Abstract
Exposure to acute high-altitude hypoxia is associated with an increase in cerebral blood flow (CBF) as a consequence of low arterial O2 tension. However, in response to high altitude acclimatization, CBF returns to levels similar to those at sea level, and tissue blood flow is maintained by an increase in angiogenesis. Of consequence, dysregulation of the acclimatization responses and CBF can result in acute mountain sickness, acute cerebral and/or pulmonary edema. To elucidate the signal transduction pathways involved in successful acclimatization to high altitude, in ovine carotid arteries, we tested the hypothesis that high altitude-associated long-term hypoxia results in changes in gene expression of critical signaling pathways. We acclimatized nonpregnant adult sheep to 3,801 m altitude for ∼110 days and conducted oligonucleotide microarray experiments on carotid arteries. Of a total of 116 regulated genes, 58 genes were significantly upregulated and 58 genes were significantly downregulated (each >2-fold, P < 0.05). Major upregulated genes included suprabasin and myelin basic protein, whereas downregulated genes included BAG2. Several of these genes are known to activate the ERK canonical signal transduction pathway and the process of angiogenesis. We conclude that among other changes, the altered signal transduction molecules involved in high-altitude acclimatization are associated ERK activation and angiogenesis.
Keywords: chronic hypoxia, oligonucleotide microarray, acute mountain sickness, prolonged hypoxia
hypoxia is a complex organismal stress accompanied by an attempt to maintain homeostasis that initiates a multitude of physiological responses. Two major responses to hypoxic stress are hematopoiesis with an increase in blood volume and induction of angiogenesis in many organs including the brain. Dysregulation of acclimatization to prolonged hypoxia can lead to acute mountain sickness, high-altitude cerebral and/or pulmonary edema, chronic migraine, and other high altitude-associated disorders (3, 22, 25–27).
At high altitude a major detrimental factor is the relatively lower oxygen tension associated with hypobaric pressure. At an altitude of 3,801 m, we observed a fall in arterial Po2 from 102 ± 2 to 64 ± 2 Torr (21). Furthermore, the adult hemoglobin concentration increased from 8.9 ± 0.5 to 10.0 ± 0.5 g/dl within 24 h where it remained till the end of the study (110 days). In contrast, erythropoietin concentration increased from 16.6 ± 2.1 to 39.1 ± 7.8 mU/ml at 24 h but then returned to near-control levels within few days. Accompanying the reduced Po2 in these animals, cardiac output decreased 14% from 7.15 ± 0.46 to 6.13 ± 0.46 l/min (7). Importantly, this decreased cardiac output was redistributed, resulting in normal blood flow to the brain and several other vital organs (14, 16, 17, 21). Despite continued hypoxic conditions, the basic mechanisms that account for successful acclimatization and normal blood flow to the brain are not known.
The carotid arteries have been shown to be crucial for the regulation and maintenance of cerebral blood flow (CBF) (10). Especially with increased circulatory demand, studies have demonstrated a significant hydrostatic pressure gradient from carotid to cerebral arteries, presumably to prevent the exposure of delicate cerebral arteries to excessive pressure (4). Along this line, it is well established that much of the change in systemic blood pressure results from vasomotion (dilation or contraction) in the large arteries that supply the brain (18). Thus, a considerable body of evidence suggests that carotid arteries constitute valid resistance vessels and are vital for the regulation of CBF. Moreover, failure of carotid arteries to regulate effectively the pressure of blood reaching the delicate cerebral arteries may result in their rupture with hemorrhage. In response to long-term hypoxia (LTH), a possible adaptive mechanism could be a shift in the structure and/or composition of carotid arteries favoring larger diameters with increased dilation and reduced hydraulic resistance. We have discovered that LTH had no significant effects on an average artery wall thicknesses or water content (23). Importantly, unstressed inside diameter of the major carotid arteries was significantly greater in normoxic than hypoxic sheep (21). Furthermore, we observed significant alterations in the protein content of the carotid arteries with LTH exposure (23). These findings suggest a substantial changes in vascular gene expression in response to LTH.
In an attempt to explore the mechanistic pathways of successful acclimatization responses, we tested the hypothesis that high altitude-associated LTH leads to changes in gene expression of critical signaling pathways in ovine carotid arteries. In the present report, we used custom ovine oligonucleotide microarrays and signaling pathway analysis to identify changes in the gene expression and the pathways involved in LTH acclimatization.
METHODS
Experimental animals and tissues.
All experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Guidelines of the American Physiological Society and were approved by the Animal Care and Use Committee of Loma Linda University.
We examined carotid arteries from adult female sheep (about 24 mo of age) maintained near sea level (300 m; arterial Po2 = 100 ± 5 Torr) and those acclimatized to high altitude (3,801 m, 12,470 ft, PaO2 = 60 ± 5 Torr) at White Mountain Research Station, Bishop, CA, for 110 days (n = 4 in each group). Ewes were obtained from Nebeker Ranch (Lancaster, CA). To avoid variability due to sex and nutrition, we conducted studies on the carotid arteries from female sheep, and both oxygenation groups were fed alfalfa pellets ad libitum. Both normoxic ewes and those acclimatized at high altitude were transported to the Center for Perinatal Biology at Loma Linda University, the latter of which is a ∼6 h trip. Shortly after the sheep were brought to the Center, a tracheal catheter was placed in the ewe, through which N2 flowed at a rate adjusted to maintain her PaO2 at ∼60 Torr, i.e., that at high altitude (17, 21). At the time of experimental study, ewes were euthanized with an overdose of the proprietary euthanasia solution Euthasol (100 mg/kg pentobarbital sodium and 10 mg/kg phenytoin sodium; Virbac, Ft. Worth, TX). In vitro studies were performed in isolated carotid arteries cleaned of endothelium, adipose, and connective tissue.
Tissue collection and microarray processing.
Ovine carotid arteries were cleaned of adventitia, and the endothelium was denuded in a phosphate-free balanced salt solution (BSS) of the following composition (mM): 126 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 2 CaCl2, 10 glucose, pH 7.4 (adjusted with NaOH). Complete endothelial removal was confirmed by confocal microscopy. Following cleaning the tissue was snap-frozen in liquid nitrogen and kept in a −80°C freezer until further analysis. Ovine oligonucleotide microarray was obtained from Agilent Technologies (Santa Clara, CA), and we conducted analysis by utilizing commercial services of GenUs Biosystems (Northbrook, IL). In brief, arterial tissue samples were lysed in Tri-reagent (Ambion, Austin, TX), and total RNA was isolated by phenol-chloroform extraction followed by purification over spin columns (Ambion). The concentration and purity of total RNA were measured by spectrophotometry at optical density 260/280, and the quality of the total RNA sample was assessed with an Agilent Bioanalyzer with the RNA6000 Nano Lab Chip (Agilent Technologies).
Labeled cRNA was prepared by linear amplification of the Poly(A)+ RNA population within the total RNA sample. In brief, we reverse-transcribed 1 μg of total RNA after priming it with a DNA oligonucleotide containing the T7 RNA polymerase promoter 5′ to a d(T)24 sequence. After second-strand cDNA synthesis and purification of double-stranded cDNA, in vitro transcription was performed with T7 RNA polymerase. The quantity and quality of the labeled cRNA were assayed by spectrophotometry and the Agilent Bioanalyzer.
We fragmented 1 μg of purified cRNA to uniform size and applied it to Agilent Sheep Gene Expression Microarray, 8 × 15K (Design ID 019921, Agilent Technologies) in hybridization buffer. Arrays were hybridized at 65°C for 17 h in a shaking incubator and washed at 37°C for 1 min. Rinsed and dried arrays were scanned with an Agilent G2565 Microarray Scanner (Agilent Technologies) at 5 μm resolution. Agilent Feature Extraction software was used to process the scanned images from arrays (gridding and feature intensity extraction), and the data generated for each probe on the array were analyzed with GeneSpring GX v7.3.1 software (Agilent Technologies). Annotations are based on the Agilent eArray annotation file dated January, 2010.
Pathway/network analysis.
Each gene was annotated/checked manually with National Center for Biotechnology Information Blast Search, Unigene, Entrez, or other databases. Genes with no known sequences for Ovis aries were annotated if >90% sequence homology was identified with Bos taurus. We then analyzed the annotated genes with Ingenuity Pathway Analysis Program (IPA; Ingenuity Systems, Redwood City, CA). IPA downstream effects analysis was utilized to predict increases (activation) or decreases (inactivation) in downstream biological activities occurring in the tissues being studied. This analysis is based on prior knowledge of expected effects of gene upregulation or downregulation on a particular biological process stored in Ingenuity Knowledge Base (studies indexed in PubMed). The analysis examines how many known targets of each transcription regulator are present in the user's dataset and also compares their direction of change [i.e., expression in the experimental sample(s) relative to control] to what is expected from the literature in order to predict likely relevant transcriptional regulators. If the observed direction of change is mostly consistent with a particular activation state of the transcriptional regulator (“activated” or “inhibited”), then a prediction is made about that activation state. For each potential transcriptional regulator (“TR”) two statistical measures, an overlap P value and an activation z score are computed. The purpose of the overlap P value is to identify transcriptional regulators that are able to explain observed gene expression changes. It is calculated by Fisher's exact test, and significance is attributed to P values < 0.01. The primary purpose of the activation z-score is to infer the activation states of predicted transcriptional regulators. The basis for inference are relationships in the molecular network that represent experimentally observed gene expression or transcription events and are associated with a literature-derived regulation direction that can be either activating or inhibiting. However, genes can be modulated by several upstream regulators with possibly opposing effects, and it is not known which will dominate in a particular system. Therefore, we take a statistical approach by defining a quantity (z-score) that determines whether an upstream transcription regulator has significantly more activated predictions than inhibited predictions (z > 0) or vice versa (z < 0). Here, significance means that we reject the hypothesis that predictions are random with equal probability.
Hypoxia inducible factor-1α response element analysis.
To examine the presence of hypoxia inducible factor-1α (HIF1α) binding site in the upregulated and downregulated genes in response to LTH exposure, we analyzed the 1800 base pair upstream to the transcription start site for the presence of HIF1α response element (HRE) sequence (5′-A/GCGTG-3′).
Real-time PCR validation.
To validate the results of the microarray analysis, we chose APOBEC2, Bcl2-associated athanogene 2 (BAG2), and suprabasin. Also, we examined ERK1, ERK2, and HIF1α genes, which did not show significant change with LTH. Using the same probe sequences as those on the microarray chip, we designed primers with the use of Primer 3 web-based software (http://frodo.wi.mit.edu/primer3/). The primers were synthesized by Integrated DNA Technologies (Coralville, CA). Total RNA (1 μg per reaction) was reverse-transcribed with the Quantitect reverse transcriptase kit (Qiagen, Valencia, CA). Relative expression was normalized to 18S RNA, and fold-changes were calculated by the ΔΔCt method with normalization of individual PCR efficiencies (28). Samples were analyzed on the Roche LightCycler 1.5 (Roche, Indianapolis, IN).
Western immunoblot validation.
As noted, ovine carotid arteries were cleaned of adventitia, and the endothelium was denuded in a phosphate-free BSS of the following composition (mM): 126 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 2 CaCl2, 10 glucose, pH 7.4 (adjusted with NaOH). The arteries were homogenized with a tissue grinder in ice-cold cell lysis buffer (Cell Signaling Technology, Danvers, MA), as described previously (8). Protein concentrations were measured with a protein assay kit (Bio-Rad Laboratories, Hercules, CA), and bovine serum albumin was used as a reference protein standard. A Mini Trans-Blot Electrophoretic Transfer Cell system (Bio-Rad Laboratories) was used to transfer proteins from the gel to a nitrocellulose membrane at 100 V for 3 h. We then performed an overnight incubation of subtype specific primary antibodies (1:500 dilution) for c-MYC, myelin basic protein (MBP), and BAG2. We used total ERK and α-smooth actin as an internal control for equal protein loading, as well as the blocking peptide for each subtype-specific antibody as a negative control. All antibodies and peptides were obtained from Abcam (Cambridge, MA). The membrane was then incubated in the secondary antibodies obtained from LI-COR Biosciences (Lincoln, NE) for 45 min. The protein bands were detected with the Odyssey Infrared Imaging System (LI-COR Biosciences).
Measurement of capillary density.
Frontal lobe segments at specific coronal and sagittal planes cortex were sliced to 10 μm with a Leica Cryostat (Leica Microsystems, Buffalo Grove, IL). The slices were fixed by 4% paraformaldehyde, and the capillaries were stained with FITC-conjugated lectin from Bandeiraea simplicifolia (Griffonia simplicifolia). Tissue sections were then sealed on microscopic slides with Vectashield mounting medium containing DAPI (H-1200; Vector Labs, Burlingame, CA). The capillaries were imaged by use of an Evos florescence microscope (Life Sciences Technologies, Grand Island, NY). Images were analyzed with the ImageJ software (NIH), and the capillary density was measured as counts/unit area normalized to the nuclei/unit area.
Statistics.
The raw intensity data from each gene were normalized to the 75th percentile intensity of each array to compare individual gene expression values across arrays. Only those genes with values greater than background intensity for all samples within each group were used for further analysis. Differentially expressed genes were identified by twofold change and Welch t-test P values < 0.05 between each treatment group and its age-specific normoxic control. Statistical significance in the real-time PCR data was determined by one-way analysis of variance and post hoc Newman-Keul test.
RESULTS
To determine the molecular mechanisms involved in LTH acclimatization responses, we sheltered sheep at high altitude (3,801 meters) for 3.5 mo. At this elevation, we observed that sheep arterial blood oxygen partial pressure fell from 95 to 100 Torr to 55 to 60 Torr.
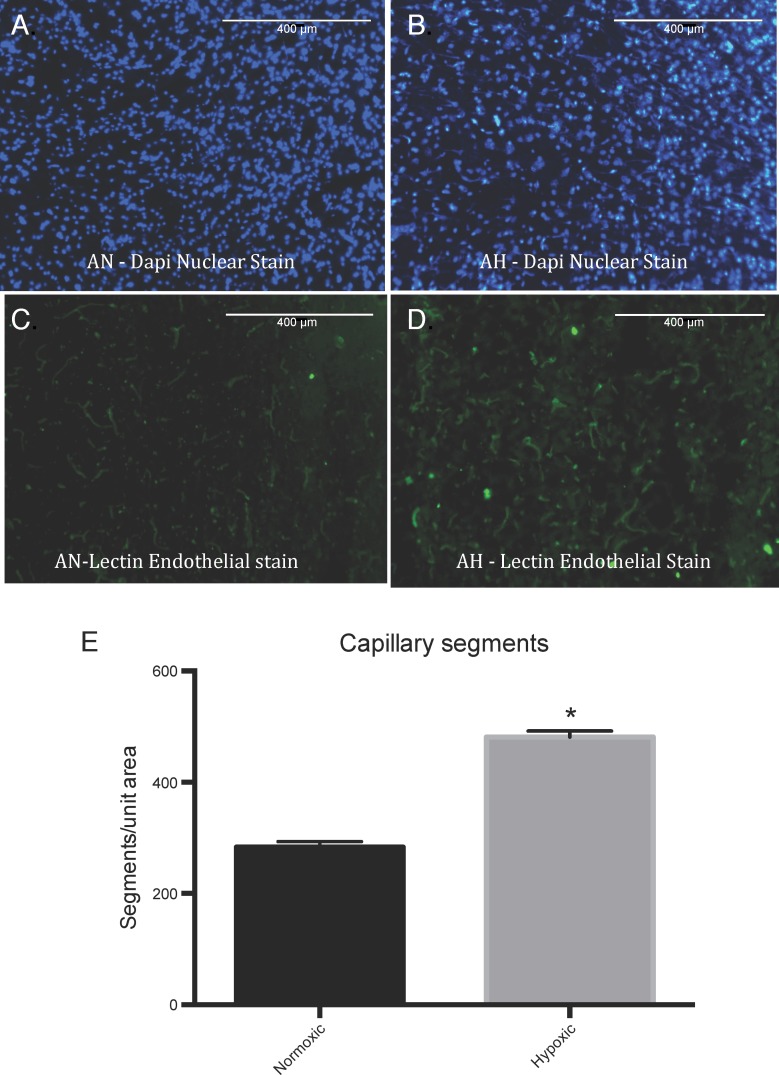
In response to high-altitude LTH exposure for 110 days, in these sheep carotid arteries, we observed increased expression of 58 genes (>2-fold, P < 0.05; Table 1) and reduced expression of 58 genes (>2-fold, P < 0.05; Table 2). Importantly, HRE was present in 44 and 40 upregulated and downregulated genes in response to LTH. Next, we conducted a functional pathway analysis to identify the major gene pathways altered with LTH acclimatization. Most of altered genes were those associated with cellular movement, growth and proliferation, and angiogenesis. To examine the extent to which LTH is indeed associated with increased angiogenesis, we also examined capillary density in frontal cortex of control and LTH-acclimatized sheep. As shown in Fig. 1, there was a significant increase in frontal cortex capillary density in LTH-acclimatized sheep.
Table 1.
Upregulated genes in hypoxic carotid arteries
| Gene Symbol | Gene Name | Fold Change | P Value | HRE |
|---|---|---|---|---|
| IMUP2 | chromosome 19 open reading frame 33 | 12.5 | 0.007 | − |
| LOC10010123 | regakine 1-like protein | 10.9 | 0.035 | − |
| SBSN | suprabasin | 9.9 | 0.002 | + |
| MBP | myelin basic protein | 9.1 | 0.029 | + |
| APOBEC2 | apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 2 | 8.4 | 0.003 | − |
| SFXN1 | sideroflexin 1 | 4.5 | 0.018 | − |
| PTN | pleiotrophin | 4.4 | 0.020 | + |
| BRB | brain ribonuclease | 4.1 | 0.025 | − |
| IL18 | interleukin 18 (interferon-gamma-inducing factor) | 4.0 | 0.045 | + |
| APOA1 | apolipoprotein A-I | 3.9 | 0.040 | − |
| POMC | proopiomelanocortin | 3.8 | 0.045 | + |
| RIN3 | Ras and Rab interactor 3 | 3.7 | 0.020 | + |
| FST | follistatin | 3.7 | 0.028 | − |
| CES1 | carboxylesterase 1 | 3.6 | 0.009 | − |
| F3 | coagulation factor III (thromboplastin, tissue factor) | 3.6 | 0.011 | unknown |
| TIMM8A | translocase of inner mitochondrial membrane 8 homolog A (yeast) | 3.5 | 0.018 | + |
| NQO2 | NAD(P)H dehydrogenase, quinone 2 | 3.5 | 0.001 | + |
| CXCL14 | chemokine (C-X-C motif) ligand 14 | 3.5 | 0.034 | + |
| CD200 | CD200 molecule | 3.4 | 0.016 | + |
| OXT | oxytocin/neurophysin 1 prepropeptide | 3.3 | 0.008 | − |
| NPDC1 | neural proliferation, differentiation and control | 3.2 | 0.002 | + |
| CPPED1 | calcineurin-like phosphoesterase domain containing 1 | 3.1 | 0.005 | − |
| LAPTM5 | lysosomal protein transmembrane 5 | 3.1 | 0.028 | + |
| BREH1 | retinyl ester hydrolase type 1 | 3.1 | 0.005 | + |
| ALDH1A1 | aldehyde dehydrogenase 1 family, member A1 | 3.0 | 0.028 | − |
| MCM6 | minichromosome maintenance complex component 6 | 3.0 | 0.026 | + |
| TMEM134 | transmembrane protein 134 | 2.9 | 0.019 | + |
| LOC782921 | ferritin, light polypeptide pseudogene | 2.8 | 0.049 | + |
| AlF1 | allograft inflammatory factor 1 | 2.7 | 0.045 | + |
| CRIP1 | cysteine-rich protein 1 (intestinal) | 2.7 | 0.003 | + |
| MFAP5 | microfibrillar associated protein 5 | 2.7 | 0.024 | + |
| ESR1 | estrogen receptor 1 | 2.6 | 0.001 | + |
| FAM89B | family with sequence similarity 89, member B | 2.6 | 0.006 | + |
| TNFAIP8L3 | tumor necrosis factor, alpha-induced protein 8-like 3 | 2.6 | 0.005 | − |
| PCOLCE2 | procollagen C-endopeptidase enhancer 2 | 2.5 | 0.029 | + |
| CRYAB | crystallin, alpha B | 2.5 | 0.019 | + |
| MYC | v-myc myelocytomatosis viral oncogene homolog (avian) | 2.5 | 0.049 | + |
| CMTM3 | CKLF-like MARVEL transmembrane domain containing 3 | 2.4 | 0.011 | + |
| CMTM5 | CKLF-like MARVEL transmembrane domain containing 5 | 2.4 | 0.004 | + |
| LOC10013891 | ribosomal protein L36a-like | 2.4 | 0.032 | − |
| LAYN | layilin | 2.4 | 0.048 | + |
| BMP4 | bone morphogenetic protein 4 | 2.4 | 0.002 | + |
| UCHL3 | ubiquitin carboxyl-terminal esterase L3 (ubiquitin thiolesterase) | 2.4 | 0.044 | + |
| MUC12 | mucin 12, cell surface associated | 2.3 | 0.019 | + |
| PSMB9 | proteasome (prosome, macropain) subunit, beta type, 9 | 2.3 | 0.039 | + |
| SOCS2 | suppressor of cytokine signaling 2 | 2.2 | 0.005 | + |
| DCN | decorin | 2.2 | 0.036 | + |
| FMN1 | formin 1 | 2.2 | 0.035 | + |
| GLT8D2 | glycosyltransferase 8 domain containing 2 | 2.2 | 0.027 | + |
| RTP4 | receptor transporter protein 4 | 2.2 | 0.042 | + |
| LOC529036 | transmembrane protein 45A-like | 2.2 | 0.009 | + |
| MX2 | myxovirus (influenza virus) resistance 2 (mouse) | 2.1 | 0.032 | + |
| DDI2 | DNA-damage inducible 1 homolog 2 (S. cerevisiae) | 2.1 | 0.020 | + |
| GYPC | glycophorin C (Gerbich blood group) | 2.1 | 0.044 | + |
| FOLR2 | folate receptor 2 (fetal) | 2.1 | 0.044 | + |
| KDELR3 | KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 3 | 2.1 | 0.008 | + |
| LOC654400 | GATA binding protein 6 | 2.0 | 0.012 | + |
| AEBP1 | AE binding protein 1 | 2.0 | 0.032 | + |
HRE, hypoxia inducible factor-1α response element.
Table 2.
Downregulated genes in hypoxic carotid arteries
| GeneSymbol | Gene Name | Fold Change | P Value | HRE + |
|---|---|---|---|---|
| ZNF146 | zinc finger protein 146 | 18.1 | 0.003 | + |
| POMP | proteasome maturation protein | 7.5 | 0.004 | + |
| BAG2 | BCL2-associated athanogene 2 | 6.3 | 0.019 | + |
| TM9SF1 | transmembrane 9 superfamily member 1 | 5.2 | 0.007 | + |
| CALCOCO1 | calcium binding and coiled-coil domain 1 | 4.9 | 0.044 | − |
| TSPAN14 | tetraspanin 14 | 4.2 | 0.043 | + |
| B3GNT2 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 2 | 4.0 | 0.011 | + |
| INTS7 | integrator complex subunit 7 | 3.8 | 0.016 | + |
| C1QTNF1 | C1q and tumor necrosis factor related protein 1 | 3.8 | 0.025 | + |
| LOC100125916 | uncharacterized protein 100125016 | 3.6 | 0.011 | − |
| NPY1R | neuropeptide Y receptor Y1 | 3.6 | 0.026 | + |
| SLC35D2 | solute carrier family 35, member D2 | 3.4 | 0.046 | + |
| LOC607775 | uncharacterized LOC607775 | 3.3 | 0.023 | unknown |
| C3H1orf85 | chromosome 3 open reading frame, human C1orf85 | 3.3 | 0.002 | − |
| EGR1 | early growth response 1 | 3.2 | 0.011 | + |
| TNFAIP8L1 | tumor necrosis factor, alpha-induced protein 8-like 1 | 3.1 | 0.035 | − |
| SSFA2 | sperm specific antigen 2 | 3.0 | 0.002 | + |
| GJA5 | gap junction protein, alpha 5 | 3.0 | 0.040 | − |
| ART4 | ADP-ribosyltransferase 4 (Dombrock blood group) | 2.9 | 0.029 | − |
| TSPAN7 | tetraspanin 7 | 2.8 | 0.006 | + |
| BANP | BTG3 associated nuclear protein | 2.8 | 0.029 | + |
| PRMT7 | protein arginine methyltransferase 7 | 2.8 | 0.040 | + |
| MAPRE2 | microtubule-associated protein, RP/EB family, member 2 | 2.7 | 0.017 | + |
| DCAF8 | DDB1 and CUL4 associated factor 8 | 2.6 | 0.049 | + |
| SIRT3 | sirtuin 3 | 2.6 | 0.037 | − |
| GFRA2 | glial cell line derived neurotrophic factor family receptor alpha 2 | 2.6 | 0.026 | + |
| FTSJ1 | FtsJ RNA methyltransferase homolog 1 (E. coli) | 2.5 | 0.045 | − |
| CLMP | CXADR-like membrane protein | 2.5 | 0.007 | + |
| ELN | elastin | 2.4 | 0.045 | + |
| SLC26A11 | solute carrier family 26, member 11 | 2.4 | 0.033 | + |
| CD82 | CD82 molecule | 2.4 | 0.014 | + |
| DCAF11 | DDB1 and CUL4 associated factor 11 | 2.4 | 0.045 | + |
| OLFML1 | olfactomedin-like 1 | 2.4 | 0.032 | − |
| PIGS | phosphatidylinositol glycan anchor biosynthesis, class S | 2.4 | 0.034 | + |
| DPH2 | DPH2 homolog (S. cerevisiae) | 2.3 | 0.037 | + |
| MSX2 | msh homeobox 2 | 2.3 | 0.002 | + |
| IER3 | immediate early response 3 | 2.3 | 0.036 | + |
| BCL6 | B-cell CLL/lymphoma 6 | 2.3 | 0.037 | − |
| FDFT1 | farnesyl-diphosphate farnesyltransferase 1 | 2.3 | 0.043 | + |
| ACVR1 | activin A receptor, type I | 2.3 | 0.026 | + |
| FAM113B | family with sequence similarity 113, member B | 2.3 | 0.029 | − |
| ABI1 | abl-interactor 1 | 2.3 | 0.045 | + |
| TMEM64 | transmembrane protein 64 | 2.3 | 0.013 | − |
| PRKCD | protein kinase C, delta | 2.3 | 0.003 | − |
| RAD23B | RAD23 homolog B (S. cerevisiae) | 2.2 | 0.037 | + |
| CLC4 | chloride channel ClC4 | 2.2 | 0.006 | − |
| IL6ST | interleukin 6 signal transducer (gp130, oncostatin M receptor) | 2.1 | 0.024 | + |
| RDH11 | retinol dehydrogenase 11 (all-trans/9-cis/11-cis) | 2.1 | 0.030 | + |
| SUB1 | SUB1 homolog (S. cerevisiae) | 2.1 | 0.031 | + |
| PTPLAD2 | protein tyrosine phosphatase-like A domain containing 2 | 2.1 | 0.014 | − |
| TOB1 | transducer of ERBB2, 1 | 2.1 | 0.041 | − |
| NDUFV3 | NADH dehydrogenase (ubiquinone) flavoprotein 3 | 2.1 | 0.031 | + |
| DPF2 | D4, zinc and double PHD fingers family 2 | 2.1 | 0.032 | + |
| ENDOV | endonuclease V | 2.1 | 0.011 | + |
| LOC718351 | RING finger protein 185-like | 2.0 | 0.042 | − |
| RPRD1A | regulation of nuclear premRNA domain containing 1A | 2.0 | 0.037 | + |
| BHLHE40 | basic helix-loop-helix family, member e40 | 2.0 | 0.005 | + |
| CDK5RAP3 | CDK5 regulatory subunit associated protein 3 | 2.0 | 0.022 | + |
Fig. 1.
Capillary density in frontal cortex from normoxic and hypoxic sheep brain. A and B: nuclei staining in the normoxic (AN) and hypoxic (AH) frontal cortex section, respectively. C and D: green fluorescent lectin staining of the capillaries from normoxic and hypoxic tissue, respectively. E: quantitative analysis of visible lectin-stained segments from normoxic and hypoxic tissue by ImageJ analysis. *P < 0.05; n = 6 in each group.
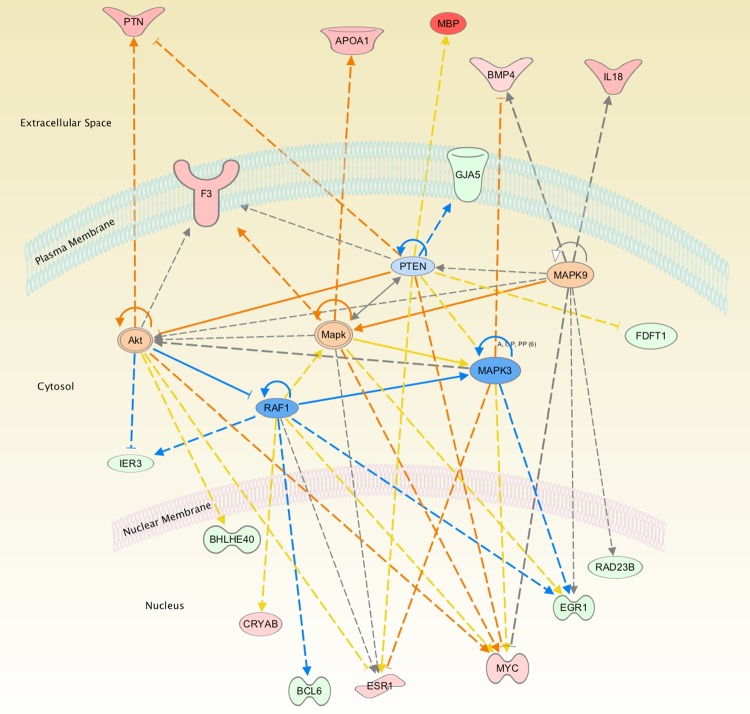
Next, we conducted a network analysis of the different pathways to examine if there are common downstream canonical signal transduction pathways that link these various functional groups. As shown in Fig. 2 bioinformatic analysis suggests activation of MAPK canonical pathways, with significant inhibition of the PTEN and RAF1 signaling cascade. However, ERK1/2 mRNA were unaltered in the microarray analysis as well as by real-time PCR (data not shown).
Fig. 2.
The network of genes identified by Ingenuity Pathway Analysis in ovine carotid arteries as a consequence of long-term hypoxia (LTH). The color red represents the upregulated genes, and green represents the downregulated genes. Orange and blue represent the activated and inhibited pathways, respectively, based on the Ingenuity Pathway Analysis. Yellow lines denote that the observed activation state was opposite to the activation state predicted by the available literature. Gray lines denote insufficient available data to predict activation state between the 2 molecules.
To explore the possibility that ERK1/2 mRNA does not change but that there is a significant increase in the activation of these molecules, we examined the phosphorylated levels of ERK1/2 in carotid arteries of control sheep and those acclimatized to LTH. As shown in Fig. 3A, we observed a significant increase in phosphorylated ERK1/2 with LTH acclimatization in the sheep carotid arteries. There was no increase in total ERK1/2 levels. Similarly, Fig. 3B demonstrates a significant increase in the levels of phospho-AKT with no change in total AKT in response to LTH in ovine carotid arteries.
Fig. 3.
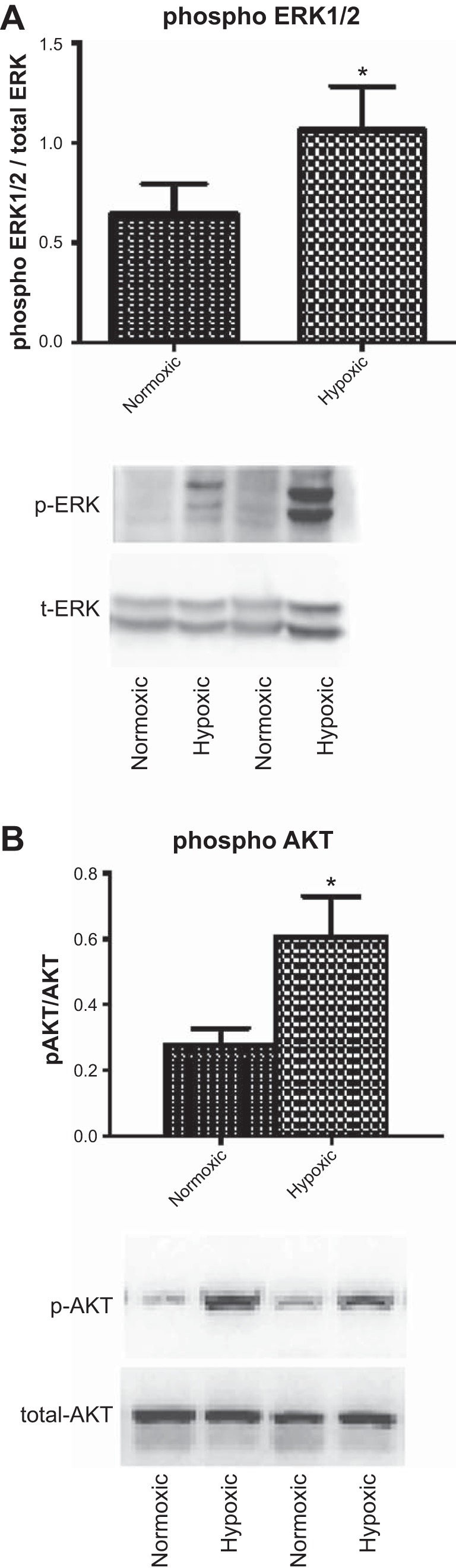
Western immunoblot analysis of phosphorylated ERK (p-ERK) and p-AKT levels in normoxic and LTH-acclimatized sheep carotid arteries. A: bar graph of densitometric analysis of phospho-ERK/total (t) ERK and Western blots from normoxic and hypoxic carotid arteries. B: bar graph of densitometric analysis of phospho-AKT/total AKT and Western blots from normoxic and hypoxic carotid arteries. Representative Western blots are shown below the bar graphs.
Importantly, to elucidate the regulators that can produce a similar change in gene expression profile, as produced by LTH acclimatization, we examined upstream regulators by IPA. We observed that LTH-associated changes in gene expression resemble those genes activated by lipopolysaccharides (Fig. 4).
Fig. 4.
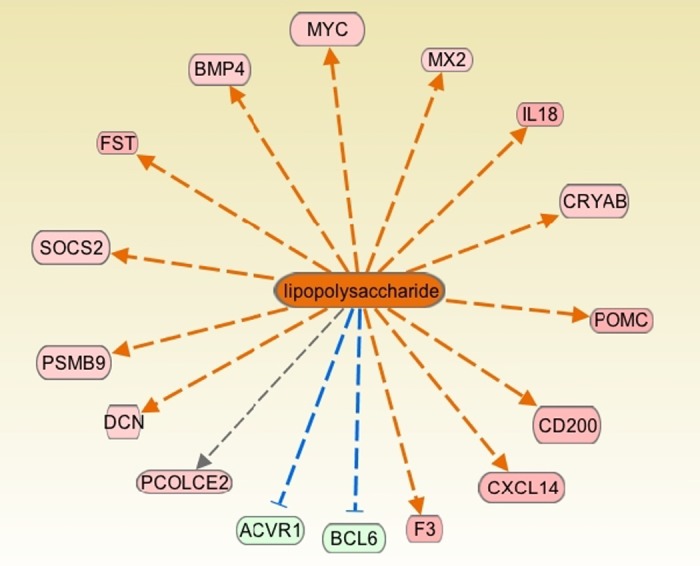
Genes known to be altered by lipopolysaccharide that were found to be altered in the present study as a consequence of LTH. The color red represents the upregulated genes, and green represents the downregulated genes. Orange and blue represent the activated and inhibited pathways, respectively, based on the Ingenuity Pathway Analysis. Yellow lines denote that the observed activation state was opposite to the activation state predicted by the available literature. Gray lines denote insufficient available data to predict activation state between the 2 molecules.
For Western immunoblot validation, we chose genes with moderate changes in gene alterations; these were MBP and C-myc from upregulated and BAG2 from downregulated genes. As demonstrated in Fig. 5, the protein levels correlated well with the microarray expression data. Also, we observed no significant difference in the protein levels of HIF1α with LTH (Fig. 6).
Fig. 5.
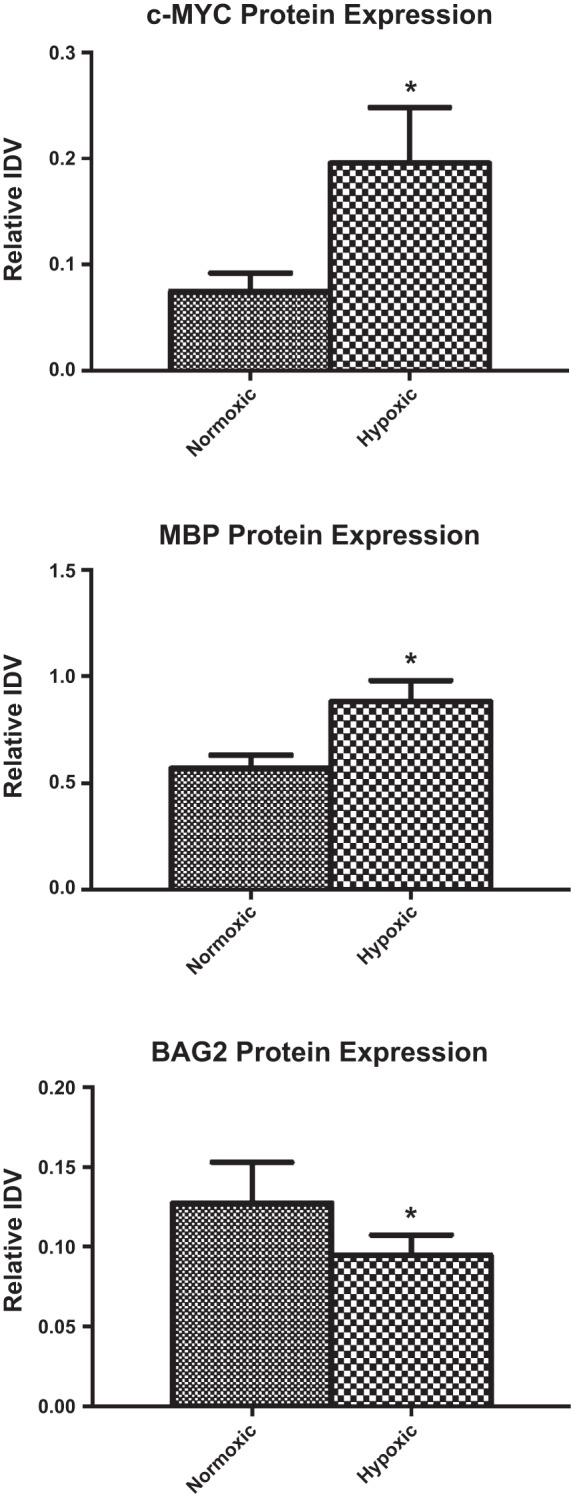
Integrated density values (IDV). Western immunoblot validation of the changes in gene expression of c-MYC (A), myelin basic protein (MBP) (B), and Bcl2-associated athanogene 2 (BAG2) (C). Relative IDV was determined by dividing densitometric value of the test protein with that of total ERK. *P < 0.05.
Fig. 6.
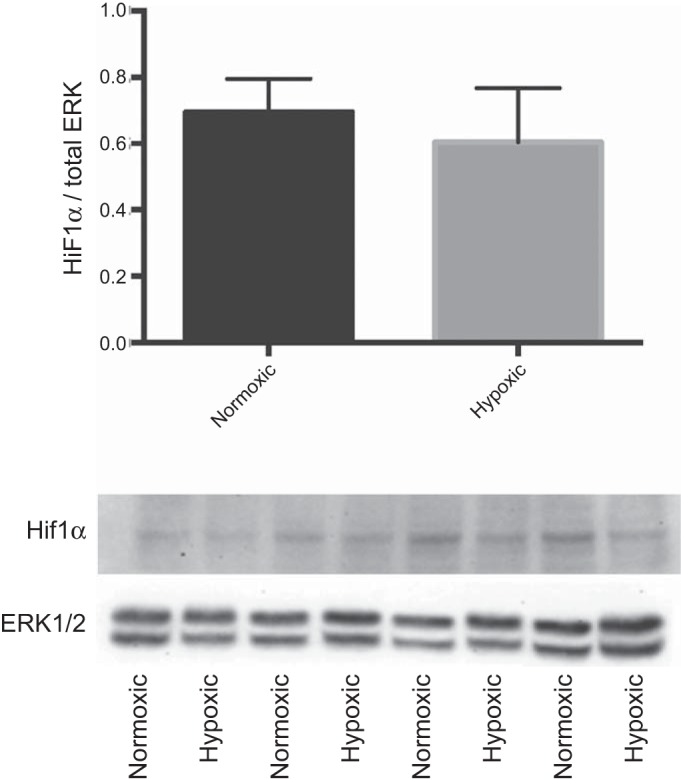
Western immunoblot validation of the changes in gene expression of hypoxia inducible factor-1 alpha (HIF1α). Relative IDV was determined by dividing densitometric value of the test protein with that of total ERK.
For PCR validation we chose genes with moderate expression, and these correlated well with the values observed by microarray analysis (Fig. 7). Real-time PCR analysis demonstrated upregulation of APOBEC2 (5 ± 1.3-fold) and suprabasin was upregulated 6.1 ± 0.9-fold. ERK1, ERK2, and HIF1α showed no significant changes as a consequence of LTH according to either real-time PCR or microarray analysis. Also, BAG2 showed 6.3 ± 0.4-fold downregulation on LTH exposure with real-time PCR.
Fig. 7.
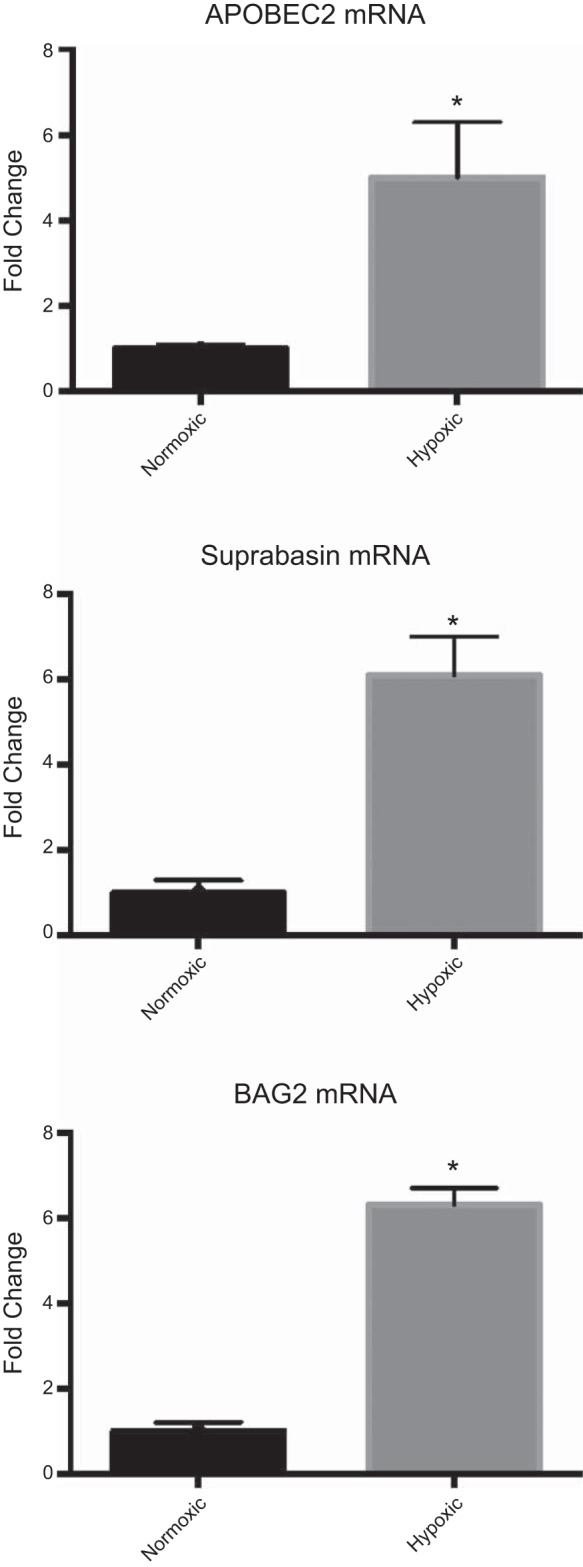
Bar graphs demonstrate mRNA levels of APOBEC2 (A), suprabasin (B), and BAG2 (C) following real-time PCR analysis. Ribosomal 18s RNA was used as control. *P < 0.05.
DISCUSSION
LTH is a complex problem and an important factor associated with a multitude of physiological and pathological conditions. With acute hypoxia, one of the initial changes is an increase in blood supply to the vital organs including the brain. As hypoxia continues, the blood flow returns to the normoxic basal levels; however, the mechanisms of this response are not known.
In the present study, we have identified a number of genes up- and downregulated by prolonged hypoxia that may play a critical role in the acclimatization response. We observed a significant upregulation of the cytokine IL-18 with LTH exposure. Importantly, studies have demonstrated that IL-18 can induce angiogenesis through phosphorylation of the SRC and JNK pathway (1). Also, bone morphogenetic protein 4 (BMP4) was upregulated, while activin A receptor was downregulated. This correlates well with a study demonstrating that activation by activin inhibits angiogenesis (15). Similarly, activation by BMP4 can lead to increased angiogenesic responses (29).
With LTH, we also observed a significant upregulation of immortalization upregulated protein-2 (IMUP-2) as has been shown in previous studies (12, 13). Although we do not know the role IMUP2 upregulation plays in carotid arteries, it is known to be upregulated in cancers. Thus, it also may play a role in hypoxia-induced angiogenesis. Similarly, MBP was upregulated as a consequence of LTH, which agrees with other studies demonstrating an increase in MBP with ischemia (5).
Importantly, with IPA we observed a significant LTH-induced activation of the pathways involved in angiogenesis (Tables 3 and 4). Also, with LTH we observed a significant increase in the capillary density in sheep brain. Furthermore, to elucidate common moieties that may be activated by functional pathways, we conducted a network analysis. We observed that AKT and ERK1/2 are the common downstream molecules that can be activated by several of the LTH-activated pathways. Upon further examination, we observed a significant increase in the phosphorylated levels of both these proteins in response to LTH exposure. Additionally, other studies support these findings that AKT and ERK play an important role in cell survival and angiogenesis (20, 31). On upstream bioinformatic analysis, we observed that lipopolysaccharides can produce changes in gene expression profiles similar to that observed in the present study. Lipopolysaccharides are well known to induce angiogenesis, and it appears that angiogenesis is a critical component of LTH acclimatization. Importantly, lipopolysaccharides are also known to mediate their effect through ERK1/2 (6) and AKT (24) signaling pathways.
Table 3.
Functional pathways represented by the genes altered in hypoxic carotid arteries
| Function | P Value | Molecules |
|---|---|---|
| Cell proliferation | 1.52E-06 | ABI1, ACVR1, AEBP1, ALDH1A1, APOA1, B3GNT2, BCL6, BHLHE40, BMP4, CD82, CRIP1, CRYAB, CXCL14, DCN, EGR1, ELN, ESR1, F3, FDFT1, FOLR2, FST, GFRA2, GJA5, IER3, IL18, IL6ST, LAPTM5, MAPRE2, MBP, MSX2, MYC, NPDC1, NPY1R, NQO2, OXT, POMC, PRKCD, PTN, SIRT3, SOCS2, TOB1, UCHL3 |
| Cell necrosis | 7.33E-04 | ALDH1A1, APOA1, BCL6, BHLHE40, BMP4, CD200, CES1, CRYAB, DCN, EGR1, ESR1, F3, FDFT1, FST, GFRA2, IER3, IL18, IL6ST, MBP, MSX2, MYC, NQO2, POMC, PRKCD, PTN, RAD23B, SIRT3, TNFAIP8L1 |
| Cell movement | 7.40E-05 | ACVR1, APOA1, BHLHE40, BMP4, CD200, CD82, CES1, CRYAB, CXCL14, DCN, EGR1, ELN, ESR1, F3, FST, IL18, IL6ST, MBP, MSX2, MYC, NQO2, POMC, PRKCD, PTN, RPRD1A, SOCS2 |
| Cell differentiation | 1.77E-04 | ABI1, ACVR1, BCL6, BHLHE40, BMP4, CD200, CD82, CDK5RAP3, DCN, EGR1, ESR1, FST, IL18, IL6ST, MBP, MSX2, MYC, NPY1R, OXT, PRKCD, PTN, SFXN1, SOCS2, TOB1, UCHL3 |
| Cell migration | 2.63E-04 | ACVR1, APOA1, BHLHE40, BMP4, CD200, CD82, CES1, CRYAB, CXCL14, DCN, EGR1, ELN, ESR1, F3, IL18, IL6ST, MSX2, MYC, NQO2, POMC, PRKCD, PTN, RPRD1A |
| Cellular development | 8.21E-08 | ABI1, ACVR1, APOA1, BCL6, BMP4, CRIP1, CRYAB, CXCL14, DCN, ELN, ESR1, F3, GJA5, IL18, IL6ST, MSX2, MYC, NPY1R, OXT, PRKCD, PTN, RAD23B |
| Transcription | 3.31E-03 | ACVR1, AEBP1, BCL6, BHLHE40, BMP4, CALCOCO1, CD82, CDK5RAP3, EGR1, ESR1, FST, IL18, IL6ST, MSX2, MYC, NPDC1, POMC, PRKCD, SOCS2, SUB1, ZNF146 |
| Cell size | 4.39E-06 | AEBP1, BCL6, BMP4, CXCL14, DCN, ESR1, F3, FST, GFRA2, IL6ST, MYC, NPY1R, POMC, PRKCD, RAD23B, SOCS2, SSFA2, UCHL3 |
| Vasculogenesis | 2.55E-06 | ABI1, ACVR1, APOA1, BMP4, CRYAB, CXCL14, DCN, ELN, ESR1, F3, IL18, MYC, OXT, PRKCD, PTN, RAD23B |
| Cell invasion | 6.88E-05 | BHLHE40, BMP4, CD82, CDK5RAP3, DCN, ESR1, F3, FST, IER3, IL18, MSX2, MYC, PRKCD, RPRD1A |
| Inflammatory response | 2.52E-04 | APOA1, BCL6, CD200, CXCL14, EGR1, ELN, IER3, IL18, IL6ST, NPY1R, POMC, PRKCD, PTN |
| Cellular morphology | 1.02E-03 | BMP4, CD200, EGR1, ESR1, FST, GFRA2, IL18, IL6ST, MBP, MSX2, MYC, NPY1R, UCHL3 |
Table 4.
Canonical pathways represented by the genes altered in hypoxic carotid arteries
| Canonical Pathways | −Log(P Value) | Ratio | Molecules |
|---|---|---|---|
| Aryl hydrocarbon receptor signaling | 2.27E +00 | 2.48E-02 | MYC, ALDH1A1, NQO2, ESR1 |
| Factors promoting cardiogenesis in vertebrates | 2.01E +00 | 3.19E-02 | BMP4, PRKCD, ACVR1 |
| Acute phase response signaling | 1.98E +00 | 2.23E-02 | IL6ST, IL18, APOA1, SOCS2 |
| Embryonic stem cell pluripotency | 1.91E +00 | 3.03E-02 | IL6ST, MYC, BMP4 |
| ERK/MAPK Signaling | 1.17E +00 | 1.46E-02 | MYC, PRKCD, ESR1 |
A limitation of many studies that investigated transcriptomic changes to LTH is that they were conducted on in vitro cell lines away from their normal environment of the tissue within the organism. However, we found few in vivo studies that have demonstrated changes in gene expression that vary with how different tissues experience hypoxia within the organisms (2, 11, 19, 30). In one such study (2), mice were acclimatized for 32 days to hypoxic conditions that simulated altitudes of 1,400, 3,000, and 4,500 m, and microarrays were conducted on liver tissue. Similar to our observation, this study demonstrated that HIF1α was not elevated, and the angiogenesis pathway was significantly upregulated. Moreover, on comparing the genes with altered expression in response to LTH, we observed significantly different sets of gene alterations with studies in liver (2), lung (19), and heart (11, 30). Even though different genes were activated in different organs, each study demonstrated that HIF1α levels were normalized with LTH exposure, and MAPK pathway activation was one of the major findings. Surprisingly, in the present study >70% of the altered genes have HRE. Thus, HIF1α, which is vital in the acute phase of survival under hypoxic stress, may be responsible for some epigenetic changes that keep these genes altered even when its own levels return to the basal state. However, further investigation are needed to elucidate the mechanisms of LTH-induced gene regulation in the absence of HIF1α.
In an earlier study, we examined the effect of LTH on gene expression in fetal carotid arteries (9). In contrast to the present findings in the adult carotid arteries, LTH in fetal carotid arteries was associated with significant activation of AKT and Bcl2 signaling pathways. Moreover, a completely different set of genes were altered in fetal carotids compared with adult carotids in response to LTH. Thus, it appears that LTH leads to differential regulation of genes based on maturational age as well as tissue examined.
Speculation and Perspective
Exposure to the stress of LTH occurs in a number of disorders such as heart and lung disease, chronic anemia, bone marrow disorders, and other diseases. Acclimatization to such a stress is critical for organismal survival and well-being. The present study demonstrates that LTH acclimatization is associated with activation of several genetic pathways, including those involved in angiogenesis. Moreover, the present and previous studies demonstrate that although important in acute hypoxic acclimatization, HIF1 may not be the major pathway involved in LTH acclimatization. Furthermore, these studies suggest that the LTH response differs in the specific tissue or maturational age examined. Thus, one should focus on the canonical or functional pathways altered rather than the individual genes involved. We speculate that individuals who fail to acclimatize to high altitude-associated LTH may benefit from therapy to stimulate angiogenesis and/or other signaling pathways.
GRANTS
The work was supported by National Institute of Child Health and Human Development Grant PO1 HD-031226 to L. D. Longo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.G. and L.D.L. conception and design of research; R.G. performed experiments; R.G. analyzed data; R.G. interpreted results of experiments; R.G. prepared figures; R.G. drafted manuscript; R.G. and L.D.L. edited and revised manuscript; R.G. and L.D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nina Chu for help with tissue collection.
REFERENCES
- 1.Amin MA, Rabquer BJ, Mansfield PJ, Ruth JH, Marotte H, Haas CS, Reamer EN, Koch AE. Interleukin 18 induces angiogenesis in vitro and in vivo via Src and Jnk kinases. Ann Rheum Dis 69: 2204–2212, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Baze MM, Schlauch K, Hayes JP. Gene expression of the liver in response to chronic hypoxia. Physiol Genomics 41: 275–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JK, Purvis RJ, Forfar JO, Cockburn F. Neurological aspects of perinatal asphyxia. Dev Med Child Neurol 16: 567–580, 1974 [DOI] [PubMed] [Google Scholar]
- 4.Dieckhoff D, Kanzow E. On the location of the flow resistance in the cerebral circulation. Pflügers Arch 310: 75–85, 1969 [DOI] [PubMed] [Google Scholar]
- 5.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res 4: 189–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, Alnemri ES, Gavrilin MA, Wewers MD. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol 192: 3881–3888, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert RD, Pearce WJ, Longo LD. Fetal cardiac and cerebrovascular acclimatization responses to high altitude, long-term hypoxia. High Alt Med Biol 4: 203–213, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 297: H2242–H2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal R, Van Wickle J, Goyal D, Matei N, Longo LD. Antenatal maternal long-term hypoxia: acclimatization responses with altered gene expression in ovine fetal carotid arteries. PLoS One 8: e82200, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heistad DD, Marcus ML, Abboud FM. Role of large arteries in regulation of cerebral blood flow in dogs. J Clin Invest 62: 761–768, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobas DA, Fan C, Iacobas S, Haddad GG. Integrated transcriptomic response to cardiac chronic hypoxia: translation regulators and response to stress in cell survival. Funct Integr Genomics 8: 265–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon SY, Lee HJ, Na KH, Cha DH, Kim JK, Park JW, Yoon TK, Kim GJ. Hypoxia-induced downregulation of XIAP in trophoblasts mediates apoptosis via interaction with IMUP-2: Implications for placental development during pre-eclampsia. J Cell Biochem 114: 89–98, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Jeon SY, Lee HJ, Park JM, Jung HM, Yoo JK, Lee HJ, Lee JS, Cha DH, Kim JK, Kim GJ. Increased immortalization-upregulated protein 2 (IMUP-2) by hypoxia induces apoptosis of the trophoblast and pre-eclampsia. J Cell Biochem 110: 522–530, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kamitomo M, Longo LD, Gilbert RD. Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol Regul Integr Comp Physiol 266: R1778–R1785, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Kaneda H, Arao T, Matsumoto K, De V, MA, Tamura D, Aomatsu K, Kudo K, Sakai K, Nagai T, Fujita Y, Tanaka K, Yanagihara K, Yamada Y, Okamoto I, Nakagawa K, Nishio K. Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer. Br J Cancer 105: 1210–1217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256: R1348–R1354, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Kitanaka T, Gilbert RD, Longo LD. Maternal responses to long-term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256: R1340–R1347, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol Heart Circ Physiol 234: H371–H383, 1978 [DOI] [PubMed] [Google Scholar]
- 19.Leonard MO, Howell K, Madden SF, Costello CM, Higgins DG, Taylor CT, McLoughlin P. Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. Am J Respir Crit Care Med 178: 977–983, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Fu GB, Zheng JT, He J, Niu XB, Chen QD, Yin Y, Qian X, Xu Q, Wang M, Sun AF, Shu Y, Rui H, Liu LZ, Jiang BH. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim Biophys Acta 1833: 3375–3385, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 264: R65–R72, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Longo LD, Packianathan S. Hypoxia-ischaemia and the developing brain: hypotheses regarding the pathophysiology of fetal-neonatal brain damage. Br J Obstet Gynaecol 104: 652–662, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Longo LD, Pearce WJ. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp Biochem Physiol Part A Mol Integr Physiol 119: 683–694, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol 180: 4218–4226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulligan JC, Painter MJ, O'Donoghue PA, MacDonald HM, Allan AC, Taylor PM. Neonatal asphyxia. II. Neonatal mortality and long-term sequelae. J Pediatr 96: 903–907, 1980 [DOI] [PubMed] [Google Scholar]
- 26.Naeye RL, Peters EC. Antenatal hypoxia and low IQ values. Am J Dis Child 141: 50–54, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Nelson KB. Can we prevent cerebral palsy? N Engl J Med 349: 1765–1769, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339: 62–66, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene 26: 4158–4170, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Singh M, Arya A, Kumar R, Bhargava K, Sethy NK. Dietary nitrite attenuates oxidative stress and activates antioxidant genes in rat heart during hypobaric hypoxia. Nitric Oxide 26: 61–73, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Liu S, Xi S, Yan L, Wang H, Song Y, Sun G. Arsenic induces the expressions of angiogenesis-related factors through PI3K and MAPK pathways in SV-HUC-1 human uroepithelial cells. Toxicol Lett 222: 303–311, 2013 [DOI] [PubMed] [Google Scholar]