Abstract
Survival of head and neck cancer patients has not improved in several decades despite advances in diagnostic and therapeutic techniques. Tumor hypoxia in head and neck cancers is a critical factor that leads to poor prognosis, resistance to radiation and chemotherapies, and increased metastatic potential. Magnetic nanoparticle hyperthermia (mNPHT) is a promising therapy for hypoxic tumors because nanoparticles (NP) can be directly injected into, or targeted to, hypoxic tumor cells and exposed to alternating magnetic fields (AMF) to induce hyperthermia. Magnetic NPHT can improve therapeutic effectiveness by two modes of action: 1) direct killing of hypoxic tumor cells; and 2) increase in tumor oxygenation, which has the potential to make the tumor more susceptible to adjuvant therapies such as radiation and chemotherapy. Prior studies in breast cancer cells demonstrated that a hypoxic microenvironment diminished NP uptake in vitro; however, mNPHT with intratumoral NP injection in hypoxic tumors increased tumor oxygenation and delayed tumor growth. In this study, head and neck squamous cell carcinoma (HNSCC) cell lines were incubated in normoxic, hypoxic, and hyperoxic conditions with iron oxide NP for 4–72 hours. After incubation, the cells were analyzed for iron uptake by mass spectrometry, Prussian blue staining, and electron microscopy. In contrast to breast cancer cells, uptake of NPs was increased in hypoxic microenvironments as compared to normoxic conditions in HNSCC cells. In future studies, we will confirm the effect of the oxygen microenvironment on NP uptake and efficacy of mNPHT both in vitro and in vivo.
Keywords: magnetic nanoparticle hyperthermia, hypoxia, head and neck cancer
1. INTRODUCTION
1.1. Background
Head and neck cancers (oral cavity, nasopharynx, other pharynx, larynx) are the sixth most common cancer in the world with 643,000 new cases and 351,000 deaths/yr.1 Despite improvements in diagnosis and treatment, the survival rates have not changed significantly for head and neck cancer patients in several decades. One of the factors that have been shown to be poor prognostic indicators is tumor hypoxia. Tumor cells that are hypoxic are radiotherapy and chemotherapy resistant and have increased malignant potential. 2 Innovative therapeutic strategies directed toward hypoxic tumors are needed to improve the clinical outcome for head and neck cancer patients.
1.2. Magnetic nanoparticle hyperthermia
Magnetic nanoparticle hyperthermia (mNPHT) is a promising new cancer treatment technology that is being heavily studied by a number of investigators at Dartmouth as part of its Center for Cancer Nanotechnology Excellence (CCNE) award. Hyperthermia to treat cancer has been explored for centuries, but its clinical use has been limited because of its poor selectivity for tumor cells.3 With the development of nanoparticles (NP) to target tumor cells, interest in hyperthermia has been revitalized as a cancer treatment.4,5 Magnetic iron oxide NP (IONP) can be injected directly into the tumor or administered systemically and excited with alternating magnetic field (AMF) to produce heat. The effectiveness of the therapy is dependent upon: 1) the ability to deliver the NPs to the tumor; 2) the ability to concentrate NPs into the tumor; and 3) the ability of particles to heat effectively (specific absorption rate or SAR value) in an AMF that is safe with respect to the generation of unacceptable eddy currents. Iron oxide NPs, which have excellent biocompatibility and high SAR values, can be placed in all locations, and the exciting AMF can be applied locally or to anywhere in the body, making mNPHT highly versatile and a potentially effective treatment regardless of the tumor size or location. An algorithm known as Cumulative Equivalent Minutes (CEM) has been used to quantify the amount of physiologically relevant heat deposited in a living tissue (estimating equivalent cytotoxicity for various temperatures and heating times).
The ability of hyperthermia to enhance the effectiveness of radiation therapy is well known and accepted6, as is its ability to kill chronically hypoxic cells preferentially,7,8 although direct and serial oxygen measurement of tumors and tissue has not been done during hyperthermia treatment. While several groups are conjugating antibodies, directed toward tumor markers, onto NPs in order to improve selectivity for tumor cells, we propose the use of hypoxia-targeted NPs to treat hypoxic tumors specifically. We hypothesize that mNPHT for hypoxic tumors can improve therapeutic effectiveness by two modes of action: 1) direct killing of hypoxic tumor cells; and 2) increase in tumor oxygenation, which has the potential to make the tumor more susceptible to adjuvant therapies such as radiation and chemotherapy.
1.3. Hypoxia assessment techniques
The “gold standard” for measuring tumor hypoxia is the Eppendorf™ polarographic electrode. Dr. Harold Swartz and colleagues at Dartmouth have developed electron paramagnetic resonance (EPR) oximetry as an alternative method for direct, quantitative oxygen measurements. EPR is the only technology that is capable of measuring tissue oxygen reliably, repeatedly, and non-invasively. The EPR technology is based on the placement of an inert paramagnetic probe, such as lithium phthalocyanine (LiPc) crystals. When the animal/tissue is placed in a non-invasive electromagnetic field, the signal registered from the tissue probe can be accurately correlated with oxygen concentration (pO2). Due to its repeatable and non-invasive nature, EPR oximetry has the potential to become a unique and powerful clinical tool in the study of tumor and tissue hypoxia.9,10 Eppendorf and EPR oximetry give in vivo direct and quantitative measurements of oxygen, but neither gives imaging or spatial resolution of hypoxia. Endogenous hypoxia markers such as hypoxia-inducible factor-1 alpha (HIF-1α) and carbonic anhydrase IX (CA-IX), and exogenous hypoxia markers such as 2-nitroimidazoles (pimonidazole and EF5) have been utilized to localize hypoxia in ex vivo tissue specimens, but are indirect and non-repeatable assessments of oxygen. Pimonidazole and EF5, which are approved for clinical use, are bioreductive hypoxia markers that bind to cellular macromolecules (mostly proteins) when reduced by hypoxia-dependent nitroreductases.11 Antibodies directed against the reduced and bound nitroimidazole adduct enable detection of hypoxic cells using enzyme-linked immunosorbent assay (ELISA), flow cytometry, or immunohistochemistry. Radiolabeled nitroimidazoles, such as 18F-misonidazole or EF5, are used to detect hypoxic cells with non-invasive positron emission tomography (PET) imaging, but PET has poor spatial resolution and limited capability for quantitative analysis. The use of radiolabeled tracers requires a radiation-approved facility, and tracer preparation is convoluted and time-limited.
1.4. Hypothesis
Prior studies performed in breast cancer cells as part of a Dartmouth CCNE project demonstrated that a hypoxic microenvironment diminished NP uptake in vitro, and mNPHT with intratumoral NP injection in hypoxic tumors increased tumor oxygenation and delayed tumor growth. In this study, the effect of oxygen microenvironment on NP uptake in head and neck squamous cell carcinoma (HNSCC) cell lines was investigated. These cells were incubated in normoxic (21% oxygen), hypoxic (1% oxygen), and hyperoxic (95% oxygen) conditions with iron oxide NP for 4–72 hours. Ectopic subcutaneous HNSCC tumors were grown and intratumoral oxygen measurements were made with EPR oximetry. After incubation with NPs, the cells and tumors were analyzed for iron uptake by mass spectrometry, Prussian blue staining, and electron microscopy and for oxygen levels by EPR oximetry and pimonidazole staining. Based on our prior observations in breast cancer cells, we expected that hypoxia would decrease NP uptake in vitro and in vivo in head and neck cancer cells.
2. METHODS AND MATERIALS
2.1. Cell lines and cell culture
Head and neck squamous cell carcinoma cell lines (FaDu and SCC-25) cells were obtained from ATCC (Manassas, VA). Cells were cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium containing 1.2 g/L sodium bicarbonate, 2.5 mM L-glutamine, 15 mM HEPES and 0.5 mM sodium pyruvate and supplemented with 400 ng/ml hydrocortisone and 10% fetal bovine serum (SCC-25) or Eagle’s Minimum Essential Medium with 10% fetal bovine serum (FaDu). For normoxic conditions, cells were incubated in a humidified atmosphere at 37°C in 5% CO2. For hypoxic conditions, cells were incubated at 37°C in an anaerobic chamber (Bactron I, Sheldon, Inc., Cornelius, OR) maintained with 1% O2, 5% CO2, and balanced N2. For hyperoxic conditions, cells were placed in a sealed container continuously flushed with carbogen (95% O2, 5% CO2) and set into a humidified incubator at 37°C.
2.2. Nanoparticle incubation and injection
Iron oxide nanoparticles (BNF-Starch Plain, Micromod, Rostock, Germany) were incubated in cell media at the concentration of 0.2 mg/ml for 4, 24, 48, and 72 hours. For tumors, the nanoparticles were injected directly into the tumors aiming for a concentration of 5–7.5 mg Fe/cm3 of tumor for 4, 24, and 48 hours.
2.3. Inductively coupled plasma mass spectrometry (ICP-MS)
After incubation with NPs, cells were washed, harvested and counted manually by use of a hemocytometer. The cells were placed in 15 ml conical tubes and weighed with and without samples. One ml trace metal grade HCl is added to each tube, weighed and left at room temperature for digestion. Samples were sent out for ICP-MS analysis after they were completely digested (Agilent 7700x, Trace Element Lab, Dartmouth College). All conditions were performed in triplicate.
2.4 In vitro Prussian blue staining
FaDu cells (a hypopharyngeal carcinoma cell line) were cultured on four-well chamber slides until subconfluent (after about 24hrs). Nanoparticles were added at the concentration of 0.2mg/ml. After various time points (4, 24, 48, and 72 hours), the slides were washed, fixed in graded alcohol solutions, and stained with Prussian blue solution for iron particles. The staining was scored as a percentage of cells which are positive for Prussian blue by a blinded pathologist.
2.5 Transmission Electron Microscopy (TEM)
Cells or tissues were fixed in 4% glutaraldehyde in 0.1 M sodium cacodylate buffer. At least 10 times volume of the buffer was added to a 10–12 × 106-cell pellet or 1–2 mm3 tissue pieces, which were stored at 4°C immediately until submission to the TEM lab (JEM 1010 transmission electron microscope, Electron Microscope Facility, Dartmouth College).
2.6. Tumor implantation
For implantation of FaDu cells, 4 × 106 cells suspended in 0.2 ml media containing Matrigel (1 mg/ml) were injected subcutaneously in the right and left lower flank area of NOD-SCID mice (Charles River, Wilmington, MA). Tumor dimensions were recorded twice weekly and tumor volumes calculated according to the formula L × (W)2 × 0.523. Treatments started when the tumors reached approximately 100–150 mm3.
2.7. Immunohistochemistry (IHC)
For pimonidazole IHC, mice were injected with pimonidazole (100 mg/kg; Hypoxyprobe-1, HPI, Inc., Burlington, MA) prior to sacrifice. Resected tumors were fixed in 10% buffered formalin and embedded in paraffin. Four to five micron sections were made. Tumor sections were incubated with an anti-pimonidazole antibody (HPI, Inc., Burlington, MA) followed by Mouse-on-Mouse-AP Polymer (Biocare Medical, Concord, CA) as secondary antibody and then Warp Red Chromogen Kit (Biocare Medical, Concord, CA). Prussian Blue staining was performed following a routine protocol. Briefly, slides were incubated for 20 minutes in equal parts of hydrochloric acid and potassium ferrocyanide prepared immediately before use. They were then washed, counterstained, and mounted.
2.8. Electron paramagnetic resonance (EPR)
Lithium phthalocyanine (LiPc) implants were placed in the tumor using a 22–25 gauge sterile hypodermic needle with wire stylus as a plunger. For the EPR measurements, the animals were anesthetized using 1–2% isoflurane with FiO2 of 30% or 95%. The external loop resonator was placed close to the surface of where the LiPc implants were located. An L-band (1.2 GHz) EPR spectrometer in Rubin 664L1 was used. Two EPR spectra were collected with magnetic field gradients between 5 and 15 G/cm. The EPR spectra were recorded at optimal power to avoid power saturation with scan times varying from 10–30 seconds. The spectra acquired at each time point were accumulated for 5–7 minutes to increase the signal to noise ratio and improve the precision of the pO2 measurement.
2.9. Statistical analysis
The ICP-MS data, Prussian blue cell counts, and EPR data were expressed as median and range, and compared between hypoxic treatment and normoxic control groups using Students t-test. Statistical software (Excel, Microsoft, Redmond, WA) was used for all analyses.
3. RESULTS
Head and neck cancer cells (FaDu and SCC-25) were incubated with iron oxide nanoparticles under varying oxygen conditions for 24 and 48 hours. Prior studies demonstrated minimal uptake after 4 hrs of NP incubation. After 24 hrs of incubation, no difference in NP uptake was seen between air or 1% oxygen environments (Figure 1). However, after 48 hours, a significant increase in NP uptake was found in both FaDu and SCC-25 cells incubated under hypoxia compared to the aerobic condition. In addition, incubation in a hyperoxic (carbogen 95% oxygen) environment also augments NP uptake in head and neck cells.
Figure 1.
NP uptake in head and neck cancer cells as quantified by ICP-MS. A, Quantification of NP uptake in FaDu cells after 24 and 48 hrs of incubation in air and 1% oxygen conditions. B, Quantification of NP uptake in SCC-25 cells after 48 hrs of incubation in air, 1% oxygen, and 95% oxygen (carbogen) conditions. Error bars=1 STD.
Examination of head and neck cells by transmission electron microscopy (TEM) reveals increased NP uptake in SCC-25 cells incubated under hypoxic (1% oxygen) conditions. Intracellular vesicles filled with nanoparticles are found in the cytoplasm of SCC-25 cells (Figure 2). The cells incubated under hypoxia conditions appeared morphologically different from the aerobic cells with dilated mitochondria and vesicles.
Figure 2.
Transmission electron microscopy of SCC-25 cells after 48 hrs of NP incubation under air (A, B) and 1% oxygen (C, D) conditions. Scale bars=2 micron.
FaDu cells which were grown in chamber slides and incubated with NPs for 4, 24, 48, and 72 hours under air and 1% oxygen conditions showed a consistent threshold between 24 and 48 hrs in which nanoparticle uptake increased (Figure 3). NP uptake appeared higher at 48 hrs under a hypoxic environment, but by 72 hrs, NP uptake was similar between cells grown in aerobic and hypoxic conditions. Figure 4 shows the quantification of the percentage of stained cells in these chamber slides.
Figure 3.
Prussian blue staining in FaDu cells after incubation of NP at various time points under normoxic and hypoxic (1% O2) conditions. Scale bar=10 microns.
Figure 4.
Quantification of Prussian blue staining in FaDu cells incubated at various time points under air and 1% oxygen conditions. Each condition was scored by a blinded pathologist three separate times and expressed as a percentage of stained to total cells. Error bars=1 STD.
The FaDu cells were implanted in the subcutaneous tissue of the flank. When the tumors reached approximately 100 mm3, tumor oxygen measurements were made with EPR oximetry and OxyLite probe. All of these ectopic tumors were hypoxic (<5 mm Hg) at this size. When the mice were switched to breathing carbogen (95% O2+5% CO2), the tumor oxygen levels increased (Figure 5). Tumor growth was similar between those injected with NPs or PBS control (Figure 6). The hypoxic FaDu tumors were directly injected with NPs and incubated for varying amounts of time. Ultrastructural location was determined by transmission electron microscopy. By 4 hours of incubation, the NP can be seen in the intracellular compartment within vesicles. With increasing incubation time (24 hours), the NPs appear to become more organized within the intracytoplasmic vesicles. By 2 weeks of incubation, the cytoplasm contains several vesicles, some of which are densely packed with NP while others appear to hold partially degraded NP (Figure 7). The FaDu tumors were examined histologically for localization of nanoparticles and areas of hypoxia. Figure 8 demonstrates serial tumor sections which have been stained with Prussian blue and pimonidazole, revealing some areas of colocalization of the nanoparticles with hypoxic regions.
Figure 5.
Tumor oxygen measurements in ectopic FaDu tumors using EPR oximetry and OxyLite. During measurements, the FiO2 was switched from 30% oxygen to 95% oxygen and back to 30% oxygen for about 30 minutes each.
Figure 6.
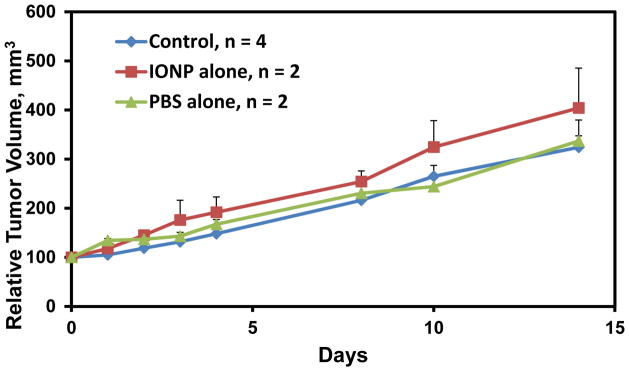
FaDu ectopic tumor growth over 14 days after the start of oxygen measurements. Control, no injection. IONP, iron oxide nanoparticle injection (5mg Fe/cm3 tumor). PBS, phoaphate buffered saline vehicle control. Error bars=1 STD
Figure 7.
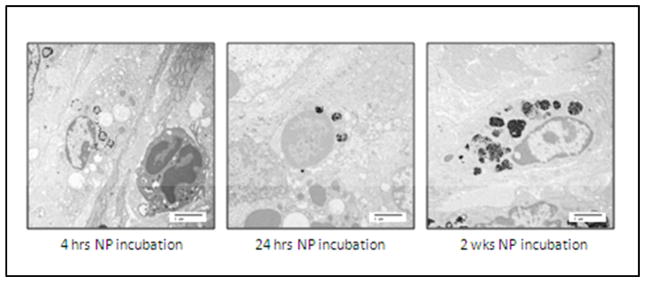
Transmission electron microscopy of FaDu tumors showing intracellular accumulation of NP. Scale bars=2 micron.
Figure 8.

Histological analysis of NP and hypoxia localization in FaDu tumors. Serial sections stained with pimonidazole antibody to indicate hypoxic regions of tumor and Prussian blue to identify NPs. Magnification 10X
4. DISCUSSION
4.1. Hypoxia-targeted therapies
This project started as a pilot grant proposal to develop hypoxia-targeted nanoparticles to use as a diagnostic and therapeutic (theragnostic) strategy. Initial experiments revealed differential uptake of NP depending on oxygen environments. As part of the Dartmouth CCNE award, which focuses on breast and ovarian cancer, breast cancer cell lines were examined in vitro and in vivo under various oxygen conditions. In these cells, ICP-MS and TEM revealed that iron oxide NP had poor uptake in cells which were exposed to hypoxia; however, these breast cancer cells in orthotopic tumors, which were measured as hypoxic (approx 5 mm Hg) at a volume of 200 mm3, showed adequate NP intracellular uptake and efficacious hyperthermia treatment with increased tumor oxygenation and decreased tumor growth.
In head and neck cancer cells, iron oxide nanoparticles appear to have increased uptake in hypoxic microenvironments both in vitro and in vivo. If nanoparticles are preferentially taken up by hypoxic cells, this can be exploited to target hypoxic tumor for any nanoparticle directed therapies. The in vivo experiments will be repeated with systemic administration of NPs. Currently several groups including the Dartmouth CCNE are working to conjugate NP with antibodies or antibody fragments to tumor markers. The challenge is to deliver enough NP systemically into the tumor to be able to create enough therapeutic heat upon exposure to AMF. If the increased NP uptake by hypoxic tumor cells is not adequate with systemic administration, the NP can be conjugated with antibodies to hypoxia-specific markers such as CA-IX or pimonidazole.
Knowing the tumor oxygen microenvironment is essential to direct any hypoxia-targeted therapies. EPR oximetry is a non-invasive method of measuring oxygen repeatedly and reliably. OxyLite is also a quantitative method for oxygen measurement but the probe is invasive and cannot be done without traumatizing tissue. Over the course of a treatment, tumor oxygenation likely changes and tracking the oxygen dynamics could be used to optimize timing and dosing of adjuvant therapies.
The results in head and neck cancer cells demonstrate that different cell types may have varying response to hypoxia challenges and therefore have varying ability to accumulate NP. As several investigators are trying to develop strategies to target NP and improve the efficacy of NPHT or other NP directed therapies, attention must be paid to the specific cell lines used in each study as the results in one cell line may not be applicable to another cell line. Similarly, one patient’s tumor may not necessarily respond the same as another patient’s tumor. Prior to any NP directed therapy, it might be beneficial to test the patient’s tumor cells for their ability to take up NP in various oxygen environments both in an in vitro culture system as well as in an in vivo patient-derived xenograft model.
4.2. Future directions
The ultimate goal of this proposed research is to identify hypoxic tumors and to stratify tumors according to their “hypoxic index” in order to improve treatment efficacy by specifically targeting hypoxic cells and adjusting dosing and timing of treatments (hyperthermia, radiation). Although this project focuses on head and neck tumors, the results are applicable to all types of malignant as well as non-malignant tumors. This novel and innovative theragnostic approach has potential clinical application in not only cancer treatment but also wound healing, peripheral vascular disease, and cardiovascular disease.
5. CONCLUSION
In this study, iron oxide nanoparticles have been shown to have increased uptake in head and neck cancer cells in a hypoxic microenvironment. After 48 hrs of nanoparticle incubation, head and neck cancer cells grown in 1% oxygen take up NP more than aerobic cells in vitro, as assessed by ICP-MS, Prussian blue histology, and TEM. When these cells are grown as an ectopic tumor in mice, the tumors are hypoxic when they reach 100 mm3. The cells in these hypoxic tumors internalize NP starting at 4 hrs of incubation and continue to contain NP-packed vesicles at 2 weeks after NP injection. This information can be used to improve the efficacy of NPHT by exploiting the predilection of hypoxic head and neck cancer cells to accumulate NP.
Acknowledgments
This study is supported by the Dartmouth Center for Clinical and Translational Sciences (Synergy) Pilot Award and Dartmouth Center for Cancer Nanotechnology Excellence Pilot Award.
References
- 1.Parkin D, Bray F, Ferlay J, Pisani P. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P. Oncologist. 2008;13:21–6. doi: 10.1634/theoncologist.13-S3-21. [DOI] [PubMed] [Google Scholar]
- 3.Oleson J, Dewhirst M. Curr Probl Cancer. 1983;8:1–62. doi: 10.1016/s0147-0272(83)80004-x. [DOI] [PubMed] [Google Scholar]
- 4.Giustini A, Petryk A, Hoopes P. Nanomedicine. 2012;8:818–21. doi: 10.1016/j.nano.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng X, Qian X, Mao H, et al. Int J Nanomed. 2008;2:311–21. doi: 10.2147/ijn.s2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overgaard J, Gonzalex D, Hulshof M, et al. Lancet. 1995;345:540–3. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 7.Horsman M, Chaplin D, Overgaard J. Cancer Res. 1990;50:7430–4. [PubMed] [Google Scholar]
- 8.Horsman M, Overgaard J. Clin Oncol. 2007;19:418–26. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Hou H, Lariviere J, Demidenko E, Gladstone D, Swartz H, Khan N. Radiother Oncol. 2009;91:126–31. doi: 10.1016/j.radonc.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz H, Khan N, Buckey J, et al. NMR Biomed. 2004;17:335–51. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 11.Ljungkvist A, Bussink J, Kaanders J, van der Kogel A. Radiat Res. 2007;167:127–45. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]







