Summary
Background
Unnecessary hospital readmissions are costly for the U.S. health care system. An automated algorithm was developed to target this problem and proven to predict elderly patients at greater risk of rehospitalization based on their medication regimens.
Objective
Improve the algorithm for predicting elderly patients’ risks for readmission by optimizing the sensitivity of its medication criteria.
Methods
Outcome and Assessment Information Set (OASIS) and medication data were reused from a study that defined and tested an algorithm for assessing rehospitalization risks of 911 patients from 15 Medicare-certified home health care agencies. Odds Ratio analyses, literature reviews and clinical judgments were used to adjust the scoring of patients’ High Risk Medication Regimens (HRMRs). Receiver Operating Characteristic (ROC) analysis evaluated whether these adjustments improved the predictive strength of the algorithm’s components.
Results
HRMR scores are composed of polypharmacy (number of drugs), potentially inappropriate medications (PIM) (drugs risky to the elderly), and Medication Regimen Complexity Index (MRCI) (complex dose forms, dose frequency, instructions or administration). Strongest ROC results for the HRMR components were Areas Under the Curve (AUC) of .68 for polypharmacy when excluding supplements; and .60 for PIM and .69 for MRCI using the original HRMR criteria. The “cut point” identifying MRCI scores as indicative of medication-related readmission risk was increased from 20 to 33.
Conclusion
The automated algorithm can predict elderly patients at risk of hospital readmissions and its underlying criteria is improved by a modification to its polypharmacy definition and MRCI cut point.
Keywords: Patient readmission, polypharmacy, medication adherence, home care agencies, ROC curve
1. Introduction
Medications can both enhance health and cause adverse events, particularly for older adults, whose prescription regimens increase with age and chronic health problems [1]. Nine in ten older adults take at least one prescription medication and most take more than five [2]. The combination of health conditions and chemical ingredients in medications can increase older adults’ risk of adverse events and need for emergency medical care [3]. Avoidable readmissions to hospitals have been linked to problems with medication usage [4–6], but efforts to identify and predict which patients suffer this adverse event have been mixed. Studies have explored a connection between readmissions and the numbers of drugs patients take (polypharmacy) [7, 8], their use of potentially inappropriate medications (PIM) [9–11], and the complexities of the doses or forms of their medications (Medication Complexity Index [MRCI]) [6, 12]. Mary Dierich theorized that limitations of these individual medication measurements might be addressed by constructing them into a combined measurement, the High Risk Medication Regimen (HRMR). In an initial study of 911 elderly home health care patients, HRMRs accounted for 10 percent of the variance in hospital readmissions, making them more predictive than comorbidity [13].
The potential utility of HRMR as a clinical decision support tool to prevent avoidable readmissions – which can now result in federal Medicare penalties if hospitals report too many of them [14] – was tempered by the labor-intensive process in the original study for calculating the scores. Further research subsequently developed an automated tool that maps medication data to RxNorm coding standards and created an algorithm with the coded medication data to calculate patients’ HRMR scores [15]. The standardized format of the coded data addressed some of the practical challenges of using HRMR for clinical decision support, and also made the algorithm potentially useable across different electronic health record (EHR) systems and health care organizations. Automating the calculation also allowed for more rapid testing of the criteria underlying this new combined measurement and the “cut points,” which were manually selected based on the researchers’ clinical expertise and literature review, that distinguish patients at high and low risk of rehospitalization. This study sought to take advantage of that advancement by testing adjustments to the HRMR criteria and to the cut points to determine the optimal calculation for predicting medication-related rehospitalizations of elderly home health care patients.
1.1 Objectives
The objective of this study was to improve the automated algorithm for predicting hospital readmissions by optimizing the underlying criteria within the algorithm and determining the optimal cut points for HRMR scores. Optimizing the algorithm’s criteria is a key next step in advancing the HRMR concept toward clinical utility.
2. Methods
2.1 Data Set
This study used Outcome and Assessment Information Set (OASIS) and medication records for 911 adults from 15 Medicare-certified home health care agencies that were used in previous studies [13, 15]. The medication records included both prescription and over-the-counter medications taken by patients in their homes and recorded by home care clinicians in their EHRs. Medication data included the medication names, doses, dose forms, frequencies and special instructions. OASIS data for the patients, all of whom were at least 65 and were admitted from the hospital to the home health care agencies in 2004, included demographic, environmental, support system, health and functional status, and health service utilization information [16].
2.2 Data Analysis
Dierich operationalized the medication data by first calculating polypharmacy, PIM and MRCI scores based on patients’ drug regimens, and then using summative factor analysis to construct those weighted scores into a combined HRMR measurement [13]. The original HRMR research defined polypharmacy as nine or more medications. Scores of “0” were assigned for patients with fewer than 9 medications, and “1” for patients with 9 or more medications.
Scores for PIM were based on the 2003 version of the Beers’ criteria, a list of 48 drugs and 20 drug classes that the elderly should avoid. In defining the Beers’ criteria, Fick et al. [17] differentiated drugs by whether or not they posed risks of severe adverse outcomes, and whether they were inappropriate for older adults regardless of diagnosis (PIM schedule 1) or inappropriate depending on the diagnosis (PIM schedule 2). The initial HRMR research assigned weighted scores of 2.5 to medications that were always inappropriate and carried the greatest risks, 2 for medications with lower risks of severe outcomes, 1.5 for medications with the highest risks for certain diagnoses, and 1.0 for medications with lower risks for certain diagnoses. (Drugs that met multiple criteria received the higher score.) The medication scores were then summed to provide a total PIM risk level score for each patient.
The original HRMR research used a modified version of the Medication Regimen Complexity Index developed by George et al. [18] that weighted drugs by three subscales – by the complexity of their route (MRCI Schedule A), their dosing frequency (MRCI Schedule B), and the complexity of their directions or preparation (MRCI Schedule C) – and then combined the subscale scores into a summary score (▶ Figure 4). A summary score cut point of 20 or above was set in the original HRMR research as an indication of high medication regimen complexity, though it was an “arbitrary” distinction due to the lack of prior research [13].
Fig. 4.
Medication Regimen Complexity Index A) The Medication Regimen Complexity Index, Section A (George et al., 2004, p. 1374), used with permission. B) The Medication Regimen Complexity Index, Sections B and C (George et al., 2004, p. 1375), used with permission.
This method of assigning weighted scores to predictive variables is similar to what was used in the development of the Charlson index of comorbidity for predicting mortality risks [19], and another recent analysis that identified factors for predicting early and preventable rehospitalizations after kidney transplants [20].
2.2.1 ROC Analysis
Receiver Operating Characteristic (ROC) curves were used in this study to evaluate optimization of the algorithm and determine optimal cut points for the HRMR components (Polypharmacy, PIM, and MRCI) associated with rehospitalization. The ability to identify cut points is considered an advantage of ROC analysis [21]. The area under the ROC curves (AUC) can be interpreted in this study as the probability of correctly predicting rehospitalization, based on sensitivity and specificity. The closer the AUC is to 1, the better the measure. An AUC resultabove 0.7 is considered meaningful by one generic value scale [22], but studies have characterized results between 0.6 and 0.7 as “moderate” or “good”[23–27]. ROC curves are frequently used to assess the value of predictive measures, and have been used to optimize the analysis of patients who had poor outcomes after hospitalization for inflammatory pelvic disease [28], and to create a prognostic index of patient mortality after intensive care [29].
In using the ROC results to select cut points for the HRMR components, the authors reviewed common mathematical approaches such as the Youden index [30] but opted on a customized approach in an attempt to account for the prevalence of hospital readmissions and also the expense of testing overall and of false positive results. The authors had to fundamentally decide whether to err in the selection of cut points on the side of sensitivity (the ability of a test to correctly identify people with a medical condition) or on specificity (the ability to rule out people who don’t have a particular disease or medical problem). The dilemma has been described, respectively, as whether a test should “rule in” patients for further consideration of a medical issue, or “rule out” their risks [31]. A “rule in” approach was adopted here, with the presumption that clinicians would use an HRMR screening to evaluate patients at risk and then conduct further clinical assessments of their needs. This favored cut points weighing more heavily on sensitivity, at the expense of specificity and a higher rate of false positive results. An initial target of 0.75 for sensitivity and 0.50 for specificity was chosen for the revision of cut points for the HRMR components.
2.2.2 Odds Ratio
Odds ratio (OR) computations were used to test the strength of the relationship between HRMR and rehospitalization risks and compare the original scoring criteria with newly derived HRMR scoring criteria using ORs. Odds ratios indicated whether the relative odds of the occurrence of rehospitalization were different for each of the independent variables that make up PIM (disease and medication class, and medications) and MRCI (dose form, instructions, and frequency). The intent was for the relative odds of the independent variables to be applied to the HRMR algorithm to see if they generated better AUC curve results and more optimal cut points for predicting rehospitalization rather than the original scoring criteria.
2.3 Data Transformations
Adjustments to the original HRMR scoring criteria were made based on clinical observations and expertise of the authors – a doctorally prepared informatician, a geriatric nurse practitioner, a nurse researcher with expertise in geriatrics and home health care data, and a physician who is also a clinical pharmacist. These transformations were attempted to optimize the criteria of the algorithm and the HRMR cut points, and the methodologies behind them are described below.
2.3.1 Polypharmacy
PRN medications (taken as needed), over-the-counter medications, and medications with limited dosing time such as antibiotics were included in the original HRMR research, while other more benign items such as oxygen or saline to dilute IV medications were excluded. Combination and variable dosed drugs were counted as one drug.
Based on clinical judgment and polypharmacy criteria in other recent publications [32, 33], this study modified the polypharmacy scoring for HRMR calculations by excluding acetaminophen, vitamins, supplements, and PRN medications from the medication count. ROC curves were used to compare the predictive strength of the original HRMR scoring with these modified scores.
2.3.2 Potentially Inappropriate Medications
This analysis modified the PIM scoring criteria, based on clinical observation and a review of recent publications regarding adverse drug events related to certain drug classes.Two additional higher-risk categories were created for selected drugs in PIM schedule 1 (those always inappropriate regardless of diagnosis) and assigning them greater scoring weights (▶ Table 1).
Table 1.
Potentially Inappropriate Medications: Independent of Diagnoses or Conditions, from Fick et al. (2003, p. 2719), used with permission.
| Drug/Drug Combinations with the Active Ingredient | Risk | |
|---|---|---|
| Low | High | |
| Propoxyphene (Darvon) | X | |
| Indomethacin (Indocin) | X | |
| Pentazocine (Talwin) | X | |
| **Muscle relaxants and antispasmodics: methocarbamol (Robaxin), carisoprodol (Soma), chlorzoxazone (Paraflex), metaxalone (Skelaxin), cyclobenzaprine (Flexeril), and oxybutynin (Ditropan). Do not consider the extended-release Ditropan XL. | X | |
| **Flurazepam (Dalmane) | X | |
| **Amitriptyline (Elavil), chlordiazepoxide-amitriptyline (Limbitrol), and perphenazine-amitriptyline (Triavil) | X | |
| Doxepin (Sinequan) | X | |
| Meprobamate (Miltown and Equanil) | X | |
| Doses of short-acting benzodiazepines: doses greater than lorazepam (Ativan), 3 mg; oxazepam (Serax), 60 mg; alprazolam (Xanax), 2 mg; temazepam (Restoril), 15 mg; and triazolam (Halcion), 0.25 mg | X | |
| **Long-acting benzodiazepines: chlordiazepoxide (Librium), chlordiazepoxide-amitriptyline (Limbitrol), clidinium-chlordiazepoxide (Librax), diazepam (Valium), quazepam (Doral), halazepam (Paxipam), and chlorazepate (Tranxene) | X | |
| Disopyramide (Norpace and Norpace CR) | X | |
| *Digoxin (Lanoxin) (should not exceed _0.125 mg/d except when treating atrial arrhythmias) | X | |
| Short-acting dipyridamole (Persantine). Do not consider the long-acting dipyridamole (which has better properties than the short-acting in older adults) except with patients with artificial heart valves | X | |
| Methyldopa (Aldomet) and methyldopa-hydrochlorothiazide (Aldoril) | X | |
| Reserpine at doses > 0.25 mg | X | |
| Chlorpropamide (Diabinese) | X | |
| *Gastrointestinal antispasmodic drugs: dicyclomine (Bentyl), hyoscyamine (Levsin and Levsinex), propantheline (Pro-Banthine), belladonna alkaloids (Donnatal and others), and clidiniumchlordiazepoxide (Librax) | X | |
| **Anticholinergics and antihistamines: chlorpheniramine (Chlor-Trimeton), diphenhydramine (Benadryl), hydroxyzine (Vistaril and Atarax), cyproheptadine (Periactin), promethazine (Phenergan), tripelennamine, dexchlorpheniramine (Polaramine) | X | |
| **Diphenhydramine (Benadryl) | X | |
| Ergot mesyloids (Hydergine) and cyclandelate (Cyclospasmol) | X | |
| Ferrous sulfate >325 mg/d | X | |
| **All barbiturates (except phenobarbital) except when used to control seizures | X | |
| **Meperidine (Demerol) | X | |
| Ticlopidine (Ticlid) | X | |
| **Ketorolac (Toradol) | X | |
| **Amphetamines and anorexic agents | X | |
| Long-term use of full-dosage, longer half-life, non–COX-selective NSAIDs: naproxen (Naprosyn, Avaprox, and Aleve), oxaprozin (Daypro), and piroxicam (Feldene) | X | |
| Daily fluoxetine (Prozac) | X | |
| Long-term use of stimulant laxatives: bisacodyl (Dulcolax), cascara sagrada, and Neoloid except in the presence of opiate analgesic use | X | |
| *Amiodarone (Cordarone) | X | |
| Orphenadrine (Norflex) | X | |
| Guanethidine (Ismelin) | X | |
| Guanadrel (Hylorel) | X | |
| Cyclandelate (Cyclospasmol) | X | |
| Isoxsurpine (Vasodilan) | X | |
| *Nitrofurantoin (Macrodantin) | X | |
| *Doxazosin (Cardura) | X | |
| Methyltestosterone (Android, Virilon, and Testrad) | X | |
| *Thioridazine (Mellaril) | X | |
| Mesoridazine (Serentil) | X | |
| Short acting nifedipine (Procardia and Adalat) | X | |
| Clonidine (Catapres) | X | |
| Mineral oil | X | |
| Cimetidine (Tagamet) | X | |
| Ethacrynic acid (Edecrin) | X | |
| Desiccated thyroid | X | |
| Amphetamines (excluding methylphenidate hydrochloride and anorexics) | X | |
| Estrogens only (oral) | X | |
New Scoring: **Highest (10)
*Medium (5) Note: Remaining PIM Table 1 drugs retained their assigned weights (2.5 and 2).
Highest (assigned weight of 10) included antispasmodics and long-acting benzodiazepines due to adverse central nervous system effects and dementia and increased sensitivity with age. Antispasmodics also have uncertain effectiveness and are highly anticholinergic while the benzodiazepines present an elevated risk of falls [34].
Medium (assigned weight of 5) included digoxin due to potential toxic effects and nitrofurantoin and thioridazine due to known risks and the availability of safer alternatives for the treatments, respectively of infections and psychosis.
Remaining PIM schedule 1 drugs retained their assigned weights (2.5 and 2) from the original analysis as did schedule 2 drugs (1.5 and 1).
Odds ratio analysis also was applied to PIM schedules 1 and 2 using the independent variables of high-risk medications and medications with disease-specific risks in the elderly. The intent of this analysis was to apply the relative odds of rehospitalization for each of the independent variables to the algorithm to determine if they were stronger than the weighted scores in the original HRMR research.
ROC analysis then was used to see if either of the modified PIM scoring criteria – one derived from clinical judgment and literature review, the other from the OR analysis – were better at identifying patients needing rehospitalization than the original scoring criteria.
2.3.3 Medication Regimen Complexity Index (MRCI)
ROC analysis then compared the predictive strength of MRCI in identifying patients who will be rehospitalized against modified criteria, including MRCI schedules A, B and C individually; and schedules A and C together only. The latter was done to address a theory that schedule B (dosing frequency) might be redundant with polypharmacy.
In addition, odds ratio analyses were applied to schedules A, B, and C using independent variables of dose form, frequency and special dosing instructions to understand the relative odds of rehospitalization. The intent of this analysis was to apply the relative odds of rehospitalization for each of the independent variables to the algorithm instead of George’s original weighted scores. ROC analysis was again used to test the independent variables and whether they optimized the algorithm.
3. Results
▶ Table 2 summarizes results of the ROC analyses.
Table 2.
Summary Results – ROC Analysis
| Polypharmacy | AUC |
|---|---|
| Original Dierich – Manual & Automated | 0.66 |
| PRN Medications Only | 0.65 |
| All Medications except PRN | 0.64 |
| All Medications except acetaminophen | 0.66 |
| All Medications except vitamins and supplements | 0.68 |
| PIM | AUC |
|---|---|
| Original PIM Manual | 0.6 |
| Original PIM Automated | 0.59 |
| Clinical Expertise – Modified 4 Scale | 0.59 |
| MRCI | AUC |
|---|---|
| Original Dierich | 0.69 |
| Table A&C Only | 0.69 |
| Table A | 0.68 |
| Table B | 0.68 |
| Table C | 0.69 |
| Odds Ratio | 0.69 |
3.1 Polypharmacy
Removing vitamins and supplements from the medication counts improved the AUC slightly (0.66 vs. 0.68) (▶ Figure 1). Removing PRN medications did not improve the AUC (0.66) and removing acetaminophen caused the AUC to decrease (0.64). Using the criteria that produced an AUC of 0.68 (the analysis in which vitamins and supplements were removed), the optimal cut point remained 9. This was based on a true positive rate of 0.77 and a false positive rate of 0.53.
Fig. 1.
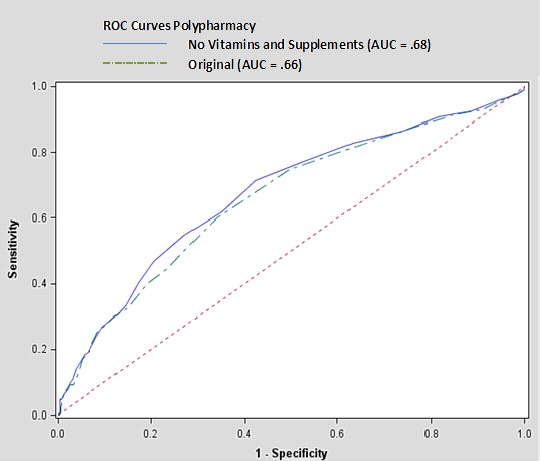
ROC Curves for Polypharmacy
3.2. Potentially Inappropriate medications (PIM)
The original automated PIM algorithm produced an AUC curve of 0.6 (▶ Figure 2). When weights based on clinical observation were applied to the algorithm, there was no improvement to the original HRMR weights, producing a curve of 0.59.
Fig. 2.
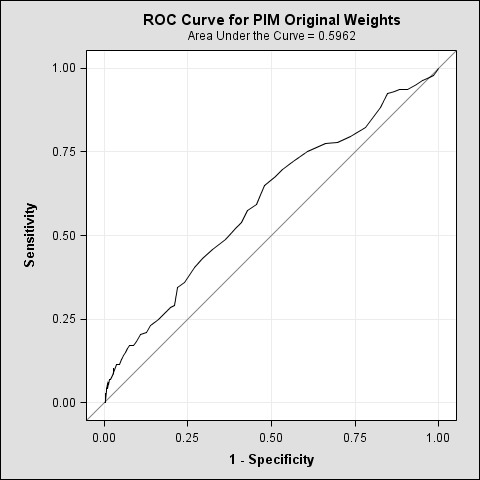
ROC Curve for PIM
When the odds ratio analysis was applied to each independent variable (risky medications) in PIM schedule 1 (▶ Table 3) and each independent variable (risky medications considering diagnosis) in PIM schedule 2 (▶ Table 4), the resulting models produced confidence intervals which contained one for each independent variable, meaning the model was not valid.
Table 3.
PIM Table 1– Sample Odds Ratio Analysis Results: Odds Ratio Estimates
| Effect | Point Estimate | 95% Wald | |
|---|---|---|---|
| Confidence Limits | |||
| alprazolam | 0.90 | 0.29 | 2.75 |
| amitriptyline | 0.51 | 0.18 | 1.47 |
| bisacodyl | 1.42 | 0.81 | 2.49 |
Table 4.
PIM Table 2– Sample Odds Ratio Analysis Results: Odds Ratio Estimates
| Effect | Point Estimate | 95% Wald | |
|---|---|---|---|
| Confidence Limits | |||
| Chronic Constipation and CCB | 0.91 | 0.68 | 1.22 |
| Clot Disorder and NSAID | 0.78 | 0.48 | 1.25 |
| Parkinson’s and Antipsychotics | 2.93 | 0.59 | 14.53 |
Therefore, there was no support of an independent PIM effect on the odds of the outcome (rehospitalization). As a result, adjusted weights based on odds ratio analysis were not applied to the algorithm to improve the AUC curve of 0.60.
3.3 Medication Complexity Index (MRCI)
MRCI schedules A, B, and C, when calculated separately, showed similar results (0.68, 0.68, 0.69) as when all MRCI schedules were calculated together (0.69). (▶ Figure 3) A cut point of 33, higher than the original 20, produced a true positive rate of 0.76 and a false positive rate of 0.49 – meeting the goal in the study for establishing HRMR as a rule-in test for readmission risks. When the odds ratio analysis was run on each component of schedule A, B, and C, the only schedule which produced a statistically valid model was C. Schedules A and B produced models in which each of the independent variables had confidence intervals which contained 1. Therefore, dose form and frequency were not supported to have an independent effect on the relative odds of the outcome (rehospitalization). Schedule C’s model produced valid confidence intervals for 7 of 10 independent variables. (▶ Table 5) The other three variables were removed from the model as their confidence intervals also were weak.
Fig. 3.
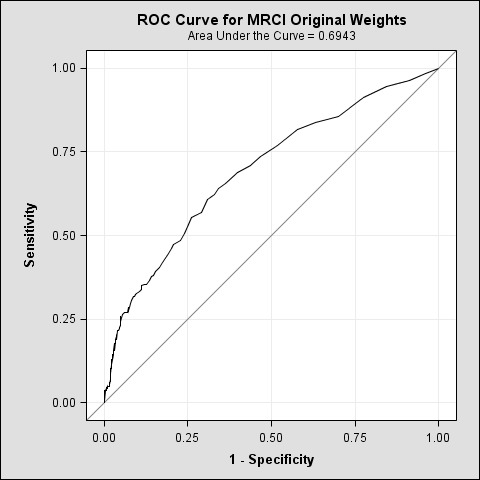
ROC Curve for MRCI
Table 5.
MRCI Odds ratio analysis for Table C: Odds Ratio Estimates
| Effect | Point Estimate | 95% Wald | |
|---|---|---|---|
| Confidence Limits | |||
| Variable dose | 1.34 | 1.09 | 1.65 |
| Take/use at specified time/s | 1.33 | 1.11 | 1.58 |
| Tapering/increasing dose | 2.52 | 2.27 | 2.79 |
| Alternating dose | 1.69 | 1.31 | 2.18 |
| Take/use as directed | 2.39 | 2.19 | 2.60 |
| Relation to food | 1.51 | 1.35 | 1.70 |
| Multiple units at one time | 1.85 | 1.56 | 2.20 |
| Dissolve tablet/powder** | 1.29 | 0.83 | 1.99 |
| Break or crush tablet** | 1.23 | 0.84 | 1.81 |
| Take with specific fluid** | 1.75 | 0.32 | 9.54 |
**Variables removed from model due to weak confidence intervals.
Rounding to the nearest whole number, each point estimate is identical to George’s original weights for the MRCI variables (▶ Figure 4). The only exception is the variable for “multiple units at one time”; the odds ratio analysis gave that a greater rounded weight (2 points) than George’s original analysis (1 point). After rerunning the ROC curve for MRCI with these modified weights, the AUC remained unchanged at 0.69. Using the actual results from the Odds Ratio analysis, instead of rounding to match George’s methodology, produced a slightly stronger 0.7 AUC result for schedule C’s influence on rehospitalization risks.
4. Discussion
This study determined optimal criteria for an algorithm using HRMR scores to predict elderly patients at risk for rehospitalization, and contributed to an acceleration of research in the area of medications and hospital readmissions. Two other studies both attributed hospital readmissions in the elderly to polypharmacy [7, 8] – though they used different criteria – while a third concluded that both polypharmacy and PIM are “under recognized causes of readmissions to the hospital”[9]. But while the components of HRMR draw increasing research interest, there has been little follow-up to the initial discovery that HRMR is uniquely associated with hospital readmission risks [13]. This could owe to the fact that HRMR and the MRCI component itself are relatively new to medical research. PubMed shows only 33 studies referring to MRCI, with one associating it with hospital read-missions in the elderly [6].
The ROC analysis supported that polypharmacy is a strong component of the HRMR model, and was slightly more predictive of rehospitalizations when vitamins and supplements were removed from patients’ drug counts. This exclusion mimics approaches used in other studies[32] and argues in favor of removing vitamins and supplements from future studies linking polypharmacy to rehospitalization and related outcomes. Supplements are not risk-free for seniors,[35] but they are widely taken for general health.[36] Removing them might have sharpened the algorithm’s ability to identify rehospitalizations by focusing on sicker patients whose high polypharmacy counts consisted of more prescription medications. The results were weakened by the removal of acetaminophen, which also is taken broadly by seniors for general pain relief,[36] but has documented risks such as drug-induced liver injury[37, 38] that could make it more relevant to this HRMR analysis.
An ancillary benefit of the study is its contribution to the global definition of polypharmacy. The original HRMR cut point for polypharmacy was 9 or more drugs, one that is commonly but not exclusively used in research, and further analysis showed a polypharmacy cut point of 9 optimized the algorithm and the prediction of patients at risk for rehospitalization. This could serve as a guide for future research.
Results for PIM schedules showed they were weaker components of the HRMR calculation in estimating patient rehospitalization risks. PIM in other studies has had a dependent relationship with polypharmacy, in that the more drugs elderly patients have, the more likely they are to have inappropriate prescriptions in their regimens [39, 40]. Attempts to strengthen PIM by revising cut points were unsuccessful in this study as the AUC curves produced were only slightly better than chance. While at least one study has associated PIM with readmissions [10], our findings agree with other studies that have found PIM alone to be predictive of other problems, such as inpatient falls, but not rehospitalization [41]. Despite its weak relationship to rehospitalizations on its own, PIM nonetheless appears an important component of the HRMR construct. Dierich’s original study found HRMR to be “more than the sum of its parts” and that PIM played a role in its predictive strength.The original MRCI scoring weights from George’s research also proved optimal, though adjustments based on an odds ratio analysis did modestly improve the predictive strength of schedule C (drugs with special instructions). ROC results for both HRMR components approached 0.7, which is a statistical threshold. This analysis also adjusted the cut point that distinguishes patients at greater risk of rehospitalization to 33 for MRCI (the original cut point in the HRMR calculation was 20). This is one of the first attempts in research literature at establishing such a cut point for the use of MRCI in predictive tests.
This study suggests a need for more targeted research on HRMR scores and whether they can predict adverse outcomes among the elderly in ways that other measures of medications and medication regimens cannot.
4.1 Limitations
Odds ratio and ROC analysis are common validation tools in medical research for the development of predictive tools and indexes, but they are ultimately dependent upon the criteria and information selected for analysis. Medical researchers have not arrived on a common definition for polypharmacy, with cut points often ranging from 2 to 9 [42], and have varied in their inclusion of over-the-counter medications. This study used a PubMed literature search and clinical judgments of its authors to decide which medications and medication classes to exclude from the weighted scoring of both polypharmacy and PIM in the calculations of HRMR scores. Due to the broad number of drug inclusion and exclusion combinations, it is possible that relevant adjustments to the weighted scores were not tested and identified in this research. For continuity with Dierich’s original HRMR research, it was necessary to use the original 2003 Beers criteria, though a significant update was produced in 2012 [34]. Although the two lists have “substantial agreement” [43], nineteen classes of drugs were removed in the latest update – in some cases because the drugs were removed from the U.S. market – while other common medications such as atypical antipsychotics were added. Further research using the updated criteria and its inclusion of antipsychotics and other medications could alter how PIM counts contribute to research involving HRMRs and to the strength of HRMRs in predicting readmission risks.
5. Conclusion
HRMR calculations are optimized by adjusting the underlying criteria of polypharmacy to exclude supplements and vitamins from the count of medications, and by increasing the MRCI cut point that distinguishes patients by their medication-related risks for hospital readmissions. While modest, the changes strengthen the case for an HRMR algorithm that clinicians can use to assess elderly patients’ risks for avoidable readmissions.Next steps include testing the automated HRMR algorithm with the prescription and OASIS data of different populations to see if can be optimized further.
Footnotes
Clinical Relevance Statement
This report is the next step in operationalizing for clinicians and researchers an automated algorithm which has proven to predict elderly home care patients at risk for unnecessary hospital read-missions.
Conflict of Interest
The authors report no conflicts of interest in the production of this paper.
Human Subjects Protections
This study was reviewed by a university Institutional Review Board and meets the human protections criteria set forth by the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects.
References
- 1.Hung WW, Ross JS, Boockvar KS, Siu AL.Recent trends in chronic disease, impairment and disability among older adults in the united states. BMC Geriatr. 2011; 11: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST.Use of prescription and over-the-counter medications and dietary supplements among older adults in the united states. JAMA. 2008; 300(24): 2867-2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adults and Older Adult Adverse Drug Event. U.S. Centers for Disease Control and Prevention 10/02/2012 cited 04/22/2014 [Google Scholar]
- 4.Freund T, Campbell SM, Geissler S, Kunz CU, Mahler C, Peters-Klimm F, Szecsenyi J.Strategies for reducing potentially avoidable hospitalizations for ambulatory care-sensitive conditions. Ann Fam Med. 2013; 11(4): 363-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kripalani S, Theobald CN, Anctil B, Vasilevskis EE.Reducing hospital readmission rates: Current strategies and future directions. Annu Rev Med. 2013; 65: 471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willson MN, Greer CL, Weeks DL.Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother 2014; 48(1): 26–32 [DOI] [PubMed] [Google Scholar]
- 7.Morandi A, Bellelli G, Vasilevskis EE, Turco R, Guerini F, Torpilliesi T, Speciale S, Emiliani V, Gentile S, Schnelle J, Trabucchi M.Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: The role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013; 14(10): 761-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sganga F, Landi F, Ruggiero C, Corsonello A, Vetrano DL, Lattanzio F, Cherubini A, Bernabei R, Onder G.Polypharmacy and health outcomes among older adults discharged from hospital: Results from the CRIME study. Geriatr Gerontol Int 2014January28 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal V, Bajwa SJ, Sehgal R, Bajaj A, Khaira U, Kresse V.Polypharmacy and potentially inappropriate medication use as the precipitating factor in readmissions to the hospital. J Family Med Prim Care. 2013; 2(2): 194-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price SD, Holman CD, Sanfilippo FM, Emery JD.Association between potentially inappropriate medications from the beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother 2014; 48(1): 6–16 [DOI] [PubMed] [Google Scholar]
- 11.Price SD, Holman CD, Sanfilippo FM, Emery JD.Impact of specific beers criteria medications on associations between drug exposure and unplanned hospitalisation in elderly patients taking high-risk drugs: A case-time-control study in western australia. Drugs Aging. 2014; 31(4): 311-325 [DOI] [PubMed] [Google Scholar]
- 12.Wimmer BC, Dent E, Bell JS, Wiese MD, Chapman I, Johnell K, Visvanathan R.Medication regimen complexity and unplanned hospital readmissions in older people. Ann Pharmacother 2014May27 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Dierich M.High risk medication regimens and medication related predictors of hospital readmission in elderly home care patients. [Doctor of Philosophy]. Minneapolis, MN: University of Minnesota; 2010 [Google Scholar]
- 14.Abelson R.Hospitals question medicare rules on readmissions. New York Times 2013 March 29, 2013 [Google Scholar]
- 15.Olson CH.Automation of a high risk medication regime algorithm in a home health care population. Journal of Biomedical Informatics 2014April13 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Data Set. Baltimore: Centers for Medicare and Medicaid Services [updated 2012 Aug 21; cited 2014 Aug 3]. Available from: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/OASIS/DataSet.html
- 17.Fick D, Cooper J, Wade W, Waller J, Maclean JR, Beers M.Updating the beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch Intern Med. 2003; 163(22): 2716-2724 [DOI] [PubMed] [Google Scholar]
- 18.George J, Phun YT, Bailey MJ, Kong D, Stewart K.Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004; 38(9): 1369-1376 [DOI] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J.Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47(11): 1245-1251 [DOI] [PubMed] [Google Scholar]
- 20.Harhay M, Lin E, Pai A, Harhay MO, Huverserian A, Mussell A, Abt P, Levine M, Bloom R, Shea JA, Troxel AB, Reese PP.Early rehospitalization after kidney transplantation: Assessing preventability and prognosis. Am J Transplant. 2013; 13(12): 3164-3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajian-Tilaki K.Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013; 4(2): 627-635 [PMC free article] [PubMed] [Google Scholar]
- 22.Tape TG.The Area Under an ROC Curve. Omaha: University of Nebraska Medical Center; [cited 2014 Aug 30] Available from: http://gim.unmc.edu/dxtests/roc3.htm [Google Scholar]
- 23.Heng EL, Bolger AP, Kempny A, Davlouros P, Davidson S, Gatzoulis MA, Babu-Narayan SV.46 serum BNP and clinical outcomes prediction in tetralogy of fallot: A prospective analysis. Heart 2014; 100(Suppl. 3): A25-A26 [Google Scholar]
- 24.Hiersch L, Yogev Y, Domniz N, Meizner I, Bardin R, Melamed N.The role of cervical length in women with threatened preterm labor – is it a valid predictor at any gestational age? Am J Obstet Gynecol 2014June4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Malik N, Banning A, Gershlick A.69 development and validation of a stent thrombosis risk scoring system. Heart 2014; 100(Suppl. 3): A39-A40 [Google Scholar]
- 26.Akyuz A, Alpsoy S, Akkoyun DC, Degirmenci H, Guler N.Heart rate recovery may predict the presence of coronary artery disease. Anadolu Kardiyol Derg 2014; 14(4): 351–356 [DOI] [PubMed] [Google Scholar]
- 27.Cheung MR.Optimization of predictors of ewing sarcoma cause-specific survival: A population study. Asian Pac J Cancer Prev. 2014; 15(10): 4143-4145 [DOI] [PubMed] [Google Scholar]
- 28.Terao M, Koga K, Fujimoto A, Wada-Hiraike O, Osuga Y, Yano T, Kozuma S.Factors that predict poor clinical course among patients hospitalized with pelvic inflammatory disease. J Obstet Gynaecol Res 2013; 40(2): 495–500 [DOI] [PubMed] [Google Scholar]
- 29.Cardoso LG, Chiavone PA.The APACHE II measured on patients’ discharge from the intensive care unit in the prediction of mortality. Rev Lat Am Enfermagem. 2013; 21(3): 811-819 [DOI] [PubMed] [Google Scholar]
- 30.Greiner M, Pfeiffer D, Smith RD.Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 2000; 45(1–2): 23–41 [DOI] [PubMed] [Google Scholar]
- 31.Florkowski CM.Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. Clin Biochem Rev 2008; 29 (Suppl. 1): S83-S87 [PMC free article] [PubMed] [Google Scholar]
- 32.Beloosesky Y, Nenaydenko O, Gross Nevo RF, Adunsky A, Weiss A.Rates, variability, and associated factors of polypharmacy in nursing home patients. Clin Interv Aging. 2013; 8: 1585-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdulraheem I.Polypharmacy: A risk factor for geriatric syndrome, morbidity & mortality. Journal of Aging Science 2013; 1(e103) [Google Scholar]
- 34.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012; 60(4): 616-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr.Dietary supplements and mortality rate in older women: The iowa women’s health study. Arch Intern Med. 2011; 171(18): 1625-1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA.Recent patterns of medication use in the ambulatory adult population of the united states: The slone survey. JAMA. 2002; 287(3): 337-344 [DOI] [PubMed] [Google Scholar]
- 37.Yuan L, Kaplowitz N.Mechanisms of drug-induced liver injury. Clin Liver Dis. 2013; 17(4): 507-518, vii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leise MD, Poterucha JJ, Talwalkar JA.Drug-induced liver injury. Mayo Clin Proc 2014; 89(1): 95–106 [DOI] [PubMed] [Google Scholar]
- 39.Vieira de Lima TJ, Garbin CA, Garbin AJ, Sumida DH, Saliba O.Potentially inappropriate medications used by the elderly: Prevalence and risk factors in brazilian care homes. BMC Geriatr. 2013; 13: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng MC, Tsai CF, Sheu KL, Lee YT, Lee HC, Tzeng SL, Ueng KC, Chen CC, Chen SC.The impact of number of drugs prescribed on the risk of potentially inappropriate medication among outpatient older adults with chronic diseases. QJM. 2013; 106(11): 1009-1015 [DOI] [PubMed] [Google Scholar]
- 41.Borenstein J, Aronow HU, Bolton LB, Choi J, Bresee C, Braunstein GD.Early recognition of risk factors for adverse outcomes during hospitalization among medicare patients: A prospective cohort study. BMC Geriatr. 2013; 13: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajjar ER, Cafiero AC, Hanlon JT.Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007; 5(4): 345-351 [DOI] [PubMed] [Google Scholar]
- 43.Baldoni AD, Ayres LR, Martinez EZ, Dewulf ND, Dos Santos V, Pereira LR.Factors associated with potentially inappropriate medications use by the elderly according to beers criteria 2003 and 2012. Int J Clin Pharm 2013; 36(2): 316–324 [DOI] [PubMed] [Google Scholar]



