Abstract
A reduction or loss of plasma membrane aquaporin 2 (AQP2) in kidney principal cells due to defective vasopressin (VP) signaling through the VP receptor causes excessive urine production, i.e., diabetes insipidus. The amount of AQP2 on the plasma membrane is regulated by a balance of exocytosis and endocytosis and is the rate limiting step for water reabsorption in the collecting duct. We describe here a systematic approach using high-throughput screening (HTS) followed by in vitro and in vivo assays to discover novel compounds that enhance vasopressin-independent AQP2 membrane expression. We performed initial chemical library screening with a high-throughput exocytosis fluorescence assay using LLC-PK1 cells expressing soluble secreted yellow fluorescent protein and AQP2. Thirty-six candidate exocytosis enhancers were identified. These compounds were then rescreened in AQP2-expressing cells to determine their ability to increase AQP2 membrane accumulation. Effective drugs were then applied to kidney slices in vitro. Three compounds, AG-490, β-lapachone, and HA14-1 increased AQP2 membrane accumulation in LLC-PK1 cells, and both AG-490 and β-lapachone were also effective in MDCK cells and principal cells in rat kidney slices. Finally, one compound, AG-490 (an EGF receptor and JAK-2 kinase inhibitor), decreased urine volume and increased urine osmolality significantly in the first 2–4 h after a single injection into VP-deficient Brattleboro rats. In conclusion, we have developed a systematic procedure for identifying new compounds that modulate AQP2 trafficking using initial HTS followed by in vitro assays in cells and kidney slices, and concluding with in vivo testing in an animal model.
Keywords: cell signaling, diabetes insipidus, drug screening, vasopressin, water channels, EGFR and JAK-2 inhibitor
diabetes insipidus (DI) is characterized by a reduction in urine concentrating ability; the main symptoms of DI are polyuria with associated polydipsia, dehydration, and hypernatremia. Both central DI [due to loss of vasopressin (VP)] or nephrogenic DI (NDI) can be hereditary or acquired. In adults, acquired NDI is more common and it is most often due to chronic lithium use or hypercalcemia. In patients with hereditary NDI, symptoms usually appear soon after birth; the most severe complications are renal insufficiency and mental retardation. Hereditary NDI is caused by mutations in two genes, the V2 vasopressin receptor (V2R) and the aquaporin 2 (AQP2) water channel (11, 39). Ninety percent of hereditary NDI is caused by inactivating mutations in V2R, and the other 10% results from AQP2 mutations (13, 34). While central DI is usually manageable by administration of desmopressin (modified vasopressin) by nasal spray, for example, treating NDI patients is more problematic because of their resistance to vasopressin. They are frequently submitted to salt restriction, thiazide diuretics, and prostaglandin inhibitors (12, 22), but these therapies have only limited benefit.
VP-induced trafficking and membrane accumulation of the water channel AQP2 plays a critical role in maintaining water balance. Our understanding of this process at the cellular level has increased considerably over the past few years and has informed directed attempts to bypass defective VP signaling to increase urinary concentrating ability in DI. We now know, for example, that AQP2 recycles constitutively between cytosolic vesicles and the apical membrane, and the amount of AQP2 on the apical membrane at any given time is determined by a balance of exocytosis and endocytosis (7, 18, 24, 25, 27). We also know that phosphorylation of AQP2 is required to maintain this channel at the cell surface following its exocytotic insertion. AQP2 membrane accumulation then allows water to permeate through the plasma membrane, resulting in urine concentration.
Recent studies from our group and others have suggested that bypassing the defective V2R (which accounts for 90% of hereditary NDI cases) to activate AQP2 membrane accumulation in kidney principal cells is a feasible and promising therapeutic strategy. Some compounds, for example a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil) (5), a phosphodiesterase type 4 (PDE4) inhibitor (rolipram) (35), statins (simvastatin, fluvastatin, and lovastatin) (21, 32), calcitonin (4), and selective agonists for E-prostanoid receptors (20, 30), successfully increased AQP2 plasma membrane accumulation. However, it remains uncertain whether these compounds can be translated into humans as a therapeutic strategy. For example, use of rolipram did not help patients with hereditary X-linked NDI (1). Therefore, additional approaches and new drugs are still needed to bypass the defective V2R/VP signaling pathway in both acquired and hereditary DI. The compounds discussed above were identified rationally on the basis of our understanding of known AQP2 signaling/trafficking pathways. However, this approach is limited and has tended to focus on mechanisms related to the cAMP signaling pathway. In contrast, nonbiased screening of chemical libraries using appropriate assays may provide unexpected novel therapeutic strategies.
In the present report we have, therefore, established and validated a nonbiased high-throughput chemical screening method which, when coupled to additional downstream assays, was designed to identify compounds that might modulate principal cell water permeability by affecting AQP2 membrane accumulation. Initial chemical screening was performed by adapting our fluorescence-based exocytosis assay (29) to a high-throughput format. The purpose of this screening was to quickly identify compounds that modulate exocytosis in renal epithelial cells. From the initial candidates we next identified those compounds that regulate specifically AQP2 trafficking first in cultured renal epithelial cells, then in kidney slices in vitro, and finally using in vivo whole animal studies.1
MATERIALS AND METHODS
High-throughput screening assay.
This screening assay was developed from the exocytosis assay that was described in detail in a previous study (29). LLC-PK1 cells expressing both AQP2 and soluble secreted yellow fluorescent protein (ssYFP) were seeded at a density of 2 × 103 cells/well on 384-well microplates using a Multidrop Combi reagent dispenser (Thermo Scientific, Waltham, MA) and then cultured until confluence (3 days). Cells were washed with Hank's balanced salt solution HBSS (containing 20 mM HEPES and 2 g/l glucose) using a BioTek ELx 405 microplate washer (BioTek US, Winooski, VT) and then incubated in HBSS for 1 h before chemical delivery. Compounds were from the Prestwick Chemical Library of agency-approved (including the FDA), off-patent drugs (Prestwick Chemical, Illkirch, France), Spectrum Collection (Microsource Discovery Systems, Gaylordsville, CT), and the supplemented ICCB (Harvard Institute of Chemistry and Cell Biology) known bioactives collection libraries. Compounds were delivered to each well using the 100 nl 384-pin head attachment of a CyBio-Well Vario robotic pipettor (CyBio US, Woburn, MA). After pinning, the cells were incubated for 30 min at 37°C, then the extracellular medium was removed and delivered to 384 black low-volume nonbinding surface microplates (Corning, Tewksbury, MA). Plates were spun at 100 g for 1 min to collect medium at the bottom of the plates, and fluorescence was read on a SafireII multiwavelength fluorimeter (Tecan Systems, San Jose, CA). Concomitantly, the remaining cells were lysed by lysis buffer (20 mM Tris·HCl, 2 mM EGTA, 2 mM EDTA, 30 mM NaF, 30 mM Na4O7P2, 2 mM Na2VO4, 1% Triton X-100, 0.03% SDS, pH 7.4, Roche complete-mini cocktail) and the well fluorescence was measured. The exocytosis (Ex) score was defined as the secreted ssYFP fluorescence divided by the sum of the secreted ssYFP fluorescence and the ssYFP fluorescence remaining in the lysates. Ex scores were then normalized by the DMSO (or HBSS for those compounds dissolved in aqueous solutions) to give the normalized exocytosis score (NEx) score. The NEx scores of the triplicate wells were averaged. Z-scores are defined as follows: Z = [(NEx − μo)/σo], where μo and σo are the DMSO NEx population mean and standard deviation, respectively. Positive hits listed in Table 1 were chemicals for which the absolute value of the Z-score was greater than 2.5.
Table 1.
Candidate AQP2 exocytosis enhancer compounds
| Compound Name | Final Concentration, μM | NEx ± SD | Description | IF Result |
|---|---|---|---|---|
| Neurotransmitter modulators | ||||
| 1) Salsoline | 50 | 1.37 ± 0.09 | Dopamine-related compound | NA |
| 2) S(-)-lisuride | 10 | 1.45 ± 0.03 | MAO-A inhibitor, serotonin | NA |
| 3) Ergonovine | 10 | 1.45 ± 0.03 | Dopamine/serotonin agonist | NA |
| 4) Benserazide hydrochloride | 7.77 | 1.54 ± 0.01 | AAAD decarboxylase inhibitor, antiparkinsonian | NA |
| 5) Agroclavine | 10 | 1.59 ± 0.02 | D2 agonist, D1 antagonist, serotonin agonist | NA |
| 6) Fluphenazine | 10 | 1.64 ± 0.03 | Adrenergic or serotonergic receptor | CT |
| 7) Trifluoperazine | 10 | 1.65 ± 0.81 | Calmodulin inhibitor, antiadrenergic/dopaminergic | AK |
| 8) Menadione | 50 | 1.78 ± 0.08 | DOPA decarboxylase inhibitor | AF |
| 9) Dobutamine hydrochloride | 50 | 1.85 ± 0.04 | A-β-2 agonist | NC |
| Lipid receptor ligands | ||||
| 10) U-46619 | 5 | 1.41 ± 0.18 | Thromboxane TP receptor agonist | NC |
| 11) Prostaglandin E2 | 5 | 1.65 ± 1.22 | Prostaglandin EP receptor agonist | AK |
| Kinase inhibitors | ||||
| 12) Emodin | 50 | 1.46 ± 0.12 | Tyrosine kinase inhibitor, CK2 inhibitor, natural | AF |
| 13) AG-490 | 85 | 1.46 ± 0.06 | JAK-2 kinase and EGFR inhibitor | PH |
| 14) Y-27632 | 100 | 1.46 ± 0.20 | ROCK-1 kinase inhibitor | AK |
| 15) Tyrphostin AG-825 | 63 | 1.55 ± 0.26 | HER-1,2 tyrosine kinase inhibitor | NC |
| 16) RO31-8220 | 54.5 | 1.66 ± 0.11 | PKC inhibitor | AF |
| 17) AG-213 | 115 | 1.97 ± 0.01 | EGFR tyrosine kinase inhibitor | NC |
| 18) GF-109203X | 60.5 | 2.96 ± 0.12 | PKC inhibitor | AF |
| Other inhibitors | ||||
| 19) β-Lapachone | 105 | 1.46 ± 0.03 | Topoisomerase I inhibitor, apoptosis inducer | PH |
| 20) NSC-95397 | 80.5 | 1.75 ± 0.28 | CDC25 phosphatase inhibitor | CT |
| 21) Dipyridamole | 3.96 | 2.09 ± 0.09 | Phosphodiesterase inhibitor | AK |
| Cytotoxic/apoptosis-inducing compounds | ||||
| 22) 1,4-Naphthoquinone | 50 | 1.36 ± 0.06 | Antibacterial, anticancer | NA |
| 23) Dalbergione | 50 | 1.45 ± 0.09 | Severe skin irritant, anti-cancer | NA |
| 24) Quinalizarin | 50 | 1.49 ± 0.04 | Cytotoxic dye, inhibitor of CK2, induces apoptosis | NC |
| 25) Shikonin | 86.5 | 1.55 ± 0.07 | Apoptosis inducer, p53-dependent | AF |
| 26) Pyrromycin | 50 | 1.58 ± 0.03 | Anthracycline antibiotic, inhibits RNA synthesis | NA |
| 27) Aklavine hydrochloride | 50 | 1.60 ± 0.07 | Cytotoxic activityagainst multidrugresistant cells | NA |
| 28) Antimycin a | 50 | 1.73 ± 0.05 | Induces apoptosis, inhibits mitochondria function | NC |
| 29) HA14-1 | 61 | 1.76 ± 0.06 | Bcl-2 ligand induces apoptosis | PH |
| 30) Doxorubicin hydrochloride | 3.68 | 1.97 ± 0.06 | DNA-intercalating agent | AF |
| Other natural products | ||||
| 31) Brazilin | 50 | 1.48 ± 0.05 | Haematoxylin-like may protect from oxidative stress | NA |
| 32) Brazilein | 50 | 1.50 ± 0.01 | Anti-inflammatory, lowers cytokines TNF-α, IL-6 | NA |
| 33) Epigallocatechin 3,5-digallate | 50 | 1.54 ± 0.01 | Tea extract polyphenol, inhibits insulin secretion | PH |
| 34) Purpurin | 50 | 1.67 ± 0.06 | Red/yellow dye, unknown | AF |
| 35) 7-Hydroxy-2-methoxyisoflavone | 50 | 1.88 ± 0.06 | Isoflavonoid, unknown | NA |
| 36) 10Dimenthyl bicycle [7:2:0] undecane | 50 | 2.68 ± 0.14 | Unknown | NA |
Thirty-six candidate enhancers of aquaporin 2 (AQP2) exocytosis were identified from primary screening of 3,464 compounds with a soluble secreted yellow fluorescent protein (ssYFP) exocytosis assay. These candidates were evaluated by secondary screening with immunofluorescence (IF). NEx, normalized exocytosis score; NA, not available commercially; CT, cytotoxic; AK, already known as enhancer of AQP2 membrane accumulation (compounds 7, 11, 14, 21); AF, autofluorescence; NC, no change of localization of AQP2; PH, positive hit with immunofluorescence (compound 13, 19, 32). AK and PH compounds are highlighted in bold. AAAD, aromatic L-amino acid decarboxylase; EGFR, epidermal growth factor receptor; ROCK-1, Rho-associated protein kinase 1.
Immunofluorescence staining.
Immunofluorescence with LLC-PK1 cells and Madin Darby canine kidney (MDCK) cells stably expressing AQP2 (17, 42) was performed as previously described (40, 41). LLC-PK1 cells and MDCK cells were cultured on glass coverslips and polyester filters (Corning), respectively. The MDCK cells were treated with 50 μM indomethacin overnight as previously described (10, 41). After 2 h of serum starvation, the cells were incubated with compounds for 30 min and fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100-PBS, and blocked in 1% BSA-PBS. Goat anti-AQP2 C-17 antibody (0.5 μg/ml, Santa Cruz Biotechnology, Dallas, TX) and donkey anti-goat IgG conjugated to Cy3 (Jackson ImmunoResearch, West Grove, PA) were used as a primary antibody and a secondary antibody, respectively. Images were acquired with a Zeiss Radiance 2000 confocal laser-scanning microscope or a Nikon 80i microscope. AG-490 was obtained from Selleck Chemicals (Houston, TX). β-Lapachone and epigallocatechin gallate (EGCG) were obtained from Enzo Life Sciences (Farmingdale, NY) and Sigma-Aldrich (St. Louis, MO), respectively.
Animal experiments.
Animal experiments were approved by the Massachusetts General Hospital Institutional Committee on Research Animal Care in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For in vitro kidney slice experiments, kidney slices were prepared as described previously (4, 5). Briefly, adult Sprague-Dawley rats were anesthetized using isoflurane. Kidneys were harvested, and slices of ∼0.5 mm were cut using a Stadie-Riggs microtome. All of the sliced kidneys were incubated at 37°C for 15 min in equilibrated HBSS (pH 7.4, with 5% CO2). After equilibration, the slices were incubated in HBSS containing chemicals (100 μM arginine vasopressin and 10 μM forskolin, 40 μM AG-490, 50 μM β-lapachone, or 0.1% DMSO as control) for 30 min. After incubation, all of the samples were immersed in 4% paraformaldehyde-lysine-periodate (PLP) fixative. For metabolic cage studies, each adult Brattleboro rat (from the Rat Resource and Research Center, Columbia, MO) was placed individually in a metabolic cage. Before the experiment, rats were allowed to acclimate to metabolic cages for 5 days. AG-490, β-lapachone, or the same amount of control solution (50% DMSO and 50% ethanol) was injected into rats intraperitoneally. The initial dose of each drug was determined by reference to previous studies (9, 19, 26, 38). We used different doses of chemicals and finally we chose a dose that had no significant side effects on animal behaviors such as food intake, grooming behavior, and general appearance of well-being. The metabolic cage study was repeated two times with a 10 days break period between treatments. Data from each rat were, therefore, determined by the average of two independent experiments. For blood sampling and kidney fixation, rats were anesthetized with isoflurane 2 h after drug injection. Blood was collected from the abdominal vena cava, and kidneys were immediately perfused through the left ventricle with PBS then with the PLP fixative. Cryosections were prepared as described previously (4). These specimens were stained with anti-AQP2 antibody (see above). Serum and urine were analyzed at the Massachusetts General Hospital Clinical Pathology Laboratory facility. Urine osmolality was analyzed with a Vapor Pressure Osmometer 5520 (Wescor, Logan, UT).
Quantification of AQP2 plasma membrane accumulation.
The fluorescence of AQP2 labeling at the plasma membrane was quantified using Volocity software (PerkinElmer, Santa Clara, CA). For the quantification, LLC-PK1 cells were costained with wheat germ agglutinin (WGA 2 μg/l; Lectin Kit, Vector Labs, Burlingame, CA) to clearly delineate the plasma and nuclear membranes. The mean fluorescence intensity of at least 30 regions of plasma membrane and cytosol from each of 5 different images from each sample taken at ×40 magnification were analyzed. The plasma membrane fluorescence was divided by the mean cytosolic value of each sample to normalize the values among different experiments. For quantification in rat kidney tissue, the fluorescence level of apical AQP2 and total cellular AQP2 was determined by measuring the intensity levels of a region of interest (ROI) encompassing only the apical membrane, and the whole cell area including the apical membrane domain, respectively. At least four tubules and three cells in each tubule were measured in each of three different areas of kidney (cortex, outer medulla, and inner medulla). Each experiment was repeated at least three times.
cAMP and cGMP assays.
Total cellular cAMP and cGMP levels were measured with the BioTrak EIA system (GE Healthcare Life Sciences, Piscataway, NJ) as described in detail previously (4, 5). Cells treated with VP (10 nM) or sodium nitroprusside (NTP; 1 mM) for 10 min were used as positive controls. Each assay was performed in triplicate and repeated three times independently.
Statistical analysis.
Statistical analyses were performed using the unpaired t-test. P values <0.05 were considered statistically significant.
RESULTS
High-throughput screening assay.
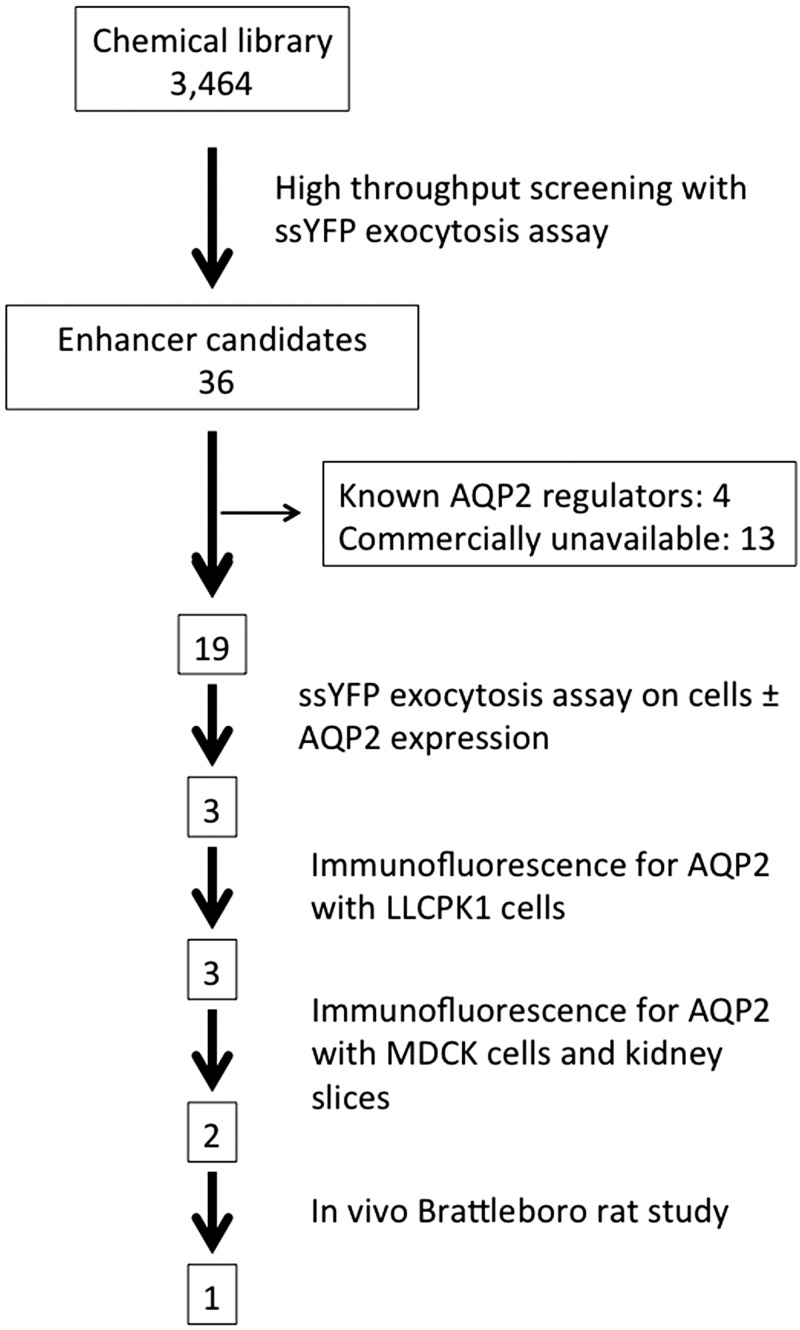
The total amount of ssYFP secreted into the extracellular medium was measured in LLC-PK1 cells expressing both ssYFP and AQP2 (LLC-AQP2-ssYFP cells) (29) in the presence of 3,464 bioactive molecules. We identified 36 candidate exocytosis enhancers that increased ssYFP medium fluorescence (Table 1). In our unbiased study, we also detected some compounds already known to regulate AQP2 trafficking: prostaglandin E2 (20, 30), dipyridamole (5, 35, 36), and Y-27632 (37). These results, which serve as positive controls, indicate that our screening system worked effectively. However, one of the compounds picked up as an exocytosis enhancer was trifluoperazine, which has been reported to inhibit VP-dependent water permeability and AQP2 trafficking (8). This indicates the need to perform careful confirmatory follow-up experiments after initial high-throughput screening of chemical libraries. Such studies are reported below, and a flow chart of the entire screening and testing procedure is illustrated in Fig. 1. The chart shows the number of compounds surviving each step of the procedure.
Fig. 1.
Flow chart showing the screening and testing procedures used in this study. The boxes show the number of compounds that remained after each screening step. From an initial pool of 3,464, only one compound remained that increased urine concentration in rats while activating membrane accumulation of aquaporin 2 (AQP2) in vivo. ssYFP, soluble secreted yellow fluorescent protein.
Testing candidate compounds on cultured cells and kidney tissue slices in vitro.
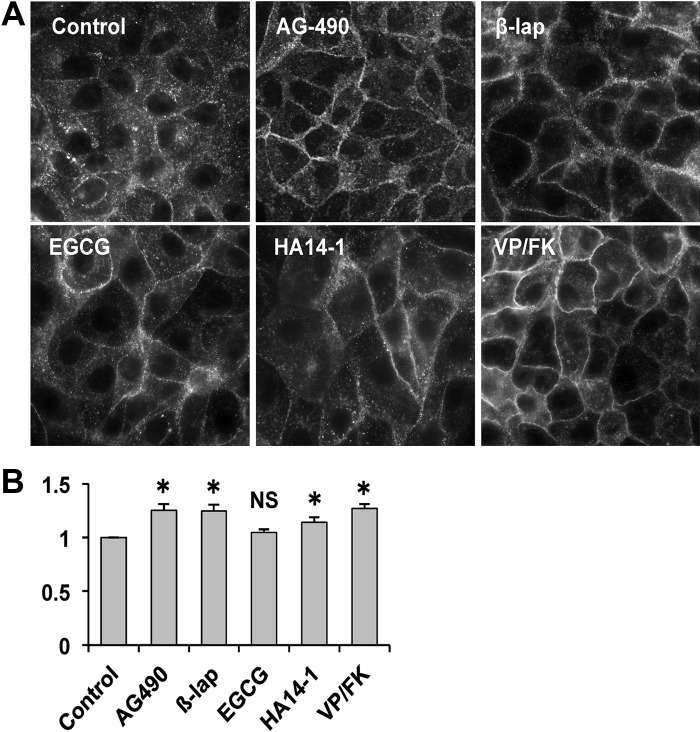
The ssYFP exocytosis assay indicated whether a given compound could stimulate or inhibit the accumulation of ssYFP in the culture medium, but it did not prove directly its effect on membrane accumulation of AQP2. “False positives” can occur if a compound is autofluorescent or causes cell death or lysis. Therefore, we performed immunofluorescence studies to determine AQP2 localization in cultured cells exposed to candidate enhancer compounds. We excluded 4 drugs (prostaglandin E2, dipyridamole, trifluoperazine, and Y-27632) that were already known to affect AQP2 trafficking, and 13 drugs were not commercially available. Finally, we tested 19 enhancer candidates by immunofluorescence with AQP2 expressing LLC-PK1 cells. Treatment with 50 μM β-lapachone, 40 μM AG-490, and 60 μM HA14-1 caused significant plasma membrane accumulation of AQP2 (Fig. 2A). Epigallocatechin gallate (EGCG, 50 μM) caused some apparent membrane accumulation by visual inspection, but the effect did not reach statistical significance upon quantification (Fig. 2B), even after increasing the concentration of EGCG to 200 μM (data not shown).
Fig. 2.
AQP2 accumulates on the plasma membrane after treatment of LLC-PK1 cells with candidate compounds. A: representative AQP2 staining in LLC-PK1 cells stably expressing AQP2 after treatment with AG-490 (40 μM), β-lapachone (β-lap, 50 μM), epigallocatechin gallate (EGCG, 50 μM), HA14-1 (60 μM), and lysine-vasopressin/forskolin (VP/FK, 10 nM/10 μM) for 30 min. B: quantification of membrane AQP2 accumulation in LLC-PK1 cells. Mean fluorescence intensity in the plasma membrane was divided by mean fluorescence intensity in the cytosol. Data are shown as means ± SE; n = 4; NS, not significant. *P < 0.05 vs. control. The images presented here were all subjected to enhancement and sharpening of the entire field using the levels command and/or the high-pass filter in Adobe Photoshop. Images for quantification were all used without any enhancement.
Seven compounds initially identified (menadion, emodin, RO31-8220, GF-109203X, shikonin, doxorubicin hydrochloride, and purpurin) were autofluorescent and had no effect on AQP2 trafficking (Table 1). Two compounds (fluphenazine and NSC-95397) were cytotoxic (Table 1). Six others (dobutamine hydrochloride, U-46619, tyrphostin AG-825, AG-213, quinalizarin, and antimycin A) had no significant effect on the localization of AQP2 in LLC-PK1 cells (Table 1).
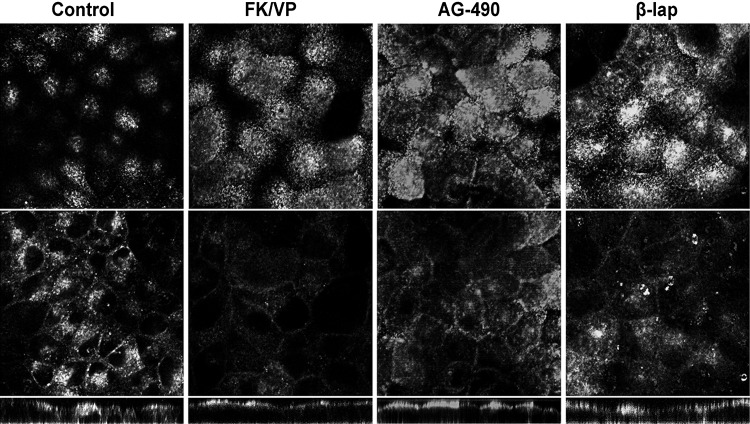
We further examined the effect of candidate compounds using MDCK cells stably expressing AQP2 to determine the generality of the effect of enhancer compounds on AQP2 trafficking. Both β-lapachone and AG-490 caused a marked accumulation of AQP2 on the apical membrane that mimicked the effect of VP (Fig. 3). However, the effect of EGCG and HA14-1 on AQP2 membrane accumulation was not significant in MDCK cells (data not shown).
Fig. 3.
Confocal immunofluorescence localization of AQP2 in Madin-Darby canine kidney (MDCK) cells stably expressing AQP2. The cells were cultured on filters. Top: apical slice of cells (i.e., the apical plasma membrane domain). Middle: middle portion of the cells. Bottom: xz-stack slice. AQP2 was located in the cytosol under basal conditions. After 30 min of incubation with VP/FK, β-lapachone, and AG-490, AQP2 accumulated at the apical pole (compare top panels with control). The concentrations of compounds were as same as in Fig. 2A with LLC-PK1 cells. The images presented here were all subjected to enhancement and sharpening of the entire field using the levels command and/or the high-pass filter in Adobe Photoshop.
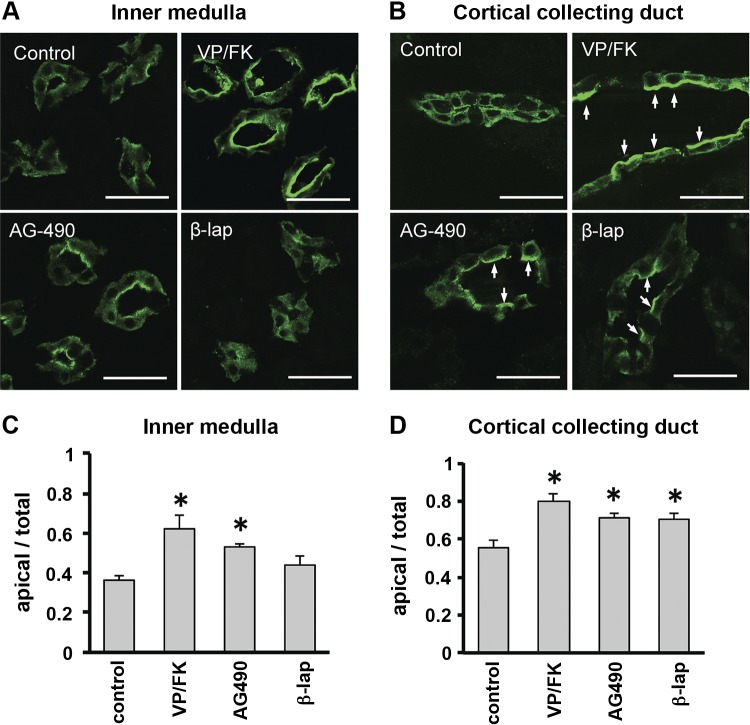
We then tested these compounds on rat kidney slices in vitro. After AG-490 treatment, AQP2 accumulated on the apical membrane of principal cells in both inner medulla (Fig. 4, A and C) and cortex (Fig. 4, B and D). β-Lapachone treatment induced AQP2 accumulation on the apical membrane in the cortex only, and not in the medulla. We also applied EGCG and HA14-1 to kidney tissue slices, but as in MDCK cells, these compounds had no significant effect on AQP2 localization (data not shown).
Fig. 4.
AQP2 accumulates on the apical plasma membrane in rat kidney slices after treatment with candidate compounds in vitro. Kidney slices from a Sprague-Dawley rat were incubated for 30 min with VP/FK (10 nM/10 μM), AG-490 (40 μM), or β-lapachone (50 μM). A: in inner medulla, AQP2 accumulated on apical membranes after VP/FK or AG-490 treatment. β-Lapachone had no detectable effect. B: in cortical collecting duct, AQP2 accumulated on apical membranes of principal cells (arrows) after VP/FK, AG-490, or β-lapachone treatment. Scale bars, 20 μm. C and D: quantitative analysis of AQP2 in inner medulla (C) and cortex (D). Fluorescence intensity of the apical area was divided by fluorescence of the whole cell area. Data are shown as means ± SE; n = 3. *P < 0.05 vs. control.
Effect of compounds on urinary concentration in VP-deficient Brattleboro rats.
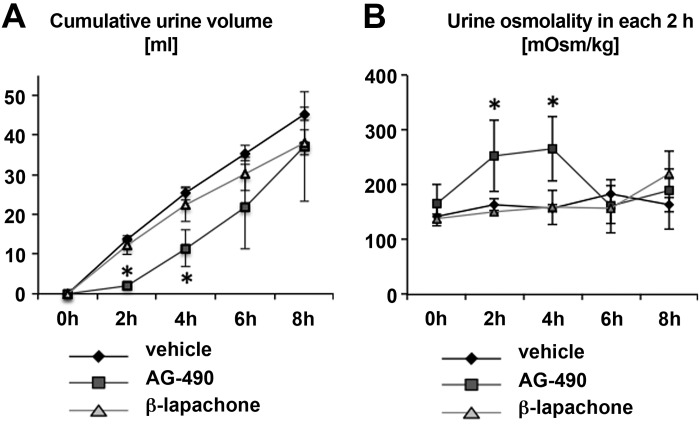
To evaluate the effect of compounds on urine volume and osmolality in vivo, VP-deficient Brattleboro rats were housed individually in metabolic cages. These rats, which model central DI, have been used in the past to identify drugs that can increase urine concentration in the complete absence of the antidiuretic hormone VP. Rats were injected intraperitoneally with AG-490 (35 mg/kg), β-lapachone (17 mg/kg), or control solution. After AG-490 injection, urine volume was significantly decreased and urine osmolality was significantly increased within 2 h after injection (Fig. 5, A and B). However, this effect of AG-490 did not continue after 6 h, and urine volume and osmolality after 8 h was almost the same as in control rats (Fig. 5). Despite a potent effect on AQP2 trafficking in all of the in vitro systems we examined, β-lapachone failed to show any significant effect on urine volume and osmolality in the time period tested (Fig. 5).
Fig. 5.
Effect of acute in vivo treatment with AG-490 and β-lapachone. A and B: cumulative urinary volume (A) and time course of change in urinary osmolality (B) in rats housed in metabolic cages. Brattleboro rats were injected with AG-490 (35 mg/kg), β-lapachone (17 mg/kg), or vehicle intraperitoneally. Urine was collected every 2 h. Data are shown as means ± SE; n = 3. *P < 0.05 vs. vehicle. The experiment was performed twice, and the average of the two experiments was used for each rat. AG-490 treatment caused a highly significant reduction in urine volume and an increase in urine osmolality 2 and 4 h after injection.
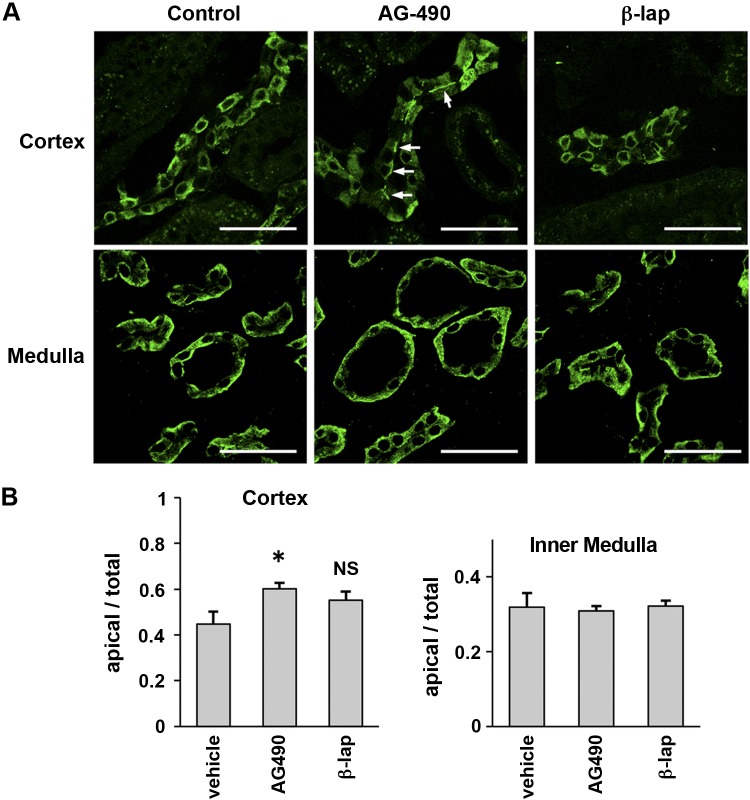
We next determined the subcellular distribution of AQP2 after in vivo drug treatment. Kidneys from each group of treated Brattleboro rats were harvested 2 h after injection and were immunostained for AQP2 (Fig. 6A). There was a significant accumulation of AQP2 at the apical membrane in the cortex of AG-490-treated rats, in contrast to the more diffuse distribution in vehicle-treated rats (Fig. 6, A and B). However, there was no significant enhancement of AQP2 apical localization in principal cells from the medulla in vivo, in contrast to the effect on kidney tissue slices in vitro. β-Lapachone had no detectable effect on AQP2 localization in any area of the kidney in vivo. These observations suggest that there are potential differences in bioavailability and drug delivery to target cells in vivo and in vitro.
Fig. 6.
A: immunofluorescence staining of AQP2 in Brattleboro rat kidney. At 2 h after injection, rats were perfused via the left ventricle and kidneys were fixed. In cortical collecting ducts (top), AQP2 accumulated on apical membranes of principal cells after AG-490 treatment. Arrows indicate AQP2 apical membrane accumulation. However, there was no significant difference in medullary collecting ducts (bottom). AQP2 localization after β-lapachone treatment was not different from the controls. Scale bars, 40 μm. B: quantification of AQP2 fluorescence intensity. Fluorescence intensity of the apical area was divided by fluorescence of the whole cell area. Data are shown as means ± SE; n = 3. *P < 0.05 vs. control.
Potential side effects of these chemicals were also examined. After the treatment, the rats looked normal and showed normal behaviors. There were no significant differences in food intake and body weight change after normalization compared with the control group (Table 2). There was no significant difference in blood chemistries, including kidney and liver function tests (Table 3). Finally, there was no significant difference in creatinine clearance (Table 3), suggesting adequate renal function in these animals. Therefore, the reduction in urine volume seen in AG-490-treated animals was unlikely to have resulted from a reduction of the glomerular filtration rate.
Table 2.
Twenty-four hour metabolic cage study data
| Time 0 | 24 h | Ratio | |
|---|---|---|---|
| Food intake, g | |||
| Control | 17.4 ± 0.4 | 16.7 ± 0.7 | 0.96 ± 0.04 |
| AG-490 | 14.5 ± 2.1 | 14.7 ± 1.8 | 1.02 ± 0.12 |
| β-Lap | 14.3 ± 3.5 | 10.9 ± 2.6 | 0.75 ± 0.18 |
| Feces, g | |||
| Control | 8.6 ± 1.2 | 10.0 ± 2.5 | 1.17 ± 0.29 |
| AG-490 | 7.8 ± 2.2 | 7.4 ± 1.9 | 0.94 ± 0.24 |
| β-Lap | 6.4 ± 0.4 | 6.4 ± 2.3 | 1.00 ± 0.36 |
| Body wt, g | |||
| Control | 468 ± 20 | 463 ± 19 | 0.99 ± 0.04 |
| AG-490 | 475 ± 24 | 472 ± 25 | 0.99 ± 0.05 |
| β-Lap | 494 ± 29 | 485 ± 29 | 0.98 ± 0.06 |
Values are means ± SE; n =3. Experiments were repeated twice, and an average for each rat was obtained. Ratio was defined as after-treatment (24 h) values divided by before-treatment (time 0) values. There were no significant differences between ratio values of treated animals compared with control untreated animals. β-Lap, β-lapachone.
Table 3.
Serum biochemistries show no significant differences after treatment
| BUN | Creatinine | TP | Glucose | ALT | ALP | CCr | |
|---|---|---|---|---|---|---|---|
| Control | 17.5 ± 2.7 | 0.3 ± 0.1 | 5.3 ± 0.1 | 217 ± 57 | 39 ± 7 | 277 ± 19 | 3.8 ± 0.7 |
| AG-490 | 15.4 ± 5.3 | 0.3 ± 0.1 | 5.0 ± 0.1 | 187 ± 7 | 32 ± 7 | 239 ± 51 | 3.5 ± 1.8 |
| β-Lapachone | 15.9 ± 0.8 | 0.3 ± 0.0 | 5.2 ± 0.3 | 187 ± 45 | 42 ± 8 | 293 ± 105 | 4.3 ± 0.3 |
Values are means ± SD; n = 3. BUN, blood urea nitrogen (mg/dl); creatinine (mg/dl); TP, total protein (g/dl); glucose (mg/dl); ALT, alanine aminotransferase (U/l); ALP, alkaline phosphatase (U/l); CCr, creatine clearance (mg/min). CCr was obtained from 4 h urine collection after injection of compounds.
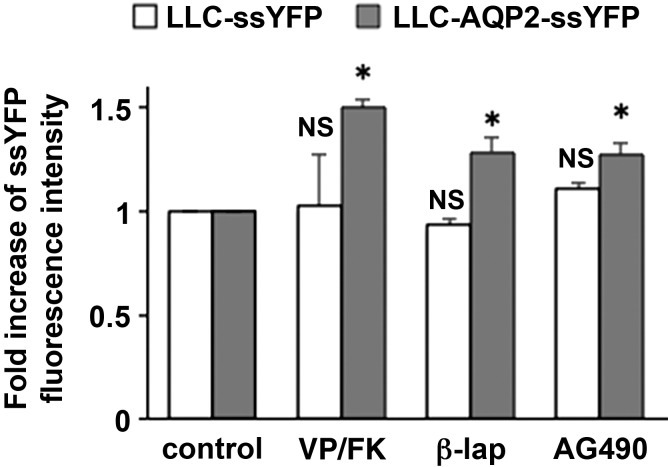
AG-490 and β-lapachone increase exocytosis only when AQP2 is expressed.
To determine the specificity of AG-490 on AQP2 exocytosis, we performed additional ssYFP exocytosis assays (Fig. 7). AG-490 increased exocytosis in LLC-AQP2-ssYFP cells, but it did not increase exocytosis in LLC-PK1 cells that do not express AQP2. This indicates that the increase in exocytosis induced by AG-490 requires expression of AQP2. These data are similar to those seen after VP treatment (Fig. 7) which, as we previously showed, increases exocytosis only in cells that express AQP2 (29, 41). Interestingly, β-lapachone, a compound that increased AQP2 membrane accumulation in cell cultures and in tissue slices (see above), also required AQP2 expression to stimulate ssYFP exocytosis. This again indicates that its apparent lack of effect in vivo could result from a bioavailability problem.
Fig. 7.
AG-490 significantly increases exocytosis only in AQP2-expressing cells. Exocytosis assay with LLC-PK1 cells stably expressing ssYFP is shown. In AQP2-expressing cells (AQP2 + ssYFP), exocytosis of ssYFP was increased after treatment with VP/FK, AG-490, and β-lapachone. In cells without AQP2 (ssYFP only), exocytosis was not significantly increased after treatment with any of the compounds. Data are shown as means ± SE; n = 4. *P < 0.05 vs. control.
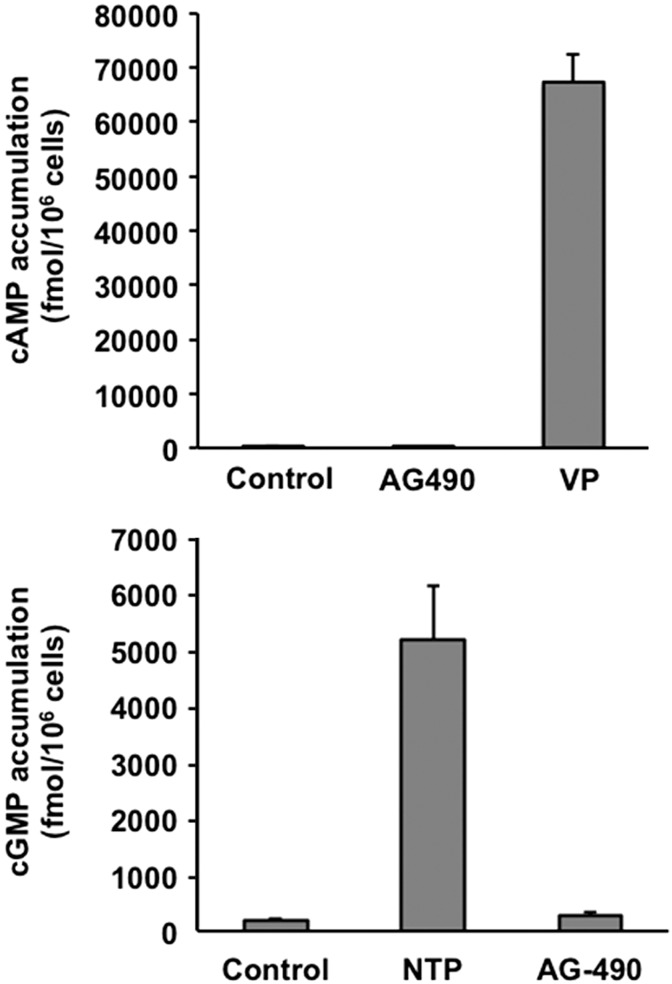
AG-490 does not increase cAMP or cGMP levels.
The significant effect of AG-490 on AQP2 trafficking prompted us to look at the effect of AG-490 on cAMP and cGMP levels to determine whether its effect on AQP2 trafficking might occur via the canonical cAMP/PKA pathway or the alternative cGMP pathway that we have previously described (3, 5). As shown in Fig. 8, AG-490 treatment with LLC-PK1 cells did not induce a measurable elevation of cAMP or cGMP levels, in contrast to the expected and strong effect of VP and NTP, respectively.
Fig. 8.
AG-490 does not increase cAMP or cGMP levels in LLC-PK1 cells. There was no significant elevation of cAMP in response to 10 min of treatment with 40 μM AG-490, in contrast to the large increase with 10 nM vasopressin (top). Similarly, AG-490 did not increase cGMP levels, whereas 1 mM sodium nitroprusside (NTP) had a highly significant effect (n = 3). Data are expressed in femtomoles of cAMP or cGMP generated per 106 cells and are shown as means ± SE. P < 0.05 vs. control for VP and NTP effects.
DISCUSSION
We describe here an unbiased screening procedure to identify novel chemicals that stimulate VP-independent AQP2 membrane trafficking. We interrogated selected libraries, including off-patent, FDA-approved drugs and known bioactive compounds, using a high-throughput modification of our ssYFP exocytosis assay (29). By following the initial screening with in vitro and in vivo studies, we identified AG-490 as a stimulator of AQP2 membrane accumulation both in cell culture systems and in principal cells in situ. AG-490 is marketed as a JAK-2 kinase and an EGF receptor (EGFR) inhibitor. Importantly, AG-490-treated, VP-deficient Brattleboro rats showed a significant and acute increase in urine osmolality and a significant decrease in urine volume. There was no significant difference in creatinine clearance between AG-490 treatment and controls groups, indicating that a reduction in glomerular filtration rate was not a major contributor to the decreased urine output. In vivo, an increased membrane accumulation of AQP2 was detectable only in the cortical collecting duct but not in medullary principal cells. This effect still resulted in a significant increase in urine concentration, because 60–70% of water reabsorption occurs in the cortical region of the collecting duct under antidiuretic conditions (33).
Our initial screening also detected some known stimulators of AQP2 trafficking (see Table 1), validating the methodology. A recent study using a different screening approach identified inhibitors of AQP2 membrane accumulation (2). There, too, the approach was validated by the unbiased identification of a blocker of V-ATPase (proton pumping ATPase) activity, which we had previously shown to prevent AQP2 trafficking (15). However, some of the compounds identified in our original screen proved to be false positives. Some were autofluorescent, and some were cytotoxic, resulting in cell damage and nonexocytotic release of ssYFP. Furthermore, some compounds may have increased medium ssYFP via exocytosis of vesicles that do not also contain AQP2. Others, including β-lapachone, seemed promising in vitro but had no detectable effect in vivo. This suggests a potential problem with bioavailability that needs to be addressed in the future. Importantly, however, neither AG-490 nor β-lapachone increased exocytosis in cultured cells that do not express AQP2, indicating that their effect depends on the presence of AQP2. Indeed, we have previously reported that VP increases exocytosis only in cells that express AQP2 (29), suggesting a relationship between AQP2 and the exocytotic machinery activated by VP. This result was repeated here (see Fig. 7). Our recent report showed that this is correlated with VP-induced actin depolymerization, which occurs only in AQP2-expressing cells (41). Previous studies have also revealed a close relationship between AQP2 and the actin cytoskeleton (23, 28, 37).
It is now appreciated that VP-independent AQP2 trafficking can be modulated by various intracellular pathways that include cGMP (5), MAP kinases (16), calcium-activated pathways (8), prostaglandins (20, 30), and the actin cytoskeleton (21, 32). Further studies will be required to clarify the precise mechanism by which AG-490 bypasses the V2R signaling pathway, but it is interesting that previous reports showed that epidermal growth factor (EGF) inhibits the VP effect and induces diuresis in vivo (6, 14, 31). A logical extension of this result is that the reverse effect, i.e., an increase in urinary concentration, might occur when the EGFR is inhibited by AG-490. Whether the activity of this drug on the JAK-2 pathway or on EGFR signaling is involved in the mechanism by which AQP2 membrane accumulation is stimulated remains to be determined. However, our data do rule out an involvement of cAMP elevation—the canonical pathway of VP signaling—as well as cGMP signaling, which we had previously described as an alternative pathway stimulating AQP2 membrane accumulation (3, 5). AG-490 had no detectable effect on the intracellular levels of these cyclic nucleotides.
In conclusion, we developed and applied a systematic screening procedure that identified AG-490 as a compound that stimulates AQP2 exocytosis, induces AQP2 membrane accumulation, and stimulates urine concentration in a VP-independent manner. High-throughput chemical screening with our cell-based assay followed by in vitro and in vivo testing has, therefore, been effective in identifying unsuspected strategies for the potential treatment of water balance disorders, and in identifying novel signaling pathways for the regulation of intracellular AQP2 trafficking that can be pursued in future studies.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-096586 (to D. Brown). H. A. Lu was supported by NIDDK Grants DK-075940 and DK-092619 and an MGH/ECOR interim support fund. P. Nunes was supported by a doctoral-level scholarship from the Natural Sciences and Engineering Research Council of Canada. N. Nomura was supported by a grant for “Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation program” from Japan Society for the Promotion of Science. Additional support for the Program in Membrane Biology Microscopy Core comes from the Boston Area Diabetes and Endocrinology Research Center (DK-57521) and the MGH Center for the Study of Inflammatory Bowel Disease (NIDDK Grant DK-43351). N. Pathomthongtaweechai was supported during his stay in D. Brown's laboratory by the Medical Scholar's Program of the Faculty of Science, Mahidol University, Bangkok, Thailand.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.N., P.N., R.B., A.V.N., S.S., N.P., H.A.J.L., and D.B. conception and design of research; N.N., P.N., R.B., A.V.N., E.U., and N.P. performed experiments; N.N., P.N., R.B., A.V.N., S.S., E.U., N.P., H.A.J.L., and D.B. analyzed data; N.N., P.N., R.B., A.V.N., S.S., N.P., H.A.J.L., and D.B. interpreted results of experiments; N.N., A.V.N., N.P., and D.B. prepared figures; N.N. and D.B. drafted manuscript; N.N., P.N., R.B., A.V.N., S.S., E.U., H.A.J.L., and D.B. edited and revised manuscript; N.N., P.N., R.B., A.V.N., S.S., E.U., N.P., H.A.J.L., and D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Maggie Ma for technical assistance, and Dr. S. Sasaki and Dr. S. Uchida (Tokyo Medical and Dental University) for providing MDCK clones.
Footnotes
This article is the topic of an Editorial Focus by Jeff M. Sands and Mitsi A. Blount (33a).
REFERENCES
- 1.Bichet DG, Ruel N, Arthus MF, Lonergan M. Rolipram, a phosphodiesterase inhibitor, in the treatment of two male patients with congenital nephrogenic diabetes insipidus. Nephron 56: 449–450, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Bogum J, Faust D, Zühlke K, Eichhorst J, Moutty MC, Furkert J, Eldahshan A, Neuenschwander M, von Kries JP, Wiesner B, Trimpert C, Deen PM, Valenti G, Rosenthal W, Klussmann E. Small-molecule screening identifies modulators of aquaporin-2 trafficking. J Am Soc Nephrol 24: 744–758, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouley R, Lu HA, Nunes P, Da Silva N, McLaughlin M, Chen Y, Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol 22: 59–72, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Breyer MD, Jacobson HR, Breyer JA. Epidermal growth factor inhibits the hydroosmotic effect of vasopressin in the isolated perfused rabbit cortical collecting tubule. J Clin Invest 82: 1313–1320, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Davoodi-Semiromi A, Hassanzadeh A, Wasserfall CH, Droney A, Atkinson M. Tyrphostin AG490 agent modestly but significantly prevents onset of type 1 in NOD mouse; implication of immunologic and metabolic effects of a Jak-Stat pathway inhibitor. J Clin Immunol 32: 1038–1047, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Deen PM, Rijss JP, Mulders SM, Errington RJ, van Baal J, van Os CH. Aquaporin-2 transfection of Madin-Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. J Am Soc Nephrol 8: 1493–1501, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264: 92–95, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest 41: 1988–1997, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol 16: 2836–2846, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Gow CB, Phillips PA. Epidermal growth factor as a diuretic in sheep. J Physiol 477: 27–33, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Hasler U, Nunes P, Bouley R, Lu HA, Matsuzaki T, Brown D. Acute hypertonicity alters aquaporin-2 trafficking and induces a MAPK-dependent accumulation at the plasma membrane of renal epithelial cells. J Biol Chem 283: 26643–26661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA 92: 7212–7216, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knepper MA, Nielsen S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F214–F224, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Li CJ, Li YZ, Pinto AV, Pardee AB. Potent inhibition of tumor survival in vivo by beta-lapachone plus taxol: combining drugs imposes different artificial checkpoints. Proc Natl Acad Sci USA 96: 13369–13374, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119: 3115–3126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Zhang Y, Bouley R, Chen Y, Matsuzaki T, Nunes P, Hasler U, Brown D, Lu HA. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol 301: F309–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr 108: 305–311, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Loo CS, Chen CW, Wang PJ, Chen PY, Lin SY, Khoo KH, Fenton RA, Knepper MA, Yu MJ. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci USA 110: 17119–17124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379: 645–648, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, Kinjo M, Okabe S, Sasaki S. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol 182: 587–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunes P, Hasler U, McKee M, Lu HA, Bouley R, Brown D. A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am J Physiol Cell Physiol 295: C1476–C1487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 108: 12949–12954, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips PA, Grant SL, Davidson AF, Risvanis J, Stephenson J, Gow CB. Epidermal growth factor antagonizes vasopressin in vivo and in vitro. Kidney Int 45: 1028–1036, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Procino G, Barbieri C, Carmosino M, Tamma G, Milano S, De Benedictis L, Mola MG, Lazo-Fernandez Y, Valenti G, Svelto M. Fluvastatin modulates renal water reabsorption in vivo through increased AQP2 availability at the apical plasma membrane of collecting duct cells. Pflügers Arch 462: 753–766, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Sands JM, Layton HE, Fenton RA. Urine concentration and dilution. In: Brenner & Rector's The Kidney, edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM. Philadelphia, PA: Elsevier/Sauders, 2012, p. 326–352 [Google Scholar]
- 33a.Sands JM, Blount MA. Novel activators of aquaporin 2 membrane expression for the treatment of nephrogenic diabetes insipidus: less is more. Focus on “High-throughput chemical screening identifies AG-490 as a stimulator of aquaporin 2 membrane expression and urine concentration.” Am J Physiol Cell Physiol (June 18, 2014). 10.1152/ajpcell.00184.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki S, Chiga M, Kikuchi E, Rai T, Uchida S. Hereditary nephrogenic diabetes insipidus in Japanese patients: analysis of 78 families and report of 22 new mutations in AVPR2 and AQP2. Clin Exp Nephrol 17: 338–344, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Sohara E, Rai T, Yang S, Uchida K, Nitta K, Horita S, Ohno M, Harada A, Sasaki S, Uchida S. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc Natl Acad Sci USA 103: 14217–14222, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, Furkert J, Santamaria K, Nedvetsky P, Hundsrucker C, Beyermann M, Krause E, Pohl P, Gall I, MacIntyre AN, Bachmann S, Houslay MD, Rosenthal W, Klussmann E. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol 18: 199–212, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Tzeng HP, Ho FM, Chao KF, Kuo ML, Lin-Shiau SY, Liu SH. beta-Lapachone reduces endotoxin-induced macrophage activation and lung edema and mortality. Am J Respir Crit Care Med 168: 85–91, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol 27: 2183–2204, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Yui N, Lu HA, Chen Y, Nomura N, Bouley R, Brown D. Basolateral targeting and microtubule-dependent transcytosis of the aquaporin-2 water channel. Am J Physiol Cell Physiol 304: C38–C48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yui N, Lu HJ, Bouley R, Brown D. AQP2 is necessary for vasopressin- and forskolin-mediated filamentous actin depolymerization in renal epithelial cells. Biol Open 1: 101–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yui N, Okutsu R, Sohara E, Rai T, Ohta A, Noda Y, Sasaki S, Uchida S. FAPP2 is required for aquaporin-2 apical sorting at trans-Golgi network in polarized MDCK cells. Am J Physiol Cell Physiol 297: C1389–C1396, 2009 [DOI] [PubMed] [Google Scholar]