Abstract
Background and Purpose
Intracerebral hemorrhage (ICH) results in high mortality and morbidity for patients. Previous retrospective studies correlated the spot sign score (SSSc) with ICH expansion, mortality and clinical outcome among ICH survivors. We performed a prospective study to validate the SSSc for the prediction of ICH expansion, mortality and clinical outcome among survivors.
Methods
We prospectively included consecutive patients with primary ICH presenting to a single institution over a 1.5-year period. All patients underwent baseline non-contrast CT (NCCT) and Multi-detector CT angiography (MDCTA) performed within 24 hours of admission and a follow-up NCCT within 48 hours after the initial CT. The ICH volume was calculated on the NCCT images using semi-automated software. The SSSc was calculated on the MDCTA source images. We assessed in-hospital mortality and modified Rankin Scale (mRS) at discharge and at 3 months among survivors. A multivariate logistic regression analysis was performed to determine independent predictors of hematoma expansion, in-hospital mortality and poor clinical outcome.
Results
131 patients met the inclusion criteria, in which a spot sign was detected in 31 patients(24%). In a multivariate analysis, the spot sign score predicted significant hematoma expansion (OR, 3.1;95% CI, 1.77–5.39;P=<0.0001), in-hospital mortality (OR, 4.1; 95% CI, 2.11–7.94;P=<0.0001)and poor clinical outcome (OR, 3; 95% CI, 1.4–4.42;P=0.004)Additionally, the spot sign score was an accurate grading scale for ICH expansion, mRS at discharge and in-hospital mortality.
Conclusion
The spot sign score demonstrated a strong stepwise correlation with hematoma expansion and clinical outcome in patients with primary ICH.
Keywords: Intracerebral Hemorrhage, Stroke, CT angiography, Spot Sign, Mortality, Modified Rankin scale
INTRODUCTION
Primary intracerebral hemorrhage (ICH) is a subtype of stroke that affects over 1 million people worldwide annually and accounts for 10%–15% of all strokes.1ICH has a mortality of 30–50%, which exceeds the mortality of ischemic stroke.2Many scoring systems have been developed, integrating demographic, clinical and radiological features to stratify mortality risk in patients with ICH. 3–5Radiological findings such as larger ICH volume, 3, 6presence of intraventricular hemorrhage, 7–9, higher spot sign score (SSSc), 10, 11and ICH expansion12–14may help predict which patients will suffer clinical deterioration and worse outcome. A better method for early detection of patients with increased risk of ICH expansion could identify a group at high risk that would be most likely to benefit from hemostatic therapy, intensive blood pressure reduction, or rapid surgical evacuation. Multiple groups have shown that the MDCTA Spot Sign predicts hematoma expansion and poor outcome.(10, 11, 16, 19, 21) A recent large, multicenter, prospective study demonstrated that while Spot Sign is a validated predictor of hematoma expansion and clinical outcome, sensitivity and specificity were imperfect. (20) The spot sign score incorporates radiological markers (spot sign number, density and size) that yield information over the simple presence or absence of contrast extravasation. These characteristics represent larger concentrations of extravasated contrast and may well identify patients with higher bleeding rate. The spot sign score had a strong stepwise correlation with hematoma expansion and clinical outcome.(10)Therefore, in order to validate the ability of Spot Sign Score to provide more information than the dichotomous presence/absence of a spot sign, we performed a prospective single center study.
MATERIALS AND METHODS
Patient selection/enrollment
Our study was approved by the hospital's Institutional Review Board and complied with HIPAA regulations. From January 2009 to June 2010, we prospectively collected data on all patients with primary ICH admitted to the emergency department of Massachusetts General Hospital. The patient eligibility criteria included: (1) evidence of non-traumatic ICH based on a non-contrast CT examination (NCCT) of the head performed at time of admission; (2) a MDCTA performed within 24 hours of admission; and (3) a follow-up NCCT within 48 hours of the baseline image. Patient exclusion criteria included the presence of (1) an associated subarachnoid hemorrhage in the basal cisterns; (2) a vascular lesion or neoplasia determined as the etiology for the ICH, identified through CT angiography, conventional angiography or MRI; (3) a loss of gray-white matter differentiation in a vascular territory suggesting a pre-established acute ischemic stroke or venous infarct; (4) a hematoma drainage between the baseline NCCT and the follow-up NCCT; or (5) anon-diagnostic CT images.
Image Acquisition
NCCT acquisitions were performed according to standard departmental protocols on a 64-section, General Electric helical CT scanners (LightSpeed; GE Medical Systems, Waukesha, Wisc). NCCT examinations were performed using helical technique with 120 to 140 kVp, auto mA (10–500), and 5-mm slice thickness reconstruction. MDCTA was subsequently performed by scanning from the base of the C1 vertebral body to the vertex using axial technique, 0.5pitch, 1.25 mm collimation, 235 mA, 120kVp, 22 cm field of view, and 65 to 85 mL of iodinated contrast material administered by a power injector at 4 to 5 mL per second into an antecubital vein with either a fixed 25-second delay between the onset of contrast injection and the start of scanning, or Smart-Prep, an semiautomatic contrast bolus triggering technique. Internal guidelines of our stroke service recommend an immediate follow up with a NCCT of the head if there is neurological deterioration (more than 2 points on the NIHSS) and/or a follow up NCCT in 24 hours if the patient is stable. MDCTA and NCCT acquisition were both performed on the same hardware platform (Light speed; GE Healthcare, Milwaukee, Wis.) and using the same protocol as previously published. 15
Image Analysis
The NCCT images were reviewed by 2 experienced neuroradiologists. (JMR and JJL) Determination of the initial and follow-up volumes of intraparenchymal hematoma and intraventricular hematoma (IVH) were performed independently with Analyze 10.0 software. (Mayo Clinic, Rochester, Minn) Volumes were measured with manual tracing of the ICH outline on the baseline and first follow-up NCCT images. A 6ml or 33% ICH enlargement was considered significant expansion.16, 17 Mean average rate of expansion was calculated by subtracting the initial ICH volume from the follow up ICH volume divided by the interval (hrs) between the two exams.
Spot sign detection and score calculation
MDCTA source images were independently reviewed in “spot windows” (width 200, level 110) by the same two neuroradiologists to determine the presence of active contrast extravasation, the spot sign, according to the following strict radiological criteria: (1) ≥1 focus of contrast pooling within the ICH; (2) with an attenuation ≥120 Hounsfield units (HU); (3) discontinuous from normal or abnormal vasculature adjacent to the ICH; and (4) of any size and morphology. (10) The spot sign score was calculated based the number of spot signs, maximum dimension in a single axial MD CTA source image and maximum absolute attenuation.10(Supplement I)
Independent variables
Patient medical records were reviewed upon admission for age, gender, mean arterial blood pressure to screen for hypertension which was verified by evidence of a documented history of hypertension either from 2 physician generated measurements or patients use of antihypertensive medication. Patients were divided into 2 groups based on blood glucose levels, either above or below 170mg/dL. In addition, coagulation status was evaluated with the International Normalized Ratio (INR), PT and PTT and modifying treatments such as anti-platelet therapy, anticoagulation therapy, administration of fresh-frozen plasma, vitamin K, and platelet transfusion on admission. Patients underwent a full neurological examination and a mRS was determined. This exam was repeated at discharge and at 3 months to determine the mRS. If the mRS at three month follow-up was not available, the last clinical observation or discharge mRS was used.
Patients with a mRS score of less than three were considered to have a good outcome, whereas those with a mRS of equal to or greater than three were classified in the poor outcome category.16This scale also included mortality, with expired patients receiving the worst possible score of 6.
Statistical Analysis
Statistical analysis was performed using SAS 9.1 software package (SAS Institute Inc).All variables including age, gender, hypertension, high blood glucose, warfarin or aspirin use, IVH, spot sign score and ICH volume were recorded and compared using univariate analysis to find possible significant predictors for the outcome under evaluation. The level of significance was set at 2-sided P<0.05 for all statistical analyses. Those variables that reached P<0.05 in univariate analysis were considered for multivariable analysis. Multivariate logistic regression analysis and linear regression for continuous variables were performed to determine independent predictors of ICH expansion and poor clinical outcome. The receiver operating characteristic (ROC) analysis was used to determine the area under the curve for the average rate of expansion in the prediction of poor clinical outcome at the 3-month follow-up.
RESULTS
During a period of 1.5 years, a total of 213 patients presented to our emergency department with non-traumatic ICH on a NCCT. 82 Patients were excluded from the study: 52 had a vascular lesion or neoplasia as ICH etiology, 4 showed loss of gray–white matter differentiation in a vascular territory suggesting a pre-established acute ischemic stroke or venous infarct, 24 underwent ICH drainage immediately after NCCT, and 2 had incomplete hematoma imaging.
A total of 131 patients met our eligibility criteria, with a mean age of 71.5 years (median, 74 years; range, 26 to 99 years). 24 (18 %) of the patients were using warfarin at time of presentation, and 46 (35%) patients were using antiplatelet. 48 patients (36.6%) had intraventricular extension of their ICH. ICH growth of 6ml or more than 33% was detected in 25 patients (19%). A total of 28 patients expired during the hospital stay (21%). Among the 106 survivors, 52 patients had poor outcome at 3-month follow-up (49%). (Table 1)
Table 1.
Baseline characteristics of the population (n=131).
| Parameters | |
|---|---|
| Age (mean +/− sd), y | 71.5 +/− 15 (Median 74) |
| Sex | Female n=52(39%), Male n=79(61%) |
| History of Hypertension | 92 (70%) |
| Admission MABP(mean +/− sd), mm Hg | 113 +/− 28 |
| Glucose (mean +/− sd), mg/dL | 143.5 +/− 51.7 |
| Glucose<= 170 | 69(53%) |
| Glucose> 170 | 62(47%) |
| Platelets (mean +/− sd), th/cumm | 246 +/− 78 |
| Anticoagulation | Yes 24(18%), No 107(82%) |
| INR (mean +/− sd) | 1.28 +/− 0.96 |
| Antiplatelet Medication | Yes 46(35%), No 85(65%) |
| Infusion of platelets on admission | Yes 12(9%) |
| Administration of Vitamin K on admission | Yes 19(14%) |
| Infusion of Fresh Frozen Plasma on admission | Yes 17(13%) |
| Initial ICH Volume (mean +/− sd), mL | 26.1 +/− 28 |
| Presence of IVH | 48(36.6%) |
| Time of ED arrival to CT (mean +/− sd), h | 2.1 +/− 2.8 |
| Time of onset to followup CT(mean +/− sd), h | 13.7 +/− 8.7 |
| Admission ICH Volume (median), mL | 14.9 |
| Followup ICH volume (mean +/− sd), mL | 29.2 +/− 33.6 |
| Follow up ICH volume (median), mL | 15.1 |
| In-hospital mortality | 28(21%) |
| Discharge mRS | 3.54 +/− 1.67 |
| Three month mRS | 3.14 +/− 2.01 |
| Average rate of ICH expansion (mean +/− sd), mL/ h | 0.62 +/− 2.8 |
| Spot sign | 31 (24%) |
| Score 0 | 100 (76%) |
| 1 | 14 (11%) |
| 2 | 11 (8%) |
| 3 | 4 (3%) |
| 4 | 2 (1.5%) |
Notes: MABP indicates Mean arterial blood pressure. ICH indicates Intracerebral hemorrhage. IVH indicates intraventricular hemorrhaae. SD indicates standard deviation. mRS indicates modified Rankin scale.
A spot sign was detected in 31 (24%) patients, 18 (58%) males and 13 (42%) females. A spot sign was detected in 19 (68%) of the 28 patients that died in the hospital and in 28 (36%) of the patients that had poor clinical outcome at 3 months. The presence of any spot sign had an overall sensitivity of 64%, specificity of 86, positive predictive value (PPV) =0.52, negative predictive value (NPV) =0.91 for significant ICH expansion. The spot sign demonstrated a sensitivity of 68%, a specificity of 88%, a PPV of 0.61 and a NPV of 0.91 for in hospital mortality. There was a 34% sensitivity for the spot sign and the prediction of a poor clinical outcome among survivors (mRS>3), a specificity of 94%, PPV of 0.90 and a NPV of 0.5.
Predictors of in-hospital mortality and mRS at 3 months
Table 2 shows univariate analyses of predictors of in-hospital mortality and mRSat3 months. Age, prolonged INR at admission, admission ICH volume, follow up ICH volume, spot sign, spot sign score, presence of IVH, average rate of ICH expansion, and time from Emergency Department (ED) admission to first CT were associated with in-hospital mortality.
Table 2.
Potential predictors of in-hospital mortality and three months' outcome
| Comparison of patients survived vs deceased at discharge | Comparison of patients with good vs poor outcomes | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Survived (n=103) | Deceased (n= 28) | p-value | Good outcome (mRS<3) (n= 53) | Poor outcome (mRS≥3) (n= 78) | p-value | |
| Age (mean +/− sd), y | 70 +/− 16 | 77 +/− 16 | p = 0.05 * | 70 +/− 16 | 72 +/− 16 | p = 0.45 * |
| 59% Male | 64% Male | 64% Male | 58% Male | |||
| Sex | 41% Female | 36% Female | p = 0.63 ** | 36% Female | 42% Female | p = 0.46 ** |
| History of Hypertension | 68/103 (66%) | 22/28 (79%) | p = 0.20 ** | 33/53 (62%) | 57/78 (73%) | p = 0.15 ** |
| SBP at admission, mmHg | 165 +/− 38 | 180 +/− 46 | p = 0.09 * | 156+/− 38 | 176+/− 40 | p = 0.01 * |
| DBP at admission, mmHg | 84 +/− 23 | 92 +/− 32 | p = 0.13 * | 80 +/− 21 | 90 +/− 28 | p = 0.03 * |
| Glucose (mean +/− sd), mg/dL | 145 +/− 56 | 139 +/− 39 | p = 0.59 * | 138 +/− 47 | 147 +/− 57 | p = 0.30 * |
| Platelets (mean +/− sd) | 245 +/− 69 | 252 +/− 103 | p = 0.65 * | 238 +/− 68 | 252 +/− 83 | p = 0.30 * |
| Anticoagulation | 14/103 (14%) | 8/28 (29%) | p = 0.09 ** | 7/53 (13%) | 15/78 (19%) | p = 0.65 ** |
| Antiplatelet Medication | 34/103 (33%) | 12/28 (43%) | p = 0.33 ** | 19/53 (36%) | 27/78 (35%) | p = 0.89 ** |
| INR (mean +/− sd) | 1.2 +/− 0.5 | 1.6 +/− 0.8 | p = 0.05 * | 1.2 +/− 0.4 | 1.3 +/− 0.7 | p = 0.11 * |
| Vitamin K | 12/103 (12%) | 7/28 (25%) | p = 0.13 + | 5/53 (9%) | 14/78 (18%) | p = 0.21 + |
| Platelet transfusion | 8/103 (8%) | 4/28 (14%) | p = 0.28 + | 3/53 (6%) | 9/78 (12%) | p = 0.36 + |
| Fresh Frozen Plasma | 11/103 (11%) | 6/28 (21%) | p = 0.20 + | 7/53 (13%) | 10/78 (13%) | p = 1.00 + |
| Admission ICH Volume (mean +/− sd), mL | 20.0 +/− 20.7 | 48.6 +/− 39.1 | p = 0.001 * | 15.3 +/− 14.3 | 33.5 +/− 32.6 | p < 0.0001 * |
| Follow-up ICH Volume (mean +/− sd), mL | 20.5 +/− 21.1 | 61.6 +/− 48.9 | p < 0.0001 * | 15.1 +/− 14.3 | 38.9 +/− 39.3 | p < 0.0001 * |
| Presence of IVH | 29/103 (28%) | 19/28 (68%) | p < 0.0001 ** | 8/53 (15%) | 40/78 (51%) | p < 0.0001 ** |
| Time of admission to first CT (mean +/− sd), h | 2.4 +/− 3.0 | 0.9 +/− 0.6 | p<0.0001 * | 2.7 +/− 3.2 | 1.7 +/− 2.4 | P=0.02 * |
| Time of first CTto follow up CT (mean +/− sd), h | 14.7 +/− 8.7 | 11.7 +/− 8.6 | p = 0.12 * | 14.1 +/− 7.3 | 14.0 +/− 9.7 | p = 0.97 * |
| Admission ICH Volume (median), mL | 12.7 | 38.5 | p < 0.0001 ++ | 9.5 | 18.6 | p < 0.0001 ++ |
| Followup ICH volume (median), mL | 13.0 | 64.9 | p < 0.0001 ++ | 10.5 | 18.7 | p < 0.0001 ++ |
| Spot sign presence | 12/103 (12%) | 19/28 (68%) | P< 0.0001 ** | 3/53 (6%) | 28/78 (36 %) | P= 0.0002 ** |
| SS Score | ||||||
|
|
||||||
| 0 | 91 | 9 | P = <.0001 ** | 50 | 50 | P = 0.1483 ** |
|
|
||||||
| 1 | 7 | 7 | P = 0.0057 ** | 2 | 12 | P = 0.0347 ** |
|
|
||||||
| 2 | 4 | 7 | P = 0.0004 ** | 1 | 10 | P = 0.0269 ** |
|
|
||||||
| 3 | 1 | 3 | P = 0.0079 ** | 0 | 4 | P = 0.0943 ** |
|
|
||||||
| 4 | 0 | 2 | P = 0.0063 ** | 0 | 2 | P = 0.2401 ** |
|
|
||||||
| Average rate of ICH expansion (mean +/− sd), mL/h | 0.03 +/− 0.39 | 2.81 +/− 5.55 | p = 0.01 * | −0.04 +/− 0.33 | 1.07 +/− 3.55 | p = 0.01 * |
Key:
Student's t-test
Chi- Square Test
Fisher Exact Test
Mann Whitney Test
Multivariate analysis of ICH expansion
The multivariate model for ICH expansion included age, ICH volume, IVH, Glucose >170, SSSc, high blood pressure and anticoagulation. Initial ICH volume (P=0.0002), Spot sign score (P=<0.0001) and glucose > 170 (P=0.01) were the remaining independent predictors of ICH expansion. (Table 3) Higher spot sign scores demonstrate increased specificity to predict significant hematoma expansion. (Table 4)
Table 3.
Multivariate analysis of predictors (n=131)
| Variable | ICH Expansion | In Hospital-Mortality | (mRS ≥3) at 3 months | |||
|---|---|---|---|---|---|---|
| OR (95% Wald CI) | P value | OR (95% Wald CI) | P value | OR (95% Wald CI) | P value | |
| Age | 1.0 (0.76 to 1.43) | 0.76 | 1.61(1.10 to 2.38) | 0.002 | 1.49, (1.06 to 2.10) | 0.02 |
| Average rate of expansion | N/A * | N/A * | 3.69 (1.55 to 8.77) | 0.0032 | 12.99, (2.68 to 62.5) | 0.0015 |
| Spot sign score | 3.1, (1.77 to 5.39) | <0.0001 | 4.1 (2.11 to 7.94) | <0.0001 | 3, (1.4 to 4.42) | 0.004 |
| IVH | 1.0 (0.25 to 4.1) | 0.9671 | 4.89 (1.74 to 13.33) | 0.002 | 4.89(1.74 to 13.33) | 0.002 |
| Glucose >170 | 3.5 (1.069 to 11.74) | 0.03 | 0.487 (0.08 to 2.68) | 0.40 | 0.739(0.18 to 2.91) | 0.66 |
| Anticoagulation | 1.02(0.25 to 4.18) | 0.97 | 1.99(0.46 to 8.55) | 0.35 | 1.51(0.41 to 5.62) | 0.53 |
| Hypertension | 3.1(0.68 to 14.84) | 0.1416 | 1.405 (0.19 to 10.30) | 0.73 | 2.022 (0.42 to 9.62) | 0.37 |
| Admissions ICH volume | 0.0002 | 0.0002 | 0.0002 | |||
| <30mL | 1 | 1 | 4.89(1.74 to 13.33) | |||
| 30–60mL | 3.5(3.21 to 3.8) | 3.5(3.21 to 3.8) | 1 | |||
| >60mL | 15.68(4.18 to 58.89) | 15.68(4.18 to 58.89) | 1.05(0.93 to 1.18) |
Notes: ICH indicates Intracerebral hemorrhage. IVH indicates intraventricular hemorrhage.
40 patients did not have a 3 month mRS evaluation and the last in-hospital mRS was included in this model.
only measured on patients that expanded.
Table 4.
Correlation of the spot sign score and ICH expansion (>6ml or 33%):
| SCORE: | SENS: | 95% CI | SPEC: | 95% CI |
| ≥ 0 | 100 | 93.6–100 | 0 | 0–1.2 |
| ≥ 1 | 87.5 | 75.9–94.8 | 92.9 | 89.5–95.5 |
| ≥ 2 | 76.8 | 63.6–87 | 96.8 | 94.2–98.4 |
| ≥ 3 | 60.7 | 46.8–73.5 | 99.7 | 98.2–100 |
| ≥ 4 | 30.4 | 18.8–44.1 | 100 | 98.8–100 |
| AUC: | 0.93 | 0.89–0.95 | ||
| p-value: | <0.0001 |
Independent predictors of in-hospital mortality in primary ICH in multivariate analysis
Patient age (P=0.0002), average rate of ICH expansion (P=0.0032), spot sign score (P=< 0.0001), IVH (P= 0.01), and admission ICH volume (P=0.0002) were independent predictors of in-hospital mortality of patients with primary ICH. (Table3)
Independent predictors of poor clinical outcome (mRS>3) at 3 months
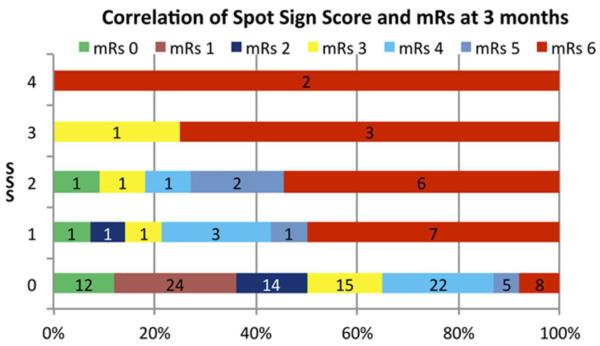
In a multivariate analysis, an association was found between age (P=0.02), average rate of ICH expansion (P=0.0015), spot sign score (P= 0.04), IVH (P=0.002), and initial ICH volume (P=0.047) and mRS at 3 months. (Table3) Figure 1 demonstrates a stepwise association of the spot sign score and the mRS at 3 months. Furthermore, all patients with a SSSc of 3 or 4 had a poor clinical outcome.
Figure 1.
Correlation of Spot Sign Score and mRs at 3 months after discharge
Spot sign score and the risk of ICH expansion and mortality
A stepwise risk of ICH expansion and mortality was seen with an increasing spot sign score. (Supplement II and III)
DISCUSSION
This study prospectively validates the spot sign score as a predictor of ICH expansion, and mortality and poor clinical outcome after primary ICH. Our data suggest that the spot sign score categorizes expansion and mortality risk in a stepwise fashion for those patients with a positive spot sign.
The recent failure of pro-coagulant medications (17) and surgery18, 19 for ICH treatment, has sparked interest in methods that detect ICH patients that are actively bleeding in order to select those patients at highest a priori risk of mortality and poor functional outcome. Unfavorable results from the recent INTERACT II trial demonstrated no significant reduction in the primary outcome of rate of death or severe disability. The trial did demonstrate improvement of the ordinal modified Rankin scores in the intensive lowering of blood pressure group.20 ICH contrast extravasation21, later known as the spot sign 16, has been associated in multiple studies with ICH expansion, likely reflecting active bleeding. In 2012, Demchuk et al, 22 prospectively demonstrated the positive correlation of the presence of a spot sign and the risk of ICH expansion and poor clinical outcome in a cohort of 228 adult patients. The sensitivity of the spot sign to predict ICH expansion in this study was lower (51%) than previous retrospective results (88–93%)23–25. We found that the spot sign had a sensitivity of 68% for the prediction of ICH expansion, which is slightly higher than the results of the PREDICT trial. Decreased sensitivity of the spot sign in these prospective trials may be technical and secondary to differences in scanner speed. Most of the previous retrospective studies scanned a large proportion of their patients on 4, 16 and 64 slice scanners16, 21, 26 and not on the new faster scanners with 128 and 320 slice scanners. Recent articles highlight the importance of the delay between contrast administration and imaging on the appearance of the spot sign.23, 27 Future research should take into account delayed imaging to improve sensitivity in the detection of the spot sign. We detected a spot sign in 31 (24%) patients, which is similar to the percent detected in previous studies 24, 26 including the PREDICT trial,22 in which a spot sign was detected in 30% of their patients. Although, multiple studies had different time intervals between ictus and imaging, the presence of the spot sign remained constant within a 20–30% range. 25 (20) The spot sign scoring system captures the morphologic and physical properties of iodinated contrast that reflect the concentration and volume of contrast extravasated. This method was previously evaluated in a retrospective study 24 that demonstrated not only a stepwise risk of hematoma expansion but also predicted the degree of the ICH expansion and mortality and poor functional outcome. We have now prospectively replicated these results demonstrating that increased spot sign scores reflect higher risk of hematoma expansion and mortality. Therefore, spot sign scores may allow early detection of patients with ICH that may be an ideal target for hemostatic therapy and/or acute surgical intervention, particularly when a reliable neurological exam is unavailable. With the likelihood of new hemostatic drugs and minimally invasive surgical treatments being evaluated in prospective clinical trials for ICH28, the identification of a surrogate marker for poor clinical outcome and mortality based on CT angiography upon admission is an important objective. In contrast to the simple presence or absence of the spot sign, the SSSc is able to stratify patients' risk of poor clinical outcome and increase the specificity of the spot sign (Table 4).
Among the multiple variables we evaluated, initial ICH volume is considerably the strongest predictor of mortality within the group. This is particularly robust in patients with ICH of >60 cc as demonstrated by Broderick and colleagues 6. Our study, as many previous ones, also detected a positive predictive value for initial ICH volume,3, 5, 6, 13 and intraventricular hemorrhage 11, 14, 29 for mortality risk.
The spot sign score improves the differentiation of patients that will expand as well as those that will have poor outcome. This is especially important for patients that may require invasive treatments where certainty of active bleeding is critical. In this study, we validate the spot sign score as an accurate grading scale for ICH expansion and clinical outcome.
CONCLUSION
Our results show a strong stepwise association of the spot sign score with both hematoma expansion and poor clinical outcome among patients with primary ICH. The spot sign score provides a dynamic selection tool for clinical decision-making and patient selection for trials and treatment.
Supplementary Material
Acknowledgments
Source of Funding Dr. Jing was funded by China Scholarship Council (Program number: [2009]3004).
Dr. Brouwers was supported by the NIH – National Institute of Neurological Disorders and Stroke (NINDS) SPOTRIAS fellowship grant P50NS051343.
Dr. Goldstein receives a research grant from NIH–NINDS and works as a consultant /on the advisory board of CSL Behring; Dr. Romero is on the Imaging Committee Desmoteplase in Acute Ischemic Stroke Trial (DIAS) trial and is on the advisory board of Lundbeck Pharmaceuticals; Dr. Rosand receives research grants from NIH.
Footnotes
Disclosures The other author reports no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 2.Zhang LF, Yang J, Hong Z, Yuan GG, Zhou BF, Zhao LC, et al. Proportion of different subtypes of stroke in china. Stroke. 2003;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- 3.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 4.Leira R, Davalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage: Predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 5.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The func score. Stroke. 2008;39:2304–2309. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 7.Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34:1717–1722. doi: 10.1161/01.STR.0000078657.22835.B9. [DOI] [PubMed] [Google Scholar]
- 8.Hallevi H, Dar NS, Barreto AD, Morales MM, Martin-Schild S, Abraham AT, et al. The ivh score: A novel tool for estimating intraventricular hemorrhage volume: Clinical and research implications. Crit Care Med. 2009;37:969–974. e961. doi: 10.1097/CCM.0b013e318198683a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: Risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor vii. Neurosurgery. 2006;59:767–773. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 10.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2009 doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Smith A, Hemphill JC, 3rd, Smith WS, Lu Y, Dillon WP, et al. Contrast extravasation on ct predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol. 2008;29:520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson CS, Huang Y, Arima H, Heeley E, Skulina C, Parsons MW, et al. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: The intensive blood pressure reduction in acute cerebral haemorrhage trial (interact) Stroke. 41:307–312. doi: 10.1161/STROKEAHA.109.561795. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, et al. Determinants of intracerebral hemorrhage growth: An exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 14.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 15.Delgado Almandoz JE, Schaefer PW, Forero NP, Falla JR, Gonzalez RG, Romero JM. Diagnostic accuracy and yield of multidetector ct angiography in the evaluation of spontaneous intraparenchymal cerebral hemorrhage. AJNR Am J Neuroradiol. 2009;30:1213–1221. doi: 10.3174/ajnr.A1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. Ct angiography “Spot sign” Predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 17.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 18.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): A randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 19.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (stich ii): A randomised trial. Lancet. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 21.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 22.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the ct-angiography spot sign (predict): A prospective observational study. Lancet Neurol. 11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 23.Ederies A, Demchuk A, Chia T, Gladstone DJ, Dowlatshahi D, Bendavit G, et al. Postcontrast ct extravasation is associated with hematoma expansion in cta spot negative patients. Stroke. 2009;40:1672–1676. doi: 10.1161/STROKEAHA.108.541201. [DOI] [PubMed] [Google Scholar]
- 24.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: The spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on ct angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Wang Y, Wang W, Ma L, Xue J, Weissenborn K, et al. Contrast extravasation on computed tomography angiography predicts clinical outcome in primary intracerebral hemorrhage: A prospective study of 139 cases. Stroke. 42:3441–3446. doi: 10.1161/STROKEAHA.111.623405. [DOI] [PubMed] [Google Scholar]
- 27.d'Esterre CD, Chia TL, Jairath A, Lee TY, Symons SP, Aviv RI. Early rate of contrast extravasation in patients with intracerebral hemorrhage. AJNR Am J Neuroradiol. 32:1879–1884. doi: 10.3174/ajnr.A2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtpa for intracerebral hemorrhage evacuation (mistie) clinical trial. Acta Neurochir Suppl. 2008;105:147–151. doi: 10.1007/978-3-211-09469-3_30. [DOI] [PubMed] [Google Scholar]
- 29.Cheung RT, Eliasziw M, Meldrum HE, Fox AJ, Barnett HJ. Risk, types, and severity of intracranial hemorrhage in patients with symptomatic carotid artery stenosis. Stroke. 2003;34:1847–1851. doi: 10.1161/01.STR.0000080523.29138.5F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.