Abstract
Activation of the cytosolic inflammasome machinery is responsible for acute and chronic liver inflammation, but little is known about its regulation. The N-methyl-d-aspartate (NMDA) receptor families are heterotetrameric ligand-gated ion channels that are activated by a range of metabolites, including aspartate, glutamate, and polyunsaturated fatty acids. In the brain NMDA receptors are present on neuronal and nonneuronal cells and regulate a diverse range of functions. We tested the role of the NMDA receptor and aspartate in inflammasome regulation in vitro and in models of acute hepatitis and pancreatitis. We demonstrate that the NMDA receptor is present on Kupffer cells, and their activation on primary mouse and human cells limits inflammasome activation by downregulating NOD-like receptor family, pyrin domain containing 3 and procaspase-1. The NMDA receptor pathway is active in vivo, limits injury in acute hepatitis, and can be therapeutically further activated by aspartate providing protection in acute inflammatory liver injury. Downregulation of inflammasome activation by NMDA occurs via a β-arrestin-2 NF-kβ and JNK pathway and not via Ca2+ mobilization. We have identified the NMDA receptor as a regulator of inflammasome activity in vitro and in vivo. This has identified a new area of immune regulation associated by metabolites that may be relevant in a diverse range of conditions, including nonalcoholic steatohepatitis and total parenteral nutrition-induced immune suppression.
Keywords: aspartate
the cytosolic machinery termed the inflammasome is responsible for sensing a wide range of cellular stressors and for activating and releasing inflammatory and tissue modeling cytokines, including IL-1β and IL-18 (1, 8, 13). Activation of inflammasomes in immune cells is required in a range of tissue injury and repair, including alcoholic and nonalcoholic hepatitis and liver fibrosis (7, 11, 16, 23, 34). The minimum activation requirements for the inflammasome machinery have been identified and consist of two signals. The first signal is initiated by pattern recognition receptors such as Toll-like receptors (TLRs), and the second originates from a diverse range of cell stressors (14). The importance of the inflammasome makes it vital to understand the regulatory steps that limit its activation and thus limit tissue injury and fibrosis.
Any molecule that undergoes a change in concentration during an inflammatory response is a potential candidate for regulating inflammasome activity. Metabolites are of particular interest, since they have recently been shown to be tightly regulated during an inflammatory response, and there are clear links between metabolic dysregulation and inflammation (20, 35). The N-methyl-d-aspartate (NMDA) receptors are heterotetrameric ligand-gated ion channels and are activated by a range of metabolites, including aspartate, glutamate, and polyunsaturated fatty acids such as docosahexaenoic acid (19, 32). NMDA receptors are well characterized at a molecular and functional level and consist of four large transmembrane subunits that form a central ion pore (30). A functional NMDA receptor requires two GluN1 subunits that can combine with two GluN2 subunits, or a GluN2 and a GluN3 subunit (2). Glutamate and aspartate bind the GluN2 subunits and activate the receptor, which results in opening of a cation selective channel (30). NMDA functional capacities have been extensively studied in the central nervous system, but very little is known about them in other cell systems. We investigated the NMDA receptor as an inflammasome regulator because during lipopolysaccharide (LPS) d-galactosamine-induced hepatitis a significant elevation in serum concentrations of a wide range of amino acids, including the NMDA ligands aspartate and glutamate, has been reported (6).
In this study we demonstrate that NMDA receptors are expressed on monocyte macrophage cells, including Kupffer cells. NMDA activation by aspartate downregulates inflammasome activity in vitro by reducing Pro-Il1β and Pro-caspase-1 transcripts. Inhibition of this pathway results in a decreased degree of acute liver injury, and NMDA ligands such as glutamate can provide significant hepatoprotection.
MATERIALS AND METHODS
Cell culture.
Two hundred nanograms per milliliter of LPS (Sigma) were added to peritoneal macrophages, and 1,000 ng/ml for RAW 264.7 cells and Kupffer cells and 2,000 ng/ml for human peripheral mononucleocytes. ATP (5 mM) was added for 15 min and washed out, and cells were incubated for an additional 1 h before collection of supernatant.
Mice.
C57BL/6N male (National Cancer Institute) and caspase-1-deficient male mice 6–12 wk were housed with a 12:12-h light-dark cycle and given free access to food and water. All experiments were in accordance with protocols approved by the Yale University Institutional Animal Care and Use Committee.
Peritoneal macrophages.
C57BL/6N mice were administered sterile 3 ml of 4% thioglycollate (Sigma), and peritoneal elicited cells were harvested by lavage after 72 h. Cells were plated, and nonadherent cells were removed by washing after 1 h to enrich for peritoneal macrophages. Adherent cells were incubated for 3 h in DMEM supplemented with 10% fetal calf serum, penicillin, and streptomycin before experiments.
Human peripheral mononucleocytes.
Human peripheral mononucleocytes were isolated from blood drawn from two normal subjects under sterile conditions using leucosep (Greiner Bio one) as per the manufacturer's protocol.
Cells were plated in 24-well polystyrene dishes. Nonadherent cells were removed by washing after 1 h to enrich for human peripheral mononucleocytes. Adherent cells were then incubated for 3 h in DMEM supplemented with 10% fetal calf serum, penicillin, and streptomycin before experiments.
Mouse kupffer cells.
Liver were harvested from C57BL/6N mice. Livers were digested using collagenase perfusion of liver in vivo and OptiPrep gradient (D1556; Sigma). Cells were plated, and nonadherent cells were removed by washing after 1 h to enrich for Kupffer cells. Adherent cells were incubated for 3 h in DMEM supplemented with 10% fetal calf serum, penicillin, and streptomycin before experiments, and purity was confirmed to be >90% by flow cytometry for F4/80.
Aspartate assay.
Supernatant was collected from peritoneal macrophages stimulated with LPS in vitro and assessed for aspartate by a fluorometric kit (Abcam). Serum was also collected from animals 60 min after intraperitoneal administration of aspartate and assessed for aspartate levels as above.
Quantitative polymerase chain reaction assessment of transcript levels.
RNA was isolated from cells and tissues using an RNeasy Mini Kit from Qiagen and cDNA reverse transcribed using a Multiscribe Reverse Transcriptase from Applied Biosystems. qPCR was used to quantify transcripts using Taqman primer-probes (Applied Biosystems) to quantify expression of Pro-IL1β, NOD-like receptor family, pyrin domain containing 3 (Nlrp3), Pro-caspase-1, Pro-Il18, Il10, Nr2a, Arrb2, and Gapdh. Real-time PCR reactions were performed in a LightCycler 480 PCR machine from Applied Biosystems. Expression of target genes was normalized relative to Gapdh. Gapdh transcript levels in cDNA were not significantly affected by LPS stimulation in vitro or by LPS/GalN, APAP, or LPS/caerulein treatment in vivo.
Immunoprecipitation.
RAW 264.7 cells were treated with or without aspartate for 1 h. RIPA Buffer (10×; Cell Signaling) was used for cell lysis. NR2A was immune precipitated using anti-NR2A antibody (catalog no. 07632; Millipore) and protein A/G plus agarose immunoprecipitation reagent (sc-2003; Santa Cruz). Western blots were immune stained with anti-β-arrestin-2 antibody (C16D9; Cell Signaling) and anti-NR2A antibody (catalog no. 07632; Millipore). Quantization of immunostained Western blot was done by using PC Image Software and an Analyst Imager from FOTODyne. Serum goat IgG was used as a negative control, and whole cell lysate was used as positive control.
Western blots.
Western blots were immunostained with anti-phospho-IKKαβ antibody (CS-176/180; Cell Signaling), anti-IKKβ (L5-70; Cell Signaling), anti-caspase-1 p 10 (Santa Cruz Biotechnology) antibody, and anti-β-actin antibody (Santa Cruz Biotechnology). Quantization of immune-stained Western blot was done by using PC Image Software and an Analyst Imager from FOTODyne.
IL-1β ELISA.
Peritoneal macrophages were treated with LPS and ATP as above, and supernatant was collected. Serum was assessed for IL-1β using a commercially available mouse IL-1β ELISA reagent from R&D Systems. Supernatant levels of IL-1β are expressed as picograms per milliliter.
In vitro siRNA treatment.
RAW 264.7 cells were plated in 24-well dishes and treated with Silencer select small-interfering RNA (siRNA) targeting NR2a, Arrb2, or scramble siRNA (Invitrogen) complexed with Lipofectamine from Invitrogen according to the manufacturer's instructions. siRNA treatment was repeated 24 h later, and the cells were used in experiments 24 h after the second treatment.
In vivo siRNA treatment.
Silencer select siRNA targeting Arrb2 or scramble siRNA (Invitrogen) were complexed with Invivofectamine according to the manufacturer's instructions and dialyzed with sterile Dulbecco's phosphate-buffered saline using Float-A-Lyzer G2 dialyzers from Spectrum Labs. Mice were administered siRNA at 7 μg/g body wt by intraperitoneal injection at doses 24 h apart. Forty eight hours after the last injection, mice were administered LPS and d-galactosamine as detailed below.
Experimental immune hepatitis models.
C56BL/6N 6- to 8-wk male mice were administered LPS at 5–7.5 μg/g and d-galactosamine at 300–400 μg/g body wt by intraperitoneal injection in sterile Dulbecco's phosphate-buffered saline in a volume of 10 μl/g. Mice were killed at 5 h postadministration for analysis. Fifteen minutes after administration of either, mice were given an intraperitoneal injection of Dulbecco's phosphate-buffered saline, 5 mM HEPES, pH 7.40, or 300 mM aspartate, 5 mM HEPES, pH 7.40, at 30 μl/g body wt. Mice were killed at 5 h postadministration of LPS with liver and serum then harvested for analysis. Livers were sectioned and stained using eosin and hematoxylin stains, and hemorrhage was scored based on the percentage of hemorrhage on at least seven different ×10 views.
Alanine aminotransferase assay.
Serum alanine aminotransferase (ALT) was measured by the standard assays.
Experimental pancreatitis with LPS/caerulein hyperstimulation.
C56BL/6N young adult male mice were administered caerulein sulfate at 100 pg/g body wt by intraperitoneal injection for six hourly injections. A single LPS (10 mg/kg) injection was given with the first dose of caerulein. Concurrent with the first and third dose of caerulein, mice were given intraperitoneal injections of sterile saline, 2.5 mM HEPES, pH 7.40, or 300 mM aspartate, 2.5mM HEPES, pH 7.40, at 30 μl/g body wt. Mice were killed 1 h after the last injection of caerulein.
Statistical analysis.
To test for significance, a two-tailed t-test was used. P < 0.05 was considered a significant difference. If not stated otherwise, data were taken from three to five individual experiments and expressed as means ± SE. The analysis of the qPCR data was performed by normalizing to one control sample that did not receive a stimulus. All data are shown in relation to this normalized untreated population.
Aspartic acid.
l- and d-aspartate were bought from Sigma with the reagent grade >98% thin-layer chromatography.
RESULTS
Aspartate downregulates inflammasome components and IL-1β in mouse macrophages and KC and in human monocytes.
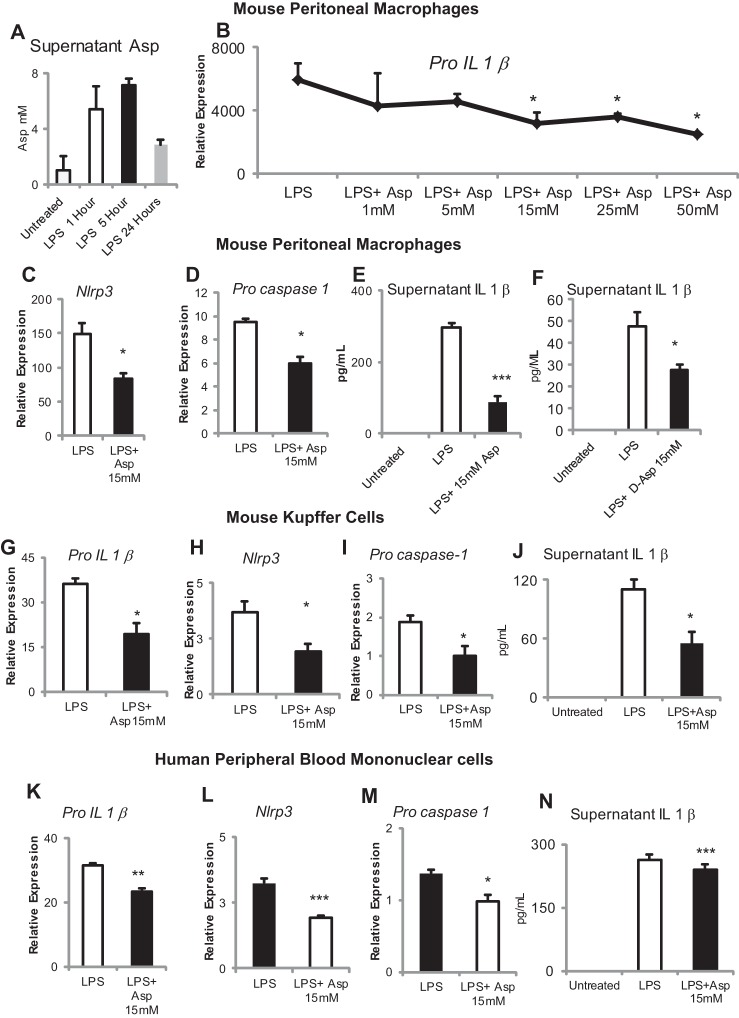
All experiments were performed with l-aspartate unless specifically stated otherwise. LPS stimulation of mouse peritoneal macrophages results in the rapid production of aspartate within 1 h, which has reduced by 24 h (Fig. 1A). Aspartate reduces transcript levels of Pro-Il-1β with an increasing dose response over the range of concentrations from 1 to 50 mM (Fig. 1B). In vitro aspartate supplementation (15 mM) of mouse peritoneal macrophages, KC, and human monocytes downregulates transcripts of inflammasome components (Pro-Il-1β, Nlrp3, Pro-caspase-1) and reduces production of active IL-1β. d-Aspartate is the isomer of the l-form that is almost exclusively present in mammals, and d-aspartate (15 mM) also significantly downregulates LPS-induced production of IL-1β (Fig. 1, C–N).
Fig. 1.
Aspartate suppresses Toll-like receptor-4 (TLR4)-mediated inflammasome signaling. A: lipopolysaccharide (LPS) treatment induces aspartate production in mouse peritoneal macrophages as measured in the supernatant by enzymatic assay. B: TLR4 agonist LPS induction of Pro-Il1β expression is significantly and dose dependently suppressed by supplementation with aspartate. Aspartate supplementation at 15 mM significantly suppresses LPS-mediated induction of NOD-like receptor family, pyrin domain containing 3 (Nlrp3, C) and Pro-caspase-1 (D). E: aspartate supplementation at 15 mM suppresses release of IL-1β in the supernatant of peritoneal macrophages in the presence of LPS priming and 5 mM ATP. F: d-Aspartate downregulates LPS-stimulated production of IL-1β in mouse peritoneal macrophages. Aspartate supplementation at 15 mM significantly suppresses LPS-mediated induction of Pro-Il1β (G), Nlrp3 (H), and Pro-caspase-1 (I) in Kupffer cells. J: aspartate supplementation at 15 mM suppresses release of IL-1β into the supernatant of Kupffer cells in the presence of LPS priming and 5 mM ATP. Aspartate supplementation at 15 mM significantly suppresses LPS-mediated induction of Pro-Il1β (K), Nlrp3 (L), and Pro-caspase-1 (M) in human peripheral mononucleocytes. N: aspartate supplementation at 15 mM suppresses release of IL-1β into the supernatant of human peripheral mononucleocytes in the presence of LPS priming and 5 mM ATP. *P < 0.05, **P < 0.01, and ***P < 0.001.
Aspartate supplementation reduces hepatic inflammasome levels and protects from acute inflammatory liver injury.
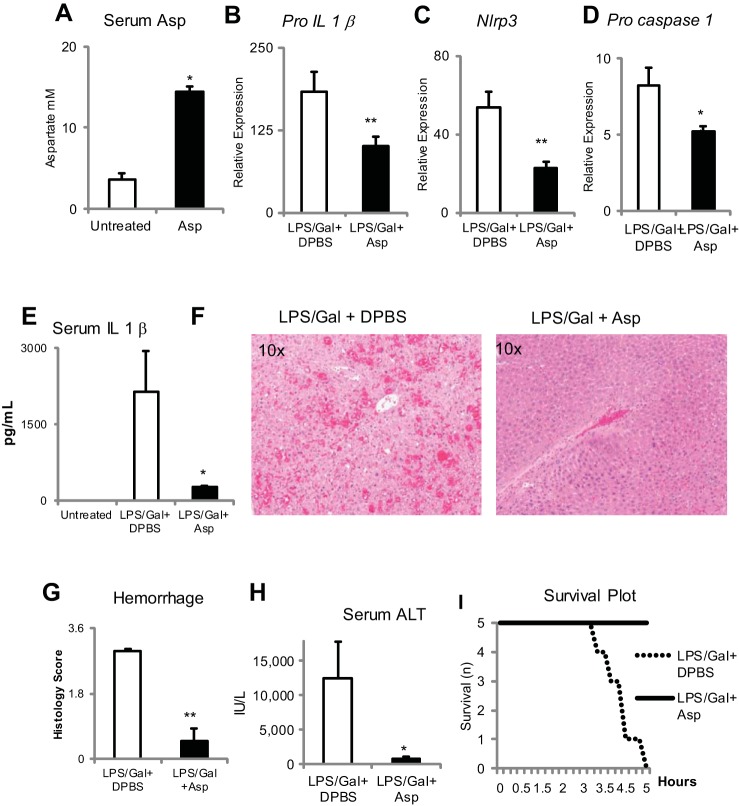
Aspartate supplementation in wild-type mice by a single intraperitoneal injection of 300 mM aspartate results in detectable increases in serum aspartate 60 min after the injection (Fig. 2A). Aspartate supplementation (single ip injection of 300 mM) 15 min after initiation of LPS/d-galactosamine injury results in the significant reduction in Pro-Il-1β, Nlrp3, and Pro-caspase-1 and reduces serum levels of IL-1β at 5 h after LPS (Fig. 2, B–E). This is associated with a reduction in liver hemorrhage and serum ALT and an improvement in survival (Fig. 2, F–I).
Fig. 2.
Aspartate supplementation can reduce TLR4-mediated proinflammatory responses in vivo in LPS d-galactosamine-induced acute hepatitis. A: aspartate supplementation increases serum aspartic acid concentration. Aspartate supplementation at 9.6 μM/g body wt by ip administration 15 min after administration of LPS and d-galactosamine results in significantly decreased induction of Pro-Il1β (B), Nlrp3 (C), and Pro-caspase-1 (D) expression in the liver and decreases serum IL-1β release (E). F: on hematoxylin and eosin stain there is decreased (G) hemorrhage. H: serum alanine aminotransferase (ALT) is also decreased. I: aspartate protects from mice mortality because of liver failure. *P < 0.05 and **P < 0.01.
Aspartate supplementation reduces pancreas inflammasome levels and protects from caerulein-induced pancreatitis.
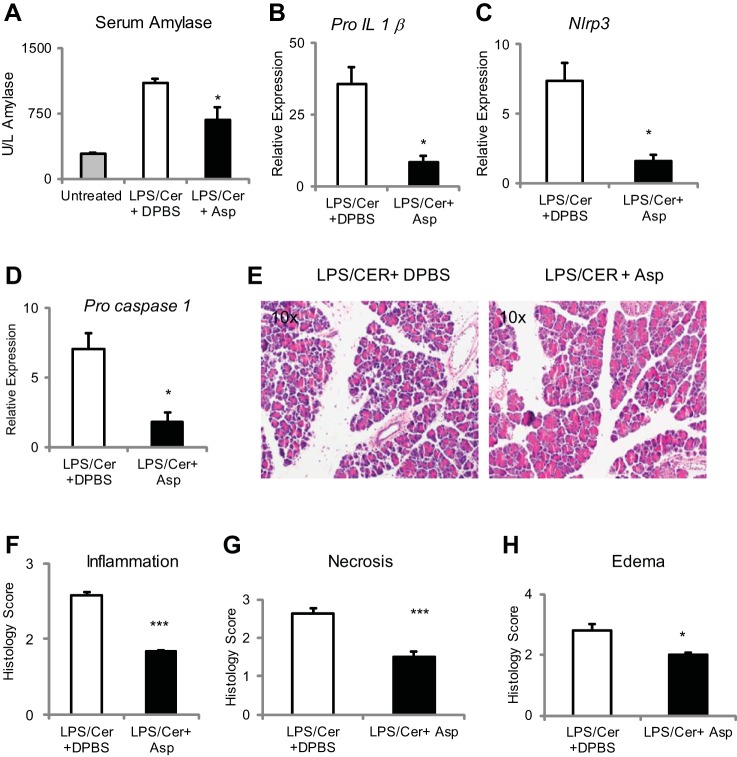
To confirm these results and to test the breadth of their applicability, the ability of aspartate to reduce inflammasome activity was tested in a second model of pancreas injury. As can be seen from Fig. 3, A-H, aspartate supplementation at 15 mM, equivalent to 9.6 μM/g body wt, by intraperitoneal injection with the first and third dose of six hourly intraperitoneal injections of caerulein sulfate at 100 pg/g body wt significantly decreased serum amylase and pancreatic Pro-IL-1β, Nlrp3, and Pro-caspase-1 induction and improved histology.
Fig. 3.
Aspartate supplementation suppresses inflammation in the LPS caerulein hyperstimulation model of acute pancreatitis. Aspartate supplementation at 9.6 μM/g body wt by ip injection with the first and third dose of six hourly intraperitoneal injections of caerulein sulfate at 100 pg/g body wt significantly decreased serum amylase (A) and pancreatic Pro-IL-1β (B), Nlrp3 (C), and Pro-caspase-1 (D) induction. On E, hematoxylin and eosin stain aspartate decreases inflammation (F), necrosis (G), and edema (H). *P < 0.05 and ***P < 0.001.
Aspartate-mediated suppression of TLR4 signaling requires the plasma membrane receptor NMDA and β-arrestin 2.
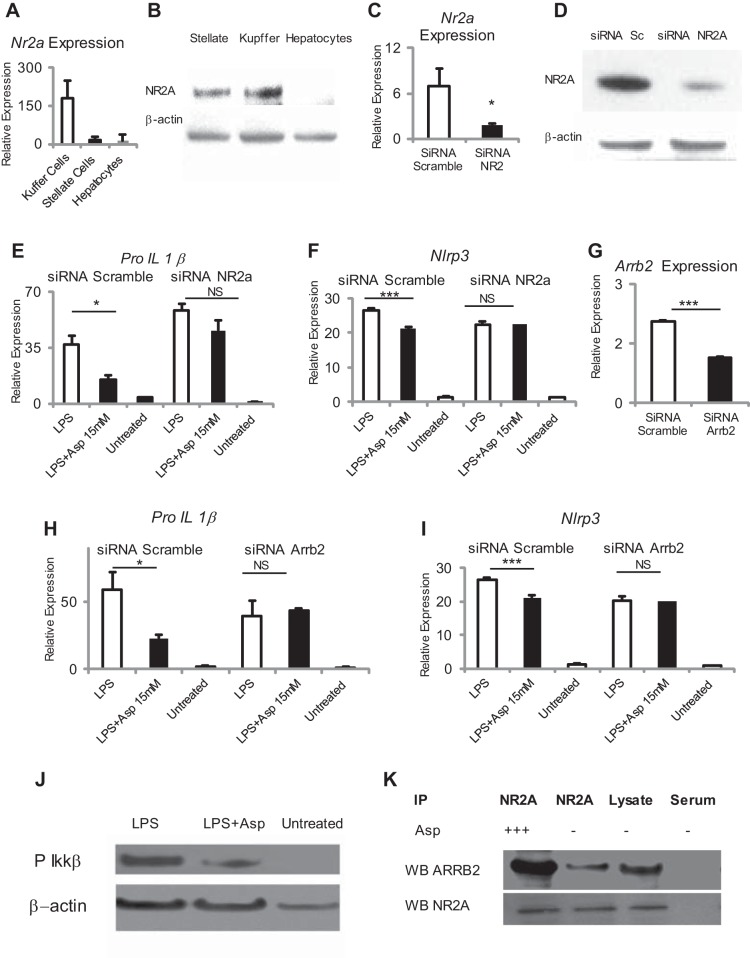
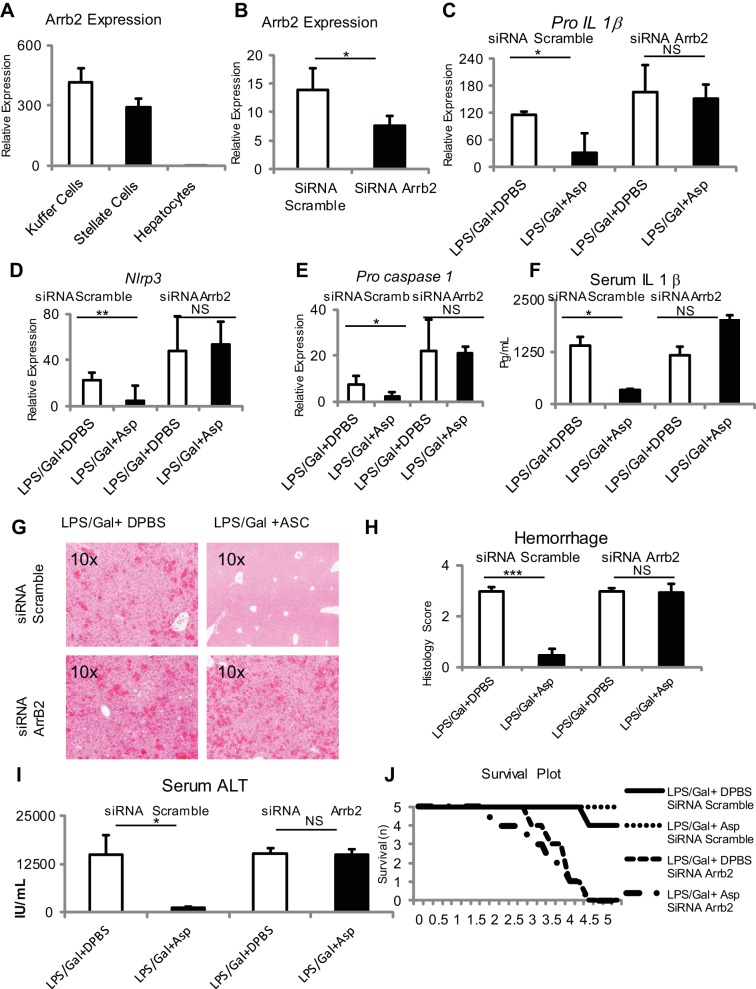
Aspartate is known to be a ligand for the NMDA receptor, which is widely expressed in the central nervous system, with limited expression in other tissues. The NMDA receptor was found to be strongly expressed in Kupffer cells and stellate cells with minimal expression in hepatocytes (Fig. 4, A and B). NMDA specific but not control siRNA successfully induced reduction in NMDA message in RAW 264.7 cells and inhibited the ability of aspartate to downregulate Pro-Il-1β and Nlrp3 (Fig. 4, C–F).
Fig. 4.
Aspartate-mediated suppression of TLR4 signaling requires the plasma membrane aspartate receptor N-methyl-d-aspartate (NMDA) and β-arrestin 2 (ARRB2). A and B: NMDA is strongly expressed in Kupffer cells and stellate cells but not hepatocytes. C and D: NMDA transcript and protein was knocked down by using targeting small-interfering RNA (siRNA) and Lipofectamine in the RAW 264.7 macrophage cell line. Selective silencing of NMDA results in loss of aspartate-mediated suppression of LPS-induced Pro-Il1β (E) and Nlrp3 (F) in RAW 264.7 cells. G: Arrb2 transcript was knocked down by using targeted siRNA and Lipofectamine in the RAW 264.7 macrophage cell line. Selective silencing of arrb2 results in loss of aspartate-mediated suppression of LPS-induced Pro-Il1β (H) and Nlrp3 (I) in RAW 264.7 cells. J: aspartate supplementation at 15 mM suppresses LPS-mediated phosphorylation of PIKKβα in peritoneal macrophages. K: NMDA binds with ARRB2; there is increased binding in the presence of aspartate. *P < 0.05 and ***P < 0.001.
To identify alternative pathways downstream from NMDA, we tested if the scaffolding protein β-arrestin-2 was required for the inhibition of Pro-Il1β using Arrb2 specific siRNA in the RAW 264.7 cell line. The specific siRNA induced a reduction in Arrb2 message (Fig. 4G). This resulted in inhibition of the ability of aspartate to downregulate Pro-Il1β and Nlrp3, demonstrating a requirement for Arrb2 (Fig. 4, H and I). LPS-induced upregulation of Pro-Il1β and Nlrp3 is known to require NF-κβ activation, and Arrb2 is required for endogenous suppression of NF-kβ activation in other systems. By using phospho-Ikkβα we established that aspartate decreases NF-κβ activity phosphorylation of phospho-Ikkβα (Fig. 4J). Finally, to demonstrate a direct binding interaction between NR2A and β-arrestin-2, we immunoprecipitated NR2A from the RAW 264.7 cell line and demonstrated that β-arrestin-2 is increased by aspartate and is associated NR2A (Fig. 4K). Collectively, these data show that aspartate-induced downregulation of TLR-induced signal 1 is via a NMDA-β-arrestin-2 and resulted in downregulation of the well-known downstream NF-κβ pathway.
β-Arrestin-2-induced immune regulation is providing a significant degree of downregulation of the inflammatory response, and supplementation with aspartate can further increase this protective effect.
β-Arrestin-2 is strongly expressed in Kupffer and stellate cells (Fig. 5A). To test the in vivo role of β-arrestin-2, β-arrestin-2 expression was reduced using Invivofectamine and siRNA. Five hours after initiation of fulminant hepatitis by LPS and d-galactosamine, whole liver expression of Pro-Il1β, Nlrp3, and Pro-caspase-1, liver hemorrhage, and serum transaminase were significantly greater in the β-arrestin-2 knockdown group (Fig. 5, B–I). In addition, aspartate was unable to downregulate Pro-Il1β, Nlrp3, and Pro-caspase-1 and was unable to reduce liver hemorrhage and to downregulate serum transaminase (Fig. 5, B–I). The β-arrestin-2 knockdown group also showed increased mortality in the presence of LPS and d-galactosamine (Fig. 5J).
Fig. 5.
β-Arrestin is required for dampening proinflammatory responses in vivo, and aspartate supplementation can further dampen proinflammatory responses in vivo. A: β-Arrestin is strongly expressed in Kupffer cells and stellate cells relative to hepatocytes. B: Selective knockdown of β-arrestin is achieved in whole liver using Invivofectamine and β-arrestin targeting siRNA as determined by β-arrestin transcript. In vivo knockdown of β-arrestin results in significant loss of aspartic acid-mediated suppression of LPS d-galactosamine-induced Pro-Il1β (C), Nlrp3 (D), and Pro-caspase-1 (E) expression in the liver, serum IL-1β release in the serum (F), liver hemorrhage (G and H), serum ALT values (I), and progression of injury to mortality (J) in mice treated with LPS and d-galactosamine at 5 h posttreatment. *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
In the setting of an acute infection or insult, the inflammatory response is activated in minutes and provides protection during the few days that it takes adaptive immunity to develop. In many diseases states, there is chronic inflammation, and these include significant liver diseases such as alcoholic hepatitis, nonalcoholic steatohepatitis, and fibrosis. It is now clear that acute and chronic inflammatory responses can add to the total tissue injury that occurs, making it important to identify how it is regulated. Complex interactions are occurring between inflammation and metabolism, and their importance is increasingly appreciated with the identification of mechanistic pathways (10, 27, 29). One aspect of this interrelationship is the change in metabolism that occurs with the initiation of inflammation. In macrophages, activation by TLR ligands results in a large shift of the flux through metabolic pathways, with a reduction in oxidative phosphorylation, and many times increases in glycolysis (20). This results in a rapid increase in the rate of production of ATP and the precursors required for synthesis of various molecules. The complementary aspect of this relationship is the effect of metabolites on the regulation of inflammatory responses, and a large number of metabolites have been identified as ligands for orphan G protein-coupled receptors (31).
The cytosolic complex consisting of the sensor NLRP3, the adaptor ASC, and caspase-1 (NLRP3 inflammasome) is central to the development of inflammation and is required for experimental drug-induced liver injury, alcoholic and nonalcoholic hepatitis, and liver fibrosis. Because of the increase in aspartate in acute inflammation, we investigated the ability of aspartate, via the NMDA receptor, to regulate inflammasome activity (6). In the set of in vitro experiments using primary mouse peritoneal macrophages and Kupffer cells as well as human peripheral blood monocytes, we demonstrate that aspartate can reduce the expression level of inflammasome components and production of IL-1β (Fig. 1). In vivo this translates to reduced inflammation and injury in two models of liver injury and one model of pancreatitis and is due to a NMDA, β-arrestin-2, NF-κβ pathway. In addition to providing protection by supplementing with aspartate, knockdown of this pathway results in increased liver injury.
The structure and function of the NMDA receptor in the central nervous system has been well characterized (32). The four subunits of the NMDA receptor assemble to form a ligand-gated ion channel containing an agonist recognition site, a transmembrane ion permeation pathway, and gating elements. NMDA activation-induced dendrite spine remodeling in neurons is dependent on the scaffolding protein β-arrestin-2 (24). This was of interest because β-arrestin-2 is known to modulate LPS-induced inflammatory responses, but its role in inflammasome activation is not known (5, 22, 25, 33). Our results clearly show that aspartate-dependent downregulation of inflammasome function in vitro and in vivo is dependent on β-arrestin-2.
The demonstration of the ability of NMDA receptor activation to downregulate inflammasome activation has broad implications for liver pathology. In acute inflammatory liver injury, for example, there is a significant increase in glutamate and aspartate (6). The importance of this is evidenced by increased liver injury upon loss of the NMDA signaling pathway and the hepatoprotection provided with aspartate supplementation. Furthermore, ethanol has been proposed to be a noncompetitive antagonist of the NMDA receptor in neurons (9, 18). If the same effect is present in Kupffer cells, it will result in loss of NMDA-induced downregulation of inflammasome activity and may provide another mechanism of the hepatic immune dysregulation induced by ethanol. Not surprisingly, serum levels of the NMDA ligands glutamine and aspartate are elevated by the administration of total parenteral nutrition (TPN) to patients (4). In addition, use of TPN enriched with glutamine has been shown to be associated with reduced inflammation in inflammatory bowel disease (21). Of great interest is the further possibility that the general immunosuppression seen with TPN may be due to activation of this pathway.
The NMDA receptor is unusual, since it is activated by l- and d-isomers of amino acids, and we have confirmed that the d-isomer of aspartate can downregulate inflammasome activity (Fig. 1F) (15, 26). This may be irrelevant for most organs, since d-isomers of amino acids are very rare in vertebrates. Bacteria, however, make large amounts of d-amino acids (3). In particular, the cell wall of bacteria is rich in d-glutamate, with the stem peptides of bacterial peptidoglycan being rich in d-amino acids. This is thought to be of functional benefit to bacteria, since it provides resistance to mammalian proteases (17). In addition, bacteria can release d-isomers of amino acids (12). Recently, the ability of the intestinal microbiome to regulate liver inflammation was established and was due in part on the ability of bacterial components in the portal blood (7). A similar effect of gut microbiome on adiposity has been demonstrated via the G protein-coupled receptor Gpr41 (28). This established that bacterial components can enter the portal circulation and regulate liver inflammation. The demonstration that l- and d-isomers of amino acids can induce inflammasome suppression provides another potential mechanism by which the intestinal microbiome can regulate liver inflammation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-076674-01A2, a Veterans Affairs Merit Award (W. Z. Mehal), NIDDK Grant K08-DK-092281 (R. Hoque), and the Yale Liver Centre.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Ahmad Farooq and W.Z.M. conception and design of research; Ahmad Farooq, R.H., X.O., Ahsan Farooq, A.G., M.K.A., M.G., and W.Z.M. performed experiments; Ahmad Farooq, Ahsan Farooq, and W.Z.M. analyzed data; Ahmad Farooq and W.Z.M. interpreted results of experiments; Ahmad Farooq, Ahsan Farooq, and W.Z.M. prepared figures; Ahmad Farooq and W.Z.M. drafted manuscript; Ahmad Farooq and W.Z.M. edited and revised manuscript; Ahmad Farooq and W.Z.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Michael Nathanson for help in the project. We also acknowledge Dr. Khalid J. Qazi and Dr. Henry Woodman, Program Directors at Catholic Health System, University at Buffalo, for support in the project.
REFERENCES
- 1.Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci 68: 765–783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brothwell SL, Barber JL, Monaghan DT, Jane DE, Gibb AJ, Jones S. NR2B- and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol 586: 739–750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cava F, Lam H, de Pedro MA, Waldor MK. Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell Mol Life Sci 68: 817–831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 18: 761–766, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. Beta-arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol 44: 3092–3099, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng B, Wu S, Lv S, Liu F, Chen H, Yan X, Li Y, Dong F, Wei L. Metabolic profiling analysis of a d-galactosamine/lipopolysaccharide-induced mouse model of fulminant hepatic failure. J Proteome Res 6: 2161–2167, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482: 179–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol 13: 321–324, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology 33: 1379–1390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115: 4742–4749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubes P, Mehal WZ. Sterile Inflammation in the liver. Gastroenterology 143: 1158–1172, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. d-Amino acids govern stationary phase cell wall remodeling in bacteria. Science 325: 1552–1555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, Mehal WZ. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci USA 108: 20095–20100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7: 31–40, 2007 [DOI] [PubMed] [Google Scholar]
- 15.McBain CJ, Kleckner NW, Wyrick S, Dingledine R. Structural requirements for activation of the glycine coagonist site of N-methyl-d-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol 36: 556–565, 1989 [PubMed] [Google Scholar]
- 16.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139: 323–334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata Y, Fujiwara T, Kawaguchi-Nagata K, Fukumori Y, Yamanaka T. Occurrence of peptidyl d-amino acids in soluble fractions of several eubacteria, archaea and eukaryotes. Biochim Biophys Acta 1379: 76–82, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol 6: 39–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-d-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol 475: 83–93, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493: 346–355, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Ockenga J, Borchert K, Stuber E, Lochs H, Manns MP, Bischoff SC. Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur J Clin Nutr 59: 1302–1309, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, Benovic JL. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem 281: 34159–34170, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 122: 3476–3489, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontrello CG, Sun MY, Lin A, Fiacco TA, DeFea KA, Ethell IM. Cofilin under control of beta-arrestin-2 in NMDA-dependent dendritic spine plasticity, long-term depression (LTD), and learning. Proc Natl Acad Sci USA 109: E442–451, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter KJ, Gonipeta B, Parvataneni S, Appledorn DM, Patial S, Sharma D, Gangur V, Amalfitano A, Parameswaran N. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by beta-arrestins. J Cell Physiol 225: 406–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pullan LM, Olney JW, Price MT, Compton RP, Hood WF, Michel J, Monahan JB. Excitatory amino acid receptor potency and subclass specificity of sulfur-containing amino acids. J Neurochem 49: 1301–1307, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 185: 605–614, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105: 16767–16772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462: 745–756, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonack S, Tang C, Offermanns S. Endogenous metabolites as ligands for G protein-coupled receptors modulating risk factors for metabolic and cardiovascular disease. Am J Physiol Heart Circ Physiol 304: H501–H513, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 7: 139–147, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS, Mahmood S, Jhandier MN, Shi Y, Flavell RA, Mehal WZ. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 296: G1248–G1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci 18: 638–649, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]