Abstract
High levels of cholecystokinin (CCK) can stimulate pancreatic adaptive growth in which mature acinar cells divide, leading to enhanced pancreatic mass with parallel increases in protein, DNA, RNA, and digestive enzyme content. Prolonged release of CCK can be induced by feeding trypsin inhibitor (TI) to disrupt normal feedback control. This leads to exocrine growth in a CCK-dependent manner. The extracellular signal-related kinase (ERK) pathway regulates many proliferative processes in various tissues and disease models. The aim of this study was to evaluate the role of ERK signaling in pancreatic adaptive growth using the MEK inhibitors PD-0325901 and trametinib (GSK-1120212). It was determined that PD-0325901 given two times daily by gavage or mixed into powdered chow was an effective and specific inhibitor of ERK signaling in vivo. TI-containing chow led to a robust increase in pancreatic mass, protein, DNA, and RNA content. This pancreatic adaptive growth was blocked in mice fed chow containing the MEK inhibitors. PD-0325901 blocked TI-induced ERK-regulated early response genes, cell-cycle proteins, and mitogenesis by acinar cells. It was determined that ERK signaling is necessary for the initiation of pancreatic adaptive growth but not necessary to maintain it. PD-0325901 blocked adaptive growth when given before cell-cycle initiation but not after mitogenesis had been established. Furthermore, GSK-1120212, a chemically distinct inhibitor of the ERK pathway that is now approved for clinical use, inhibited growth similar to PD-0325901. These data demonstrate that the ERK pathway is required for CCK-stimulated pancreatic adaptive growth.
Keywords: mitogen-activated protein kinase, mitogen/extracellular signal-regulated kinase inhibitors, PD-0325901, GSK-1120212, pancreatic growth, cholecystokinin, extracellular signal-related kinase
pancreatic adaptive growth occurs in response to high-protein diets or the hyperphagia seen in pregnancy and lactation when an increase in digestive enzyme secretion is required (12, 13, 23, 29). The gastrointestinal hormone cholecystokinin (CCK) is released from the duodenal I cells in response to feeding and stimulates enzyme secretion. When CCK stimulation is prolonged, CCK causes an increase in pancreatic DNA and protein synthesis (31, 40). Administration of exogenous CCK or increased endogenous CCK secretion leads to pancreatic adaptive growth in the normally quiescent adult pancreatic acini (13, 34). This can be modeled by feeding a synthetic trypsin inhibitor (TI) (35, 46). TI-induced adaptive growth is blocked by the CCKA-R antagonist lorglumide and in CCK and CCKA-R-deficient mice (5, 40, 41, 46). However, the intracellular mechanisms regulating pancreatic adaptive growth are not fully established.
G protein-coupled CCKR1 receptors on pancreatic acinar cells bind CCK and signal via Gq/11 to activate multiple signaling pathways, including mTOR, calcineurin (CN)/NFAT, JNK, STAT, and MAPK (4, 6, 19, 45, 48). The role of mTOR and CN/NFAT in pancreatic adaptive growth was described previously (2, 17, 18). However, the significance of MAPK signaling in this process has not been established. The extracellular signal-related kinase (ERK) pathway has been shown to play an integral role in many proliferative processes in other tissues (27, 32). G protein-coupled receptors activate MEK via various stimuli, and MEK phosphorylates ERK on T202/Y204 promoting translocation to the nucleus and regulation of early response genes, including c-Fos and Egr-1. These transcription factors control multiple cell-cycle proteins stimulating mitogenesis and growth.
Pharmacological inhibitors have been developed to inhibit the ERK pathway because of its importance in regulating the growth of many cell types, including cancer cells. Two well-characterized MEK inhibitors, U-0126 and PD-98059, that are frequently used in in vitro studies have a short half-life and low solubility leading to low in vivo potency (7, 30). In the present study, the MEK inhibitors PD-0325901 and GSK-1120212, which are known to suppress phosphorylation of ERK in vivo, were assessed for their effects on pancreatic adaptive growth. PD-0325901 and GSK-1120212 are chemically distinct and bind to the catalytic site of MEK with high potency and specificity in vivo (11, 30). Both of these inhibitors maintain MEK in an inactive state inhibiting phosphorylation and activation of ERK. The interaction between ERK and MEK is essential to regulating the nuclear translocation of ERK (37). In the present study PD-0325901 and GSK-1120212 specifically blocked ERK signaling with low systemic toxicity. Pancreatic adaptive growth as assessed by pancreatic mass, protein, DNA, and RNA content was robustly blocked by inhibition of ERK signaling. Furthermore, PD-0325901 was found to inhibit ERK-regulated early response genes, cell-cycle proteins, and mitogenesis impacting pancreatic adaptive growth.
METHODS
Materials.
The TI camostat mesylate S was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PD-0325901 and GSK-1120212 were obtained from LC Laboratories (Woburn, MA).
Experimental design.
Male ICR mice (Harlan, Indianapolis, IN) 25–35 g were housed in a 12:12-h light-dark room at 22–24°C and fed 5001 chow (LabDiet, St. Louis, MO). Mice were fasted overnight before the start of each study and refed at 9:00 A.M. For studies at 2, 5, 7, and 8 days, animals were given either powdered chow, chow containing 0.1% TI, chow containing 0.3 mg/g PD-0325901, or chow containing both TI and PD-0325901. For PD-0325901 studies at 2 and 24 h mice were given vehicle or 10 mg/kg PD-0325901 by oral gavage two times daily starting 1 day before refeeding. For GSK-1120212 studies, animals were gavaged with vehicle or GSK-1120212 dissolved in corn oil at 9:00 A.M. daily (3 mg/kg) starting 2 days before refeeding. Tissue was harvested and either placed in RNAlater, fixed in formalin, homogenized using a Polytron homogenizer, or snap-frozen. All experiments and procedures were approved by the University of Michigan Committee on Use and Care of Animals.
Growth determinations.
Mice were killed by CO2 asphyxiation followed by cardiac puncture blood withdrawal. The pancreas was carefully dissected, weighed, and homogenized using a Polytron homogenizer at 100 mg pancreas/2 ml homogenization buffer containing protease inhibitors for 15 s (2). Protein concentrations were determined by Bradford assay (Bio-Rad Laboratories, Richmond, VA) with bovine serum albumin as a standard. For DNA and RNA measurements, the snap-frozen pancreas was homogenized using a Polytron homogenizer at 100 mg/2 ml buffer containing 5 mM MgCl2 and 0.1% Triton X-100 for 15 s and sonicated for 10 s (46). DNA and RNA were measured using the Quibit 2.0 Fluorometer (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Quantitative real-time PCR.
TRIzol reagent and RNeasy spin columns (Qiagen, Gaithersburg, MD) were used to isolate pancreatic RNA following homogenization; purity and concentration were determined by the ratios of optical density at 280 nm to that at 260 nm and agarose gel electrophoresis. The protocol used for quantitative real-time PCR was previously described (38). Briefly, 200 ng/μl of isolated RNA was reverse transcribed using TaqMan reverse transcription reagents and random hexamers as primers. The Bio-Rad C1000 Thermal Cycler was used for quantitative PCR purposes with Absolute Blue SYBR Green ROX (Thermo Scientific, Waltham, MA). Quantitative PCR primers for c-Jun, JunB, c-Fos, and Egr-1 were described previously (15). The GAPDH and 18S RNA primers have been described (38). The resulting threshold cycle (CT) obtained from the fluorescence was used to find a mean CT value, and the relative amounts of mRNA were determined as 2ΔCT where ΔCT is the mean CT minus the individual CT.
Western blotting.
Phospho (p)-ERK (T202, Y204), pS6 (S240/244), pSTAT3 (Y705), p-c-Jun (S63), and cyclin D1 antibodies were purchased from Cell Signaling (Beverly, MA). Cyclin D3, cyclin E, cyclin A, proliferating cell nuclear antigen (PCNA), and GAPDH antibodies were purchased from Santa Cruz Biotechnology. Pancreatic tissue was homogenized in lysis buffer using a Polytron homogenizer as previously described (17). Following centrifugation to remove debris, proteins were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted using specific antibodies (14).
Immunohistochemistry.
Ki-67 antibody was obtained from Vector Laboratories (Burlingame, CA), and pERK (T202, Y204) and cyclin D1 antibodies were purchased from Cell Signaling. Paraffin-embedded pancreatic sections were rehydrated, and antigen retrieval was performed using CITRA (BioGenex, Fremont, CA). Samples were quenched using 1 ml 30% H2O2 in 50 ml methanol and blocked using 1% BSA. Incubation with pERK, Ki-67, or cyclin D1 primary antibody was carried out overnight at 4°C. Immunoperoxidase localization was accomplished using Vectastain ABC kits (Vector Laboratories) followed by counterstaining with hematoxylin (36). Pancreatic tissue was visualized using an Olympus BX-51 light microscope, and images were recorded digitally and processed using Photoshop software.
5′-Bromo-2′-deoxyuridine incorporation and quantification.
5′-Bromo-2′-deoxyuridine (BrdU) was purchased from Sigma (St. Louis, MO). BrdU (0.8 mg/ml) was administered in water also containing 10 mg/ml glucose at the time of refeeding following fasting overnight. BrdU was administered for 48 h after which the pancreas was dissected and BrdU incorporation was determined by immunofluorescence (2, 17). Sections were blocked in 5% normal goat serum containing 0.3% Triton in PBS. Sections were exposed to BrdU antibody (1:10 dilution) (Accurate Chemical and Scientific, Westbury, NY) and mounted with prolong gold antifade reagent containing DAPI (Molecular Probes, Carlsbad, CA). Five separate ×40 fields were quantitated for each pancreas sample. Analysis was performed using Metamorph Offline version 6.1 software (Olympus Optical, Melville, NY). DAPI-stained nuclei and BrdU-positive nuclei of a predetermined size were quantitated within a field. BrdU-labeled acinar cell nuclei were determined by dividing the BrdU-positive nuclei by the number of DAPI nuclei.
Statistical analysis.
Data are reported as means ± SE and analyzed using GraphPad Prism software (GraphPad Software, San Diego). Statistical significance was reported using one-way or two-way ANOVA and Bonferroni's posttest except for Fig. 7 where Dunnett's test for multiple comparison was used. P values <0.05 were considered significant.
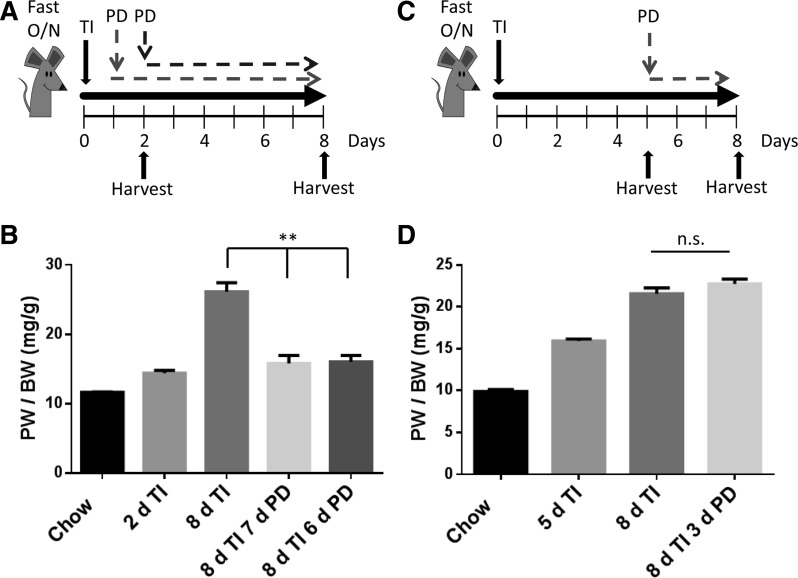
Fig. 7.
Effect of MEK inhibition on the initiation and maintenance of pancreatic adaptive growth. A: schematic diagram of experimental procedure. Mice were fasted overnight and refed 0.1% TI continuously. The pancreas was harvested at 2 and 8 days following refeeding. At 1 or 2 days a cohort of mice was also given PD-0325901 continuously in the chow, and PW/BW was measured at 8 days. B: quantitated results of PW/BW. C: schematic of experimental procedure. Mice were fasted overnight and refed 0.1% TI continuously. The pancreas was harvested at 5 and 8 days following refeeding. At 5 days, a cohort of mice was given PD-0325901 continuously in the chow, and the pancreas was harvested at 8 days. The PW/BW was measured and quantitated in D. All results shown are means ± SE of n = 6–10 mice. NS, not significant. **P < 0.01.
RESULTS
ERK signaling is activated by TI and blocked by PD-0325901 in vivo.
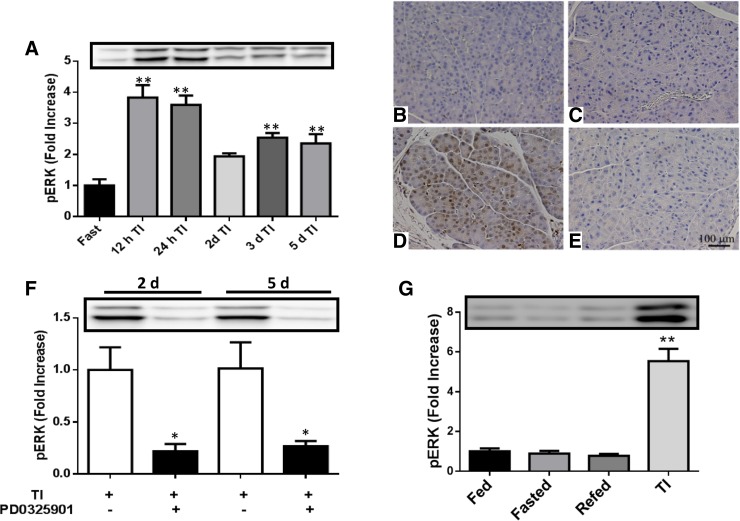
Pancreatic adaptive growth was initiated by TI feeding. ERK activation was maximal at 2 h following TI refeeding and remained elevated over 5 days compared with fasted animals (Fig. 1A). To address whether PD-0325901 could be used to effectively block ERK signaling in vivo, pERK immunohistochemistry was assessed. ERK signaling was not activated in animals that were fasted (Fig. 1B) or treated with PD-0325901 (Fig. 1C). However, mice given TI for 2 h during refeeding exhibited a strong induction of pERK (Fig. 1D) in pancreatic acinar cells that was completely blocked by PD-0325901 (Fig. 1E). Consistent with these results, PD-0325901 blocked ERK phosphorylation as assessed by Western blot analysis at 48 h and 5 days following TI-induced adaptive pancreatic growth (Fig. 1F). Mice that were fed, fasted, or refed chow alone for 2 h had similar pERK levels, whereas refeeding chow containing TI for 2 h following fasting caused a significant increase in ERK activation (Fig. 1G). However, fasting and refeeding chow alone stimulated other pathways, including mTOR (data not shown). Thus, TI feeding resulted in activation of ERK signaling that was effectively blocked by PD-0325901 in mice.
Fig. 1.
Effect of trypsin inhibitor (TI) and PD-0325901 on extracellular signal-related kinase (ERK) signaling. A: Western blot (top) and signal quantitation (bottom) of pancreatic phospho (p)-ERK levels following TI feeding. Mice were fasted overnight and refed 0.1% TI in the chow for the indicated time. Pancreatic pERK immunoperoxidase staining of mice fasted overnight (B), fasted mice administered PD-0325901 by gavage (C), TI fed for 2 h following fasting (D), or treated with both TI and PD-0325901 (E). F: mice were fasted overnight and refed TI and PD-0325901 for the indicated times. G: phosphorylated ERK expression of fed, fasted, refed, and TI-fed mice. Values are represented as fold increase of control mice. Results in A and F are means ± SE of n = 6–10 mice. *P < 0.05 and **P < 0.01.
PD-0325901 is a specific and potent inhibitor of ERK signaling in vivo.
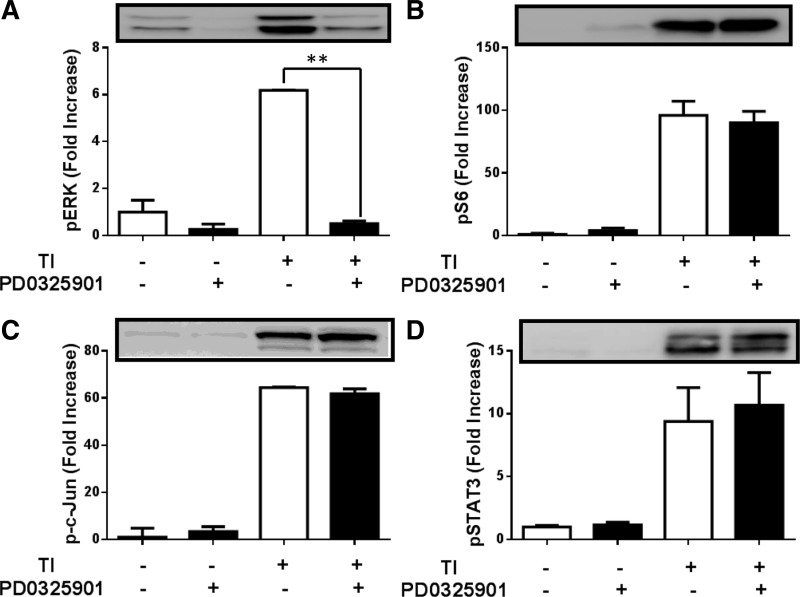
To test the potency and specificity of the MEK inhibitor PD-0325901, Western blot analysis for ERK phosphorylation and multiple signaling cascades known to be activated by TI feeding were assessed. It had previously been established that TI feeding activates the mTOR, JNK, and STAT pathways (2, 18, 19). TI treatment for 2 h following fasting led to a sixfold increase in phosphorylated ERK that was completely blocked by the addition of PD-0325901 (Fig. 2A). Refeeding TI induced the mTOR pathway 100-fold (Fig. 2B), the JNK pathway 60-fold (Fig. 2C), and the STAT pathway 10-fold (Fig. 2D). However, these pathways were not affected by PD-0325901 administration. These data show that ERK signaling is effectively blocked by PD-0325901, which is highly specific for MEK, as reflected by its lack of effect on other CCK-activated signaling cascades.
Fig. 2.
Effect of PD-0325901 on ERK, mTOR, JNK, and STAT signaling pathways following 2 h TI treatment. Western blots of pERK (A), pS6 (B), p-c-Jun (C), and pSTAT3 (D) in mice. Values are represented as fold increase of control mice. All results shown are means ± SE of n = 6–10 mice. **P < 0.01.
PD-0325901 inhibits pancreatic adaptive growth as measured by mass, protein, DNA, and RNA content.
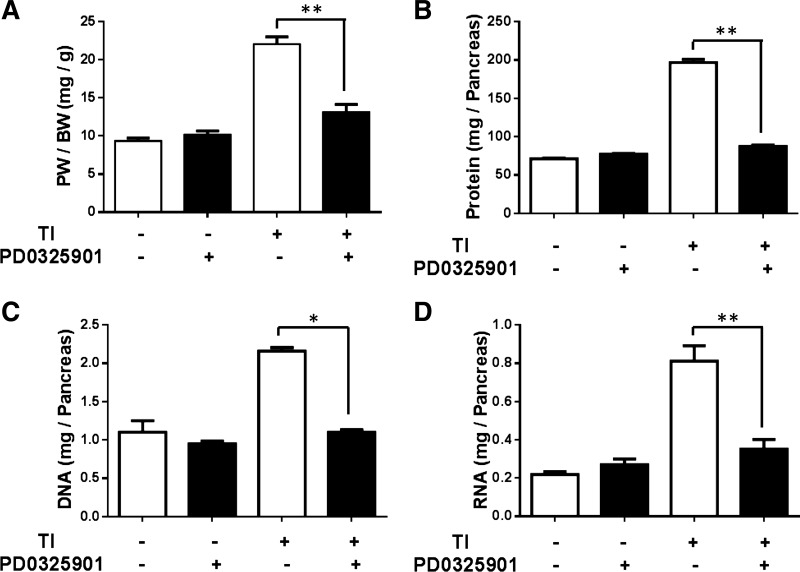
PD-0325901 treatment extending over 5 days did not result in a significant decrease in pancreatic mass as evidenced by the lack of significant difference in pancreatic weight/total body weight when comparing animals on a normal diet with those given chow and PD-0325901. Mice treated with TI demonstrated a 2.35-fold increase in pancreatic weight/total body weight that was effectively suppressed by treatment with PD-0325901, leading to a 77% inhibition of the increase in pancreatic mass induced by TI (Fig. 3A).
Fig. 3.
Effect of PD-0325901 on pancreatic adaptive growth following 5 days TI treatment. Pancreas weight (PW)/body weight (BW) (A), protein content (B), DNA content (C), and RNA content (D) in mice treated with 0.1% TI and PD-0325901. All results shown are means ± SE of n = 8–12 mice. *P < 0.05 and **P < 0.01.
Because pancreatic mass can be affected by changes in pancreatic protein, DNA, and RNA content, potential changes in these components were evaluated. There was no significant change in any of these macromolecules in mice given chow containing PD-0325901 compared with control mice (Fig. 3). TI-induced adaptive growth led to a significant increase in protein, DNA, and RNA content in the pancreas that was robustly blocked by PD-0325901 (Fig. 3, B–D). These data provide further support for the role of ERK signaling in mediating pancreatic adaptive growth induced by TI.
PD-0325901 specifically blocks ERK-regulated early response genes.
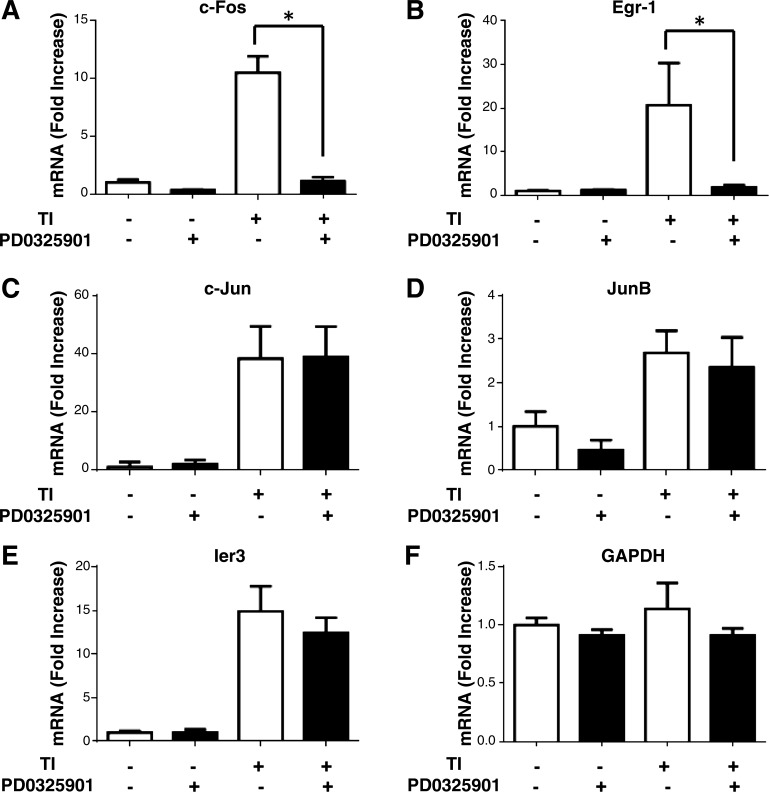
To determine whether downstream targets of ERK signaling were affected by ERK inhibition, mRNA levels of early response genes regulated by ERK were assessed by qRT-PCR. In addition, other transcription factors known to be upregulated by TI that are not believed to be part of the ERK pathway were examined. c-Fos, Egr-1, c-Jun, JunB, and Ier3 have been reported to be rapidly upregulated following induction of pancreatic growth by TI, and c-Jun/AP-1 is required for acinar cell growth in vitro (15, 16). Consistent with these data, a 10.5- and 20.6-fold increase in the ERK-regulated early response genes c-Fos (Fig. 4A) and Egr-1 (Fig. 4B) in TI-fed mice was observed compared with fasted mice. PD-0325901 significantly inhibited c-Fos and Egr-1 mRNA compared with mice given TI alone. Similar results were seen in Egr-2 mRNA (data not shown).
Fig. 4.
Effect of PD-0325901 on the expression of early response genes following 2 h TI treatment. qRT-PCR analysis of c-Fos (A), Egr-1 (B), c-Jun (C), JunB (D), and Ier3 (E) in mouse pancreas. F: GAPDH was included as a control. Values are represented as fold increase of control mice. All results shown are means ± SE of n = 8–10 mice. *P < 0.05.
c-Jun, JunB, and Ier3 are known to be regulated in an ERK-independent manner (15). c-Jun, JunB, and Ier3 mRNA expression was induced following 2 h TI treatment compared with fasted mice and was unaffected by PD-0325901 (Fig. 4, C–E). Similar results were observed for Egr-3 expression (data not shown). GAPDH was not affected by either TI or PD-0325901 administration (Fig. 4F). Taken together, these results show that PD-0325901 is specific in blocking ERK-regulated early response genes during TI feeding.
Pancreatic DNA synthesis is blocked by ERK inhibition following TI feeding.
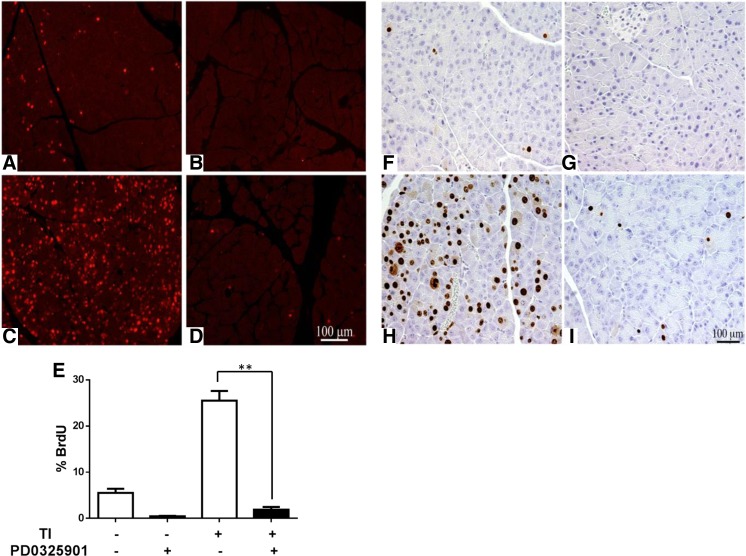
The ERK signaling pathway and the ERK regulated early response genes c-Fos and Egr-1 have been shown to be involved in many proliferative processes, including the regulation of cell-cycle proteins and mitogenesis (43). To understand if ERK inhibition would lead to decreased pancreatic DNA synthesis and mitogenesis, BrdU incorporation analysis was performed. Mice given BrdU in the drinking water over 48 h exhibited BrdU incorporation in 5% of the cell population (Fig. 5, A and E), whereas animals given chow and PD-0325901 exhibited <1% incorporation in the acinar cell nuclei (Fig. 5, B and E). Mice given TI for 2 days (Fig. 5, C and E) showed a 4.6-fold increase in BrdU incorporation that was completely inhibited by PD-0325901 treatment (Fig. 5, D and E). To further examine mitogenesis, the same mice were evaluated for Ki-67 immunoperoxidase staining. Similar to BrdU incorporation, very few Ki-67-positive nuclei appeared in the control groups (Fig. 5, F and G). Mice refed with TI for 2 days showed a significant increases in Ki-67-positive nuclei (Fig. 5H), an effect that was nearly abolished by treatment with PD-0325901 (Fig. 5I). These results indicate that DNA synthesis and mitogenesis are dependent on ERK signaling following TI feeding.
Fig. 5.
Effect of PD-0325901 on pancreatic DNA synthesis and mitogenesis. 5′-Bromo-2′-deoxyuridine (BrdU) immunofluorescence of control (A), PD-0325901 (B), and TI (C) for 48 h or TI and PD-0325901 (D). E: quantitated results. K-i67 immunoperoxidase staining in control (F), PD-0325901 (G), and 0.1% TI (H) for 48 h or TI and PD-0325901 (I). Results are means ± SE of n = 8–12 mice. **P < 0.01 compared with TI.
PD-0325901 blocks cell-cycle proteins and mitogenesis.
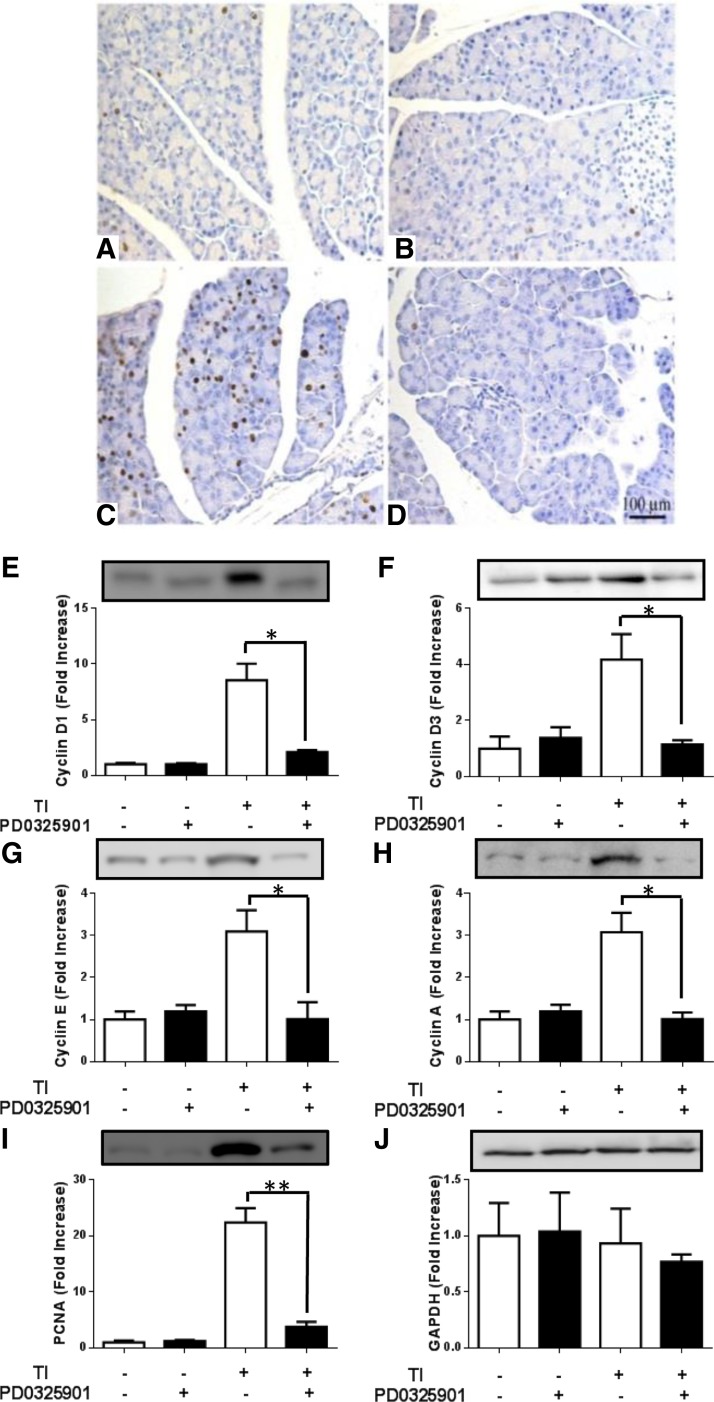
To examine the mechanism by which cell proliferation is blocked by ERK inhibition, cell-cycle proteins were studied by immunohistochemistry and Western blotting. Nuclear cyclin D1 expression was very low in the acinar cell nuclei of the control (Fig. 6A) and PD-0325901-treated (Fig. 6B) mice. The pancreas of mice treated with TI exhibited a significant increase in cyclin D1 staining (Fig. 6C) that was blocked by PD-0325901 (Fig. 6D). Western blotting analysis showed no difference between the chow control and chow plus PD-0325901 treatment groups. However, TI refeeding resulted in an 8.6-fold increase in cyclin D1 expression that was inhibited 75% by PD-0325901 treatment compared with the TI group (Fig. 6E). Other cell-cycle proteins, including cyclin D3 (Fig. 6F), cyclin E (Fig. 6G), and cyclin A (Fig. 6H), were increased 3.1- to 4.2-fold with TI treatment, and their expression was completely blocked by PD-0325901 administration. The DNA polymerase δ clamp PCNA, which is only expressed when cells are in S phase, was upregulated 22.4-fold during TI refeeding but was reduced 89% when mice were also given PD-0325901 (Fig. 6I). There was no significant difference in GAPDH expression between groups (Fig. 6J).
Fig. 6.
Effect of PD-0325901 on the expression of cell-cycle proteins in the mouse pancreas. Immunohistochemistry of cyclin D1 on mice that were refed chow (A), PD-0325901 (B), or 0.1% TI (C) for 24 h or TI and PD-0325901 (D). Western blots of cyclin D1 (E), cyclin D3 (F), cyclin E (G), cyclin A (H), and proliferating cell nuclear antigen (PCNA, I). J: GAPDH was included as a control. Values are represented as fold increase of control mice. Results are means ± SE of n = 8–10 mice. *P < 0.05 and **P < 0.01.
ERK signaling is required to initiate adaptive growth but is not required for maintenance of growth.
Mice were fasted overnight and refed either chow or chow containing 0.1% TI, and the pancreas was harvested at 2 and 8 days following refeeding. In addition, treatment with PD-0325901 was initiated 1 or 2 days following TI refeeding, and pancreas tissue was harvested at 8 days to assess whether ERK signaling was necessary to initiate adaptive growth (Fig. 7A). Mice treated with only TI for 2 days exhibited an increase in pancreatic mass that was further increased at 8 days. However, the animals given PD-0325901 at 1 or 2 days following TI refeeding did not show a significant difference in pancreatic mass compared with the mice treated with TI alone for 2 days (Fig. 7B). These data demonstrate that, during adaptive growth, ERK activation is required in the early initiation of growth.
To test whether ERK signaling is necessary to maintain pancreatic adaptive growth, ERK was similarly blocked at a later time point following TI-induced adaptive growth. Mice were treated with TI alone, and an increase in pancreatic mass was observed at 5 and 8 days (Fig. 7C). Another cohort of mice was treated with PD-0325901 5 days following TI-induced pancreatic growth, and the pancreas was harvested on day 8. These mice showed no significant change from the 8-day TI-induced mice (Fig. 7D). These results show that ERK signaling is not necessary to maintain growth.
The MEK inhibitor GSK-1120212 blocks TI-induced adaptive growth.
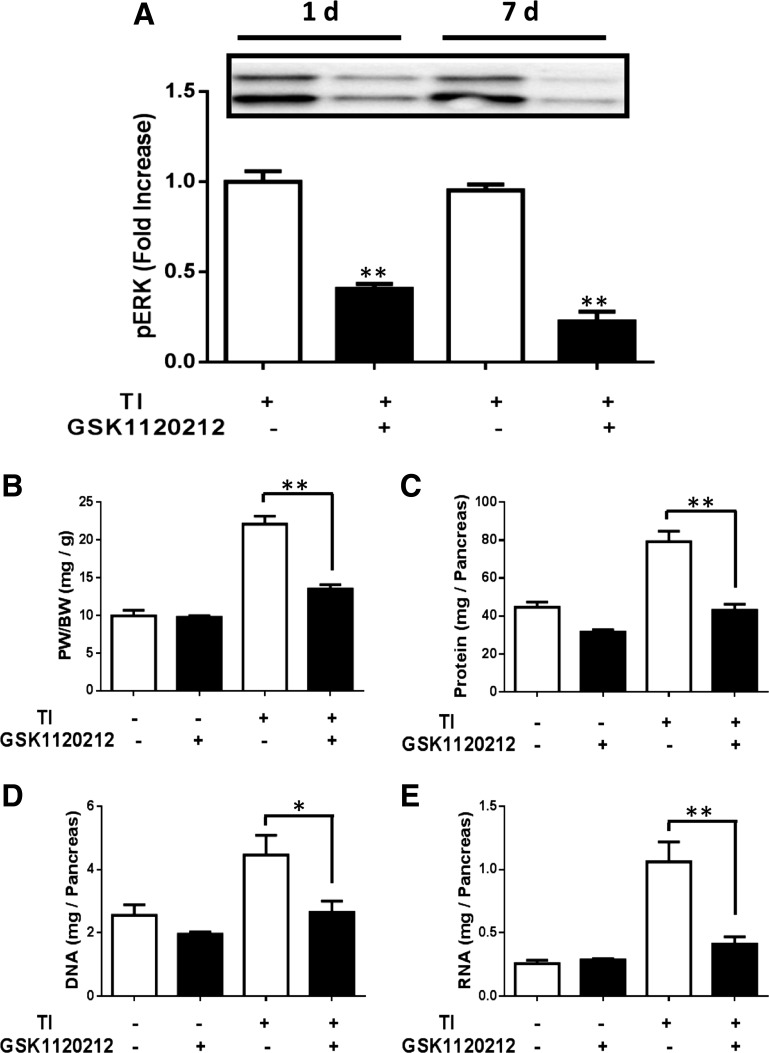
To show that inhibition of pancreatic adaptive growth was not unique to PD-0325901, the chemically distinct MEK inhibitor GSK-1120212 was assessed. GSK-1120212 was effective at blocking phosphorylation of ERK over 24 h and 7 days (Fig. 8A). To test whether the inhibitor blocked adaptive growth, mice were treated with GSK-1120212 and/or TI for 7 days. TI-induced pancreatic growth was blocked by GSK-1120212, as measured by pancreatic mass, protein, DNA, and RNA content (Fig. 8, B–E). These results show that GSK-1120212, like PD-0325901, blocks pancreatic adaptive growth induced by TI.
Fig. 8.
Effect of GSK-1120212 on ERK signaling and pancreatic adaptive growth following TI feeding. A: Western blot of ERK activation on mice that were treated with TI and/or GSK at the indicated times. Values are represented as fold increase of control mice. PW/BW (B), protein content (C), DNA content (D), and RNA content (E) were measured following 7 days treatment. All results shown are means ± SE of n = 6–10 mice. *P < 0.05 and **P < 0.01.
DISCUSSION
This study evaluated the role of ERK signaling in CCK-mediated pancreatic adaptive growth modeled using TI feeding. It was determined that the pharmacological inhibitors PD-0325901 and GSK-1120212 are effective at inhibiting the ERK pathway in vivo and that ERK exhibits a prolonged upregulation in response to endogenous CCK release induced by TI feeding. By inhibiting ERK signaling, pancreatic adaptive growth was effectively blocked, which we determined most likely occurred through ERK-regulated early response genes and cell cycle proteins. Thus, we conclude that ERK activation is required for CCK-mediated pancreatic adaptive growth.
A sustained, elevated release of CCK is necessary for pancreatic adaptive growth, which can occur during hyperphagia or ingestion of a high-protein diet (3, 12, 13). However, CCK is not necessary for the maintenance of pancreatic size, and CCK-deficient mice have normal pancreatic mass (46). Previous studies demonstrated that the mTOR and CN/NFAT pathways are required for adaptive growth induced by TI (Fig. 9) (2, 17, 18). In addition, other studies suggest that the STAT, JNK, and MAPK pathways are activated by CCK (15, 19). With the use of the MEK inhibitor PD-0325901, ERK activation was nearly abolished during TI feeding, which increases endogenous CCK levels. The fact that the mTOR, STAT, and JNK pathways were unaffected following TI feeding shows the specificity of the inhibitor and also that the inhibitor did not block CCK release. Similar inhibition could be obtained by gavage feeding two times daily or adding PD-0325901 to the powdered chow. It is evident that PD-0325901 or GSK-1120212 do not affect basal levels of CCK-mediated pancreatic growth and that these inhibitors do not cause pancreatic atrophy. This could be because of the limited time scale and slow rate of cell turnover of pancreatic acini. However, CCK-induced signaling does not appear to be necessary for basal pancreatic size regulation, which is minimally affected in CCK-deficient mice (29, 46).
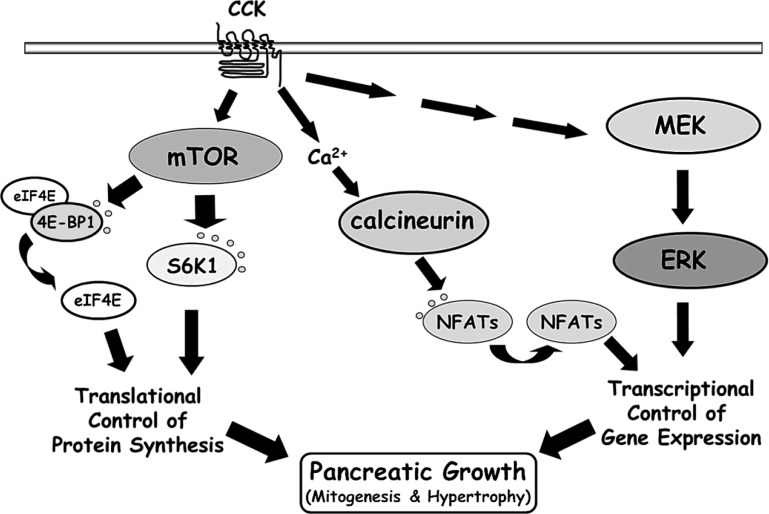
Fig. 9.
Model of the signaling pathways required for TI-induced cholecystokinin (CCK)-mediated pancreatic adaptive growth. Inhibition of the mTOR, calcineurin/NFAT, and MEK/ERK pathways by chemical inhibitors or genetic models indicates that each is independently necessary for pancreatic adaptive growth to occur.
It was necessary to fast the mice before the start of each experiment to ensure an adequate and reproducible food intake that allowed time-dependent analysis. Refeeding normal chow stimulates transient CCK release; however, pancreatic growth does not occur. Our data (not shown) as well as others demonstrate that fasting and refeeding activates other pathways that are triggered by CCK (38). However, ERK is not activated by the fasting-refeeding cycle (Fig. 1G), and basal levels of pERK are relatively low. We use TI to stimulate sustained CCK release (46). It is possible that TI could have other non-CCK actions; however, such an effect does not appear important for pancreatic growth, since growth is blocked in the absence of CCK or the CCK receptor (40, 46).
TI feeding causes a doubling of pancreatic mass with parallel increases in protein, DNA, and RNA content over 5–7 days. Our results show that, when ERK signaling is blocked by PD-0325901 or GSK-1120212, this increase in pancreatic mass, protein, DNA, and RNA content is nearly completely inhibited compared with TI-fed mice. It seems unlikely that compensation or upregulation from the mTOR, STAT, or JNK pathways contributes to this lack of complete inhibition because no increase in the activation of downstream targets of these pathways during ERK inhibition was observed. However, in pancreatic acinar cells, mTOR signaling is known to control protein synthesis during translation and regulate G1 cell cycle progression and G1 cyclins in some cancers (10, 39). The CN/NFAT pathway is required for adaptive growth, and NFATs are known to be coactivators with Jun and Fos to regulate proliferation (21, 22, 47). Furthermore, CN has also been known to be involved in regulating the expression of cyclin D1 and cyclin A (24–26). The JAK/STAT and JNK pathways are upregulated in response to CCK and also regulate many mitotic genes, including c-myc and cyclin D1 expression (42, 44). It is conceivable that cross talk of feedback loops on these pathways may contribute to incomplete inhibition of pancreatic adaptive growth shown with these MEK inhibitors. However, the simplest explanation is that each signaling pathway activates an essential step.
In cultured cell models when ERK is activated by MEK, it translocates to the nucleus where it activates multiple early response genes involved in mitogenesis (37). In our studies, MEK inhibition blocks TI-induced gene expression of ERK-regulated early response genes (c-Fos, Egr-1, Egr-2) but does not affect transcription factors from other signaling cascades, including c-Jun, JunB, JunD, or Ier3. By contrast, Rcan-1, a downstream target of CN/NFAT, is partially inhibited by MEK inhibitor treatment (∼25%) (data not shown). These results indicate that the ERK and CN/NFAT pathways may share some common transcription factors that may affect mitogenesis. However, taken together, PD-0325901 is effective at inhibiting the increase in expression of ERK-regulated early response genes caused by TI.
To test how mitogenesis was affected by MEK inhibition, BrdU incorporation and Ki-67 immunohistochemistry were analyzed. A marked increase in both BrdU incorporation and Ki-67 staining by TI refeeding was observed, which was blocked by PD-0325901. The rate of BrdU incorporation was higher in this study compared with previous studies because of the fact that BrdU was continuously present in the drinking water over 2 days as opposed to being given intraperitoneally one time 2 h before tissue collection (2, 17). These results indicate that mitogenesis is dependent on ERK phosphorylation in pancreatic adaptive growth.
Although we have determined that ERK is necessary for adaptive growth, the mechanism by which ERK stimulates pancreatic acinar cells to leave G0 and enter the cell cycle has not been established. The early response genes c-Fos and Egr-1 are known to have binding sites on the promoters of cell-cycle proteins, including cyclin D1 (9, 28, 50). It has also been established that c-Fos regulates cyclin D1 expression in other tissues (1). Furthermore, ERKs regulate the G1 to S phase transition in mammalian somatic cells (32). To address when ERK regulates pancreatic adaptive growth, we tested the cell-cycle proteins and determined that TI upregulates cyclin D1, cyclin D3, cyclin E, cyclin A, and PCNA, which are all blocked by PD-0325901 administration. Thus, it can be concluded that, since cyclin D1 and cyclin D3 are the first cell-cycle proteins transcribed in G1, ERK signaling is essential in initiating cell-cycle progression and may be involved in stimulating the exit of acinar cells from G0. To confirm that ERK signaling is necessary to initiate early cell-cycle events, we found that, if ERK is blocked early on in the adaptive growth process, mitogenesis ceases. However, when ERK is inhibited at later time points during pancreatic adaptive growth, blocking ERK does not change pancreatic mass. These results suggest that ERK is necessary to get acinar cells past a critical checkpoint in the cell-cycle process; once this happens, cells continue to undergo mitogenesis independent of ERK.
CCK and the ERK signaling pathway have been suggested to play a role in other pancreatic proliferative processes. Pancreatic recovery occurs following acute pancreatitis in rats (8, 20). Morisset et al. showed that, in pancreatic regeneration following pancreatectomy, an upregulation of ERK signaling occurred followed by an overexpression of cell-cycle proteins, including cyclin D and E (33). It is not known, however, if ERK signaling is required for this process. In isolated rat pancreatic acini, ERK signaling was enhanced following CCK treatment, and in primary cell culture exogenous CCK activated ERK-regulated early response genes (6, 16). Furthermore, the MEK inhibitor PD-0325901 has been shown to elicit therapeutic activity in reducing tumor volume in pancreatic cancer in mice (49).
In conclusion, our results demonstrate that the ERK pathway is necessary for pancreatic mitogenesis and adaptive growth in response to feeding TI. ERK is necessary to initiate early stages of the cell cycle, possibly causing acinar cells to exit G0 or enabling them to pass a critical checkpoint in the cell-cycle process. It seems likely that activation of multiple signaling cascades, including the ERK, mTOR, and CN/NFAT pathways, are required for pancreatic adaptive growth to occur (Fig. 9). Interestingly, these pathways are all distinct from pathways that regulate the acute secretion of digestive enzymes. Thus, the extracellular messenger CCK, known to activate multiple cellular processes, does this through the activation of multiple signaling pathways.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-59578 (J. A. Williams) and the Protein Identification and Localization Core of the Michigan Gastrointestinal Peptide Center, P30-DK-34933.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.J.H., J.S.S.-L., S.A.E., and J.A.W. conception and design of research; B.J.H. and K.B.L. performed experiments; B.J.H. and K.B.L. analyzed data; B.J.H., S.A.E., and J.A.W. interpreted results of experiments; B.J.H. and S.A.E. prepared figures; B.J.H. drafted manuscript; B.J.H., J.S.S.-L., S.A.E., and J.A.W. edited and revised manuscript; B.J.H. and J.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brad Nelson for assistance with BrdU staining, Marina Pasca di Magliano for reagents and advice on immunohistochemistry, and Yatrik Shah for careful revisions of the manuscript.
REFERENCES
- 1.Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F, Pestell RG, Greenberg ME. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol 18: 5609–5619, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crozier SJ, Sans MD, Guo L, D'Alecy LG, Williams JA. Activation of the mTOR signalling pathway is required for pancreatic growth in protease-inhibitor-fed mice. J Physiol 573: 775–786, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crozier SJ, Sans MD, Wang JY, Lentz SI, Ernst SA, Williams JA. CCK-independent mTORC1 activation during dietary protein-induced exocrine pancreas growth. Am J Physiol Gastrointest Liver Physiol 299: G1154–G1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabrowski A, Grady T, Logsdon CD, Williams JA. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitro and in vivo. J Biol Chem 271: 5686–5690, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Douglas BR, Woutersen RA, Jansen JB, Rovati LC, Lamers CB. Comparison of the effect of lorglumide on pancreatic growth stimulated by camostate in rat and hamster. Life Sci 46: 281–286, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Duan RD, Williams JA. Cholecystokinin rapidly activates mitogen-activated protein kinase in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 267: G401–G408, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Duncia JV, Santella JB, 3rd, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC, Teleha CA, Blom KF, Favata MF, Manos EJ, Daulerio AJ, Stradley DA, Horiuchi K, Copeland RA, Scherle PA, Trzaskos JM, Magolda RL, Trainor GL, Wexler RR, Hobbs FW, Olson RE. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett 8: 2839–2844, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Elsasser HP, Adler G, Kern HF. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas 1: 421–429, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Eto I. Molecular cloning and sequence analysis of the promoter region of mouse cyclin D1 gene: implication in phorbol ester-induced tumour promotion. Cell Prolif 33: 167–187, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol 287: C281–C291, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, Rominger CM, Erskine S, Fisher KE, Yang J, Zappacosta F, Annan R, Sutton D, Laquerre SG. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res 17: 989–1000, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Green GM, Jurkowska G, Berube FL, Rivard N, Guan D, Morisset J. Role of cholecystokinin in induction and maintenance of dietary protein-stimulated pancreatic growth. Am J Physiol Gastrointest Liver Physiol 262: G740–G746, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Green GM, Levan VH, Liddle RA. Plasma cholecystokinin and pancreatic growth during adaptation to dietary protein. Am J Physiol Gastrointest Liver Physiol 251: G70–G74, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Groblewski GE, Grady T, Mehta N, Lambert H, Logsdon CD, Landry J, Williams JA. Cholecystokinin stimulates heat shock protein 27 phosphorylation in rat pancreas both in vivo and in vitro. Gastroenterology 112: 1354–1361, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Guo L, Sans MD, Gurda GT, Lee SH, Ernst SA, Williams JA. Induction of early response genes in trypsin inhibitor-induced pancreatic growth. Am J Physiol Gastrointest Liver Physiol 292: G667–G677, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Sans MD, Hou Y, Ernst SA, Williams JA. c-Jun/AP-1 is required for CCK-induced pancreatic acinar cell dedifferentiation and DNA synthesis in vitro. Am J Physiol Gastrointest Liver Physiol 302: G1381–G1396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurda GT, Crozier SJ, Ji B, Ernst SA, Logsdon CD, Rothermel BA, Williams JA. Regulator of calcineurin 1 controls growth plasticity of adult pancreas. Gastroenterology 139: 609–619, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurda GT, Guo L, Lee SH, Molkentin JD, Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell 19: 198–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurda GT, Wang JY, Guo L, Ernst SA, Williams JA. Profiling CCK-mediated pancreatic growth: the dynamic genetic program and the role of STATs as potential regulators. Physiol Genomics 44: 14–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman L, Fitzgerald PJ. Restitution of pancreatic acinar cells following ethionine. J Cell Biol 12: 297–312, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity 2: 461–472, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 365: 352–355, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Jolicoeur L, Asselin J, Morisset J. Trophic effect of gestation and lactation on rat pancreas. Biomed Res 1: 482–488, 1980 [Google Scholar]
- 24.Kahl CR, Means AR. Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts. Mol Biol Cell 15: 1833–1842, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpurapu M, Wang D, Singh NK, Li Q, Rao GN. NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis. J Biol Chem 283: 26577–26590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karpurapu M, Wang D, Van Quyen D, Kim TK, Kundumani-Sridharan V, Pulusani S, Rao GN. Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J Biol Chem 285: 3510–3523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kehat I, Molkentin JD. Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann NY Acad Sci 1188: 96–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci 121: 3853–3857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol Gastrointest Liver Physiol 276: G1302–G1309, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Li R, Stafford JA. Kinase Inhibitor Drugs. Hoboken, NJ: Wiley, 2009, pp. xv, 510 [Google Scholar]
- 31.Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am J Physiol Gastrointest Liver Physiol 269: G319–G327, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26: 3227–3239, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Morisset J, Aliaga JC, Calvo EL, Bourassa J, Rivard N. Expression and modulation of p42/p44 MAPKs and cell cycle regulatory proteins in rat pancreas regeneration. Am J Physiol Gastrointest Liver Physiol 277: G953–G959, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Niederau C, Liddle RA, Williams JA, Grendell JH. Pancreatic growth: interaction of exogenous cholecystokinin, a protease inhibitor, and a cholecystokinin receptor antagonist in mice. Gut Suppl 28: 63–69, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsuki M, Ohki A, Okabayashi Y, Suehiro I, Baba S. Effect of synthetic protease inhibitor camostate on pancreatic exocrine function in rats. Pancreas 2: 164–169, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 20: 3161–3173, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranganathan A, Yazicioglu MN, Cobb MH. The nuclear localization of ERK2 occurs by mechanisms both independent of and dependent on energy. J Biol Chem 281: 15645–15652, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sans MD, Lee SH, D'Alecy LG, Williams JA. Feeding activates protein synthesis in mouse pancreas at the translational level without increase in mRNA. Am J Physiol Gastrointest Liver Physiol 287: G667–G675, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sans MD, Williams JA. Translational control of protein synthesis in pancreatic acinar cells. Int J Gastrointest Cancer 31: 107–115, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Sato N, Suzuki S, Kanai S, Ohta M, Jimi A, Noda T, Takiguchi S, Funakoshi A, Miyasaka K. Different effects of oral administration of synthetic trypsin inhibitor on the pancreas between cholecystokinin-A receptor gene knockout mice and wild type mice. Jpn J Pharmacol 89: 290–295, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Scarpignato C, Varga G, Dobronyi I, Papp M. Effect of a new potent CCK antagonist, lorglumide, on caerulein- and bombesin-induced pancreatic secretion and growth in the rat. Br J Pharmacol 96: 661–669, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology 37: 824–832, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 282: L1296–L1304, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Tashiro M, Dabrowski A, Guo L, Sans MD, Williams JA. Calcineurin-dependent and calcineurin-independent signal transduction pathways activated as part of pancreatic growth. Pancreas 32: 314–320, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286: G784–G790, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Walters RD, Drullinger LF, Kugel JF, Goodrich JA. NFATc2 recruits cJun homodimers to an NFAT site to synergistically activate interleukin-2 transcription. Mol Immunol 56: 48–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams JA, Sans MD, Tashiro M, Schafer C, Bragado MJ, Dabrowski A. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol Toxicol 91: 297–303, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Williams TM, Flecha AR, Keller P, Ram A, Karnak D, Galban S, Galban CJ, Ross BD, Lawrence TS, Rehemtulla A, Sebolt-Leopold J. Cotargeting MAPK and PI3K signaling with concurrent radiotherapy as a strategy for the treatment of pancreatic cancer. Mol Cancer Ther 11: 1193–1202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao D, Chinnappan D, Pestell R, Albanese C, Weber HC. Bombesin regulates cyclin D1 expression through the early growth response protein Egr-1 in prostate cancer cells. Cancer Res 65: 9934–9942, 2005 [DOI] [PubMed] [Google Scholar]