Abstract
Facial pallor is commonly observed at presyncope in humans, suggestive of reductions in facial skin blood flow (SkBF). Yet, cutaneous vasoconstriction is usually minimal at presyncope when measured at the forearm. We tested the hypothesis that reductions in forehead SkBF at presyncope are greater than in the forearm. Forehead and forearm SkBF (laser-Doppler) and blood pressure (Finometer or radial artery catheterization) were measured during lower body negative pressure (LBNP) to presyncope in 11 normothermic and 13 heat-stressed subjects (intestinal temperature increased ∼1.4°C). LBNP reduced mean arterial pressure from 91 ± 5 to 57 ± 7 mmHg during normothermia (P ≤ 0.001) and from 82 ± 5 to 57 ± 7 mmHg during heat stress (P ≤ 0.001). During normothermia, LBNP decreased forehead SkBF 55 ± 14% compared with 24 ± 11% at the forearm (P = 0.002), while during heat stress LBNP decreased forehead SkBF 39 ± 11% compared with 28 ± 8% in the forearm (P = 0.007). In both conditions, most (≥68%) of the decreases in SkBF were due to decreases in blood pressure. However, a greater contribution of actively mediated reductions in SkBF was observed at the forehead, relative to the forearm during normothermia (32 ± 13% vs. 11 ± 11%, P = 0.031) and heat stress (30 ± 13% vs. 10 ± 13%, P = 0.004). These data suggest that facial pallor at presyncope is due to a combination of passive decreases in forehead SkBF secondary to reductions in blood pressure and to active decreases in SkBF, the latter of which are relatively greater than in the forearm.
Keywords: heat, lower body negative pressure, skin blood flow, syncope, vasoconstriction
it is well established that the control of human skin blood flow (SkBF) differs between glabrous and nonglabrous regions. Although SkBF within these regions generally responds similarly to many stimuli, discrepancies in responses can occur within a given skin type. In particular, cutaneous vessels of forehead skin vasodilate similarly relative to other nonglabrous areas during local (11) and whole body (3, 6, 7) heating, yet forehead cutaneous vasoconstriction has been reported to be both attenuated (4) and greater (15) in response to hypocapnia, as well as nonexistent during cold stress (5, 17). Microneurographic recordings of the supraorbital nerve, which serves forehead skin, have also demonstrated an absence of sympathetic vasoconstrictor activity during cold stress (12). Thus, despite forehead skin being considered nonglabrous, it may respond differently relative to other nonglabrous regions.
Facial pallor is a typical observation in humans during profound hypotension sufficient to cause presyncope. When the hypotensive challenge is accompanied by heat stress, this occurrence is particularly noticeable. The pallor observed in the face is in contrast to what is observed in other nonglabrous areas, such as the forearm. As forehead SkBF is generally greater compared with the forearm, particularly during heat stress (6, 14, 15), facial pallor may be more noticeable due to a greater decrease in facial SkBF relative to the forearm, although the mechanisms for this presumed response have not been studied. Facial pallor could result from passive reductions in SkBF secondary to the rapid and pronounced reductions in blood pressure at the point of presyncope (1, 21), from neurally mediated vasoconstriction (should this exist in forehead skin) and/or withdrawal of active vasodilation, or a combination of these mechanisms. Although the neural control of SkBF may differ between the forehead and the forearm, the passive effect of reductions in blood pressure would be expected to similarly affect the skin circulation of both regions. Therefore, it can be hypothesized that facial pallor at presyncope is associated with a greater neurally mediated reduction in facial SkBF relative to the forearm.
The purpose of this study was to compare forehead and forearm SkBF responses during a central hypovolemic challenge performed to presyncope under normothermic, as well as heat stress conditions. Furthermore, we determined the fractional contributions by which decreases in forehead and forearm SkBF were “passive” i.e., as a result of decreases in blood pressure, or “active” i.e., due to influences on the skin vasculature, which were presumed to be of neural origin. We tested the hypotheses that 1) the magnitude of the reduction in SkBF would be greater in the forehead compared with the forearm at presyncope, while normothermic or heat stressed, which may explain facial pallor subjectively observed under such conditions, and 2) greater reductions in forehead SkBF relative to the forearm at presyncope would be due to a greater “active” component.
METHODS
Subjects.
Simultaneous measurements of forehead and forearm SkBF were retrospectively analyzed from 24 subjects who underwent incremental lower body negative pressure (LBNP) to presyncope. Eleven of these subjects (three females) underwent the protocol while normothermic, and 13 subjects (six females) performed the protocol while heat-stressed. Of the 13 subjects that completed the heat stress trial, 2 of them performed the protocol twice, on separate days. The averaged data from both trials were analyzed for these two subjects. Five of the subjects completed the protocol under both thermal conditions, although on different days. Subject characteristics are as follows: age, 37 ± 11 years; height, 172 ± 6 cm; weight, 71.1 ± 12.0 kg. All subjects were free of any known cardiovascular, respiratory, neurological or metabolic diseases. Phase of menstrual cycle was recorded, but not controlled for in female subjects. All procedures were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center and at Texas Health Presbyterian Hospital Dallas. Written informed consent was obtained from all subjects prior to their participation in the study.
Measurements.
SkBF, in arbitrary units (au), was measured by laser-Doppler flowmetry using integrated probes (Moor Instruments, Devon, United Kingdom; or Perimed, North Royalton, OHA) placed on forearm and forehead skin. The forearm SkBF probe was placed on the dorsal side of the forearm, in a region that visually was devoid of large veins. The forehead SkBF probe was placed at the center of the forehead. Local skin temperature at each site was not controlled and was, therefore, allowed to fluctuate freely. This approach was selected to maximize the practical validity of the obtained SkBF values. Blood pressure was continuously measured noninvasively using photoplethysmography (Finometer Pro, FMS, Amsterdam, Netherlands) and corrected to pressures obtained by auscultation of the brachial artery (Tango+; SunTech Medical, Morrisville, NC). In seven of the subjects who performed the heat stress trial, blood pressure was measured by direct cannulation of the radial artery (Baxter Healthcare, Irving, CA). Cutaneous vascular conductance (CVC) was calculated as SkBF divided by mean arterial pressure. Heart rate was obtained from an electrocardiogram (Agilent, Munich, Germany) that was interfaced with a cardiotachometer (CWE, Ardmore, PA). Internal body temperature was measured by an ingestible telemetric pill (HQ Palmetto, FL) that was swallowed by the subject upon arrival, ∼2 h prior to data collection. Mean skin temperature was measured as the weighted average of six thermocouples attached to the skin surface on the abdomen, calf, chest, lower back, shoulder, and quadriceps (22).
Experimental protocol.
Upon arrival to the laboratory, the subjects swallowed the telemetric pill for the measurement of internal body temperature before being dressed in a two-piece tube-lined suit that covered the entire body except for the head, hands, feet, and one forearm. While supine, the subjects were sealed at the waist within a custom-made LBNP chamber. The subjects were then instrumented for the measurement of heart rate, blood pressure, forehead and forearm SkBF, and mean skin temperature. Following a baseline rest period, incremental LBNP to presyncope was performed for the normothermic trial, while the subjects in the heat stress trial underwent incremental LBNP to presyncope after internal body temperature had increased by ∼1.4°C. The LBNP protocol began at 20 mmHg, with an increase in LBNP of 10 mmHg every 3 min until presyncope. During the normothermic condition, mean skin temperature was clamped by circulating water maintained at ∼34°C through the tube-lined suit, while whole-body heat stress was achieved by circulating water at 48°C through the tube-lined suit. Once the desired increase in internal body temperature was achieved, the temperature of the water perfusing the suit was slightly reduced to 46°C to maintain internal body temperature relatively constant during the subsequent LBNP period. Criteria for the termination of the LBNP protocol included continued self-reporting by the subject of feeling faint, sustained nausea, rapid and progressive decrease in blood pressure resulting in a sustained systolic blood pressure being <80 mmHg, and/or relative bradycardia accompanied with a narrowing of pulse pressure.
Data analysis.
Data were collected with data acquisition software (Biopac MP150, Santa Barbara, CA) at a minimum sampling frequency of 50 Hz. The data were analyzed at baseline rest, during heat stress prior to the initiation of LBNP, and during the final 100 s prior to the cessation of LBNP. Minute averages were performed for the baseline normothermic and heat stress data, while the data during the final 100 s of LBNP were averaged into 5-s segments. To account for differences in absolute values of SkBF and CVC between the forehead and forearm (see results section), the percent reduction in SkBF and CVC from pre-LBNP was calculated for each site. To determine the contribution by which decreases in SkBF occurred due to the decrease in blood pressure (i.e., passive), the ratio of the percent reduction in blood pressure to the percent reduction in SkBF during LBNP was calculated using the following formula: passive contribution (%) = [percent reduction in blood pressure from pre-LBNP ÷ percent reduction in SkBF from pre-LBNP] × 100. The remaining contribution (i.e., 100% − passive contribution) was considered to be due to active, presumably neurally mediated decreases in SkBF. In some instances, the percent reduction in mean arterial pressure was greater than the percent reduction in SkBF. In these cases, a value of 100% was attributed to the passive contribution, while a value of 0% was attributed to the active contribution, given that under such conditions, there was no evidence of a neurally mediated (i.e., active) vasoconstrictor response.
Statistical analysis.
Within each thermal condition, the percent reduction in SkBF was analyzed using a two-way repeated-measures ANOVA using the repeated factors of skin site (levels: forehead and forearm) and time (levels: pre-LBNP and every 5 s during the final 100 s of LBNP). Mean arterial pressure was analyzed within each thermal condition using a one-way repeated-measures ANOVA, using the repeated factor of time (pre-LBNP and every 5 s during the final 100 s of LBNP). Differences between sites in absolute values of SkBF at baseline and heat stress, in the overall reduction in SkBF and CVC from pre-LBNP to presyncope, as well as in the relative contributions of passive vs. active reductions in SkBF, were analyzed using paired-samples Student's t-test. The level of significance was set at an alpha of P ≤ 0.05, and a Holm-Bonferroni correction was applied when multiple comparisons were made. Statistical analyses were performed using commercially available statistical software (SPSS 20.0 for Windows, SPSS, Chicago, IL). All variables are reported as means ± 95% confidence intervals. Confidence intervals were calculated as 1.96 × standard error of the mean.
RESULTS
Normothermic condition.
Baseline SkBF was greater at the forehead (96.3 ± 19.7 au) compared with the forearm (16.1 ± 4.7 au, P ≤ 0.001). LBNP time and level at presyncope averaged 1,251 ± 173 s and 75 ± 9 mmHg, respectively, while in this thermal condition. Mean arterial pressure decreased during the final 100 s of LBNP (P ≤ 0.001), averaging 57 ± 7 mmHg at presyncope compared with 91 ± 5 mmHg at baseline rest (P ≤ 0.001). Relative to pre-LBNP, forehead, and forearm SkBF decreased over the final 100 s of LBNP (P ≤ 0.001, Fig. 1), with the magnitude of the reduction in SkBF over time being different between skin sites (site × time interaction; P = 0.009). At presyncope, forehead SkBF was reduced 55 ± 14% compared with 24 ± 11% at the forearm (P ≤ 0.001; Fig. 3). At the forehead, 68 ± 13% of that decrease in SkBF was attributed to decreases in blood pressure, compared with 89 ± 11% at the forearm (P = 0.031 between sites; Fig. 4). As such, 32 ± 13% and 11 ± 11% of the decrease in forehead and forearm SkBF, respectively, were active in origin (P = 0.031 between sites, Fig. 4). At presyncope, forehead CVC decreased by 31 ± 15% (P = 0.003), while forearm CVC increased by 27 ± 25% although this increase was not statistically significant (P = 0.061). The difference in CVC between the forehead and forearm at presyncope was significant (P = 0.002).
Fig. 1.

Relative decreases in mean arterial pressure (left), as well as forehead and forearm skin blood flows (right) during the last 100 s of incremental lower body negative pressure (LBNP) performed to presyncope in a normothermic condition. The values are presented as a percentage of the values measured prior to LBNP (i.e., pre-LBNP). Values are expressed as means ± confidence intervals. *Significantly different from the forehead.
Fig. 3.

The percent reduction in forehead and forearm skin blood flow (SkBF) at presyncope, relative to the period just prior to LBNP, under normothermic and heat stress conditions. The open circles represent individual data points, while the solid circles represent the means ± confidence intervals. *Significantly different from the forehead.
Fig. 4.

The relative contribution of passive (i.e., due to reductions in blood pressure, gray bars) and active (i.e., presumably neurally mediated, open bars) reductions in forehead and forearm SkBF at presyncope in normothermic and heat stressed subjects. Values are expressed as means ± confidence intervals. *Significantly different from the forehead for the indicated contribution.
Heat stress condition.
Prior to heat stress, SkBF was greater at the forehead (121.5 ± 37.5 au) compared with the forearm (20.6 ± 3.7 au; P ≤ 0.001). Whole body heat stress increased mean skin temperature from 34.5 ± 0.4°C to 38.9 ± 0.5°C (P ≤ 0.001) and internal body temperature by 1.42 ± 0.12°C. Forehead SkBF (311.1 ± 36.5 au) remained greater compared with the forearm (149.8 ± 33.0 au; P ≤ 0.001) with heat stress. Heat stress also increased heart rate from 63 ± 4 bpm to 110 ± 9 bpm (P ≤ 0.001) and reduced mean arterial pressure from 90 ± 5 mmHg to 82 ± 5 mmHg (P ≤ 0.001). LBNP time and LBNP level at presyncope averaged 682 ± 159 s and 47 ± 8 mmHg, respectively. Mean arterial pressure decreased over the last 100 s of LBNP during heat stress (P ≤ 0.001), averaging 57 ± 7 mmHg at presyncope (P ≤ 0.001 relative to baseline). Relative to pre-LBNP, forehead and forearm SkBF decreased over the last 100 s of LBNP (P ≤ 0.001, Fig. 2), with the pattern of response differing between sites (site × time interaction; P = 0.003). Overall, forehead SkBF decreased by 39 ± 11% during LBNP compared with a reduction in forearm SkBF of 28 ± 8% (P = 0.007, Fig. 3). At the forehead, 70 ± 13% of that decrease in SkBF was due to the decrease in mean arterial pressure, compared with 90 ± 13% at the forearm (P = 0.004 between sites, Fig. 4). As such, 30 ± 13% of the decrease in forehead SkBF and 10 ± 13% of the decrease in forearm SkBF was active in nature (P = 0.004 between sites, Fig. 4). The relative contribution of passive and active decreases in SkBF did not differ between thermal conditions at both sites (all P > 0.05). Forehead CVC decreased by 15 ± 13% (P = 0.047) during LBNP, while it increased 5 ± 9% at the forearm, although this change was not statistically significant (P = 0.334). The difference in CVC between the forehead and forearm at presyncope was significant (P = 0.018).
Fig. 2.
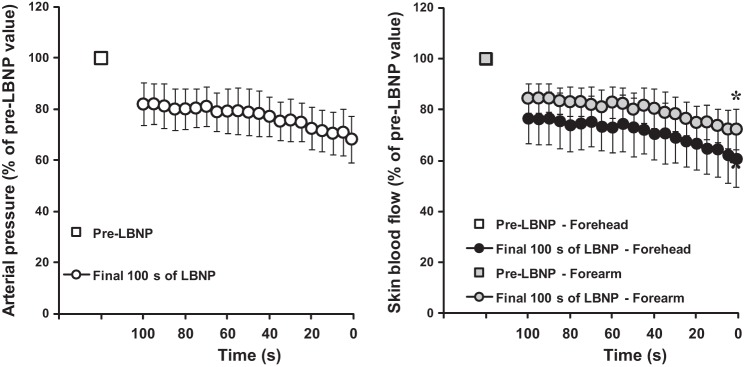
Relative decreases in mean arterial pressure (left), as well as forehead and forearm skin blood flows (right) during the last 100 s of incremental LBNP performed to presyncope during a heat stress condition. The values are presented as a percentage of the values measured prior to LBNP (i.e., pre-LBNP). Values are expressed as means ± confidence intervals. *Significantly different from the forehead.
DISCUSSION
This study examined forehead and forearm SkBF responses during incremental LBNP to presyncope in both normothermic and heat-stressed humans. Regardless of the thermal condition, the relative decrease in forehead SkBF at presyncope was greater compared with that in the forearm. While the primary mechanism for these decreases in SkBF is accounted for by decreases in blood pressure, a greater proportion of actively mediated decreases in forehead SkBF (relative to forearm) was observed in both conditions. These findings provide a potential mechanism for the facial pallor commonly observed at presyncope in humans.
Previous studies have noted a lack of decrease in forehead SkBF during the local application of cold to the forehead (5), as well as during whole body cold stress (17). Furthermore, attenuated decreases in forehead SkBF relative to the forearm have been observed during hyperventilation-induced hypocapnia (4). Together, these studies suggest that the forehead skin circulation may not be responsive to sympathetic vasoconstriction stimuli. In fact, Nordin (12) performed direct microneurographic recordings of cutaneous sympathetic activity in the supraorbital nerve and observed little background activity when individuals rested at normal ambient temperatures and no increase in nerve activity during body cooling. Although the present results do not support or refute sympathetic vasoconstrictor control of forehead SkBF, they clearly demonstrate that forehead SkBF is capable of decreasing during a sympathoexcitatory stimulus, regardless of whether it is performed under normothermic or heat stress conditions. Furthermore, the relative decreases in forehead SkBF were greater compared with those observed in the forearm. It should be noted that the stimulus employed in the current study (i.e., LBNP) elicited substantial reductions in blood pressure, whereas most of the previous stimuli used to examine the control of forehead SkBF (e.g., local application of cold, cold stress) likely resulted in either no change or an increase in blood pressure. Given that decreases in blood pressure were the main determinant for the reduction in forehead SkBF under both thermal conditions, the lack of change in forehead SkBF in previous studies could have been due to a lack of decrease in blood pressure. To this effect, a more recent study has reported decreases in forehead SkBF during acute and pronounced reductions in blood pressure under normothermic conditions (13).
Overall, reductions in blood pressure during LBNP explained the majority of the reductions in forehead and forearm SkBF under both thermal conditions. However, a novel finding of the current study is the greater contribution of active influences upon the reduction in forehead SkBF relative to the forearm. The mechanisms by which a larger fraction of forehead SkBF is “actively” decreased during presyncopal LBNP include greater cutaneous vasoconstriction and/or greater withdrawal of active vasodilation. Since active vasodilation occurs only after core and skin temperatures have increased beyond an onset threshold (10, 18), it is unlikely that a withdrawal of active vasodilation can explain the greater proportion of active influences upon the reduction in forehead SkBF during the normothermic condition. As such, the greater actively mediated reduction in forehead SkBF during the normothermic condition was most likely driven by greater vasoconstrictor activity, should it exist in forehead skin. Although Nordin (12) did not report any increase in nerve activity from the supraorbital nerve during body cooling, it was acknowledged that vasoconstrictor fibers supplying forehead skin may run through other nerves than the one measured. It should also be considered that absolute values of SkBF were greater at the forehead compared with the forearm, which could contribute to a greater relative decrease in blood flow, since there was more “reserve” for SkBF to decrease. This could potentially account for a greater relative decrease in mean arterial pressure, which exceeded that for SkBF in a few subjects. This was particularly noticeable in the CVC values, as attenuated decreases in forearm SkBF combined with a continued decrease in mean arterial pressure led to CVC values that indicated a vasodilation (i.e., increase in conductance) in the forearm as opposed to vasoconstriction (i.e., a decrease in conductance) in the forehead at presyncope. Although this possibility may especially be true for the normothermic condition, nonetheless, we observed a greater relative decrease in forehead SkBF during the heat stress condition when forearm SkBF was substantially elevated, thereby minimizing any potential for its decrease to be limited.
Minor reductions in forearm CVC at presyncope have been reported previously during heat stress (1). The cutaneous vasculature represents the greatest reservoir from which blood volume, as well as vascular conductance, can be drawn upon to maintain blood pressure during a central hypovolemic challenge under heat stress conditions. However, heat stress itself adversely affects the responsiveness of the forearm cutaneous vasculature to constrict to adrenergic agonists (23), in part, because of an inhibitory effect of nitric oxide (2, 19, 20). As such, the current results in the forearm support previous observations of minimal reductions in forearm CVC at the point of presyncope in heat-stressed humans and suggest that the reductions in SkBF that do occur are primarily passive in nature due to the decrease in blood pressure. In contrast, the current results suggest that active influences contribute to the relatively greater decrease in forehead SkBF during LBNP to presyncope in heat-stressed humans. Since the forehead cutaneous vasculature is under the control of the active vasodilator system (3), the greater actively mediated reduction in forehead SkBF during the heat stress condition could be due to a greater cutaneous vasoconstriction and/or a greater withdrawal of active vasodilation. Regardless of the potential mechanism(s), the results of the current study suggest that facial pallor observed at presyncope is associated with relatively greater decreases in forehead SkBF (compared to the forearm), which are primarily related to the decreases in blood pressure, as well as with an added active influence upon the cutaneous vasculature.
Limitations.
Hyperventilation-induced hypocapnia commonly occurs during LBNP performed to presyncope under both normothermic and heat stress conditions (16). Although hypocapnia itself has been shown to reduce forehead but not forearm SkBF during heat stress (15), these reductions were small (∼5%) relative to the decreases in SkBF observed at presyncope in the current study (∼39%). Furthermore, Fujii et al. (4) reported that hypocapnia (∼20 mmHg reduction in end-tidal CO2) induced by voluntary hyperventilation similarly affected the forehead and forearm cutaneous circulations during passive heat stress sufficient to elevate internal body temperature by 1°C. Therefore, it is unlikely that the greater decreases in forehead SkBF observed in the current study can be attributed to regional differences in the sensitivity of the cutaneous vasculature to hypocapnia. Further work is needed to determine the exact mechanism by which forehead SkBF decreases to a greater extent compared with forearm SkBF during LBNP performed to presyncope. Potential mechanisms could be addressed by the application of drugs (e.g., via microdialysis, intradermal injection, iontophoresis, etc.) that block the sympathetic vasoconstrictor (8) and active vasodilator (9, 19) systems. For cosmetic reasons, we chose to refrain from using such techniques and rather sought to first identify whether differences in forehead and forearm SkBF responses exist, prior to seeking cosmetically favorable approaches to investigate more mechanistic answers. It should also be noted that the current study only examined forehead SkBF, and therefore, the results might not be applicable to SkBF in other areas of the face, particularly those that are considered glabrous in nature (e.g., ears, nose, lips, etc.).
In conclusion, the results of the current study show that relative decreases in SkBF are greater in the forehead compared with the forearm during incremental LBNP to presyncope in normothermic and heat-stressed humans. Although reductions in blood pressure explain the majority of the decrease in SkBF at both skin sites, a significantly greater proportion of actively mediated decreases in forehead SkBF was observed under both thermal conditions. Overall, these results suggest that the forehead cutaneous vasculature is more responsive relative to that of the forearm during incremental LBNP, which could explain the commonly observed facial pallor in individuals at the point of presyncope.
GRANTS
This project was funded in part by National Institutes of Health grants HL-61388 and HL-84072 and Department of Defense Grant W81XWH-12-1-0152.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.G. and C.G.C. analyzed data; D.G., R.M.B., M.S.G., J.L.H., and C.G.C. interpreted results of experiments; D.G. prepared figures; D.G. drafted manuscript; D.G., R.M.B., M.S.G., J.L.H., and C.G.C. edited and revised manuscript; D.G., R.M.B., M.S.G., J.L.H., and C.G.C. approved final version of manuscript; R.M.B., M.S.G., J.L.H., and C.G.C. conception and design of research; R.M.B., M.S.G., J.L.H., and C.G.C. performed experiments.
REFERENCES
- 1.Crandall CG, Shibasaki M, Wilson TE. Insufficient cutaneous vasoconstriction leading up to and during syncopal symptoms in the heat stressed human. Am J Physiol Heart Circ Physiol 299: H1168–H1173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durand S, Davis SL, Cui J, Crandall CG. Exogenous nitric oxide inhibits sympathetically mediated vasoconstriction in human skin. J Physiol 562: 629–634, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox RH, Goldsmith R, Kidd DJ. Cutaneous vasomotor control in the human head, neck and upper chest. J Physiol 161: 298–312, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii N, Honda Y, Delliaux S, Tsuji B, Watanabe K, Sugihara A, Kondo N, Nishiyasu T. Effect of voluntary hypocapnic hyperventilation on cutaneous circulation in resting heated humans. Am J Physiol Regul Integr Comp Physiol 303: R975–R983, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Hertzman AB, Roth LW. The absence of vasoconstrictor reflexes in the forehead circulation. Effects of cold. Am J Physiol 136: 692–697, 1942 [Google Scholar]
- 6.Inoue Y, Shibasaki M. Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating. Eur J Appl Physiol Occup Physiol 74: 78–84, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Inoue Y, Tanaka Y, Omori K, Kuwahara T, Ogura Y, Ueda H. Sex- and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur J Appl Physiol 94: 323–332, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Kellogg DLJr Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Kellogg DLJr Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Lewis T, Pickering GW. Vasodilation in the limbs in response to warming the body; with evidence for sympathetic vasodilator nerves in man. Heart 16: 33–51, 1931 [Google Scholar]
- 11.Metzler-Wilson K, Kellie LA, Tomc C, Simpson C, Sammons D, Wilson TE. Differential vasodilatory responses to local heating in facial, glabrous and hairy skin. Clin Physiol Funct Imaging 32: 361–366, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Nordin M. Sympathetic discharges in the human supraorbital nerve and their relation to sudo- and vasomotor responses. J Physiol 423: 241–255, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogoh S, Lericollais R, Hirasawa A, Sakai S, Normand H, Bailey DM. Regional redistribution of blood flow in the external and internal carotid arteries during acute hypotension. Am J Physiol Regul Integr Comp Physiol 306: R747–R751, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Morimoto K, Shibasaki M. Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab 33: 1915–1920, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Shibasaki M. Hyperthermia modulates regional differences in cerebral blood flow to changes in CO2. J Appl Physiol 117: 46–52, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Pearson J, Ganio MS, Lucas RA, Babb TG, Crandall CG. Heat stress does not augment ventilatory responses to presyncopal limited lower body negative pressure. Exp Physiol 98: 1156–1163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasch W, Cabanac M. Vasomotor response of the human face: laser-Doppler measurements during mild hypo- and hyperthermia. Acta Physiol Scand 147: 431–436, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Roddie IC, Sheperd JT, Whelan RF. The vasomotor nerve supply to the skin and muscle of the human forearm. Clin Sci 16: 67–74, 1957 [PubMed] [Google Scholar]
- 19.Shibasaki M, Davis SL, Cui J, Low DA, Keller DM, Durand S, Crandall CG. Neurally mediated vasoconstriction is capable of decreasing skin blood flow during orthostasis in the heat-stressed human. J Physiol 575: 953–959, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibasaki M, Durand S, Davis SL, Cui J, Low DA, Keller DM, Crandall CG. Endogenous nitric oxide attenuates neutrally mediated cutaneous vasoconstriction. J Physiol 585: 627–634, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens PM, Lamb LE. Effects of lower body negative pressure on the cardiovascular system. Am J Cardiol 16: 506–515, 1965 [DOI] [PubMed] [Google Scholar]
- 22.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci 97: 122–128, 2002 [DOI] [PubMed] [Google Scholar]


