Abstract
Activation of NF-κB signaling in the heart may be protective or deleterious depending on the pathological context. In diabetes, the role of NF-κB in cardiac dysfunction has been investigated using pharmacological approaches that have a limitation of being nonspecific. Furthermore, the specific cellular pathways by which NF-κB modulates heart function in diabetes have not been identified. To address these questions, we used a transgenic mouse line expressing mutated IκB-α in the heart (3M mice), which prevented activation of canonical NF-κB signaling. Diabetes was developed by streptozotocin injections in wild-type (WT) and 3M mice. Diabetic WT mice developed systolic and diastolic cardiac dysfunction by the 12th week, as measured by echocardiography. In contrast, cardiac function was preserved in 3M mice up to 24 wk of diabetes. Diabetes induced an elevation in cardiac oxidative stress in diabetic WT mice but not 3M mice compared with nondiabetic control mice. In diabetic WT mice, an increase in the phospholamban/sarco(endo)plasmic reticulum Ca2+-ATPase 2 ratio and decrease in ryanodine receptor expression were observed, whereas diabetic 3M mice showed an opposite effect on these parameters of Ca2+ handling. Significantly, renin-angiotensin system activity was suppressed in diabetic 3M mice compared with an increase in WT animals. In conclusion, these results demonstrate that inhibition of NF-κB signaling in the heart prevents diabetes-induced cardiac dysfunction through preserved Ca2+ handling and inhibition of the cardiac renin-angiotensin system.
Keywords: nuclear factor-κB, renin-angiotensin system, diabetic cardiomyopathy, IκB-α transgenic mice
nf-κb signaling has a central role in cardiac pathophysiology (10). NF-κB regulates the expression of several cellular pathways that are important in cardiac development as well as postnatal function. In a pathological context, NF-κB activation has been described in cardiac hypertrophy, ischemia-reperfusion injury, myocardial infarction, heart failure, and diabetic cardiomyopathy (18, 23). Given the multiple roles of NF-κB in different tissues and disease conditions, questions have been raised as to whether its activation is protective or detrimental for heart function in disease states (8). A generally accepted view is that acute activation of NF-κB, such as in preconditioning, is required for cardioprotection, whereas chronic activation leads to heart failure. The lack of consensus among different studies is likely due to differences in components of NF-κB signaling investigated and the cell type and pathological conditions studied (18).
Diabetes is a condition of chronic inflammation, and prolonged activation of NF-κB has been reported in the diabetic heart. Cardioprotective effects of a pharmacological agent, pyrrolidine dithiocarbamate, which are associated with reduced NF-κB activity in the diabetic heart, have been reported (26). Since pyrrolidine dithiocarbamate also reduces oxidative stress, it is not clear whether the protection provided by this compound comes from NF-κB inhibition or an attenuation of oxidative stress. Diabetes also increases the activity of the cardiac renin-angiotensin system (RAS). Significantly, ANG II, the bioactive peptide of the RAS, has been reported to stimulate NF-κB activity in several cell types (3, 28, 33, 34, 45). Clinical and experimental studies have shown cardioprotective effects of RAS inhibition in diabetes, which might partly be due to a reduction in NF-κB-mediated inflammation (12, 37, 47). Thus, evidence exists, albeit indirect, to suggest the involvement of NF-κB in the development of diabetic cardiomyopathy and heart failure. However, a direct causative role of NF-κB in diabetes-induced cardiac pathology and putative downstream cellular pathways has not been established.
The NF-κB signaling pathway is a multicomponent system with diverse mechanisms of activation (18). The most common mechanism is the canonical pathway, in which a heterodimer of p65 and p50 subunits is held in the cytoplasm in an inactive state by the IκB α-subunit. Phosphorylation of the latter, as a result of the appropriate stimuli, leads to degradation and translocation of the heterodimer to the nucleus. Approaches using overexpression, deletion, or mutations of the above subunits in transgenic animals have been used to study the role of NF-κB in various pathophysiological states but not in diabetic cardiomyopathy (18, 27).
In this study, we used a cardiac-specific transgenic mouse line that overexpresses IκB-α protein with a triple mutation (3M) to prevent its phosphorylation (5). 3M mice have been well characterized in several studies and reported to have a normal phenotype with lack of NF-κB activation in the heart in several pathological states and in response to proinflammatory cytokines (5, 20, 48). We tested the hypothesis that suppression of NF-κB in the heart would protect these animals from developing diabetes-induced cardiac dysfunction. Furthermore, we tested whether the lack of NF-κB activation attenuates the cardiac RAS, thereby providing a mechanistic explanation of its protective effects.
MATERIALS AND METHODS
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee and conformed with National Institutes of Health guidelines. The generation of 3M mice has been previously described (5). These mice have been back crossed to C57bl/6 mice for more than five generations; therefore, C57bl/6J mice (The Jackson Laboratory, Bar Harbor, ME) were used as wild-type (WT) mice for comparison. Twelve-week-old male WT and 3M mice were randomized into control and diabetic groups. Animals in the diabetic group were injected with streptozotocin (STZ; 50 mg·kg−1·day−1, Zanosar) intraperitoneally for 5 consecutive days, whereas animals in the control group received vehicle (0.1 M sodium citrate buffer, pH 4.5). This multiple low-dose STZ regimen does not produce nonspecific toxicity, and we did not observe any mortality in WT and 3M animals (11). After 2 wk, mice with a blood glucose value of ≥250 mg/dl were considered diabetic. Heart function was measured by echocardiography, which was performed every 4 wk from the beginning of diabetes (0 wk) until the end of the study (24 wk). At 24 wk, animals were anesthetized with isoflurane/O2 (2%/2%), and hearts collected for further analysis.
Echocardiographic measurements.
Transthoracic echocardiography was performed using a VisualSonics Vevo 2100 with a 35-MHz probe, as previously described (37). Briefly, mice were anesthetized with 3–5% isoflurane (with 2% O2), which was reduced to 1.5% to maintain heart rate in the range of 400–500 beats/min. The heart was imaged in the two-dimensional, short-axis, and four-chamber view to measure multiple parameters of systolic and diastolic function.
Urine collection and albumin-to-creatinine ratio.
Mice were individually housed in metabolic cages (Techniplast) for 24 h with free access to food and water. Excreted urine was collected over a 24-h period, and volume was measured. Urinary albumin excretion was determined using the albumin-to-creatinine ratio. Urinary albumin and creatinine were measured using a commercially available Albuwell M and creatinine companion kit, respectively, according to the manufacturer's instructions (Exocell).
Real-time PCR.
Gene expression of α-myosin heavy chain (MHC), β-MHC, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), ryanodine receptor 2 (Ryr2), angiotensinogen (AGT), and renin was determined using TaqMan assays (Applied Biosystems), as previously described (47). Data were normalized to 18S mRNA. In the case of renin and AGT, WT and 3M samples were analyzed separately after a gap of time, resulting in the change of reagents. To allow baseline comparison, we reanalyzed WT and 3M samples from control animals together.
Western blot analysis.
Protein expression was assessed with the standard Western immunoblot technique. Briefly, hearts were lysed in ice-cold lysis buffer (Cell Signaling) supplemented with protease and phosphatase inhibitor cocktails (Roche Applied Science). Equal amounts of cell lysate (60 μg protein) were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Blots were probed with antibodies for AGT (IBL-America), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2; Santa Cruz Biotechnology), and phospholamban (PLB; Thermo Scientific). Equal protein loading was confirmed by β-actin levels. Protein bands were detected using secondary antibodies labeled with infrared dye 680/800 and an enhanced Odyssey Infrared Imaging System (LI-COR, Biosciences). Samples from control and diabetic animals were run on the same blot, resulting in WT and 3M samples being analyzed separately. In the case of SERCA2 and PLB, a common sample was run along all blots to correct for interexperimental variation and to allow comparison. In the case of AGT, we reanalyzed WT and 3M control samples together on the same gel to allow baseline comparison.
ROS staining.
Hearts were fixed in 4% paraformaldehyde and frozen in OCT compound (Tissue-Tek). Frozen sections (20 μm) were incubated with 10 μM dihydroethidium (DHE; Sigma-Aldrich) at 37°C for 30 min in a humidified chamber in the dark. Fluorescent images (×60) were obtained with a Leica TCS SP5X confocal microscope and analyzed using ImageJ. DHE fluorescence was quantified by dividing the integrated density of the fluorescent staining by the area for 10 fields/heart (5 hearts/group).
Immunofluorescence staining.
Heart sections (5 μm) were fixed with 4% formaldehyde and permeabilized using 0.1–0.3% Triton X-100. After being blocked, sections were incubated with anti-ANG II (Bachem, 1:500) and anti-p65 (Cell Signaling, 1:50) antibodies followed by an incubation with the respective secondary antibodies (1:500). The specificity of staining was determined using secondary antibody alone. Sections were costained with phalloidin (1:100), 4′,6-diamidino-2-phenylindole, and/or wheat germ agglutinin to visualize the cytoplasm, nuclei, and cell boundaries, respectively. Images (×60) were acquired with a confocal fluorescence microscope (Leica TCS SP5X). Cardiac myocyte area and fluorescence intensities were determined using ImageJ.
Statistical analysis.
This study was designed to test the hypothesis that there is no difference in cardiac function and other parameters between diabetic and nondiabetic 3M mice. Two-way ANOVA with a Fisher's least-significant difference post hoc test or Student's t-test (Fig. 7) were used for statistical analysis (GraphPad Prism). P values of <0.05 were considered statistically significant.
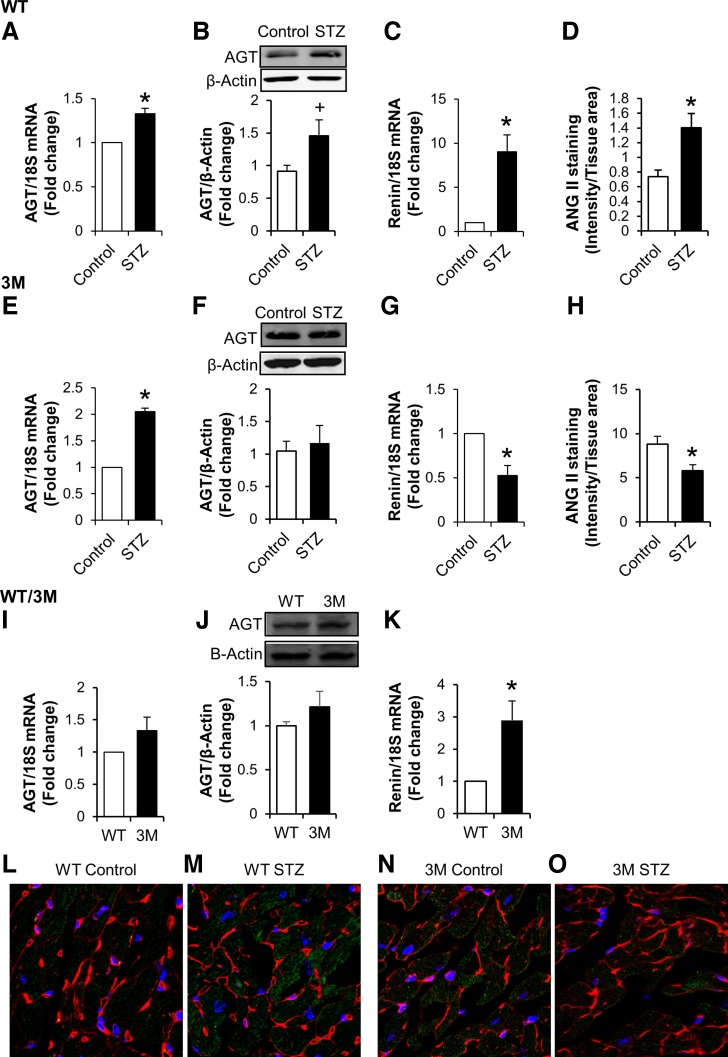
Fig. 7.
Measurement of renin-angiotensin system (RAS) components in the heart. A–H: hearts from control and diabetic (STZ) WT (A–D) and 3M (E–H) mice were used to determine the expression of angiotensinogen (AGT), renin, and ANG II. AGT expression was determined by real-time PCR (A and E) and Western blot analysis (B and F) and normalized to 18S mRNA or β-actin levels, respectively. Renin expression was determined by real-time PCR (C and G). I–K: control samples from WT and 3M mice were analyzed together for baseline comparison. Values are expressed as fold changes compared with control ± SE; n = 4–8. *P < 0.05 and +P < 0. 1 vs. control mice. L–O: immunostaining of heart sections for ANG II (green), WGA (red), and DAPI (blue) from WT and 3M mice. ANG II staining intensity was quantified, normalized to tissue area, and plotted for WT (D) and 3M (H) mice. *P < 0.05 vs. control mice.
RESULTS
NF-κB signaling may produce protective or deleterious effects in the heart depending on the pathological condition (18, 27). To investigate its role in diabetic cardiomyopathy, we used 3M mice in which NF-κB canonical signaling is suppressed in the heart in response to multiple pathological stimuli (5, 20, 48).
Induction of diabetes and cardiac morphology.
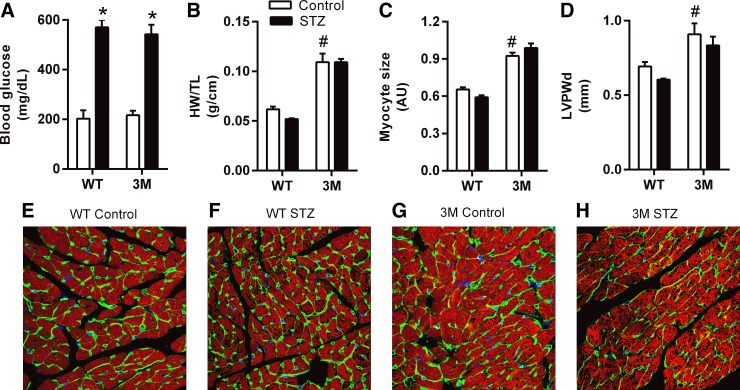
Both WT and 3M mice developed robust, sustained, and equivalent hyperglycemia (Fig. 1A). After 24 wk of diabetes, the heart weight-to-tibia length ratio, cardiac myocyte size, and left ventricular posterior wall thickness were not significantly affected in WT or 3M mice compared with their respective nondiabetic controls (Fig. 1, B–H). However, 3M mice had significantly increased baseline values for these morphological parameters compared with WT mice.
Fig. 1.
Blood glucose and heart morphology parameters after 24 wk of diabetes. Twelve-week-old male C57BL6/J mice [wild-type (WT) mice] and cardiac-specific transgenic mice expressing mutated IκB-α (3M mice) were injected with either 0.1 M citrate buffer (pH 4.5, control) or streptozotocin (STZ; 50 mg/kg) for 5 days. A: blood glucose levels after 24 wk of diabetes in control and diabetic (STZ) WT and 3M mice. B–D: parameters of cardiac morphology in control and diabetic WT and 3M mice. HW/TL, heart weight-to-tibia length ratio; AU, arbitrary units; LVPWd, left ventricular posterior wall thickness. E–H: confocal images (×60) of immunostaining of heart sections with phalloidin (red), wheat germ agglutinin (WGA; green), and 4′,6-diamidino-2-phenylindole (DAPI; blue) to demonstrate cardiac morphology in control and diabetic WT and 3M mice. The myocyte size plotted in C was calculated from these images using ImageJ. Values presented in bar charts are means ± SE; n = 5–7. *P < 0.05 vs. respective control mice; #P < 0.05 vs. control WT mice.
Cardiac function was preserved in 3M mice.
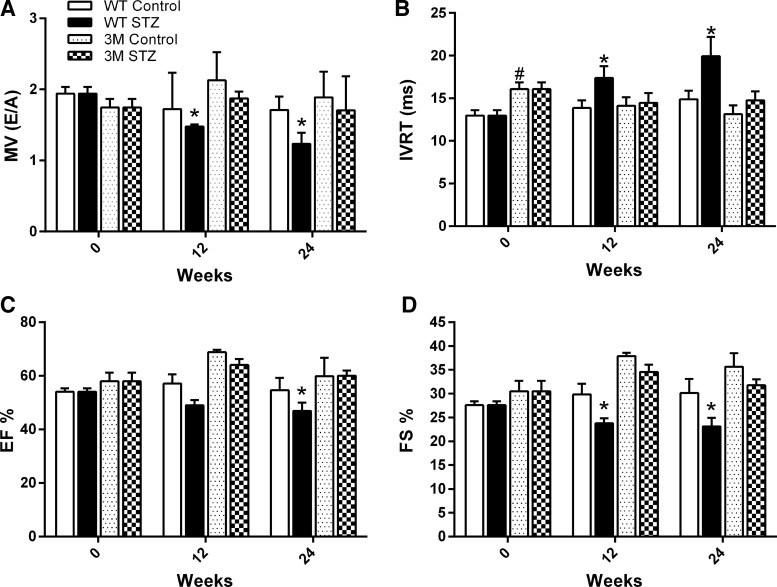
Echocardiography was used to measure cardiac systolic and diastolic function every 4 wk after the development of diabetes (only 0-, 12-, and 24-wk data are shown). After 12 wk, a significant reduction in the ratio of mitral valve flow velocities and an increase in the isovolumic relaxation time, both parameters of diastolic dysfunction, were evident in diabetic WT mice (Fig. 2, A and B). Systolic function, as measured by ejection fraction and fractional shortening, was also reduced in WT mice beginning at 12 wk of diabetes (Fig. 2, C and D). Cardiac function further declined with the duration of diabetes, until the last study point of 24 wk. However, 3M mice did not show any signs of cardiac dysfunction through the end of the study (Fig. 2, A–D). There were no significant differences in baseline (0 wk, control) heart function between WT and 3M mice except for isovolumic relaxation time, which appeared to be an artifact considering the values at other time points.
Fig. 2.
Measurement of diastolic and systolic function by echocardiography. Values are echocardiographic parameters in control and diabetic (STZ) WT and 3M mice performed on day 0, week 12, and week 24 after the induction of diabetes. A: ratio of mitral valve flow velocities [MV (E/A)]. B: isovolumic relaxation time (IVRT). C: ejection fraction (EF). D: fractional shortening (FS). Values are expressed as means ± SE; n = 5–7. *P < 0.05 vs. the respective 0-wk time point; #P < 0.05 vs. 0-wk control WT mice.
Molecular markers of cardiac function are preserved in 3M mice.
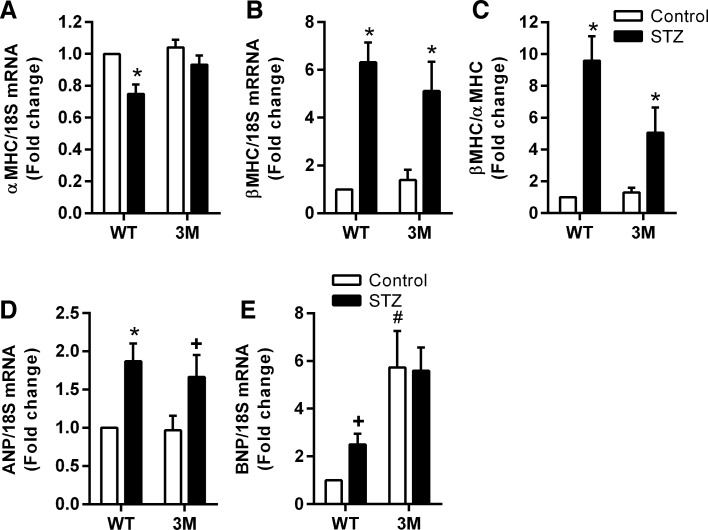
To confirm echocardiographic measurements of cardiac function, we measured the expression of α-MHC, β-MHC, ANP, and BNP in the hearts of WT and 3M mice (Fig. 3). Expression of α-MHC was significantly reduced, whereas that of other markers was increased in diabetic WT animals compared with nondiabetic control animals. A 2.5-fold increase in the expression of BNP in diabetic WT animals compared with control animals did not reach statistical significance upon multiple comparison, due to the large baseline difference between WT and 3M animals (Fig. 3E). In 3M diabetic mice, other than β-MHC (and, thus, the β-MHC-to-α-MHC ratio), the expression of these pathological markers did not change significantly, confirming that diabetes did not induce cardiac dysfunction in these animals.
Fig. 3.
Measurement of molecular parameters of cardiac dysfunction. Gene expression of α-myosin heavy chain (MHC; A), β-MHC (B), atrial natriuretic peptide (ANP; D), and brain natriuretic peptide (BNP; E) was measured by real-time RT-PCR and normalized to 18S mRNA. C: α-MHC-to-β-MHC ratio. Values are expressed as fold changes compared with control ± SE; n = 4–8. *P < 0.05 and +P < 0.2 vs. respective control mice; #P = 0.05 vs. control WT mice.
The NF-κb p65 subunit does not show increased translocation to the nucleus in diabetic 3M mice.
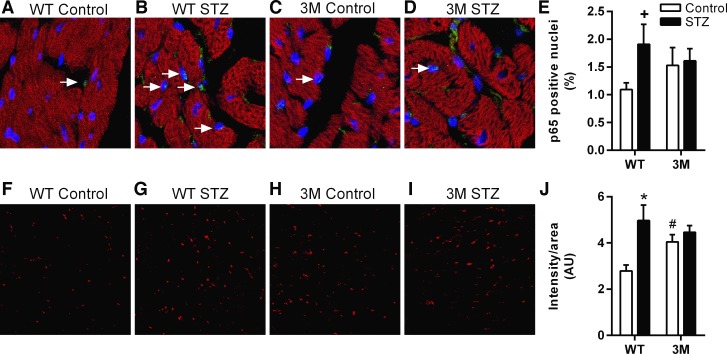
Expression of mutated IκB-α in 3M mice prevents the translocation of the p65:p50 dimer to the nucleus and NF-κB activation in the heart under different pathological conditions (5). To confirm that in diabetes there is no enhanced NF-κB activation in 3M mice, we quantified the number of nuclei in the heart that were positive for p65 immunostaining (Fig. 4, A–E). WT mice had increased numbers of p65-positive nuclei in diabetic mice compared with control mice, whereas no increase was observed in diabetic 3M mice, which was consistent with previous reports (5, 20, 48) of a lack of NF-κB activation in these transgenic mice.
Fig. 4.
Measurement of p65 nuclear translocation and oxidative stress. A–D: immunostaining of heart sections for p65 to demonstrate nuclear localization, as a measure of NF-κB activity, in control and diabetic (STZ) WT and 3M mice. Green color represents p65, blue represents nuclei, and red represents phalloidin staining. E: numbers of p65-positive nuclei, indicated by white arrows, were counted (∼4,000 nuclei from 5–6 images/heart, 4–5 animals/group) and plotted as percentage of total nuclei. +P = 0. 06 vs. respective control mice. F–I: oxidative stress was measured by dihydroethidium (DHE) staining (red) of heart sections. Representative confocal images of DHE staining for WT and 3M mice are shown. J: DHE staining intensity was normalized to section area and plotted. *P < 0.05 vs. respective control mice; #P = 0.05 vs. control WT mice.
3M mice are protected from diabetes-induced oxidative stress.
We measured oxidative stress in the heart by DHE staining. Diabetic WT mice had a significant increase in ROS staining, which was not observed in 3M mice compared with their respective nondiabetic controls (Fig. 4, F–J). Basal oxidative stress levels were higher in control 3M mice compared with control WT mice, the reason for which was not clear.
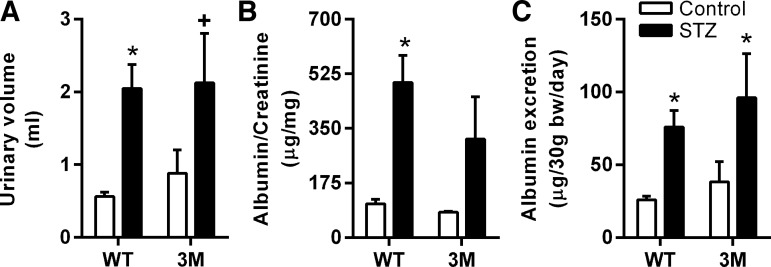
Renal function is not preserved in diabetic 3M mice.
Since 3M mice express mutated IκB-α in a cardiac-specific manner, the protection provided by NF-κB inhibition should be restricted to the heart. To confirm that 3M mice are not protected from diabetes-induced renal impairment, we evaluated renal function in these animals by determining the 24-h urine volume, albumin-to-creatinine ratio, and 24-h albumin excretion. These parameters were similarly increased in both diabetic WT and 3M mice compared with their respective nondiabetic controls, suggesting that renal function was not preserved in 3M mice after hyperglycemia (Fig. 5). However, the difference in urinary volume and albumin-to-creatinine ratio between control and diabetic 3M mice did not reach statistical significance due to the large variation in these mice.
Fig. 5.
Measurement of renal function. Twenty-four-hour urine collection from WT and 3M was obtained from mice individually housed in metabolic cages. Urinary volume, albumin, and creatinine levels were determined. A: urinary volume. B: albumin-to-creatinine ratio. C: albumin excretion. Values are expressed as means ± SE; n = 3–6. *P < 0.05 and +P ≤ 0.07 vs. respective control mice.
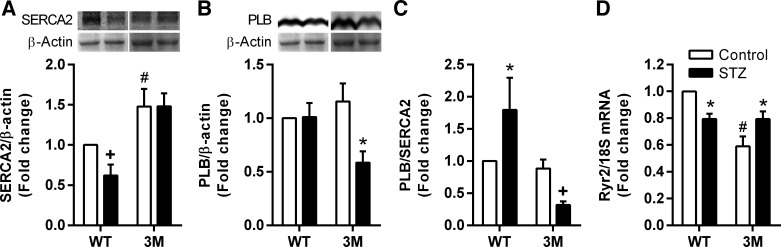
Ca2+-handling proteins in cardiac myocytes of 3M mice are preserved in diabetes.
Cardiac dysfunction in diabetes is associated with detrimental changes in expression of the sarcoplasmic reticulum Ca2+ uptake pump SERCA2 and its inhibitory protein PLB in cardiac myocytes (15, 36). We determined whether these changes were prevented in 3M mice in response to diabetes. SERCA2 expression was reduced in diabetic WT mice, without any change in PLB expression, resulting in a significantly increased PLB-to-SERCA2 ratio, which is an important indicator of inefficient Ca2+ uptake to the sarcoplasmic reticulum from the cytosol (Fig. 6, A–C). Significantly, SERCA2 expression was preserved and PLB expression was reduced in diabetic 3M mice, thereby reducing the PLB-to-SERCA2 ratio. We also measured gene expression of Ryr2, which mediates the release of Ca2+ from the sarcoplasmic reticulum to the cytosol. Ryr2 expression was significantly downregulated in WT mice and upregulated in 3M mice in response to hyperglycemia (Fig. 6D). 3M mice had significantly higher baseline expression of SERCA2 and lower expression of Ryr2 compared with WT mice (Fig. 6, A and D).
Fig. 6.
Measurement of parameters of Ca2+ handling in the heart. A and B: protein expression of sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2; A) and phospholamban (PLB; B) was determined by Western blot analysis in hearts from control and diabetic (STZ) WT and 3M mice. WT and 3M samples were run on separate blots, with a common sample on all blots to allow for interblot comparison. Intensity of protein bands was quantified by densitometry, normalized to β-actin expression, and plotted as the fold change compared with the control WT sample. C: the ratio of PLB to SERCA2 was plotted separately. D: expression of ryanodine receptor 2 (Ryr2) was measured by real-time PCR and normalized to 18S mRNA expression. Values are expressed as fold changes compared with the control WT sample ± SE; n = 4–8. *P < 0.05 and +P ≤ 0.1 vs. respective control mice; #P < 0.05 vs. control WT mice.
Activity of the RAS was suppressed in diabetic 3M mice.
We (37, 47) have previously demonstrated activation of the cardiac RAS in diabetes, inhibition of which prevents the development of diabetic cardiomyopathy. To understand the mechanism of cardiac protection through NF-κB inhibition, we measured changes in components of the RAS in hearts of WT and 3M mice in response to hyperglycemia. Both AGT and renin expression were increased in diabetic WT mice compared with nondiabetic control mice (Fig. 7, A–C). In diabetic 3M mice, AGT mRNA expression was increased, but protein expression did not change significantly, likely due to secretion (Fig. 7, E and F). However, renin expression was significantly reduced in diabetic 3M mice compared with nondiabetic control mice (Fig. 7G). Renin protein levels are not presented as we could not identify (with certainty) a renin band on Western blots, due to the nonspecificity of commercially available antibodies. The above-described changes in the expression of RAS genes resulted in increased ANG II production in the myocardium of diabetic WT mice but reduced levels in 3M mice compared with their respective nondiabetic controls (Fig. 7, D, H, and L–O). WT and 3M samples were analyzed after a time gap, resulting in a change in experimental conditions (new batch of reagents). Whether the difference in baseline ANG II staining intensity between WT and 3M mice was due to interexperimental variation as a result of the time gap is not clear. For comparison between baseline values of AGT and renin gene expression, we reanalyzed samples from control WT and 3M animals together (Fig. 7, I–K). AGT expression was similar, whereas renin expression was increased about threefold in 3M mice compared with WT mice.
DISCUSSION
In the present study, we investigated the role of NF-κB canonical signaling in diabetes-induced cardiac dysfunction using a genetic model of NF-κB inactivation. We observed that lack of NF-κB activation protects animals from developing diabetic cardiomyopathy. We also reported that the mechanism of protection, by NF-κB inhibition, involves an improvement in Ca2+ handling and inhibition of RAS activity in the diabetic myocardium. We and others (5, 20, 48) have previously described that 3M mice are resistant to NF-κB activation and exhibit preserved cardiac structure and function in response to multiple stimuli. This is the first report to demonstrate cardiac protection by NF-κB inhibition in diabetes using a genetic approach.
In the heart, activation of NF-κB has been reported in numerous disease contexts, including hypertrophy, ischemia-reperfusion injury, myocardial infarction, myocarditis, and diabetes (10, 27). With regard to the STZ model of diabetes, we observed an increased percentage of p65-positive nuclei in WT mice treated with STZ after 24 wk, indicating activation of NF-κB signaling (Fig. 4). 3M mice had higher basal levels of p65-positive nuclei compared with WT mice (statistically nonsignificant, P = 0.3); however, diabetes did not cause a further increase in p65-positive nuclei in 3M mice. Although we did not perform temporal measurements, other investigators have reported activation of NF-κB signaling at different time points in the myocardium of STZ-treated animals, e.g., in rats at 4 and 12 wk of diabetes by electrophoretic mobility shift assay (19, 22) and increased p65 expression (46); in mice at 8 and 12 wk by inflammatory cytokine expression (30, 42, 43) and IκB-α degradation, p65 expression, and electrophoretic mobility shift assay (31). To determine the significance of the NF-κB signaling pathway in various cardiac pathological conditions, investigators have used genetic models that target different members of this pathway (17, 18, 25, 49). These studies have produced confounding data, demonstrating both protective and detrimental functions of NF-κB. A broader view has emerged that suggests that the mechanisms and effects of NF-κB activation are disease and cell type specific. While having a role in the development of cardiac hypertrophy, this signaling pathway is also required for preconditioning benefits of myocardial ischemia-reperfusion (44).
We observed that STZ-treated 3M mice developed sustained hyperglycemia similar to WT mice (Fig. 1). Additionally, an increase in urinary volume and albumin excretion after 24 wk of diabetes was observed in both WT and 3M mice (Fig. 5), indicating that cardiac-specific expression of mutated IκB-α did not influence systemic parameters, such as hyperglycemia, and distant organ function, such as diabetic nephropathy. However, all measures of heart function were completely preserved in diabetic 3M mice (Fig. 2), suggesting that activation of the canonical NF-κB pathway in the diabetic myocardium is required for cardiac remodeling in this pathological state. Based on the cardiac morphological data, control 3M mice appeared hypertrophic compared with control WT mice (Fig. 1), which was in contrast to the first description of these animals (5), the reason for which is not clear. Whether this phenotype of 3M mice contributed to cardiac protection in diabetes is not known. Whereas WT mice displayed a significant reduction in systolic and diastolic function after 12 wk of diabetes, no cardiac dysfunction was observed even after 24 wk in 3M mice. Therefore, inhibition of NF-κB activation did not simply delay the onset of cardiac dysfunction but rather provided long-term protection, suggesting that this pathway is a major determinant of cardiac remodeling in diabetes.
Diabetes is considered a condition of chronic inflammation, which might contribute significantly to cardiac dysfunction. However, the levels in plasma and expression in tissues of proinflammatory cytokines have been shown to change with time in both type 1 and 2 diabetes models (2, 35), making it difficult to define the inflammatory status and role of individual cytokines in this disease process. In the heart, although increased early in diabetes, no increase in the expression of several inflammatory cytokines was observed after 22 wk of diabetes in STZ-treated rats (2). Similarly, we observed no increase or decreased expression of IL-1β, IL-6, TNF-α, and interferon-γ in 24-wk diabetic hearts compared with nondiabetic control hearts (data not shown). Thus, local expression of inflammatory mediators at this time point did not appear to be mechanistically linked to cardiac function, corroborating the above-described rat study (2). To provide a mechanistic explanation for cardiac protection in 3M mice, we investigated local mechanisms that have a direct role in cardiac function, such as the cardiac RAS, oxidative stress, and Ca2+ handling, which we hypothesized to be preserved in the absence of NF-κB activation.
Oxidative stress in the diabetic myocardium is widely accepted as the initiating mechanism that modulates multiple metabolic and signaling pathways, leading to cardiac dysfunction and diabetic cardiomyopathy (9, 16). NF-κB signaling is a redox-sensitive pathway that enhances inflammation and other cellular pathways, such as the RAS, which, in turn, may lead to a further increase in oxidative stress. Consistent with previous studies, we observed increased ROS in diabetic hearts of WT mice compared with nondiabetic control hearts (26, 37). In 3M mice, although baseline oxidative stress was higher than in WT mice, no further increase was observed in diabetic mice (Fig. 4, G–L). The reason for the increased basal oxidative stress is not clear; however, it did not cause any apparent phenotype in 3M animals, likely due to lack of NF-κB activation in these animals.
Ca2+ has multiple roles in the heart, including electrical activation, excitation-contraction coupling, and regulation of intracellular signaling (24). An aberration in Ca2+ handling by cardiac myocytes can lead to cardiac hypertrophy and failure. In diabetic cardiomyopathy, changes in the expression of regulators of intracellular calcium concentration and cycling, SERCA2, PLB, and Ryr2, have been associated with decreased cardiac function (36, 38, 41, 49). Consistent with these findings, we observed a decrease in SERCA2 and Ryr2 expression in diabetic WT mice, which was completely prevented in 3M mice (Fig. 6). In another study (49) on the role of NF-κB in the heart after ischemia-reperfusion injury, a complete recovery of calcium transients was observed in cardiac-specific p65 NF-κB knockout mice, suggesting a role of this transcription factor in calcium homeostasis. A difference in the basal expression of SERCA2 and PLB in p65 knockout mice compared with WT mice was not observed (49). We also did not find a significant change in PLB protein levels between control WT and 3M mice, indicating that this gene was not a direct target of the NF-κB transcriptional program. However, levels of SERCA2 protein were higher by 47%, and Ryr2 expression was lower by 40% in 3M mice compared with control WT mice. More studies would be required to determine if SERCA2 and Ryr2 transcription are directly regulated by NF-κB or not. Previously, it has been reported that the Ca2+ release from intracellular stores through Ryr2 and Ca2+/calmodulin-dependent protein kinase II mediate NF-κB activation (21, 41). It is clear from this study that NF-κB, directly or indirectly, also has a role in maintaining Ca2+ homeostasis in the heart, thus preserving cardiac function in diabetes.
Activation of the tissue RAS has a major role in the development of diabetes-induced cardiac dysfunction that leads to diabetic cardiomyopathy. We and others (13, 37, 47) have shown that inhibition of this system using angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or a renin inhibitor prevents the development of diabetic cardiomyopathy in experimental animals and retards the progression of cardiovascular events in patients. Previously, it has been shown that NF-κB controls the expression of AGT and that ANG II activates NF-κB signaling (1, 6, 14). In addition, angiotensin receptor blockers and angiotensin-converting enzyme inhibitors have been demonstrated to reduce inflammation through inhibition of the NF-κB pathway (4). Therefore, we investigated whether lack of NF-κB activation in 3M mice prevents diabetes-induced stimulation of the cardiac RAS, thus providing a mechanism for cardiac protection in these animals. Surprisingly, in the present study, we did not observe an attenuation of AGT expression in 3M mice in response to diabetes (Fig. 7, E and F). This finding suggests that AGT gene expression by NF-κB was mediated by one of the noncanonical pathways previously described by other investigators in noncardiac cells (3, 7, 32). In the latter studies, it was reported that NF-κB signaling regulated AGT expression in a cell type- and stimulus- dependent manner, either by forming p50:p50 or p50:p65 dimers or by IκB-α-independent DNA binding of different p50 isoforms in renal proximal tubular cells, vascular smooth muscle cells, and hepatocytes, respectively (3, 7, 32). The formation of p50:p50 homodimers was shown to inhibit TNF-α-mediated AGT expression, in contrast to the other two mechanisms (32). The mechanism of AGT expression by NF-κB has not been studied in the heart. The reason for no increase in AGT protein levels is not clear, although it is unlikely due to increased conversion to ANG II, as renin expression was reduced in these animals (Fig. 7G). It is possible that we could not capture a change in AGT levels due to its secretion from the tissue. Importantly, ANG II levels, which were significantly elevated in diabetic WT mice, were reduced in diabetic 3M mice, as a result of a decrease in the expression of renin. The conversion of AGT to ANG I by renin is the rate-limiting step in ANG II synthesis (29). With regard to the regulation of renin expression by NF-κB, it has been described only in juxtaglomerular cells, in response to TNF-α stimulation (39, 40). Although an NF-κB consensus binding sequence is present on the mouse renin promoter, it has been reported that activation of NF-κB, by TNF-α, reduced renin gene expression in a juxtaglomerular cell line (As4.1) by an indirect mechanism. The latter mechanism did not involve p65 binding to the NF-κB consensus sequence but rather was mediated by reduced transactivation capacity of p65 and attenuated cAMP-responsive element-binding protein 1 binding to a cAMP response element on the renin promoter (39). Whether NF-κB is required for basal renin expression is not known. When we compared AGT and renin expression between WT and 3M mice, we did not observe a statistically significant difference in AGT expression, further suggesting that AGT gene expression is independent of the canonical NF-κB pathway. However, renin expression was increased threefold in 3M mice compared with WT mice, suggesting that renin was negatively regulated by the NF-κB canonical pathway, consistent with the study discussed above (39). However, hyperglycemia reduced renin expression in 3M mice in this study. These results suggest that NF-κB regulates AGT and renin expression in a gene-specific manner and that these mechanisms may differ in normal and diabetic conditions. However, it is clear that lack of NF-κB activation in diabetic 3M mice reduced the activity of the cardiac RAS, as evidenced by reduced ANG II levels, which likely contributed significantly to the cardiac protective mechanism in diabetic 3M mice.
Significance.
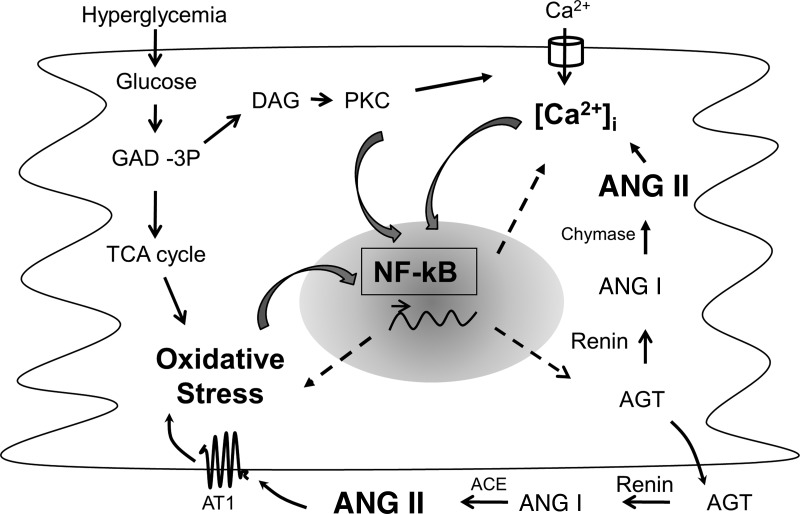
Cardiovascular diseases are a major cause of morbidity and mortality in the diabetic population. Several mechanisms, including oxidative stress, inflammation, impaired Ca2+ homeostasis, and activation of the RAS, have a major role in the development of diabetes-induced cardiac dysfunction that leads to the specific condition referred to as diabetic cardiomyopathy. A unifying molecular mechanism that would explain the development of the above pathological markers is lacking. NF-κB is a pleiotropic transcription factor that not only is activated by oxidative stress, intracellular Ca2+ release, and ANG II but also controls these events in a continuous feedback manner. Figure 8 shows a putative position of NF-κB in the cellular mechanism of diabetic cardiomyopathy that needs further investigation. This study provides evidence of NF-κB as a focal point of major pathological changes in the diabetic myocardium and might provide an attractive therapeutic target.
Fig. 8.
Schematic representation of the putative cross-talk between NF-κB, oxidative stress, intracellular Ca2+, and the RAS in cardiac myocytes exposed to hyperglycemia. Hyperglycemia increases oxidative stress as a byproduct of glucose metabolism and intracellular Ca2+ levels through intermediates of the glycolytic pathway. Oxidative stress and intracellular Ca2+ have been shown to activate NF-κB signaling. NF-κB controls the transcription of genes of RAS components, enzymes that modulate oxidative stress (e.g., NADPH oxidase), and Ca2+-handling proteins. ANG II, the bioactive peptide of the RAS, in turn increases oxidative stress and intracellular Ca2+ levels and activates NF-κB signaling. Thus, NF-κB appears to be the nodal point for the convergence of major cellular events that contribute to cardiac dysfunction in diabetes. This study provides evidence that lack of NF-κB activation prevents diabetic cardiomyopathy. GAD-3P, glyceraldehyde 3-phosphate; DAG, diacylglycerol; TCA cycle, tricarbocylic acid cycle; AT1, ANG II type 1 receptor; ACE, angiotensin-converting enzyme; [Ca2+]i, intracellular Ca2+ concentration.
GRANTS
This work was supported by funding by National Heart, Lung, and Blood Institute Grant 5-R01-HL-090817.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.T., Q.C.Y., R.M.R., and R.S. performed experiments; C.M.T., Q.C.Y., R.M.R., R.S., S. Gopal, and R.K. analyzed data; C.M.T., Q.C.Y., S. Gopal, D.E.C., W.K.J., S. Gupta, K.M.B., and R.K. interpreted results of experiments; C.M.T., Q.C.Y., and R.K. prepared figures; C.M.T., Q.C.Y., D.E.C., W.K.J., S. Gupta, K.M.B., and R.K. edited and revised manuscript; K.M.B. and R.K. approved final version of manuscript; R.K. conception and design of research; R.K. drafted manuscript.
ACKNOWLEDGMENTS
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System (Temple, TX).
Present affiliation of R. Seqqat: Escuela Politécnica del Ejército, Sangolquí, Ecuador.
REFERENCES
- 1.Acres OW, Satou R, Navar LG, Kobori H. Contribution of a nuclear factor-κB binding site to human angiotensinogen promoter activity in renal proximal tubular cells. Hypertension 57: 608–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ares-Carrasco S, Picatoste B, Benito-Martin A, Zubiri I, Sanz AB, Sanchez-Nino MD, Ortiz A, Egido J, Tunon J, Lorenzo O. Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am J Physiol Heart Circ Physiol 297: H2109–H2119, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-κB (NF-κB) transcription factor. Mol Cell Biochem 212: 155–169, 2000 [PubMed] [Google Scholar]
- 4.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257–1266, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK. Cardiac-specific blockade of NF-κB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am J Physiol Heart Circ Physiol 289: H466–H476, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Choudhary S, Lu M, Cui R, Brasier AR. Involvement of a novel Rac/RhoA guanosine triphosphatase-nuclear factor-κB inducing kinase signaling pathway mediating angiotensin II-induced RelA transactivation. Mol Endocrinol 21: 2203–2217, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-κB pathway. Am J Physiol Renal Physiol 296: F1212–F1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhingra R, Shaw JA, Aviv Y, Kirshenbaum LA. Dichotomous actions of NF-κB signaling pathways in heart. J Cardiovasc Transl Res 3: 344–354, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res 108: 1122–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 142: 375–415, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Ismail H, Mitchell R, McFarlane SI, Makaryus AN. Pleiotropic effects of inhibitors of the RAAS in the diabetic population: above and beyond blood pressure lowering. Curr Diab Rep 10: 32–36, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Jamaluddin M, Meng T, Sun J, Boldogh I, Han Y, Brasier AR. Angiotensin II induces nuclear factor (NF)-κB1 isoforms to bind the angiotensinogen gene acute-phase response element: a stimulus-specific pathway for NF-κB activation. Mol Endocrinol 14: 99–113, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Karakikes I, Kim M, Hadri L, Sakata S, Sun Y, Zhang W, Chemaly ER, Hajjar RJ, Lebeche D. Gene remodeling in type 2 diabetic cardiomyopathy and its phenotypic rescue with SERCA2a. PLOS ONE 4: e6474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol 88: 233–240, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Kratsios P, Huth M, Temmerman L, Salimova E, Al Banchaabouchi M, Sgoifo A, Manghi M, Suzuki K, Rosenthal N, Mourkioti F. Antioxidant amelioration of dilated cardiomyopathy caused by conditional deletion of NEMO/IKKγ in cardiomyocytes. Circ Res 106: 133–144, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Yong QC, Thomas CM. Do multiple nuclear factor κB activation mechanisms explain its varied effects in the heart? Ochsner J 13: 157–165, 2013 [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Prasad S, Sitasawad SL. Multiple antioxidants improve cardiac complications and inhibit cardiac cell death in streptozotocin-induced diabetic rats. PLOS ONE 8: e67009, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Wei C, Thomas CM, Kim IK, Seqqat R, Kumar R, Baker KM, Jones WK, Gupta S. Cardiac-specific genetic inhibition of nuclear factor-κB prevents right ventricular hypertrophy induced by monocrotaline. Am J Physiol Heart Circ Physiol 302: H1655–H1666, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Ling H, Gray CB, Zambon AC, Grimm M, Gu Y, Dalton N, Purcell NH, Peterson K, Brown JH. Ca2+/Calmodulin-dependent protein kinase IIδ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ Res 112: 935–944, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke M, Anderson J. NF-κB activation in organs from STZ-treated rats. Appl Physiol Nutr Metab 36: 121–127, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo O, Picatoste B, Ares-Carrasco S, Ramirez E, Egido J, Tunon J. Potential role of nuclear factor κB in diabetic cardiomyopathy. Mediators Inflamm 2011: 652097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo M, Anderson ME. Mechanisms of altered Ca2+ handling in heart failure. Circ Res 113: 690–708, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier HJ, Schips TG, Wietelmann A, Kruger M, Brunner C, Sauter M, Klingel K, Bottger T, Braun T, Wirth T. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci USA 109: 11794–11799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-κB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85: 473–483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemchenko A, Hill JA. NEMO nuances NF-κB. Circ Res 106: 10–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-κB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol 293: F100–F109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson PB. Renin: origin, secretion and synthesis. J Physiol 552: 667–671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61: 716–727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horvath B, Mukhopadhyay B, Becker L, Hasko G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher P. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56: 2115–2125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satou R, Miyata K, Katsurada A, Navar LG, Kobori H. Tumor necrosis factor-α suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am J Physiol Cell Physiol 299: C750–C759, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High glucose induced regulation of intracellular angiotensin II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H939–H948, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57: 3297–3306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG. Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes 55: 1252–1259, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Suarez J, Scott B, Dillmann WH. Conditional increase in SERCA2a protein is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 295: R1439–R1445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas CM, Yong QC, Seqqat R, Chandel N, Feldman DL, Baker KM, Kumar R. Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor. Clin Sci (Lond) 124: 529–541, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian C, Shao CH, Moore CJ, Kutty S, Walseth T, DeSouza C, Bidasee KR. Gain of function of cardiac ryanodine receptor in a rat model of type 1 diabetes. Cardiovasc Res 91: 300–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todorov VT, Volkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, Haegeman G, Schmitz ML, Kurtz A. Role of CREB1 and NFκB-p65 in the down-regulation of renin gene expression by tumor necrosis factor α. J Biol Chem 280: 24356–24362, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Todorov VT, Volkl S, Muller M, Bohla A, Klar J, Kunz-Schughart LA, Hehlgans T, Kurtz A. Tumor necrosis factor-α activates NFκB to inhibit renin transcription by targeting cAMP-responsive element. J Biol Chem 279: 1458–1467, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Valdes JA, Hidalgo J, Galaz JL, Puentes N, Silva M, Jaimovich E, Carrasco MA. NF-κB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am J Physiol Cell Physiol 292: C1960–C1970, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschope C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56: 641–646, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschope C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia 49: 2507–2513, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Wilhide ME, Tranter M, Ren X, Chen J, Sartor MA, Medvedovic M, Jones WK. Identification of a NF-κB cardioprotective gene program: NF-κB regulation of Hsp70.1 contributes to cardioprotection after permanent coronary occlusion. J Mol Cell Cardiol 51: 82–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu S, Zhi H, Hou X, Cohen RA, Jiang B. IκBβ attenuates angiotensin II-induced cardiovascular inflammation and fibrosis in mice. Hypertension 58: 310–316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yar AS, Menevse S, Alp E. The effects of resveratrol on cyclooxygenase-1 and -2, nuclear factor kappa beta, matrix metalloproteinase-9, and sirtuin 1 mRNA expression in hearts of streptozotocin-induced diabetic rats. Genet Mol Res 10: 2962–2975, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Yong QC, Thomas CM, Seqqat R, Chandel N, Baker KM, Kumar R. Angiotensin type 1a receptor-deficient mice develop diabetes-induced cardiac dysfunction, which is prevented by renin-angiotensin system inhibitors. Cardiovasc Diabetol 12: 169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young D, Popovic ZB, Jones WK, Gupta S. Blockade of NF-κB using IκBα dominant-negative mice ameliorates cardiac hypertrophy in myotrophin-overexpressed transgenic mice. J Mol Biol 381: 559–568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang XQ, Tang R, Li L, Szucsik A, Javan H, Saegusa N, Spitzer KW, Selzman CH. Cardiomyocyte-specific p65 NF-κB deletion protects the injured heart by preservation of calcium handling. Am J Physiol Heart Circ Physiol 305: H1089–H1097, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]