Abstract
Intracerebroventricular injection of stable somatostatin (SST) agonists stimulates food and water intake in rats. We investigated the receptor subtype(s) involved in the dipsogenic effect of intracerebroventricular injection of SST agonists, mechanisms of action, and role. In nonfasted and non-water-deprived male rats with chronic intracerebroventricular cannula, intake of water without food or food without water was monitored separately to avoid any interactions compared with intracerebroventricular vehicle. SST-14 and cortistatin (CST-14) (1 μg/rat icv) increased water intake by 3.1- and 2.7-fold, respectively, while both peptides did not alter food intake at 1 h postinjection in the light phase. By contrast, the stable pan-somatostatin agonist ODT8-SST (1 μg/rat icv) increased both water and food intake by 4.9- and 3.7-fold, respectively. S-346-011, a selective receptor 2 (sst2) agonist (1 μg/rat icv) induced water ingestion, while sst1 or sst4 agonist, injected under the same conditions, did not. The sst2 antagonist S-406-028 (1 μg/rat icv) prevented the 1-h water intake induced by intracerebroventricular ODT8-SST and CST-14. Losartan (100 μg/rat icv), an angiotensin receptor 1 (AT1) antagonist, completely blocked the water consumption induced by intracerebroventricular ODT8-SST, whereas intracerebroventricular injection of S-406-028 did not modify the intracerebroventricular ANG II-induced dipsogenic response. The sst2 antagonist reduced by 40% the increase of the 3-h water intake in the early dark phase. These data indicate that SST-14 and CST-14 interact with sst2 to exert a potent dipsogenic effect, which is mediated downstream by angiotensin-AT1 signaling. These data also indicate that sst2 activation by brain SST-14 and/or CST-14 may play an important role in the regulation of drinking behavior.
Keywords: angiotensin receptor 1, brain, cortistatin, somatostatin agonists, water intake
somatostatin (SST) is a cyclic neuropeptide that is expressed in the central nervous systems (21, 62). The two endogenous isoforms of somatostatin, SST-14 and SST-28, interact with five distinct Gi protein-coupled receptors (sst), named sst1 to sst5 (49, 53). In addition to the originally described physiological action to inhibit growth hormone release, somatostatin exerts several extrapituitary actions (29, 62) in keeping with the wide brain distribution of SST-14 and its receptors outside of the median eminence (21, 62). Namely, SST-14 or the stable pan-somatostatin agonist, ODT8-SST (1, 18) early on were reported to act in the brain to influence behavior, including food intake (20), sympathetic outflow, and visceral functions, namely, heart rate, blood pressure, and gastric secretion, and motility (7, 59). With regard to food intake, we recently showed that acute intracerebroventricular injection of ODT8-SST induces a potent sst2-mediated orexigenic effect in rodents (55–57), which was associated with an increase in drinking behavior assessed by the number of drinking approaches during the second hour postinjection (55, 57). Previous reports in rats also showed that the stable somatostatin agonist octreotide injected intracerebroventricularly increased water consumption when assessed without food for the 10-min period postinjection (30). In the presence of food, the peptide induced first a robust drinking within 1 min followed by eating after 10 min (4). These data are indicative of a dipsogenic effect, independent of food intake, induced by the activation of brain sst, which so far has been little documented outside of these reports (4, 30).
In particular, it is still unknown whether the native peptide SST-14 and the structurally related endogenous peptide cortistatin (14) act in the brain to stimulate drinking. Cortistatin is widely expressed in the brain with a selective distribution pattern, although regional overlaps exist with somatostatin (13). Cortistatin also activates sst1–sst5 with comparable binding affinities to those of SST-14 and SST-28 (53). However, cortistatin exerts distinct biological actions compared with somatostatin (6, 14), which have been ascribed to its specific binding affinity to ghrelin receptor (growth hormone secretagogue receptor 1a) (15), the orphan G protein-coupled receptor MrgX2 (51), or yet uncharacterized cortistatin receptor(s).
In the present study, we compared the orexigenic and dipsogenic effects of endogenous sst1–sst5 ligands, namely SST-14 and cortistatin, and of the long-acting pan-somatostatin agonist, ODT8-SST (18) injected intracerebroventricularly. As the feeding and drinking behaviors are closely related and usually occur concomitantly with 80% of spontaneous daily water intake being temporally associated with feeding in rats (3, 23), changes in ingestion of food or water can affect each other. In previous studies (4, 55–57) except one (30), food intake after intracerebroventricular injection of somatostatin agonists was assessed under normal conditions of feeding that include free access to food and water, and the observed food intake response could have been influenced by changes in water intake. Therefore, we first determined whether somatostatin agonists injected intracerebroventricularly induce orexigenic or dipsogenic effect in the light phase without access to water or food, respectively, to dissociate these two components. Then, we determined the sst subtype(s) involved in the dipsogenic effect using sst subtype-specific peptide analogs, namely the sst1 agonist S-406-062, sst2 agonist S-346-011, sst4 agonist S-315-297, and the sst2 antagonist S-406-028 recently developed (9, 17, 19, 27). The brain angiotensinergic mechanism is well known to regulate thirst and fluid balance (22), and one report indicates that this pathway plays a role in the dipsogenic effect of intracerebroventricular octreotide (30). Therefore, we examined the interaction between the brain sst2 signaling system and the angiotensin system using the intracerebroventricular injection of the ANG II receptor type 1 (AT1) antagonist losartan (47) and whether the dipsogenic response to intracerebroventricular ODT8-SST activates brain sites previously shown to be responsive to intracerebroventricular ANG II or octreotide to stimulate water intake in rats (22, 31). Lastly, we tested the physiological relevance of brain sst2 signaling system during the spontaneous circadian water intake in the dark phase using the selective sst2 antagonist injected intracerebroventricularly.
METHODS
Animals.
Adult male Sprague-Dawley rats were purchased from Harlan Laboratories (San Diego, CA) at an initial body weight of 230–250 g. Animals were kept under controlled illumination (12:12-h light-dark cycle, lights on/off: 6:00 AM/6:00 PM) and temperature (22 ± 2°C). Animals were fed standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO), and tap water was available ad libitum. Animal care and experimental procedures followed institutional ethical guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at the Veterans Affairs Greater Los Angeles Healthcare System (animal protocol no. 11047-09).
Peptides and compounds.
Structure, molecular weight, and binding affinity to human sst1–5 of SST-14, cortistatin-14 (CST-14), ODT8-SST (compound 1 in Ref. 18), selective peptide sst1 agonist (S-406-062, compound 25 in Ref. 19), selective sst2 agonist (S-346-011, compound 2 in Ref. 27), selective sst4 agonist (S-315-297, compound 14 in Ref. 17), and selective sst2 antagonist (S-406-028, compound 4 in Ref. 9) are detailed in Table 1. Peptides were designed, synthesized, purified, and characterized at the Clayton Foundation Peptide Biology Laboratories Salk Institute (La Jolla, CA). ANG II and the selective angiotensin AT1 receptor antagonist losartan were purchased from Sigma-Aldrich (St. Louis, MO). All compounds were stored at −80°C and dissolved in sterile saline before the start of the experiment.
Table 1.
Structure, molecular weight, and human receptor binding affinity of somatostatin-related peptides used in this study
| Human Receptor Binding Affinity (IC50, nM)* |
|||||||
|---|---|---|---|---|---|---|---|
| Peptide | Structure | MW | sst1 | sst2 | sst3 | sst4 | sst5 |
| SST-1411 | H-Ala-Gly-c[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys]-OH | 1638 | 2.3 | 0.2 | 1.2 | 1.7 | 1.4 |
| CST-1411 | H-Pro-c[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Ser-Ser-Cys]-Lys-OH | 1721 | 2.1 | 0.5 | 3.8 | 18 | 0.9 |
| ODT8-SST18 | H-c[Cys-Phe-Phe-DTrp-Lys-Thr-Phe-Cys]-OH | 1079 | 27 | 41 | 13 | 1.8 | 46 |
| sst1 agonist19 S-406-062 | H-DTyr-c[Cys-DAgl(NMe,2naphthoyl)-IAmp-Phe-Cys]-Thr-NH2 | 1238 | 0.2 | >1000 | 158 | 27 | >1000 |
| sst2 agonist27 S-346-011 | H2N-CO-DPhe-c[Cys-Aph(CONH2)-DTrp-Lys-Thr-Cys]-Thr-NH2 | 1133 | >1000 | 6.2 | 394 | 494 | 100 |
| sst4 agonist17 S-315-297 | H2N-CO-c[Cys-Phe-Aph-Trp-Lys-Thr-Phe-Cys]-OH | 1137 | 650 | >1000 | 780 | 1.5 | >1000 |
| sst2 antagonist9 S-406-028 | H2N-pNO2-Phe-c[DCys-Tyr-DAph(Cbm)-Lys-Thr-Cys]-2Nal-NH2 | 1208 | >1000 | 2.6 | 384 | >1000 | >1000 |
Superscript numbers in the first column denote the references.
Derived from competitive ligand displacement assays in cells expressing the cloned human sst1–sst5 using 125I-[Leu8d-Tryp22Tyr25]-SST-289, 17–19, 27 or 125I-SST-1411. It is to note that binding affinity of the used sst agonists/antagonists on cloned rat sst1–sst5 is yet not available.
Intracerebroventricular cannulation and injection.
Intracerebroventricular cannulation and injection were performed as previously described (58). Rats were anesthetized by an intramuscular injection of ketamine hydrochloride (75 mg/kg im, Ketanest; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg/kg im, Rompun; Mobay, Shawnee, KS). They were placed in a stereotaxic apparatus and implanted with a chronic guide cannula (22-gauge, Plastics One, Roanoke, VA) into the right lateral brain ventricle. Stereotaxic coordinates were selected according to the Paxinos and Watson's rat brain atlas (50) as follows: 0.8 mm posterior, 1.5 mm right lateral, and 4.0 mm ventral from the bregma. The guide cannula was secured by dental cement and anchored by screws fixed to the skull and occluded. After surgery, individually housed animals had a recovery period of 1 wk and were handled for 2–3 min/day for 3 days prior to the experiments performed within 7–28 days postsurgery. The intracerebroventricular injection was done in lightly hand-restrained conscious rats in a 10-μl volume delivered over 1 min for single injection or 5 μl in 30 s each for two consecutive injections. The injection cannula remained in place for an additional 1 min after each injection. The correct placement of the cannula into the right lateral ventricle was verified at the end of the experiment. No animal had to be excluded from data analysis.
Food and water intake measurements.
Food intake and water intake were determined by measuring the weight of food and water bottle before and after each time period and calculating the amount consumed. To facilitate the collection of food spillage, rats were placed in the home cage with a grid floor and paper under the grid. To avoid the spill of water, we used water bottles with ball-pointed sipper tube. Spillage of food was recovered and weighed. Food and water intake were expressed as grams per rat and milliliters per rat, respectively.
Immunohistochemistry.
The procedure was essentially as described in our previous report (26). Briefly, rats were deeply anesthetized with pentobarbital sodium (100 mg/kg ip; Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) and perfused transcardially with saline followed by ice-cold fixative (4% paraformaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer). The brain was removed, postfixed overnight, and cryoprotected in 10% sucrose for 1–2 days. Frozen coronal brain sections (30 μm) were cut using a cryostat (Microm International, Walldorf, Germany) from the bregma level −0.20 mm to the end of the hypothalamus (−4.30 mm), according to Paxinos and Watson's rat brain atlas (50). The immunoreactivity for c-Fos was visualized by the avidin-biotin-peroxidase complex (ABC) immunohistochemical technique, as previously described (62). Free-floating sections were incubated for two nights with rabbit polyclonal anti-Fos antibody (1:10,000; PC38, Millipore, Billerica, MA). Then, sections were incubated at room temperature for 1 h with biotinylated secondary goat anti-rabbit IgG (1:1,000; cat no. 111-067-003; Jackson ImmunoResearch Laboratories, West Grove, PA) followed by 1-h incubation with the ABC (1:200; Vector Laboratories, Burlingame, CA). The chromogen was diaminobenzidine tetrachloride (0.025%) with hydrogen peroxide (0.01%). Then, sections were mounted, air-dried, dehydrated in ethanol, cleared in xylene, and coverslipped. c-Fos-immunoreactive cells were observed by light microscopy (Axioscop II; Carl Zeiss, Jena, Germany). Coordinates of selected brain nuclei were identified with the Paxinos and Watson's rat brain atlas (50) and are provided in millimeters from bregma: median preoptic nucleus (MnPO), +0.24 to −0.24; lateral hypothalamus (LH): −1.80 to −3.60; supraoptic nucleus (SON), −0.60 to −1.56; paraventricular nucleus (PVN), −1.44 to −1.80; and subfornical organ (SFO), −0.84 to −1.08.
Experimental protocols.
Except otherwise stated, singly housed chronically intracerebroventricularly cannulated rats were placed in a cage with a grid floor 24 h before the experiment. Rats had free access to food and water, and the intracerebroventricular injection started between 3 and 4 h after the onset of lights on.
Experiment 1: effects of intracerebroventricular ODT8-SST, SST-14, and CST-14 on food intake assessed in rats without access to water.
Vehicle (saline), ODT8-SST (1 μg/rat, 0.93 nmol), SST-14 (1 μg/rat, 0.61 nmol), or CST-14 (1 μg/rat, 0.58 nmol) was injected intracerebroventricularly, after which rats had free access to food without water. Food intake was monitored for 1 h after the intracerebroventricular injection. The dose of peptides was based on our previous studies showing a robust feeding response following intracerebroventricular ODT8-SST in rats with free access to food and water (55).
Experiment 2: effects of intracerebroventricular ODT8-SST, SST-14, CST-14, and subtype-selective sst agonists on water intake measured without food.
Rats were intracerebroventricularly injected with vehicle (saline), ODT8-SST (1 μg/rat), SST-14 (1 μg/rat), CST-14 (1 μg/rat), or subtype-selective agonists to sst1, sst2, and sst4 as listed in Table 1 (all 1 μg/rat = 0.81, 0.88, and 0.88 nmol, respectively). Rats were placed back in their home cages with access to water without food. Water intake was measured at 10 and 60 min after the intracerebroventricular injection. The peptide doses were based on our previous studies on food intake with water in rats (55, 57, 58).
Experiment 3: effects of intracerebroventricular sst2 antagonist on water intake induced by intracerebroventricular injection of ODT8-SST and CST-14.
Rats received an intracerebroventricular injection of vehicle (saline) or the selective sst2 antagonist, S-406-028 (1 μg/rat, 0.83 nmol) immediately followed by an intracerebroventricular injection of either saline, ODT8-SST (1 μg/rat), or CST-14 (1 μg/rat). Rats were placed back in their home cages with access to water only. Water intake was measured at 10 and 60 min after intracerebroventricular injection. The intracerebroventricular sst2 antagonist dose was based on a previous study showing the blockade of intracerebroventricularly administered sst2 agonist-induced stimulation of food intake in rats with free access to food and water (55).
Experiment 4: effects of intracerebroventricular AT1 antagonist, losartan, and sst2 antagonist, S-406-028 on water intake induced by intracerebroventricular ODT8-SST and ANG II.
Rats received an intracerebroventricular injection of saline, losartan (100 μg/rat), or S-406-028 (1 μg/rat) immediately followed by an intracerebroventricular injection of saline, ODT8-SST (1 μg/rat), or ANG II (52 ng/rat, 50 pmol). Rats were placed back in their home cages with free access to water only, and water intake was measured at 10 and 60 min after the intracerebroventricular injection. The losartan dose was based on a previous study (30) and that of ANG II was the minimal effective dose inducing a reproducible dipsogenic effect in our settings based on preliminary data.
Experiment 5: effects of intracerebroventricular ODT8-SST on c-Fos expression in brain areas involved in water intake.
Rats were injected intracerebroventricularly with saline or ODT8-SST (1 μg/rat) and placed back in their home cages without food or water to avoid any confounding effect of differences in food and water intake between the two groups. At 90 min after the intracerebroventricular injection, rats were euthanized, and brains were processed for immunohistochemistry, and c-Fos immunoreactivity was assessed in the MnPO, LH, SON, PVN, and SFO.
Experiment 6: basal water intake before and after the lights off.
Basal spontaneous water intake was measured for two consecutive 3-h periods starting 4 h before and immediately after the lights off in intracerebroventricularly cannulated untreated rats without access to food. During the 1-h period just before the lights off, rats had no access to food or water.
Experiment 7: effects of selective sst2 antagonist on water intake during the lights off period.
One week after experiment 6 was performed, the same experiment was repeated except that during the 30-min interval before the lights off, rats were injected intracerebroventricularly with vehicle (saline) or S-406-028 (1 μg/rat) and returned to their home cages. Water bottles were placed back just before lights off at 6:00 PM, and water intake without food was measured at 1 and 3 h thereafter. Rats were refed at 3 h after the lights off, and food and water intake were measured 13 h later (4 h after the lights on). The experiment was performed in a cross-over design using six animals with a 4-day washout period between the measurements.
Statistical analysis.
Data are shown as means ± SE. Comparison between two groups was performed by Student's or Welch's t-test following the F-test. Comparison among multiple groups was performed by 1-way or 2-way ANOVA followed by post hoc Tukey multiple comparisons. P < 0.05 was considered significant.
RESULTS
Experiment 1: intracerebroventricular ODT8-SST increased food intake assessed without water, while SST-14 and CST-14 did not.
In rats with free access to food without water after the intracerebroventricular injection, ODT8-SST (1 μg/rat) significantly increased the 60-min cumulative food intake compared with intracerebroventricular vehicle (3.7 ± 0.8 vs. 1.0 ± 0.3 g; P < 0.05), while intracerebroventricular SST-14 or CST-14 (both 1 μg/rat) under the same conditions had no effect (Fig. 1).
Fig. 1.
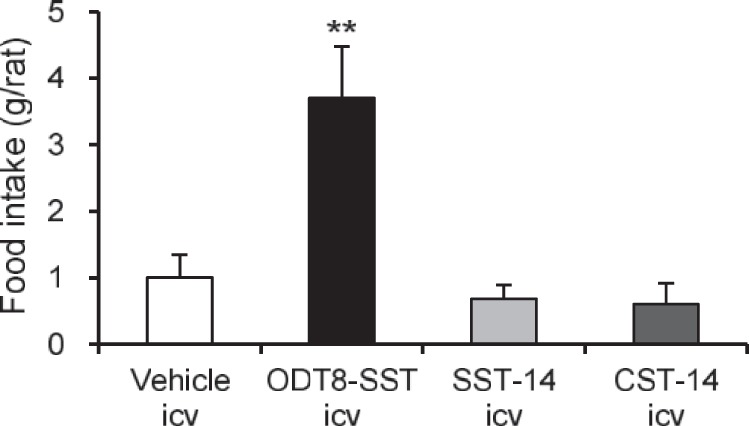
Effects of intracerebroventricular injection of ODT8-somatostatin (SST), SST-14, and cortistatin (CST)-14 on food intake without water. Nonfasted and non-water-deprived rats were injected (10 μl/rat icv) with vehicle (saline), ODT8-SST, SST-14, or CST-14 (all 1 μg/rat icv), and food intake was measured for 1 h in rats that had no access to water. Data are expressed as means ± SE of 5 or 6 rats per group. **P < 0.01 vs. vehicle.
Experiment 2: ODT8-SST, SST-14, CST-14, and sst2 agonist increased water intake measured without food.
In rats with free access to water without food, the 10- and cumulative 60-min water intake was significantly increased by the intracerebroventricular injection of ODT8-SST, SST-14, and CST-14 compared with vehicle (3.3 ± 1.2, 4.9 ± 0.6, and 4.0 ± 0.3 ml vs. 0.7 ± 0.3 ml at 10 min, respectively; 8.5 ± 1.2, 5.6 ± 0.5, and 4.8 ± 0.4 ml vs. 1.8 ± 0.4 ml at 60 min, respectively; P < 0.05; Fig. 2). However, only intracerebroventricular ODT8-SST maintained a significant increase in water intake during the 10–60-min period compared with intracerebroventricular vehicle (Fig. 2).
Fig. 2.
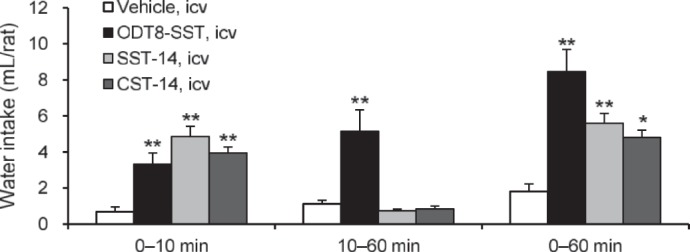
ODT8-SST, SST-14, and CST-14 injected intracerebroventricularly increased water intake without food. Nonfasted and non-water-deprived rats were injected (10 μl/rat icv) with vehicle (saline), ODT8-SST, SST-14, or CST-14 (all 1 μg/rat icv), and water intake was measured in rats without access to food during the 0–10- and 10–60-min periods postinjection; then data were cummulated over the 60-min period. Data are expressed as means ± SE of 5 or 6 rats per group. *P < 0.05, **P < 0.01 vs. vehicle.
To elucidate the sst subtype responsible for the water intake occurring in the absence of food, we tested the subtype-selective peptide sst1, sst2, or sst4 agonists. The selective sst1, sst2, and sst4 agonists did not influence water intake monitored at 10 min postinjection (Fig. 3). However, during the 10–60-min period, the sst2 agonist, S-346-011 (1 μg/rat icv) significantly increased water intake (6.5 ± 1.2 vs. 1.1 ± 0.2 ml icv in vehicle; P < 0.01). The sst1 agonist and the sst4 agonist showed a trend to reduce the 10–60-min water intake, which did not reach statistical significance (Fig. 3). When the cumulative 60-min period of water intake was analyzed after the intracerebroventricular injection, there was a significant 4.7-fold increase induced by the sst2 agonist (8.5 ± 1.2 ml vs. 1.8 ± 0.4 ml in intracerebroventricular vehicle, P < 0.01) and no significant changes induced by intracerebroventricular sst1 and sst4 (Fig. 3).
Fig. 3.
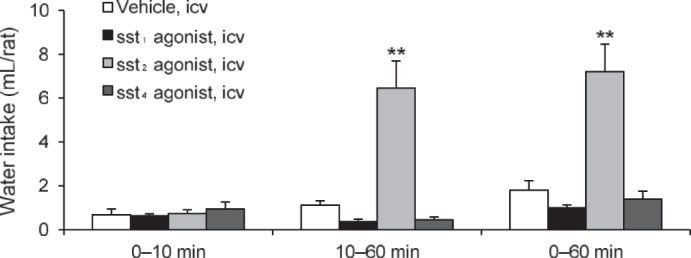
The intracerebroventricular injection of selective the sst2 agonist, unlike the sst1 or sst4 agonists increased water intake without food. Nonfasted and non-water-deprived rats were injected (10 μl/rat icv) with vehicle (saline) or selective sst1, sst2, or sst4 agonists (all 1 μg/rat icv). Water intake was measured in rats without access to food during the 0–10- and 10–60-min periods postinjection; then data were cummulated over the 60-min period. Data are expressed as means ± SE of 5 or 6 rats per group. **P < 0.01 vs. vehicle.
In experiments 1 and 2, we also analyzed the food and water intake by normalizing values to individual body weight and found that it did not influence the significance of the results (body weight of rats before the start of experiments: 298 ± 20 g, means ± SD).
Experiment 3: the sst2 antagonist injected intracerebroventricularly blocked water intake induced by intracerebroventricular ODT8-SST and CST-14.
To further establish the involvement of the sst2 receptor in the water intake response, we examined the influence of pretreatment with the sst2 antagonist, S-406-028 (1 μg/rat icv) on the drinking response to ODT8-SST and CST-14 (both 1 μg/rat icv). The antagonist by itself under these conditions did not influence the early light-phase water intake compared with intracerebroventricular saline (Fig. 4). However, the intracerebroventricular injection of S-406-028 just before that of ODT8-SST or CST-14 prevented the increased 10-min water intake (both P < 0.05; Fig. 4), and the cumulative water intake at 60 min in ODT8-SST-treated rats. Values did not differ significantly from those in rats treated with vehicle plus saline at either time. Two-way ANOVA showed a significant influence of antagonist treatment (F1,29 = 23.4; P < 0.001), agonist treatment (F2,29 = 18.5; P < 0.001), and antagonist × agonist treatment (F2,29 = 5.9; P < 0.01) on 10-min water intake; and a significant influence of antagonist treatment (F1,29 = 21.0; P < 0.001), agonist treatment (F2,29 = 27.8, P < 0.001), and antagonist × agonist treatment (F2,29 = 7.8; P < 0.01) on the cumulative 60-min water intake.
Fig. 4.
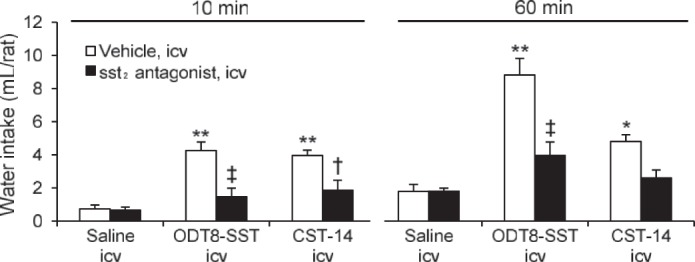
The selective sst2 antagonist injected intracerebroventricularly blocked water intake induced by intracerebroventricular ODT8-SST and CST-14. Nonfasted and non-water-deprived rats were pretreated (5 μl/rat icv) with vehicle (saline) or the selective sst2 antagonist (1 μg/rat icv) followed by vehicle (saline), ODT8-SST, or CST-14 (both 1 μg/rat icv) and water intake without food was measured during the 0–10- and 10–60-min periods after the intracerebroventricular injection. Data are expressed as means ± SE of 5 or 6 rats per group. *P < 0.05, **P < 0.01 vehicle plus agonists vs. vehicle plus saline. †P < 0.05, ‡P < 0.01 sst2 antagonist plus agonists vs. vehicle plus respective agonists.
Experiment 4: the selective AT1 antagonist losartan injected intracerebroventricularly blocked intracerebroventricularly administered ODT8-SST-induced water intake, while the sst2 antagonist did not modify water intake induced by ANG II.
The intracerebroventricular injection of losartan (100 μg/rat icv) given before that of ODT8-SST (1 μg/rat icv) completely blocked the water intake induced by ODT8-SST at 10 min (0.5 ± 0.1 vs. 4.3 ± 0.8 ml; P < 0.01) and at cumulative 60-min periods (1.2 ± 0.4 vs. 9.2 ± 0.5 ml; P < 0.01) after treatment (Fig. 5). In contrast, the selective sst2 antagonist S-406-028 (1 μg/rat icv) had no effect on the water intake induced by ANG II (52 ng/rat icv) either at 10 min or the cumulative 60 min (Fig. 5).
Fig. 5.
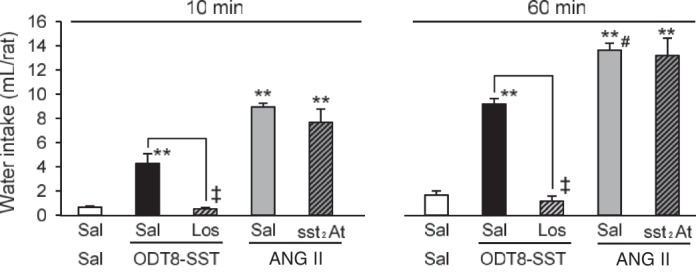
Intracerebroventricular injection of the selective AT1 antagonist losartan blocked the ODT8-SST-induced water intake, while the selective sst2 antagonist did not block the water intake induced by ANG II. Nonfasted and non-water-deprived rats were pretreated (5 μl/rat icv) with saline or losartan (100 μg/rat icv) followed by ODT8-SST (1 μg/rat icv), or were pretreated with saline or selective sst2 antagonist (1 μg/rat icv) followed by ANG II (52 ng/rat icv). Water intake without food was measured at 10 and 60 min post injection. Data are expressed as means ± SE; n = 6 rats per group. **P < 0.01 saline plus agonists vs. saline plus saline. ‡P < 0.01 losartan plus ODT8-SST vs. saline plus ODT8-SST. #P < 0.01 saline plus ANG II vs. saline plus ODT8-SST. Sal, saline; Los, losartan; ODT8, ODT8-SST; sst2At, sst2 antagonist.
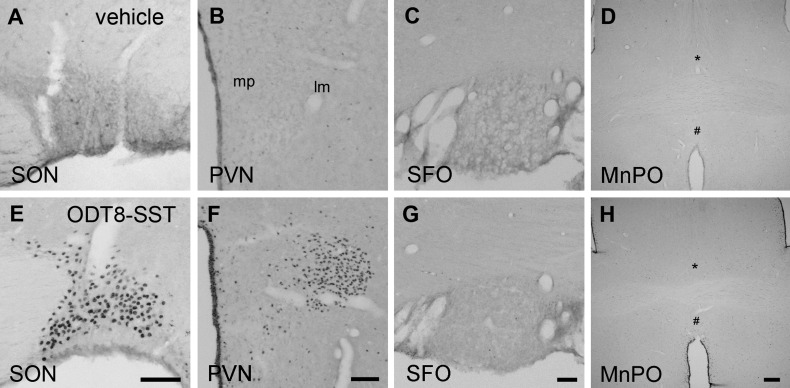
Experimental 5: ODT8-SST injected intracerebroventricularly increased c-Fos immunoreactivity in the PVN and SON of the hypothalamus, but not in the SFOs, MnPO, or LH.
As we reported previously (26), intracerebroventricularly administered ODT8-SST induced a significant c-Fos immunoreactivity in the SON and magnocellular neurons of the PVN (Fig. 6, E and F); however, no clear c-Fos induction was observed in the SFO, MnPO (Fig. 6, G and H), or LH (data not shown). The c-Fos immunoreactivity in rats injected intracerebroventricularly with vehicle showed subtle or almost no c-Fos expression in these brain nuclei (Fig. 6, A–D).
Fig. 6.
ODT8-SST injected intracerebroventricularly induced c-Fos expression in the supraoptic nucleus (SON) and magnocellular paraventricular nucleus (PVN), but not in subfornical organ (SFO) or median preoptic nucleus (MnPO). Nonfasted and non-water-deprived rats were injected (10 μl icv) with vehicle (saline) or ODT8-SST (1 μg/rat) and euthanized 90 min later for brain immunohistochemical analysis. The representative images of the SON (A and E), PVN (B and F), SFO (C and G), and MnPO (D and H) following saline (A--D) or ODT8-SST (E--H) are shown. * and # indicate the MnPO areas divided by the anterior commissure. Scale bars: 100 μm, except for MnPO (300 μm). SON, supraoptic nucleus; PVN, paraventricular nucleus of the hypothalamus; mp, medial parvocellular; lm, lateral magnocellular; SFO, subfornical organ; MnPO, median preoptic nucleus.
Experiment 6: basal water intake increases after the onset of the lights off.
The 3-h water intake following the lights off was increased by 4.4-fold compared with the 3-h period starting 4 h before the lights off in rats without food (7.7 ± 0.9 ml vs. 1.7 ± 0.5 ml; P < 0.01, n = 6).
Experiment 7: the selective sst2 antagonist suppressed the spontaneous water intake after the onset of lights off.
The assessment of S-406-028, the selective sst2 antagonist, on the spontaneous water intake in the early dark phase without food showed that the S-406-028 (1 μg/rat icv) significantly suppressed water intake by 55% and by 40% at 1 and 3 h after the lights off, respectively, compared with saline-treated rats at the same time period (1.3 ± 0.3 vs. 2.8 ± 0.4 ml, and 3.2 ± 0.6 vs. 5.3 ± 0.6 ml, P < 0.05, respectively; Fig. 7). After refeeding, the subsequent 13-h water and food intake in S-406-028-treated rats was comparable to that in vehicle-treated rats (data not shown).
Fig. 7.
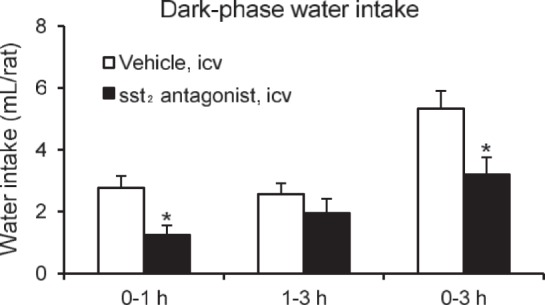
The selective sst2 antagonist blocked the physiological water intake in the early dark phase. Rats were injected (10 μl/rat icv) with vehicle (saline) or sst2 selective antagonist (1 μg/rat icv) 30 min before lights were turned off at 6:00 PM. Water intake without food was measured during the 0–1- and 1–3-h periods; then data were cumulated over the 0–3-h period. Data are expressed as means ± SE of 6 rats per group. *P < 0.05 vs. vehicle.
DISCUSSION
In the present study, we showed that the activation of brain somatostatin receptors by the native peptides SST-14 and CST-14 and the stable peptide agonist ODT8-SST injected intracerebroventricularly elicits a prompt stimulation of water consumption independently of food intake in rats. The dipsogenic action is primarily mediated by sst2 with the downstream involvement of ANG II-AT1 receptor signaling. We also demonstrated that the selective sst2 antagonist, S-406-028 injected intracerebroventricularly dampened the spontaneous 1-h water intake occurring in the early dark phase by 55%, while not modifying ANG II's dipsogenic effect in the light phase. These findings point to a physiological role of brain somatostatin-sst2 signaling in the stimulation of water ingestion.
SST-14 or CTS-14 injected acutely at ∼0.60 nmol into the lateral brain ventricle in chronically cannulated rats increased water intake by 3.1- and 2.7-fold compared with intracerebroventricularly vehicle-treated rats during the first 1 h postinjection in the morning of the light phase. This reflects independent mechanisms of those associated with food intake since rats had no access to food after intracerebroventricular injection. In addition, both SST-14 and CST-14 had no effect on food intake assessed without water under otherwise similar conditions. These data provide the first evidence that endogenous somatostatin ligands act in the brain to induce a potent dipsogenic effect in rats. The time course response indicates that both SST-14 and CTS-14 action was rapid in onset and short-lasting, as shown by the increase of water intake occurring during the first 10 min and the lack of significant changes in the subsequent 10–60-min period after intracerebroventricular injection. Likewise, the stable pan-somatostatin agonist ODT8-SST (18) induces a dipsogenic response of similar magnitude to that of SST-14 or CST-14 at 10 min; however, the effect was maintained during the 10–60-min period with a 4.7-fold increase compared with intracerebroventricular vehicle. The ODT8-SST long duration of action may be related to the stability of the peptide agonist compared with the short half-life of the native ligands (1), although differences in intracerebroventricular nanomolar doses between ODT8-SST (0.93 nmol) and SST-14 and CTS-14 (∼0.60 nmol) may also play a role.
SST-14 and CST-14 and ODT8-SST display affinity to sst1–5 in the nanomolar range (Table 1) (53), and CST-14 can, in addition, bind to other receptors, including the ghrelin receptor (15, 51). However, the implication of the ghrelin receptor in CST-14 dipsogenic response can be ruled out since ghrelin intracerebroventricularly inhibits water intake in dehydrated rats monitored in the absence of food (32) and the dipsogenic response to intracerebroventricularly administered ANG II in rats (33, 44). The present study provides convergent pharmacological evidence of a primary role of brain sst2 in mediating the stimulation of water intake by intracerebroventricular CST-14 and the stable somatostatin agonist. First, the intracerebroventricular injection of the sst2 agonist, S-346-011 (27) (0.88 nmol) induces water intake, reaching a similar magnitude to that of intracerebroventricular ODT8-SST or CST-14 at 1 h postinjection. Second, the intracerebroventricular injection of the sst2 antagonist, S-406-028 (9) blocked the increase in water intake induced by both ODT8-SST and CST-14, as monitored at either 10 or 60 min after intracerebroventricular injection, while having no effect on basal light phase water intake. However, it has to be noted that the selective sst2 agonist enhanced drinking during the 10–60 min after injection but failed to do so during the first 10 min. Possible distinct physicochemical profiles between the sst2 agonist and native peptides or ODT8-SST that influence their brain diffusion may account for the differential time course in the onset of water intake. It is unlikely that it reflects the involvement of additional sst subtype(s) since the selective sst2 antagonist completely blocked the 10-min water intake stimulated by intracerebroventricular ODT8-SST and CST-14. Third, the selective sst1 (S-406-062) or sst4 (S-315-297) agonist (17, 19) injected under the same conditions showed a trend toward decrease in water intake. In line with these findings, previous reports showed that the stable sst agonist octreotide, which is devoid of sst1 and sst4 affinity, while binding to sst2 > sst5 >> sst3 (28), increased water consumption within the first 10 min after intracerebroventricular injection in picomolar range in rats that had no access to food (30).
The present study also showed that the nonpeptide AT1 receptor antagonist losartan (47) injected intracerebroventricularly blocked the drinking response induced by intracerebroventricular injection of ODT8-SST either at 10 or 60 min postinjection. Consistent with these findings, a previous report indicated that intracerebroventricular injection of saralasin, an antagonist of the AT1/AT2 receptors, and losartan prevented the initial 10-min drinking elicited by intracerebroventricular injection of octreotide in rats without food (30). There is also evidence that ANG II is released from the rat hypothalamus within 10 min of intracerebroventricular octreotide injection (25). By contrast the sst2 antagonist injected intracerebroventricularly at a dose blocking the dipsogenic effect of intracerebroventricularly administered ODT8-SST did not interfere with the drinking response to intracerebroventricularly administered ANG II. Collectively, these data support the involvement of brain ANG II-AT1 transmission downstream of the activation of sst2 in the dipsogenic action of intracerebroventricularly administered somatostatin.
The exact underlying neuroanatomical substrate(s) through which the activation of sst2 signaling by intracerebroventricular ODT8-SST recruits the brain ANG II-AT1 system (22, 43) will need to be further delineated. The SFO, organum vasculosum laminae terminalis (OVLT), and MnPO are among the major brain structures involved in the drinking response to ANG II acting on AT1 receptors (43). In particular, the dipsogenic effect of intracerebroventricular ANG II is abolished by the ablation of the anteroventral third ventricle (AV3V) (8), unlike SFO (42), suggesting the importance of AT1 receptors in the MnPO in the AV3V region. It may be speculated that intracerebroventricular ODT8-SST acts on sst2 receptors in the SFO and induce water ingestion by activating angiotensinergic pathways innervating the MnPO within the AV3V region. In support of this contention, microinjection of octreotide into the SFO induces water ingestion in rats (31). Somatostatin binding sites have been reported in the SFO (38, 39), although the receptor subtype(s) are still to be determined, and AT1 receptors are expressed in the MnPO (43, 46). However, ODT8-SST, as well as the sst2 agonist injected intracerebroventricularly under similar conditions did not induce c-Fos expression in the SFO or MnPO, while a strong c-Fos expression is observed in the magnocellular neurons of the PVN and SON (Ref. 26, present study). These hypothalamic nuclei are part of angiotensin pathways descending from the SFO and OVLT (22, 45). Convergent studies have highlighted that ANG II injected intracerebroventricularly induced c-Fos expression selectively in four areas of the forebrain in rats: the SFO, MnPO, SON and PVN (34, 41, 46). Moreover, the AT1 receptor is coexpressed with c-Fos/c-Jun in those areas, prominently in the PVN and SON after intracerebroventricularly ANG II (46). Collectively, these data would be compatible with a network that involves intracerebroventricular ODT8-SST, stimulating the angiotensinergic drinking system by acting in the PVN and SON. This is also consistent with the report that octreotide microinjected into or around the PVN induces water ingestion (31). In addition, there is a robust expression of sst2 in the PVN, where the neuronal localization of this receptor subtype can mediate both postsynaptic and presynaptic actions (12, 16). ODT8-SST activating sst2 may target primary inhibitory neurons (40) that are directly or indirectly suppressing the angiotensinergic signals.
Although each of ODT8-SST-, SST-14-, or CST-14-induced dipsogenic effects, only ODT8-SST stimulated food intake when assessed separately without water. The orexigenic action of intracerebroventricular ODT8-SST or the sst2 agonist was well documented in rats with access to food and water (55–57). The present study clearly established that the orexigenic and dipsogenic effects of intracerebroventricular ODT8-SST can be regarded as parallel and independent mechanisms at least during the first hours postinjection, although they may act in concert thereafter. This is further supported by the similar 1-h food intake increment induced by intracerebroventricular ODT8-SST in the presence or absence of water tested under otherwise similar conditions [present and previous studies (Ref. 57)]. The understanding of the differential selective dipsogenic effects of intracerebroventricular injection of SST-14 and CTS-14 vs. dipsogenic and orexigenic actions of intracerebroventricular injection of ODT8-SST may be related to differential pharmacokinetics of the peptides. The short half-life (2–3 min) of SST-14 and CST-14 (2) may not allow the peptide to reach brain sites regulating feeding behavior, while reaching those involved in thirst. We observed that rats injected intracerebroventricularly with ODT8-SST with access to both food and water, tend to drink water first, and, thereafter, start to eat (data not shown), similar to the pattern of the other stable sst agonist, octreotide, injected intracerebroventricularly in rats (4, 30).
One major finding of the present study is that the activation of brain sst2 contributes to the early nocturnal water intake. Physiologically, rats drink primarily in the dark phase (64), and this nocturnal rhythm in water intake is considered to be driven by the nocturnal rhythm in food intake (36, 37). However, as reported previously (23, 48) and observed in the present study, rats are keeping the nocturnal pattern of water ingestion, even without food, as shown by the 4.4-fold increase in the amount of water intake during the first 3 h after the lights off compared with the same period before the dark phase in rats without access to food. Convergent evidence supports the implication of brain somatostatin-sst2 signaling in the early dark-phase drinking. First, the native peptides SST-14 and CST-14 injected intracerebroventricularly at a low dose were devoid of an orexigenic effect, and the selective sst2 agonist elicited water intake of a similar magnitude during the light phase as the spontaneous water drinking occurring during the first hours of the dark phase. Furthermore, the selective sst2 antagonist injected intracerebroventricularly reduced the increase in spontaneous water intake occurring during the first 1 h by 55%, while not influencing 1-h water intake monitored at 4 h after the lights on. The partial suppression of the dark-phase water intake is unlikely to reflect submaximal dosing of the sst2 antagonist, since the peptide normalized a similar rise in water intake induced by intracerebroventricular ODT8-SST in the light phase. Lastly, convergent reports demonstrated that somatostatin content and release in the rat hypothalamus showed diurnal variations consistent with a surge of peptide release at the early onset of the dark phase compared with low levels of the peptide in the light phase (24, 35, 52). With regard to the involvement of cortistatin, the gene expression is mainly localized in the rat cerebral cortex and hippocampus, unlike the hypothalamus (54), and mRNA levels showed a circadian fluctuation with highest levels of prepro-cortistatin in the cortex recorded in the late dark phase (5). On the basis of the brain distribution and time course of diurnal variations of both peptides, it is suggested that hypothalamic somatostatin may be more prominently involved in the early nocturnal drinking response than cortistatin.
In conclusion, our study showed that the endogenous sst ligands, SST-14, CST-14, and the stable agonist ODT8-SST injected intracerebroventricularly elicited a prompt drinking response in rats without access to food. The dipsogenic effect of the peptides results mainly from the activation of brain sst2 signaling and requires downstream the integrity of the brain angiotensinergic AT1 transmission. There was no reciprocal interaction between these systems, as the ANG II drinking response was not altered by the sst2 antagonist injected intracerebroventricularly. Importantly, the 55% blockade of spontaneous water intake in the first 1 h after the lights off under conditions of selective blockade of the brain sst2 is indicative that the brain somatostatin-sst2 pathway plays an important role in the control of early nocturnal stimulation of water intake occurring without food intake.
Perspectives and Significance
The present findings open new venues in the understanding of mechanisms regulating drinking behavior pointing toward the importance of brain somatostatin-sst2 signaling upstream of the brain ANG II-AT1 transmission. However, the neuroanatomical circuitries that underlie the link between sst2-mediated dipsogenic action and the recruitment of brain ANG II-AT1 pathway are still to be elucidated. In particular, neuronal activation by intracerebroventricularly administered ODT8-SST or sst2 agonist in the magnocellular PVN and SON (26), unlike other neuroanatomical substrates (SFO, MnPO) responsive to ANG II (34, 41, 46), suggest an action via these hypothalamic sites. On the basis of the well-documented inhibitory action of somatostatin on neurotransmission (40), whether the sst2 pathway may inhibit inhibitory mechanisms regulating thirst (60), most likely GABAergic neurons around the PVN (10) will need to be assessed.
As SST-14 is one of the most robustly conserved peptides throughout the evolution of vertebrates (61), it may be hypothesized that its biological action to stimulate fluid intake may have a bearing with primitive adaptive dipsogenic mechanisms to maintain body fluid homeostasis during vertebrate phylogeny.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants P30-DK-41303 (Animal Core, to Y. Taché and L. Wang), and R01-30110, and by the Veterans Administration Research Career Scientist Award (to Y. Taché).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.K. and Y.T. conception and design of research; H.K., S.Y., and L.W. performed experiments; H.K. and Y.T. analyzed data; H.K. and Y.T. interpreted results of experiments; H.K. prepared figures; H.K. and Y.T. drafted manuscript; H.K., A.S., J.E.R., and Y.T. edited and revised manuscript; H.K., S.Y., L.W., A.S., J.E.R., and Y.T. approved final version of manuscript.
ACKNOWLEDGMENT
The authors are grateful to Honghui Liang for her excellent technical support. J. Rivier is the Dr. Frederik Paulsen Chair in Neuroscience Professor.
REFERENCES
- 1.Barnes AJ, Long RG, Adrian TE, Vale W, Brown MR, Rivier JE, Hanley J, Ghatei MA, Sarson DL, Bloom SR. Effect of a long-acting octapeptide analogue of somatostatin on growth hormone and pancreatic and gastrointestinal hormones in man. Clin Sci (Lond) 61: 653–656, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, Petcher TJ, Pless J. SMS 201–995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci 31: 1133–1140, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Bealer SL, Johnson AK. Preoptic-hypothalamic periventricular lesions after food-associated drinking and circadian rhythms. J Comp Physiol Psychol 94: 547–555, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Beranek L, Hajdu I, Gardi J, Taishi P, Obal F, Jr, Krueger JM. Central administration of the somatostatin analog octreotide induces captopril-insensitive sleep responses. Am J Physiol Regul Integr Comp Physiol 277: R1297–R1304, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bourgin P, Fabre V, Huitron-Resendiz S, Henriksen SJ, Prospero-Garcia O, Criado JR, de Lecea L. Cortistatin promotes and negatively correlates with slow-wave sleep. Eur J Neurosci 26: 729–738, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Broglio F, Papotti M, Muccioli G, Ghigo E. Brain-gut communication: cortistatin, somatostatin and ghrelin. Trends Endocrinol Metab 18: 246–251, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Brown M, Taché Y. Hypothalamic peptides: central nervous system control of visceral functions. Fed Proc 40: 2565–2569, 1981 [PubMed] [Google Scholar]
- 8.Buggy J, Johnson AK. Angiotensin-induced thirst: effects of third ventricle obstruction and periventricular ablation. Brain Res 149: 117–128, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Cescato R, Erchegyi J, Waser B, Piccand V, Maecke HR, Rivier JE, Reubi JC. Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J Med Chem 51: 4030–4037, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chigr F, M'Hamed SB, Najimi M. Allosteric modulation of the GABA(A) receptor in rat hypothalamus by somatostatin is altered by stress. Clin Exp Pharmacol Physiol 28: 329–330, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Criado JR, Li H, Jiang X, Spina M, Huitron-Resendiz S, Liapakis G, Calbet M, Siehler S, Henriksen SJ, Koob G, Hoyer D, Sutcliffe JG, Goodman M, de Lecea L. Structural and compositional determinants of cortistatin activity. J Neurosci Res 56: 611–619, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Csaba Z, Simon A, Helboe L, Epelbaum J, Dournaud P. Targeting sst2A receptor-expressing cells in the rat hypothalamus through in vivo agonist stimulation: neuroanatomical evidence for a major role of this subtype in mediating somatostatin functions. Endocrinology 144: 1564–1573, 2003 [DOI] [PubMed] [Google Scholar]
- 13.de Lecea L, Criado JR, Prospero-Garcia O, Gautvik KM, Schweitzer P, Danielson PE, Dunlop CL, Siggins GR, Henriksen SJ, Sutcliffe JG. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature 381: 242–245, 1996 [DOI] [PubMed] [Google Scholar]
- 14.de Lecea L. Cortistatin—functions in the central nervous system. Mol Cell Endocrinol 286: 88–95, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Deghenghi R, Papotti M, Ghigo E, Muccioli G. Cortistatin, but not somatostatin, binds to growth hormone secretagogue (GHS) receptors of human pituitary gland. J Endocrinol Invest 24: RC1–RC3, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody. J Neurosci 16: 4468–4478, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erchegyi J, Waser B, Schaer JC, Cescato R, Brazeau JF, Rivier J, Reubi JC. Novel sst(4)-selective somatostatin (SRIF) agonists. 3. Analogues amenable to radiolabeling. J Med Chem 46: 5597–5605, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Erchegyi J, Grace CR, Samant M, Cescato R, Piccand V, Riek R, Reubi JC, Rivier JE. Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity. J Med Chem 51: 2668–2675, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erchegyi J, Cescato R, Grace CR, Waser B, Piccand V, Hoyer D, Riek R, Rivier JE, Reubi JC. Novel, potent, and radio-iodinatable somatostatin receptor 1 (sst1) selective analogues. J Med Chem 52: 2733–2746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feifel D, Vaccarino FJ. Central somatostatin: a re-examination of its effects on feeding. Brain Res 535: 189–194, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Finley JC, Maderdrut JL, Roger LJ, Petrusz P. The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 6: 2173–2192, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Fitzsimons TJ, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol 67: 273–283, 1969 [DOI] [PubMed] [Google Scholar]
- 24.Gardi J, Obal F, Jr, Fang J, Zhang J, Krueger JM. Diurnal variations and sleep deprivation-induced changes in rat hypothalamic GHRH and somatostatin contents. Am J Physiol Regul Integr Comp Physiol 277: R1339–R1344, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Gardi J, Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. The somatostatin analog, octreotide, causes accumulation of growth hormone-releasing hormone and depletion of angiotensin in the rat hypothalamus. Neurosci Lett 315: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Goebel M, Stengel A, Wang L, Coskun T, Alsina-Fernandez J, Rivier J, Taché Y. Pattern of Fos expression in the brain induced by selective activation of somatostatin receptor 2 in rats. Brain Res 1351: 150–164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grace CR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst2-selective somatostatin agonists. Three-dimensional consensus structure by NMR. J Med Chem 49: 4487–4496, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grace CR, Erchegyi J, Samant M, Cescato R, Piccand V, Riek R, Reubi JC, Rivier JE. Ring size in octreotide amide modulates differently agonist versus antagonist binding affinity and selectivity. J Med Chem 51: 2676–2681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillemin R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J Endocrinol 184: 11–28, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hajdu I, Obal F, Jr, Gardi J, Laczi F, Krueger JM. Octreotide-induced drinking, vasopressin, and pressure responses: role of central angiotensin and ACh. Am J Physiol Regul Integr Comp Physiol 279: R271–R277, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Different brain structures mediate drinking and sleep suppression elicited by the somatostatin analog, octreotide, in rats. Brain Res 994: 115–123, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, Takei Y, Ueta Y. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology 148: 1638–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto H, Otsubo H, Fujihara H, Suzuki H, Ohbuchi T, Yokoyama T, Takei Y, Ueta Y. Centrally administered ghrelin potently inhibits water intake induced by angiotensin II and hypovolemia in rats. J Physiol Sci 60: 19–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbert J, Forsling ML, Howes SR, Stacey PM, Shiers HM. Regional expression of c-fos antigen in the basal forebrain following intraventricular infusions of angiotensin and its modulation by drinking either water or saline. Neuroscience 51: 867–882, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa M, Mizobuchi M, Takahashi H, Bando H, Saito S. Somatostatin release as measured by in vivo microdialysis: circadian variation and effect of prolonged food deprivation. Brain Res 749: 226–231, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Johnson RF, Johnson AK. Light/dark cycle modulates food to water intake ratios in rats. Physiol Behav 48: 707–711, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Kissileff HR. Food-associated drinking in the rat. J Comp Physiol Psychol 67: 284–300, 1969 [DOI] [PubMed] [Google Scholar]
- 38.Krantic S, Martel JC, Quirion R. Brain somatostatin receptors in spontaneously hypertensive rats: an autoradiographic study. Peptides 12: 81–87, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Krisch B. Somatostatin-binding sites on structures of circumventricular organs. Prog Brain Res 91: 247–250, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Kumar U, Grant M. Somatostatin and somatostatin receptors. Results Probl Cell Differ 50: 137–184, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Lebrun CJ, Blume A, Herdegen T, Seifert K, Bravo R, Unger T. Angiotensin II induces a complex activation of transcription factors in the rat brain: expression of Fos, Jun, and Krox proteins. Neuroscience 65: 93–99, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Lind RW, Thunhorst RL, Johnson AK. The subfornical organ and the integration of multiple factors in thirst. Physiol Behav 32: 69–74, 1984 [DOI] [PubMed] [Google Scholar]
- 43.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Mietlicki EG, Nowak EL, Daniels D. The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav 96: 37–43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230: 1–23, 1981 [DOI] [PubMed] [Google Scholar]
- 46.Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun CJ, Unger T. Effect of repetitive icv injections of ANG II on c-Fos and AT1-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol 280: R1095–R1104, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Naik P, Murumkar P, Giridhar R, Yadav MR. Angiotensin II receptor type 1 (AT1) selective nonpeptidic antagonists—a perspective. Bioorg Med Chem 18: 8418–8456, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Oatley K. Dissociation of the circadian drinking pattern from eating. Nature 229: 494–496, 1971 [DOI] [PubMed] [Google Scholar]
- 49.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 2007 [Google Scholar]
- 51.Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem 278: 44400–44404, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Shinohara K, Isobe Y, Takeuchi J, Inouye ST. Circadian rhythms of somatostatin-immunoreactivity in the suprachiasmatic nucleus of the rat. Neurosci Lett 129: 59–62, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Siehler S, Nunn C, Hannon J, Feuerbach D, Hoyer D. Pharmacological profile of somatostatin and cortistatin receptors. Mol Cell Endocrinol 286: 26–34, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Spier AD, de Lecea L. Cortistatin: a member of the somatostatin neuropeptide family with distinct physiological functions. Brain Res Brain Res Rev 33: 228–241, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Selective central activation of somatostatin receptor 2 increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol 61: 399–407, 2010 [PMC free article] [PubMed] [Google Scholar]
- 56.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav 101: 614–622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology 151: 4224–4235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stengel A, Goebel-Stengel M, Wang L, Luckey A, Hu E, Rivier J, Taché Y. Central administration of pan-somatostatin agonist ODT8-SST prevents abdominal surgery-induced inhibition of circulating ghrelin, food intake and gastric emptying in rats. Neurogastroenterol Motil 23: e294–e308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stengel A, Rivier J, Taché Y. Modulation of the adaptive response to stress by brain activation of selective somatostatin receptor subtypes. Peptides 42: 70–77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka J, Fujisawa S, Nomura M. GABAergic modulation of the ANG II-induced drinking response in the rat medial preoptic nucleus. Pharmacol Biochem Behav 76: 43–51, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Tostivint H, Ocampo Daza D, Bergqvist CA, Quan FB, Bougerol M, Lihrmann I, Larhammar D. Molecular evolution of GPCRs: Somatostatin/urotensin II receptors. J Mol Endocrinol 52: T61–T86, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol 286: 75–87, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Martinez V, Larauche M, Taché Y. Proximal colon distension induces Fos expression in oxytocin-, vasopressin-, CRF- and catecholamines-containing neurons in rat brain. Brain Res 1247: 79–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zucker I. Light-dark rhythms in rat eating and drinking behavior. Physiol Behav 6: 115–126, 1971 [DOI] [PubMed] [Google Scholar]