nephrogenic diabetes insipidus (NDI) is a disease characterized by the production of very large quantities of dilute urine resulting from an inability of the kidney to respond to vasopressin (9). In the US, approximately 41,000 people are diagnosed with NDI each year. NDI can result from either congenital or acquired etiologies. Mutations in the type 2 vasopressin receptor (V2R) occur in 90% of families (in which the mutation is known) with congenital NDI and in the aquaporin 2 (AQP2) water channel in the other 10% (9). Congenital NDI has a profound impact on children with the disease who produce up to 20 liters of urine per day and thus must drink 20 liters of water daily to avoid dehydration. Children who suffer multiple episodes of severe dehydration often end up with mental retardation (1). However, mental retardation can be prevented with adequate water intake to prevent dehydration (1). Acquired NDI can result from multiple causes, most commonly by chronic use of lithium, a medication that interferes with cAMP production and signaling, and which is used to treat bipolar disorders (10).
AQP2 increases the water permeability of the collecting duct apical plasma membrane in response to vasopressin, facilitating water reabsorption in the presence of an osmotic gradient (3). AQP2 has four vasopressin-sensitive phosphorylation sites: S256, S261, S264, and S269. Both S256 and S261 are involved in AQP2 trafficking to the apical plasma membrane. Phosphorylation at S269 is important for AQP2 retention in the apical plasma membrane. In patients with V2R mutations or taking lithium, there are no mutations in AQP2, suggesting that if it were possible to increase AQP2's apical plasma membrane accumulation and function, independent of vasopressin, one might be able to treat, or at least lessen the severity of, the NDI.
Brown and colleagues have been leaders in looking for compounds that can increase AQP2 expression in the apical plasma membrane, as such compounds may be therapeutically useful in treating NDI. The elucidation of AQP2 signaling and trafficking pathways has led to the testing of compounds that could increase AQP2 expression in the apical plasma membrane when the V2R is either absent or defective. The knowledge that has been gained is an elegant example of the importance of fundamental physiological research that sets the stage for pharmacological and translational studies. Sildenafil, through its ability to inhibit cyclic nucleotide phosphodiesterase type 5 (PDE5), increases AQP2 accumulation in the apical plasma membrane by increasing cGMP levels in Brattleboro rats, which have central DI, and also reduces polyuria in rats with lithium-induced NDI (8). The HMG-CoA reductase inhibitor simvastatin also increases urine concentrating ability in Brattleboro rats (5). Calcitonin has a vasopressin-like effect on AQP2 trafficking and urine concentrating ability (2). A selective EP4 PGE2 receptor agonist improves urine concentrating ability in a V2R knockout mouse (4). However, none of these compounds has yet to be translated into a therapy for patients with NDI.
In the present study, Nomura et al. (6) report on the development of a novel assay that permits the use of high-throughput screening as a unique means to identify chemical compounds that increase AQP2 expression in the apical plasma membrane. The screening assay was developed from an exocytosis assay that they previously developed (7) and utilizes LLC-PK1 (renal epithelial) cells that express both AQP2 and a soluble secreted yellow fluorescent protein. In the present study, the authors modified this assay for use in 384-well plates, making it possible to perform high-throughput screening.
The authors tested positive hits in two renal epithelial cell lines, LLC-PK1 and MDCK, and then on rat kidney slices. With each step, the test system became more physiologic, and the number of potential positive compounds decreased. The use of these approaches yielded a single compound, AG-490, which is an EGFR and JAK-2 kinase inhibitor (Fig. 1). Nomura et al. show that a single injection of AG-490 into Brattleboro rats decreases urine volume and increases urine osmolality 2–4 hours after injection, although these beneficial changes were gone by 8 hours (6). The authors find that the decrease in urine volume is not due to renal toxicity. The increase in urine osmolality is around 100 mosmol/kgH2O, which is somewhat less than is observed following a single injection of vasopressin in people with central DI (9).
Fig. 1.
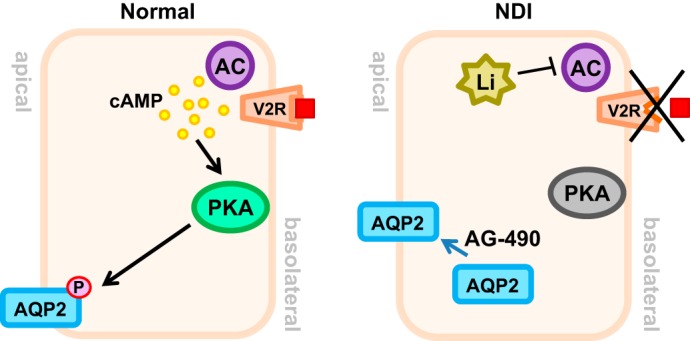
Vasopressin (AVP, red square) binds to the type 2 vasopressin receptor (V2R) receptor on the basolateral membrane, resulting in increased cellular cAMP via adenylyl cyclase (AC) activation. Increased cAMP activates PKA, which in turn, phosphorylates aquaporin 2 (AQP2), increases its apical membrane expression, and increases water reabsorption. Nephrogenic diabetes insipidus (NDI), either congenital (V2R defect) or acquired (lithium), results in lowered cellular cAMP levels and lack of PKA activation and decreases AQP2 in the membrane. AG-490 increases the membrane expression of AQP2.
The present study contains both an important technical advance, the development of a high-throughput screening method, and a suggestion that the EGFR signaling and/or the JAK-2 pathway may play a previously unrecognized role in AQP2 accumulation in the plasma membrane (6). Future studies will be needed to test the role of EGFR and/or JAK-2, and to determine whether the decrease in urine volume can be sustained by repeated administration of AG-490. It is also possible that combination therapy with other agents that have different mechanisms of action, such as sildenafil or simvastatin, will yield a better or more sustained increase in urine concentrating ability.
Ultimately, a therapeutically useful compound for the treatment of NDI will need to lead to a sustained improvement in urine concentrating ability. However, it does not need to normalize urine output since patients with NDI do well if they can drink as much fluid as they urinate. If a therapy can reduce urine output from 10–20 liters per day to 5 liters per day or less, it would reduce the amount of water that individuals must drink and would dramatically improve their quality of life. Thus, with respect to therapies for patients with NDI, less urine is more (better) quality of life.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK89828 (to J. M. Sands) and K01-DK82733 (to M. A. Blount).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.S. and M.A.B. prepared figure; J.M.S. and M.A.B. drafted manuscript; J.M.S. and M.A.B. edited and revised manuscript; J.M.S. and M.A.B. approved final version of manuscript.
REFERENCES
- 1.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis 13: 96–104, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bouley R, Lu HA, Nunes P, Da Silva N, McLaughlin M, Chen Y, Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol 22: 59–72, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juul KV, Bichet DG, Nielsen S, Norgaard JP. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol 306: F931–F940, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119: 3115–3126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Zhang Y, Bouley R, Chen Y, Matsuzaki T, Nunes P, Hasler U, Brown D, Lu HA. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol 301: F309–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomura N, Nunes P, Bouley R, Nair AV, Shaw S, Ueda E, Lu HA, Brown D. High-throughput chemical screening identifies AG-490 as a stimulator of aquaporin 2 membrane expression and urine concentration. Am J Physiol Cell Physiol (June 18, 2014). 10.1152/ajpcell.00154.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunes P, Hasler U, McKee M, Lu HA, Bouley R, Brown D. A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am J Physiol Cell Physiol 295: C1476–C1487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanches TR, Volpini RA, Massola Shimizu MH, Bragança AC, Oshiro-Monreal F, Seguro AC, Andrade L. Sildenafil reduces polyuria in rats with lithium-induced NDI. Am J Physiol Renal Physiol 302: F216–F225, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Sands JM, Bichet DG. Nephrogenic diabetes insipidus. Ann Intern Med 144: 186–194, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 [DOI] [PubMed] [Google Scholar]


