Abstract
Recent evidence from humans and rats indicates that nitrite is a vasodilator under hypoxic conditions by reacting with metal-containing proteins to produce nitric oxide (NO). We tested the hypothesis that near-physiological concentrations of nitrite would produce vasodilation in a hypoxia- and concentration-dependent manner in the hind limb of sheep. Anesthetized sheep were instrumented to measure arterial blood pressure and femoral blood flows continuously in both hind limbs. Nitrite was infused into one femoral artery to raise the nitrite concentration in the femoral vein by 10 to 15-fold while the sheep breathed 50%, 14% or 12% oxygen in inspired air. In contrast to reports in humans and rats, the nitrite infusion had no measurable effect on mean femoral blood flows or vascular conductances, regardless of inspired O2 levels. In vitro experiments showed no significant difference in the release of NO from nitrite in sheep and human red blood cells. Further experiments demonstrated nitrite is converted to NO in rat artery homogenates faster than sheep arteries, and that this source of NO production is attenuated in the presence of a heme oxidizer. Finally, western blots indicate that concentrations of the heme-containing protein cytoglobin, but not myoglobin, are markedly lower in sheep arteries compared with rats. Overall, the results demonstrate that nitrite is not a physiological vasodilator in sheep. This is likely due to a lack of conversion of nitrite to NO within the vascular smooth muscle, perhaps due to deficient amounts of the heme-containing protein cytoglobin.
Keywords: nitric oxide, nitrite, femoral artery, hypoxic vasodilation, sheep
the inorganic anion nitrite (NO2−) is typically present at concentrations of ∼100 to 500 nM in the plasma of humans, sheep, and many other mammals studied (21, 30), although 10-fold higher levels have been found in high-altitude Tibetans (15). It has also been measured in arterial smooth muscle tissue of rats at concentrations of ∼10 μM (8, 33). Increased plasma nitrite is associated with protection against ischemia-reperfusion injury in several organs and animal models [reviewed by Dezfulian et al. (14)], increased exercise performance (20, 28, 38), and decreased vascular resistance to blood flow (9, 13, 27). The mechanisms underlying these effects are not completely understood but are likely to involve the conversion of nitrite to nitric oxide (NO), a reaction that can occur nonenzymatically by chemical disproportionation (39), or by a redox reaction between nitrite and a number of metal-containing proteins including deoxyhemoglobin, deoxymyoglobin, cytoglobin, xanthine oxidase, aldehyde dehydrogenase, and others [reviewed by Lundberg and Weitzberg (25)]. Deoxyhemoglobin was the first such protein described to reduce nitrite to NO (7) and has recently been proposed to facilitate hypoxia-sensitive local production of vasodilating concentrations of NO from nitrite (16). The reaction between nitrite and deoxyhemoglobin is under allosteric control, with the production of NO being maximal at 50% to 70% oxyhemoglobin saturation (19), making the hemoglobin-reductase theory especially attractive as a feedback mechanism for matching oxygen delivery with oxygen need (16). However, the hemoglobin-nitrite reductase reaction produces NO at a rate six to seven orders of magnitude slower than the rate at which NO itself is scavenged by both oxy- and deoxyhemoglobin, raising the yet-unanswered question of how vasoactive quantities of nitrite-derived NO might escape hemoglobin scavenging inside the red blood cells.
The demonstration that infusion of nitrite into the brachial artery of human subjects increases forearm blood flow rate unilaterally is a main pillar of the hemoglobin-reductase hypothesis (9, 13, 26, 27). It was reported that a ∼10% increase in forearm blood flow could be obtained by a 0.35 μM increase in blood nitrite concentration (12), a level clearly within the physiological range (17). These reports also provide evidence that the vasodilatory effects of nitrite are potentiated by either local (9) or systemic (27) hypoxia, consistent with the theory that nitrite is reduced to NO by deoxyhemoglobin. However, the forearm blood flow studies are technically limited since the methodology (strain-gauge plethysmography) has a low resolution in time. Furthermore, the results are not unanimous, with one study unable to detect local vasodilation during normoxia until nitrite concentrations were above 30 μM (27) and another finding no effects even at concentrations approaching three orders of magnitude above baseline levels (22). Although there have been a number of animal studies demonstrating protective effects of near-physiological concentrations of nitrite against ischemic injury (25), most in vivo assessments of the effects of nitrite on vascular tone have been in the setting of pharmacological and/or systemic doses of nitrite and thus reveal little about the extent and mechanism of local vasodilation within the range of physiological nitrite concentrations.
We recently observed that nitrite infusion into the carotid artery of near-term fetal sheep does not increase the conductance of the cephalic vascular bed (35), a result that was unexpected particularly given that fetal deoxyhemoglobin reduces nitrite to NO twice as fast as adult deoxyhemoglobin (6). We therefore decided to measure the effect of nitrite on resistance to flow in the femoral artery of adult sheep. Using perivascular transit-time flow probes for continuous measurement of blood flow, we test the hypothesis that exogenous intra-arterial nitrite acts as a hypoxia-sensitive vasodilator at near-physiological concentrations. The failure of this vascular bed to respond to nitrite confirmed our findings in the fetal sheep and led us to perform additional ex vivo studies to test whether the vasodilatory effects of nitrite are species dependent. In so doing, we compared the release of nitrite-derived NO from sheep and human blood and from homogenates prepared from rat and sheep arteries. We also compared the relaxing capacity of nitrite on arteries isolated from rats and sheep, free of blood, thus permitting separation of responses dependent on hemoglobin from those dependent on the direct effects of nitrite on vascular smooth muscle. Finally, we measured in arterial vessels from rats and sheep the content of myoglobin and cytoglobin, two proteins proposed to play a role in the vasodilatory effects of nitrite (2, 31).
METHODS
All animal experiments were preapproved by the Animal Care and Use Committee and conformed to the US National Institutes of Health (NIH) guidelines on the use of animals in research. Collection of human blood was carried out under a protocol preapproved by the Institutional Review Board of Loma Linda University and conformed to Declaration of Helsinki guidelines. In experiments involving the use of human blood, written consent was obtained from four male volunteers.
Preparation of NO Gas in Solution
Ten milliliters of PBS (pH 7.4) in a 20-ml glass syringe filled with nitrogen was shaken vigorously for 5 min. The headspace gas was ejected and replaced with nitrogen, and the procedure repeated three times to remove dissolved oxygen from saline. The headspace was then filled with pure NO gas (Matheson TriGas, Riverside, CA) sparged through sodium hydroxide (1 M) to remove higher nitrogen oxides. The syringe was shaken for 5 min. The headspace gas was replaced by fresh NO, and the procedure repeated four times. The syringes were kept at room temperature until use, within 1 h of preparation. The NO concentration was estimated to be 2 mM/l based on a Bunsen solubility coefficient for NO dissolved in water of 0.0454 ml STPD·ml−1·atm−1.
Nitrite Infusion Experiments
Surgical procedures.
Female and neutered male sheep 9 to 10 mo old and weighing 37.2 ± 1.7 kg were sedated with diazepam (0.5 mg/kg) and ketamine (10 mg/kg; Ketaject, Phoenix Pharmaceutical, Burlingame, CA) given intravenously and then intubated and ventilated with 1.5% to 2.5% isoflurane (Baxter U. S., Deerfield, IL) in oxygen during surgery. Catheters were inserted into a carotid artery to measure systemic blood pressure (ArgoTrans; Argon Medical Devices, Plano, TX) and to withdraw blood. Catheters were also inserted into an external jugular vein for drug injections and into a brachial vein for continuous infusion of hexamethonium bromide (Sigma Aldrich, St Louis, MO).
The femoral arteries and veins, including their branches (the lateral femoral circumflex artery and vein), were exposed in the inguinal region of both hind limbs. These arterial and venous branches were catheterized (catheter tips: ID 0.58 mm, OD 0.99 mm). The tip of the venous catheter was advanced into the main femoral vein, whereas the tip of the arterial catheter was advanced only to within 1 to 2 mm of the main femoral artery to ensure it did not impede blood flow through the femoral artery. The arterial catheter (total volume, 0.4 to 0.6 ml) in one leg was connected to a syringe infusion pump (Harvard Apparatus; No. 55-5920). This side is designated the ipsilateral side, whereas the other side is termed the contralateral side. Ipsilateral and contralateral sides were alternated (left or right legs) sequentially between animals. Finally, transit-time flow probes (Transonic PS series; Ithaca, NY) were placed around the femoral arteries of both legs at a position 0.5 to 1.0 cm upstream of the entry points of the catheterized arterial branches. The probes were connected to a dual-channel flow meter (Transonic System T402; Transonic) with the upper frequency limit set to 160 Hz.
Pulsatile pressures, pulsatile flows, and mean flows (based on the temporal mean of each flow pulse) were recorded continuously beat-to-beat by an analog-to-digital data acquisition system (Powerlab; ADInstruments, Grand Junction, CO) and software (Labchart v6.0 for OS X; ADInstruments) at acquisition rates of at least 400 Hz. A signal from the infusion pump indicating its on/off status was also recorded. The rectal temperature of the animals was monitored continuously (YSI 4000 Thermometer; Yellow Springs Instrument, Yellow Springs, OH).
In vivo experimental procedures.
After completion of instrumentation, the animals were connected to a mechanical ventilator (V.I.P. Bird; Bird Technologies, Solon, OH). The concentration of oxygen in the inspired air, which initially had been 50%, was monitored continuously thereafter (O2 Analyzer; Analytical Industries, Pomona, CA). Tidal volume and breathing frequency were adjusted to maintain arterial carbon dioxide tensions in the range of 35 to 45 mmHg and pH from 7.35 to 7.45. Isoflurane anesthesia was discontinued and replaced by intravenous infusion of ketamine (Ketaject; 1 mg/kg) and vecuronium bromide, a relaxant of skeletal muscles (0.1 mg/kg; Sun Pharmaceutical, Mumbai, India), supplemented hourly, or as required. Next, NG-nitro-l-arginine methyl ester (l-NAME; Sigma-Aldrich), dissolved in 9 to 12 ml saline, was infused intravenously (45 mg/kg) over 3 to 5 min to block endogenous synthesis of NO. Fifteen to 30 min later when blood pressure was stable, an intravenous bolus of hexamethonium (1.0 mg/kg) was given, followed by a continuous infusion (2.0 mg·h−1·kg−1) that was maintained throughout the experiment. Hexamethonium (Sigma-Aldrich) was given to remove spontaneous fluctuations in pressure and flow by blocking sympathetic and parasympathetic influences on vascular tone. When the hexamethonium bolus was repeated at the end of the experiment (in 3 animals), no further significant effects on flow, heart rate, or blood pressure were observed, indicating the infusion had been effective throughout the experiments.
Continuous nitrite infusion protocol.
The vasodilatory effects of nitrite were assessed with a standard protocol consisting of a continuous 15–17 min infusion of sodium nitrite (dissolved in saline to concentrations of 1.0 mM or 10 mM) into the ipsilateral femoral artery at a rate of 1 ml/min. To test the effects of hypoxia, the infusion period was divided into a normoxic phase (inspired O2 = 50%) and two subsequent hypoxic phases (inspired O2 = 14% and 12%, in this order) each lasting 5 to 7 min. At the end of each phase, carotid artery samples were collected for measurement of arterial blood gases, and carotid artery and femoral vein samples were collected for measurement of plasma nitrite concentrations.
Continuously recorded data were resampled at 40 Hz. Mean systemic blood pressure was calculated as the average pressure for 3-s intervals. As an index of femoral artery vascular tone, mean femoral artery conductance (ml/min/mmHg), the reciprocal of resistance to flow, was calculated for both hind limbs by dividing mean flow rates by mean systemic pressure.
Immediately before each nitrite infusion, average values for flow and pressure were calculated during a 20- to 40-s time interval as baseline values. Then at the end of each phase of the protocol the average values were calculated for a similar time interval, and finally postbaseline values were calculated 15 to 25 min after the nitrite (or vehicle control) infusion was discontinued. Because the vasoactive actions of nitrite were expected to be restricted to the ipsilateral side, the ratio of flows (or conductances) (ipsilateral side/contralateral side) was considered a critical index of the local vasoactivity of nitrite, as described previously by Maher et al. (27). A comparison of ratios has the further advantage that systemic influences on flows and conductances during the course of experiments do not obscure the side-specific effects.
Bolus injections.
To assess the capacity of the femoral vasculature to respond to NO and supra-physiological doses of nitrite, 1 ml of deoxygenated saline containing dissolved NO (∼2 mM) or nitrite (1, 5, 10, 50 mM) was injected manually over a period of 5 s into the femoral artery. The catheter then was flushed immediately with 1 ml of saline. Inspired O2 during these bolus experiments was always 50%. To assess the possibility of injection artifacts, 1 ml of saline was injected as a negative control. It may be noted that the bolus injections were equivalent to a short lasting infusion at an average rate of 10 to 15 ml/min. Responses of femoral flows and conductances to bolus injections were quantified based on peak increases from baseline and on time-averaged values over the duration of the changes observed after each injection. Changes in flows and conductances were expressed relative to 20-s averages of the immediately preceding baseline values as absolute or relative (percentage) values.
To assess the possible influence of l-NAME and hexamethonium on responses to nitrite infusion, the standard 15-min protocol was performed in four animals before the use of these drugs. In addition, to assess the possible effects of hypoxia alone on femoral vascular tone, the hypoxia protocol was repeated in four animals during infusion of saline (vehicle) without nitrite. l–NAME and hexamethonium were administered in these animals.
At the end of experiments, while still under anesthesia, the animals were euthanized with an intravenous injection of 15 ml of Euthasol (a formulation of pentobarbital sodium and phenytoin sodium; Virbac, Fort Worth, TX).
Determination of Blood Gases and Nitrite in Blood
Heparinized arterial blood samples (0.5 ml each) were collected to measure hemoglobin concentration, oxyhemoglobin saturation (OSM3; Radiometer Copenhagen), and blood gases (ABL 5; Radiometer Copenhagen). One milliliter of blood was collected for measurement of plasma nitrite concentrations. The blood was centrifuged immediately (8,000 rpm for 30 s), and the plasma was separated and frozen in liquid nitrogen and then stored at −80°C until assayed.
Nitrite concentrations were determined by triiodide chemiluminescence using a nitrogen oxide analyzer (NOA 280i; Sievers, Boulder CO) as described previously in detail (5). Plasma (50 to 200 μl) was injected into 5.0 ml of triiodide solution that reduces nitrite to NO. The triiodide was purged continuously with argon that carried the NO into the NOA for detection. This method also detects S-, N-, and Fe- nitroso species, but treatment of aliquots of the plasma with acid sulfanilamide, which selectively removes the nitrite (37), resulted in a >98% decrease in the signal, indicating that the contribution of these species to the total signal was nominal.
The nitrite concentration in the perfused femoral artery was calculated taking allowance for dilution by blood flow measured in the artery at the time of infusion. The increase in femoral artery nitrite concentration was calculated as infusate concentration × infusion rate/(ipsilateral blood flow rate + infusion rate).
Ex Vivo Experiments
The production of NO from nitrite mediated by red blood cells or tissue homogenates, the relaxing effect of nitrite on preconstricted vascular rings (wire myography), and the content of myoglobin and cytoglobin in vascular tissue (Western blotting) were investigated in ex vivo experiments as described below.
Production of NO From Nitrite
Red blood cells.
In order to compare the release of NO from human and sheep red blood cells, experiments were initially performed using the methods of Cosby et al. (9). A membrane gas exchanger, used clinically as an extra-corporal membrane oxygenator (Quadrox-ID Pediatric, Maquet, Rastatt, Germany), was next adapted to measure the release of nitrite-derived NO from human and sheep blood. From healthy adult volunteers and adult sheep, 60 ml of blood was collected in a syringe containing 3,000 IU of heparin. The blood was then diluted with physiological saline to a hematocrit of 5% to 6%. Oxyhemoglobin percentage and pH were adjusted to 45% to 55% and 7.3 to 7.4, respectively, by partial tonometric equilibration with 3% CO2 in a balance of N2. With the use of a syringe pump, the diluted blood was then infused through the gas exchanger at a rate of 10 ml/min. The gas phase of the exchanger was continuously supplied with nitrogen that was drawn directly into a chemiluminescence NO analyzer (280i; Sievers) with a lower limit of detection at 5 ppb. The gas exchanger was kept at 37°C. At predetermined time points, ascending concentrations of nitrite dissolved in PBS (pH 7.4) were infused into the blood at a point immediately proximal to the gas exchanger at a rate of 1.0 ml/min to achieve final concentrations of 0.1, 1, 10, and 100 μM. In addition, to assess the sensitivity of the system to detect free NO in an aqueous solution, experiments were conducted in which the gas exchanger was perfused with PBS (10 ml/min) whereas the NO donor 1-(hydroxy-NNO-azoxy)-L-proline (PROLI-NO; dissolved in 1 mM NaOH) was admixed into the saline immediately proximal to the gas exchanger to achieve final NO concentrations of 0.1, 1, 10, and 100 μM based on the assumption that each unit of PROLI-NO decomposed with a half-life of 1.8 s to produce 1.5 units of NO. The principal advantage of the membrane gas exchanger is that it provides a high surface area to volume ratio in the liquid phase for gas exchange without causing foaming of the blood solution.
Tissue Homogenates
Tissue collection and homogenization.
Adult (6 to 12 mo of age) rats were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg) followed by thoracotomy and removal of the heart and descending aorta. Adult sheep were anesthetized with an intravenous injection of ketamine (10 mg/kg) and midazolam (5 mg/kg) for immediate intubation and ventilation with 5% isoflurane, and the lateral femoral circumflex arteries were removed. With anesthesia maintained, the sheep were then euthanized by exsanguination from the jugular vein followed by thoracotomy and removal of hearts. All tissues collected were placed in HEPES buffer on ice and dissected free of blood and adhering tissue within 30 min after euthanization of animals. Samples were homogenized using a rotor-stator homogenizer (TissueRuptor; Qiagen, Hilden, Germany), sonicated in ice-cold lysis buffer containing (in mM) 150 NaCl, 50 Tris, 10 EDTA, 1 DTT, and 1 PMSF and 2 μg/ml Aprotinin (pH 7.4), centrifuged to remove unhomogenized tissue, and diluted in lysis buffer to a protein concentration of 4 μg/μl after protein assay (No. 500-0006; Bio-Rad, Hercules, CA).
NO production from homogenates.
The NO production from nitrite was compared in homogenates prepared from rat descending aorta, sheep femoral artery, and ventricular myocardium from each species. Homogenate (100 μl) was introduced into 20 ml of 100 μM nitrite (in PBS; pH = 6.2) in a vessel continuously purged with argon flowing into a chemiluminescence NO analyzer (Sievers, 280i). Lysis buffer was injected as a blank control. To test the possible role of heme-containing proteins, xanthine oxidase, and aldehyde dehydrogenase in NO production, 10 μM [1H-[1,2,4]oxadiazolo-[4, 3-a]quinoxalin-1-one] (ODQ), 100 μM oxypurinol, and 0.5 μM daidzin, respectively, were introduced into the purge vessel at 1 min before the addition of homogenates. Cumulative NO generation was expressed as the area under the voltage versus time curve for 100 s following addition of the homogenate to the purge vessel.
Wire Myography
Both rat aorta and the lateral femoral circumflex artery from sheep were isolated and cut into 5-mm rings. Three to five arterial rings from each species were mounted in wire myography organ bath systems (Radnoti Glass Instruments, Monrovia, CA). The baths contained Krebs-Henseleit solutions aerated with 5% CO2-95% N2 (pH = 7.4) at room temperature. l-NAME (100 μM final concentration) was added to inhibit the endogenous production of NO. The baths were capped with paraffin film to maintain a hypoxic environment, and oxygen tensions were measured to be 6.2 ± 1.0 Torr. The rings were stretched with 1 g of baseline tension and subjected to high potassium concentrations (120 mM KCl) to verify the ability of the vessel to constrict. The KCl solution was washed out, and the solution in the bath was replaced with PBS and 10 μM serotonin (5-hydroxy-tryptamine). The rings were then exposed to stepwise increasing concentrations of sodium nitrite (30 nM to 10 mM in half-log steps) or the NO donor PROLI-NO (100 pM to 0.1 mM) to measure vasodilating potencies. PROLI-NO prepared in 1 mM NaOH stock solutions for handling before injections into the vessel bath had no significant effect on the bath pH.
For each experiment, two vessel rings from each species were subjected to saline (nitrite controls) or 1 mM NaOH in saline (PROLI-NO controls). The time-matched tension changes observed in the controls were subtracted from the tension changes observed following nitrite or PROLI-NO application, to correct for spontaneous or vehicle-induced decreases in tension.
Dose-response curves were constructed using percentages of the serotonin-induced maximum tension. The EC50 (concentration that results in 50% vasodilation) and maximal response values were calculated by fitting the data to the Gaddum/Schild EC50 equation in Prism v5.0c (Graphpad Software, La Jolla, CA).
Western Blotting
The expression of myoglobin and cytoglobin were compared in homogenates prepared from rat descending aorta and sheep lateral femoral circumflex artery. To account for the possibility of interspecies variations in antibody:target avidities, samples of ventricular myocardium were also prepared from each species as a reference for the arterial samples. Proteins were electrophoretically separated in 15% polyacrylamide gel and transferred to nitrocellulose membranes (No. 162-0115; Bio-Rad, Hercules, CA). Polyclonal antibodies of myoglobin (bs-3805R; Bioss, Woburn, MA) and cytoglobin (ab127028; Abcam, Cambridge, MA) were used after 400- and 1,000-fold dilution, respectively. Anti-rabbit secondary antibody (No. 401393; Calbiochem, San Diego, CA) was applied after 2,000-fold dilution. Due to the significant variation of housekeeping proteins in homogenates from different tissue types, stains of total protein in each lane of the Western blot were used as loading controls, as described previously (1). Protein band intensities were quantified by ImageJ (v10.2; NIH, Bethesda, MD).
Statistical Analysis
Mean values ± SE are given. The effect of infused nitrite or saline was assessed by one-way ANOVA applied to data from the preinfusion baseline period (inspired O2 = 50%), nitrite infusion periods with inspired O2 values of 50%, 14%, and 12%, and postinfusion recovery period (no nitrite infusion, 50% inspired O2). T-tests were used for two sample comparisons as appropriate. Statistical analyses were performed using Statistica (StatSoft, Tulsa, OK). Significance level was accepted at P < 0.05.
RESULTS
No Effect of l-NAME and Hexamethonium on Responses to Nitrite
It was observed that administration of l-NAME and hexamethonium did measurably affect flows and conductances, as demonstrated in Fig. 1. Consistent with constitutive production of NO by endothelial NO synthase, femoral flow and conductance tended to decrease after infusion of l-NAME (ns), whereas, by contrast, infusion of hexamethonium increased femoral flow and conductance (P < 0.005), indicating attenuation of sympathetic contribution to basal vascular tone. Therefore, in six animals we infused nitrite (1 mM) before or after the application of l-NAME/hexamethonium (2 animals both before and after, 2 animals only before, 2 animals only after). There were no significant responses to nitrite regardless of the presence of the blockers or of the level of oxygenation (ANOVA, P = 0.9). Mean flow ratios before and after l-NAME and hexamethonium were 1.07 ± 0.09 vs. 1.13 ± 0.13 before nitrite infusion, 1.15 ± 0.11 vs. 1.14 ± 0.12 during nitrite infusion at 50% inspired O2, 1.04 ± 0.11 vs. 1.14 ± 0.12 at 14% O2, and 1.11 ± 0.06 vs. 1.16 ± 0.13 at 12% O2. Thus no local vasodilatory effects of nitrite infusion were observed in the absence of l-NAME and hexamethonium.
Fig. 1.
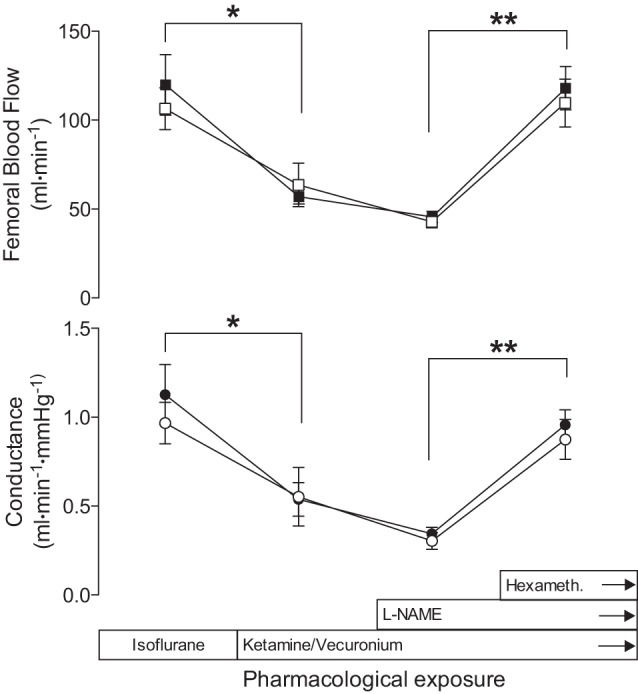
Responses of femoral blood flows and vascular conductances to ketamine, vecuronium, NG-nitro-l-arginine methyl ester (l-NAME), and hexamethonium (hexameth). After isoflurane was discontinued and ketamine and vecuronium were given, flows and conductances decreased (*P < 0.01). l-NAME administration to block nitric oxide (NO) synthesis slightly reduced flows (not significant) and conductances further, which then doubled when the ganglion blocking agent hexamethonium was given (**P < 0.001). The results indicate femoral blood flow is under strong sympathetic control in anesthetized sheep. Open symbols show the ipsilateral leg, and closed symbols show the contralateral leg. The time interval covered is about 45 to 60 min. Mean values are ± SE.
Nitrite Infusion In Vivo
The vasodilatory effects of nitrite were first assessed by infusing nitrite into one femoral artery of eight anesthetized sheep while continuously measuring arterial blood pressure and flow in both hind limbs. Nitrite (1 mM in saline) was infused for 15 min into the femoral artery. To assess the effects of hypoxia, the infusion period consisted of three 5-min phases of ventilation with 50%, 14%, and 12% inspired O2 in sequence. Calculated arterial nitrite concentrations, based on dilution of the infusate into measured femoral artery flows, averaged 12.4 ± 0.8 μM, whereas measured venous concentrations increased from 0.12 ± 0.04 μM to 8.3 ± 1.8 μM in response to the infusion. Baseline systemic nitrite concentrations in blood collected from the carotid artery averaged 0.12 ± 0.06 μM and increased to 0.52 ± 0.18 μM at the end of the 15-min infusion period (t-test, P < 0.03).
Arterial oxyhemoglobin saturation decreased from 99 ± 1% at an inspired O2 of 50% to 67 ± 3% and 44 ± 3% at inspired O2 levels of 14% and 12%, respectively. The corresponding average Po2 values were 202 ± 7 mmHg, 51 ± 3 mmHg, and 37 ± 2 mmHg. pH, pCO2, bicarbonate, and base excess did not vary with nitrite concentration and averaged 7.43 ± 0.01, 39.8 ± 0.6 mmHg, 25.6 ± 0.3 mM, and 2.5 ± 0.3 mM, respectively.
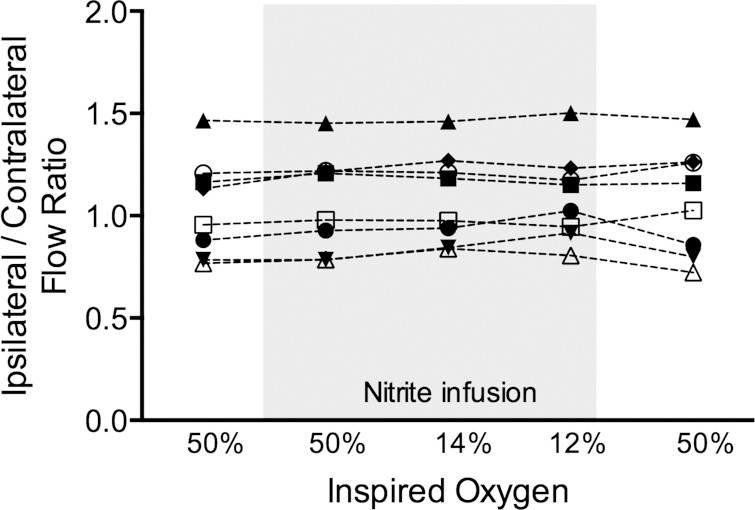
There were no significant changes in arterial blood pressure or blood flows and conductances in either hind limb with respect to baseline during the nitrite infusion while inspired O2 was 50% (Table 1). In addition, the ratio of ipsilateral/contralateral femoral artery flow was unchanged during the nitrite infusion (Fig. 2), indicating a lack of local vasodilatory effect of the infused nitrite. At reduced oxygen levels while breathing 14% or 12% inspired O2, there were again no significant changes in blood flow or conductance in either hind limb, nor was there a change in the ipsilateral/contralateral flow ratio (ANOVA, P = 0.69; Table 1). Thus there were no detectable local vasodilatory effects of nitrite even with oxyhemoglobin saturations lowered to be in favor of the deoxyhemoglobin-nitrite reduction reaction.
Table 1.
Flow, conductance, and systemic blood pressure responses to 1.0 mM nitrite infusion into one hind limb of anesthetized sheep at different levels of oxygenation
| Flow, ml/min |
Conductance, ml·min−1·mmHg−1 |
|||||||
|---|---|---|---|---|---|---|---|---|
| [Nitrite], mM | Inspired O2, % | n | Ipsilateral | Contralateral | Ipsilateral | Contralateral | Blood Pressure, mmHg | Flow Ratio |
| Pre | 50 | 8 | 82.3 ± 5.9 | 80.1 ± 4.5 | 0.61 ± 0.04 | 0.60 ± 0.05 | 136.0 ± 6.2 | 1.05 ± 0.09 |
| 1 | 50 | 8 | 83.4 ± 6.5 | 78.2 ± 4.1 | 0.63 ± 0.04 | 0.61 ± 0.05 | 133.2 ± 6.1 | 1.07 ± 0.08 |
| 1 | 14 | 8 | 82.4 ± 6.4 | 76.9 ± 5.1 | 0.66 ± 0.04 | 0.62 ± 0.04 | 124.8 ± 4.5 | 1.09 ± 0.08 |
| 1 | 12 | 7 | 75.6 ± 4.7 | 71.7 ± 5.2 | 0.66 ± 0.05 | 0.62 ± 0.04 | 114.8 ± 4.9 | 1.08 ± 0.09 |
| Post | 50 | 8 | 78.7 ± 7.4 | 75.4 ± 6.1 | 0.62 ± 0.04 | 0.60 ± 0.06 | 128.5 ± 4.9 | 1.07 ± 0.04 |
Values are means ± SE.
Fig. 2.
Ratio of ipsilateral to contralateral femoral artery flow during each of the 5 protocol phases for the 1 mM nitrite infusion. Nitrite infusion results in a ∼10-fold increase in ipsilateral arterial nitrite concentrations (see results). Responses for each of 8 animals are shown, with each animal represented by a different symbol (n = 8). There were no differences between mean values at each level of inspired O2 (1-way ANOVA; see Table 1).
In five animals, the infusion protocol was repeated with 10 mM sodium nitrite in the infusate instead of 1 mM, resulting in calculated femoral artery concentrations of 188 ± 35 μM, measured femoral venous concentrations of 92 ± 29 μM, and measured systemic arterial concentrations of 9.6 ± 0.5 μM at the end of the 15-min infusion period. Changes in arterial blood gases were similar to those observed during the 1-mM infusion experiments described above. Arterial blood pressure tended to decrease during the 15-min infusion from 109 ± 10 to 87 ± 11 mmHg although the change did not reach significance (P = 0.49). Similar to the 1-mM infusion experiments, there were no significant increases in the ratio of flow to the two hind limbs (ANOVA, P = 0.99) during normoxia or hypoxia, again suggesting no local vasodilatory effects of nitrite.
We considered the possibility that the ability of hypoxia to potentiate the local vasodilatory effects of nitrite was being masked by an opposing systemic vasoconstrictive response, such as might have occurred, for example, by release of catecholamines into the circulation. To test this possibility, physiological saline was infused without nitrite into four animals under the standard 15-min protocol. No significant increases in arterial blood pressure (129 ± 7 mmHg during saline infusion before hypoxia, 120 ± 8 mmHg at the end of 12% hypoxia; P = 0.13, paired t-test) or decreases in conductances were observed. Thus vasodilation from nitrite was not being obscured by hypoxia-induced vasoconstriction.
We also considered the possibility that the vasodilatory effect of nitrite infusion was being masked by administration of l-NAME and hexamethonium. However, we observed no significant vasodilatory effects of nitrite infusion when experiments were repeated while l-NAME and hexamethonium were omitted from the protocol (see Supplemental Material), making this possibility unlikely.
Bolus Injection of NO
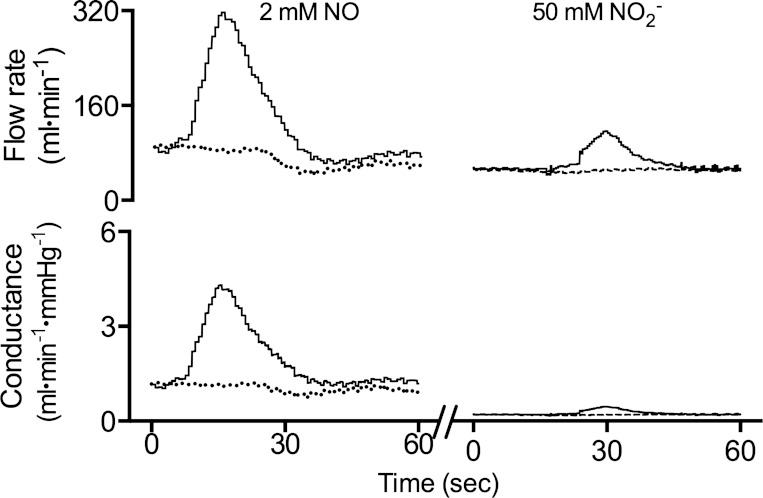
The question arises whether the hind limb vessels were capable of vasodilatory response at all. To assess the ability of the femoral vasculature to respond to NO, boluses of NO dissolved in saline (∼2.0 mM) were injected into the femoral artery. In contrast to the nitrite infusions, dissolved NO uniformly increased flows and conductances (representative response shown in Fig. 3). The time-averaged flow increased by 37 ± 3 ml/min from baseline (71 ± 4 ml/min) in 11 animals (28 injections total) and reached an average peak value of 176 ± 9 ml/min above baseline levels. Time-averaged conductance in the ipsilateral leg increased by 0.40 ± 0.04 ml·min−1·mmHg−1 from a baseline of 0.75 ± 0.05 ml·min−1·mmHg−1 and reached a peak value of 1.94 ± 0.15 ml·min−1·mmHg−1 above baseline levels. The changes were significant in the leg receiving the bolus (t-test, P < 0.0001) regardless of whether it was the right or left leg, whereas there were no measurable changes in the contralateral leg. The mean systemic arterial pressure was not affected by the injections.
Fig. 3.
Representative results of a bolus injection. A bolus of 1.0 ml of either 2 mM NO gas or 50 mM nitrite dissolved in saline was injected into the left femoral artery over a period of 5 s. Flow and conductance increased in the left leg (solid line) but not in the right leg (dashed line), demonstrating the capacity of the resistance vessels to vasodilate in response to NO and supraphysiological concentrations of nitrite.
Bolus Injection of Sodium Nitrite
To test whether the femoral vessels would respond to exceptionally high concentrations of nitrite, bolus injections containing large amounts of nitrite were infused in four sheep (total of 17 injections). Bolus injections with 1, 5, 10, and 50 mM sodium nitrite in saline were given. Significant effects on flow and conductance were observed only at the highest concentration (8 injections, representative response shown in Fig. 3), providing arterial concentrations estimated to be about 700-fold higher than baseline. Mean ipsilateral conductance increased to 120 ± 5% of baseline (P = 0.002). The corresponding increase in time-averaged flow rate was 14 ± 2 ml/min (P < 0.001), and the peak flow rate was 133 ± 10 ml/min (P < 0.001). The calculated nitrite concentration during the 50 mM bolus injection was 755 ± 120 μM, far higher than during constant infusion with 1.0 mM or even 10 mM nitrite.
Ex Vivo Production of NO From Nitrite in Blood
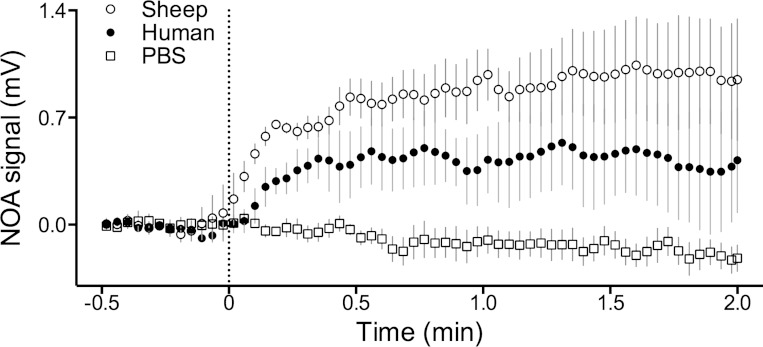
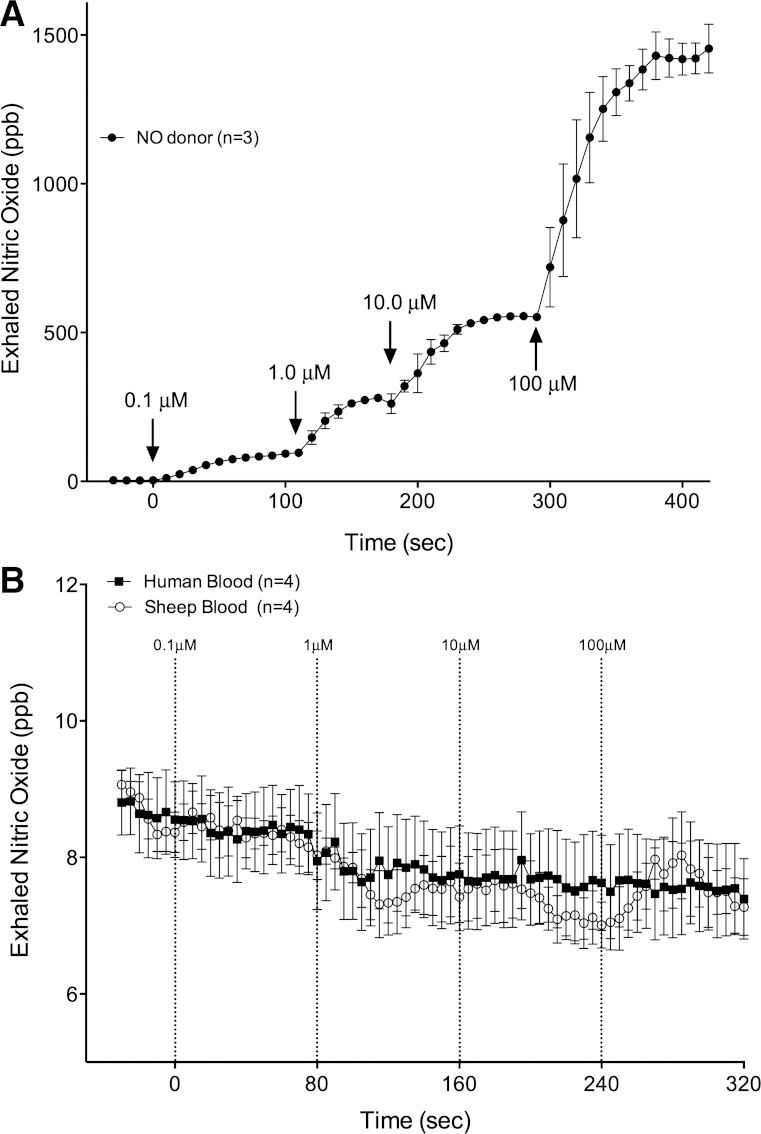
The lack of vasodilatory effect of nitrite in the hypoxic sheep hind limb conflicted with previous human studies and failed to support the hypothesis that vasodilating amounts of NO are derived from the reduction of nitrite by deoxyhemoglobin. Our initial experiments comparing the release of NO from human and sheep red blood cells demonstrated no significant difference between the amount of NO released into the headspace after addition of nitrite to the red blood cells (Fig. 4). Thus we undertook additional experiments to test whether less NO would be derived from sheep blood than from human blood using a membrane gas exchanger. The apparatus was configured to perfuse one side of a NO-permeable membrane with a liquid phase and to continuously measure NO concentrations in the gas on the other side of the membrane with a chemiluminescence analyzer (Fig. 4). The sensitivity of the apparatus was first characterized by measuring NO derived from addition of an NO donor (PROLI-NO) into PBS as it entered the membrane exchanger (Fig. 5A). When liquid-phase NO concentrations were 100 nM in PBS, the chemiluminescence analyzer detected 93 ± 5 ppb NO in the gas phase, indicating the sensitivity of the system to detect liquid-phase NO in the low nanomolar range. In whole blood diluted to a hematocrit of 5% to 6% (estimated heme concentration 2 to 3 mM), no increase of free NO could be detected even when nitrite concentrations were increased to 100 μM. Importantly, no difference between human and sheep blood was observed (2-way ANOVA; Fig. 5B) and thus these experiments do not support the hypothesis that differences exist between the release of nitrite-derived NO from sheep or human blood within the physiological range of nitrite concentrations.
Fig. 4.
Appearance of NO in the headspace of sealed containers after injection of sodium nitrite (final concentration 0.5 mM) into PBS (☐, n = 5), human red blood cell suspensions (●, n = 3), and sheep red blood cell suspensions (○, n = 3). The heme concentration in the cell suspensions was ∼0.32 mM, and the pO2 ∼30 Torr. NO analyzer (NOA) values were normalized to average 0 mV over the baseline period. Nitrite injection at t = 0 min resulted in a measurable increase in the release of NO from sheep (P = 0.005) and human (P = 0.008) cell suspensions (1-way ANOVA with Bonferroni correction), but there was no significant difference between human and sheep blood (2-way ANOVA). Mean values are ± SE.
Fig. 5.
NO measured in the gas phase of a membrane gas exchanger perfused with a liquid phase of diluted blood and a gas phase supplied with nitrogen. A: introduction of the NO donor 1-(hydroxy-NNO-azoxy)-L-proline (PROLI-NO) into a liquid phase of PBS provided significant increases of NO in the gas phase at all concentrations of PROLI-NO infused. Concentrations of NO as low as 0.1 μM in the liquid phase result in gas phase NO concentrations of 93 ± 5 ppb. B: during perfusion with diluted blood (hematocrit 4–6%), no detectable NO increases were measured until nitrite concentrations were increased to 10 mM (not shown), and there were no differences between human and sheep blood. Note that NO scales differ by a factor of 100. Means are ± SE.
Wire Myography
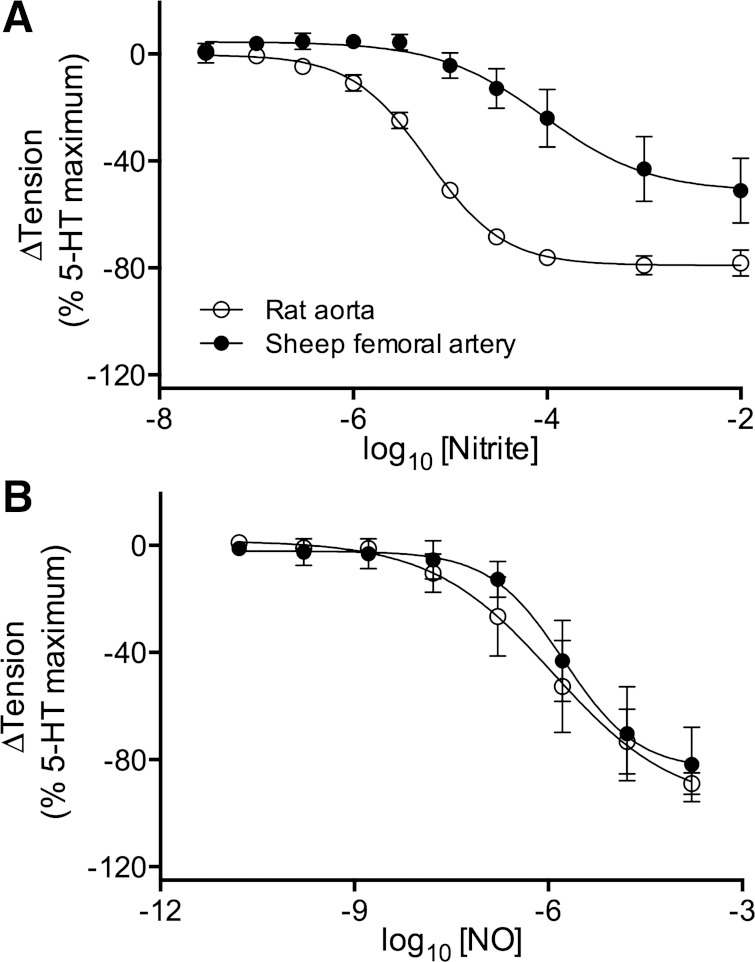
To test whether the lack of vasodilatory effect of nitrite in the sheep was because sheep arteries are less sensitive to nitrite acting on vascular smooth muscle, vessel rings were prepared from sheep femoral arteries and from the descending aorta of rats, a species previously demonstrated to vasodilate in response to nitrite both in vivo (32) and in vitro (9, 10). The maximal vasodilatory response to nitrite was significantly greater in rats (79 ± 2% decrease in vessel tension) than in sheep (51 ± 9% decrease; P = 0.05; Fig. 6A). In addition, the concentration of nitrite required to reach 50% of the maximal response (EC50, expressed as log10 of molar concentration) for rat aorta was −5.2 ± 0.1 compared with −4.0 ± 0.3 for sheep, a highly significant difference (P > 0.001). These results indicate sheep vessels are less sensitive to vasodilation by nitrite than the rat aorta and that the difference does not depend on reactions within blood. This difference is found for nitrite, not NO itself, because dose-response curves to NO demonstrated similar maximal dilation and EC50 for both rat aorta and sheep femoral arteries (Fig. 6B), suggesting the difference between rat and sheep arteries is due to handling of nitrite rather than sensitivity to NO.
Fig. 6.
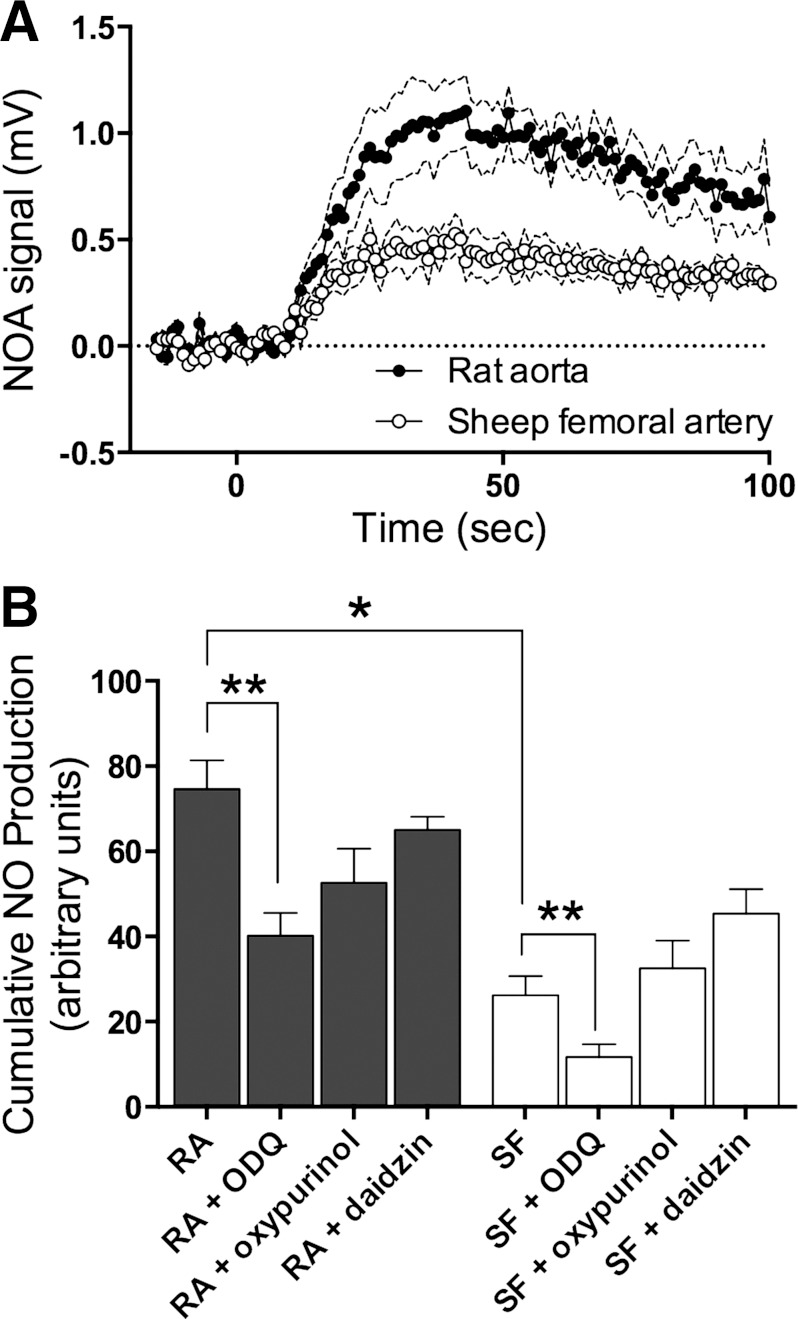
Production of free NO from nitrite in tissue homogenates from rat and sheep arteries. A: injection at time zero of 100 μl of tissue homogenate (4 μg protein/μl, n = 8 for each tissue type) into 20 ml of PBS (pH 6.2) containing 100 μM nitrite resulted in release of detectable amounts of NO into argon gas being continuously purged through the buffer. B: cumulative NO production in rat aorta (RA) homogenates was greater than that in sheep femoral artery (SF) homogenates. Heme oxidizer [1H-[1,2,4]oxadiazolo-[4, 3-a]quinoxalin-1-one] (ODQ; 10 μM) significantly inhibited NO production in both homogenates (n = 4 for rat and 6 for sheep), whereas xanthine oxidase inhibitor oxypurinol (100 μM, n = 4 for rat and 7 for sheep) and aldehyde dehydrogenase inhibitor daidzin (0.5 μM, n = 3 for rat and 7 for sheep) did not. *Significant difference between rat and sheep (P < 0.05, 1-way ANOVA); **significant effect of ODQ (P < 0.05, 1-way ANOVA).
NO Production From Homogenates
To compare the ability of rat and sheep arteries to convert nitrite to free NO, we measured the NO produced when homogenates of isolated arteries were injected into a purge vessel containing 100 μM nitrite dissolved in PBS and continuously purged with argon. The production of NO from sheep femoral artery homogenates was ∼60% less than that of rat aorta (Fig. 7; P < 0.001). Addition of ODQ to oxidize heme-containing proteins resulted in a significant decrease in both rat aorta (∼46%) and sheep femoral arteries (72%). Although both oxypurinol and daidzin tended to decrease NO production, the effects of these antagonists were not statistically significant. The lesser response in sheep vessel homogenates is again consistent with the existence of a species difference in the vascular smooth muscle.
Fig. 7.
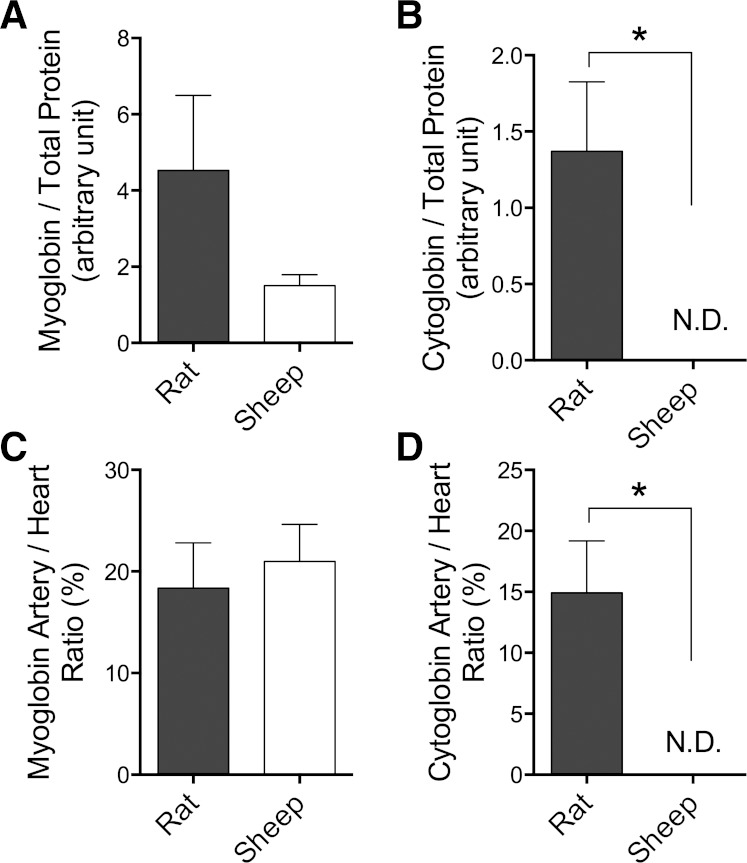
Expression of myoglobin and cytoglobin in rat descending aorta and sheep femoral artery from each species. Myoglobin levels in rat arteries did not differ significantly from those of the sheep when normalized to either total protein (A) or myoglobin levels in homogenized rat or sheep myocardium (C). In contrast, rat arteries contained significantly greater levels of cytoglobin than sheep arteries when normalized to either total protein (B) or myocardium (D), since cytoglobin levels in sheep arteries were not detectable (ND). *P < 0.05, t-test; n = 3 for each tissue type.
Western Blotting
Western blot analysis (Fig. 8) demonstrated that although arteries had less myoglobin than the myocardium in both sheep and rats, the difference between rat and sheep arteries was not statistically significant. In contrast, although cytoglobin was detected in rat arteries and in the hearts of both rats and sheep, it was undetectable in the sheep arteries. These results indicate higher concentrations of cytoglobin exist in rat arteries compared with sheep.
Fig. 8.
Vasodilatory dose-response curves of nitrite on vascular rings from rat aorta and a branch of the sheep femoral artery. A: endothelium-intact rings were preconstricted with 5-hydroxy-tryptamine (5-HT; 10 μM) in the presence of 100 μM l-NAME and then exposed to stepwise increases of nitrite in the bath solution (Krebs-Henseleit, pO2 6.2 ± 1.0 Torr). EC50 values are −4.0 ± 0.3 (sheep) and −5.2 ± 0.1 (rat), indicating ∼15-fold (P < 0.01) greater sensitivity of rat arteries to nitrite. B: in contrast, there are no significant differences between the dose response curves of sheep and rat arteries exposed to NO from the donor PROLI-NO. Relaxation is expressed as percent change from plateau tension after 5-HT-induced contraction. Data are corrected for spontaneous relaxation of rings from the same animal exposed to saline instead of nitrite. Results are for 5 animals for each group and are based on means of 3 nitrite, 3 NO, and 3 control vessel rings from each animal. Means are ± SE.
DISCUSSION
The results of this study show that, in contrast with previous studies in the human forearm but in agreement with our study on cephalic blood flow in fetal sheep (35), blood vessels in the hind limb of adult sheep do not vasodilate in response to increases in arterial nitrite concentration in the physiologic range. This observation holds true even when blood oxyhemoglobin saturations are within the optimal range for the reduction of nitrite to NO by deoxyhemoglobin. The experiments also find that the production of NO from the reaction of nitrite with deoxyhemoglobin is similar in whole blood from sheep and humans, suggesting that the lack of vasodilatory response to nitrite in sheep is not due to a species difference in this reaction. As such, the findings fail to support the hypothesis that physiological concentrations of nitrite are reduced to vasoactive NO by the deoxyhemoglobin-reductase reaction. Also, the capacity of vascular homogenates from sheep arteries to generate NO from nitrite is distinctly smaller than that of rat aorta homogenates, which may be related to the lack of cytoglobin in sheep arteries. Finally, the current experiments demonstrate that isolated sheep arteries are ∼10-fold less sensitive to the direct vasodilatory effects of nitrite than rat arteries. Taken together, these results support the idea that the primary mechanism by which nitrite produces vasodilation is through its effects on vascular smooth muscle and that this mechanism is species dependent.
Our study is the first to directly measure limb blood flow in experimental animals under the influence of intra-arterial sodium nitrite application. The ultrasound transit-time flow probes that were used to measure flow rates in both femoral arteries of the sheep enable continuous measurement of blood flow with a high level of accuracy and stability. Thus the quality of flow and pressure measurement exceeds the noninvasive plethysmographic technique used in previous human forearm experiments. The simultaneous intravascular measurement of pressure and flow enables the calculation of vascular conductance, a necessity if changes in vascular tone are to be assessed. It also enables comparison of changes in the infused and noninfused leg that avoids systemic effects obscuring local changes in vascular resistance.
Nitrite infusion experiments.
Increasing the nitrite concentration in one femoral artery to about 10 μM did not significantly increase flow or conductance in that limb or change the flow ratio between the ipsilateral and contralateral side. These findings strongly contradict the notion of a local vasodilating effect of intravascular nitrite in the sheep hind limb at concentrations that are considered to be at the higher end of the physiological range. In contrast with our results, Dejam et al. (13) reported a 10% increase of forearm blood flow rate when the nitrite concentration was raised by 0.35 μM and an increase of 50% (from 2.2 to 3.3 ml·min−1·100 g−1) with an increase of nitrite concentration to about 4.7 μM. Cosby et al. (9) described a forearm flow increase of 22% at nitrite concentrations of 2.5 μM and by 170% at about 200 μM. Changes of these magnitudes would have been easily detectable with the transit-time flow probes used in our experiments. However, although measurement of nitrite in the ipsilateral femoral vein confirmed that the femoral blood nitrite concentration had increased to about 8 μM during infusion, we never observed significant increases in flow or conductance. Instead, nitrite concentrations more than 10-fold above the physiological range were required to elicit significant vasodilation in sheep.
One proposed mechanism for the vasodilatory effects of nitrite is based on the conversion of nitrite to NO by reaction with deoxyhemoglobin (16). This reaction is under allosteric control, such that unliganded hemes in R-state (e.g., partially oxygenated) hemoglobin reduce nitrite to NO at a rate ∼60-fold faster than the hemes of T-state hemoglobin (18, 19). As a result, the maximal rate of nitrite reduction to NO occurs when oxyhemoglobin saturations are in the range of 50% to 70%, when a majority of the hemoglobin tetramers are in the R-state, but ample deoxygenated hemes are present to react with nitrite. However, in the present experiments, lowering arterial oxyhemoglobin saturations via systemic hypoxia to optimize the reduction of nitrite to NO failed to potentiate the vasodilatory effects of intra-arterial nitrite. In contrast, Maher et al. (27) observed that raising the nitrite concentration in the brachial artery of one forearm to about 5 μM did not increase the flow ratio between the ipsilateral and contralateral forearms above 1.0. When hypoxia was induced, however, the ratio increased significantly to ∼1.5. The conflicting results of our study suggest a markedly different response to nitrite in the sheep animal model.
Effects of anesthesia, l-NAME, and hexamethonium.
A major difference between previous human studies and the present study is the use of l-NAME and hexamethonium and the anesthesia required for the sheep. We have previously observed a lack of vasodilatory response to nitrite in chronically instrumented sheep fetuses that were not anesthetized (35). Although this indicates the lack of response to nitrite in the present study may be specific to sheep than due to anesthesia, studies in unanesthetized adult sheep have not been conducted to rule out this possibility.
After the start of hexamethonium infusion, femoral flows and conductances increased significantly indicating there had been strong sympathetic influences before exposure to hexamethonium. Hexamethonium was given to decrease the possibility that the local vasodilatory effects of nitrite would be masked by sympathetic input, and to reduce the brief but pronounced fluctuations of arterial pressure and flow that were observed in the baseline period. Because hind limb resistance vessels did not relax when hexamethonium was omitted from the protocol, its use seems unlikely to explain the lack of vasodilatory effect of nitrite. Also, hexamethonium did not dilate the vasculature to a maximum, as evidenced by the strong vasodilatory response to intra-arterial infusion of NO, and thus vessels retained a potential for further relaxation during nitrite infusions. Finally, hexamethonium was not used in the previous fetal sheep studies where a lack of effect of nitrite was also observed (35).
Although NO synthase has been proposed to catalyze the reduction of nitrite to NO under anoxic conditions (36), it is not likely that such a low level of oxygen tension was reached in our experiments even during the hypoxia phases. Furthermore, in the previous human forearm studies the vasodilatory effects of nitrite were not blocked by l-NAME (9), and thus NOS inhibition is not likely to explain the lack of response to nitrite in the current experiments.
Effect of NO and nitrite boluses.
Boluses of NO dissolved in saline were infused to show femoral vessels could vasodilate. With each infusion dissolved NO increased flows and conductances in the leg receiving the bolus, the peak values averaging twice as high as the preceding baseline values, whereas the contralateral side flows and conductances were unchanged. Therefore, the mechanism(s) that mediate the vasodilating effect of NO remained intact in the sympathetically blocked sheep and despite blockade of endogenous NO synthesis with l-NAME.
When sodium nitrite in concentrations of 50 mM was injected as a bolus, a vasodilating response was clearly observed. On a dose-response basis, this nitrite-induced vasodilation was less than that observed with dissolved NO (Fig. 3). With the assumption that homogeneous mixture of nitrite into blood during bolus injections, an arterial concentration of 1 to 5 mM may be estimated, several orders of magnitude higher than physiological values. At these high concentrations the hemoglobin-reductase mechanism may have produced sufficient free NO to reach the vascular smooth muscle cells. It would seem more likely, however, that nitrite exerted direct effects on vascular smooth muscle following its diffusion through the endothelium. The wire myography experiments described here support this possibility by demonstrating nitrite can relax arterial rings in tissue baths directly. The results of bolus injections confirm that intravascular nitrite does have vasodilating effects in sheep, but only at concentrations that exclude any participation in the physiological regulation of blood flow.
Free NO derived from nitrite in human and sheep blood cell suspensions.
We have demonstrated previously that the reduction of nitrite to NO by deoxyhemoglobin occurs at similar rates in human and sheep blood (6). Furthermore, the allosteric influence of hemoglobin conformation on the rate of this reaction is also similar in sheep and human hemoglobin (6). Thus the unexpected lack of vasodilation observed in the present studies is not likely to be due to species differences in the reaction between nitrite and deoxyhemoglobin. However, because the rate at which NO is scavenged by hemoglobin is many orders of magnitude greater than the rate at which it is produced from the deoxyhemoglobin-nitrite reductase reaction, the production of vasoactive NO by this reaction would likely be highly dependent on a putative concerted export of NO from the red blood cell to avoid hemoglobin scavenging. The present experiments find no difference in the release rate for sheep and human red blood cells (Figs. 4 and 5). This is suggested by the current experiments with the membrane gas exchanger. The exchanger provided an NO-permeable liquid-gas interface without foaming that is capable of detecting less than 10 nM NO in the liquid phase. Introduction of nitrite into diluted human or sheep blood entering the exchanger did not elicit any measurable NO signal even when blood nitrite concentrations were elevated to 100 μM, more than 10-fold above the physiological range. This result suggests that the relative lack of vasodilation in sheep compared with humans cannot be attributed to a different rate of release of NO from human and sheep blood cells. There remains the possibility that the reduction of nitrite by deoxyhemoglobin results in a vasodilatory NO-adduct that was not detected by the gas analysis in the current study, such as dinitrogen trioxide (N2O3) (4) or nitrosothiols (3). Further experiments comparing sheep and human blood are needed to rule out this possibility.
Responses of isolated rat aorta and sheep femoral artery to nitrite.
Preconstricted vessel rings from rat aorta reduced tension with ∼15-fold higher sensitivity to nitrite than those from branches of sheep femoral arteries (Fig. 8A). However, responses to PROLI-NO, a donor of free NO, were similar, indicating there was no difference in the sensitivity of these vessels to NO itself. Our observations of greater NO production from nitrite in homogenates of rat aorta compared with sheep femoral artery (Fig. 6) are also consistent with the idea that conversion of nitrite to NO is attenuated in sheep arteries. If a similar species difference exists between humans and sheep, it would explain the lack of vasodilation during nitrite infusions in the current experiments compared with previous human forearm studies. This notion has important consequences since it suggests the vasodilatory effects of nitrite are due to its direct effects on vessel walls as opposed to reactions occurring in blood.
NO generation from tissue homogenates.
Consistent with the idea that nitrite can be converted to NO within the vascular smooth muscle, the addition of artery homogenates to deoxygenated buffer containing 100 μM nitrite resulted in NO production. This production was significantly greater with rat artery homogenates compared with sheep, suggesting sheep arteries may have decreased capacity for reduction of nitrite to NO. The NO production from both rat and sheep homogenates was attenuated by ∼50% in the presence of the heme-oxidizer ODQ, implicating a role for heme-based nitrite reduction. This finding is consistent with recent evidence that myoglobin (34), cytoglobin (2, 23), and cytochrome c (23) all play a role in nitrite reduction within vascular smooth muscle. In contrast, the role of xanthine oxidase and aldehyde dehydrogenase was minimal since neither oxypurinol nor diadzin had significant effects on NO production. The lack of a role for xanthine oxidase is consistent with previous reports that oxypurinol does not attenuate nitrite-mediated dilation of isolated hypoxic rat aorta (11), but conflicts with more recent evidence that xanthine oxidase inhibition decreases nitrite-mediated cGMP production by ∼33% in cultured human vascular smooth muscle cells (23).
Given recent reports that both myoglobin (34) and cytoglobin (23) in vascular smooth muscle cells play a role in the vasodilatory effects of nitrite, we compared the concentrations of these two proteins in the sheep and rat arteries of interest in this study. Although levels of myoglobin were similar between the two species, we were unable to detect cytoglobin in the sheep arteries. These results raise the possibility that the lack of vasodilatory response of sheep arteries to nitrite is due to a deficiency of cytoglobin-mediated nitrite reduction.
Conclusions.
Although unilateral increases of forearm blood flow following infusion of sodium nitrite into one brachial artery suggest a role for nitrite as a local vasodilating agent of physiological importance in humans, this phenomenon is not observed in sheep. Our current and previous (5) experiments indicate that NO is produced from the reaction of nitrite and deoxyhemoglobin at similar rates in human and sheep blood, and we conclude that this reaction does not underlie the differential effect of nitrite on human and sheep vascular resistance.
An alternative and perhaps more likely possibility is that a species difference exists in the direct effects of nitrite on vascular smooth muscle. In this instance nitrite would react with heme-containing proteins, such as cytoglobin (2, 24) and myoglobin (29, 34), expressed directly within the vascular smooth muscle. Whether differences between smooth muscle of resistance vessels in this respect account also for the difference between in vivo responses to nitrite in humans and sheep is, however, speculative at present.
GRANTS
This work was supported by the NIH National Heart, Lung, and Blood Institute Grant HL-095973 (to A. B. Blood) and the National Institute of General Medical Sciences Grant 2 R25 GM060507.
DISCLOSURES
G. G. Power is listed as a co-inventor on a NIH patent for the use of inhaled nitrite for cardiovascular conditions. The remaining authors report no conflicts.
AUTHOR CONTRIBUTIONS
Author contributions: T.L., H.J.S., S.M.W., G.G.P., and A.B.B. conception and design of research; T.L., H.J.S., L.B., S.L.B., M.H.T., and A.B.B. performed experiments; T.L., H.J.S., L.B., S.L.B., and A.B.B. analyzed data; T.L., H.J.S., S.M.W., G.G.P., and A.B.B. interpreted results of experiments; T.L., H.J.S., L.B., S.L.B., and A.B.B. prepared figures; T.L. and H.J.S. drafted manuscript; T.L., H.J.S., M.H.T., S.M.W., G.G.P., and A.B.B. edited and revised manuscript; T.L., H.J.S., L.B., S.L.B., M.H.T., S.M.W., G.G.P., and A.B.B. approved final version of manuscript.
REFERENCES
- 1.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172: 250–254, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzawahra WF, Talukder MA, Liu X, Samouilov A, Zweier JL. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol 295: H499–H508, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA 103: 8366–8371, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol 3: 785–794, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. Am J Physiol Heart Circ Physiol 293: H1508–H1517, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol 296: H237–H246, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks J. The action of nitrite on haemoglobin in the absence of oxygen. Proc R Soc Lond B Biol Sci 123: 368–382, 1937. [Google Scholar]
- 8.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1: 290–297, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol 292: H3072–H3078, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood 106: 734–739, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116: 1821–1831, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res 75: 327–338, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, Beall CM. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA 104: 17593–17598, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol 291: H2026–H2035, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hall CN, Garthwaite J. What is the real physiological NO concentration? Nitric Oxide 21: 92–103, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem 280: 31126–31131, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 115: 2099–2107, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol 110: 1582–1591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35: 790–796, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Hemann C, Abdelghany TM, El-Mahdy MA, Zweier JL. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem 287: 36623–36633, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Tong J, Zweier JR, Follmer D, Hemann C, Ismail RS, Zweier JL. Differences in oxygen-dependent nitric oxide metabolism by cytoglobin and myoglobin account for their differing functional roles. FEBS J 280: 3621–3631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun 396: 39–45, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Mack AK, McGowan Ii VR, Tremonti CK, Ackah D, Barnett C, Machado RF, Gladwin MT, Kato GJ. Sodium nitrite promotes regional blood flow in patients with sickle cell disease: a phase I/II study. Br J Haematol 142: 971–978, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 117: 670–677, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sports Exerc 46: 143–150, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Ormerod JO, Ashrafian H, Maher AR, Arif S, Steeples V, Born GV, Egginton S, Feelisch M, Watkins H, Frenneaux MP. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc Res 89: 560–565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med 41: 541–548, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Rifkind JM, Nagababu E, Barbiro-Michaely E, Ramasamy S, Pluta RM, Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: a role for red cell NO. Nitric Oxide 16: 448–456, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez J, Maloney RE, Rassaf T, Bryan NS, Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc Natl Acad Sci USA 100: 336–341, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 126: 325–334, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong GT, Schroder HJ, Liu T, Zhang M, Kanda E, Bragg S, Power GG, Blood AB. Role of nitrite in regulation of fetal cephalic circulation in sheep. J Physiol 592: 1785–1794, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci 64: 96–103, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci USA 101: 11477–11482, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol 115: 325–336, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta 1411: 250–262, 1999. [DOI] [PubMed] [Google Scholar]