Abstract
Fe/Fe oxide nanoparticles, in which the core consists of metallic Fe and the shell is composed of Fe oxides, were obtained by reduction of an aqueous solution of FeCl3 within a NaBH4 solution, or, using a water-in-oil micro-emulsion with CTAB as the surfactant. The reduction was performed either in an inert atmosphere or in air, and passivation with air was performed to produce the Fe/Fe3O4 core/shell composite. Phase identification and particle size were determined by X-ray diffraction and TEM. Thermal analysis was performed using a differential scanning calorimeter. The quasistatic magnetic properties were measured using a VSM, and the specific absorption rates (SARs) of both Fe oxide and Fe/Fe3O4 composite nanoparticles either dispersed in methanol or in an epoxy resin were measured by Luxtron fiber temperature sensors in an alternating magnetic field of 150 Oe at 250 kHz. It was found that the preparation conditions, including the concentrations of solutions, the mixing procedure and the heat treatment, influence the particle size, the crystal structure and consequently the magnetic properties of the particles. Compared with Fe oxides, the saturation magnetization (MS) of Fe/Fe3O4 particles (100–190 emu/g) can be twice as high, and the coercivity (HC) can be tunable from several Oe to several hundred Oe. Hence, the SAR of Fe/Fe3O4 composite nanoparticles can be much higher than that of Fe oxides, with a maximum SAR of 345 W/g. The heating behavior is related to the magnetic behavior of the nanoparticles.
Keywords: Fe nanoparticles, SAR, magnetic nanoparticles, chemical reduction, hyperthermia
1. INTRODUCTION
Magnetic materials were shown to be capable of producing hyperthermia in tumors nearly 50 years ago through in vitro experiments when Fe2O3 nanoparticles injected into lymph nodes were shown to produce a temperature rise of 14°C in an alternating magnetic field [1]. Since then, researchers have demonstrated that polymer-coated superparamagnetic iron oxide (SPIO) nanoparticles can be used to localize the hyperthermia to a tumor by tagging the nanoparticles with an antibody [2].
For clinical use, in addition to the need for biocompatibility, it is useful for nanoparticles to be observable in vivo prior to initiating treatment both to ensure productive therapy and to avoid normal tissue toxicity. Fine (<10 nm) SPIO nanoparticles serve the latter purpose since they can be observed by Magnetic Resonance Imaging (MRI) [3]. In selecting nanoparticles, ones with the highest specific absorption rate (SAR) value are key. This points to the use of the largest single domain particles possible. Having a large SAR value not only minimizes the dose of nanoparticles required for hyperthermia treatment, but is also a key parameter for the minimum size of tumor that can be treated. There is also presumably a limit to the concentration of nanoparticles that a cell can take up [4].
The magnitude of the magnetic fields that have to be applied to SPIO nanoparticles to produce hyperthermia, at least in nude mice, can cause morbidity [5]. It has been suggested [6] that for human use the product H•f should not be more than about 6×106Oe•Hz, where H is the applied field strength and f the frequency of the applied field. Thus, the ideal response of magnetic nanoparticles to an external field for clinical applications would be to develop sufficient heat at the lowest possible frequency and the smallest external magnetic field strength. Iron nanoparticles are possible candidates for this goal. Iron has a high MS (> 210 emu/g) while those of Fe oxides are ≤ 90 emu/g. Theoretically, the hysteresis power loss to heat is given by the frequency times the integral of B•dH over a closed loop where B is the inductive magnetization. Fe nanoparticles can have high enough coercivities for hyperthermia with limited applied field amplitudes, and since B for iron is more than twice that of iron oxides, the power losses of a single domain Fe particle can be more than twice that of an iron oxide particle.
While ferromagnetic particles such as Fe can be imaged with MRI, the contrast is much less than the contrast that can be achieved with SPIO nanoparticles. In this paper we describe the production of advanced nanoparticles, with a single-domain core of pure iron covered with 3–4 nm of iron oxide. The idea is to exploit the likely higher SAR of pure iron (compared to iron oxides) for heating, while using the film of superparamagnetic iron oxide for imaging of the nanoparticles.
2. EXPERIMENTAL
Fe2O3 nanoparticles were purchased from Alfa Aesar. Fe/Fe oxide nanoparticles were synthesized by reduction of aqueous solutions of FeCl3 within a NaBH4 solution, with or without the presence of a micro-emulsion. For synthesis of Fe/Fe oxide nanoparticle without a micro-emulsion, a typical procedure (carried out in an inert atmosphere or in aerobic conditions, at room temperature and ambient pressure) was started with dropwise addition of NaBH4 into a vigorously stirred FeCl3 solution. At the beginning of the reaction, the solution turned to a blackish color due to the precipitation of particles. The precipitates were washed with de-ionized (DI) water and acetone. Prior to use, DI water and acetone were purged with Ar for several hours to get rid of the oxygen. Anhydrous FeCl3 purchased from Alpha Aesar was stored in glove box until used. Aqueous solutions of FeCl3 were prepared immediately before nanoparticle synthesis using prepurged DI water.
After washing, the specimens were subjected to a few hours in an Ar + air atmosphere to passivate the surface. Since the particles were strongly pyrophoric, care was taken to spread the particles gently. Passivation or further annealing at low temperature (150–300°C) produced a Fe/Fe3O4 core/shell structure. Some powder samples were heated in a gas flow of Ar between 400°C – 600°C in order to make the particles grow and/or crystallize.
Coated Fe/Fe oxide nanoparticles were prepared using water-in-oil micro-emulsion with cetyl trimethyl ammonium bromide (CTAB) as the surfactant, n-butanol as the co-surfactant, n-octane as the oil phase [7], and an aqueous FeCl3 or NaBH4 solution as the water phase. Micro-emulsions were prepared by dissolving the two salt solutions into a CTAB/n-butanol/n-octane solution. Two micro-emulsions (I and II) with identical compositions (see table 1) but different aqueous phases were used. These two micro-emulsions were then mixed under constant stirring. Due to the frequent collisions of aqueous cores of water-in-oil micro-emulsions, the reacting species in the two micro-emulsions came in contact with each other, leading to the precipitation of Fe within the aqueous micro-droplets of the micro-emulsion [8]. Since the two micro-emulsions were of identical compositions, differing only in the nature of the aqueous phases, the micro-emulsion did not destabilize upon mixing. As the surfactant monolayer provided a barrier restricting the growth of the particles, it also hindered coagulation of the particles and therefore monodisperse particles might be obtained.
Table 1.
Composition of the micro-emulsion system used for the synthesis reactions.
| Micro-emulsion I | Micro-emulsion II | Weight percentage (%) | |
|---|---|---|---|
| Aqueous phase | 0.08 M FeCl3 | 0.2M NaBH4 | 34 |
| Surfactant | CTAB | CTAB | 12 |
| Co-surfactant | n-butanol | n-butanol | 1 |
| Oil phase | n-octane | n-octane | 44 |
The precipitated particles were separated using high speed centrifugation. The precipitate was then washed in methanol to remove any oil and surfactant from the particles. The particles were then re-dispersed in methanol. The concentration of dispersed solution was determined from the measured Ms of the solution sample using the Ms of uncoated dry powders. Powder samples were obtained by coagulating the colloids with acetone then washing with distilled water and acetone several times to totally remove the CTAB. The precipitates were then dried in flowing Ar at 100°C.
Phase analysis and the crystallite size were determined via a Siemens D5000 diffractometer using Cu-Kα radiation. The particle size and shape as well as the core-shell structure were determined by an_FEI F20 field emission gun transmission electron microscopy (TEM). Thermal analysis was performed using a Perkin Elmer DSC 7 differential scanning calorimeter. The quasi-static magnetic properties of the nanoparticles were measured using a Lakeshore model 7300 vibrating sample magnetometer (VSM).
The SARs of the particles were studied by placing either 0.4 ml solutions or solid samples in a well-insulated, non-metallic container, which was then placed in an air-cooled, 11 mm dia. × 35 mm long magnetic excitation coil. For solid samples, the nanoparticles were dispersed uniformly in Epofix resin and the resulting mixture solidified at room temperature. The dispersion generally resulted in a particle/resin ratio of less than 4% in weight, making the dipoledipole interparticle interaction negligible. Although the dimension of the specimens was much shorter than the homogeneous magnetic zone along the z-axis of the coil, care was taken to maintain the suspension in a constant field zone within the coil. Heating tests were performed using a Hafler P7000 power amplifier to drive a resonant network comprised of the magnetic coil and polypropylene capacitors, which were used to achieve a real input impedance matched to the amplifier capability for maximum efficiency. A Tektronix 60 MHz AC current probe was used with an Agilent Infinium digital oscilloscope to measure the current. The field strength was determined from the peak current. An alternating magnetic peak field strength of 150 Oe and a frequency of 250 kHz were applied. These field parameters were chosen to satisfy the criteria noted earlier for use on the human body [9].
The increase in solution temperature was recorded as a function of time by a fiber temperature sensor (Luxtron Corporation., Santa Clara, CA). As a control, the temperature rise of the same amount of DI water and pure resin without nanoparticles present was also measured and subtracted from the temperature rise measured for the nanoparticles. SAR (W/g) per unit mass of ferromagnetic material was defined by:
| (1) |
where c is the specific heat capacity of the specimen, mp is the mass of the particles, mt is the total mass of the specimen. T is temperature, and t is time. This means the data were normalized with respect to the particle mass. For the particle/resin composite, the heat capacity of the system was calculated as follows:
| (2) |
where Wp is the mass of Fe oxides or Fe. We used the following values of c: cresin = 1.4 J/(k.g); cFe2O3 = 0.75 J/(k.g); cFe = 0.44 J/(k.g); cmethanol = 2.55 J/(k.g) [10].
3. RESULTS AND DISCUSSION
3.1 Fe/Fe oxides particles without coating
Particles with different sizes and magnetic properties were obtained by varying the flow rate of the NaBH4 addition into a FeCl3 solution (0.75 ml/min, 5 ml/min and 50 ml/min) while keeping the concentration of FeCl3 and NaBH4 solutions constant at 0.08 M and 0.2 M, respectively. Typical TEM electron micrographs of particles resulting from the 50ml/min, 5 ml/min and 0.75 ml/min flow rates are shown in Fig. 1a, b, 1c and 1d, respectively. The particles have a nearly spherical shape with a mean size of ~40 nm when the flow rate was 50 ml/min (Fig. 1a). The tendency of the particles to form a long chain-like structure was also observed (Fig. 1b, c). The chain could be due to dipolar coupling, favoring a head-to-tail orientation [11]. It was found that decreasing the flow rate increased the particle size substantially. A very slow flow rate (0.75 ml/min) was found to decrease the HC, as shown later, but it also increased the particle size to more than 200 nm. The latter samples also demonstrated a particle-aggregate morphology (see Fig. 1d). Thus, decreasing the addition rate of NaBH4 was not a suitable way to vary magnetic properties to obtain a better heating effect.
Fig. 1.
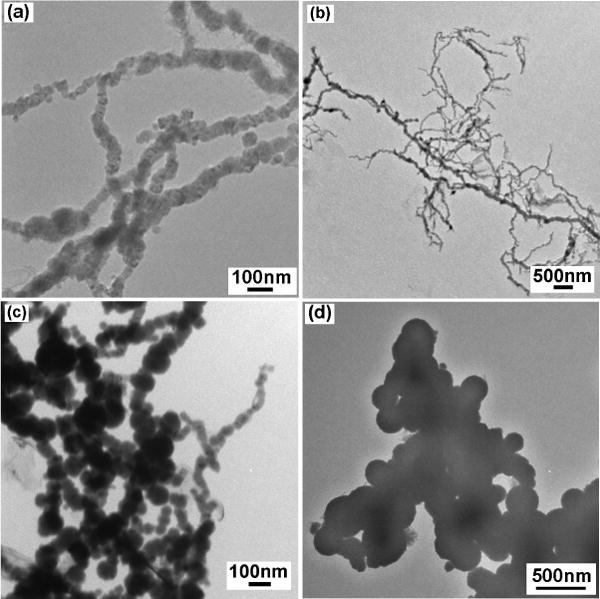
TEM images of Fe/Fe oxides particles with flow rate of 50 ml/min (a, b), 5 ml/min (c), and 0.75 ml/min (d). Concentration of NaBH4 = 0.2 M.
The XRD patterns of the three as-made samples are shown in Fig. 2. The slowest flow rate sample showed a typical amorphous or extremely fine nanocrystalline structure. With increasing flow rate the peaks became sharper. When the flow rate was reached 50 ml/min, the sample showed a pure nanocrystalline phase with grain size of ~ 25 nm (determined by Scherrer formula from x-ray line broadening) and the peaks could be clearly identified as b.c.c. α-Fe. This grain size measured by XRD was smaller than the particle size determined by TEM (40 nm), which indicated that the particles are polycrystalline. None of Fe oxide peaks were definitely detected by XRD possibly because they were too broad and had low intensities. However, when the reduction reaction was performed in air, and the passivation was undertaken for a very long time, XRD results shown in Fig. 3 indicated that these particles were composed of Fe and Fe3O4.
Fig. 2.

XRD patterns of nanocomposite particles produced using indicated flow rates with the NaBH4 concentration of 0.2 M. Peaks corresponding to α–Fe and a possible Fe3O4 peak are indicated.
Fig. 3.
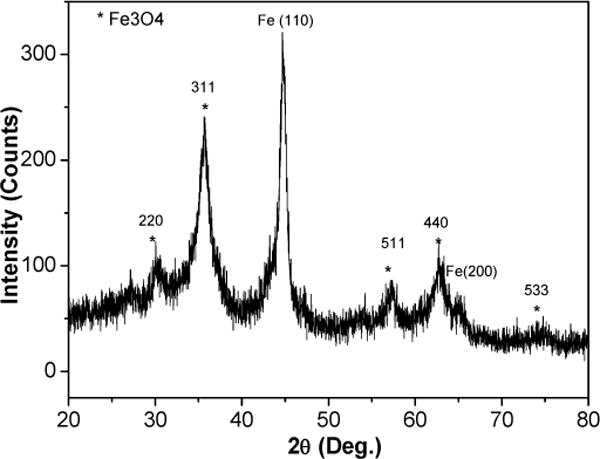
XRD pattern for passivated nanocomposite particles with flow rate of 0.75 ml/min.
DSC curves of the as-produced materials are shown in Fig. 4. The slow and medium flow rate materials showed a sharp exothermic peak at 471°C and 497°C upon heating, respectively. The high flow rate (50 ml/min) sample showed more complicated behavior with several phase transformations. In addition, the transition temperatures tended to increase and transition energy tended to decrease with increasing flow rate. However, it is not clear, at the moment, what are these transformations.
Fig. 4.
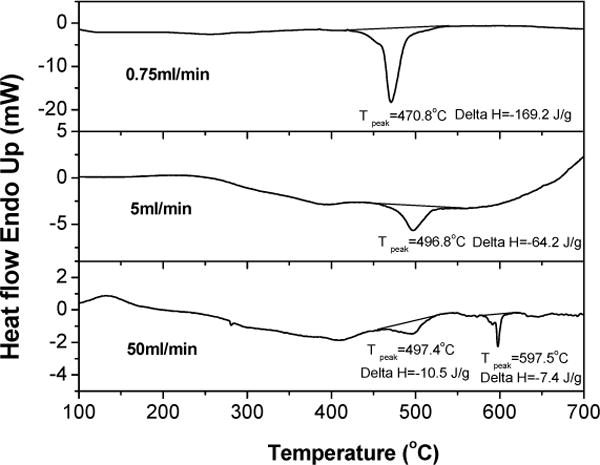
DSC curves for three NaBH4 addition rates
Efforts to vary the particle size and, hence, the magnetic properties were undertaken by varying the concentration of NaBH4 while keeping the concentration of FeCl3 constant (0.8 M), or through subsequent heat treatment. Although the crystalline grain size decreased when decreasing the NaBH4 concentration from 0.5 M to 0.025 M, the particle size did not change much (40–50 nm), see Table 2. However, the particle size distribution increased. Fig. 5a, b show TEM images after decreasing the NaBH4 concentration to 0.025 M. Some particles had a size of more than 100 nm. Heat treatment can vary HC dramatically as shown in Table 2, while, most importantly, the particle size can be still maintained at nano-scale. It is possible that the Fe3O4 coating prevents form coarsening. Fig. 5c, d show TEM images of the sample after 600°C for 5 min annealing. The particle size was less than 100 nm.
Table 2.
Quasi-static magnetic properties, particle size and SAR for particles dispersed in resin.
| Conditions* | Ms(8kOe) (emu/g) | Hc (Oe) | Particle size (nm) | Wt % | SAR (W/g) (150Oe/250kHz) |
|---|---|---|---|---|---|
| 0.025M | 133 | 288 | 40–50 | 10.02 | 1.9 |
| 0.05M | 146 | 329 | 40–50 | 7.14 | 3.3 |
| 0.1M | 148 | 451 | 40–50 | 2.58 | 5.1 |
| 0.2M (0.75ml/min) | 157 | 66 | 200 | 3.38 | 11.6 |
| 0.2M (5ml/min) | 169 | 580 | 40 | 4.05 | 4.5 |
| 0.2M (50ml/min) | 137 | 581 | 40 | 2.98 | 3.9 |
| 0.5M | 133 | 617 | 40 | 2.58 | 6.4 |
| 0.2M(5ml/min) 600°C/5min | 168 | 74 | 80 | 1.0 | 31.3 |
| 0.2M(5ml/min) 500°C/5min | 194 | 462 | 50 | 3.72 | 5.7 |
| Fe2O3 (20nm) | 57 | 101 | 25 | 1.84 | 8.8 |
| Fe2O3 (9nm) | 51 | 3.5 | 9 | 3.33 | 6.9 |
the molar concentration is for NaBH4, the flow rate is indicated in parentheses.
Fig. 5.
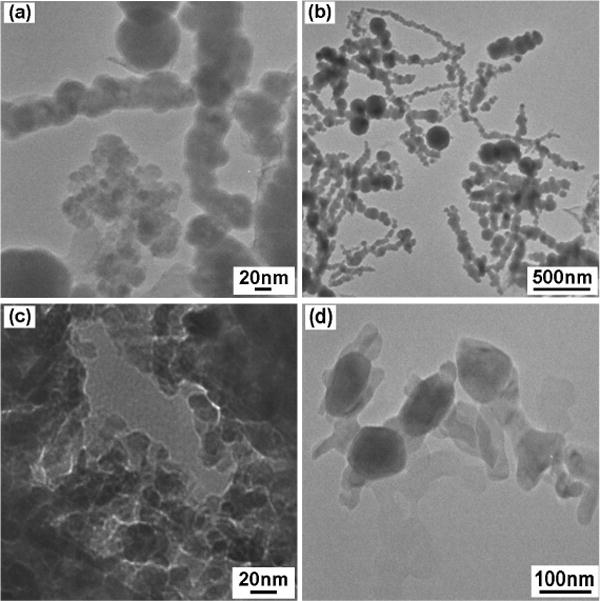
TEM images of particles with NaBH4 concentration of 0.025 M (a, b), and 0.5 M specimen after annealing at 600°C for 5 min (c, d).
Table 2 summarizes the effects of concentration, flow rate and heat treatment on both the magnetic properties and SAR under a field of 150 Oe at 250 kHz. Data for Fe oxide are also given for comparison. It can be seen from Table 2 that the magnetic properties and particle size can, to a large extent, be altered continuously by varying the preparation conditions and thermal treatments, thus making it easier to “engineer” the particles to have a certain set of end-properties. The MS of Fe/Fe3O4 particles (130–190 emu/g) is twice as high as Fe oxide alone, and the HC can be tunable from several Oe to several hundred Oe.
The difference in magnetization of the Fe/Fe3O4 nanoparticles from the Fe bulk value (210 emu/g) may be due to either the presence of nonmagnetic surface oxides dead layers [11] or the canting of moments in the oxide coating. Except for the slow flow rate sample with a large particle size, all the as-produced particles with sizes of 40–50 nm had high HC values from 288–617 Oe, which is almost an order of magnitude larger than the bulk Fe and Fe oxides values [12]. The HC of the fine particles can not be explained by assuming the average values of magnetization and magnetocrystalline anisotropy for Fe and Fe3O4. The origin of such a large HC could be partly due to the shell-type particle morphology where the oxide coating is believe to interact strongly with the Fe core and partly due to the large surface effects which are expected in small particles [13].
It can be also seen from the Table 2 that, regardless of higher MS, only 600 °C-annealed particles and particles produced at a slow flow rate had low HC (74 Oe and 66 Oe respectively) and higher SARs than pure Fe oxide. Heating from ferromagnetic particles is essentially due to hysteresis losses and Brownian relaxation losses. For immobilized dry particles, the influence of Brownian losses is negligible. Therefore, the particles that undergo significant magnetization reversal will have high hysteresis losses, and also high SAR. In general high HC particles, although producing wide B–H loops and, consequently, high heating capability, do so only at high values of the external field (at least the coercive field value), whereas very low HC particles, although responsive to low field strengths, produces low heating. For hysteresis heating therapy under the physiological restrictions, we estimate an HC value of ~ 80 Oe is desirable.
Although a very slow NaBH4 flow rate (0.75ml/min) produced a suitable HC value for a high SAR, its large particle size (more than 200nm) is not desirable. On the other hand, annealing at 600°C to decrease HC and retain the nanoscale of the particles seems to be a good way to achieve a suitable HC. Nevertheless, since the single domain size of Fe is ~ 20 nm [13], and all the samples had particle size ≥ 40nm, the particles most probably had a magnetic multi-domain structure. Thus, the magnetization reversal can not be described by Stoner-Wohlfarth model [14] for single domain particles overcoming of a single energy barrier. Switching instead occurred by a nucleation/propagation process, and so less energy was absorbed. Therefore, smaller single domain particles are needed for better heating effects.
3.2 CTAB-coated particles
Micro-emulsions were used to synthesize very small, single domain particles. Fig. 6 shows XRD patterns for as-made and annealed powders. The as-made particles showed a large band centered at 2θ = 44°, the fundamental {110} peak of bcc α-Fe, as well as a possible peak at 2θ = 35°, the fundamental {311} peak of f.c.c. Fe oxide. After the powder was annealed under Ar at 500°C/5min, the XRD spectrum showed only the characteristic pattern of bcc Fe metal, no other peaks or impurities were detected. The disappearance of Fe oxide peak could be because of its very low intensity compared with those of the Fe peaks.
Fig. 6.
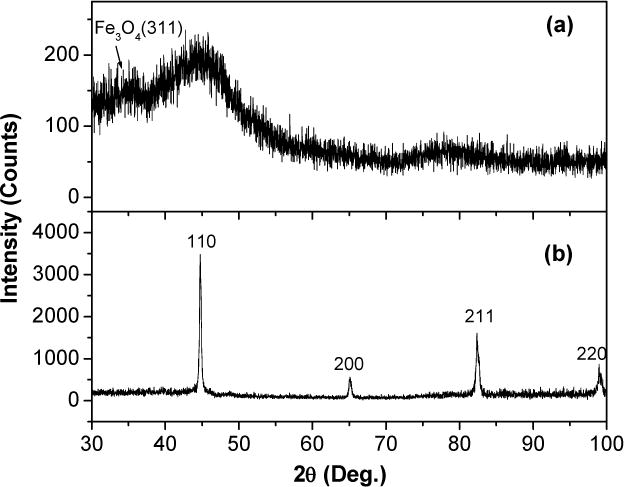
X-ray diffraction patterns on powders obtained after total washing of CTAB: (a) as-made particles prepared in the presence of air and passivated; (b) after annealed at 500C for 5 min under Ar. α–Fe peaks are shown.
A representative bright-field TEM micrograph of this material is presented in Fig. 7. The particles had a narrow size distribution ranging from ~10–15 nm, which is common in the micro-emulsion techniques. Since the particles are smaller than the critical domain size for Fe [13], all the particles should be magnetically single domain. One of the more noteworthy features on the micrograph is the presence of the shell structure revealed as concentric rings on the particles and that many of the particles appear not to touch their neighbors. This could be attributed either a thin surfactant coating, or due to Fe/Fe oxide core shell type of structure. Nevertheless, EAD pattern from only several individual particles gave a composite diffraction pattern, bcc α-Fe + f.c.c. Fe3O4.
Fig. 7.
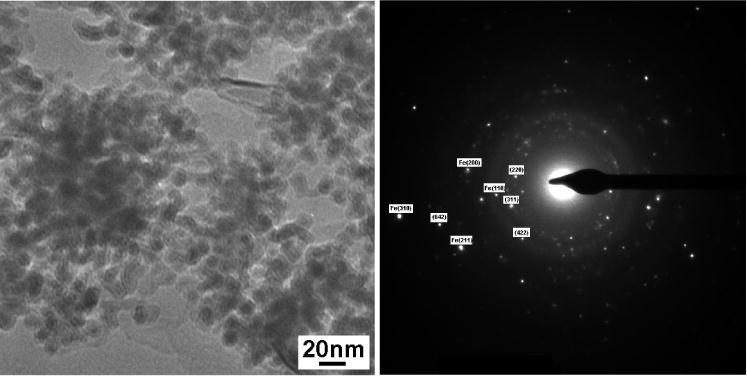
Bright field TEM image and indexed diffraction patterns for CTAB coated particles. Note that the indexed but not labeled phase is Fe3O4.
Fig. 8 shows the hysteresis loops for CTAB coated Fe/Fe3O4 powders and Dextran-coated Fe2O3 powders at room temperature. The 10–15 nm CTAB coated dry powder showed obvious hysteresis, i.e. ferromagnetic behavior, as opposed to just superparamagnetic behavior. This again indicated that the Fe/Fe3O4 composite had a large effective magnetic anisotropy, i.e. the energy barrier, KV (K the anisotropic constant, V the particle volume), can override the thermal energy, kT (k the Boltzmann constant, T the absolute temperature). Compared to 40–50 nm uncoated (measured earlier) Fe/Fe3O4 particles, a relatively small HC of 89 Oe for the 10–15 nm coated Fe/Fe3O4 particles was obtained. The origin of this soft magnetic behavior can be explained based on Herzer’s so-called random anisotropy model [15] when the particle size is less than the magnetic exchange length. Compared with Fe2O3 particles, one can see from Fig. 8 that Fe/Fe3O4 particles have higher MS, Mr (magnetic remanence) as well as higher susceptibility. The higher susceptibility could be due to the narrower size distribution, since particles with different sizes have different magnetic anisotropy values.
Fig. 8.
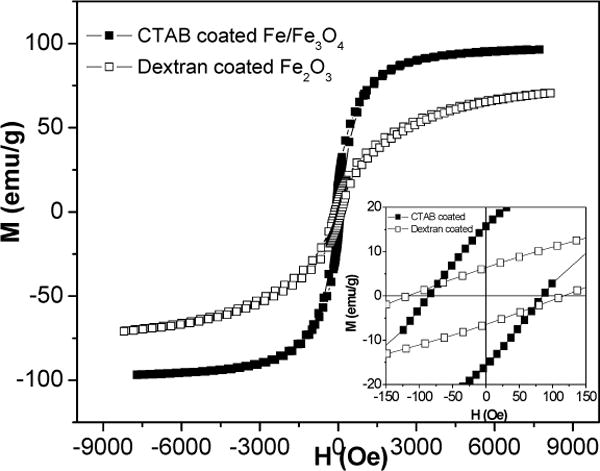
Hysteresis loops for CTAB coated Fe/Fe3O4 and Dextran coated Fe2O3 dried powders, inset is the central part of the same loops.
Fig. 9 shows a plot of the temperature rise as a function of time for Fe/Fe3O4 and Fe2O3 particles dispersed in methanol with the same concentration of 5mg/ml and under an alternating magnetic field of 150 Oe and 250 kHz. The temperature rise of CTAB coated Fe/Fe3O4 particles is much larger than that for Fe2O3 particles, correspondingly, the calculated SARs for Fe/Fe3O4 and Fe2O3 are 345 W/g and 122 W/g, respectively. The SAR of 345 W/g was also found to be twice as high as that for the Dextran coated Fe oxides [16]. We believe that the difference in SAR between Fe/Fe3O4 and Fe2O3 lies in two factors: high MS and narrow particle size distribution for the Fe/Fe3O4 particles. The former directly means high hysteresis loop area, the latter leads to a more square loop and therefore larger loop area for a certain field amplitude.
Fig. 9.

Temperature versus time for CTAB coated Fe/Fe3O4 and Fe2O3 particles dispersed in methanol and under an alternating field of 150 Oe and 250 kHz. The drop of temperature was due to magnetic field being turned off. Inset is the corresponding dT/dt versus time plots.
4. CONCLUSIONS
Fe/Fe3O4 core-shell nanoparticles were synthesized by reduction of aqueous FeCl3 within a NaBH4 solution with or without micro-emulsions. Particles obtained without micro-emulsions showed larger particle sizes and higher HC values. A slow NaBH4 flow rate or annealing decreased HC to a value that is suitable for higher SAR. In the presence of micro-emulsions, smaller, single domain particles (10–15 nm) with a narrow size distribution were obtained. Due to high MS and narrow particle size distribution, Fe/Fe3O4 core-shell nanoparticles showed larger SAR than Fe oxide. The maximum SAR of 345 W/g was obtained for CTAB coated Fe/Fe3O4 nanoparticles at an alternating field of 150Oe and 250 kHz.
Acknowledgments
This work was supported by Cancer Nanotechnology Working Group (CNWG) at Dartmouth College and NIST grant 60NANB2D0120. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing official policies, either expressed or implied, of the National Institute of Standards and Technologies, or the U.S. Government.
References
- 1.Gilchrist RK, et al. Ann Surgery. 1957;146:596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinkai M. J Biosci Bioeng. 2002;94:606. [PubMed] [Google Scholar]
- 3.Josephson L, et al. Mag Reson Imag. 1988;6:564–653. [Google Scholar]
- 4.Hergt R, Hiergeist R, Hilger I, et al. J Magn Magn Mater. 2004;270:345–357. [Google Scholar]
- 5.Ivkov R, et al. Clin Cancer Res. 2005;11(19 Supppl):7093s–7103s. doi: 10.1158/1078-0432.CCR-1004-0016. [DOI] [PubMed] [Google Scholar]
- 6.Andrä W. In: Magnetism in Medicine: A Handbook. Andrä W, Nowak H, editors. Wiley-VCH; Berlin: 1998. p. 455. [Google Scholar]
- 7.Pillai V, Shah DO. J Magn Magn Mater. 1996;163:243. [Google Scholar]
- 8.Eicke HF, Shepherd JCW, Steinemann A. J Colloid Interface Sci. 1976;56:168. [Google Scholar]
- 9.Baker I, Zeng Q, Li W, Sullivan CR. J Appl Phys. 2006;99(8):08H106. [Google Scholar]
- 10.Specific heat capacity, From Wikipedia, the free encyclopedia.
- 11.Chantrell RW, Bradbury A, Popplewell J, Charles SW. J Phys D: Appl Phys. 1980;13:l119. [Google Scholar]
- 12.Chen CW. Magnetism and Metallurgy of Soft Magnetic Materials. North-Holland: 1977. p. 132. [Google Scholar]
- 13.Gangopadhyay S, Hadjiopanayis GC, Dale B, et al. Phy Rev B. 1992;45:9778. doi: 10.1103/physrevb.45.9778. [DOI] [PubMed] [Google Scholar]
- 14.Stoner EC, Wohlfarth EP. Philos Trans R Soc London Ser A. 1948;240:599. [Google Scholar]
- 15.Herzer G. IEEE Trans Magn. 1990;26:1397. [Google Scholar]
- 16.Zeng Q, Baker I. The Heating Effects of Dextran Coated Iron Oxide. 2006 accepted, MRS. [Google Scholar]


