Abstract
Social stress may play a role in urinary bladder dysfunction in humans, but the underlying mechanisms are unknown. In the present study, we explored changes in bladder function caused by social stress using mouse models of stress and increasing stress. In the stress paradigm, individual submissive FVB mice were exposed to C57BL/6 aggressor mice directly/indirectly for 1 h/day for 2 or 4 wk. Increased stress was induced by continuous, direct/indirect exposure of FVB mice to aggressor mice for 2 wk. Stressed FVB mice exhibited nonvoiding bladder contractions and a decrease in both micturition interval (increased voiding frequency) and bladder capacity compared with control animals. ELISAs demonstrated a significant increase in histamine protein expression with no change in nerve growth factor protein expression in the urinary bladder compared with controls. Unlike stressed mice, mice exposed to an increased stress paradigm exhibited increased bladder capacities and intermicturition intervals (decreased voiding frequency). Both histamine and nerve growth factor protein expression were significantly increased with increased stress compared with control bladders. The change in bladder function from increased voiding frequency to decreased voiding frequency with increased stress intensity suggests that changes in social stress-induced urinary bladder dysfunction are context and duration dependent. In addition, changes in the bladder inflammatory milieu with social stress may be important contributors to changes in urinary bladder function.
Keywords: nerve growth factor, stress, inflammation, voiding frequency, cystometry
stress affects urinary bladder function and has been reported to exacerbate signs/symptoms of urinary bladder dysfunction in overactive bladder, interstitial cystitis/bladder pain syndrome, bladder outlet obstruction, and spinal cord injury-induced bladder dysfunction. Clinicians have long surmised that stress plays a role in bladder dysfunction, especially in children, but the underlying mechanisms have remained obscure. Treatment options for stress-induced bladder dysfunction include behavioral modification and anticholinergic medications (38). However, these interventions merely treat symptoms and have a variable success rate. The frequent ineffectiveness of these treatments stems from a failure to understand how stress affects the mechanics of human urine storage and emptying and highlights the need for relevant preclinical animal models for testing treatments for stress-induced bladder dysfunction.
Animal models based on intravesical instillation of inflammatory agents or surgical ligations of the bladder outlet have recently been created to study overactive bladder (20, 31). However, these models are less effective in mirroring human bladder function disorders in children than in adults and have limited validity in studies of stress-related bladder dysfunction. To overcome this problem, researchers have developed a number of rodent stress models that mimic human bladder dysfunction. In 1973, Desjardins et al. (12) reported wide differences in the micturition patterns of dominant versus submissive mice during direct and barrier exposure. An alternative mouse model, the chronic water avoidance stress paradigm demonstrated enhanced bladder nociceptive responses as well as alterations in micturition frequency, interval, and volume (26). Most recently, Chang et al. (7) reported that social stress leads to remodeling and hypertrophy of the bladder in addition to abnormal voiding patterns. Findings from murine models of social stress appear to closely correlate with the symptoms of stress-induced bladder overactivity/underactivity observed in children (G. C. Mingin, personal observations).
Nerve growth factor (NGF), a potent neurotrophin that exerts pleiotropic effects in the peripheral and central nervous system, has been shown to regulate sensory and sympathetic neuronal development and maintenance (22) and plays a role in painful somatic and visceral inflammation (1, 8, 17, 27, 32). Notably, NGF contributes to inflammation of the bladder, colon, and lung (11, 16, 35), and there is increasing evidence showing that NGF plays a role in increased voiding frequency (8, 19, 14, 29, 21, 30, 44). Recently, increased NGF expression in the urinary bladder was demonstrated in rats exposed to a repeated variate stress paradigm (28). Additional inflammatory changes in the urinary bladder were also demonstrated with the repeated variate stress model, including increases in histamine, chemokine, and myeloperoxidase expression, suggesting that markers of inflammation might be useful biochemical indicators of the effects of social stress (2).
In the present study, we examined the role that social stress plays in bladder function using a stress and increased stress paradigm in mice. In this study, the difference between the stress and increased stress protocols relates to the duration of time that a submissive mouse is exposed to an aggressor mouse via a permeable barrier allowing for visual and olfactory contact. We demonstrated that social stress produced a significant increase in histamine protein expression in the urinary bladder from both stressed and increased stress groups. In addition, we observed a significant increase in NGF protein expression in the urinary bladder from the increased stress group. Notably, changes in bladder function depended on the stress paradigm: stress induced decreased bladder capacity and increased voiding frequency, whereas increased stress produced an elevation in bladder capacity and a decrease in voiding frequency. Collectively, our findings reveal that changes in bladder function in response to stress are context and duration dependent.
MATERIALS AND METHODS
Animal Care and Use
Male C57BL/6 mice and FVB mice (Charles River Canada, St. Constant, PQ, Canada, 20–30 g) were used in these experiments. Mice had free access to food and water while housed in animal facilities. All procedures that involved animals were performed in accordance with the Institutional Animal Care and Use Committee at the Univerity of Vermont College of Medicine and were consistent with the Guide for the Care and Use of Laboratory Animals (8th ed.).
Social Stress Protocol
On consecutive days, submissive 6-wk-old FVB mice were placed in direct contact with C57BL/6 retired breeder mice (aggressor mice; Charles River Canada) for a period of 5 min or until C57BL/6 mice initiated aggressive behavior (e.g., biting sufficient to break the skin). After the 5-min period or demonstrated aggressive behavior, a clear plastic barrier with small holes allowing for olfactory stimulation was placed in the cage separating the FVB mouse from the aggressor mouse for a total time of 1 h. FVB mice were then returned to their own cages for 23 h. The process was repeated the next day with a different aggressor mouse. Age-matched control FVB mice were placed in barrier cages without exposure to aggressor mice for a total of 1 h before being returned to their own cage. Submissive mice were exposed to the stress protocol for either 2 or 4 wk.
Increased Social Stress Protocol
Submissive 6-wk-old FVB mice were placed in direct contact with C57BL/6 retired breeder mice (aggressor mice) for 5 min or until aggressor mice initiated traumatic aggressive behavior. After the 5-min period, a clear plastic barrier with small holes allowing for olfactory stimulation was placed separating the FVB mouse from the aggressor mouse for 23 h. The process was repeated the next day with a different aggressor mouse. Submissive mice were exposed to this protocol for a 2-wk period.
Assessment of Additional Mouse Strain in Stress Protocols
Both stress and increased stress protocols were repeated as above substituting 6-wk-old C57BL/6 mice for 6-wk-old FVB submissive mice. The 6-wk-old C57/BL/6 mice were exposed to C57/BL/6 retired breeder aggressor mice.
Measurement of Urinary Bladder NGF and Histamine Protein Content by ELISAs
The whole bladder was put in T-PER Tissue Protein Extraction Reagent (Thermo Scientific, Waltham, MA) with an added protease inhibitor cocktail (Roche, Indianapolis, IN). Total bladder proteins were then extracted using Lysing Matrix D (MP Biomedicals, Santa Ana, CA), and the protein concentration was measured using a Coomassie Plus (Bradford) Assay Kit (Thermo Scientific). This extract was then used for ELISAs. Histamine protein content was measured using a Histamine EIA Kit (Oxford Biomedical Research) with a detection range from 2.5 to 50.0 ng/ml. The histamine standard curve was calculated using a 4-PL curve fit (R2 = 0.9975). Mouse NGF content was measured using a ChemiKine Nerve Growth Factor Sandwich ELISA kit (Millipore) with a detection range of 10–1,000 pg/ml. The mouse NGF standard curve was generated using a 2-PL curve fit (R2 = 0.9961). Samples did not fall below the minimum detection limits of either assays. Measured histamine and NGF contents were normalized to total protein concentration.
Quantitative RT-PCR for NGF and Histidine Decarboxylase
There is no specific gene coding for histamine; therefore, we substituted histidine decarboxylase (HDC), the enzyme that catalyzes the reaction producing histamine from histidine. Total RNA was extracted using STAT-60 total RNA/mRNA isolation reagent (Tel-Test'B', Friendswood, TX), as previously described. Two micrograms of RNA per sample were used to synthesize cDNA using a mix of random hexamer and oligo dT primers with Moloney murine leukemia virus reverse transcriptase (Promega) in a 25-μl final reaction volume. Quantitative PCR standards for all transcripts were prepared with amplified cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). Nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically. cDNA templates, diluted 10-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using HotStart-IT SYBR Green qPCR Master Mix (USB, Cleveland, OH) and 300 nM of each primer in a 25-μl final reaction volume. HDC primer sequences were as follows: forward 5′-GGATTCTGGGTCAAGGACAAG-3′ and reverse 5′-GTCCGTGGCTGCACCAGAG-3′. L32 primer sequences were as follows: forward 5′-AGTCGCCGTGCCTACCAT-3′ and reverse 5′-GCCTGCTGCCTTCCTTG-3′. NGF and L32 primer sequences have been previously published (39).
The real-time quantitative PCR was performed (Applied Biosystems 7500 Fast real-time PCR system, Foster City, CA) using the following standard conditions: 1) serial heating at 94°C for 2 min and 2) amplification over 45 cycles at 94°C for 15 s and 60–65°C depending on the primer sets for 30 s. The amplified product from these amplification parameters was subjected to SYBR green I melting analysis by ramping the temperature of the reaction samples from 60 to 95°C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using sequence detection software (Sequence Detection software, version 1.3.1, Applied Biosystems). In standard assays, default baseline settings were selected. The increase in SYBR green I fluorescence intensity (ΔRn) was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the ribosomal protein reference gene (L32).
Open Voiding Cystometry in Conscious, Unrestrained Mice
Open voiding cystometry in conscious, unrestrained mice was conducted on all animals 3 days after stress exposure. In anesthetized FVB/C57BL/6 mice and control mice, the urinary bladder was exposed through a lower midline abdominal incision under general anesthesia (2–3% isoflurane). A saline-filled polyethylene-10 cannula with the end flared by heat was inserted into the dome of the bladder and secured with a 6-0 nylon purse string suture. The distal end of the cannula was sealed. Muscle and skin layers were closed separately using absorbable and nonabsorbable sutures, respectively. The distal part of the cannula was placed in the subcutaneous space, and mice were returned to normal caging for 72 h to ensure complete recovery. Postoperative analgesics were given for a period of 48 h. Mice were anesthetized, and the cannula was exteriorized. Mice were placed conscious and unrestrained in recording cages with a balance and pan for urine collection and measurements placed below the cage. Intravesical pressure changes were recorded using a Small Animal Cystometry System (Med Associates, St. Albans, VT). The cannula was exteriorized and connected to one port of a pressure transducer; the other port of the pressure transducer was connected to a syringe pump. Room temperature saline was infused at a rate of 10 μl/min to elicit repetitive urinary bladder contractions. At least four reproducible micturition cycles were recorded after an initial stabilization period of 25–30 min. Voided saline was collected to determine void volume. Intercontraction interval, maximal voiding pressure, pressure threshold for voiding, and baseline resting pressure were measured. The number of nonvoiding urinary bladder contractions (NVC) per voiding cycle, maximal NVC pressure, and frequency of NVC were assessed. For these experiments, NVCs were defined as rhythmic intravesical pressure rises (>5 cmH2O from baseline pressure) without a release of fluid from the urethra. Mice were excluded from experiments when adverse events occurred, such as a ≥20% reduction in body weight postsurgery, lethargy, pain or distress not relieved by our Institutional Animal Care and Use Committee-approved regimen of postoperative analgesics, or a significant postoperative adverse event. In the present study, three mice were excluded due to iatrogenic bladder outlet obstruction. Behavioral movements such as grooming, standing, ambulation, and defecation also rendered bladder pressure recordings during these events unusable, and these were excluded from analysis. In the majority of animals, experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements. Mice were euthanized at the conclusion of the study by isoflurane (5%) and thoracotomy, and the bladder was harvested and weighed.
Statistical Analyses
All values are represented as means ± SE. Data were compared with ANOVA. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F-ratios exceeded the critical value (P ≤ 0.05 by two-tailed test), the Newman-Keuls post hoc test was used to compare group means.
RESULTS
Mice Evaluated
Fifty-eight mice of a total of 64 mice were studied. Five mice were discounted due to bladder obstruction (n = 3) or unstable cystometric recordings (n = 2). One animal died after the survival surgery of unknown causes.
Effects of Social Stress on Urinary Bladder Function in Mice
To determine whether exposure to stress results in changes in urinary bladder function, we performed conscious urodynamic experiments on stressed, increased stressed, and control mice.
Stressed.
Whereas age-matched control mice exhibited similar urinary bladder function (Fig. 1A), mice exposed to the stress protocol for 2 wk exhibited significantly reduced intermicturition intervals (increased voiding frequency) and bladder capacities (Fig. 1B and Table 1). They also showed an increase in baseline and micturition threshold pressure with no change in maximum voiding pressure (Table 2). Moreover, NVCs were increased (5.8 NVCs/100 s) in mice exposed to the 2-wk stress protocol compared with the control group (1.2 NVCs/100 s). In mice exposed to the stress protocol for 4 wk, intermicturition interval and bladder capacity tended to be lower than those in the control group, but these differences did not reach statistical significance. The recovery of baseline resting pressure, micturition threshold pressure, and maximal voiding pressures at 4 wk were similar to those in the control group (Table 2).
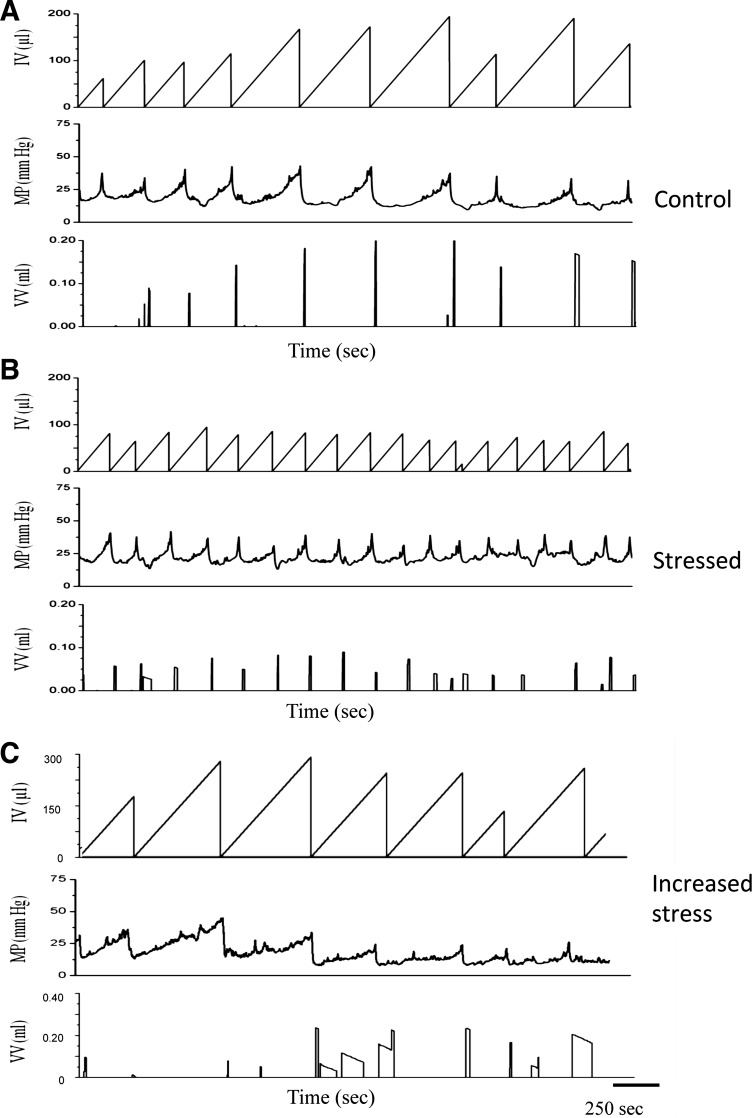
Fig. 1.
Cystometrogram traces of stress (S) and increased stress (IS) protocols in FVB mice. A: representative cystometry traces from control mice. B: representative cystometry traces after stress (2 wk). C: representative cystometry traces after increased stress (2 wk). IV, infused volume; MP, mean pressure; VV, voided volume. Social stress (B) significantly (P ≤ 0.01) increased voiding frequency and decreased bladder capacity (total infused saline at the start of micturition, 1.65-fold) and intercontraction intervals (1.65-fold) relative to control measurements (A).
Table 1.
Intermicturition interval and bladder capacity of mice exposed to social stress at 2 and 4 wk compared with controls
| 2-wk Control | 2-wk Stress | 4-wk Control | 4-wk Stress | 2-wk Increased Stress Control | 2-wk Increased Stress | |
|---|---|---|---|---|---|---|
| FVB mice | ||||||
| Intermicturition interval, s | 321.2 ± 26.4 | 193.2 ± 10.2* | 311.5 ± 19.5 | 264.2 ± 18.3n.s. | 395.2 ± 17.3 | 568.7 ± 24.3* |
| Bladder capacity, μl | 133.9 ± 11.0 | 80.7 ± 8.5* | 129.5 ± 10.2 | 110.3 ± 5.2n.s. | 131.6 ± 8.2 | 236.7 ± 18.2* |
| n | 5 | 5 | 6 | 6 | 6 | 6 |
| C57BL/6 mice | ||||||
| Intermicturition interval, s | 284.2 ± 18.4 | 208.5 ± 12.5* | 272.6 ± 17.5 | 495.4 ± 19.2* | ||
| Bladder capacity, μl | 118.5 ± 13.0 | 87.2 ± 7.2* | 113.8 ± 12.4 | 205.5 ± 12.4* | ||
| n | 5 | 8 | 5 | 6 |
Values are means ± SE; n, number of mice (FVB and C57BL/6) in each group. The intermicturition interval and bladder capacity for mice exposed to increased stress (2 wk) compared with controls are shown.
P ≤ 0.01.
Table 2.
Micturition pressure (threshold, baseline, and maximum) of mice exposed to social stress at 2 and 4 wk compared to controls
| 2-wk Control | 2-wk Stress | 4-wk Control | 4-wk Stress | 2-wk Increased Stress Control | 2-wk Increased Stress | |
|---|---|---|---|---|---|---|
| FVB mice | ||||||
| Threshold micturition pressure | 16.1 ± 1.0 | 24.1 ± 3.3* | 20.3 ± 4.0 | 18.6 ± 1.7 | 14.8 ± 3.0 | 16.6 ± 1.5 |
| Baseline micturition pressure | 14.7 ± 0.9 | 20.7 ± 2.2* | 17.0 ± 3.9 | 15.1 ± 1.4 | 13.1 ± 2.4 | 13.0 ± 1.2 |
| Maximum micturition pressure | 28.5 ± 3.0 | 37.0 ± 2.9 | 30.4 ± 3.8 | 33.9 ± 3.1 | 23.9 ± 3.6 | 25.5 ± 1.6 |
| n | 5 | 5 | 6 | 6 | 6 | 6 |
| C57BL/6 mice | ||||||
| Threshold micturition pressure | 15.4 ± 1.9 | 16.2 ± 1.3 | 18.0 ± 3.3 | 17.6 ± 1.9 | ||
| Baseline micturition pressure | 12.5 ± 1.0 | 13.4 ± 0.8 | 17.3 ± 3.2 | 15.7 ± 1.5 | ||
| Maximum micturition pressure | 33.5 ± 5.0 | 33.0 ± 1.9 | 29.7 ± 4.2 | 28.8 ± 1.7 | ||
| n | 5 | 8 | 5 | 6 |
Values are means ± SE; n, number of mice (FVB and C57BL/6) in each group. Micturition pressures (threshold, baseline, and maximum) of mice exposed to increased stress (2 wk) compared with controls are also shown.
P ≤ 0.01.
Increased stress.
Exposure of mice to the increased stress protocol produced changes in bladder function that were qualitatively different from those exposed to the stress protocol. Unlike the case with stress, which decreased intermicturition interval (increased voiding frequency) and bladder capacity, mice exposed to increased stress exhibited significantly increased intermicturition intervals (reduced voiding frequency) and increased bladder capacities compared with age-matched control mice (Fig. 1C and Table 1). No differences in baseline resting pressure, micturition threshold pressure, or maximal voiding pressures were observed between mice exposed to the increased stress protocol and control mice (Table 2).
Effects of Social Stress on Mouse Body and Urinary Bladder Weights
Urinary bladder weight.
Weights of bladders from FVB mice exposed to the stress protocol for 2 wk (0.018 ± 0.002 g) were not significantly different from control mice (0.018 ± 0.002 g). Weights of bladders from FVB mice exposed to the increased stress protocol were not significantly increased (0.023 ± 0.002 g). Weights of bladders from C57BL/6 mice exposed to the stress protocol for 2 wk (0.062 ± 0.002 g) were significantly (P ≤ 0.01) increased compared with bladders from control mice (0.044 ± 0.001 g). In contrast, weights of bladders were unchanged in C57Bl/6 mice exposed to increased stress.
Body weight.
Body weights of FVB mice exposed to both the stress and increased stress protocol (25 ± 0.63 g) were similar to those of control mice. Body weights of C57BL/6 mice were significantly (P ≤ 0.01) increased at the end of both stress (27 ± 0.16 g) and increased stress (22 ± 0.13 g) protocols compared with those of control mice (14 ± 0.14 g).
Effects of Social Stress on NGF and Histamine Protein Expression in Mouse Urinary Bladders
Previous studies (19, 20, 21, 33, 39) have demonstrated that urinary bladder NGF expression is increased with increased voiding frequency. Given that voiding frequency increased in mice exposed to stress but decreased in mice exposed to increased stress, we hypothesized that NGF expression would be selectively increased in the bladders of stressed mice. We evaluated both NGF and histamine protein expression using ELISAs. For these experiments, we used an additional 17 FVB mice; 5 for controls and 6 in each experimental group (stress and increased stress). Mice were exposed to the previously described stress and increased stress protocols and, for consistent comparison, underwent tube implantation before being euthanized. We observed no changes in NGF protein expression in stressed bladders compared with controls. In increased stressed bladders, we observed a significant (P ≤ 0.05) increase in NGF protein content in urinary bladders (Fig. 2A). Histamine protein expression was significantly increased in both stressed and increased stressed bladders compared with controls (Fig. 2B).
Fig. 2.
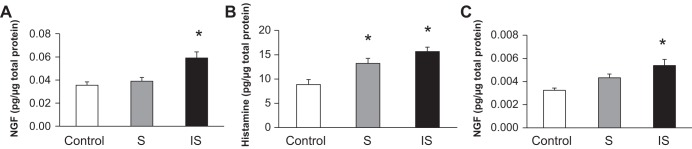
Social stress (2 wk) increases nerve growth factor (NGF) and histamine protein in the urinary bladder of mice. A: NGF protein expression was unchanged in stressed bladders compared with control bladders. NGF protein was significantly (*P ≤ 0.01) elevated in increased stressed bladders compared with control and stressed bladders (with tube implantation). B: histamine protein expression was significantly increased in both stressed and increased stressed bladders compared with control bladders (with tube implantation, *P ≤ 0.01). C: NGF was unchanged in stressed mice and significantly elevated (*P ≤ 0.01) in increased stressed mice compared with control mice (without tube implantation).
To demonstrate that elevations in protein expression were not related to postimplantation surgery, we repeated the above experiments to determine NGF protein expression in the urinary bladder without tube implantation. Fifteen additional mice were distributed equally among control, stressed, and increased stress groups (n = 5 mice/group). NGF protein expression was significantly (P ≤ 0.05) increased in the urinary bladder of mice (no tube implant) exposed to increased stress, but the magnitude of expression was reduced compared with that observed in mice with tube implants (Fig. 2C).
Effects of Social Stress on NGF and HDC (Histamine) Transcript Expression
Twenty-one additional FVB mice, divided equally into control, stressed, and increased stressed groups (n = 7 mice/group), were used for quantitative RT-PCR. These mice were exposed to the previously described stress and increased stress protocols and, for consistent comparison, underwent tube implantation before being euthanized. In each group, the urinary bladder was separated into both the urothelium and detrusor using a dissecting microscope, fine forceps, and iris scissors.
We observed a decrease in NGF transcript expression in detrusor and urothelial tissues in both stress and increased stress bladders compared with controls (Fig. 3A).
Fig. 3.
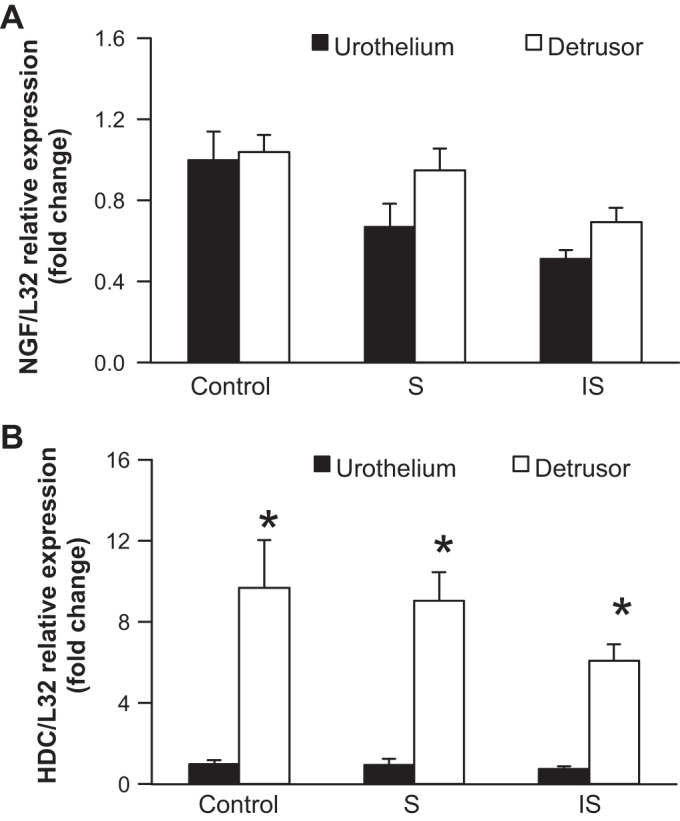
Social stress decreases histidine decarboxylase (HDC) and NGF transcripts expression in the urinary bladder. A: NGF mRNA was decreased in both the urothelium and detrusor in stressed and increased stressed mice compared with control mice. B: HDC mRNA was decreased in the urothelium and significantly (*P ≤ 0.01) decreased in the detrusor in stressed and increased stressed mice compared with control mice and with each other. L32, ribosomal protein used as a reference gene.
Urothelial and detrusor HDC transcript expression were also decreased compared with controls (Fig. 3B).
DISCUSSION
In the present study, we expanded on the current knowledge of the effects of social stress on bladder function. Functionally, stressed mice (2 wk) exhibit decreased bladder capacity and intermicturition intervals (increased voiding frequency) in addition to the presence of NVCs. At 4 wk, baseline and micturition threshold pressures, which were increased at 2 wk with this paradigm, had completely normalized, whereas bladder capacity and intermicturition interval were not different from control animals. Mice exposed to increased stress had increased bladder capacity leading to prolonged periods between urination. Thus, both stress and increased stress exposure affected voiding frequency; however, these paradigms produced opposing results. Other findings of interest included an increase in histamine protein expression in stressed mice consistent with an inflammatory milieu in the urinary bladder. In mice exposed to increased stress, there was both significantly increased NGF and histamine protein expression in the urinary bladder. Collectively, our results demonstrate differences in urinary bladder function and expression of inflammatory mediators that are dependent on the context and duration of the stress exposure.
Few studies in the literature have described the functional effects of this stress model in the bladder. Our work extends that of Chang et al. (7), who reported functional findings consistent with urinary retention. In contrast, we demonstrate that stress induced both retention and increased voiding frequency (i.e., overactivity). The difference is likely attributable to differences in the intensity of the stress stimulus, as determined by the type and degree. To overcome the challenges involved with a social stress model (expense involved due to single cage housing and the need for aggressor male mice to be periodically exposed to female mice), water avoidance stress protocols were adopted to characterize the voiding phenotype, only one of which was carried out using male mice instead of rats (26). In the study by McGonagle et al. (26), cystometry revealed increased intermicturition intervals and bladder volumes. This pattern of retention is also similar to that reported by Chang et al. (7). Comparing the intensity of stress in the water avoidance model to our protocol is difficult. These findings are similar to what we see with increased stress. Both the studies by Chang et al. and McGongle et al. are concerned with comparing their modals with the hypertrophy and bladder remodeling seen with partial bladder outlet obstruction. These models do not fully mimic the urological conditions seen in children; thus, our model of social stress may have more translational applicability as it is likely that perturbations in micturition reside on a spectrum with overactivity and retention at opposite ends. The underlying mechanisms that trigger this transition are not known, but our data suggest that this transition point is not time dependent but rather influenced by the intensity and duration of stress exposure. This is clearly evident when one considers that the functional micturition pattern did not worsen with a doubling of the exposure time from 2 to 4 wk in stressed mice but actually showed evidence of recovery. In contrast, increasing the intensity of the stress through prolonged direct/indirect contact resulted in changes in bladder function and a shift in the spectrum. The intensity threshold for this transition and the physiological mechanism responsible for it warrant further investigation in future studies using the social stress model.
We also extended the scope of our work to include an examination of the neurotrophin NGF in stress-induced urinary bladder dysfunction. Altered NGF content in the urinary bladder is associated with urinary bladder inflammation and dysfunction in both rodents and humans and may underlie neurochemical organizational and electrophysiological changes that can affect the micturition reflex pathway (13, 19, 20, 21, 28, 33, 35, 36). In addition, inflammation induced by noxious chemical or mechanical stimulation in the urinary bladder of rodents increases the expression of NGF mRNA as well as protein throughout the bladder, causing morphological changes in both bladder sensory and motor neurons (6, 23, 39, 40). Increases in NGF protein content have also been reported in hypertrophied bladders after spinal cord injury and bladder outlet obstruction, where expression correlates with neuronal hypertrophy and detrusor overactivity (41, 43). In contrast to the findings of Schnegelsberg et al. (33), who reported an increase in voiding frequency in a transgenic mouse model of NGF overexpression, we observed an increase in voiding frequency but no increase in NGF in mice in the stress protocol.
NGF has been implicated in altering the expression of membrane ion channels such as the transient receptor potential vanilloid (TRPV) family of channels, large-conductance Ca2+-sensitive K+ (BK) channels, and small-conductance Ca2+-sensitive K+ (SK) channels (18, 25, 29). Thus, it is possible that the effects of increased NGF bladder expression are indirect, including effects of altered expression and function of TRPV, BK, and SK channels that may directly contribute to the demonstrated urinary bladder dysfunction. We have recently demonstrated cystometrically that the TRPV1 antagonist capsaicin decreases bladder overactivity in stressed mice (Mingin et al., unpublished observations). Although this work is preliminary, it appears that stress may play a role in overactive bladder by way of increased inflammation or by altering the activity of the TRPV1 ion channel; however, additional studies are necessary to address these possibilities.
Of note, our results demonstrated a difference in mice subjected to increased stress, where they exhibited a decrease in voiding frequency compared with stressed or control mice. A significant increase in NGF protein in mice exposed to increased stress was noted compared with control or stressed mice. The difference between the stress and increased stress group in terms of NGF expression is intriguing. Interesting, preliminary studies in our laboratory have shown a significant upregulation of the BK channel in bladders of mice subjected to increased stress (data not shown). As BK channels are important regulators of bladder smooth muscle activity, it is possible that this channel may play a role in modulating bladder activity in increased stress (34). Thus, NGF as well as changes in BK channel expression/function may contribute to changes in bladder function in mice exposed to increased stress. In contrast, the present experiments do not implicate NGF expression in the urinary bladder as a contributor to urinary bladder dysfunction in mice exposed to stress.
It is interesting to speculate on the etiology of the increased NGF and histamine expression. The results from the quantitative RT-PCR experiments do not demonstrate an increase in either NGF or HDC mRNA, suggesting that changes in posttranscriptional processing contribute to increased NGF and HDC protein expression in the urinary bladder with increased stress. Reports of upregulated protein expression in the absence of a concomitant increase in mRNA in animal models of stress and inflammation have been previously demonstrated (42). Degranulation of mast cells releases NGF (3), and NGF itself may be a mast cell degranulator (4). However, recent data in a stress model of maternal separation suggests that in the setting of stress, NGF is mast cell derived (4, 5). These same authors determined that there is a closer association between mast cells and nerves in the setting of neonatal stress. The mechanisms underlying stress induction of mast cell degranulation and release of NGF are not completely understood. It is possible that this is due to a central mediator such as corticotropin-releasing hormone (CRH). Several investigators have shown that stress-induced visceral hypersensitivity and perturbations in colonic barrier function induced by mast cell degranulation could be inhibited by CRH antagonists (24, 37). In the present study, we did not investigate the contributions of CRH in the stress models used.
This speculation will have to await further studies, which have been planned to investigate the activity of NGF along the voiding spectrum, in particular, blockade of NGF/TrKA receptor interactions or using intravesical anti-inflammatory agents. Future studies will also investigate the role of various ion channels known to be important in bladder contractility, including TRPV1, TRPV4, BK, and SK channels. Studies examining bladder afferent nerve activity in both stressed and increased stressed mice in response to urinary bladder distention will also be pursued.
We acknowledge certain limitations in this study, namely, that the social stress model can be subjective when assessing the aggressiveness of retired breeder mice. The failure to subject all of the mice to each of the protocols for period of 4 wk is another limitation. Future experiments are planned to examine the effect of social stress over increased duration as well as examining the durability of these findings to determine if they are permanent or transient and normalize at some time point after exposure. The majority of mice in the study were subjected to tube implantation and although we acknowledge that this may be a confounding source of inflammation, control animals were also subjected to survival surgery. To remove this variable, we further analyzed bladder samples from animals that were subjected to stress and increased stress protocols but did not undergo tube implantation. The findings for NGF protein were similar in all of the groups. Yet another limitation is the inability to completely control for other confounding sources of stress, such as variations in handling, different animal care technicians, and seasonal effects. The former may be partially responsible for the difference in bladder intermicturition intervals between C57BL6 control mice.
The present findings increase our understanding of the role of social stress in bladder dysfunction, in particular how activation of neurotrophins may underlie the condition. Based on the above results, we propose that social stress represents a useful animal model for the study of both urinary bladder hyperactivity as well as bladder underactivity. These findings, using the social stress model, represent a novel foundation for future studies that will identify potential lower urinary tract targets for treatments for children with stress-related bladder disorders.
Perspectives and Significance
Stress may be directly causative or exacerbate the symptoms (i.e., urinary frequency) seen in disorders of the urinary bladder, such as interstitial cystitis and overactive bladder. The current experiments characterized the effects of social stress on bladder function as well as the possible mechanisms underlying the changes in function with the long-term goal of identifying potential targets for pharmacological intervention. Social stress produces changes in the bladder over a spectrum ranging from increased urinary frequency with reduced bladder capacity to decreased voiding and an increase in bladder capacity (urinary retention). The increased expression of NGF and histamine suggest either an inflammatory component and/or upregulation of neurotrophins leading to possible increased membrane channel activation. Future studies will be aimed at exploring the mechanisms underlying social stress-induced bladder dysfunction by determining the contributions of inflammatory mediators (e.g., NGF) and ion channels (e.g., transient receptor potential channels) as well as exploring the role of central mediators (e.g., CRH).
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R37-DK-053832, P01-HL-095488, DK-051369, DK-065989, and DK-060481, the Fondation Leducq, and Totman Medical Research Trust. NIH Grant P20 RR-16435 from the Centers of Biomedical Research Excellence Program of the National Center also supported the project with core resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.C.M., M.T.N., and M.A.V. conception and design of research; G.C.M., A.P., and C.S.E. performed experiments; G.C.M. and C.S.E. analyzed data; G.C.M., M.T.N., and M.A.V. interpreted results of experiments; G.C.M., A.P., C.S.E., and M.A.V. prepared figures; G.C.M. drafted manuscript; G.C.M., M.T.N., and M.A.V. edited and revised manuscript; G.C.M., A.P., C.S.E., M.T.N., and M.A.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical expertise and support provided by Susan Malley, Shannon Wright, and the Vermont Cancer Center DNA Analysis Facility. Gratitude is also expressed to Dr. Steve Zderic and Dr. Rita Valentino for assistance in establishing the social stress model.
REFERENCES
- 1.Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci 110: 175–191, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Arms L, Girard BM, Vizzard MA. Expression and function of CXL12/CXR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589–F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbara G, Wang B, Stanghellini V. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterologyn 132: 26–27, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 127: 524–34, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barreau F, Cartier C, Leveque M. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol 580: 347–356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorling DE, Jacobsen HE, Blum JR, Shih A, Beckman M, Wang ZY, Uehling DT. Intravesical escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int 87: 697–702, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chang A, Butler S, Silwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol 297: F1101–F1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang YC, Fraser MO, Yu Y, Chancellor MB, De Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975–979, 2001 [PubMed] [Google Scholar]
- 9.Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 275: R1279–R1286, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-related kinases (pERK) in urinary bladder in rats with cyclophosphamide (CYP)-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Delafoy L, Gelot A, Aridid D, Eschalier A, Bertrand C, Doherty AM, Diop L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 55: 940–945, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973 [DOI] [PubMed] [Google Scholar]
- 13.Dmitrieva N, McMahon SB. Senitization of visceral afferents by nerve growth factor in the adult rat. Pain 66: 87–97, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449–459, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Dopont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol 166: 2119–2120, 2001 [PubMed] [Google Scholar]
- 16.Freund-Michel V, Frossard N. The nerve growth factor and its receptors in airway inflammatory disease. Pharmacol Ther 117: 52–76, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Frossard N, Freund V, Advenier C. Nerve growth factor and its receptors in asthma and inflammation. Eur J Pharmacol 500: 453–465, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Girard BM, Merrill L, Malley S, Vizzard MA. Increased TRPV4 expression in urinary bladder ans lumbosacral dorsal root ganglia in mice with chronic over expression of NGF in urothelium. J Mol Neurosci 51: 602–614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Homan T, Tsuzuki T, Dogishi K, Shirakawa H, Oyama T, Nakagawa T, Kaneko S. A novel mouse model of chronic inflammatory and overactive bladder by a single intravesical injection of hydrogen peroxide. J Pharmacol Sci 121: 327–337, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Hu VY, Zavara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stoemer RP, Vizzard MA. Decrease in bladder over activity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Huang EJ, Reichardt LF. Neurtrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larauche MH, Bradesi S, Million M. Corticotropin releasing factor type1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol 294: G1033–G1040, 2008 [DOI] [PubMed] [Google Scholar]
- 25.McDowell TS, Wang ZY, Singh R, Bjorling D. CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPv1 in sensory neurons. Neurosci Lett 13: 34–38, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGongle E, Smith A, Butler S, Siliwoski J, Valentino R, Canning D, Zderic SA. Water avoidance stress results in an altered voiding phenotype in male mice. Neurourol Urodyn 31: 1185–1189, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Mcmahon SB, Dmitrieva N, Koltzenburg M. Visceral pain. Br J Anaesth 75: 132–144, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Merrill L, Malley S, Vizzard MA. Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol 305: R147–R156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain 17: 469–479, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9: 1455–1458, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Parsons BA, Drake MJ. Animal models in overactive bladder research. Handb Exp Pharmacol 202: 15–34, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford A, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyper innervation, pelvic sensitivity and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprossmann F, Pankert P, Sausbier U, Wirth A, Zhou XB, Madlung J, Zhao H, Bucurenciu I, Jakob A, Lamkemeyer T, Neuhuber W, Offermanns S, Shipston MJ, Jorth M, Nordheim A, Ruth P, Sausbier M. Inducible knockout mutagenesis reveals compensatory mechanisms eliceteed by constitutive BK channel deficiency in overactive murine bladder. FEBS J 276: 1680–1697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanzel RD, Lourenssen S, Blennerhassett MG. Inflammation causes expression of NGF in epithelial cells of the rat colon. Exp Neurol 211: 203–213, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest 88: 1709–1715, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tache Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastrenterol Motil 16: 137–142, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Van Gool JD, de Jong TPVM, Winkler-Seinstra P, Tamminen-Mobius T, Lax H, Hirche H, Nijman RJ, Hjalmas K, Jodal U, Bachmann H, Hoebeke P, Vande Walle J, Misselwitz J, John U, Bael A. Multi-center randomized controlled trail of cognitive treatment, placebo, oxybutynin, bladder training, and pelvic floor training in children with functional urinary incontinence. Neurourol Urodyn 15: 2–6, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol 279: R295–R305, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Winston JH, Li Q, Sarna S. Paradoxical regulation of ChAT and nNos expression in animal models of Crohn's colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol 305: G295–G302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zvara P, Kliment J, Jr., DeRoss AL, Irwin BH, Malley SE, Plante MK, Vizzard MA. Differential expression of bladder neurotrophic factor mRNA in male and female rats after bladder outflow obstruction. J Urol 168: 2682–2688, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Zavara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces over activity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]