Abstract
We have previously demonstrated that adenosine-mediated H2O2 production and opening of ATP-sensitive K+ (KATP) channels contributes to coronary reactive hyperemia. The present study aimed to investigate the roles of adenosine, H2O2, and KATP channels in coronary metabolic hyperemia (MH). Experiments were conducted on isolated Langendorff-perfused mouse hearts using combined pharmacological approaches with adenosine receptor (AR) knockout mice. MH was induced by electrical pacing at graded frequencies. Coronary flow increased linearly from 14.4 ± 1.2 to 20.6 ± 1.2 ml·min−1·g−1 with an increase in heart rate from 400 to 650 beats/min in wild-type mice. Neither non-selective blockade of ARs by 8-(p-sulfophenyl)theophylline (8-SPT; 50 μM) nor selective A2AAR blockade by SCH-58261 (1 μM) or deletion affected MH, although resting flow and left ventricular developed pressure were reduced. Combined A2AAR and A2BAR blockade or deletion showed similar effects as 8-SPT. Inhibition of nitric oxide synthesis by N-nitro-l-arginine methyl ester (100 μM) or combined 8-SPT administration failed to reduce MH, although resting flows were reduced (by ∼20%). However, glibenclamide (KATP channel blocker, 5 μM) decreased not only resting flow (by ∼45%) and left ventricular developed pressure (by ∼36%) but also markedly reduced MH by ∼94%, resulting in cardiac contractile dysfunction. Scavenging of H2O2 by catalase (2,500 U/min) also decreased resting flow (by ∼16%) and MH (by ∼24%) but to a lesser extent than glibenclamide. Our results suggest that while adenosine modulates coronary flow under both resting and ischemic conditions, it is not required for MH. However, H2O2 and KATP channels are important local control mechanisms responsible for both coronary ischemic and metabolic vasodilation.
Keywords: coronary metabolic hyperemia, adenosine receptor knockouts, A2A adenosine receptor, A2B adenosine receptor, hydrogen peroxide, ATP-sensitive K+ channels
myocardial function is dependent on a constant O2 supply from the coronary circulation to myocytes. Myocardial O2 consumption (mvo2) increases whenever there is enhanced cardiac work. Because the myocardium has a very limited anerobic capacity with a high O2 extraction (75%) (46) under resting conditions, the increased O2 delivery during exercise mostly relies on increased coronary blood flow, termed as metabolic hyperemia. Numerous mechanisms are responsible for coronary metabolic hyperemia, allowing a balance between myocardial O2 demand and supply. Disturbance of these mechanisms results in decreased cardiac output, hypotension, arrhythmia, heart failure, and death.
Metabolic coronary vasodilation has been found to be regulated by both local metabolic feedback and adrenergic feedforward mechanisms (46). For decades, adenosine, a locally released metabolite from the myocardium, has been postulated as one of the important mechanisms responsible for metabolic coronary hyperemia (3, 4, 6, 16, 17, 19, 28). However, recent evidence suggests that adenosine is not required for metabolic coronary vasodilation (4, 14, 47–49), and its role in regulating resting coronary flow (CF) still remains controversial (14, 25–27, 49). The discrepancy may be due to different animal models, differences in species, and/or the different agonists and antagonists applied in these studies. It is well established that adenosine exerts its effect through activation of four receptor subtypes, namely, A1 and A3 adenosine receptors (ARs), which exert constrictive effects, and A2A and A2BARs, which have dilatory effects (31). The majority of these studies to characterize the functional role of adenosine in coronary hyperemia have relied heavily on adenosine-related pharmacological compounds, which may lead to misinterpretation of the data due to the mixed specificity of the agonists and antagonists for the four different ARs. In addition, the complexity of the combined intrinsic (e.g., myogenic, shear-mediated, metabolic, and endothelium-originated mediators) with extrinsic (neural hormonal regulatory mechanism) blood flow control mechanisms make whole animal study a nonoptimal model for the mechanistic exploration in local metabolic coronary blood flow regulation. Therefore, there is a need to reexamine the functional role of adenosine in coronary metabolic hyperemia using combined AR knockout (KO) mice with traditional pharmacological agents in isolated Langendorff-perfused hearts.
Increasing evidence suggests that H2O2, an endogenous metabolite, acts as an important mediator responsible for metabolic coronary vasodilation (37, 42, 53, 54). We (42, 57) have previously demonstrated that A2AAR-mediated H2O2 production opens ATP-sensitive K+ (KATP) channels in coronary smooth muscle cells, contributing to the coronary ischemic vasodilation (42, 57). While the KATP channel is known to be important for coronary reactive hyperemia (1, 2, 10, 11, 42), it remains controversial regarding its role in metabolic hyperemia (13, 15, 16, 32, 34, 35, 47, 51). Therefore, the present study further examined whether H2O2 and/or KATP channels play an important role in coronary metabolic hyperemia using isolated Langendorff-perfused mouse hearts in the presence of catalase (an enzyme that decomposes H2O2 to water) or glibenclamide (a selective KATP channel blocker).
MATERIALS AND METHODS
Animals.
The Institutional Animal Care and Use Committee of West Virginia University School of Medicine approved all experimental protocols. We followed guidelines set forth by the American Physiological Society and National Institutes of Health regarding the care and use of laboratory animals. A2AAR and A2BAR single KO mice (A2A KO and A2B KO mice, respectively) were generously provided by Dr. C Ledent (Universite Libre de Bruxelles, Brussels, Belgium) and Stephen Tilley (University of North Carolina, Chapel Hill, NC), respectively. A2A and A2B KO mice, both backcrossed 12 generations to the wild-type (WT) C57BL/6 background (Jackson Laboratory, Bar Harbor, ME), were bred to generate A2A/A2B double-KO (DKO) heterozygotes. Double heterozygotes were intercrossed, and 1/16 of the offspring were A2A/A2B DKO mice. A2A/A2B DKO breeding pairs were then established. Mice were caged in a 12:12-h light-dark cycle with free access to standard chow and water. The absence of A2AAR and A2BAR at both mRNA and protein levels in A2A KO, A2B KO, and A2A/2B DKO mice has been confirmed by our previous studies using PCR, Western blot analysis, and immunohistochemistry in the aorta, mesenteric arteries, and coronary arteries (30, 41, 43–45, 57). Our functional data also confirmed the lack of a CGS-21680 (a selective A2AAR agonist)-induced CF response in A2A KO and A2A/2B DKO mice, the lack of a BAY 60-6583 (selective A2BAR agonist) response in A2B KO and A2A/2B DKO mice, and the lack of an adenosine response in A2A/2B DKO mice (41, 45).
Langendorff-perfused mouse heart preparations.
Mice (14–17 wk) of either sex (equal ratio) were anesthetized with pentobarbital sodium (50 mg/kg body wt ip), and hearts were excised into heparinized (5 U/ml) ice-cold Krebs-Henseleit buffer containing (in mM) 119 NaCl, 11 glucose, 22 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 2 pyruvate, and 0.5 EDTA. After removal of the lung and surrounding tissue, the aorta was rapidly cannulated with a 20-gauge, blunt-ended needle and continuously perfused with 37°C buffer bubbled with 95% O2 and 5% CO2 at a constant perfusion pressure of 80 mmHg. The left atrium was removed, and a fluid-filled balloon made of plastic wrap was inserted into the left ventricle (LV) across the mitral valve. The balloon was connected to a pressure transducer for the continuous measurement of LV developed pressure (LVDP). The heart was then immersed in a water-jacketed perfusate bath maintained at 37°C and allowed to beat spontaneously. LV diastolic pressure was adjusted to 2–5 mmHg. CF was continuously measured with an ultrasonic flow probe (Transonic Systems, Ithaca, NY) placed in the aortic perfusion line. A Power Lab Chart data-acquisition system (AD Instruments, Colorado Springs, CO) was used for data acquisition. After stabilization for 20 min, hearts were then paced at 400 beats/min by two platinum wires connected to the stainless steel cannula and through the apical myocardium. Function was allowed to stabilize for another 10 min before baseline functional data were collected. The heart was then paced at gradually increasing frequencies (500, 550, 600, and 650 beats/min) to induce metabolic hyperemia. Hearts with persistent arrhythmias or LVDP of <80 mmHg were excluded from the study.
Metabolic hyperemia protocol.
After stabilization, hearts were subjected to a 2 min of pacing at each frequency of 500, 550, 600, and 650 beats/min, respectively. The reason why we paced the heart from 400 to 650 beats/min (1.62-fold increase) was based on the fact that heart rate (HR) has been reported to increase by ∼1.9-, 1.8-, 1.8-, and 1.7-fold in humans, swine, dogs, and mice, respectively, upon maximum exercise (13, 17, 29). At the end of each pacing, the heart was allowed to stabilize for 5 min at a HR of 400 beats/min before the heart was paced at a different frequency. 8-(p-Sulfophenyl)theophylline (8-SPT; nonselective adenosine receptor antagonist, 50 μM, Sigma), N-nitro-l-arginine methyl ester [l-NAME; nitric oxide (NO) synthase inhibitor, 100 μM, Sigma, based on our previous study (45) showing that l-NAME at ≥10−5 reached its maximum inhibition on resting CF and 5′-(N-ethylcarboxamido)adenosine (nonselective adenosine agonist)-induced CF responses], SCH-58261 (selective A2AAR antagonist, Ki = 1.3 nM, 1 μM, Tocris Bioscience), CVT-6883 (selective A2BAR antagonist, Ki = 22 nM, 1 μM, Tocris Bioscience), catalase (the enzyme that decomposes H2O2 to water, 2,500 U/ml, Sigma), or glibenclamide (KATP channel blocker, 5 μM, Sigma) were delivered into the aortic perfusion line for 15 min using a microinjection pump (Harvard Apparatus, Holliston, MA) as 1% of CF. In the presence of each pharmacological reagent, a second pacing-induced functional hyperemia was elicited. Time-matched control experiments with two consecutive inductions of functional hyperemia showed no differences in baseline function as well as the magnitude of pacing-induced changes in CF (n = 3 animals).
Data analysis and statistics.
All data collected were analyzed by LabChart 7.0 software. Baseline CF, LVDP, and dP/dt were calculated as mean values after each heart was paced at 400 beats/min for 10 min. Mean CF was calculated during the 2-min pacing at each frequency. Because absolute CF rates changed proportionally with heart mass, CF was normalized by heart mass and presented as milliliters per gram of wet heart weight per minute. Because mvo2 is a function of HR, cardiac contractility, ventricular wall tension, and muscle shortening (9, 12, 20), it is represented by the rate-pressure product (RPP). To examine the relationship between mvo2 and RPP, Po2 of the inflow (Pi{O2}; an indication of arterial O2 tension) and outflow perfusate (Po{O2}; an indication of venous O2 tension collected through a tube inserted in the right atrium close to the coronary sinus) were measured using a GEM Premier 4000 gas analyzer (n = 2). Because of the absence of hemoglobin in the perfusion buffer, O2 content is dependent solely on the O2 solubility in the buffer at 37°C, which was 0.023 ml O2·ml buffer−1·atm−1 (39). mvo2 was calculated using the following equation: mvo2 = CF × (Pi{O2} − Po{O2}) × 0.023/760, as previously described (39). As expected, mvo2 linearly correlated with RPP as hearts were paced at graded frequencies (mvo2 =1.49 × RPP + 101.5, R2 = 0.9596, P < 0.01), indicating that mvo2 is a function of the HR and LVDP product.
Least-square linear regression analysis was conducted between HR or RPP, and CF to calculate the slope of the relationship. The slope of each curve was compared before and after drug treatment or between groups. All data are presented as means ± SE; n represents the number of animals unless otherwise indicated. Paired t-test was used for paired data analysis. One-way ANOVA was used to compare the data between groups. The effect of treatment on the relation between two variables was analyzed by analysis of covariance using GraphPad Prism5. P values of <0.05 were considered as statistically significant.
RESULTS
Baseline function from WT, A2A KO, A2B KO, and A2A/2B DKO mouse hearts.
Baseline functional parameters of isolated mouse hearts were recorded at 10 min of pacing (400 beats/min) after 20-min stabilization. There were no statistically significant differences in the heart-to-body weight ratio and maximum −dP/dt among all animal strains, although +dP/dt had a trend to be less in A2A KO, A2B KO, or A2A/2B DKO mice compared with WT mice. Baseline CF as well as LVDP were significantly lower in KO mice compared with WT mice (P < 0.05 vs. WT mice; Table 1).
Table 1.
Baseline functional data in isolated WT, A2A KO, A2B KO, and A2A/2B DKO mouse hearts
| WT | A2A KO | A2B KO | A2A/2B DKO | |
|---|---|---|---|---|
| No. of animals | 23 | 12 | 6 | 6 |
| Age, wk | 14.8 ± 0.17 | 14.8 ± 0.20 | 14.3 ± 0.25 | 15.0 ± 0.10 |
| Body weight, g | 24.3 ± 0.82 | 25.1 ± 1.14 | 22.3 ± 0.96 | 25.0 ± 1.49 |
| Heart weight, mg | 112.4 ± 2.74 | 109 ± 3.9 | 103 ± 4.7 | 113 ± 8.1 |
| Heart-to-body weight ratio, % | 0.46 ± 0.005 | 0.43 ± 0.010 | 0.46 ± 0.001 | 0.45 ± 0.020 |
| Coronary flow, ml·min−1·g−1 | 17.1 ± 0.54 | 14.4 ± 0.56* | 14.1 ± 1.78* | 13.4 ± 0.52* |
| Left ventricular developed pressure, mmHg | 105 ± 4.0 | 90 ± 3.9* | 93 ± 5.3 | 88 ± 2.5* |
| +dP/dt, mmHg/s | 4,704 ± 233.7 | 4,158 ± 278.6 | 3,878 ± 313.9 | 4,078 ± 249.0 |
| −dP/dt, mmHg/s | 3,582 ± 169.5 | 3,743 ± 272.5 | 3,167 ± 164.1 | 3,245 ± 404.4 |
All values are means ± SE. WT, wild type; A2A KO, A2A adenosine receptor knockout; A2B KO, A2B adenosine receptor knockout; A2A/AB DKO, A2A/2B adenosine receptor double knockout.
P < 0.05 compared with WT.
Pacing-induced coronary hyperemia in the absence or presence of 8-SPT.
The CF response in isolated hearts of WT mice was measured before and after the addition of 8-SPT (50 μM, a nonselective adenosine antagonist, n = 6). Under control conditions, mean CF increased significanlty from 14.4 ± 1.2 to 16.8 ± 1.1, 18.5 ± 1.3, 19.5 ± 0.9, and 20.6 ± 1.2 ml·min−1·g−1 when hearts were paced from 400 to 500, 550, 600, and 650 beats/min, respectively (Fig. 1A). The increased CF linearly correlated with the enhanced mvo2, indicated as RPP, which increased from 41 ± 5.1 to 56 ± 5.1, 65 ± 6.7, 70 ± 6.1, and 75 ± 7.0 ×103 beats·min−1·mmHg, respectively (P < 0.05; Fig. 1B). LVDP increased significanlty from 102 ± 12.7 to 112 ± 10.0, 117 ± 12.1, 117 ± 10.0, and 115 ± 10.7 mmHg, respectively (P < 0.05; Fig. 1C). The linear relationship between CF and RPP suggests a balance between O2 delivery and consumption in pacing-induced metabolic hyperemia in isolated Langendorff-perfused mouse hearts.
Fig. 1.
Effect of 8-(p-sulfophenyl)theophylline (8-SPT; 50 μM) on the pacing-induced coronary flow (CF) response. A–C: relationship between heart rate [HR; in beats/min (bpm)] and CF (A), rate-pressure product (RPP) and CF (B), and HR and left ventricular developed pressure (LVDP; C) before and after the addition of 8-SPT (n = 6). D: adenosine (10−9–10−5M)-induced coronary response before and after the infusion of 8-SPT (50 μM, n = 3). *P < 0.05, significant difference between the curves.
In the presence of 8-SPT, resting CF significantly decreased from 14.4 ± 1.20 to 10.8 ± 0.91 ml·min−1·g−1 (P < 0.05 by paired t-test). The decreased CF was associated with a correlated decrease in baseline LVDP from 102 ± 12.7 to 80 ± 6.5 mmHg (P < 0.05; Fig. 1C). However, the pacing-induced increase in CF was not prevented by 8-SPT, although the CF at each given HR or RPP was decreased (Fig. 1, A and B). CF increased from 10.8 ± 0.91 to 16.0 ± 0.61 ml·min−1·g−1 when hearts were paced from 400 to 650 beats/min, and the linear curve of the relationship between HR or RPP and CF was shifted downward in a parallell manner without significant changes in the slope before or after 8-SPT treatment (0.187 ± 0.001 vs. 0.185 ± 0.002, P > 0.05; Fig. 1, A and B).
To confirm the antagonistic ability of 8-SPT against adenosine, we examined the effect of 8-SPT at the same concentration (50 μM) on adenosine (10−9∼10−5 M)-induced CF responses. 8-SPT significantly decreased both baseline CF (from 13.2 ± 0.55 to 10.8 ± 0.52 ml·min−1·g−1, n = 3, P < 0.05) as well as the adenosine-induced CF increase (maximal coronary flow was reduced from 35 ± 1.4 to 15.4 ± 1.5 ml·min−1·g−1, P < 0.05; Fig. 1D).
Combined effect of l-NAME and 8-SPT on pacing-induced coronary hyperemia.
Baseline CF was significantly decreased by l-NAME (10−4 M) from 18.0 ± 0.86 to 14.8 ± 0.78 ml·min−1·g−1 (P < 0.05, n = 8; Fig. 2A). However, the pacing-induced CF increase was not affected by l-NAME (CF increased linearly from 14.8 ± 0.78 to 22.9 ± 1.53 ml·min−1·g−1 when hearts were paced from 400 to 650 beats/min; Fig. 2, A and B). The slope of the relationship between HR or RPP and CF was not significantly changed before or after the addition of l-NAME (P > 0.05), and the linear curve was shifted downward in a parallell manner. Concomitant with the decreased baseline flow, LVDP was signficantly diminshed by l-NAME from 110 ± 5.9, 130 ± 4.9, 136 ± 3.5, 135 ± 3.5, and 134 ± 3.4 to 88 ± 6.1, 104 ± 6.4, 112 ± 44.8, 112 ± 4.8, and 116 ± 4.1 mmHg when hearts were paced at 400, 500, 550, 600, and 650 beats/min, respectively (P < 0.05; Fig. 2C).
Fig. 2.

Effect of N-nitro-l-arginine methyl ester (l-NAME) on the pacing-induced CF response in the absence or presence of 8-SPT. A–C: relationship between HR and CF (A), RPP and CF (B), and HR and LVDP (C) under control conditions (n = 8), in the presence of l-NAME (nitric oxide synthase inhibitor, 100 μM, n = 4), and in the combined presence of 8-SPT (50 μM) and l-NAME (100 μM, n = 4). *P < 0.05, significant difference from the control curve (A and B) and versus control (C).
In four of eight animals, 8-SPT (50 μM) was added in the presence of l-NAME. 8-SPT did not further decrease baseline CF, nor did it affect pacing-induced hyperemia (P > 0.05 vs. l-NAME; Fig. 2, A–C).
Effect of A2AAR blockade or deletion on pacing-induced coronary hyperemia.
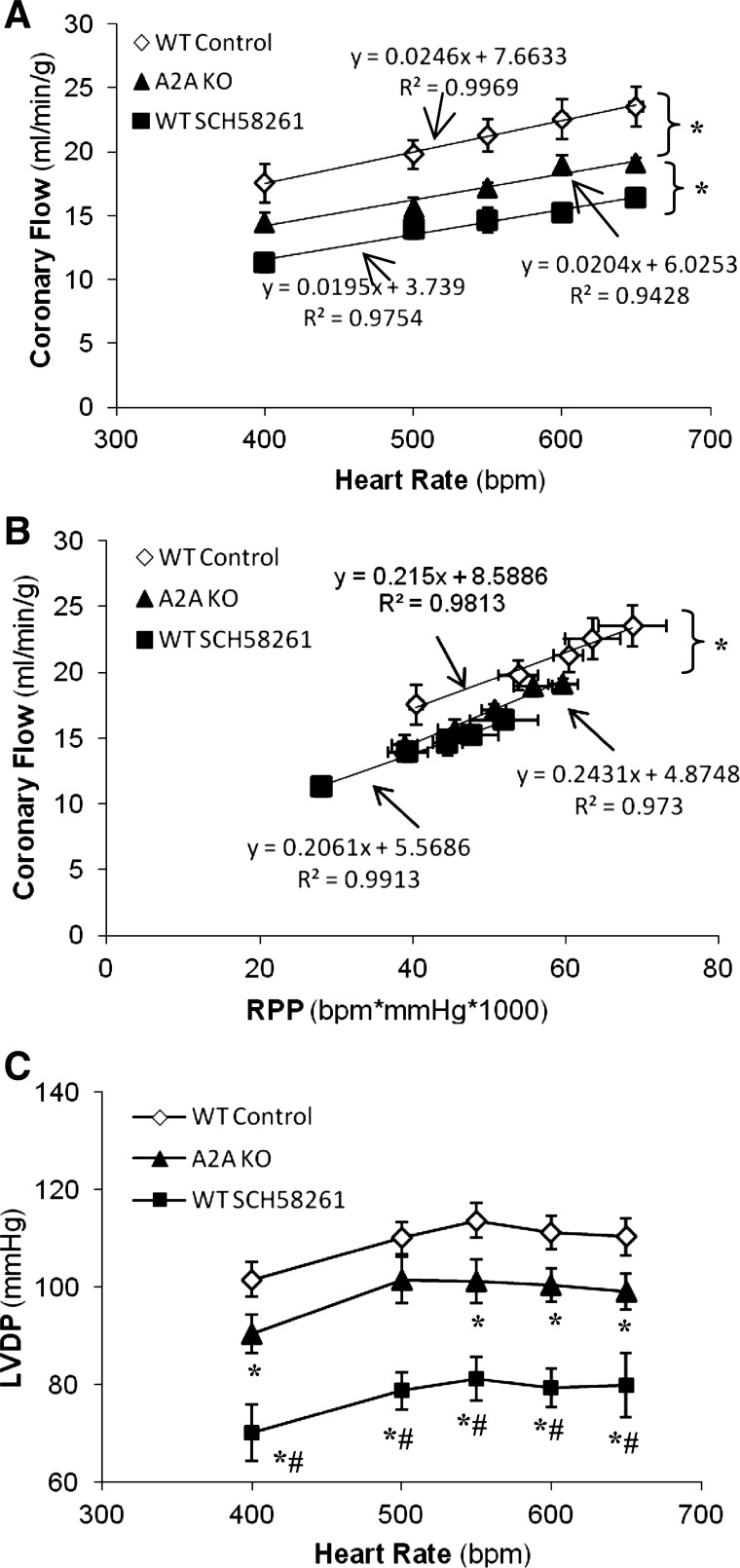
The role of A2AARs in coronary functional hyperemia was examined in WT mice (n = 6) in the presence of SCH-58261 (1 μM, a selective A2AAR antagonist). SCH-58261 significantly decreased baseline CF from 17.5 ± 1.54 to 11.3 ± 0.70 ml·min−1·g−1 (P < 0.05; Fig. 3, A and B) and LVDP from 101 ± 3.5 to 70 ± 5.8 mmHg, respectively (P < 0.05; Fig. 3C). However, after A2AAR blockade, CF still increased from 11.3 ± 0.70 to 16.3 ± 0.54 ml·min−1·g−1 when hearts were paced from 400 to 650 beats/min. The slope of the relationship between HR or RRP and CF was not different before or after A2AAR blockade (P > 0.05; Fig. 3, A and B).
Fig. 3.
Effect of A2A adenosine receptor (AR) blockade or deletion on pacing-induced coronary hyperemia. A–C: relationship between HR and CF (A), RPP and CF (B), and HR and LVDP (C) in control wild-type (WT) mice (n = 6), A2AAR knockout (A2A KO) mice (n = 9), and WT mice treated with SCH-58261 (selective A2AAR antagonist, 1 μM, n = 6). *P < 0.05, significant difference between the curves or significant difference versus control; #P < 0.05, significant difference versus A2A KO mice.
We further examined the effect of A2AAR deletion on pacing-induced coronary hyeremia using A2A KO mice (n = 9). Baseline CF and LVDP were 14.4 ± 0.56 ml·min−1·g−1 and 90 ± 3.9 mmHg, respectively, which were significantly lower than those in WT mice (Table 1 and Fig. 3) but were higher than those in WT mice treated with SCH-58261 (P < 0.05; Fig. 3, A and C). Consistent with the effect of SCH-58261, A2AAR deletion did not affect the pacing-induced CF increase, although receptor deletion resulted in a parallel downward shift of the relationship between RPP or HR and CF (Fig. 3, A and B).
Effect of double blockade or deletion of A2AAR and A2BAR on pacing-induced coronary hyperemia.
Both A2AARs and A2BARs are involved in coronary vasodilation (8, 41, 45). Therefore, we examined the effect of double blockade or deletion of both ARs on pacing-induced coronary hyperemia. In A2A KO mice (n = 4), CVT-6883 (1 μM) further decreased baseline CF from 13.5 ± 1.03 to 11.6 ± 0.75 ml·min−1·g−1 (P < 0.05) and LVDP from 91 ± 2.3 to 71 ± 5.7 mmHg (P < 0.05; Fig. 4D). Similarly, in A2B KO mice, which have lower baseline CF (14.1 ± 1.78 ml·min−1·g−1, n = 6) and LVDP (93 ± 5.3 mmHg) than WT mice (P < 0.05; Table 1 and Fig. 4D), combined blockade of A2AARs by SCH-58261 (1 μM) further decreased baseline CF and LVDP to 10.6 ± 0.65 ml·min−1·g−1 and 74 ± 3.3 mmHg, respectively (P < 0.05; Fig. 4D). However, in both cases, pacing-induced hyperemia was not affected (CF increased from 11.0 ± 0.58 and 10.6 ± 0.65 to 16.0 ± 0.81 and 15.7 ± 0.77 ml·min−1·g−1, respectively when hearts were paced from 400 to 650 beats/min; Fig. 4, A–C). The slope of the relationship between HR and/or RPP and CF under each experimental condition was not significantly different from WT mice (Fig. 4, A–C).
Fig. 4.
Effect of double blockade or deletion of A2AARs and A2BARs on the pacing-induced CF response. A–D: relationship between HR and CF (A), RPP and CF (B and C), and HR and LVDP (D) in WT mice (n = 6), A2A KO mice (n = 4), A2BAR knockout (A2B KO) mice (n = 6), A2A KO mice with CVT-6883 (selective A2BAR antagonist, 1 μM, n = 4), and A2B KO mice with SCH-58261 (1 μM, n = 6). E and F: the relationship between HR and CF (E) and the relationship between RPP and CF (F) were compared between WT mice (n = 6) and A2A/2BAR double-knockout (A2A/2B DKO) mice (n = 6). *P < 0.05, significant difference between the curves or significant different versus control.
To confirm the role of A2AARs and A2BARs in coronary metabolic hyperemia, we further examined the hyperemic response in A2A/2B DKO mice (n = 6). Baseline CF (13.4 ± 0.52 ml·min−1·g−1) and LVDP (88 ± 2.5 mmHg) were lower than those in WT mice (P < 0.05) and were less than those in either A2A KO or A2B KO mice, although they did not reach statistical significance (Table 1 and Fig. 4, E and F). However, the pacing-induced hyperemic response was not affected (CF increased from 13.3 ± 0.52 to 19.9 ± 0.73 ml·min−1·g−1, linearly correlated with the increased HRs from 400 to 650 beats/min; Fig. 4E). The slope of the relationship between HR and/or RPP and CF in A2A/2B DKO mice was not significantly different from that in WT mice, suggesting that neither A2AARs nor A2BARs are required for the pacing-induced increase in CF.
Effect of catalase on pacing-induced coronary hyperemia.
The role of H2O2 in pacing-induced hyperemia was examined in WT mice (n = 6) before and after the addition of catalase (2,500 U/ml). Catalase significantly decreased CF from 16.5 ± 1.22 to 13.8 ± 1.40 ml·min−1·g−1 (Fig. 5A) and significantly decreased LVDP from 96 ± 7.4 to 88 ± 7.1 mmHg (Fig. 5C), respectively (P < 0.05 by paired t-test). Additionally, catalase significantly attenuated the pacing-induced increase in CF by ∼37% (the net increase in CF of hearts paced from 400 to 650 beats/min was decreased from 7.4 ± 0.12 to 4.6 ± 0.49 ml·min−1·g−1, P < 0.05). The slope of the relationship between HR and/or RPP and CF in the presence of catalase was significantly lower than that under control conditions (P < 0.05 by paired t-test; Fig. 5, A and B).
Fig. 5.

Effect of catalase on pacing-induced coronary hyperemia. A–C: relationship between HR and CF (A), RPP and CF (B), and HR and LVDP (C) before and after the addition of catalase (the enzyme that decomposes H2O2 to water, 2,500 U/ml, n = 6). *P < 0.05, significant difference in the slope of the linear curves (A and B) and significant difference versus control (C, paired t-test).
Effect of glibenclamide on pacing-induced coronary hyperemia.
The role of KATP channels in pacing-induced coronary hyperemia was determined in isolated WT mouse hearts (n = 6) in the absence or presence of glibenclamide (KATP channel blocker, 5 μM). In the absence of glibenclamide, CF increased by 6.2 ml·min−1·g−1 after hearts were paced from 400 to 650 beats/min (Fig. 6, A and B). After glibenclamide infusion, CF decreased significantly by ∼ 45% (from 20 ± 1.6 to 11 ± 0.9 ml·min−1·g−1, P < 0.05; Fig. 6, A and B) and LVDP was reduced by ∼36% (from 112 ± 13.3 to 72 ± 7.0 mmHg, P < 0.05; Fig. 6C). Moreover, pacing-induced hyperemia was dramatically decreased by ∼94% (the net CF increase before and after glibenclamide was 6.1 and 0.39 ml·min−1·g−1, respectively; Fig. 6, A and B). The dramatic reduction in functional hyperemia was concomitant with a significant decrease in LVDP during pacing (LVDP decreased from 72 ± 7.0 to 63 ± 9.0, 57 ± 4.8, and 59 ± 9.2 mmHg when hearts were paced at 550, 600, and 650 beats/min, respectively; Fig. 6C), suggesting compromised heart function as a result of an imbalance between O2 supply and demand after KATP channel blockade.
Fig. 6.
Effect of ATP-sensitive K+ channel blockade on pacing-induced coronary hyperemia. A–C: relationship between HR and CF (A), RPP and CF (B), and HR and LVDP (C) before and after the addition of glibenclamide (ATP-sensitive K+ channel blocker, 5 μM, n = 6). *P < 0.05, significant difference in the slope of the linear curves (A and B) and significant difference versus control; #P < 0.05 vs. LVDP at a HR of 400 beats/min.
DISCUSSION
In the present study, using targeted AR KO mice combined with pharmacological approaches, we further examined the role of adenosine, H2O2, and KATP channels in coronary metabolic hyperemia. In isolated Langendorff-perfused mouse hearts from A2A KO, A2B KO, and A2A/2B DKO mice, we demonstrated that adenosine and its receptor subtypes (A2A and A2B) are not required for pacing-induced coronary metabolic hyperemia, although they exert important roles in maintaining CF under resting conditions. Similarly, broad pharmacological blockade of ARs by 8-SPT in the absence or presence of a NO synthase inhibitor or selective A2AAR and A2BAR blockade did not affect pacing-induced increase in CF, although baseline flow was reduced. However, KATP channel blockade by glibenclamide remarkably reduced both resting flow and pacing-induced hyperemia, resulting in a dramatic decrease in LVDP. Scavenging of H2O2 by catalase also decreased resting flow and pacing-induced coronary hyperemia but to a lesser extent than glibenclamide. Our results suggest that while adenosine modulates coronary vascular tone under both resting and ischemic conditions, it is not required for metabolic hyperemia even when the NO pathway is inhibited. However, H2O2 and KATP channels are important local control mechanisms responsible for both coronary ischemic and metabolic vasodilation.
Since Berne (6) proposed that adenosine is an important metabolite that links the changes in CF to mvo2, there have been numerous studies that have examined the adenosine hypothesis in coronary metabolic hyperemia (3, 4, 7, 14, 21, 47–49). Early studies supported this hypothesis in that the augmentation in cardiac interstitial adenosine concentration was observed to be correlated with increased mvo2 in chronically instrumented dogs (3, 19, 21) and pharmacological blockade of ARs was shown to decrease coronary metabolic hyperemia in isolated guinea pig hearts (21). However, more recent studies in awake dogs have reported that there was no significant increase in cardiac interstitial adenosine levels that was sufficient to cause coronary vasodilation (49, 50, 52) and that AR blockade (49, 50) or triple blockade of adenosine, KATP channels, and NO (47, 48) did not affect exercise or norepinephrine-induced coronary hyperemia, suggesting that adenosine is not required in coronary functional hyperemia. Interestingly, Duncker and colleagues (16, 17) suggested that adenosine is an important mediator for coronary metabolic hyperemia only when the KATP channel is blocked or under conditions where coronary perfusion pressure is reduced. The discrepancies among these studies may be due to the mixed drug specificity or to the complexity of mixed local and neuronal control mechanisms responsible for coronary blood flow regulation under in vivo conditions. Using targeted AR KO mice combined with an isolated heart preparation to solve the specificity issue as well as to exclude the neurohormonal effect on CF regulation, the present study showed that A2AARs and A2BARs are important in modulating coronary vascular tone under resting conditions because baseline CF in A2A KO, A2B KO, and A2A/2B DKO mice was reduced (by ∼16%, ∼18%, and ∼24%, respectively) compared with WT mice (Figs. 3 and 4). Of interest, although double deletion of A2AARs and A2BARs further reduced baseline CF from either A2A KO or A2B KO mice, the extent of reduction in A2A/2B DKO mice (by ∼22%) was much less than the summed effect of A2AAR and A2BAR deletion (∼34%), suggesting that other unknown compensatory mechanisms might be turned on to maintain resting CF when both ARs are deleted. Consistent with the results observed in AR KO mice, broad AR blockade by 8-SPT and selective A2AAR or A2BAR blockade by SCH-58261 (1 μM) or CVT-6883 (1 μM) also resulted in a significant reduction (by ∼25%, ∼35%, and ∼18%, respectively) in resting CF (Figs. 1, 3, and 4). In agreement with our findings, other studies in humans (18), pigs (14), dogs (49), and isolated guinea pig hearts (21) have also reported that adenosine is involved in modulating CF under resting condition. Of note, a lower reduction in CF was observed in A2A KO mice (by ∼18%) versus SCH-58261-treated animals (by ∼35%; Fig. 3, A and C). The lower reduction in baseline CF might be due to upregulated A2BARs in A2A KO mice compared with WT mice, as previously reported (45). Regarding the downstream mechanisms of adenosine in modulating resting CF, our results (45) and those of others (56) suggest that A2AAR-mediated NO production may be responsible for resting tone regulation because 8-SPT (nonselective adenosine antagonist; Fig. 2) or SCH-58261 (selective A2AAR antagonist) (56) failed to further decrease baseline CF in the presence of a NO synthase inhibitor. Concomitant with the decreased baseline flow, a significant reduction in LVDP (Table 1 and Figs. 1C, 3C, and 4D) and a relatively but not statistically lower +dP/dt (Table 1) were observed upon blockade of adenosine either pharmacologically or genetically. The correlated decrease in cardiac contraction with the decreased CF suggest that adenosine is an important mediator involved in modulating resting CF and that the decreased cardiac function is associated with the decreased CF.
Despite the decrease in resting CF, pacing-induced hyperemia was not affected after AR blockade or deletion, in as much as the linear curve of the relationship between HR and/or RPP and CF was downward shifted in a parallel manner and the slope of the curve was not altered before or after AR blockade or deletion (Figs. 1, 3, and 4). These results, obtained from targeted AR KO mice, add further evidence to the previous findings that an AR blocker failed to inhibit exercise-induced coronary hyperemia in chronically instrumented dogs, although it lowered the balance between O2 supply and delivery under resting conditions (47, 49). The approach of using targeted AR KO mice allowed us to rule out the possibility that increased endogenous adenosine overcomes the competitive receptor blockade, thus masking the direct AR blockade effect. Additionally, in the presence of a NO synthase inhibitor, 8-SPT did not further decrease resting flow as well as pacing-induced coronary hyperemia, indicating both NO and adenosine act as tonic vasodilator but are not required for coronary metabolic hyperemia.
Increasing evidence suggests that H2O2, an endogenous metabolite, acts as a pivotal mediator responsible for metabolic coronary vasodilation (37, 42, 53, 54). In support of this idea, our results showed that catalase significantly decreased resting CF (by ∼16%) as well as the pacing-induced increase in CF (by ∼37%; Fig. 5, A and B). It should be pointed out that although A2AAR-mediated H2O2 production in the coronary endothelium and smooth muscle cells has been previously reported to play an important role in coronary ischemic hyperemia (42, 57), the failure of AR blockade or deletion to reduce metabolic hyperemia (discussed above) suggest that other mechanisms than adenosine-mediated signaling are responsible for H2O2 production, thus contributing to coronary metabolic vasodilation. Regarding the source of H2O2, we speculate that mitochondrial electron transport in cardiomyocytes might be an important source for pacing-induce H2O2 production (37).
The KATP channel has been reported to be involved in coronary ischemic vasodilation (24, 41, 42, 57), although its role in metabolic vasodilation remains controversial (13, 15, 23, 35, 47, 55). In isolated mouse hearts, the present study showed that glibenclamide (5 μM) significantly reduced CF (by ∼45%; Fig. 6A) and LVDP (by ∼36%; Fig. 6C) at rest. In agreement with our results, studies in dogs have reported that intracoronary infusion of glibenclamide (50–80 μg·kg−1·min−1) significantly decreased baseline flow (by ∼20–50%) (38) and was associated with cardiac contractile dysfunction (systolic wall thickening decreased by ∼43%) (15). Importantly, recovery of the CF rate to preglibenclamide levels after the addition of sodium nitroprusside reversed the cardiac dysfunction, suggesting that KATP channels exert essential roles in maintaining resting CF and that the decreased CF upon KATP channel blockade in the coronary vasculature causes ischemia-induced cardiac contractile dysfunction (15). It is important to note, however, that KATP channels at concentrations of >10 μM have been reported to inhibit other K+ and Ca2+ channels (5, 36). The concentration we used in the present study (5 μM) is within its selectivity range (5, 36), thus strengthening our conclusion that the KATP channel is important in modulating resting CF. Interestingly, in disagreement with the majority of studies showing that blockade of KATP channels did not attenuate the increase in CF during treadmill exercise or paired cardiac pacing in dogs and pigs (13, 15, 34, 35, 47), the present study on isolated mouse hearts demonstrated that blockade of KATP channels not only reduced resting flow but also markedly inhibited pacing-induced functional hyperemia by ∼94% (Fig. 6, A and B). The discrepancy among the studies could be due to 1) species differences (mice vs. pigs and dogs) or 2) the difference in animal models (isolated hearts vs. whole animals). In isolated hearts from mice deficient in Kir6.1 (one of the KATP subunits), isoprenaline-induced coronary vasodilation was significantly attenuated, indicating an important role of KATP channels in coronary metabolic hyperemia (55). Additionally, the redundant mechanisms responsible for CF regulation in whole animals, including local and neurohormonal control mechanisms, may complicate the explanation of the data, e.g., the reduction of coronary metabolic hyperemia by the KATP channel blocker might be compensated by an enhanced neurohormonal effect or other unknown mechanisms to maintain the balance between O2 supply and demand, thus resulting in unchanged metabolic hyperemia in vivo even after KATP channel blockade. Concomitant with the reduced metabolic hyperemia in isolated mouse hearts, we also observed dramatic cardiac contractile dysfunction. In contrast to significant increases in LVDP upon pacing in control condition, KATP channel blockade resulted in a significant reduction in LVDP upon pacing (Fig. 6C), suggesting potential ischemic cell damage related to a decrease in CF.
It is well established the KATP channels play important roles in adenosine-mediated coronary reactive hyperemia (1, 8, 22, 41, 42, 57). Our recent studies (42, 58) have demonstrated that A2AAR-mediated H2O2 production opens KATP channels, partially contributing to coronary reactive hyperemia. In these studies, although adenosine-induced KATP current was reduced in A2A KO and A2A/2B DKO animals, pinacidil (KATP opener)-induced current was not affected after deletion of both A2AARs and A2BARs, suggesting that A2AARs and A2BARs are not required for KATP activation and that the absence of ARs does not affect channel expression, as we previously reported (33, 42). Additionally, we demonstrated that KATP channel blockade inhibited coronary reactive hyperemia (57) to a higher extent than A2AAR blockade, indicating that other factors, including adenosine, can also activate KATP channels, thus contributing to coronary vasodilation. In the present study, KATP channel blockade dramatically decreased metabolic hyperemia, whereas only a parallel downward shift of the relationship between CF and RPP was observed upon A2AAR and/or A2BAR blockade or ablation, suggesting that other unknown mediators than adenosine released during increased cardiac metabolic demand can activate KATP channels, contributing to the increased flow during metabolic hyperemia. One of the mediators appears to be H2O2, because scavenging of H2O2 by catalase significantly decreased metabolic hyperemia by ∼37% (Fig. 5, A and B) and to a lesser extent than KATP channel blockade (by ∼94%; Fig. 6, A and B). Taken together, our results suggest that while adenosine-mediated H2O2 production and KATP channel opening are important for coronary reactive hyperemia, adenosine appears to be not required for metabolic hyperemia, and other unknown mediators act together with H2O2 to open KATP channels and are thus responsible for metabolic hyperemia in isolated mouse hearts.
It should be pointed out that there are some limitations regarding the translation of our findings based on our present experimental model into the clinical applications regarding the pathophysiology of CF regulation. First, although the effects of neurohomonal and blood components were excluded to scrutinize the metabolic control mechanism in CF regulation, buffer-perfused isolated hearts did not allow us to clearly separate the contribution of shear- and/or pressure-induced CF changes from local metabolic effects. Second, the continuously oxygenated (95% O2) Krebs buffer used in isolated hearts has much less O2 carrying capacity than blood, which might cause hypoxia and relatively lower cardiac contractile function compared with blood-perfused hearts (40). However, in buffer-perfused hearts paced from 400 to 600 beats/min, we observed a correlated increase in mvo2 from 160 to 230 μl·min−1·g−1 as well as CF (increased by ∼40%). More importantly, CF increased more than twofold over baseline after 15-s flow occlusion (42, 57), indicating that buffer-perfused hearts have a minimum hypoxia and coronary vessels have the capacity to further dilate. However, due to the relatively lower myoglobin O2 saturation in buffer-perfused hearts (39), the cardiac interstitial adenosine level might be higher compared with blood-perfused hearts, which may overestimate the role of adenosine in modulating resting coronary blood flow in our model. Future studies are needed to compare adenosine levels in buffer- versus blood-perfused hearts. Finally, to extend our study to an atherosclerotic model, where blunted metabolic hyperemia has been reported, it should be noted that other factors, such as phenotypic changes of coronary vessels (more stiff and fibrotic) and hemodynamic alterations due to collateral circulation and/or coronary steal (less flow to the ischemic region where the vascular network is already maximally dilated), need to be considered for studies.
In conclusion, blockade of ARs, in particular, A2AARs and A2B ARs using combined pharmacological compounds with targeted gene deletion, decreased CF at rest but failed to reduce the pacing-induced increase in CF. Inhibition of NO synthase decreased resting CF without an effect on pacing-induced hyperemia, which was not altered by combined blockade of ARs, suggesting both NO and adenosine are not required for coronary metabolic hyperemia. The mechanisms responsible for pacing-induced coronary hyperemia involve mostly KATP channels and, to a lesser extent, H2O2, since blockade of KATP channels by glibenclamide almost abolished pacing-induced coronary vasodilation, whereas scavenging of H2O2 by catalase only partially inhibited the response. Thus, although adenosine-mediated H2O2 production and the subsequent opening of KATP channels in the coronary vasculature play an important role in coronary ischemic vasodilation, other mechanisms, but not adenosine, are responsible for H2O2 production and opening of KATP channels, leading to coronary metabolic vasodilation in isolated mouse hearts.
A compromised coronary vasodilation may not necessarily cause ischemia under resting condition. However, during increased myocardial demand, e.g., exercise, the coronary vascular dysfunction will lead to a mismatch between O2 demand and supply, manifesting the clinical symptom known as angina. Although mice differ significantly from humans in many aspects, which may limit the extrapolation of present study to clinical situations, our model, using paced mouse hearts to gradually increase rates that are within the range of HR change during exercise in humans, will provide an important basis for further mechanistic studies in humans. The different mechanisms regarding the roles of adenosine, H2O2, and KATP channels in CF regulation during ischemic versus metabolic hyperemia may add new knowledge to the understanding of pathophysiology of coronary artery diseases.
GRANTS
This work was supported by National Institutes of Health Grants HL-027339, HL-09444, HL-071802, and U54-GM-104942.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Z. and S.J.M. conception and design of research; X.Z. performed experiments; X.Z. analyzed data; X.Z. interpreted results of experiments; X.Z. prepared figures; X.Z. drafted manuscript; X.Z. and S.J.M. edited and revised manuscript; X.Z., B.T., S.L.T., C.L., and S.J.M. approved final version of manuscript.
REFERENCES
- 1.Akatsuka Y, Egashira K, Katsuda Y, Narishige T, Ueno H, Shimokawa H, Takeshita A. ATP sensitive potassium channels are involved in adenosine A2 receptor mediated coronary vasodilatation in the dog. Cardiovasc Res 28: 906–911, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Aversano T, Ouyang P, Silverman H. Blockade of the ATP-sensitive potassium channel modulates reactive hyperemia in the canine coronary circulation. Circ Res 69: 618–622, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Bacchus AN, Ely SW, Knabb RM, Rubio R, Berne RM. Adenosine and coronary blood flow in conscious dogs during normal physiological stimuli. Am J Physiol Heart Circ Physiol 243: H628–H633, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Bache RJ, Dai XZ, Schwartz JS, Homans DC. Role of adenosine in coronary vasodilation during exercise. Circ Res 62: 846–853, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Beech DJ, Zhang H, Nakao K, Bolton TB. Single channel and whole-cell K-currents evoked by levcromakalim in smooth muscle cells from the rabbit portal vein. Br J Pharmacol 110: 583–590, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204: 317–322, 1963 [DOI] [PubMed] [Google Scholar]
- 7.Berne RM. The role of adenosine in the regulation of coronary blood flow. Circ Res 47: 807–813, 1980 [DOI] [PubMed] [Google Scholar]
- 8.Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A2A and A2B receptors to ischemic coronary dilation: role of KV and KATP channels. Microcirculation 17: 600–607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boerth RC, Covell JW, Seagren SC, Pool PE. High-energy phosphate concentrations in dog myocardium during stress. Am J Physiol 216: 1103–1106, 1969 [DOI] [PubMed] [Google Scholar]
- 10.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 247: 1341–1344, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 235: 10–22, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of KATP+ channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol 280: H22–H33, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Duncker DJ, Stubenitsky R, Verdouw PD. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am J Physiol Heart Circ Physiol 275: H1663–H1672, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of KATP+ channels in coronary vasodilation during exercise. Circulation 88: 1245–1253, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Duncker DJ, van Zon NS, Ishibashi Y, Bache RJ. Role of KATP+ channels and adenosine in the regulation of coronary blood flow during exercise with normal and restricted coronary blood flow. J Clin Invest 97: 996–1009, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncker DJ, van Zon NS, Pavek TJ, Herrlinger SK, Bache RJ. Endogenous adenosine mediates coronary vasodilation during exercise after KATP+ channel blockade. J Clin Invest 95: 285–295, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edlund A, Sollevi A. Theophylline increases coronary vascular tone in humans: evidence for a role of endogenous adenosine in flow regulation. Acta Physiol Scand 155: 303–311, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Ely SW, Knabb RM, Bacchus AN, Rubio R, Berne RM. Measurements of coronary plasma and pericardial infusate adenosine concentrations during exercise in conscious dog: relationship to myocardial oxygen consumption and coronary blood flow. J Mol Cell Cardiol 15: 673–683, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Graham TP, Jr, Covell JW, Sonnenblick EH, Ross J, Jr, Braunwald E. Control of myocardial oxygen consumption: relative influence of contractile state and tension development. J Clin Invest 47: 375–385, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Headrick JP, Ely SW, Matherne GP, Berne RM. Myocardial adenosine, flow, and metabolism during adenosine antagonism and adrenergic stimulation. Am J Physiol Heart Circ Physiol 264: H61–H70, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Hein TW, Belardinelli L, Kuo L. Adenosine A2A receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J Pharmacol Exp Ther 291: 655–664, 1999 [PubMed] [Google Scholar]
- 23.Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res 82: 346–359, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Kingsbury MP, Robinson H, Flores NA, Sheridan DJ. Investigation of mechanisms that mediate reactive hyperaemia in guinea-pig hearts: role of KATP channels, adenosine, nitric oxide and prostaglandins. Br J Pharmacol 132: 1209–1216, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroll K, Feigl EO. Adenosine is unimportant in controlling coronary blood flow in unstressed dog hearts. Am J Physiol Heart Circ Physiol 249: H1176–H1187, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Mallet RT, Lee SC, Downey HF. Endogenous adenosine increases O2 utilisation efficiency in isoprenaline-stimulated canine myocardium. Cardiovasc Res 31: 102–116, 1996 [PubMed] [Google Scholar]
- 27.Merrill GF, Downey HF, Jones CE. Adenosine deaminase attenuates canine coronary vasodilation during systemic hypoxia. Am J Physiol Heart Circ Physiol 250: H579–H583, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Minamino T, Kitakaze M, Matsumura Y, Nishida K, Kato Y, Hashimura K, Matsu-Ura Y, Funaya H, Sato H, Kuzuya T, Hori M. Impact of coronary risk factors on contribution of nitric oxide and adenosine to metabolic coronary vasodilation in humans. J Am Coll Cardiol 31: 1274–1279, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension 39: 761–766, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 282: H437–H444, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 161–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narishige T, Egashira K, Akatsuka Y, Imamura Y, Takahashi T, Kasuya H, Takeshita A. Glibenclamide prevents coronary vasodilation induced by β1-adrenoceptor stimulation in dogs. Am J Physiol Heart Circ Physiol 266: H84–H92, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Ponnoth DS, Nayeem MA, Tilley SL, Ledent C, Mustafa SJ. CYP-epoxygenases contribute to A2A receptor-mediated aortic relaxation via sarcolemmal KATP channels. Am J Physiol Regul Integr Comp Physiol 303: R1003–R1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of KATP+ channels and adenosine in the control of coronary blood flow during exercise. J Appl Physiol 89: 529–536, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of KATP+ channels in local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 277: H2115–H2123, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Sadraei H, Beech DJ. Ionic currents and inhibitory effects of glibenclamide in seminal vesicle smooth muscle cells. Br J Pharmacol 115: 1447–1454, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol Cell Physiol 262: C1220–C1227, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Schenkman KA. Cardiac performance as a function of intracellular oxygen tension in buffer-perfused hearts. Am J Physiol Heart Circ Physiol 281: H2463–H2472, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Schenkman KA, Beard DA, Ciesielski WA, Feigl EO. Comparison of buffer and red blood cell perfusion of guinea pig heart oxygenation. Am J Physiol Heart Circ Physiol 285: H1819–H1825, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Sharifi-Sanjani M, Teng B, Krahn T, Tilley SL, Ledent C, Mustafa SJ. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double knockout mice. Am J Physiol Heart Circ Physiol 301: H2322–H2333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharifi-Sanjani M, Zhou X, Asano S, Tilley SL, Ledent C, Teng B, Dick GM, Mustafa SJ. Interactions between A2A adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol 304: H1294–H1301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talukder MA, Morrison RR, Ledent C, Mustafa SJ. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol 41: 562–570, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Teng B, Fil D, Tilley SL, Ledent C, Krahn T, Mustafa SJ. Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J Cardiovasc Pharmacol 61: 70–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985) 97: 404–415, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Tune JD, Richmond KN, Gorman MW, Feigl EO. KATP+ channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 280: H868–H875, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation 101: 2942–2948, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74–H84, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Van Bibber R, Stepp DW, Kroll K, Feigl EO. Role of adenosine in norepinephrine-induced coronary vasodilation. Am J Physiol Heart Circ Physiol 273: H557–H565, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Vanelli G, Chang HY, Gatensby AG, Hussain SN. Contribution of potassium channels to active hyperemia of the canine diaphragm. J Appl Physiol (1985) 76: 1098–1105, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Yada T, Richmond KN, Van Bibber R, Kroll K, Feigl EO. Role of adenosine in local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 276: H1425–H1433, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y, Kajiya F. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 107: 1040–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Yada T, Shimokawa H, Hiramatsu O, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Important role of endogenous hydrogen peroxide in pacing-induced metabolic coronary vasodilation in dogs in vivo. J Am Coll Cardiol 50: 1272–1278, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Yu L, Jin X, Yang Y, Cui N, Jiang C. Rosiglitazone inhibits vascular KATP channels and coronary vasodilation produced by isoprenaline. Br J Pharmacol 164: 2064–2072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zatta AJ, Headrick JP. Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol 144: 576–587, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X, Teng B, Tilley S, Mustafa SJ. A1 adenosine receptor negatively modulates coronary reactive hyperemia via counteracting A2A-mediated H2O2 production and KATP opening in isolated mouse hearts. Am J Physiol Heart Circ Physiol 305: H1668–H1679, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]