapnia relative to the response in time controls (both P < 0.05), indicating exposure to neural apnea (and iPMF induction) resulted in an enhanced hypercapnic response. By contrast, phrenic burst amplitude during hypercapnia in CRSD rats receiving vehicle prior to neural apnea was not significantly increased from time controls (P > 0.05) and was significantly lower than the response in vehicle-treated HSD rats (P < 0.05), suggesting that neural apnea did not lead to an enhanced hypercapnic response in CRSD rats. However, in CRSD rats receiving intrathecal APV prior to or following neural apnea, a significant increase in the phrenic burst amplitude response during hypercapnia was observed relative to time controls and CRSD rats receiving intrathecal vehicle prior to neural apnea (P < 0.05), indicating that spinal NMDAR inhibition in CRSD rats enabled an increased hypercapnic response following neural apnea. By contrast, in CRSD rats receiving sTNFR1 and APV-post, phrenic burst amplitude during hypercapnia was not significantly different than that of time controls or CRSD rats receiving vehicle (both P > 0.05), indicating that spinal TNF-α inhibition impaired the enhanced hypercapnic response following neural apnea in CRSD rats with NMDAR inhibition. Similar to CRSD rats receiving APV, CRSD rats receiving intrathecal APV and scrPKCζ-PS or PKCζ-PS expressed an enhanced phrenic hypercapnic response (both P < 0.05), indicating that spinal aPKC inhibition does not impair the enhanced phrenic hypercapnic response following neural apnea enabled by NMDAR inhibition. Thus with one exception (discussed below), these data indicate that rats expressing a long-lasting iPMF, but not a transient iPMF, exhibit an enhanced phrenic burst amplitude response to hypercapnia.
Fig. 5.
Long-lasting iPMF is associated with an enhanced phrenic burst amplitude response to hypercapnia. Average change in phrenic burst amplitude during hypercapnia (% baseline) following neural apnea or an equivalent duration in time controls. Shaded bars indicate significant iPMF at 60 min. As expected, all rat groups exhibited a significant increase in phrenic burst amplitude in hypercapnia. HSD rats receiving intrathecal vehicle or APV prior to neural apnea expressed a significantly enhanced phrenic burst amplitude response to hypercapnia compared with time controls exposed to hypercapnia. By contrast, phrenic burst amplitude during hypercapnia in CRSD rats receiving vehicle was not significantly increased compared with the hypercapnic response in time controls. However, CRSD rats receiving intrathecal APV or intrathecal APV and scrPKCζ-PS expressed a significantly increased phrenic burst amplitude response during hypercapnia relative to the response in time controls and CRSD rats receiving vehicle, suggesting that an enhanced hypercapnic response following reduced respiratory neural activity is enabled by NMDAR inhibition. Intrathecal sTNFR1 impaired the enhanced phrenic hypercapnic response in CRSD rats with NMDAR inhibition, suggesting that spinal TNF-α is necessary for the enhanced phrenic response to hypercapnia. By contrast, CRSD rats receiving APV-post and PKCζ-PS continued to express an increased phrenic hypercapnic response, suggesting that aPKC activity is not necessary for the enhanced phrenic response to hypercapnia following reduced respiratory neural activity (see discussion). Values are means ± SE. *Significantly different than time controls; #significantly different than CRSD + vehicle; ϕsignificantly different than CRSD + sTNFR1 and APV-post; P < 0.05.
DISCUSSION
Here we demonstrate that NMDAR activation during transient iPMF constrains long-lasting iPMF in some rat substrains. Specifically, we show that unlike HSD rats, CRSD rats express only an attenuated, transient iPMF that returns to baseline within 30 min after the resumption of respiratory neural activity. Furthermore, CRSD rats also do not express an enhanced phrenic hypercapnic response 60 min post neural apnea, which is known to accompany long-lasting iPMF expression (2, 46). Robust, long-lasting iPMF is revealed in CRSD by inhibiting spinal NMDARs shortly (∼4 min) prior to resumption of respiratory neural activity, and similar to HSD rats, this iPMF is associated with a proportional increase in the phrenic hypercapnic response. Furthermore, we demonstrate that long-lasting iPMF enabled by NMDAR inhibition requires spinal TNF-α and aPKC activity, suggesting that similar mechanisms give rise to long-lasting iPMF in CRSD with spinal NMDAR inhibition as iPMF in HSD rats (without NMDAR inhibition). Thus genetic or epigenetic variation in NMDARs in some rat substrains may restrain long-lasting iPMF by impairing TNF-α-induced activation of aPKC. Unraveling the constraints and regulatory mechanisms underlying plasticity induced by reduced respiratory neural activity may lead to novel insights regarding endogenous mechanisms that compensate for changes in motor neuron activity, such as those occurring during certain ventilatory control disorders, and may provide clinicians with novel therapeutic targets to treat these disorders when endogenous compensatory mechanisms fail.
Burst Amplitude Facilitation Is Differentially Regulated Among Rat Substrains
Although descendants from the same original colony, Sprague-Dawley rats from Charles River and Harlan Laboratories have been genetically isolated from one another for >30 yr, and several studies suggest that this separation has created genetically or epigenetically distinct groups of rats (9, 10, 15, 21, 40, 42, 53, 59). For example, cervical spinal segments in CRSD rats are more densely innervated by noradrenergic projections than in HSD rats (9, 10). Furthermore, HSD rats but not CRSD rats express hypoglossal long-term facilitation, a distinct form of respiratory plasticity induced by acute intermittent hypoxia (22). HSD rats exhibit greater functional recovery from spinal cord injury than CRSD rats (30), and HSD rats are more susceptible than CRSD rats to epileptogenesis (32). The genetic/epigenetic basis for these differences remains unknown, but it underscores the concept that different groups of genetically isolated rats may have completely different responses to a given perturbation. As such, understanding the diversity of responses in model systems is key to understanding the range of the diverse responses that might be obtained in a genetically heterogeneous human population.
We hypothesized that differential expression of iPMF between HSD and CRSD rats was due to regulatory mechanisms operating at the level of the phrenic motor pool. This hypothesis was based on several observations: 1) a reduction in spinal synaptic inputs to phrenic motor neurons without a change in brainstem respiratory neural activity is sufficient to give rise to iPMF (46); 2) pharmacological inhibitors of the cellular pathways leading to iPMF applied to spinal segments encompassing the phrenic motor nucleus blocks iPMF (7, 46, 48); and 3) distinct groups of respiratory motor neurons differentially express inactivity-induced plasticity (2, 5, 47). Consistent with this hypothesis, NMDAR activation regulates the magnitude and duration of iPMF in CRSD rats via mechanisms operating within or near the phrenic motor pool, because intrathecal application of an NMDAR antagonist revealed a robust and long-lasting iPMF. We speculate that regulation of inactivity-induced plasticity at the level of the motor pool may confer advantages in enabling stimulus-specific responses tailored to the function of the motor pool (2). Somewhat surprisingly, HSD rats did not exhibit a larger iPMF with NMDAR inhibition, suggesting that the same NMDAR constraints are not operative in HSD rats. Although this could be due to differences in expression of NMDARs (33, 37), one caveat that must be considered is that HSD and CRSD rats may have a differential response to, or a differential wear-off time of APV; nevertheless, our conclusion that NMDARs constrain iPMF in CRSD rats remains true even if a higher dose of APV results in a more robust iPMF in HSD rats.
NMDAR Activation Constrains Long-Lasting iPMF
iPMF consists of at least two phases: 1) an early, labile phase that requires TNF-α-induced aPKC activation to stabilize into 2) a long-lasting form of plasticity (7, 48). Indeed, inhibition of spinal TNF-α or aPKC activity in HSD rats results in an attenuated, transient iPMF that is phenotypically similar to the transient iPMF apparent in CRSD rats. Our working model suggests that reduced respiratory-related synaptic inputs to phrenic motor neurons results in local release of TNF-α, which activates TNF receptors on (or near) phrenic motor neurons to give rise to iPMF (7). Although TNF-α is typically thought of as a proinflammatory cytokine, it is also an important neuromodulator within the central nervous system and is necessary for increases in synaptic strength following reduced neural activity in cortical and hippocampal neurons (6, 27, 45, 54). Evidence suggests that in response to reduced phrenic neural activity, TNF receptor activation increases the activity of aPKC isoforms PKCζ and PKCι/λ (referred to as PKCζ/ι for brevity) and induces the formation of a signaling complex between PKCζ/ι and the scaffolding protein PKCζ interacting protein (p62/ZIP) (48) to stabilize a transient, labile form of plasticity to a long-lasting iPMF (48). Mechanisms that give rise to transient iPMF are not understood, but do not require spinal TNF-α or aPKC (7, 48). Cellular mechanisms downstream of TNF-α and the PKCζ/ι-p62/ZIP signaling complex are currently unknown, but may involve changes in membrane bound chloride cotransporters KCC2 and NKCC1 (20), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (16), γ-aminobutyric acid receptors (44), or a combination of these.
It is noteworthy that the specific time window that NMDAR activation inhibits long-lasting iPMF in CRSD rats is during transient iPMF expression, which also corresponds to the time in which the PKCζ/ι-p62/ZIP signaling complex converts transient iPMF into long-lasting iPMF (48). Indeed, long-lasting iPMF enabled by NMDAR inhibition in CRSD rats requires spinal TNF-α and aPKC activity, suggesting that NMDAR activation constrains TNF-α- and aPKC-dependent conversion of transient iPMF into long-lasting iPMF in CRSD rats. Mechanisms whereby NMDAR activation constrains iPMF are unknown. In the hippocampus, activation of NMDARs by miniature synaptic events (mEPSCs or minis) during global reductions in neural activity (i.e., TTX) inhibits dendritic protein synthesis and delays the expression of inactivity-induced plasticity, suggesting that tonic activation of NMDARs by minis during activity deprivation stabilizes synapses (50, 51). Indeed, when action potential blockade is coupled with local NMDAR blockade in hippocampal neurons, protein synthesis constraints are relieved and synaptic scaling occurs much faster (i.e., 4 h vs. >12 h) (50). Thus it is possible that NMDAR activation in CRSD rats impairs the synthesis of necessary proteins required to stabilize iPMF. Alternatively, differential regulation of iPMF by NMDARs may reflect substrain-specific differences in NMDAR expression, specifically in the extrasynaptic vs. synaptic pool. For example, increased expression or activation of extrasynaptic NMDARs during long-term potentiation (LTP)-inducing stimuli can override and inhibit LTP (34). Mechanisms underlying LTP inhibition by extrasynaptic NMDAR are not well understood, but may involve an inhibition of mitogen-activated protein kinase activation, cAMP response element–binding protein phosphorylation, and brain-derived neurotrophic factor expression (23, 24, 26, 34), leading to subsequent endocytosis of AMPARs (29). Because evidence suggests that activity deprivation increases extrasynaptic NMDARs in the cortex (58), differential expression of extrasynaptic NMDARs in phrenic motor neurons following prolonged activity deprivation in CRSD vs. HSD rats requires further investigation.
Although CRSD rats lack long-lasting iPMF after prolonged reductions in respiratory neural activity, it is possible that other stimulus paradigms may reveal long-lasting iPMF. For example, intermittent reductions in respiratory neural activity are more efficient at eliciting long-lasting iPMF than sustained patterns in HSD rats (2); thus future studies are necessary to address whether long-lasting iPMF may be revealed following brief, intermittent neural apnea in CRSD rats (without the need for NMDAR inhibition).
Long-Lasting iPMF Is Associated with an Increased Hypercapnic Response
In addition to maintaining eupnic breathing, the respiratory control system must respond to respiratory challenges (e.g., hypercapnia and hypoxia) and participate in nonventilatory behaviors (e.g., coughing). Because these tasks require larger diaphragmatic forces, the respiratory control system recruits specific motor units tailored to each function to mediate these behaviors (22). As such, during resting/eupnic breathing, the respiratory system operates at a fraction of its maximal dynamic range (36). A key question is how does the respiratory control system respond to these challenges, despite the already enhanced phrenic motor output apparent during iPMF expression? Similar to HSD rats (2, 46), CRSD rats expressing long-lasting, but not transient iPMF, had a significantly larger phrenic burst amplitude response to hypercapnia, with one exception: CRSD rats receiving APV-post and PKCζ-PS expressed an increased phrenic hypercapnic response, but did not express significant long-lasting iPMF. However, in these rats, a small but nonsignificant increase in phrenic burst amplitude was observed at 60 min; thus the apparent enhanced phrenic hypercapnic response may be the result of residual mechanisms that had not yet been completely abolished. Indeed, in other reports (46), an enhanced hypercapnic phrenic amplitude response after reduced respiratory neural activity is blocked by aPKC inhibition (in HSD rats).
Although the ventilatory response to hypercapnia is initiated by central and peripheral chemoreceptors (31), we suggest that the enhanced hypercapnic response is due to spinal mechanisms in or near the phrenic motor pool. Consistent with this hypothesis, spinal APV application in CRSD rats enabled long-lasting iPMF and a subsequently enhanced hypercapnic response following neural apnea. Furthermore, iPMF elicited following unilateral reduction in phrenic spinal synaptic inputs with no apparent change in brainstem neural activity is associated with a proportional increase in the ipsilateral phrenic burst amplitude response during hypercapnia (46). The specific mechanisms whereby long-lasting iPMF expression leads to an enhanced phrenic hypercapnic response are not yet known; however, one attractive possibility is that mechanisms giving rise to iPMF result in a global shift in phrenic motor neuron excitability (20, 57), enabling phrenic motor output to maintain a dynamic response in the presence of plasticity.
Burst Frequency Facilitation
With the exception of three experimental groups, the general response to central neural apnea in both substrains is transient burst frequency facilitation. Similar to burst amplitude, CRSD rats have an attenuated increase in phrenic burst frequency following central neural apnea compared with HSD rats. Although our data appear to suggest that intrathecal application of APV-post in CRSD rats gives rise to sustained burst frequency facilitation and intrathecal sTNFR1 and PKCζ-PS block burst frequency facilitation, we refrain from making these conclusions because the magnitude of burst frequency facilitation observed following central neural apnea is small (relative to amplitude changes) and highly variable among rat groups, similar to other reports (3, 4). Future studies are necessary to fully characterize phrenic burst frequency facilitation following neural apnea in these rat substrains.
Significance of Long-Lasting iPMF
We have only recently begun to appreciate that the respiratory control network monitors activity within the phrenic motor pool, and that local (phrenic) mechanisms respond vigorously and rapidly to reductions in neural activity—even without changes in blood gases (35, 46). Importantly, complete absence of phrenic neural activity is not necessary to elicit iPMF (46); thus iPMF-like mechanisms may adjust phrenic motor output during physiologically relevant situations in which synaptic inputs to phrenic motor neurons are merely reduced—not eliminated. In this sense, iPMF may be one component in a continuum of homeostatic plasticity mechanisms that enable phrenic neurons to adapt to prolonged changes in neuronal excitability and/or synaptic inputs that may occur throughout life, thereby preventing catastrophic decreases in respiratory motor output. It is possible that the apparent lack of long-lasting iPMF in CRSD rats may reflect a hyperactivity-induced reversal of iPMF. In this sense, the initial facilitation in phrenic burst amplitude observed after neural activity is restored may activate an opposing, NMDAR-dependent countermechanism that interferes with TNF-α-induced activation of aPKCs to offset the relative hyperexcitability observed after resumption of respiratory neural activity and restore baseline phrenic burst amplitude, similar to homeostatic downscaling (41). As such, iPMF and NMDA-dependent inhibition of iPMF may fine-tune and balance the need to preserve adequate, but not excessive respiratory motor output. Consistent with this hypothesis, NMDAR activation downregulates key molecules underlying iPMF, such as p62/ZIP (55). The functional significance of preventing activity from returning to baseline in HSD remains unknown, but may reflect a differential time course for hyperactivity vs. inactivity in some rat substrains.
In addition to providing insight into the basic physiology of the phrenic circuit, differential expression of iPMF may have important clinical implications. Indeed, many individuals experience transient, recurrent, or prolonged periods of reduced respiratory neural activity in both physiological and pathophysiological situations (47). However, little is known about the impact of inactivity-induced plasticity in these situations. Clearly, 30 min of central neural apnea would rarely be encountered in humans, and may be experienced only during artificial situations such as artificial ventilation during normal surgeries or prolonged ventilatory support. We recently demonstrated that iPMF is also elicited by brief, intermittent episodes of neural apnea (1); as such, iPMF-like mechanisms may stabilize breathing and prevent the reoccurrence of central neural apneas, such as those encountered by normal individuals during sleep, or during sleep at altitude (42).
Prolonged decreases in respiratory neural activity may also be experienced in certain nonphysiological circumstances (47), such as following cervical spinal injury. Strikingly, approximately 50–70% of patients with high cervical spinal cord injury (SCI) exhibit some degree of spontaneous recovery of diaphragm function in the days to weeks following SCI (8, 13, 25, 43, 56). However, mechanisms giving rise to this recovery are not yet understood. iPMF-like mechanisms may play a key role in some aspects of this spontaneous recovery because cervical spinal injury is associated with increased aPKC activity (31) as well as TNF-α (60), and there is some evidence suggesting that TNF-α promotes motor recovery after central nervous system injury (28, 38, 49).
One interesting question is why some patients with SCI recover breathing function, whereas others do not. Although the answer is likely multifactorial, it is possible that genetic/epigenetic differences in inactivity-induced plasticity may play a role. Interestingly, NMDAR inhibition enhances recovery of hemidiaphragm motor activity (1) and locomotion in rodent models of SCI (11, 12, 17, 19) in a dose- and time-dependent manner (18). Furthermore, there is some evidence suggesting that NMDAR antagonists enhance motor recovery in humans with incomplete cervical SCI (14, 52). Although NMDAR inhibition had been hypothesized to protect the injured spinal cord from secondary damage due to glutamate-induced excitotoxicity, NMDAR inhibition may also promote spontaneous functional recovery in hours (11) to days (1) postinjury, long after the surge of injury-induced glutamate release is over (39). Thus it is possible that NMDAR inhibition may promote greater spontaneous recovery after SCI by removing a constraint to an iPMF-like mechanism, at least in subpopulations of humans with SCI. This intriguing possibility requires further investigation. Understanding differential iPMF regulation among individuals may provide key insights concerning the frustratingly variable spontaneous recovery of respiratory function frequently reported in the genetically diverse (vs. laboratory rat) human population with SCI.
GRANTS
Support for this study was provide by National Heart, Lung and Blood Institute Grant HL-105511.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.A.S. and T.L.B.-H. conception and design of research; K.A.S. performed experiments; K.A.S. and T.L.B.-H. analyzed data; K.A.S. and T.L.B.-H. interpreted results of experiments; K.A.S. prepared figures; K.A.S. drafted manuscript; K.A.S. and T.L.B.-H. edited and revised manuscript; K.A.S. and T.L.B.-H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. N. Baertsch for comments on the manuscript.
REFERENCES
- 1.Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med 30: 346–354, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baertsch NA, Baker-Herman TL. Inactivity-induced phrenic and hypoglossal motor facilitation are differentially expressed following intermittent vs. sustained neural apnea. J Appl Physiol 114: 1388–1395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 179: 48–56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science 295: 2282–2285, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Broytman O, Baertsch NA, Baker-Herman TL. Spinal TNF is necessary for inactivity-induced phrenic motor facilitation. J Physiol 591: 5585–5598, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Call MS, Kutcher ME, Izenberg RA, Singh T, Cohen MJ. Spinal cord injury: outcomes of ventilatory weaning and extubation. J Trauma 71: 1673–1679, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Clark FM, Proudfit HK. Anatomical evidence for genetic differences in the innervation of the rat spinal cord by noradrenergic locus coeruleus neurons. Brain Res 591: 44–53, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Clark FM, Yeomans DC, Proudfit HK. The noradrenergic innervation of the spinal cord: differences between two substrains of Sprague-Dawley rats determined using retrograde tracers combined with immunocytochemistry. Neurosci Lett 125: 155–158, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Esposito E, Paterniti I, Mazzon E, Genovese T, Galuppo M, Meli R, Bramanti P, Cuzzocrea S. MK801 attenuates secondary injury in a mouse experimental compression model of spinal cord trauma. BMC Neurosci 12: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Faden AI, Lemke M, Simon RP, Noble LJ. N-methyl-D-aspartate antagonist MK801 improves outcome following traumatic spinal cord injury in rats: behavioral, anatomic, and neurochemical studies. J Neurotrauma 5: 33–45, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45: 190–205, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fehlings MG, Baptiste DC. Current status of clinical trials for acute spinal cord injury. Injury 36, Suppl 2: B113–B122, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics 4: 175–181, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Bereguiain MA, Gonzalez-Islas C, Lindsly C, Butler E, Hill AW, Wenner P. In vivo synaptic scaling is mediated by GluA2-lacking AMPA receptors in the embryonic spinal cord. J Neurosci 33: 6791–6799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaviria M, Privat A, d'Arbigny P, Kamenka J, Haton H, Ohanna F. Neuroprotective effects of a novel NMDA antagonist, gacyclidine, after experimental contusive spinal cord injury in adult rats. Brain Res 874: 200–209, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Gaviria M, Privat A, d'Arbigny P, Kamenka JM, Haton H, Ohanna F. Neuroprotective effects of gacyclidine after experimental photochemical spinal cord lesion in adult rats: dose-window and time-window effects. J Neurotrauma 17: 19–30, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Pinilla F, Tram H, Cotman CW, Nieto-Sampedro M. Neuroprotective effect of MK-801 and U-50488H after contusive spinal cord injury. Exp Neurol 104: 118–124, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Islas C, Chub N, Garcia-Bereguiain MA, Wenner P. GABAergic synaptic scaling in embryonic motoneurons is mediated by a shift in the chloride reversal potential. J Neurosci 30: 13016–13020, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham BA, Hammond DL, Proudfit HK. Differences in the antinociceptive effects of alpha-2 adrenoceptor agonists in two substrains of Sprague-Dawley rats. J Pharmacol Exp Ther 283: 511–519, 1997 [PubMed] [Google Scholar]
- 22.Gransee HM, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Compr Physiol 2: 1441–1462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11: 682–696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hoh DJ, Mercier LM, Hussey SP, Lane MA. Respiration following spinal cord injury: evidence for human neuroplasticity. Respir Physiol Neurobiol 189: 450–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol 572: 789–798, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58: 673–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G, Xu XM, Hsu CY. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci 21: 6617–6625, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 46: 745–760, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kjell J, Sandor K, Josephson A, Svensson CI, Abrams MB. Rat substrains differ in the magnitude of spontaneous locomotor recovery and in the development of mechanical hypersensitivity after experimental spinal cord injury. J Neurotrauma 30: 1805–1811, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahiri S, Forster RE., 2nd CO2/H(+) sensing: peripheral and central chemoreception. Int J Biochem Cell Biol 35: 1413–1435, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Langer M, Brandt C, Loscher W. Marked strain and substrain differences in induction of status epilepticus and subsequent development of neurodegeneration, epilepsy, and behavioral alterations in rats. Epilepsy Res 96: 207–224, 2011. [Erratum. Epilepsy Res 99 (1–2): 191, 2012.] [DOI] [PubMed] [Google Scholar]
- 33.Lei Y, Yaroslavsky I, Tejani-Butt SM. Strain differences in the distribution of N-methyl-D-aspartate and gamma (gamma)-aminobutyric acid-A receptors in rat brain. Life Sci 85: 794–799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci 31: 6627–6638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahamed S, Strey KA, Mitchell GS, Baker-Herman TL. Reduced respiratory neural activity elicits phrenic motor facilitation. Respir Physiol Neurobiol 175: 303–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin S, Lyupina Y, Crespo JA, Gonzalez B, Garcia-Lecumberri C, Ambrosio E. Genetic differences in NMDA and D1 receptor levels, and operant responding for food and morphine in Lewis and Fischer 344 rats. Brain Res 973: 205–213, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T. TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res 1290: 102–110, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Panter SS, Yum SW, Faden AI. Alteration in extracellular amino acids after traumatic spinal cord injury. Ann Neurol 27: 96–99, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Pecoraro N, Ginsberg AB, Warne JP, Gomez F, la Fleur SE, Dallman MF. Diverse basal and stress-related phenotypes of Sprague Dawley rats from three vendors. Physiol Behav 89: 598–610, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Siddoway B, Hou H, Xia H. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology 78: 38–44, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sluka KA, Westlund KN. Spinal projections of the locus coeruleus and the nucleus subcoeruleus in the Harlan and the Sasco Sprague-Dawley rat. Brain Res 579: 67–73, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Steeves JD, Kramer JK, Fawcett JW, Cragg J, Lammertse DP, Blight AR, Marino RJ, Ditunno JF, Jr, Coleman WP, Geisler FH, Guest J, Jones L, Burns S, Schubert M, van Hedel HJ, Curt A; EMSCI Study Group. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 49: 257–265, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci 25: 3219–3228, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature 440: 1054–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Streeter KA, Baker-Herman TL. Decreased spinal synaptic inputs to phrenic motor neurons elicit localized inactivity-induced phrenic motor facilitation. Exp Neurol 256: 46–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strey KA, Baertsch NA, Baker-Herman TL. Inactivity-induced respiratory plasticity: protecting the drive to breathe in disorders that reduce respiratory neural activity. Respir Physiol Neurobiol 189: 384–394, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strey KA, Nichols NL, Baertsch NA, Broytman O, Baker-Herman TL. Spinal atypical protein kinase C activity is necessary to stabilize inactivity-induced phrenic motor facilitation. J Neurosci 32: 16510–16520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci 19: 6248–6256, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science 304: 1979–1983, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Tadie dAP M, Mathe JF, Loubert G, Saint Marc C, Menthonnex Ph, Christen Y, Carli P. Acute spinal cord injury: early care, and treatment in a multicenter study with gacyclidine (abstract 1090). Soc Neurosci Abstract, 1999 [Google Scholar]
- 53.Turnbull AV, Rivier CL. Sprague-Dawley rats obtained from different vendors exhibit distinct adrenocorticotropin responses to inflammatory stimuli. Neuroendocrinology 70: 186–195, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Turrigiano GG. More than a sidekick: glia and homeostatic synaptic plasticity. Trends Mol Med 12: 458–460, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Wang YR, Qin S, Han R, Wu JC, Liang ZQ, Qin ZH, Wang Y. Cathepsin L plays a role in quinolinic acid-induced NF-Kb activation and excitotoxicity in rat striatal neurons. PloS One 8: e75702, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wicks AB, Menter RR. Long-term outlook in quadriplegic patients with initial ventilator dependency. Chest 90: 406–410, 1986 [DOI] [PubMed] [Google Scholar]
- 57.Wilhelm JC, Rich MM, Wenner P. Compensatory changes in cellular excitability, not synaptic scaling, contribute to homeostatic recovery of embryonic network activity. Proc Natl Acad Sci USA 106: 6760–6765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci 25: 11684–11692, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon YW, Lee DH, Lee BH, Chung K, Chung JM. Different strains and substrains of rats show different levels of neuropathic pain behaviors. Exp Brain Res 129: 167–171, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, Kim YC, Shin ML, Oh YJ, Han CT, Markelonis GJ, Oh TH. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma 20: 207–219, 2003 [DOI] [PubMed] [Google Scholar]