Abstract
Mast cells express the substance P (SP) neurokinin 1 receptor and the calcitonin gene-related peptide (CGRP) receptor in guinea pig and human small intestine. Enzyme-linked immunoassay showed that activation of intramural afferents by antidromic electrical stimulation or by capsaicin released SP and CGRP from human and guinea pig intestinal segments. Electrical stimulation of the afferents evoked slow excitatory postsynaptic potentials (EPSPs) in the enteric nervous system. The slow EPSPs were mediated by tachykinin neurokinin 1 and CGRP receptors. Capsaicin evoked slow EPSP-like responses that were suppressed by antagonists for protease-activated receptor 2. Afferent stimulation evoked slow EPSP-like excitation that was suppressed by mast cell-stabilizing drugs. Histamine and mast cell protease II were released by 1) exposure to SP or CGRP, 2) capsaicin, 3) compound 48/80, 4) elevation of mast cell Ca2+ by ionophore A23187, and 5) antidromic electrical stimulation of afferents. The mast cell stabilizers cromolyn and doxantrazole suppressed release of protease II and histamine when evoked by SP, CGRP, capsaicin, A23187, electrical stimulation of afferents, or compound 48/80. Neural blockade by tetrodotoxin prevented mast cell protease II release in response to antidromic electrical stimulation of mesenteric afferents. The results support a hypothesis that afferent innervation of enteric mast cells releases histamine and mast cell protease II, both of which are known to act in a diffuse paracrine manner to influence the behavior of enteric nervous system neurons and to elevate the sensitivity of spinal afferent terminals.
Keywords: mast cell degranulation, histamine, mast cell proteases, functional gastrointestinal disorders, irritable bowel syndrome, visceral pain
experimental antidromic stimulation of the sensory innervation of the airways or skeletal joints evokes neurogenic inflammation, associated with hypersensitivity in the respiratory system and arthritic pain in the joints (11, 21, 33, 41). O'Connor et al. (51) reviewed evidence implicating afferent release of substance P (SP) as a mediator for neurogenic inflammation in skeletal joints and the respiratory system. Aside from the airways and joints, afferent sensitization is implicated as a factor in multiple abdominal and pelvic pain syndromes, which include chronic urological pain, irritable bowel syndrome (IBS), prostatitis, fibromyalgia, and vulvodynia (25, 26, 79, 92). Most of these fall into a so-called functional pain category, because no abnormal physical or metabolic processes that explain the symptoms can be identified.
Overlapping morbidity is frequent for one or more of the functional pain syndromes. For example, symptoms associated with IBS have a high rate of overlap with symptoms of interstitial cystitis in the urinary tract (25). Francis and Whorwell (26) reported that one-third of patients attending urological clinics for symptoms of pelvic pain also had a diagnosis of IBS. Women are impacted in a majority of the cases, and the symptoms can be sufficiently severe as to compromise ability to function in daily life (20). Comorbidity of IBS and fibromyalgia attracts attention in terms of translational sensory physiology, because IBS is a visceral pain syndrome, with lowered threshold to bowel distension, while fibromyalgia is somatic, with lowered thresholds for tactile stimulation. A subset of patients with both IBS and fibromyalgia experience a combination of visceral and somatic hypersensitivity (12, 13).
Vagal and spinal afferent terminals in the digestive tract express receptors for several mast cell degranulation products, including 5-hydroxytryptamine, bradykinin, ATP, adenosine, prostaglandins, leukotrienes, histamine, and mast cell proteases (5, 6, 10, 40). Any one of these inflammatory- or ischemia-related degranulation products, when applied experimentally, stimulates the terminals to fire. This endows mast cell degranulation products with a potential for elevating the sensitivity of an afferent to its preferred stimulus modality (e.g., mechanical, chemical, temperature, or nociception), especially in disordered conditions associated with inflammation or ischemia. This possibility is reinforced by findings of a reduced threshold for painful responses to balloon distension in the large bowel associated with degranulation of mast cells in animal models (14, 54). Treatment with mast cell-stabilizing drugs prevents lowering of the pain threshold to distension during mucosal inflammation in these models.
Neurons in the enteric nervous system (ENS) express the same array of receptors for mast cell degranulation products as found on vagal and spinal afferents (9, 30, 85, 87). Stimulation of neuronal excitability, presynaptic suppression of neurotransmitter release, and activation of an ENS network that elevates mucosal secretion in concert with powerful aboral propulsive motility can result from mast cell immunoneural communication in the small and large intestine (81, 87, 91).
In view of the several kinds of evidence for functional interactions among sensory nerves, mast cells, and the ENS, we aimed to investigate the effects of antidromic stimulation of intestinal spinal afferents on mast cell degranulation and electrophysiological and synaptic behavior of ENS neurons. Our results suggest that enteric mast cells receive input from spinal afferents, which in turn evoke the release of mast cell degranulation products that might feed back as paracrine mediators at receptors expressed by the same afferent terminals and simultaneously act in a paracrine manner at receptors on ENS neurons.
MATERIALS AND METHODS
Our methods for procurement of tissues for in vitro study were the same as those described in our previous study (77). Segments of ileum 10–20 cm proximal to the ileocecal junction and segments of jejunum ∼20 cm distal from the gastroduodenal junction and from the colon were obtained from male Hartley-strain guinea pigs (300–400 g; Charles River, Wilmington, MA). The animal care and experimental protocols were approved by The Ohio State University Laboratory Animal Care and Use Committee and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Fresh preparations of healthy human small intestine were obtained from segments of jejunum discarded during Roux-en-Y gastric bypass surgeries, as described in earlier papers from our laboratory (23, 61). The Institutional Review Board of The Ohio State University Office of Research Risks Protection approved the human protocols (protocol 02H0208). Preparations from the small intestine were obtained by microdissection for immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), organ bath pharmacology, and neuronal electrophysiology.
Immunohistochemistry.
Whole-mount immunohistochemistry was carried out as we described elsewhere (23, 61). Therefore, the current presentation of methods summarizes and quotes our previous studies (23, 61). Whole-mount preparations were obtained by dissection from segments of guinea pig and human small intestine and transferred to disposable chambers filled with fixative solution containing 2% formaldehyde and 0.2% of a saturated picric acid solution in 0.1 M phosphate-buffered saline (PBS). Nonspecific immunological binding was blocked with 10% normal donkey serum in 0.01 M PBS, pH 7.4, for 1 h at room temperature. The tissues were incubated with the primary antibodies (Table 1) diluted in 0.01 M PBS containing 10% normal donkey serum, 0.3% Triton X-100, and 0.05% sodium azide overnight at 4°C after incubation with the primary antibodies (Table 1). The tissues were washed (3 times for 10 min each time) in PBS, pH 7.4, and incubated with appropriate secondary antibodies conjugated with fluorescein isothiocyanate or indocarbocyanin diluted in 0.01 M PBS. The tissues were then rinsed in PBS, and coverslips were mounted using VECTASHIELD (Vector Laboratories, Burlingame, CA). Preabsorption of the antibodies with 10 μg of receptor protein was done as described for controls. Specificity of immunostaining was evaluated further by omission of the primary or the secondary antibody.
Table 1.
Codes and sources of primary antibodies
| Antigen | Host | Code | Dilution | Sources |
|---|---|---|---|---|
| Anti-Hu | Mouse | A21271 | 1:200 | Molecular Probes |
| NK1 | Rabbit | S8305 | 1:1,000 | Sigma |
| Anti-S100 | Mouse | S2644 | 1:1,000 | Chemicon |
| Tryptase | Mouse | VP4IV433 | 1:500 | Chemicon |
| Tryptase | Mouse | MAb1222 | 1:500 | Chemicon |
| Chymase | Mouse | MAb1254 | 1:500 | Sigma |
| SP | Rat | MAb356 | 1:1,000 | EMD Millipore |
| VR1 | Goat | SC12498 | 1:200 | Calbiochem |
| VR1 | Mouse | PC420 | 1:1,000 | Chemicon |
| CGRP | Mouse | MAb317 | 1:200 | Vector Laboratories |
| CGRP-R | Rabbit | H-001-37 | 1:200 | Phoenix Pharmaceuticals |
Anti-Hu, anti-human neuronal protein HuC/HuD; NK1, neurokinin 1; anti-S100, low-molecular-weight protein; SP, substance P; VR1, transient receptor potential cation channel subfamily V member 1; CGRP, calcitonin gene-related peptide; CGRP-R, CGRP receptor.
Immunohistochemistry was carried out also with cryostat sections obtained from guinea pig and human intestine as we described in our previous study (77). The guinea pigs were anesthetized with 20% urethane and infused transcardially with chilled 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Segments of human jejunum were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer overnight at 4°C. The fixed tissues were washed in 0.2 M PBS and then dehydrated in 30% sucrose overnight at 4°C. Thereafter the tissues were frozen and embedded in optimal cutting temperature compound (Tissue-Tek OCT, Ted Pella, Redding, CA). Sequential 4-μm sections were cut on a cryostat microtome. To block endogenous peroxidase activity, the sections were treated with 0.3% hydrogen peroxide solution for 30 min and then with normal horse and goat serum at room temperature for 2 h to block nonspecific protein binding. Pretreated sections were incubated with one or the other of the primary antibodies (Table 1) overnight at 4°C. After incubation, the sections were washed with 0.01 M PBS (pH 7.4) and incubated with biotin-labeled secondary antibody for 60 min. At the end of secondary antibody incubation, the sections were rinsed with PBS and incubated in VECTASTAIN Elite ABC system reagents (Vector Laboratories) for 30 min. All experiments were done in humidified chambers. Parallel sections incubated with nonimmune serum were negative controls.
Whole-mount preparations were examined with an epifluorescence microscope (Nikon Eclipse-1000, Nikon, Melville, NY) and analyzed with filter combinations that enabled separate visualization of multiple fluorophores. Tissue sections were examined with general light microscopy. Digital images were obtained with a SPOT RT cooled CCD digital camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed with SPOT III software. Contrast in the digital images was sometimes enhanced before the JPEG file was converted to interchange format (*.jpg) for electronic transfer or printed as photomicrographs.
Electrophysiology.
Effects of electrically stimulating perivascular nerve bundles in the mesentery were recorded with intracellular “sharp” microelectrodes in ENS neurons. Electrophysiological and synaptic behavior was studied in the same manner as described elsewhere (38, 82). Therefore, the following presentation of methods summarizes and quotes from these studies.
Conventional sharp intracellular microelectrodes, filled with 4% biocytin (Sigma, St. Louis, MO) in 2 M KCl and containing 0.05 M tris-(hydroxymethyl)-aminomethane buffer (pH 7.4), had resistances of 80–200 MΩ. The preamplifiers (model M-767, World Precision Instruments, Sarasota, FL) had bridge circuitry for intraneuronal injection of electrical current. Constant-current rectangular pulses were driven by a Grass SD9 stimulator (Grass Instrument Division, Astro-Med, W. Warwick, RI). Data were digitized and stored for analysis.
At the end of each electrophysiological study, the anal end of the preparation was marked and the tissue was washed three times in cold Krebs solution, placed in cold fixative in a disposable recording chamber, and left overnight at 4°C. The fixative contained 2% formaldehyde in a 15% solution of picric acid. The preparations were cleared in three changes of dimethylsulfoxide and three 10-min washes with PBS (0.9% NaCl in 0.01 M sodium phosphate buffer, pH 7.0). They were then reacted with fluorescein isothiocyanate-conjugated streptavidin. After a thorough wash in PBS, coverslips were applied using VECTASHIELD (Vector Laboratories), and the preparations were examined with a Nikon Eclipse E600 microscope. Images were digitized with a SPOT 2 chilled color and black-and-white camera (Diagnostic Instruments) and analyzed with SPOT III software.
ELISA.
ELISAs were carried out as we described elsewhere (77). Therefore, the current presentation of methods summarizes and quotes our previous study (77). Protocols for study of SP or calcitonin gene-related peptide (CGRP) release after 40 min of equilibrium were as follows: 1) allow 10 min for basal release to occur and then withdraw three samples for analysis of SP and CGRP content and 2) electrically stimulate perivascular nerve bundles in the mesentery of intestinal segments or apply capsaicin for 15–20 s and then withdraw three samples for SP and CGRP assay. The samples were centrifuged at 10,000 rpm for 15 min, and the supernatants were stored at −20°C until assayed. Amounts of CGRP and SP in the supernatants were determined with ELISA kits designed for the purpose. Assay kits for SP (catalog no. 583751) and CGRP (catalog no. A05482) were obtained from Cayman Chemical (Ann Arbor, MI). For termination of each experiment, the segments were removed from the bathing medium and blotted on filter paper, and the weight was recorded. Data are expressed as nanograms or picograms per gram of tissue weight.
Reagents and antibodies.
SP, CGRP, CGRP-(8–37), RP 67580, capsaicin, GR15897, SB218795, capsazepine, tetrodotoxin (TTX), and SB366791 were purchased from Tocris (Ellisville, MO). Capsaicin was purchased from Sigma Biochemicals (St. Louis, MO). The antibodies and antisera for immunochemical staining are listed in Table 1. Histamine ELISA kits were obtained from SPI-BIO (catalog no. A05890, Massy Cedex, France) and Oxford Biomedical Research (catalog no. EA31, Oxford, MI). SP ELISA kits (catalog no. 583751) and CGRP ELISA kits (catalog no. A05482) were purchased from Cayman Chemical (Ann Arbor, MI). Mast cell protease II ELISA kits were purchased from Moredun Scientific (Penicuik, Midlothian, Scotland, UK).
Data analysis.
Values are means ± SE. Student's t-test and paired t-test were used for statistical analysis of significance, with P < 0.05 accepted as significant. Concentration-response relationships were determined using the following least-squares fitting routine: V = Vmax/[1 + (EC50/C)nH], where V is the observed response, EC50 is the concentration that induces the half-maximal response, C is concentration, and nH is the apparent Hill coefficient. Plots were drawn by averaging results from all experiments and fitting to single concentration-response curves with Sigma Plot software (SPSS, Chicago, IL).
RESULTS
Immunohistochemistry.
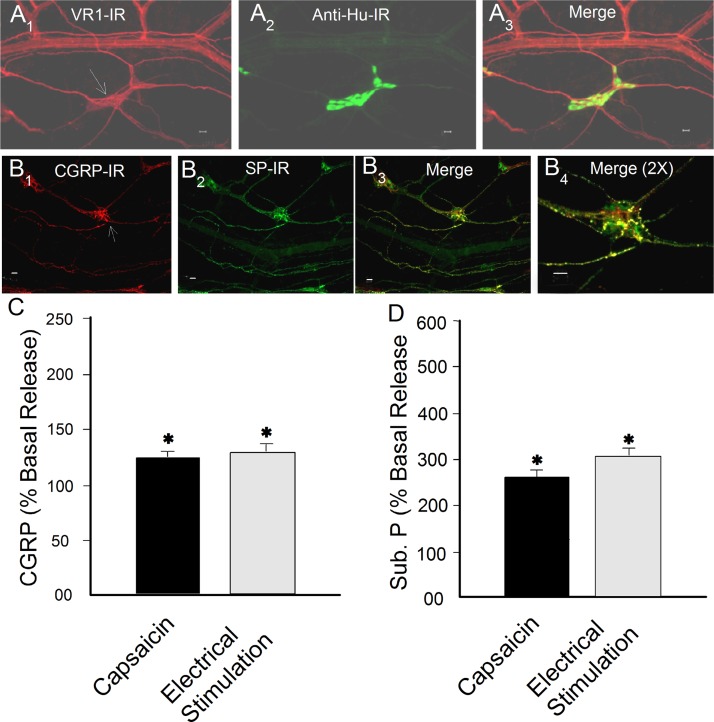
We confirmed that coexpression of immunoreactivity (IR) for the transient receptor potential cation channel subfamily V member 1 (VR1), SP, and CGRP is a reliable marker for intramural spinal afferents in our preparations, as described in recent reviews by others (Fig. 1) (5, 10). VR1-IR was expressed in 99.2% (2,370 of 2,390) of SP-IR fibers and 98.4% (1,810 of 1,840) of CGRP-IR fibers in 19 small intestinal whole-mount preparations. CGRP-IR and SP-IR were colocalized to every observable small-diameter nerve fiber in 14 whole-mount preparations. VR1-IR was not found in the neuronal cell bodies in the myenteric plexus or submucosal plexus of the jejunum or ileum from 21 guinea pigs. Small-diameter VR1-IR fibers ran parallel to blood vessels and diverged to enter ganglia (Fig. 1A). Fibers with SP- and CGRP-IR likewise entered the same ganglia (Fig. 1B).
Fig. 1.
Universal coexpression of immunoreactivity (IR) for transient receptor potential cation channel subfamily V member 1 (VR1), substance P (SP), and calcitonin gene-related peptide (CGRP) by intramural spinal afferents in the submucosal plexus of guinea pig small intestine. A1: VR1-IR. A2: IR for anti-Hu, a pan-enteric neuronal marker that labels neuronal cell bodies in a ganglion. A3: VR1-IR fibers project along blood vessels and enter the ganglion. B1, B2, and B3: afferent fibers that coexpressed CGRP-IR and SP-IR projected into a ganglion in the submucosal plexus. B4: B3 at higher magnification. Calibration = 20 μm. C and D: electrical stimulation of spinal afferents or exposure to capsaicin evoked release of CGRP and SP (Sub P) into the bathing medium of intact small intestinal segments from guinea pigs. *P < 0.05 vs. basal release.
Enteric mast cells.
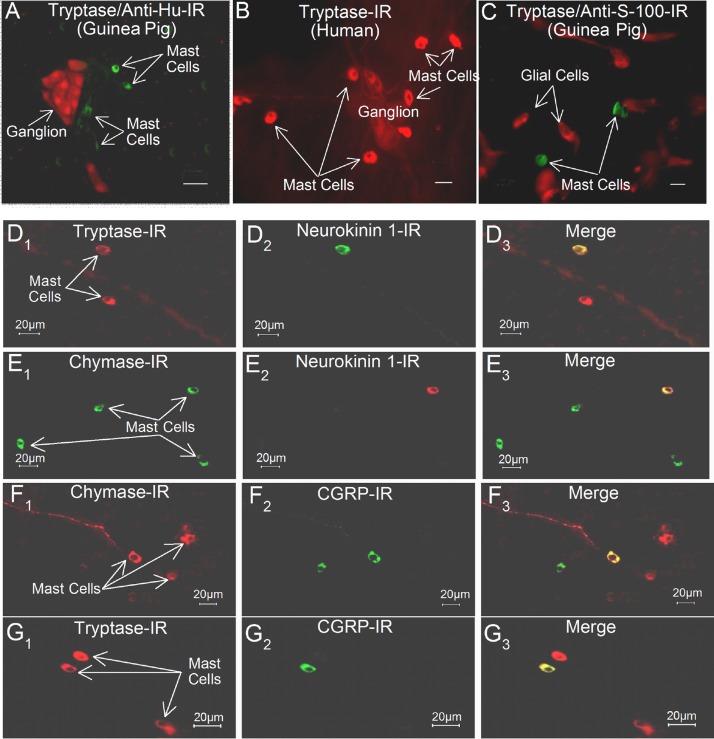
We used IR for mast cell tryptase and chymase as markers for identification of intramural enteric mast cells (Figs. 2 and 3). Primary antibodies to chymase- or tryptase-labeled (Table 1) 8- to 10-μm-diameter single cells and characteristics common for enteric mast cells (63, 77). Preabsorption of the antibodies with 10 μg of chymase or tryptase always abolished the immunostaining. Chymase- and tryptase-IR mast cells were widely distributed, with one or more in close apposition to ganglia in the myenteric or submucosal plexus (Fig. 2). Double immunolabeling revealed expression by mast cells of SP and CGRP receptor protein in guinea pig and human small intestine (Figs. 2 and 3). Expression of tryptase- or chymase-IR was never found to be associated with glial cells that were colabeled for their S-100 protein marker (Fig. 2C).
Fig. 2.
Localization of mast cell tryptase, SP neurokinin (NK) type 1 (NK1) receptor, and CGRP receptor in whole-mount preparations of the submucosal plexus of guinea pig and human small intestine. A: mast cells expressing tryptase-IR (green) are in close proximity to a submucosal ganglion in which the neurons express IR for the pan-neuronal marker anti-Hu (red) in guinea pig. B: mast cells expressing trypsin-IR (red) are in close proximity to a submucosal ganglion in human small intestine. C: mast cells expressing tryptase-IR are distinct from glial cells that express IR for S-100 protein (red). D1: tryptase-IR. D2: NK1 receptor-IR. D3: merged image. E1: chymase-IR. E2: NK1 receptor-IR. E3: merged image. F1: chymase-IR. F2: CGRP receptor-IR. F3: merged image. G1: tryptase-IR. G2: CGRP receptor-IR. G3: merged image. Calibration = 20 μm.
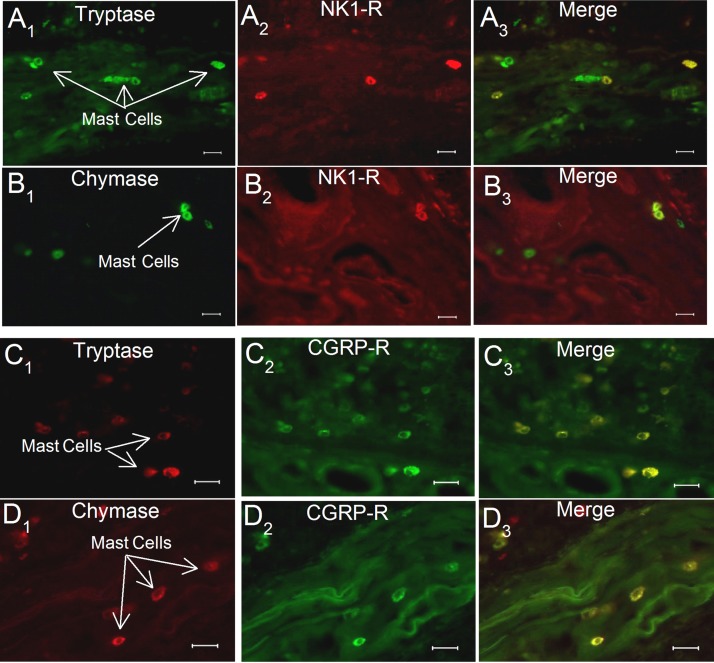
Fig. 3.
Enteric mast cells express IR for the SP NK1 receptor and the receptor for CGRP in cryostat sections of human jejunal mucosa and lamina propria. A1: tryptase-IR. A2: NK1 receptor-IR. A3: merged image. B1: chymase-IR. B2: NK1 receptor-IR. B3: merged image. C1: tryptase-IR. C2: CGRP receptor-IR. C3: merged image. D1: chymase-IR. D2: CGRP receptor-IR. D3: merged image. Calibration = 20 μm.
Expression of the neurokinin (NK) 1 (NK1) receptor for SP was found for 58% (87 of 149) of tryptase-IR cells and 47% (63 of 132) of chymase-IR cells in guinea pigs. CGRP receptor protein was expressed in 20% (37 of 182) of tryptase-IR cells and 13% (32 of 235) of chymase-IR cells in guinea pigs. In human small intestine, CGRP receptor-IR was expressed in 15% (47 of 298) of tryptase-IR cells and 12% (52 of 423) of chymase-IR cells. NK1-IR was expressed in 17% (79 of 465) of tryptase-IR cells and 11% (42 of 432) of chymase-IR cells.
Electrophysiology.
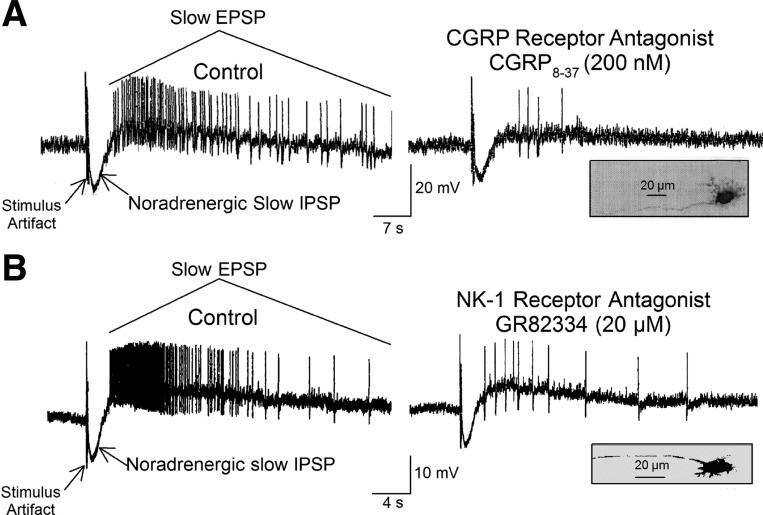
Antidromic electrical stimulation of perivascular nerve bundles in the mesentery evoked synaptic responses consisting of a canonical noradrenergic slow inhibitory postsynaptic potential followed by a characteristic slow excitatory postsynaptic potential (EPSP) in submucosal neurons (Fig. 4) (62, 83, 84, 86). The slow EPSPs in each of 11 synaptic (S)- and 2 afterspike hyperpolarization (AH)-type ENS neurons were suppressed in the presence of the CGRP receptor antagonist CGRP-(8–37) (Fig. 4A). Slow EPSPs in eight S-type and two AH-type neurons were suppressed by the NK1 receptor antagonist GR82334 in the same neurons in which the CGRP receptor antagonist suppressed stimulus-evoked EPSPs (Fig. 4B). Pretreatment with the nonpeptide NK2 receptor antagonist GR15897 or the nonpeptide NK3 receptor antagonist SB218795 did not change slow EPSP responses evoked by mesenteric nerve stimulation (data not shown). Cromolyn (5 μM) was present throughout the experiment to prevent release of excitatory mast cell mediators, the actions of which mimic slow synaptic excitation.
Fig. 4.
Slow inhibitory postsynaptic potentials (IPSPs) and slow excitatory postsynaptic potentials (EPSPs) evoked in a submucosal neuron by electrical stimulation of perivascular nerve bundles in the intestinal mesentery. A: electrical stimulation evoked a slow IPSP followed by a slow EPSP, which aborted the IPSP. Suppression of the EPSP by the CGRP receptor antagonist CGRP-(8–37) identifies the EPSP as CGRP-mediated. B: electrical stimulation evoked a slow IPSP followed by a slow EPSP, which aborted the IPSP. Suppression of the EPSP by the selective NK1 tachykinin receptor antagonist GR82334 identifies the EPSP as a SP-mediated EPSP. Insets show morphology of the uniaxonal neurons from which the recordings were made. Cromolyn (5 μM) was present throughout the experiment to prevent release of excitatory mast cell mediators. Both neurons exhibited synaptic (S)-type electrophysiological behavior.
Compound 48/80.
Application of the mast cell secretogogue compound 48/80 (80 μg/ml) in the bathing medium elevated the excitability of AH-type neurons in the myenteric and submucosal plexuses (Fig. 5A1). Elevated excitability was reflected by depolarization of the membrane potential, increased input resistance, suppression of the AH, and augmented excitability, recorded as increased frequency of action potential discharge during intraneuronal injection of rectangular, constant-current depolarizing pulses. Presence of the mast cell stabilizer cromolyn (5 μM) in the bathing medium suppressed the responses to compound 48/80 (Fig. 5A2).
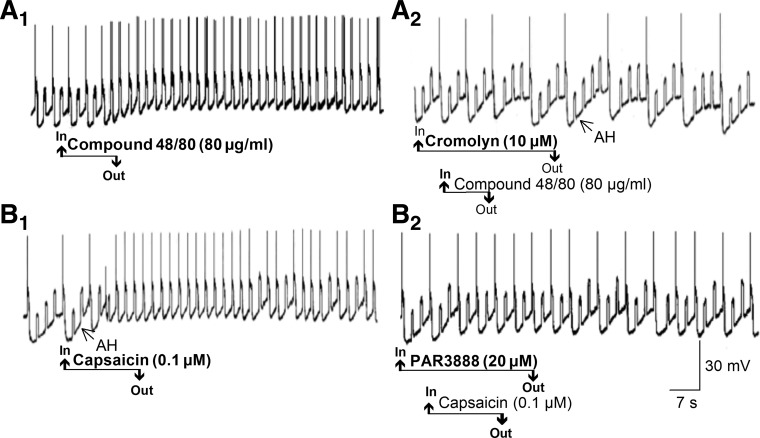
Fig. 5.
Mast cell degranulation, evoked by the secretogogue compound 48/80 or by capsaicin-induced afferent activation, released mediators that elevated excitability of ENS neurons. A1: application of compound 48/80 increased excitability in an afterhyperpolarization (AH)-type guinea pig myenteric neuron. Elevated excitability is reflected by suppression of post-action potential afterhyperpolarization (i.e., the AH), membrane depolarization, and increased frequency of action potential discharge during repetitive intraneuronal injection of constant-current depolarizing pulses. A2: application of compound 48/80 in the presence of the mast cell-stabilizing drug cromolyn did not increase excitability of the same AH-type neuron in A1. B1: application of capsaicin increased excitability in an AH-type guinea pig myenteric neuron in the same manner as in A1. B2: application of capsaicin in the presence of the protease-activated receptor type 2 (PAR2) antagonist PAR3888 did not increase the excitability of the same AH-type neurons in B1, suggesting that capsaicin acted to release a mast cell protease that reached the neuron by paracrine-like diffusion. Repetitive upward deflections on the records are electrotonic potentials evoked by intraneuronal injection of rectangular constant-current pulses.
Capsaicin.
Bath application of the VR1 agonist capsaicin (0.1–1.0 μM) mimicked the action of compound 48/80 to elevate the excitability of AH-type neurons in the myenteric plexus (Fig. 5B1). Responses to capsaicin were reduced when it was applied in the presence of PAR3888, a selective antagonist for the protease-activated receptor type 2 (PAR2; Fig. 5B2) (1).
Mesentery afferents.
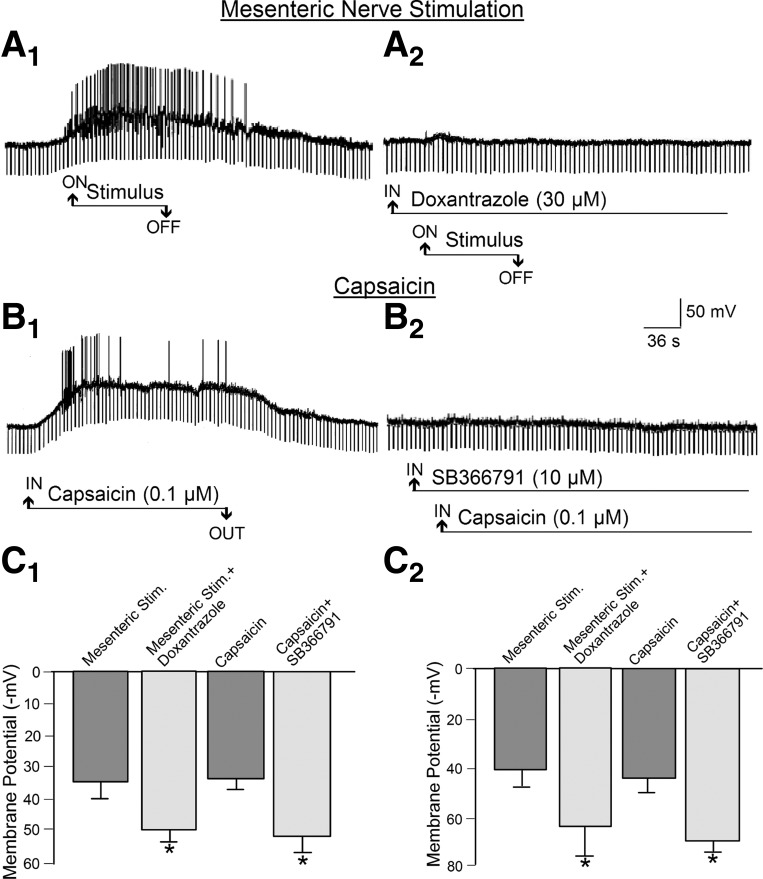
Slow EPSP-like responses, evoked by antidromic electrical stimulation of perivascular nerve trunks in the mesentery, were suppressed after addition of 30 μM doxantrazole, a mast cell stabilizer, in the bathing medium (Fig. 6, A1, A2, C1, and C2). Bath application of 0.1 μM capsaicin mimicked the electrophysiological changes during stimulus-evoked slow EPSPs (Fig. 6, B1 and B2). Capsaicin-evoked slow EPSP-like responses were suppressed when the VR1 antagonists capsazepine (10–20 μM) and SB366791 (10 μM) were present in the bathing medium (Fig. 6, B1, B2, C1, and C2). This action of capsaicin likely was due to stimulation of VR1 expressed by afferents, because neural blockade with TTX suppressed capsaicin-evoked release of mast cell protease II (see Fig. 9).
Fig. 6.
Release of mast cell degranulation products, evoked by antidromic electrical stimulation of mesenteric afferents or by capsaicin-induced activation of intramural afferents, elevated excitability in the same AH-type neuron in guinea pig myenteric plexus. A1: elevated excitability evoked by mesenteric nerve stimulation. Elevated excitability was reflected by depolarization of the membrane potential, increased input resistance, and augmented excitability reflected by increased frequency of action potential discharge. Repetitive downward deflections are electrotonic potentials evoked by intraneuronal injection of rectangular constant-current pulses. Increases in the amplitude of the electrotonic potentials are a reflection of increased neuronal input resistance. A2: mast cell stabilization by the mast cell-stabilizing drug doxantrazole suppressed the excitatory action of mesenteric nerve stimulation, suggesting that stimulation of the afferents released one or more mast cell degranulation products that reached the neuron by paracrine-like diffusion. B1: bath application of capsaicin mimicked the excitatory effects of electrical stimulation of mesentery afferents. B2: SB366791, a potent and selective antagonist for VR1, suppressed the excitatory action of capsaicin for the same neuron, suggesting that stimulation of intramural afferents by capsaicin released one or more mast cell degranulation products that reached the neuron by paracrine-like diffusion. C1: quantitative data for effects of mesenteric nerve stimulation and capsaicin for 24 AH-type myenteric neurons, 8 from the jejunum and 16 from the ileum. C2: quantitative data for effects of mesenteric nerve stimulation and capsaicin for S-type myenteric neurons, 9 from the jejunum and 5 from the ileum. *P < 0.05 vs. stimulation (mesenteric or capsaicin) alone (without SB366791).
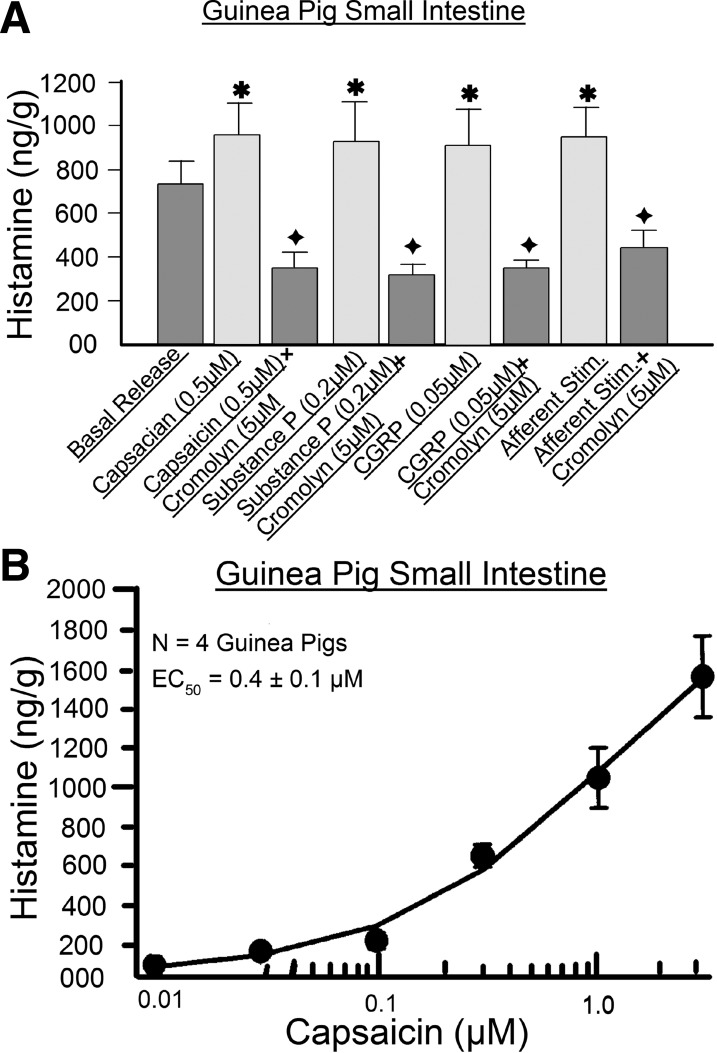
Fig. 9.
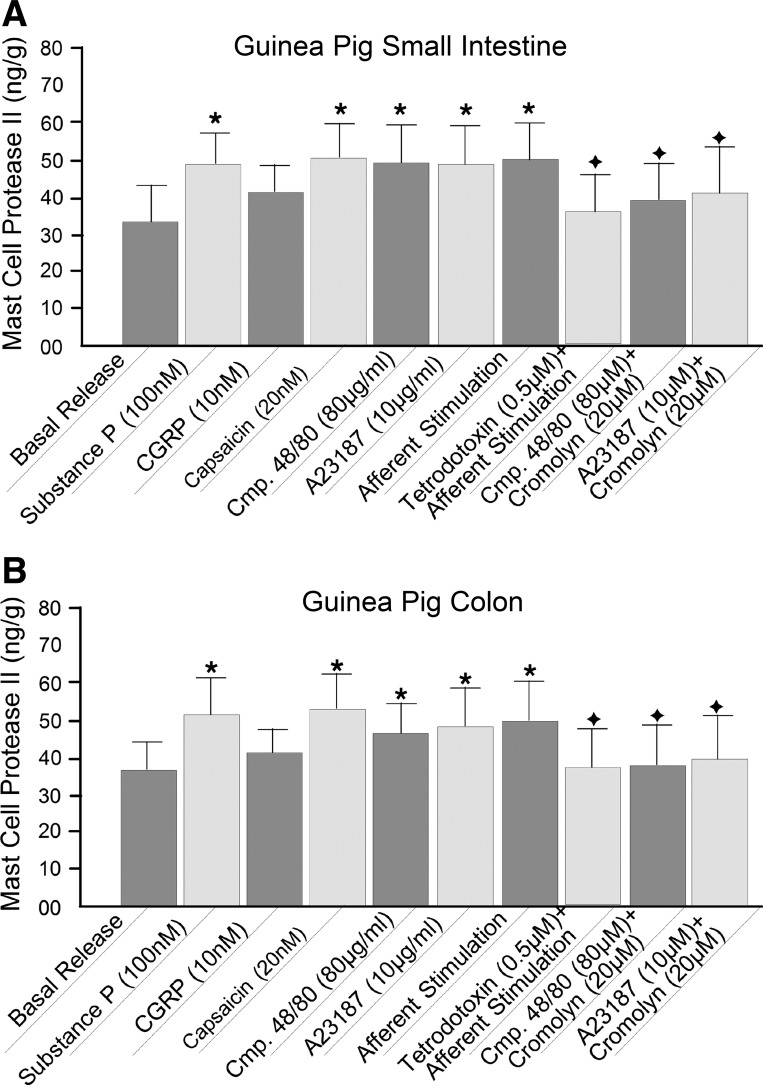
Release of protease II was used as a marker for guinea pig mast cell degranulation. A: small intestine. B: colon. Data are derived from 5 methods: 1) exposure to the putative sensory neurotransmitter SP and CGRP, 2) capsaicin-evoked firing of afferents, 3) direct degranulation evoked by compound (Cmp) 48/80, 4) elevation of intracellular Ca2+ by the ionophore A23187, and 5) antidromic electrical stimulation of afferents in perivascular nerve bundles in the intestinal mesentery. The mast cell stabilizing drug cromolyn suppressed release of protease II when evoked by compound 48/80 or A23187. Neural blockade by tetrodotoxin prevented the mast cell protease II release that occurred in response to electrical stimulation of mesenteric nerves. *P < 0.05 vs. basal release. +P < 0.05 vs. responses in the absence of cromolyn.
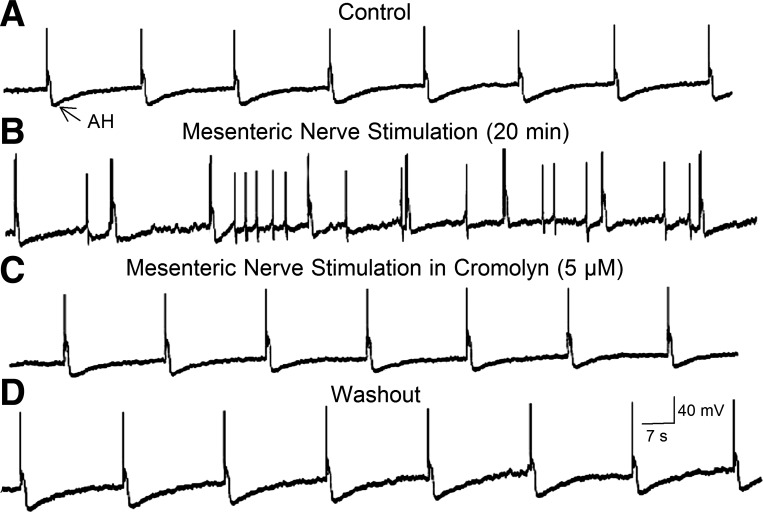
Antidromic electrical stimulation of mesenteric nerves elevated excitability of AH- and S-type neurons in the myenteric or submucosal plexus. Elevated excitability occurred in 22 of 25 AH-type neurons in the myenteric plexus (Fig. 7, A and B). Elevated excitability in AH-type neurons was reflected by depolarization of the membrane potential, increased input resistance, suppression of the AH, and augmented excitability, recorded as increased frequency of discharge of action potentials during intraneuronal injection of rectangular, constant-current depolarizing pulses. Mesenteric nerve stimulation did not elevate excitability in the AH-type neurons when applied with the mast cell-stabilizing drugs 5 μM cromolyn and 20–30 μM doxantrazole present in the bathing medium (data for doxantrazole not shown; Fig. 7C).
Fig. 7.
Electrical stimulation of perivascular nerve bundles in the intestinal mesentery released mast cell degranulation products that elevated excitability in an AH-type myenteric neuron. A: action potentials evoked by intraneuronal injection of rectangular depolarizing current pulses are followed by characteristic afterhyperpolarization (i.e., the AH). B: electrical stimulation of mesenteric nerves evoked canonical slow EPSP-like excitation marked by membrane depolarization, suppression of the AH, and augmented excitability reflected by spontaneous spike discharge and repetitive discharge during depolarizing current pulses. Application of the mast cell stabilizer cromolyn abolished the slow EPSP-like excitation, suggesting that mesenteric nerve stimulation released one or more excitatory mast cell degranulation products that reached the neuron by paracrine-like diffusion. D: return to control following washout of cromolyn.
Elevated excitability in response to mesenteric nerve stimulation occurred also in 11 of 12 S-type neurons in the myenteric plexus (Fig. 4, A and B). It was reflected by depolarization of the membrane potential, decreased input resistance, and augmented excitability, recorded as increased frequency of discharge of action potentials during intraneuronal injection of rectangular, constant-current depolarizing pulses and spontaneous action potential discharge.
Mesenteric nerve stimulation did not elevate excitability in S-type neurons when applied with cromolyn (5 μM) or doxantrazole (20–30 μM) in the tissue bath (data not shown). Ketotifen, another mast cell-stabilizing drug in concentrations up to 100 μM, did not suppress excitatory responses to mesenteric nerve stimulation or to capsaicin in AH- or S-type neurons.
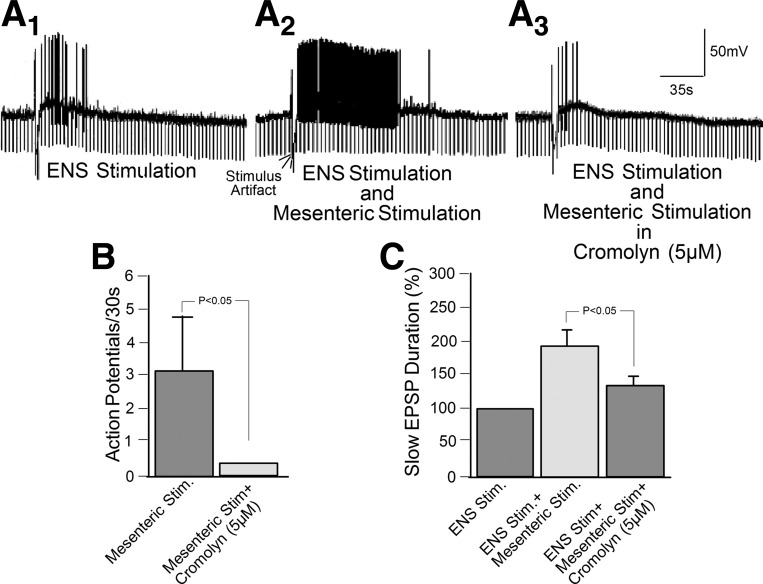
Focal electrical stimulation of interganglionic fiber tracts in the myenteric or submucosal plexus evoked characteristic slow synaptic excitation (Fig. 8A1) (38, 82–84). Simultaneous electrical stimulation of mesenteric nerves enhanced the slow EPSPs evoked by focal ENS stimulation (Fig. 8A2). Bath application of the mast cell stabilizer cromolyn counteracted the augmenting effects of mesenteric nerve stimulation on slow EPSPs evoked by stimulation in the ENS (Fig. 8, A3, B, and C).
Fig. 8.
Electrical stimulation of perivascular nerve bundles in the intestinal mesentery released mast cell degranulation products that facilitated excitatory slow synaptic transmission in guinea pig myenteric plexus networks. A1: slow EPSP evoked by focal electrical stimulation of an interganglionic fiber tract. ENS, enteric nervous system. A2: simultaneous electrical stimulation of the same interganglionic fiber tract and a perivascular nerve bundle in the intestinal mesentery enhanced the duration of the slow EPSP and the frequency of action potential discharge evoked by the EPSP. A3: simultaneous electrical stimulation of the same interganglionic fiber tract and a perivascular nerve bundle in the intestinal mesentery, in the presence of 5 μM cromolyn, did not result in facilitation of the slow EPSP. B: quantitative data for effects of cromolyn on slow synaptic excitation evoked by antidromic electrical stimulation of perivascular nerve bundles in the intestinal mesentery. Data for the mean number of action potentials during the initial 30 s of EPSP were obtained from 26 AH-type neurons, 11 in the jejunal myenteric plexus and 15 in the ileal myenteric plexus. C: quantitative data for effects of simultaneous electrical stimulation of interganglionic fiber tracts in the myenteric plexus and mesenteric nerves in the presence and absence of cromolyn. Data for duration of slow EPSPs were obtained from 12 AH-type neurons, 5 in the jejunal myenteric plexus and 7 in the jejunal myenteric plexus.
ELISA.
We used ELISA to study effects of antidromic electrical stimulation of mesentery nerves and capsaicin activation of spinal afferents on release of the mast cell degranulation products histamine and mast cell protease II from intact intestinal segments. Application of 0.5–20 μM capsaicin stimulated release of mast cell protease II and histamine into the media bathing small and large intestinal preparations from guinea pigs and segments of jejunum from human small intestine (Figs. 9–11).
Fig. 11.
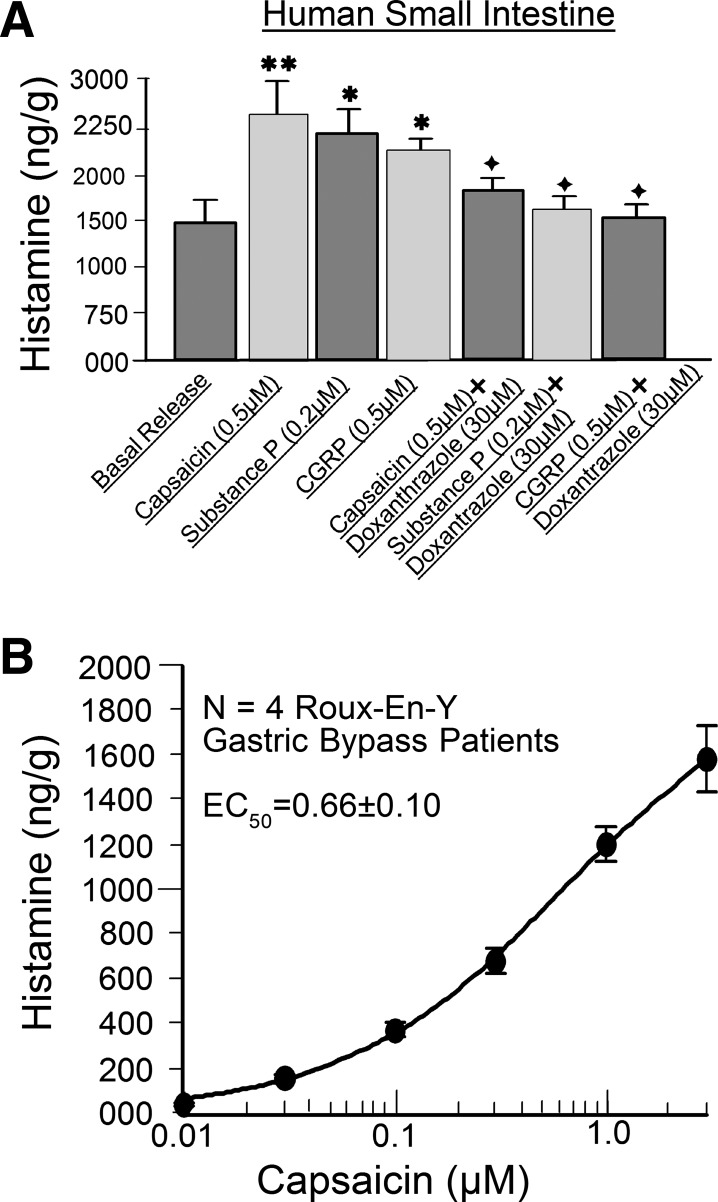
Release of histamine was used as a marker for mast cell degranulation in human small intestine. A: release data compare effects of putative capsaicin-evoked firing of intramural afferents and exposure to the putative sensory neurotransmitters SP and CGRP. The mast cell-stabilizing drug doxantrazole suppressed release of histamine when evoked by capsaicin, SP, or CGRP. B: capsaicin-evoked firing of intramural afferents was concentration-dependent. *P < 0.05, **P < 0.01 vs. basal release. +P < 0.05 vs. responses in the absence of doxantrazole.
Mast cell protease II.
Bath application of compound 48/80 (80 μg/ml) evoked release of mast cell protease II in concentrations greater than basal release in the small and large intestine of guinea pigs (Fig. 9). Preapplication of 20 μM cromolyn suppressed the release of mast cell protease II evoked by compound 48/80 (Fig. 9). Bath application of the Ca2+ ionophore A23187 (20 μM) likewise stimulated release of mast cell protease II relative to basal release, and this effect was suppressed by the presence of 20 μM cromolyn (Fig. 9).
We used release of mast cell protease II as a marker in investigation of afferent input to intramural mast cells. Application of 20 nM capsaicin, to stimulate intramural afferents, elevated release of mast cell protease II to significant levels above basal release (Fig. 9). Electrical stimulation of mesenteric nerves, to antidromically activate intramural afferents, elevated release of mast cell protease II in a manner similar to the action of capsaicin (Fig. 9). Blockade of action potential conduction in intramural afferents by TTX prevented elevation of mast cell protease II release during electrical stimulation of mesenteric afferents (Fig. 9). Placement of SP into the organ bath, as a putative spinal afferent neurotransmitter, evoked release of mast cell protease II (Fig. 9). On the other hand, application of CGRP, in the same manner as for SP, did not elevate the release of mast cell protease II to levels significantly greater than basal release (Fig. 9).
Histamine.
We studied release of histamine from intact segments of guinea pig and human small intestine in the same manner as was done for mast cell protease II. Stimulation of intramural afferents by 0.05–0.5 μM capsaicin evoked release of histamine beyond basal levels in guinea pig and human intestinal segments (Figs. 10 and 11). The action of capsaicin to stimulate histamine release was concentration-dependent, with an EC50 of 0.4 ± 0.1 μM for guinea pig small bowel from four animals and an EC50 of 0.7 ± 0.1 μM for four human jejunal preparations (Figs. 10B and 11B). Both EC50 values were calculated for a range of 0.01–1.2 μM due to desensitization at higher concentrations. Capsaicin-evoked stimulation of histamine release was suppressed by 5 μM cromolyn in guinea pig preparations and by 30 μM doxantrazole in human preparations (Figs. 10A and 11A).
Fig. 10.
Release of histamine was used as a marker for guinea pig mast cell degranulation. A: data were obtained with 1) putative capsaicin-evoked firing of intramural afferents, 2) exposure to the putative sensory neurotransmitters SP and CGRP, and 3) antidromic electrical stimulation of afferents in perivascular nerve bundles in the intestinal mesentery. The mast cell-stabilizing drug cromolyn suppressed release of histamine when evoked by SP, CGRP, capsaicin, or electrical stimulation of afferents. B: capsaicin-evoked firing of intramural afferents was concentration-dependent. *P < 0.05 vs. basal release; +P < 0.05 vs. responses in the absence of cromolyn.
Antidromic electrical stimulation of mesenteric nerves mimicked the action of capsaicin to stimulate release of histamine (Fig. 10A). SP (0.2 μM) or CGRP (0.05 μM), placed in the organ bath as putative afferent neurotransmitters, evoked increases in release of histamine relative to basal release in guinea pig and human intestinal segments (Figs. 10A and 11A). Pretreatment with 5 μM cromolyn suppressed this action of SP and CGRP in guinea pig preparations (Fig. 10A). Pretreatment with 30 μM doxantrazole suppressed SP- or CGRP-evoked release of histamine in human jejunal segments (Fig. 11A).
DISCUSSION
Immunohistochemistry.
Coexpression of VR1-IR, SP-IR, and CGRP-IR in small-diameter intramural fibers identified the fibers, in our preparations, as primary spinal afferents and was consistent with many reports in the literature that establish the coexpression as a marker for spinal afferents in the gut (5, 6, 10, 32, 37, 70). Projection of the afferents into enteric ganglia (Fig. 1) was reminiscent of retrograde tracing studies of others showing that spinal afferents in mesenteric nerve trunks enter the intestinal wall and branch to send projections into ENS ganglia. Inside the ganglia, some of the afferents appear to form synapses with ganglion cell somas, while others have specialized terminals that discharge action potentials in response to mechanical stimulation in a manner similar to mechanoreceptors elsewhere (4, 44, 71). Connections of this nature might be indicative of local axon reflex connections between afferent collaterals and enteric neurons, as well as other cell types, such as occurs in the classical “triple response of Lewis,” which involves afferent-evoked release of histamine from cutaneous mast cells.
Afferent stimulation.
Release of SP and CGRP evoked by firing of intramural afferents, either by antidromic electrical stimulation of the afferents as they entered the intestine from the mesentery or by exposure to capsaicin, was consistent with our immunohistochemical results showing coexpression of SP-, CGRP-, and VR1-IR by small-diameter intramural fibers. Capsaicin-evoked release of SP and CGRP into the bathing medium of the intestinal segments was most likely due to its well-known action to open VR1 channels, expressed by primary afferents (3, 36, 37). Opening of the VR1 channels is expected to evoke action potential discharge, which in turn would release SP and CGRP simultaneously from intramural terminals of afferent collaterals at junctions with mast cells and at synapses with ENS neurons, as we found elsewhere (19).
Electrophysiology.
Our results obtained with electrical stimulation of afferents as they entered the intestine via the mesentery closely resembled those first reported by Takaki and Nakayama (66, 67), who found that afferent stimulation evoked capsaicin-sensitive fast and slow synaptic responses mediated by acetylcholine and SP, respectively, in AH- and S-type ENS neurons. Entry of the afferents into ENS ganglia (Fig. 1) and finding that SP and CGRP receptor antagonists suppressed the slow EPSPs, evoked by mesenteric nerve stimulation in the present study, are consistent with the conclusion of Takaki and Nakayama that spinal afferents enter ENS ganglia, where they release SP and CGRP at synapses with ENS neuronal cell bodies.
Sympathetic postganglionic nerve fibers, known to release norepinephrine and ATP at synaptic connections with ENS neurons, also enter the intestine in mesenteric nerves alongside spinal afferents (38, 86). Stimulus-evoked slow inhibitory postsynaptic potentials, which preceded the stimulus-evoked slow EPSPs (Fig. 4), are known to be noradrenergic and are a marker for S-type noncholinergic submucosal secretomotor/vasodilator neurons that release vasoactive intestinal peptide as a neurotransmitter at neuroglandular junctions (7, 22, 24, 62).
Electrical stimulation of mesenteric nerves can also backfire intestinofugal axons, known to be projections from S-type ENS neuronal cell bodies to prevertebral sympathetic ganglia (29, 35, 60, 65). Antidromically propagating action potentials in these fibers can be recorded with intracellular microelectrodes in the S-type neuronal cell bodies as they invade the cell body electrotonically (66, 67, 84). Nevertheless, these are rare events in AH- or S-type neurons that usually do not reach somal spike threshold, because the electronic spread of current from the small-diameter unmyelinated C fibers is into the large-volume and low-input resistance of the cell body (66, 67). Although antidromic stimulation of intestinofugal fibers, firing of their cell bodies, and release SP or CGRP are unlikely, they cannot be fully ruled out in our study. On the other hand, most intestinofugal fibers in the mesenteric nerve trunks are projections from single-axonal S-type neurons that would not be expected to have terminal release sites inside the ganglia or at motor effector junctions (35, 60).
Slow EPSPs, evoked by focal electrical stimulation applied to ganglia or interganglionic fiber tracts in the ENS, have been investigated for over three decades in terms of electrical and synaptic behavior, with SP and serotonin being among the first putative neurotransmitters for slow synaptic excitation (83, 84).
Compound 48/80.
Compound 48/80 is a secretagogue commonly used experimentally to evoke degranulation of mast cells and release of preformed mediators, such as histamine and mast cell proteases (55). Our observations of elevated excitability in AH-type ENS neurons following application of compound 48/80 most likely reflected mast cell degranulation and a diffuse paracrine action of released mast cell mediators, because cromolyn stabilization of the mast cells suppressed this action of compound 48/80. Cromolyn, itself, is a common mast cell-stabilizing drug used in asthma therapy and other allergies. Similar to doxantrazole, another mast cell-stabilizing agent used in our studies, cromolyn acts to prevent opening of Ca2+ channels necessary for degranulation of mast cells (15, 56, 59). Histamine and mast cell proteases are known to be excitatory neuronal mediators that can reach the ENS in a paracrine fashion following antigen-evoked release from sensitized enteric mast cells (27, 28, 30, 43).
Capsaicin.
We applied capsaicin to guinea pig preparations, in view of its much reported action to stimulate VR1, fire afferent fibers, and thereby evoke release of SP and CGRP from intramural afferent nerve terminals (36, 37, 64). The action of capsaicin to elevate excitability of AH-type neurons in these preparations, similar to the action of compound 48/80 on the mast cells per se, appeared to result from mast cell degranulation and release of mast cell proteases, because the excitatory action was suppressed by antagonists for PAR2. Mast cell proteases are known to act at G protein-coupled PARs, expressed by AH-type neurons, and thereby to evoke slow EPSP-like excitation (30). We cannot exclude the possibility that the slow EPSP-like actions of capsaicin were due entirely to stimulation of afferents, because TTX suppressed only a fraction of the capsaicin-evoked release of mast cell protease II.
Mesentery afferents.
Excitation of AH- and S-type neurons during electrical stimulation of mesenteric afferents was not only sensitive to blockade of receptors for SP and CGRP, but it was reduced by prevention of mast cell degranulation by the mast cell-stabilizing drugs cromolyn and doxantrazole, but not ketotifen. This suggests that part of the excitatory effects of antidromic firing of afferents in mesenteric nerves resulted from evoked release and diffusion of mast cell mediators to bind with excitatory receptors expressed by ENS neurons in the neighborhood of the mast cells. Failure of ketotifen to suppress afferent-evoked excitation of ENS neurons in the present study differed from its suppression of membrane-depolarizing responses evoked by application of sensitizing antigen in one of our earlier studies in guinea pigs (43). How degranulation evoked by afferent nerve input might differ mechanistically from degranulation evoked by antigen binding to Fc receptors on sensitized cells remains unresolved.
Slow and fast synaptic excitation in the intrinsic microcircuitry have important significance in the integrative functioning of ENS neural networks. Several paracrine mediators, as well as pharmacotherapies for gastrointestinal disorders, produce their effects by influencing synaptic transmission. For example, histamine release from enteric mast cells acts at presynaptic receptors to suppress slow EPSPs and fast nicotinic transmission, whereas the promotor drug tegaserod (HTF919) facilitates fast nicotinic neurotransmission in the ENS (23, 43, 68). Our results suggest that firing of intramural afferents releases mast cell degranulation products, which in turn function in a paracrine fashion to facilitate slow EPSPs at synapses in the ENS neural networks (Fig. 8).
Mast cell protease II and histamine release.
Our findings suggest that effects of afferent input to enteric mast cells mimic the action of compound 48/80 to degranulate mast cells and release preformed mediators. Five lines of evidence support the hypothesis that spinal afferents innervate enteric mast cells, degranulate enteric mast cells, and release mast cell proteases and histamine into the extracellular milieu in the small intestine of guinea pigs and humans: 1) enteric mast cells expressed receptors for the sensory neurotransmitters SP and CGRP; 2) exposure to the putative sensory neurotransmitters SP and CGRP released mast cell protease II and histamine in a cromolyn/doxantrazole-sensitive manner; 3) backfiring of afferents, entering the gut in the mesentery, released mast cell protease II and histamine; 4) afferent stimulation, by capsaicin, mimicked the effects of backfiring afferents; and 5) mast cell-stabilizing drugs or neural blockade with TTX suppressed afferent-evoked release of mast cell protease II and histamine.
Translational implications.
Figure 12 is a heuristic model for interpretation of our findings in terms of how spinal afferents, mast cells, and the ENS might interact in signaling involving mast cell proteases and histamine as key paracrine mediators in normal and disordered intestinal sensitivity. Elevated levels of the proteases and histamine are expected when mastocytosis occurs in the esophagus, stomach, or intestinal tract. In fact, mast cell hyperplasia is reported to be associated with the diarrhea-predominant form of IBS, where exaggerated release of histamine and mast cell proteases, acting at PAR2, is associated with the symptoms of cramping abdominal pain, acute watery diarrhea, and fecal urgency (2, 52, 76).
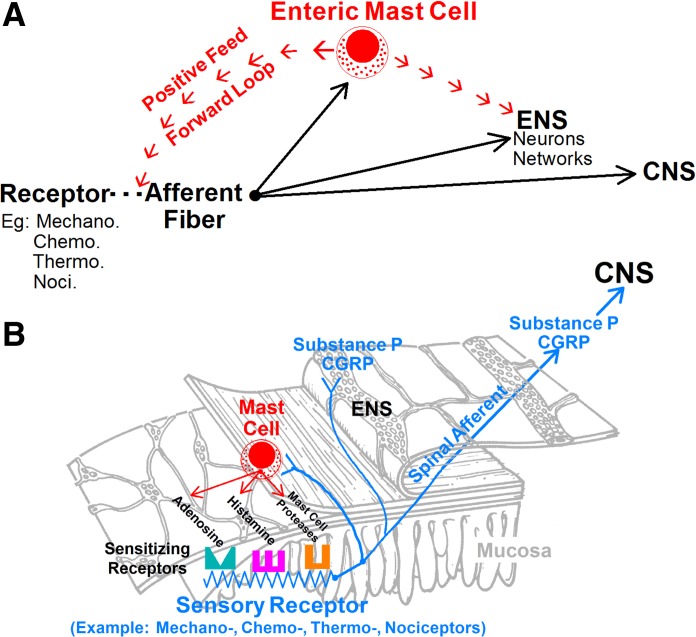
Fig. 12.
Heuristic model for connectivity of spinal afferents, enteric mast cells, and the ENS. A: enteric mast cells are a major node in the interactive induction of afferent hypersensitivity. An afferent terminal fires when its receptors and/or channels for a sensory modality respond to the preferred stimulus. Signals in the form of action potential codes release SP and CGRP at afferent-mast cell junctions and at synapses with neurons in the ENS and second-order neurons in the central nervous system (CNS). Mast cells express receptors for SP and CGRP, which degranulate the mast cells and release mediators such as mast cell protease II and histamine. The mast cell proteases, histamine, and additional mediators diffuse to their receptors on afferent terminals in a paracrine fashion and act to enhance the sensitivity for a stimulus to trigger action potentials and augment firing frequency in the terminal. When this occurs, afferent input to the mast cells, enteric neuronal networks, and the CNS is enhanced. Mast cells and afferents connected in this manner form a positive feed-forward loop that self-perpetuates in the sensitization of afferent terminals. B: functional connectivity of an interactive enteric mast cell-afferent-enteric neuronal network that might underlie sensory hypersensitivity in the gut as well as in neighboring viscera.
Stress.
Stressful life events are associated with onset and exacerbation of the symptoms of the diarrhea-predominant form of IBS (39, 47, 72, 73). Release of mast cell proteases occurs during responses to stress in human and rodent intestine (45, 58). Moreover, exposure to the stress hormone corticotropin-releasing factor stimulates mast cells to release histamine in animals (42, 43). Restraint stress in rats intensifies nociceptive sensory responses to colonic distension, which likely reflects sensitization of afferents by mast cell products, such as histamine and proteases (8, 34, 49).
Food allergy and infectious enteritis.
Secondary exposure to the offending antigen in animals with small intestinal parasitic infection (e.g., Trichinella spiralis) or food allergy (e.g., milk protein) evokes histaminergic excitatory responses in AH- and S-type ENS neurons that mimic the effects of afferent-induced degranulation of mast cells in the present study (27, 28). Earlier work that described actions of a variety of mast cell degranulation products, such as histamine and mast cell protease II, on electrical and synaptic behavior of ENS neurons provided a foundation for the work on antigen-sensitized animal models and the present study (28, 43, 50, 68, 69). Work of this nature, at the cellular level, translates to the whole organ in vitro, where bath application of histamine or histamine release evoked by application of sensitizing antigens, in food allergy or parasitic infection, activates a central pattern generator in the ENS that drives precisely timed recurrent cycles of mucosal secretion of H2O and electrolytes linked with propulsive musculomotor activity (16, 17, 18, 78, 89).
Visceral sensitization.
Frequent overlap of morbidity in one or more of the visceral pain syndromes was mentioned earlier (12, 13). How hypersensitivity in one visceral organ can be transferred to a nearby neighbor or transferred from a visceral organ to expression as tactile allodynia in the skin, in the functional pain syndromes, has not been resolved satisfactorily. Nevertheless, it is apparent, in animal models, that mast cells, spinal afferents, and neurogenic inflammation form a common denominator in the sharing of hypersensitivity between abdominal viscera (88). Uterine inflammation induced by chemical irritation or endometriosis in rat models induces inflammation and hypersensitivity in the urinary bladder and colon, and the inflammatory cross talk is interrupted by resection of sensory afferents in the hypogastric nerve (80). Similarly, colonic irritation by chemical mucosal irritants (e.g., 2,4,6-trinitrobenzenesulfonic acid) or by mechanical distension induces inflammation associated with sensitization of spinal afferents and mast cell activation in the urinary bladder of rats (46, 54, 74, 75). Firing of urinary bladder afferents, by instillation of capsaicin, evokes hypersensitivity to distension in the colon (56, 57). Blockade of afferents by instillation of a local anesthetic (e.g., lidocaine), together with capsaicin, prevents the sensitizing cross talk from the bladder to the colon. In a similar manner, experimentally induced inflammation in the urinary bladder evokes tactile cutaneous allodynia in mouse models. Release of mast cell mediators is implicated as the primary factor in the sensitization of bladder afferent innervation to distension in these studies (56, 57). Receptors for histamine and mast cell proteases are expressed by spinal afferent terminals in the intestine (10, 40). These mast cell degranulation products fire the afferent terminal, when applied experimentally, and have potential for elevating the sensitivity of the terminal to its preferred stimulus modality, especially in disordered conditions of mastocytosis and associated inflammation. This possibility is reinforced by findings that a reduced threshold for painful responses to balloon distension in the large bowel is associated with degranulation of mast cells in animal models. Treatment with mast cell-stabilizing drugs prevents lowering of the pain threshold to distension, which occurs during mucosal inflammation in the animal models (14).
A reasonable explanation for the cross talk between the intestine and neighboring pelvic organs is that information transmitted by sensitized intestinal afferents projects to the same spinal interneurons that receive afferent input from visceral neighbors. For example, the majority of spinal neurons that respond to bladder stimulation also respond to colon stimulation, and vice versa (48). Sensitization of intestinal afferents by mediators released from enteric mast cells is likely to underlie both the well-documented hypersensitivity to colonic distension associated with inflammation or stress in rodent models and the cross talk between the colon and urinary bladder.
Heuristic model.
The interactive connections between spinal afferents, enteric mast cells, and the ENS, identified in the present and earlier studies and illustrated by the model in Fig. 12, suggest how elevation of intestinal hypersensitivity for a specific stimulus modality, such as derived from a nociceptor or a mechanoreceptor, might occur. Connectivity is arranged in a positive feed-forward configuration of mast cell → afferent → mast cell. A consequence of a feed-forward interaction is that hypersensitivity becomes amplified and continues to escalate if unchecked.
Enteric mast cells are at the vortex of signaling in the induction of afferent hypersensitivity (88). Mast cells emit multiple kinds of paracrine signals when the antigen-sensitized IgE antibodies, which are bound to their surface, detect antigens associated with infectious organisms, food allergins, and toxins that might breach mucosal barrier function. Afferent nerve terminals express receptors for many of the mast cell signals (Fig. 12). Histamine and serine proteases are prominent signals for sensitization of the terminals. Sensitization can be reflected as activation of a silent afferent, as in the case of nociceptors, or as activation by release from mast cells associated with IBS (2, 31).
Mast cell function in immunoafferent communication can be viewed as a neuroimmune counterpart of sensory neurophysiology. Sensory neurons are genetically programed to express selective detection for specific stimuli (e.g., touch, temperature, pH, or pain) that are fixed throughout an individual's lifetime. Mast cells, instead, acquire specific detection specificities by virtue of flexibility of recognition functions inherent in production by the immune system of new antibodies that become attached to mast cell surfaces. Detection specificity for foreign antigens is thus acquired and reinforced throughout life due to formation of new antibodies that bind to and remain attached to immunoglobulin receptors on mast cells. Output signals from mast cells, which are triggered by antigen cross-linking with the attached antibodies, are chemical entities and analogous to chemical output signals (i.e., neurotransmitters) from primary afferents to second-order neurons in the ENS and central nervous system. Hence, mast cells and sensory neurons ultimately code information on a sensed parameter by releasing a chemical message that is decoded by information-processing circuits in the nervous system.
Conclusion.
Coexpression of IR for VR1, SP, and CGRP is a marker for intramural spinal afferent fibers that project into ENS ganglia. Firing of these intramural afferents by antidromic stimulation of perivascular nerve bundles in the mesentery or by exposure to capsaicin released SP and CGRP at excitatory synapses in the ENS and at afferent junctions with intramural mast cells. Intramural mast cells, which express SP and CGRP receptors, are in close proximity to the ENS and intramural afferent terminals. Afferent input, mediated by SP and CGRP, releases histamine and mast cell protease II. Histamine and mast cell protease II act, in a diffuse paracrine manner, to influence the behavior of ENS neurons and to elevate the sensitivity of spinal afferent terminals to their preferred modality. A heuristic model suggests that sensitization and elevated firing frequencies of the afferent terminals feed forward to amplify mast cell release, which further sensitizes the terminal and continues a forward feed that intensifies actions in the ENS and sensory input to the spinal cord. Intensified input to the spinal cord, if from nociceptors, might be interpreted as pain and/or discomfort at the level of consciousness (Fig. 12).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-37238 and K08 DK-060468.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.-D.W., S.L., Y.X., B.J.N., D.J.M., and J.D.W. are responsible for conception and design of the research; G.-D.W., X.-Y.W., F.Z., M.Q., S.L., and J.D.W. performed the experiments; G.-D.W., X.-Y.W., M.Q., S.L., Y.X., B.J.N., D.J.M., and J.D.W. analyzed the data; G.-D.W., M.Q., S.L., Y.X., B.J.N., D.J.M., and J.D.W. interpreted the results of the experiments; G.-D.W., X.-Y.W., M.Q., S.L., Y.X., B.J.N., D.J.M., and J.D.W. approved the final version of the manuscript; S.L. and J.D.W. edited and revised the manuscript; J.D.W. prepared the figures; J.D.W. drafted the manuscript.
ACKNOWLEDGMENTS
Present addresses: M. Qu, Department of Pharmacology, Weifang Medical University, Weifang, Shandong, China; S. Liu, Department of Biology, University of Wisconsin, La Crosse, Lacrosse, WI.
REFERENCES
- 1.Al-Ani B, Saifeddine M, Wijesuriya SJ, Hollenberg MD. Modified proteinase-activated receptor-1 and -2 derived peptides inhibit proteinase-activated receptor-2 activation by trypsin. J Pharmacol Exp Ther 300: 702–708, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bartho L, Holzer P, Lembeck F, Szolcsanyi J. Evidence that the contractile response of the guinea-pig ileum to capsaicin is due to release of substance P. J Physiol 332: 157–167, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16 Suppl 1: 28–33, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Beyak MJ, Bulmer DC, Jiang W, Keating C, Rong W, Grundy D. Estrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD. San Diego: Academic, 2006 [Google Scholar]
- 6.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19: 1–19, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol 398: 371–390, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 289: G42–G53, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol 583: 731–742, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brierley SM, Hughes P, Harrington A, Blackshaw A. Estrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Physiology of the Gastrointestinal Tract (5th ed.), edited by Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD. San Diego: Academic, 2012 [Google Scholar]
- 11.Butler CA, Heaney LG. Neurogenic inflammation and asthma. Inflamm Allergy Drug Targets 6: 127–132, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain 84: 297–307, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Berman S, Mayer EA, Suyenobu B, Derbyshire S, Naliboff B, Vogt B, FitzGerald L, Mandelkern MA. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol 98: 1354–1361, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Coelho AM, Fioramonti J, Bueno L. Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig Dis Sci 43: 727–737, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Cook EB, Stahl JL, Barney NP, Graziano FM. Mechanisms of antihistamines and mast cell stabilizers in ocular allergic inflammation. Curr Drug Targets Inflamm Allergy 1: 167–180, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cooke HJ, Nemeth PR, Wood JD. Histamine action on guinea pig ileal mucosa. Am J Physiol Gastrointest Liver Physiol 246: G372–G377, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Cooke HJ, Wang YZ, Rogers R. Coordination of Cl− secretion and contraction by a histamine H2-receptor agonist in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 265: G973–G978, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Cooke HJ, Wang YZ, Reddix R, Javed N. Cholinergic and VIP-ergic pathways mediate histamine H2 receptor-induced cyclical secretion in the guinea pig colon. Am J Physiol Gastrointest Liver Physiol 268: G465–G470, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Dray A. Mechanism of action of capsaicin-like molecules on sensory neurons. Life Sci 51: 1759–1765, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E, et al. US householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 38: 1569–1580, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Elekes K, Helyes Z, Nemeth J, Sandor K, Pozsgai G, Kereskai L, Borzsei R, Pinter E, Szabo A, Szolcsanyi J. Role of capsaicin-sensitive afferents and sensory neuropeptides in endotoxin-induced airway inflammation and consequent bronchial hyperreactivity in the mouse. Regul Pept 141: 44–54, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, Xia Y, Wood JD. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol 536: 113–122, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Fang X, Liu S, Wang XY, Gao N, Hu HZ, Wang GD, Cook CH, Needleman BJ, Mikami DJ, Xia Y, Fei GJ, Hicks GA, Wood JD. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil 20: 80–93, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Fei G, Fang X, Wang GD, Liu S, Wang XY, Xia Y, Wood JD. Neurogenic mucosal bicarbonate secretion in guinea pig duodenum. Br J Pharmacol 168: 880–890, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis CY, Duffy JN, Whorwell PJ, Morris J. High prevalence of irritable bowel syndrome in patients attending urological outpatient departments. Dig Dis Sci 42: 404–407, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Francis CY, Whorwell PJ. The irritable bowel syndrome. Postgrad Med J 73: 1–7, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology 107: 1602–1609, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol Gastrointest Liver Physiol 267: G1087–G1093, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Furness JB, Kuramoto H, Messenger JP. Morphological and chemical identification of neurons that project from the colon to the inferior mesenteric ganglia in the guinea-pig. J Auton Nerv Syst 31: 203–210, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Gao C, Liu S, Hu HZ, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology 123: 1554–1564, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Gebhart GF. Bonica Lecture–2000: Physiology, pathophysiology, and pharmacology of visceral pain. Reg Anesth Pain Med 25: 632–638, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett 57: 125–130, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy 59: 1139–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Gue M, Del Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 9: 271–279, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Hibberd TJ, Zagorodnyuk VP, Spencer NJ, Brookes SJ. Viscerofugal neurons recorded from guinea-pig colonic nerves after organ culture. Neurogastroenterol Motil 24: 1041-e548, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991 [PubMed] [Google Scholar]
- 37.Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol 500: 231–241, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550: 493–504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janowitz HD. Your Gut Feelings. New York: Oxford University Press, 1989, p. 14–34 [Google Scholar]
- 40.Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol 280: G787–G794, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Levine JD, Khasar SG, Green PG. Neurogenic inflammation and arthritis. Ann NY Acad Sci 1069: 155–167, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Wood JD. Enteric neurobiology of stress. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Kaunitz JD, Ghishan FK, Merchant JL, Said HM, Wood JD. San Diego: Elsevier, 2012, p. 2001–2018 [Google Scholar]
- 43.Liu S, Hu HZ, Gao N, Gao C, Wang G, Wang X, Peck OC, Kim G, Gao X, Xia Y, Wood JD. Neuroimmune interactions in guinea pig stomach and small intestine. Am J Physiol Gastrointest Liver Physiol 284: G154–G164, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125: 786–794, 2003 [DOI] [PubMed] [Google Scholar]
- 45.MacQueen G, Marshall J, Perdue M, Siegel S, Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science 243: 83–85, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience 149: 660–672, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 47: 861–899, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon SB, Morrison JF. Two groups of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J Physiol 322: 21–34, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol 302: G260–G266, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Nemeth PR, Ort CA, Wood JD. Intracellular study of effects of histamine on electrical behaviour of myenteric neurones in guinea-pig small intestine. J Physiol 355: 411–425, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol 201: 167–180, 2004 [DOI] [PubMed] [Google Scholar]
- 52.O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil 12: 449–457, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 128: 1953–1964, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Qin C, Malykhina AP, Akbarali HI, Greenwood-Van Meerveld B, Foreman RD. Acute colitis enhances responsiveness of lumbosacral spinal neurons to colorectal distension in rats. Dig Dis Sci 53: 141–148, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Rothschild AM. Mechanisms of histamine release by compound 48–80. Br J Pharmacol 38: 253–262, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 293: R1191–R1198, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLos One 3: e2096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santos J, Saperas E, Nogueiras C, Mourelle M, Antolin M, Cadahia A, Malagelada JR. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 114: 640–648, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Shapiro GG, Konig P. Cromolyn sodium: a review. Pharmacotherapy 5: 156–170, 1985 [DOI] [PubMed] [Google Scholar]
- 60.Sharkey KA, Lomax AE, Bertrand PP, Furness JB. Electrophysiology, shape, and chemistry of neurons that project from guinea pig colon to inferior mesenteric ganglia. Gastroenterology 115: 909–918, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Sun X, Wang X, Wang GD, Xia Y, Liu S, Qu M, Needleman BJ, Mikami DJ, Melvin WS, Bohn LM, Ueno R, Wood JD. Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in human small intestine. Dig Dis Sci 56: 330–338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surprenant A, North RA. Mechanism of synaptic inhibition by noradrenaline acting at α2-adrenoceptors. Proc R Soc Lond B Biol Sci 234: 85–114, 1988 [DOI] [PubMed] [Google Scholar]
- 63.Swieter M, Lee TD, Stead RH, Fujimaki H, Befus D. Mast cell pleomorphism: properties of intestinal mast cells. Adv Exp Med Biol 216A: 613–623, 1987 [DOI] [PubMed] [Google Scholar]
- 64.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999 [PubMed] [Google Scholar]
- 65.Szurszewski JH, Ermilov LG, Miller SM. Prevertebral ganglia and intestinofugal afferent neurones. Gut 51 Suppl 1: 6–10, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takaki M, Nakayama S. Effects of mesenteric nerve stimulation on the electrical activity of myenteric neurons in the guinea pig ileum. Brain Res 442: 351–353, 1988 [DOI] [PubMed] [Google Scholar]
- 67.Takaki M, Nakayama S. Electrical behavior of myenteric neurons induced by mesenteric nerve stimulation in the guinea pig ileum. Acta Med Okayama 44: 257–261, 1990 [DOI] [PubMed] [Google Scholar]
- 68.Tamura K, Palmer JM, Wood JD. Presynaptic inhibition produced by histamine at nicotinic synapses in enteric ganglia. Neuroscience 25: 171–179, 1988 [DOI] [PubMed] [Google Scholar]
- 69.Tamura K, Wood JD. Effects of prolonged exposure to histamine on guinea pig intestinal neurons. Dig Dis Sci 37: 1084–1088, 1992 [DOI] [PubMed] [Google Scholar]
- 70.Tan LL, Bornstein JC, Anderson CR. The neurochemistry and innervation patterns of extrinsic sensory and sympathetic nerves in the myenteric plexus of the C57Bl6 mouse jejunum. Neuroscience 166: 564–579, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Tassicker BC, Hennig GW, Costa M, Brookes SJ. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res 295: 437–452, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Thompson WG. The Angry Gut. New York: Plenum, 1993, p. 67–95 [Google Scholar]
- 73.Thompson WG, Longstreth GL, Drossman DA, Heaton K, Irvine EJ, Muller-Lissner S. Functional bowel disorders and functional abdominal pain. In: The Functional Gastrointestinal Disorders (2nd ed.), edited by Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. McLean VA: Degnon, 2000 [Google Scholar]
- 74.Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol 290: F1478–F1487, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol 292: F123–F130, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Valdez-Morales EE, Overington J, Guerrero-Alba R, Ochoa-Cortes F, Ibeakanma CO, Spreadbury I, Bunnett NW, Beyak M, Vanner SJ. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol 108: 1634–1643, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Wang GD, Wang XY, Zou F, Qu M, Liu S, Fei G, Xia Y, Needleman BJ, Mikami DJ, Wood JD. Mast cell expression of the serotonin1A receptor in guinea pig and human intestine. Am J Physiol Gastrointest Liver Physiol 304: G855–G863, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang YZ, Cooke HJ. H2 receptors mediate cyclical chloride secretion in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 258: G887–G893, 1990 [DOI] [PubMed] [Google Scholar]
- 79.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 122: 1140–1156, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol 291: R1592–R1601, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Wood JD. Pathophysiology underlying the irritable bowel syndrome. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Kaunitz JD, Ghishan FK, Merchant JL, Said HM, Wood JD. San Diego: Elsevier, 2012, p. 2157–2181 [Google Scholar]
- 82.Wood JD, Mayer CJ. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflügers Arch 374: 265–275, 1978 [DOI] [PubMed] [Google Scholar]
- 83.Wood JD, Mayer CJ. Slow synaptic excitation mediated by serotonin in Auerbach's plexus. Nature 276: 836–837, 1978 [DOI] [PubMed] [Google Scholar]
- 84.Wood JD, Mayer CJ. Intracellular study of tonic-type enteric neurons in guinea pig small intestine. J Neurophysiol 42: 569–581, 1979 [DOI] [PubMed] [Google Scholar]
- 85.Wood JD. Histamine signals in enteric neuroimmune interactions. Ann NY Acad Sci 664: 275–283, 1992 [DOI] [PubMed] [Google Scholar]
- 86.Wood JD. Neurotransmission at the interface of sympathetic and enteric divisions of the autonomic nervous system. Chin J Physiol 42: 201–210, 1999 [PubMed] [Google Scholar]
- 87.Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 127: 635–657, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Wood JD. Effects of bacteria on the enteric nervous system: implications for the irritable bowel syndrome. J Clin Gastroenterol 41 Suppl 1: 7–19, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol 24: 149–158, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Wood JD. Visceral pain: spinal afferents, enteric mast cells, enteric nervous system and stress. Curr Pharm Des 17: 1573–1575, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Wood JD. Nonruminant Nutrition Symposium: Neurogastroenterology and food allergies. J Anim Sci 90: 1213–1223, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci 48: 743–749, 2003 [DOI] [PubMed] [Google Scholar]