Abstract
Caldicellulosiruptor saccharolyticus is one of the most thermophilic cellulolytic organisms known to date. This Gram-positive anaerobic bacterium ferments a broad spectrum of mono-, di- and polysaccharides to mainly acetate, CO2 and hydrogen. With hydrogen yields approaching the theoretical limit for dark fermentation of 4 mol hydrogen per mol hexose, this organism has proven itself to be an excellent candidate for biological hydrogen production. This review provides an overview of the research on C. saccharolyticus with respect to the hydrolytic capability, sugar metabolism, hydrogen formation, mechanisms involved in hydrogen inhibition, and the regulation of the redox and carbon metabolism. Analysis of currently available fermentation data reveal decreased hydrogen yields under non-ideal cultivation conditions, which are mainly associated with the accumulation of hydrogen in the liquid phase. Thermodynamic considerations concerning the reactions involved in hydrogen formation are discussed with respect to the dissolved hydrogen concentration. Novel cultivation data demonstrate the sensitivity of C. saccharolyticus to increased hydrogen levels regarding substrate load and nitrogen limitation. In addition, special attention is given to the rhamnose metabolism, which represents an unusual type of redox balancing. Finally, several approaches are suggested to improve biohydrogen production by C. saccharolyticus.
Keywords: Caldicellulosiruptor saccharolyticus, biohydrogen, dark fermentation, cellulolytic thermophile, thermodynamics, rhamnose metabolism, pyrophosphate, redox balance, hydrogen inhibition, regulation
1. Introduction
The use of renewable plant biomass for the production of biofuels, chemicals or other biocommodities can provide a realistic alternative for fossil fuel based processes [1,2]. The implementation of lignocellulosic biomass for biofuel production requires the degradation of recalcitrant substrates like cellulose, hemicellulose or lignin. Lignin is either removed or modified [3], while cellulose and hemicellulose are converted into more readily fermentable mono-, di- and oligo-saccharides. Although this can be achieved by different (thermo)chemical or enzymatic pre-treatments, a more desirable process combines both substrate hydrolysis and fermentation of complex plant biomass. Such a “consolidated bioprocess” (CBP) circumvents the negative environmental impact inherent to (thermo)chemical pre-treatment and might limit overall process costs [1,4].
Hydrogen gas (H2) is considered an alternative for the non-renewable fossil fuels and can be produced in a carbon neutral process. The controlled biological production of H2 would allow for capturing the CO2 released during the process, preventing it to dissipate into the environment. In addition, compared to carbon based (bio)fuel types, H2 has the advantage that (i) during its oxidation only H2O is released and (ii) that H2 fuel cells can be used, which are more energy efficient than the presently used combustion engines [5]. Biohydrogen can be produced from renewable feedstocks in an anaerobic fermentation process, which is often referred to as dark fermentation to distinguish it from photofermentative hydrogen production.
Both plant biomass degradation and biological H2 formation appear advantageous under thermophilic conditions. Moreover, thermophiles display an extensive glycoside hydrolase inventory aiding in the lignocellulosic biomass breakdown [6,7,8]. Based on thermodynamic considerations H2 formation is more feasible at elevated temperatures [9,10]. Correspondingly, H2 yields are in general higher for (hyper)thermophiles, reaching the theoretical limit of 4 mol H2 per mol of hexose, compared to the mesophilic hydrogen producers [9,11,12].
Since its isolation in the mid-eighties it has become clear that the thermophilic anaerobic bacterium Caldicellulosiruptor saccharolyticus [13] displays both the desirable polysaccharide degrading capabilities (including cellulose) and H2 producing characteristics, making it an outstanding source for thermostable glycoside hydrolases and an excellent candidate for biohydrogen production from renewable biomass.
This review will discuss the available scientific data on C. saccharolyticus regarding its lignocellulolytic capability, substrate specificity, catabolism and H2 producing capacity, which has made C. saccharolyticus to become a model organism for the study of fermentative hydrogen formation at elevated temperatures.
2. Isolation and Initial Characterization
The foreseen commercial value of thermostable cellulolytic enzymes in biotechnological applications triggered the investigation of new sources of these types of enzymes. In the search for novel thermophilic cellulolytic micro-organisms, several anaerobic bacteria have been isolated from natural enrichment sites from the Rotorua-Taupo thermal area in New Zealand [14]. One of the isolated strains, TP8.T 6331 [14] also referred to as TP8 [15] or “Caldocellum saccharolyticum” [16], revealed thermostable cellulase activity up to 85 °C [14,15] but also lignocellulolytic biomass decomposition capabilities [16]. Strain TP8.T 6331 was assigned to a new genus Caldicellulosiruptor as Caldicellulosiruptor saccharolyticus and was characterized as a Gram-positive, asporogenous, extremely thermophilic and strictly anaerobic bacterium capable of sustaining growth at a temperature range of 45–80 °C (Topt =70 °C) and pH range of 5.5–8.0 (pHopt = 7) [13]. Acid production could be detected for a broad substrate range including different pentoses and hexoses, di-saccharides and polysaccharides like cellulose and xylan [13,14,15,16]. In particular, the capacity to use cellulose at high temperatures was exceptional.
Ever since, several cellulolytic and weakly cellulolytic Caldicellulosiruptor species have been identified, all of which are isolated from terrestrial geothermal regions. [13,14,17,18,19,20,21,22,23,24,25,26,27,28]. The availability of the fully sequenced genomes of 8 of these Caldicellulosiruptor species allows the investigation of the possible differences in their cellulolytic traits and the analysis of other remarkable features of this genus [29,30,31,32,33].
3. Hydrolytic Capacity and Complex Biomass Decomposition
For the decomposition of recalcitrant plant polysaccharides C. saccharolyticus does not employ cellulosome-like structures, as described for some Clostridium species [34], but wields a variety of free-acting endo- and exo-glycoside hydrolases (GH) capable of hydrolyzing the glycosidic bonds of α- and β-glucans like starch, pullulan and cellulose, but also xylan and hetero-polysaccharides like hemicelluloses and pectin [8,13,33,35,36]. Actually, Caldicellulosiruptor species, together with Thermoanaerobacter species, are one of the most thermophilic crystalline cellulose-degrading organisms known to date that use free-acting primary cellulases [7]. C. saccharolyticus contains 59 open reading frames (ORFs) that include GH catalytic domains [29]. Some of these ORFs code for multifunctional, multi-domain proteins that contain glycoside hydrolase domains, belonging to different GH families, and multiple carbon binding modules [6,29,36]. The catalytic properties and structural organization of some of the glycoside hydrolases from C. saccharolyticus, have been extensively investigated.
A β-glucosidase (BglA) [37,38], β-xylosidase [39], β-1,4-xylanase [40] and a type I pullulanase [41] from C. saccharolyticus have been cloned into E. coli and characterized. The majority of the genes encoding xylan degradation associated enzymes appear to be clustered on the genome (xynB-xynF, Csac_2404-2411) [6,36]. Both XynA and XynE exhibit endoxylanase and xylosidase activity [36,42], while XynB only acts as a β-D-xylosidase [43]. XynC does not contain a GH domain and was shown to be an acetyl esterase [44], XynD showed to be active on xylan [36] and although the cloning and expression of intact multi-domain XynF could not be achieved, its N- and C-terminal parts revealed catalytic activity on arabinoxylan. Hence XynF was proposed to be involved in the degradation of the arabinoxylan component of hemicellulose [36]. A second locus covers several genes coding for multidomain proteins involved in glucan and mannan hydrolysis (celA-manB, Csac_1076-1080) [6,36]. CelA is coding for a multidomain cellulase [45], the bifunctional cellulase CelB exhibited both endo-β-1,4-glucanase and exo-β-1,4-glucanase activity [36,46,47] and CelC was characterized as an endo-1,4-β-D-glucanase [48]. ManA was characterized as a β-mannanase [49], but ManB codes for an inactive mannase, which after correcting for a frame shift in the nucleotide sequence, exhibited β-mannanase activity [48]. Several ORFs of the described celA-manB and xynB-xynF loci were differentially transcribed on pretreated poplar and switch grass compared to the monosaccharides glucose and xylose, showing their involvement in the decomposition of complex carbohydrates [36].
While some of the GH proteins act intracellularly, others are excreted, allowing the decomposition of non-soluble substrates to smaller oligo- or mono-saccharides. Early findings by Reynolds et al. already indicated that a significant percentage of the cellulolytic activity was found to be associated with insoluble substrate [15]. These interactions, between GH and substrate, are facilitated by carbon binding modules (CBM). CBMs allow the positioning of the GH catalytic domains in the vicinity of the substrate, thus increasing the rate of catalysis. Interestingly, most multi-domain GHs identified in C. saccharolyticus, containing one or more CBMs, possess a signal peptide, which mark them for excretion [36]. A relative higher amount of GH related proteins could be observed in the substrate bound protein fraction, from Caldicellulosiruptor species grown on Avicel, with respect to the whole cell proteome [29]. Additionally, these proteome studies allowed the identification of specific GHs which interact with crystalline cellulose. CelA, a multi-domain GH consisting of two GH domains (GH9 and GH48) and three CBM3 modules, was found to be the most abundant substrate bound protein for strong cellulolytic Caldicellulosiruptor species [29]. The sequenced genomes of Caldicellulosiruptor species reveal differences in glycoside hydrolytic capacity, which reflects their difference in biomass degrading capabilities [29,35]. The secretome of C. saccharolyticus grown on glucose contains several carbohydrate-degrading enzymes including CelA, ManA, CelB and CelC (protein sequence ID A4XIF5/6/7/8 respectively), which indicates that these GH are constitutively expressed even under non-cellulose degrading conditions [50].
In addition to the interactions between substrate and glycoside hydrolases, interactions between whole cells and a substrate have also been observed for C. saccharolyticus. These interactionsappeared to be substrate specific. For instance, a higher degree of cell-to-substrate attachment was observed for cells grown on switch grass, compared to poplar [36]. Several S-layer homology (SLH) domain containing proteins, which have been identified in Caldicellulosiruptor species, are proposed to have a role in such cell substrate interactions. These SLH domain proteins contain both glycoside hydrolases domains and non-catalytic carbohydrate binding domains, which are utilized in lignocellulose degradation by both recruiting and degrading complex biomass via cell substrate interactions [51]. In addition, cell immobilization on a support matrix, like pine wood shavings, supports cell survival and improves the H2 evolving capacity of C. saccharolyticus [52].
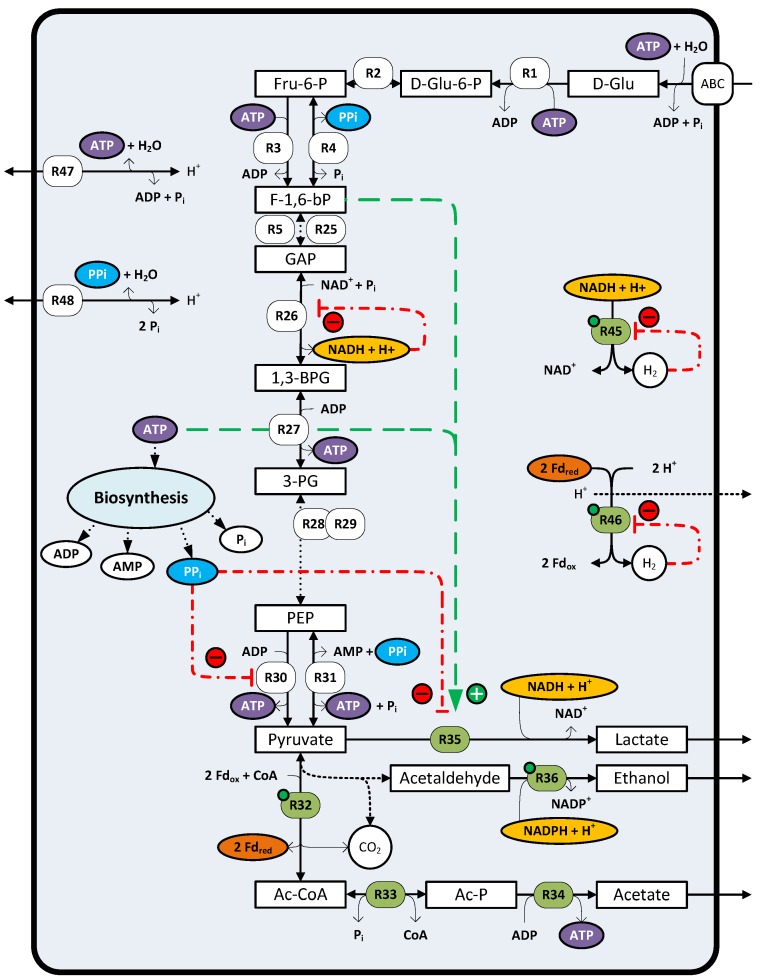
4. Sugar Catabolism and Pathway Regulation
4.1. Sugar Uptake and Fermentation
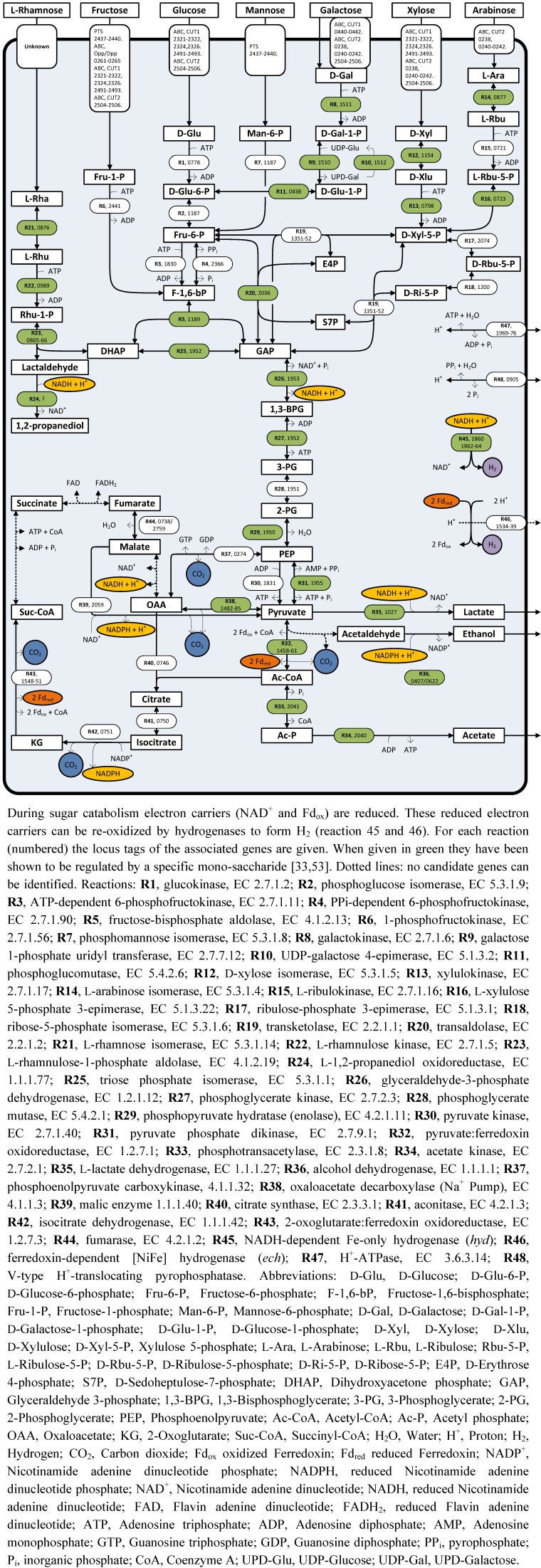
Soluble sugar substrates can enter C. saccharolyticus cells either as mono-, di- or oligo-saccharides via several ABC-transporters, which facilitate substrate transport across the membrane at the expense of ATP. In addition, C. saccharolyticus contains one fructose specific phosphotransferase system (PTS). During PTS-mediated transport the substrate is both transported and phosphorylated at the expense of phosphoenolpyruvate (PEP). The substrate specificity of the 24 sugar ABC transport systems, identified in C. saccharolyticus, has been assigned based on bioinformatic analysis and functional genomics [53]. Most of the identified ABC transporters have a broad predicted substrate specificity and for some transporters the annotated substrate specificity was confirmed by transcriptional data obtained from cells grown on different mono-saccharides [53]. Some substrates can be transported by multiple transporter systems (Figure 1).
Figure 1.
Overview of the central carbon metabolism of Caldicellulosiruptor saccharolyticus.
The growth of C. saccharolyticus on sugar mixtures revealed the co-utilization of hexoses and pentoses, without any signs of carbon catabolite repression (CCR) [33,53]. The absence of CCR is in principle a very advantageous characteristic of C. saccharolyticus, as it enables the simultaneous fermentation of hexoses and pentoses [33]. Substrate co-utilization has also been confirmed for biomass derived hydrolysates [54,55]. Despite the absence of CCR, a somewhat higher preference for the pentose sugars (xylose and arabinose) with respect to the hexose sugars (glucose, mannose and galactose) was demonstrated, but the highest preference was observed for the PTS-transported hexose fructose [53].
Once inside the cell, the sugar substrates are converted into a glycolytic intermediate. NMR analysis of the fermentation end-products of C. saccharolyticus grown on 13C-labeled glucose showed that the Embden-Meyerhof pathway is the main route for glycolysis [56]. All genes encoding components of the Embden-Meyerhof and non-oxidative pentose phosphate pathway have been identified in the genome. There is no evidence for the presence of the oxidative branch of the pentose phosphate pathway or the Entner-Doudoroff pathway [33] (Figure 1).
Each sugar substrate, with the exception of rhamnose is completely catabolized to glyceraldehyde 3-phosphate (GAP) (rhamnose catabolism will be discussed separately in more detail below). The subsequent conversion of GAP to pyruvate, via the C-3 part of glycolysis, results in the formation of the reduced electron carrier NADH. Pyruvate can be further oxidized to acetyl-CoA by pyruvate: ferredoxin oxidoreductase (POR), which is coupled to the generation of reduced ferredoxin (Fdred). Finally, acetyl-CoA can be converted to the fermentation end-product acetate (Figure 1).
Both types of reduced electron carriers (NADH and Fdred) can be used by hydrogenases for proton reduction, thus forming H2. The genome of C. saccharolyticus contains a gene cluster coding for an NADH-dependent cytosolic hetero-tetrameric Fe-only hydrogenase (hyd) and a cluster encoding a membrane bound multimeric [NiFe] hydrogenase (ech), which presumably couples the oxidation of Fdred to H2 production [33]. Under optimal cultivation conditions, when all reductants are used for H2 formation, the complete oxidation of glucose yields 4 mol of H2 per mol of glucose consumed (Equation 1).
| glucose + 4·H2O → 2 acetate− + 2·HCO3− + 4·H+ + 4·H2 | (1) |
| glucose + 2·H2O → 2 ethanol + 2·HCO3- + 2·H+ | (2) |
| glucose → 2 lactate- + 2·H+ | (3) |
However, suboptimal growth conditions lead to a mixed acid fermentation with ethanol (Equation 2) and lactate (Equation 3) as end products in addition to acetate and H2. Lactate is produced from pyruvate, using NADH as electron donor and catalyzed by lactate dehydrogenase (LDH). The corresponding ldh gene could be easily identified in the genome [33,57]. However, the identity of the enzymes and genes involved in ethanol formation, are less clear. Two alcohol dehydrogenase (ADH) genes have been identified in the genome which, based on transcriptional data, can both be involved in ethanol formation from acetaldehyde. The way acetaldehyde is produced, is however, not known. Acetaldehyde can be produced from pyruvate by a pyruvate decarboxylase, as described for yeast, or from acetyl-CoA by an acetaldehyde dehydrogenase, as is commonly seen in fermentative bacteria. In several thermophilic ethanol-producing bacteria, acetaldehyde is produced by a bifunctional acetaldehyde/ethanol dehydrogenase [58,59]. However, no candidate gene could be identified for any of these alternatives [33,60]. A third option might be that acetaldehyde is formed from pyruvate in a CoA-dependent side reaction of the pyruvate:ferredoxin oxidoreductase as described for Pyrococcus furiosus [61]. There is no experimental evidence for such a side reaction in C. saccharolyticus, but the absence of a dedicated enzymatic acetaldehyde-forming step might explain the low ethanologenic capacity of C. saccharolyticus. The difference in the observed lower ethanol to acetate ratio for C. saccharolyticus with respect to Clostridium thermocellum [16] can be explained by the fact that the C. saccharolyticus genome does not have a similar pathway for ethanol formation as identified in C. thermocellum [58]. Similar to other high yield ethanol-producing thermophiles, like Thermoanaerobacter ethanolicus [62] or Thermoanaerobacterium saccharolyticum [63], Clostridium thermocellum has a bifunctional acetaldehyde-CoA/alcohol dehydrogenase, which catalyzes ethanol formation from acetyl-coA [58,59]. Such a pathway is absent in low level ethanol-producing thermophiles like C. saccharolyticus [33], Thermoanaerobacter tengcongensis [64] and Pyrococcus furiosus [65]. Although C. saccharolyticus is able to produce some ethanol, the flux through the ethanol-forming pathway is apparently limited resulting in the lower ethanol to acetate ratio.
NADPH is assumed to be the preferred substrate for the ethanol-forming ADH reaction [33,57]. A potential source of NAPDH could be the isocitrate dehydrogenase in the oxidative branch of the incomplete TCA cycle (Figure 1). Alternatively, NADPH can be produced from NADH, but so far no candidate genes coding for such transhydrogenase have been identified in C. saccharolyticus [33,60]. With respect to H2 production, it is important to realize that only the production of acetate is coupled to H2 formation since no net reducing power remains for H2 formation when ethanol or lactate is produced.
4.2. Rhamnose Fermentation
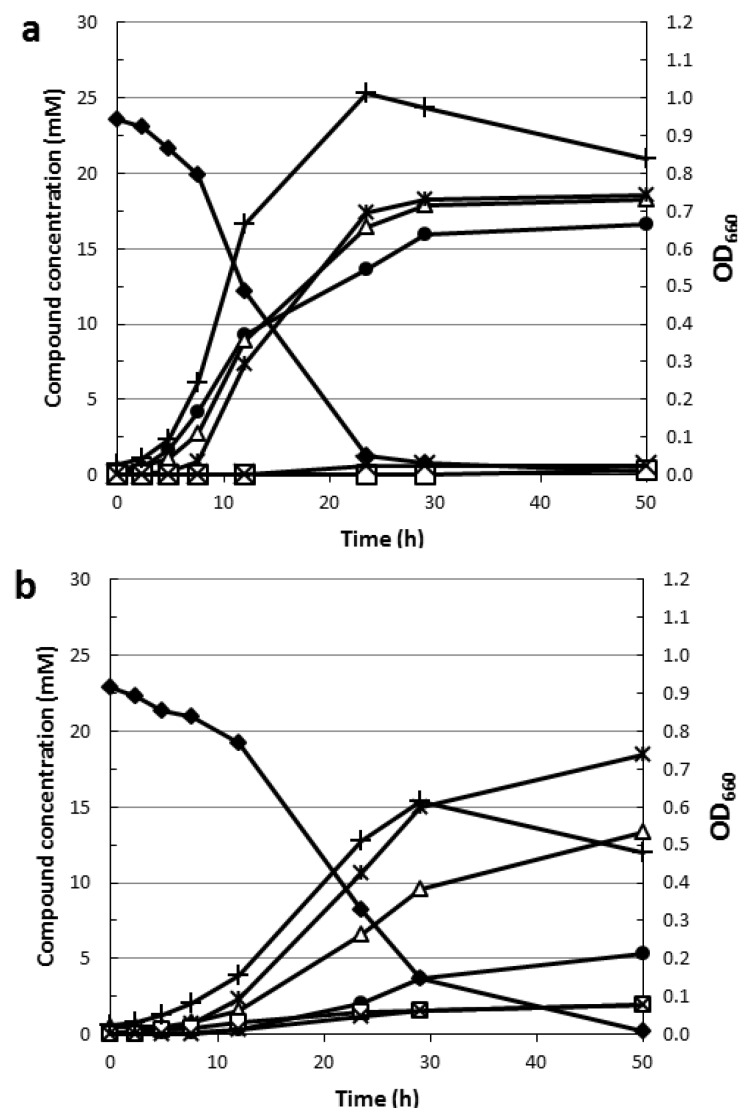
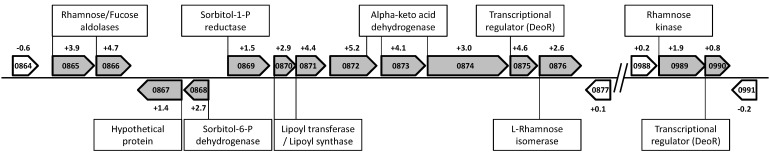
Compared to glucose, fermentative growth on the deoxy sugar rhamnose is associated with a different carbon and electron metabolism. The proposed pathway for rhamnose degradation (Figure 1) implies that during rhamnose catabolism half of the generated reduced electron-carriers is used for the reduction of lactaldehyde to 1,2-propanediol, while the other half can be recycled through H2 formation [33]. Indeed, fermentation of rhamnose by C. saccharolyticus results in the production of 1,2-propanediol, acetate, H2 and CO2 in a 1:1:1:1 ratio ([66], Figure 2a). This ratio suggests that all NADH is used for 1,2-propanediol formation and that all Fdred is used for H2 formation. However, when C. saccharolyticus is grown on rhamnose under a headspace of carbon monoxide (CO), which is an established competitive inhibitor of both NiFe- [67] and Fe-only [68] hydrogenases, H2 evolution is significantly inhibited ([66], Figure 2b). The CO cultivation condition does not affect 1,2-propanediol formation, but less H2 is produced and the remaining reduced electron-carriers are now recycled by the formation of lactate and ethanol, consequently leading to a decrease in the acetate level. These findings suggest that, based on the substrate specificity of the lactate dehydrogenase (NADH, [57]) and the ethanol dehydrogenase(s) (NADPH, [57]), electron exchange between the Fdred and NAD(P)+ is required. However, no genes have been identified in C. saccharolyticus coding for an enzyme capable of catalyzing such a reaction [33,60]. Transcript levels of the genes from the gene cluster containing both the L-rhamnose isomerase and L-rhamnulose-1-phosphate aldolase are highly upregulated during growth on rhamnose (Figure 3) [33]. Although the function of the other genes in the cluster with respect to rhamnose catabolism remains unclear, the absence of such gene cluster and the rhamnose kinase gene from the genome of other Caldicellulosiruptor species, strongly correlates with their inability of rhamnose degradation (Table 1). This difference in rhamnose degrading capability between Caldicellulosiruptor species reflects the open nature of the Caldicellulosiruptor pan genome [29].
Figure 2.
Fermentation profile of C. saccharolyticus grown on rhamnose batch cultivation: (a) without CO in the headspace (b) with a 100% CO headspace. Rhamnose (diamonds), acetate (open triangles), 1,2 propanediol (asterisks), lactate (open squares), ethanol (crosses), H2 (circles) and OD660 (plus sign).
Figure 3.
Schematic representation of two rhamnose associated gene clusters from C. saccharolyticus (Csac_0865-Csac_0876 and Csac_0989-Csac_0990). For each member of the cluster (grey arrows) the proposed function (text box) and locus tag number (four digit number) is given. Presented log2 values represent the ratio between transcription levels of the specific gene during growth on rhamnose with respect to growth on glucose; (+) upregulated, (−) downregulated on rhamnose versus glucose [33].
Table 1.
Overview of Caldicellulosiruptor species which are able to grow on rhamnose. There is a correlation between the ability to grow on rhamnose and the presence of gene clusters orthologous to the rhamnose associated gene clusters identified in C. saccharolyticus (Figure 3). nt, not tested for growth on rhamnose. (+), able to grow of rhamnose/gene cluster is present in genome. (−), no growth observed on rhamnose/gene cluster is not present in genome. ns, not sequenced.
| Strain | Growth on Rhamnose | Gene cluster Csac_0865-76 | Gene cluster Csac_0989-90 | Reference |
|---|---|---|---|---|
| C. saccharolyticus | + | + | + | [13] |
| C. bescii | + | + | + | [28] |
| C. owensensis | + | + | + | [21] |
| C. obsidiansis | nt | + | + | [20] |
| C. kronotskyensis | nt | + | + | [22] |
| C. hydrothermalis | nt | + | + | [22] |
| C. kristjanssonii | − | − | − | [17] |
| C. lactoaceticus | − | − | − | [23] |
| C. acetigenus | nt | ns | ns | [26] |
4.3. Involvement of Pyrophosphate in the Energy Metabolism
From a bioenergetics point of view, glucose fermentation is optimal when acetate is the only end product, because an additional ATP is generated during the final acetate-forming step (Figure 1). When it is assumed that the ABC-transporter mediated substrate uptake requires 1 ATP, overall ATP yields become 1.5 ATP per acetate versus only 0.5 ATP per lactate or ethanol. However, ATP yields might even be higher when the involvement of pyrophosphate (PPi) as an energy carrier is considered.
PPi is a by-product of biosynthesis reactions like DNA and RNA synthesis or is generated when the amino acids are coupled to their tRNAs during protein synthesis [69]. Since these reactions are close to equilibrium accumulation of PPi is believed to have an inhibitory effect on growth, and only the effective removal of PPi drives these biosynthetic reactions forward [70]. When PPi is hydrolysed to Piby a cytosolic inorganic pyrophosphatase (PPase), the free energy just dissipates as heat. C. saccharolyticus does not contain a cytosolic PPase but possesses a membrane bound H+-translocating PPase [71], which allows the free energy released upon PPi hydrolysis to be preserved as a proton motive force. The high-energy phosphate bond of PPi can also be used for the phosphorylation of fructose 6-phosphate, catalyzed by a PPi-dependent phosphofructokinase (Figure 1).
Furthermore, PPi is consumed during the catabolic conversion of phosphoenolpyruvate to pyruvate, catalyzed by pyruvate phosphate dikinase (PPDK). Such a catabolic role for PPDK was proposed based on the increase in transcript level of the ppdk gene under increased glycolytic fluxes [33,71]. Altogether, the use of PPi as an energy donor could be a way for the organism to deal with the relative low ATP yields which are usually associated with fermentation [72].
4.4. Mechanism Involved in Mixed Acid Fermentation
H2 has been reported as a growth inhibitor for C. saccharolyticus [16,73] and a critical dissolved H2 concentration, which leads to the complete inhibition of growth, of 2.2 mmol/L has been determined for controlled batch cultivations [74]. In addition, an elevated PH2 has been shown to cause a switch in the fermentation profile, leading to increased formation of ethanol and lactate, in both controlled batch and chemostat cultivations [12,60,74,75]. Willquist et al. reported that in controlled batch fermentations the initiation of lactate formation coincided with an increment in both the internal NADH/NAD+ ratio and the PH2 of the system [57,75].
An increase of the overall carbon flux through glycolysis results in a higher NADH production rate. To maintain a constant NADH/NAD+ ratio a subsequently higher H2 formation rate is required. However, when the H2 formation rate (volumetric H2 production rate) exceeds the H2 liquid to gas mass transfer rate, H2 will accumulate in the liquid phase. In some cases this can even lead to super saturation of the liquid, meaning that the dissolved H2 concentration exceeds the maximal theoretical H2 solubility [74,76]. Such high dissolved H2 levels inhibit H2 formation and presumably cause an increase in the NADH/NAD+ ratio. An increased NADH/NAD+ ratio has been shown to have an inhibitory effect on GAPDH activity thus limiting the glycolytic flux [75] and consequently leads to a decrease in substrate consumption and growth rate. A switch to lactate formation alleviates the inhibitory effect of an increased NADH/NAD+ ratio by causing a decrease of the NADH/NAD+ ratio through the reduction of pyruvate.
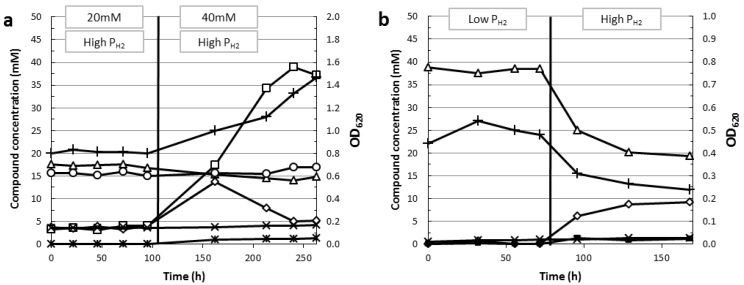
A clear switch to lactate formation caused by an increase in glycolytic flux can be observed for C. saccharolyticus grown in a chemostat under high PH2 ([77], Figure 4a). When the glucose load is increased from 20 mM to 40 mM, end-product formation is switched from mainly acetate to mainly lactate, respectively. After the switch to 40 mM glucose some adaptation time is required before a new steady state is achieved. During this adaptation period substantially higher amounts of glucose were detected in the culture effluent, probably indicating an inhibition of the glycolytic flux at the level of GAPDH. When a new steady state was achieved, residual glucose was only slightly higher with respect to the 20 mM condition. As a consequence of the increase in glycolytic flux, the lactate concentration dramatically increased while the acetate concentration hardly changed, indicating that during both substrate loads a similar volumetric H2 productivity was maintained. These data indicate that under these conditions the organism is not capable of dealing with the increased glycolytic flux by increasing its H2 productivity, but requires a switch to lactate formation. Although oxaloacetate formation was discernible under a 20 mM glucose load, significantly higher and therefore quantifiable levels of oxaloacetate were detected under the 40 mM glucose load condition. This observation together with the increased flux towards lactate suggests that in the newly achieved steady state, a bottleneck exists at the level of pyruvate.
Figure 4.
Fermentation profile of C. saccharolyticus (a) grown in a chemostat under high H2 partial pressure at two different glucose concentrations (see Section 4.4), (b) grown in a chemostat without NH4+ in the medium under low and high H2 partial pressure (see Section 6.4). The switch from 20 mM to 40 mM glucose containing-medium and the switch from low to high H2 partial pressure cultivation condition is indicated by a vertical line. Acetate (open triangles), lactate (open squares), ethanol (crosses), CO2 (open circles), oxaloacetate (asterisks), glucose out (open diamonds) and CDW (plus sign). Chemostat cultivation parameters (3 L reactor, 1 L working volume, pH = 7.0 (NaOH), temp 70 °C, D = 0.1 h−1, low PH2 [sparging (4 L/h) with N2 gas and stirring speed = 250 rpm]), high PH2 [headspace flushing (4 L/h) with H2 gas and stirring speed = 50 rpm]).
Ethanol formation can also serve as reductant sink in C. saccharolyticus. For some chemostat cultivation conditions a decrease in H2 yield is only associated with ethanol formation and not with lactate formation [56,60,75]. These conditions concern low substrate loads, and the observed increase in ethanol formation was triggered by an increase of the dilution rate [56,75] or a change in mode of gas flushing [60], both potentially generating a moderate increase in the H2 level of the system.
4.5. Regulation of Reductant Disposal Pathways
Hydrogen, ethanol and lactate formation are the main routes for reductant disposal in C. saccharolyticus. The gene expression of the hydrogenases and both alcohol dehydrogenases involved in ethanol formation are proposed to be under the control of the NADH/NAD+ sensitive transcriptional regulator Rex [60] (Figure 5). Cultivation of C. saccharolyticus under high PH2 conditions was shown to lead to an upregulation of both the hydrogenases and alcohol dehydrogenases [60]. In addition, the ldh transcript level is also upregulated under high PH2 conditions, but in silico analysis of the ldh promoter region did not reveal a likely Rex operator binding sequence [60]. Thus, the exact regulatory mechanism triggering ldh transcription remains therefore unclear. Nonetheless, lactate dehydrogenase activity has been shown to be regulated at the enzyme level, with fructose 1,6-bisphosphate and ATP acting as allosteric activators and both NAD+ and PPi as competitive inhibitors [57]. Under high PH2 cultivation conditions, enzyme activity assays revealed increased LDH activity with respect to low PH2 conditions [57,75]. A hampered glycolytic flux at the level of GAPDH, potentially triggered by NADH build-up, might lead to the accumulation of fructose 1,6-bisphosphate. In turn, the accumulated level of fructose 1,6-bisphosphate could stimulate lactate formation (Figure 5), explaining the switch to lactate under high PH2 at the enzyme level.
Figure 5.
Overview of the regulatory mechanisms involved in the central metabolism of Caldicellulosiruptor saccharolyticus. The abbreviations of the compounds and reactions (circled numbers) are given in the legend of Figure 1. For the enzyme reactions given in green (circled numbers, green) the encoding genes are upregulated under increased PH2 [60]. Those which are under the control of the REX transcriptional regulator are marked with a green dot. H2 inhibits its own formation (R45, R46) and accumulation of NADH inhibits glyceraldehyde-3-phosphate dehydrogenase (R26). PPi, a by-product of biosynthesis, acts as an inhibitor of both pyruvate kinase (R30) and lactate dehydrogenase (R35) activity. Both ATP and F-1,6-bP are activators of lactate dehydrogenase (R35) activity.
Interestingly, LDH enzyme activity can also be measured under non-lactate producing conditions, which suggest that additional factors control lactate formation [57,75]. Low levels of lactate formation can be observed at the end of growth during the transition to stationary phase for cultures grown in controlled batch systems [55,57,75,78,79]. This initiation of the lactate formation coincided with a relative increase in ATP levels and a relative decrease in PPi levels [57,71]. These observed changes in ATP and PPi levels could release the inhibitory effect of PPi and stimulate LDH activity [57] (Figure 5). For this latter mode of LDH activity regulation, the lactate formation is proposed to be coupled to the energy metabolism. Accordingly, lactate formation is prevented during exponential growth, which is associated with a high anabolic activity and high PPi levels. Whereas, during the transition to stationary phase, which is associated with low anabolic activity and relative low PPi levels, lactate formation inhibition is alleviated [12,57].
5. Thermodynamic Considerations of Glucose Conversion and H2 Formation
For C. saccharolyticus an elevated hydrogen concentration has been shown to affect fermentation performance, leading to a mixed acid fermentation [60,73,74,75]. These observed changes in the fermentation profile as a function of the H2 concentration can be conceptually explained by considering the thermodynamics of the reactions leading to H2 formation [9,10]. The Gibbs energy (ΔG') for a specific reaction can be calculated from the standard Gibbs energy (ΔG0') and the reactant concentrations by the following relation:
| ΔG' = ΔG0' + RT ln([C]c[D]d/[A]a[B]b) | (4) |
where ΔG0' is the standard Gibbs energy (J/mol, 1 mol concentration of all reactants, at a neutral pH and at a specific temperature), R is the gas constant (J/mol*K); T is the temperature (K), A and B are the substrate concentrations with respective stoichiometric reaction coefficients a, b; and C and D are the reaction products with respective stoichiometric reaction coefficients c, d.
When H2 formation occurs in the aqueous phase H2 supersaturation can occur. For instance, over-saturation of 12 to 34 times the equilibrium concentration has been reported for C. saccharolyticus cultivations [74]. This indicates that estimating the dissolved H2 concentration from the measured H2 partial pressure by using the equilibrium constant does not always give an accurate representation of the state of the system. So H2(aq), instead of the H2 partial pressure (PH2), should be used as a parameter when investigating the effect of H2 on the metabolism. Therefore, all Gibbs energy calculations discussed herein were performed using the ΔfG0 of the dissolved H2 concentration (H2(aq)).
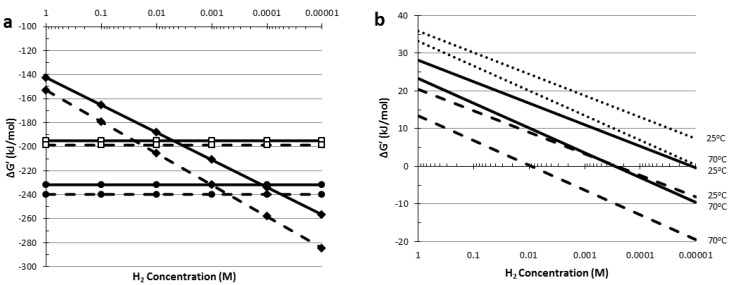
Under standard conditions (25 °C), acetate formation (Equation 1, ΔG0' = −142.6 kJ/reaction) is energetically less favorable then lactate (Equation 3, ΔG0' = −195.0 kJ/reaction) and ethanol (Equation 2, ΔG0' = −231.8 kJ/reaction) formation. The Gibbs energy (ΔG') for the complete conversion of glucose to acetate depends, however, on the H2 concentration (Equation 1), whereas the ΔG' for both ethanol and lactate formation from glucose is independent of the H2 concentration (Equations 2 and 3, respectively). This means that the complete oxidation of glucose to acetate becomes energetically more favorable when the dissolved H2 concentration is lowered. For example, acetate formation becomes energetically favorable compared to lactate or ethanol formation when the dissolved H2 concentration drops below 5.0 or 0.12 mM, respectively (Figure 6a, supplementary data S1).
Figure 6.
Effect of the H2 concentration on the Gibbs energy change of reactions involved in H2 formation: (a) ΔG' of the complete oxidation of 1 mol of glucose to acetate (closed diamonds), lactate (open squares) and ethanol (closed circles) at 25 °C (solid lines) and 70 °C (dashed lines); (b) ΔG' of H2 formation from NADH (dotted line), reduced ferredoxin (dashed line) and via the bifurcating system (50% NADH and 50% Fdred) (solid line) at 25 °C and 70 °C. Values were calculated from data presented in ([80,81,82,83,84]).
However, considering the Gibbs energies of the overall conversions can be misleading, as the thermodynamics of the involved partial reactions can be less favorable. Acetate formation is inevitably linked to H2 formation and the ΔG0' of the redox reaction coupling the reoxidation of the reduced electron carriers NADH or Fdred to proton reduction are endergonic under standard conditions (Figure 6b, supplementary data S2). Decreasing the dissolved H2 concentration will lower the ΔG' of these H2 forming reactions, but an extremely low H2 concentration is required before H2 formation from NADH becomes exergonic (0.5 µM) (Fdred, 0.2 mM) (Figure 6b). Therefore, at elevated H2 concentrations this partial reaction raises a thermodynamic barrier. NADH dependent pyruvate reduction to lactate, on the other hand, has a negative Gibbs energy (ΔG0' = −25.0 kJ/reaction) and is independent of the dissolved H2 concentration. This means that under certain conditions lactate formation is more feasible, despite the more negative ΔG' for acetate formation compared to lactate formation (Equations 1 and 3), simply because H2 formation from NADH is energetically unfavorable.
As explained, the thermodynamic barrier associated with H2 formation from NADH can be lowered by decreasing the dissolved H2 concentrations, moreover this barrier may also be tackled by an additional input of energy. The latter can be achieved by reverse electron transport catalyzed by an NADH: ferredoxin oxidoreductase, where the transfer of electrons from NADH to Fdred is coupled to a proton or ion gradient [85]. Produced Fdred can subsequently be used for H2 formation. Alternatively, the energetically more favourable oxidation of Fdred can be used to push the less favourable formation of H2 from NADH in a bifurcating system (Figure 6b). Such a bifurcating function has been identified for T. maritima and was, based on protein sequences, also attributed to the Fe-only hydrogenase (hyd) of C. saccharolyticus [86]. However, the gene-arrangement of the Fe-only hydrogenase (hyd) in C. saccharolyticus is identical to that of Thermoanaerobacter tengcongensis [33], for which a NADH-dependent hydrogenase activity has been demonstrated [64], thus arguing against a bifurcating systems in C. saccharolyticus.
In general, H2 formation is thermodynamically more favorable at elevated temperatures because (i) ΔG0' values of the involved reactions are lower at increased temperature, and (ii) the RT coefficient in Equation 4 is temperature dependent, thus enhancing the effect of a decreased H2 concentration (Figure 6a,b). Overall, thermophilic organisms have been shown to be able to produce H2 at higher yields compared to mesophilic organisms. For thermophiles yields approaching the theoretical limit of 4 H2 per hexose [83] have been reported, while for mesophiles H2 yields generally do not exceed 2 H2 per hexose [9,11,12]. These higher yields reflect the indicated thermodynamic advantage but also the lower diversity of fermentation end products observed for those thermophiles. For a specific organism the diversity of available electron acceptors, and formed end products, depends on the metabolic capabilities of the organism. Whether a specific pathway is operational depends on the regulation of that pathway at the transcription or translation level, but also depends on the kinetic properties and regulation of the enzyme activities of the specific enzymatic steps of that pathway.
6. Factors Limiting H2 Formation
With an eye to the potential use of complex biomass for H2 production a multitude of fermentability studies have been performed using C. saccharolyticus. An overview of the literature related to fermentability studies on either crop-based feedstock or industrial waste stream derived biomass is given in Table 2. Those investigations mainly focus on the fermentability of various complex substrates, associated H2 formation and the effect of pretreatment on substrate accessibility and growth, overall demonstrating the bacterium’s broad hydrolytic capacity.
Table 2.
Literature overview of fermentability studies on C. saccharolyticus using crop-based feedstock or industrial waste stream derived biomass. * Extraction methods and mechanical pre-treatments were excluded in this overview, biological pre-treatment indicated pre-treatment by pre-incubation with Bacillus amyloliquefaciens; ** Multiple, different pre-treatments used; B, batch cultivation; CB, controlled batch cultivation; $ Minimal and maximal reported H2 yields are given.
| Reference | Substrate | Pre-treatment * | Cultivation method | H2 yields $/Remarks |
|---|---|---|---|---|
| [87] | Wheat grains | Enzymatic | B | |
| Wheat straw | Acid/Enzymatic | B | ||
| [88] | Barley straw | Acid/Enzymatic ** | B | |
| [29] | Crystalline cellulose | - | B | Proteome data |
| Birchwood xylan | - | B | ||
| Switchgrass | Acid | B | ||
| Whatman no. 1 filterpaper | - | B | ||
| [89] | Wheat straw | Acid/Enzymatic | B | |
| Barley straw | Acid/Enzymatic | B | ||
| Corn stalk | Acid/Enzymatic | B | ||
| Corn cob | Acid/Enzymatic | B | ||
| [36] | Poplar | - | B | Microarray data |
| Switchgrass | Acid | B | Microarray data | |
| [90] | Beet Molasses | - | CB | 0.9–4.2 |
| [91] | Potato steam peels | Enzymatic | CB | 1.7–3.4 |
| Potato steam peels | - | CB | 1.1–3.5 | |
| [92] | Filter paper | - | B | |
| Wheat straw | Biological | B | ||
| Silphium perfoliatum leaves | Biological | B | ||
| Maize leaves | Biological | B | ||
| Sugar cane bagasse | Biological | B | ||
| Sweet sorghum whole plant | Biological | B | ||
| [93] | Sugar beet | - | CB | 3.0 |
| [94] | Sweet sorghum bagasse | Alkaline/Enzymatic ** | B/CB | 1.3–2.6 |
| [55] | Carrot pulp | Enzymatic | CB | 1.3–2.8 |
| Carrot pulp | - | CB | ||
| [35] | Crystalline cellulose | - | B | Proteome data |
| Cellobiose | - | B | Microarray data | |
| [95] | Switchgrass | - | B | |
| Poplar | - | B | ||
| [53] | Xylan | - | B | Microarray data |
| Xyloglucan | - | B | Microarray data | |
| Xyloglucan-oligosaccharides | - | B | Microarray data | |
| [96] | Barley straw | Acid/Enzymatic | B | |
| Corn stalk | Acid/Enzymatic | B | ||
| Barley grain | Enzymatic | B | ||
| Corn grain | Enzymatic | B | ||
| Sugar beet | - | B | ||
| [97] | Sweet sorghum plant | - | B | |
| Sweet sorghum juice | - | B | ||
| Dry sugarcane bagasse | - | B | ||
| Wheat straw | - | B | ||
| Maize leaves | - | B | ||
| Maize leaves | Biological | B | ||
| Silphium trifoliatum leaves | - | B | ||
| [54] | Miscanthus giganteus | Alkaline/Enzymatic | B/CB | 2.4–3.4 |
| [52] | Agarose | - | B | With different support matrixes |
| Alginic acid | - | B | ||
| Pine wood shavings | - | B | ||
| [98] | Jerusalem artichoke | - | B | Co-fermentation with natural biogas-producing consortia |
| Fresh waste water sludge | - | B | ||
| Pig manure slurry | - | B | ||
| [78] | Paper sludge | Acid/Enzymatic | CB | |
| [99] | Paper sludge | Acid/Enzymatic | B |
On the other hand, growth experiments on pure sugar substrates can be used to investigate the specific response associated with a certain substrate or to examine specific pathways involved in the metabolism of a substrate. Table 3 gives an overview of the literature related to growth experiments on pure sugars and pure sugar mixes, including the determined H2 yields and H2 productivities. The currently available fermentation data are discussed here to highlight the different factors limiting H2 formation during the fermentative H2 production by C. saccharolyticus.
Table 3.
Literature overview of fermentation studies on C. saccharolyticus grown on pure sugar substrates. * Yields in mol H2/mol hexose; nd, not determined; ** For batch cultivations the maximal productivity is given; nd, not determined; B, batch cultivation; and CB, controlled batch cultivation; Chem, chemostat cultivations.
| Reference | Substrate | Substrate | H2 Yield * | Productivity ** | Cultivation method | Dilution rate (h−1) | Remarks |
|---|---|---|---|---|---|---|---|
| load (g/L) | (mmol/(L*h)) | ||||||
| [100] | Glucose | 10 | 3.0 | 20.0 mol/(g*h) | CB | ||
| 10 | 3.4 | 23.6 mol/(g*h) | CB | No YE in medium | |||
| 4 | 3.5 | 10.1 mol/(g*h) | Chem | 0.05 | |||
| 4 | 3.5 | 10.4 mol/(g*h) | Chem | 0.05 | No YE in medium | ||
| [75] | Glucose | 5 | 3.5 | 5.2 | Chem | 0.05 | |
| 5 | 2.9 | 11.0 | Chem | 0.15 | Residual glucose (3 mM) | ||
| 5 | 1.8 | 2.5 | Chem | 0.05 | no sparging, open gas outlet | ||
| 5 | wash out | wash out | Chem | 0.15 | no sparging, open gas outlet | ||
| [101] | Glucose | 5 | nd | nd | B | Extracellular proteome | |
| [91] | Glucose | 10 | 3.4 | 12.0 | CB | ||
| 31 | 2.8 | 12.9 | CB | Residual glucose | |||
| [93] | Sucrose | 10 | 2.9 | 7.1 | CB | ||
| [94] | Glucose/Xylose/Sucrose | 10 | 3.2 | 10.7 | CB | Sugar mix | |
| (6:2.5:1.5, w/w/w) | 20 | 2.8 | 9.4 | CB | Sugar mix | ||
| [55] | Glucose | 10 | 3.2 | 11.2 | CB | ||
| 20 | 3.4 | 12.2 | CB | ||||
| Fructose | 10 | 2.6 | 13.2 | CB | |||
| 20 | 2.4 | 13.4 | CB | ||||
| Glucose/Fructose | 10 | 3.0 | 13.2 | CB | Sugar mix | ||
| (7:3, w/w) | 20 | 2.6 | 12.2 | CB | Sugar mix | ||
| [35] | Xylose | 5 | nd | nd | B | Proteome data | |
| Glucose | 5 | nd | nd | B | Proteome data | ||
| [50] | Glucose | 5 | nd | nd | B | Extracellular proteome | |
| [79] | Sucrose | 4 | 2.7 | 23.0 | CB | ||
| 4 | 3.1 | 11.8 | CB | CO2 sparging | |||
| Glucose | 4 | 3.0 | 20.0 | CB | |||
| 4 | 2.7 | 12.0 | CB | CO2 sparging | |||
| [53] | Glucose | 0.5 | nd | nd | B | Microarray data | |
| Mannose | 0.5 | nd | nd | B | Microarray data | ||
| Arabinose | 0.5 | nd | nd | B | Microarray data | ||
| Xylose | 0.5 | nd | nd | B | Microarray data | ||
| Fructose | 0.5 | nd | nd | B | Microarray data | ||
| Galactose | 0.5 | nd | nd | B | Microarray data | ||
| mix (0.5 g/L each) | 3 | nd | nd | B | Microarray data, Sugar mix | ||
| [54] | Glucose/Xylose | 10 | 3.4 | 12.0 | CB | Sugar mix | |
| (7:3 w/w) | 14 | 3.3 | 10.1 | CB | Sugar mix | ||
| 28 | 2.4 | 9.7 | CB | Sugar mix | |||
| [102] | Sucrose | 10.3 | 2.8 | 22.0 | Trickle bed | 0.2–0.3 | 400 L, non-axenic fermentation |
| [33] | Glucose | 4 | nd | nd | CB | Microarray data | |
| Xylose | 4 | nd | nd | CB | Microarray data | ||
| Rhamnose | 4 | nd | nd | CB | Microarray data | ||
| Glucose/Xylose (1:1, w/w) | 4 | nd | nd | CB | Microarray data | ||
| [56] | Glucose | 4.4 | 3.3 | 4.2 | Chem | 0.05 | |
| 4.4 | 3.6 | 8.9 | Chem | 0.10 | |||
| 4.4 | 2.9 | 9.5 | Chem | 0.15 | Residual glucose (3.3 mM) | ||
| 4.4 | 2.9 | 9.1 | Chem | 0.20 | Residual glucose (8.9 mM) | ||
| 4.4 | 3.1 | 11.0 | Chem | 0.30 | Residual glucose (12.4 mM) | ||
| 4.4 | 3.0 | 12.4 | Chem | 0.35 | Residual glucose (12.7 mM) | ||
| 1.9 | 4.0 | 4.0 | Chem | 0.09 | |||
| 1.9 | 3.3 | 9.9 | Chem | 0.30 | Residual glucose (0.6 mM) | ||
| 4.1 | 3.5 | 7.7 | Chem | 0.09 | |||
| 4.1 | 3.1 | 11.6 | Chem | 0.30 | Residual glucose (11.9 mM) | ||
| [78] | Glucose | 10 | 2.5 | 10.7 | CB | ||
| Xylose | 10 | 2.7 | 11.3 | CB | |||
| Glucose/Xylose (11:3, w/w) | 8.4 | 2.4 | 9.2 | CB | Sugar mix | ||
| [103] | Sucrose | 10 | 3.3 | 8.4 | CB |
6.1. Comparison between Hydrolysates and Pure Sugar Mixtures
Studies on biomass hydrolysates and mono-saccharide mixtures, mimicking the biomass hydrolysates, showed that, while at low substrate loads fermentation performances were comparable, at higher substrate loads H2 yields were higher on the mixed mono-saccharides compared to the biomass hydrolysates. The difference in yields was caused by a shift to lactate formation during the growth on high substrate load hydrolysates. Interestingly, for the higher substrate concentrations, the total sugar consumption was higher during growth on hydrolysates compared to growth on sugar mixtures [55,91]. For C. saccharolyticus, grown on carrot pulp hydrolysate (20 g/L), a lower cumulative H2 production was found, compared to growth on a glucose/fructose mixture (20 g/L), while a relatively higher maximal H2 productivity was observed for the hydrolysate compared to the sugar mixture. During the growth on carrot pulp hydrolysates the relative higher H2 productivity preceded the switch to lactate formation [55]. These results demonstrate the relation between high H2 productivity, lactate formation and an overall low H2 yield. However, these described phenomena were not observed for fermentations on Miscanthus hydrolysates. For each tested substrate load (10, 14 and 28 g/L) fermentation performance during growth on the Miscanthus hydrolysate was similar to the glucose/xylose sugar mix and only moderate levels of lactate were formed even at high substrate loads [54]. The differences in H2 production characteristics between the discussed hydrolysates might be related to the difference in sugar composition of the hydrolysates or the differences in pretreatment applied prior to hydrolysis.
In general, biomass derived hydrolysates might contain substances which negatively affect growth or fermentation performance. For example, the dilute-acid pretreatment of lignocellulosic biomass releases undesirable inhibiting compounds like 5-hydroxymethylfurural (HMF), furfural, phenolic compounds and acetate [89] and a growth inhibition of 50% was reported for C. saccharolyticus in the presence of 1–2 g/L HMF or furfural [54].
6.2. Incomplete Substrate Conversion
For chemostat cultivation on glucose (4.4 and 5 g/L) it was shown that a dilution rate (D) exceeding 0.1 h−1 gave rise to an incomplete substrate conversion but also to a lower H2 yield. The concomitant decrease in both biomass level and H2 yield caused the volumetric H2 productivity (mmol/L*h) to level off at higher dilution rates [56,75]. The observed lower H2 yield reflects the shift in end product formation, from mainly acetate at a low dilution rate of 0.05 h−1 to a mix of acetate and ethanol at a D = 0.15 h−1 [75]. Interestingly, no lactate was produced during these chemostat cultivations [56,75]. The observed incomplete substrate conversion indicated that another factor was limiting under those conditions. Indeed, increasing the yeast extract to glucose ratio, from 0.25 to 1 g/g, resulted in the almost complete consumption of glucose and also a doubling of cell density (D = 0.3 h−1), which led to an increase in volumetric H2 productivity (20 mmol/L*h). The H2 yield was, however, not affected [56]. This finding indicated that these observed changes in H2 yields were a function of the growth rate and did not depend on the substrate conversion efficiency.
6.3. End Product Inhibition and Osmotolerance
Incomplete substrate conversions can also be observed in controlled batch fermentations with high initial substrate levels. Growth on 10 g/L of both glucose and fructose resulted in complete substrate consumption, while higher initial substrate levels of glucose (20 and 31 g/L) and fructose (20 g/L) resulted in incomplete conversions [55,91]. Similar observations were done for different sugar mixtures [54,55,94]. These incomplete conversions were attributed to the inhibitory effect of accumulating organic acids like acetate or lactate. Inhibition experiments showed that an acetate concentration of 200 mM and higher prevented acid production by C. saccharolyticus grown on glucose (10 g/L) in batch [91], which is in line with the earlier findings of van Niel et al., who observed critical sodium acetate and potassium acetate concentrations of 192 mM and 206 mM, respectively, for batch growth on sucrose [73]. However, similar inhibitory effects were observed for NaCl and KCl, with critical concentration of 216 mM and 250 mM respectively [73], suggesting that increased osmolarity is the cause of inhibition and not the end products per se. This relative low osmotolerance in comparison to marine organisms, like Thermotoga neapolitana [91], probably reflects the terrestrial origin of C. saccharolyticus. Ljunggren et al. designed a kinetic model for the growth of C. saccharolyticus incorporating the inhibitory effect of a high osmolarity and determined a critical osmolarity (no growth) in the range of 270 to 290 mM. They also showed that osmolarity is of minor influence on fermentations with low initial glucose levels [74]. This low tolerance to osmotic pressure also prevents the application of CO2 as a cheaper and more convenient stripping gas than N2. The use of CO2 as stripping gas during C. saccharolyticus cultivations negatively affects growth rate and hydrogen productivity. CO2 sparging led to a higher dissolved CO2 concentration, which required addition of extra base to maintain a constant pH, overall leading to an increase in osmotic pressure [79].
6.4. Medium Requirements
Controlled batch cultivations were used to investigate the influence of NH4+ on the performance of C. saccharolyticus grown on molasses. These experiments revealed that the omission of NH4+ gave rise to a higher H2 yield and maximal H2 productivity [90]. Although C. saccharolyticus is able to grow on a medium without NH4+, containing only YE as a nitrogen source, it becomes very sensitive to changes in PH2 ([77], Figure 4b). Chemostat cultivations showed complete glucose (20.7 mM) consumption and high acetate yields (1.87 ± 0.02 mol/mol) under low PH2. However, when the cultivation condition changed to a high PH2 a new steady state could be achieved, but substrate consumption was incomplete (55%) and acetate yields decreased (1.68 ± 0.01 mol/mol).
Omission of yeast extract (YE) during growth of C. saccharolyticus on molasses did not affect the H2 yield but led to a lower volumetric H2 productivity [90]. Similar observations were made for controlled batch fermentations on glucose, where the absence of YE did not affect the H2 and biomass yields [100]. Contrary to the molasses study the volumetric productivity was not affected [100] but this might be due to the lower substrate load used. C. saccharolyticus is able to grow on a defined minimal medium with additional vitamins, but without additional amino acids [100]. Growth in the absence of YE helps to reduce the production costs. However, increased biomass levels and especially growth on high substrate loads might augment medium requirements. So fine-tuning of the medium composition with respect to the specific substrate and substrate load is required.
7. Future Prospects for Improving Biohydrogen Production
7.1. Improving H2 Yields and H2 Productivity
C. saccharolyticus has many properties that make it an excellent candidate for biohydrogen production via dark fermentation. However, for biohydrogen production to become economically feasible major improvements should be made with respect to the H2 productivity [104,105,106]. Productivity is maximized by improving the substrate consumption rate but also the H2 yield.
C. saccharolyticus is able to produce H2 close to the theoretical maximum of 4 H2 per hexose. In this respect, it is important to realize that the theoretical yield refers to the pure catabolic component of glucose conversion. The glucose used for anabolism should not be incorporated. Generally, this distinction is not considered in literature, and reported experimental data therefore reveal H2 yields lower than 4. For example, a yield of 3.5 H2 per consumed glucose has been reported by de Vrije et al. (chemostat cultivation with a 23.0 mM glucose load and a dilution rate of 0.1 h−1) [56]. According to their data 16% of the consumed glucose is used only for biomass formation indicating that only ~19.4 mM glucose was available for ATP generation. Given the reported H2 production this results in a theoretical conversion efficiency of 4.15 mol H2 per mol glucose, which approximates the theoretical maximum of 4 H2 per hexose as indicated by Thauer et al. [83]. These high yields are only achievable when the organism ferments the substrate solely to acetate. However, non-ideal growth conditions lead to a mixed fermentation profile (acetate, lactate and ethanol), and a consequently lower H2 yield. From the organism’s perspective switching to lactate or ethanol is profitable since it allows the organism to continue to grow under elevated PH2 conditions, albeit with a lower growth rate because ATP yields are lower under lactate and ethanol forming conditions. Obviously, the lower H2 yield under a mixed end-product fermentation, is not desirable from a biotechnological point of view. To maximize the H2 yield and productivity, the dissolved H2 concentration should be kept as low as possible, which requires an optimization of the liquid to mass transfer rate [74,76], which is mainly a matter of reactor design. In continuous stirred-tank reactor systems low dissolved H2 concentrations could be achieved by increasing the sparging rate [107,108] or the stirring speed [109,110]. In addition, reduction of internal reactor pressure [111,112,113] and enforced bubble formation [113,114] could potentially lead to a lower dissolved H2 concentration. Addition of zeolite particles, which enhances bubble formation, allowed a reduction of the N2 stripping rate from 5 L/(h*L) to 1 L/(h*L), without affecting the H2 productivity and H2 yield of C. saccharolyticus. N2 stripping could even be completely omitted when an internal reactor pressure of 0.3 bar was used, which sustained a similar fermentation performance compared to cultivation at atmospheric pressure (1 bar) using a N2 gas stripping rate of 5 L/(h*L) [113].
Most research on C. saccharolyticus has been performed in serum bottles and suspended continuous stirred-tank reactor systems, where only relatively low cell biomass levels can be achieved. Higher cell biomass levels would cause an increase in substrate consumption rates leading to an increase in H2 productivity when H2 yields are maintained. To realize higher biomass levels a deeper insight into growth limiting medium compounds should be acquired. Additionally, other reactor types, like a trickle bed reactor or a fluidized bed system might allow cell biomass accumulation. C. saccharolyticus could be cultivated in a non-axenic 400 L trickle bed reactor [102] out-competing other organisms, with H2 yields around 2.8 mol H2/mol hexose and a productivity of 22 mmol H2/L*h. These results showed that C. saccharolyticus can be used in a large scale non-sterile industrial setting.
Combining different organisms in a co-cultivation setup allows the exploitation of the hydrolytic capability of each individual species and could enhance the overall range of useable substrates. Batch co-cultivations of C. saccharolyticus with either C. owensensis, C. kristjanssonii or an enriched compost microflora were performed on a glucose-xylose mixture [115]. The co-cultivation with the enriched compost microflora resulted in a fast, simultaneous consumption of both glucose and xylose with a relatively high specific hydrogen production rate, but with a lower H2 yield [115]. A stable co-culture consisting of two closely related Caldicellulosiruptor species, C. saccharolyticus and C. kristjanssonii, could be established in a continuous cultivation system [116]. These findings demonstrate the possibility to create co-cultures for H2 formation and reveal an apparent synergistic effect of the strains, which lead to improved fermentation performances.
Overall H2 yields from biomass derived substrates can be increased when the dark fermentation is coupled to a second stage like electrohydrogenesis or photofermentation [117,118]. The former system uses a microbial electrolysis cell (MEC), in which electricity is used to convert acetate or other organic acids to hydrogen. In the latter case the main end product of the dark fermentation, acetate, is further converted by an anaerobic non-sulfur purple photosynthetic bacterium, forming a maximum of 4 mol H2 per mol acetate, giving an overall H2 yield of 12 mol H2 per mol glucose. The effluent of C. saccharolyticus has been successfully used as a feed for photofermentative growth and H2 production [90,119,120]. Alternatively, dark fermentation end-products H2 and acetate could serve as substrates for hydrogenothropic methanogens in a biogas generating system. The addition of C. saccharolyticus to natural biogas-producing consortia led to an improvement of biogas production and a stable co-cultivation could be maintained for several months [98].
7.2. Genetic Engineering of Caldicellulosiruptor Species
The first steps in the development of a genetic system for Caldicellulosiruptor species have been made. Chung et al. have shown that methylation with an endogenous unique α-class N4-Cytosine methyltransferase is required for transformation of DNA isolated from E. coli into Caldicellulosiruptor bescii [121]. Furthermore, an uracil auxotrophic C. bescii mutant strain was generated by a spontaneous deletion in the pyrBCF locus [121]. This nutritional deficiency was exploited as a selection marker of C. bescii transformants [121]. A similar strategy might be applied to develop a genetic system for the other members of this genus.
To improve the H2 producing capabilities of C. saccharolyticus or other Caldicellulosiruptor species metabolic engineering strategies could focus on improving the H2 yields. Additionally it could be aimed at altering intrinsic properties of Caldicellulosiruptor species limiting H2 productivity like the enhancement of their H2 tolerance or osmotolerance. For example, for industrial applications increased substrate concentrations are favored since it reduces the fresh water demand, thus reducing the overall costs and the environmental impact of the process. However, for C. saccharolyticus the maximum substrate load is limited by its sensitivity to osmotic stress [12,73,74].
With respect to the mixed acid fermentation, both lactate and ethanol formations lead to a lowered H2 yield. Because ethanol formation is not the major reductant sink under redox stress and in general is only produced at low levels, knocking out the alcohol dehydrogenase responsible for ethanol formation will probably not significantly alter the fermentation performance of C. saccharolyticus. However, lactate formation can be seen as the main mechanism to alleviate redox stress. Targeting the lactate formation pathway for complete knockout will probably make C. saccharolyticus less resilient to fluctuations in dissolved H2 concentration and is inadvisable.
Alternatively, one could alter glycolysis in such a way that substrate conversion is less energy efficient. So to generate the same amount of ATP, essential for biosynthesis and maintenance, a higher glycolytic flux to acetate is required. This would result in a higher H2 yield because the glycolytic flux to acetate is increased with respect to the carbon flux to biomass. A less energy efficient glycolysis can be achieved by eliminating some ATP generating steps from the central metabolic pathway. For example, exchanging the NADH-dependent GAPDH in C. saccharolyticus with the ferredoxin-dependent GAPOR, would decrease the overall ATP yield of glycolysis. In addition, since the H2 formation from the generated Fdred is energetically more favourable than H2 formation from NADH, the organism would become less sensitive to increased H2 levels [9,12].
H2 yields on rhamnose and fucose could be increased if the carbon flux from the intermediate lactaldehyde is redirected via methylglyoxal to pyruvate, by the insertion of a lactaldehyde dehydrogenase and a methylglyoxal dehydrogenase. When rhamnose is completely oxidized to acetate, via this pathway, the H2 yield increases from 1 to 5 H2 per rhamnose. With respect to alternative substrates, glycerol could serve as a good substrate for H2 production because of the relative high reduced state of its carbon atoms. A maximum H2 yield of 3 mol/mol glycerol is achieved if glycerol is completely oxidized to acetate. So far, growth or co-consumption on/of glycerol has, however, not been observed for C. saccharolyticus [122].
8. Conclusions
The bacterium Caldicellulosiruptor saccharolyticus possesses several features that make it an excellent candidate for biological hydrogen production. With an optimal growth temperature of 70 °C C. saccharolyticus is one of the most thermophilic cellulose degrading organisms known to date. The organisms diverse inventory of endo- and exo-glycoside hydrolases allow it to degrade and grow on a variety of cellulose- and hemicellulose-containing biomass substrates. Some of these glycosidases are multi-domain proteins that contain both glycoside hydrolase domains and carbon binding modules, which facilitate the efficient degradation of recalcitrant plant polysaccharides into mono-, di- or oligo-saccharides. The high diversity of transport systems present in the genome confirm the broad substrate preferences of C. saccharolyticus and its ability to co-utilization hexoses and pentoses, without any signs of carbon catabolite repression, is a desirable trait for any consolidated bioprocess.
For C. saccharolyticus sugar substrates are primarily fermented to acetate, CO2 and H2, via the Embden-Meyerhof pathway. Typically, the fermentation of hexose and pentose lead to the generation of the reduced electron carriers NADH and Fdred in a 1:1 ratio. These reduced electron carriers can be reoxidized during two distinct H2 generating steps, respectively catalyzed by an NADH-dependent cytosolic Fe-only hydrogenase (hyd) and the Fdred-dependent membrane-bound [NiFe] hydrogenase (ech). Alternatively, rhamnose catabolism is coupled to a different type of redox balancing, where the generated NADH is used for 1,2-propanediol formation and only the Fdred is available for H2 formation.
Fermentation data reveal that C. saccharolyticus is capable of producing H2 with yields close to the theoretical limit of 4 H2 per hexose. However, under non-ideal conditions both ethanol and lactate formation act as alternative redox sinks, thus reducing H2 yields. All possible redox sinks, including the hydrogenases, are upregulated during cultivation under an increased partial hydrogen pressure. The mechanism underlying transcription of the lactate dehydrogenase gene remains elusive, but the transcription of the genes coding for both hydrogenases and the alcohol dehydrogenases, potentially involved in ethanol formation, seem to be under the control of an NADH/NAD+-sensing transcriptional regulator REX.
An increased intracellular NADH/NAD+ ratio, putatively caused by the inhibition of hydrogenase activity at elevated H2 levels, can hinder glycolysis at the level of glyceraldehyde-3-phosphate dehydrogenase, resulting in the inhibition of growth. Lactate formation serves as an alternative redox sink, alleviating redox stress. Lactate dehydrogenase activity is enhanced by the glycolytic intermediate fructose-1,6-bisphosphate but also modulated by the energy carriers ATP and pyrophosphate. The latter mechanism couples lactate formation to the energy metabolism, where lactate formation is inhibited during exponential growth and inhibition is alleviated during the transition to the stationary phase.
Overall, maintaining low dissolved H2 levels in the system appeared to be one of the most important factors for optimizing H2 production. In addition, improvements should be made with respect to the H2productivity and osmotolerance of the organism to allow biohydrogen production by C. saccharolyticus to become economically feasible.
Acknowledgments
This research was supported by the IPOP program of Wageningen University and a grant from the 6th EU Framework Programme, Priority 6.1: Sustainable Energy Systems, contract 019825 (HYVOLUTION).
Supplementary Files
Supplementary Table 1 (XLSX, 19 KB)
Supplementary Table 2 (XLSX, 24 KB)
References and Notes
- 1.Lynd L.R., Wyman C.E., Gerngross T.U. Biocommodity engineering. Biotechnol. Prog. 1999;15:777–793. doi: 10.1021/bp990109e. [DOI] [PubMed] [Google Scholar]
- 2.Lynd L.R., Zyl W.H.V., McBride J.E., Laser M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005;16:577–583. doi: 10.1016/j.copbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Agbor V.B., Cicek N., Sparling R., Berlin A., Levin D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011;29:675–685. doi: 10.1016/j.biotechadv.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Olson D.G., McBride J.E., Shaw A.J., Lynd L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012;23:396–405. doi: 10.1016/j.copbio.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Hallenbeck P.C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrogen Energy. 2009;34:7379–7389. doi: 10.1016/j.ijhydene.2008.12.080. [DOI] [Google Scholar]
- 6.Bergquist P.L., Gibbs M.D., Morris D.D., Te'o V.S., Saul D.J., Moran H.W. Molecular diversity of thermophilic cellulolytic and hemicellulolytic bacteria. FEMS Microbiol. Ecol. 1999;28:99–110. doi: 10.1111/j.1574-6941.1999.tb00565.x. [DOI] [Google Scholar]
- 7.Blumer-Schuette S.E., Kataeva I., Westpheling J., Adams M.W.W., Kelly R.M. Extremely thermophilic microorganisms for biomass conversion: Status and prospects. Curr. Opin. Biotechnol. 2008;19:210–217. doi: 10.1016/j.copbio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 8.VanFossen A.L., Lewis D.L., Nichols J.D., Kelly R.M. Polysaccharide degradation and synthesis by extremely thermophilic anaerobes. Incredible Anaerobes Physiol. Genomics Fuels. 2008;1125:322–337. doi: 10.1196/annals.1419.017. [DOI] [PubMed] [Google Scholar]
- 9.Kengen S.W.M., Goorissen H.P., Verhaart M.R.A., Stams A.J.M., van Niel E.W.J., Claassen P.A.M. Biological hydrogen production by anaerobic microorganisms. In: Soetaert W., Verdamme E.J., editors. Biofuels. John Wiley & Sons; Chichester, UK: 2009. pp. 197–221. [Google Scholar]
- 10.Verhaart M.R.A., Bielen A.A.M., van der Oost J., Stams A.J.M., Kengen S.W.M. Hydrogen production by hyperthermophilic and extremely thermophilic bacteria and archaea: Mechanisms for reductant disposal. Environ. Technol. 2010;31:993–1003. doi: 10.1080/09593331003710244. [DOI] [PubMed] [Google Scholar]
- 11.De Vrije T., Claasen P.A.M. Dark hydrogen fermentations. In: Reith J.H., Wijffels R.H., Barten H., editors. Bio-methane & Bio-hydrogen. Smiet Offset; The Hague, The Netherlands: 2003. pp. 103–123. [Google Scholar]
- 12.Willquist K., Zeidan A.A., van Niel E.W. Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: An efficient hydrogen cell factory. Microb. Cell Fact. 2010;9:89. doi: 10.1186/1475-2859-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rainey F.A., Donnison A.M., Janssen P.H., Saul D., Rodrigo A., Bergquist P.L., Daniel R.M., Stackebrandt E., Morgan H.W. Description of Caldicellulosiruptor saccharolyticus gen. nov., sp. nov: An obligately anaerobic, extremely thermophilic, cellulolytic bacterium. FEMS Microbiol. Lett. 1994;120:263–266. doi: 10.1111/j.1574-6968.1994.tb07043.x. [DOI] [PubMed] [Google Scholar]
- 14.Sissons C.H., Sharrock K.R., Daniel R.M., Morgan H.W. Isolation of cellulolytic anaerobic extreme thermophiles from New Zealand thermal sites. Appl. Environ. Microbiol. 1987;53:832–838. doi: 10.1128/aem.53.4.832-838.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds P.H.S., Sissons C.H., Daniel R.M., Morgan H.W. Comparison of cellulolytic activities in Clostridium thermocellum and three thermophilic, cellulolytic anaerobes. Appl. Environ. Microbiol. 1986;51:12–17. doi: 10.1128/aem.51.1.12-17.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnison A.M., Brockelsby C.M., Morgan H.W., Daniel R.M. The degradation of lignocellulosics by extremely thermophilic microorganisms. Biotechnol. Bioeng. 1989;33:1495–1499. doi: 10.1002/bit.260331118. [DOI] [PubMed] [Google Scholar]
- 17.Bredholt S., Sonne-Hansen J., Nielsen P., Mathrani I.M., Ahring B.K. Caldicellulosiruptor kristjanssonii sp. nov., a cellulolytic extremely thermophilic, anaerobic bacterium. Int. J. Syst. Bacteriol. 1999;49:991–996. doi: 10.1099/00207713-49-3-991. [DOI] [PubMed] [Google Scholar]
- 18.Dwivedi P.P., Gibbs M.D., Saul D.J., Bergquist P.L. Cloning, sequencing and overexpression in Escherichia coli of a xylanase gene, xynA from the thermophilic bacterium Rt8.4 genus Caldicellulosiruptor. Appl. Microbiol. Biotechnol. 1996;45:86–93. doi: 10.1007/s002530050653. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs M.D., Reeves R.A., Farrington G.K., Anderson P., Williams D.P., Bergquist P.L. Multidomain and multifunctional glycosyl hydrolases from the extreme thermophile Caldicellulosiruptor isolate Tok7B.1. Curr. Microbiol. 2000;40:333–340. doi: 10.1007/s002849910066. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton-Brehm S.D., Mosher J.J., Vishnivetskaya T., Podar M., Carroll S., Allman S., Phelps T.J., Keller M., Elkins J.G. Caldicellulosiruptor obsidiansis sp. nov., an anaerobic, extremely thermophilic, cellulolytic bacterium isolated from Obsidian pool, Yellowstone national park. Appl. Environ. Microbiol. 2010;76:1014–1020. doi: 10.1128/AEM.01903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C.Y., Patel B.K., Mah R.A., Baresi L. Caldicellulosiruptor owensensis sp. nov., an anaerobic, extremely thermophilic, xylanolytic bacterium. Int. J. Syst. Bacteriol. 1998;48:91–97. doi: 10.1099/00207713-48-1-91. [DOI] [PubMed] [Google Scholar]
- 22.Miroshnichenko M.L., Kublanov I.V., Kostrikina N.A., Tourova T.P., Kolganova T.V., Birkeland N.K., Bonch-Osmolovskaya E.A. Caldicellulosiruptor kronotskyensis sp. nov. and Caldicellulosiruptor hydrothermalis sp. nov., two extremely thermophilic, cellulolytic, anaerobic bacteria from Kamchatka thermal springs. Int. J. Syst. Evol. Microbiol. 2008;58:1492–1496. doi: 10.1099/ijs.0.65236-0. [DOI] [PubMed] [Google Scholar]
- 23.Mladenovska Z., Mathrani I.M., Ahring B.K. Isolation and characterization of Caldicellulosiruptor lactoaceticus sp. nov., an extremely thermophilic, cellulolytic, anaerobic bacterium. Arch. Microbiol. 1995;163:223–230. doi: 10.1007/BF00305357. [DOI] [Google Scholar]
- 24.Morris D.D., Gibbs M.D., Ford M., Thomas J., Bergquist P.L. Family 10 and 11 xylanase genes from Caldicellulosiruptor sp. strain Rt69B.1. Extremophiles. 1999;3:103–111. doi: 10.1007/s007920050105. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen P., Mathrani I.M., Ahring B.K. Thermoanaerobium acetigenum spec. nov., a new anaerobic, extremely thermophilic, xylanolytic non-spore-forming bacterium isolated from an Icelandic hot spring. Arch. Microbiol. 1993;159:460–464. doi: 10.1007/BF00288594. [DOI] [Google Scholar]
- 26.Onyenwoke R.U., Lee Y.J., Dabrowski S., Ahring B.K., Wiegel J. Reclassification of “Thermoanaerobium acetigenum” as Caldicellulosiruptor acetigenus comb. nov and emendation of the genus description. Int. J. Syst. Evol. Microbiol. 2006;56:1391–1395. doi: 10.1099/ijs.0.63723-0. [DOI] [PubMed] [Google Scholar]
- 27.Svetlichnyi V.A., Svetlichnaya T.P., Chernykh N.A., Zavarzin G.A. Anaerocellum thermophilum gen. nov. sp. nov.: An extremely thermophilic cellulolytic eubacterium isolated from hot-springs in the Valley of Geysers. Microbiology. 1990;59:598–604. [Google Scholar]
- 28.Yang S.J., Kataeva I., Wiegel J., Yin Y., Dam P., Xu Y., Westpheling J., Adams M.W. Reclassification of “Anaerocellum thermophilum” as Caldicellulosiruptor bescii strain DSM 6725T sp. nov. Int. J. Syst. Evol. Microbiol. 2009;60:2011–2015. doi: 10.1099/ijs.0.017731-0. [DOI] [PubMed] [Google Scholar]
- 29.Blumer-Schuette S.E., Giannone R.J., Zurawski J.V., Ozdemir I., Ma Q., Yin Y.B., Xu Y., Kataeva I., Poole F.L., Adams M.W.W., et al. Caldicellulosiruptor core and pangenomes reveal determinants for noncellulosomal thermophilic deconstruction of plant biomass. J. Bacteriol. 2012;194:4015–4028. doi: 10.1128/JB.00266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumer-Schuette S.E., Ozdemir I., Mistry D., Lucas S., Lapidus A., Cheng J.F., Goodwin L.A., Pitluck S., Land M.L., Hauser L.J., et al. Complete genome sequences for the anaerobic, extremely thermophilic plant biomass-degrading bacteria Caldicellulosiruptor hydrothermalis, Caldicellulosiruptor kristjanssonii, Caldicellulosiruptor kronotskyensis, Caldicellulosiruptor owensensis, and Caldicellulosiruptor lactoaceticus. J. Bacteriol. 2011;193:1483–1484. doi: 10.1128/JB.01515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkins J.G., Lochner A., Hamilton-Brehm S.D., Davenport K.W., Podar M., Brown S.D., Land M.L., Hauser L.J., Klingeman D.M., Raman B., et al. Complete genome sequence of the cellulolytic thermophile Caldicellulosiruptor obsidiansis OB47T. J. Bacteriol. 2010;192:6099–6100. doi: 10.1128/JB.00950-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kataeva I.A., Yang S.J., Dam P., Poole F.L., Yin Y., Zhou F.F., Chou W.C., Xu Y., Goodwin L., Sims D.R., et al. Genome sequence of the anaerobic, thermophilic, and cellulolytic bacterium “Anaerocellum thermophilum” DSM 6725. J. Bacteriol. 2009;191:3760–3761. doi: 10.1128/JB.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van de Werken H.J.G., Verhaart M.R.A., VanFossen A.L., Willquist K., Lewis D.L., Nichols J.D., Goorissen H.P., Mongodin E.F., Nelson K.E., van Niel E.W.J., et al. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol. 2008;74:6720–6729. doi: 10.1128/AEM.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamed R., Bayer E.A. The cellulosome of Clostridium thermocellum. Adv. Appl. Microbiol. 1988;33:1–46. doi: 10.1016/S0065-2164(08)70203-X. [DOI] [Google Scholar]
- 35.Blumer-Schuette S.E., Lewis D.L., Kelly R.M. Phylogenetic, microbiological, and glycoside hydrolase diversities within the extremely thermophilic, plant biomass-degrading genus Caldicellulosirupt. Appl. Environ. Microbiol. 2010;76:8084–8092. doi: 10.1128/AEM.01400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanFossen A.L., Ozdemir I., Zelin S.L., Kelly R.M. Glycoside hydrolase inventory drives plant polysaccharide deconstruction by the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Biotechnol. Bioeng. 2011;108:1559–1569. doi: 10.1002/bit.23093. [DOI] [PubMed] [Google Scholar]
- 37.Bergquist P.L., Love D.R., Croft J.E., Streiff M.B., Daniel R.M., Morgan W.H. Genetics and potential biotechnological applications of thermophilic and extremely thermophilic microorganisms. Biotechnol. Genet. Eng. Rev. 1987;5:199–244. doi: 10.1080/02648725.1987.10647838. [DOI] [PubMed] [Google Scholar]
- 38.Love D.R., Streiff M.B. Molecular cloning of a beta-glucosidase gene from an extremely thermophilic anaerobe in Escherichia coli and Bacillus subtilis. BioTechnology. 1987;5:384–387. doi: 10.1038/nbt0487-384. [DOI] [Google Scholar]
- 39.Hudson R.C., Schofield L.R., Coolbear T., Daniel R.M., Morgan H.W. Purification and properties of an aryl beta-xylosidase from a cellulolytic extreme thermophile expressed in Escherichia coli. Biochem. J. 1991;273:645–650. doi: 10.1042/bj2730645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schofield L.R., Daniel R.M. Purification and properties of a beta-1,4-xylanase from a cellulolytic extreme thermophile expressed in Escherichia coli. Int. J. Biochem. 1993;25:609–617. doi: 10.1016/0020-711X(93)90670-A. [DOI] [PubMed] [Google Scholar]
- 41.Albertson G.D., McHale R.H., Gibbs M.D., Bergquist P.L. Cloning and sequence of a type I pullulanase from an extremely thermophilic anaerobic bacterium, Caldicellulosiruptor saccharolyticus. Biochimica Et Biophysica Acta-Gene Structure and Expression. 1997;1354:35–39. doi: 10.1016/S0167-4781(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 42.Luthi E., Jasmat N.B., Bergquist P.L. Xylanase from the extremely thermophilic bacterium “Caldocellum saccharolyticum”: Overexpression of the gene in Escherichia coli and characterization of the gene product. Appl. Environ. Microbiol. 1990;56:2677–2683. doi: 10.1128/aem.56.9.2677-2683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luthi E., Bergquist P.L. A beta-D-xylosidase from the thermophile “Caldocellum saccharolyticum” expressed in Escherichia coli. FEMS Microbiol. Lett. 1990;67:291–294. [Google Scholar]
- 44.Luthi E., Jasmat N.B., Bergquist P.L. Overproduction of an acetylxylan esterase from the extreme thermophile “Caldocellum saccharolyticum” in Escherichia coli. Appl. Microbiol. Biotechnol. 1990;34:214–219. doi: 10.1007/BF00166783. [DOI] [PubMed] [Google Scholar]
- 45.Te'o V.S.J., Saul D.J., Bergquist P.L. Cela, another gene coding for a multidomain cellulase from the extreme thermophile “Caldocellum saccharolyticum”. Appl. Microbiol. Biotechnol. 1995;43:291–296. doi: 10.1007/BF00172827. [DOI] [PubMed] [Google Scholar]
- 46.Park C.S., Kim J.E., Choi J.G., Oh D.K. Characterization of a recombinant cellobiose 2-epimerase from Caldicellulosiruptor saccharolyticus and its application in the production of mannose from glucose. Appl. Microbiol. Biotechnol. 2011;92:1187–1196. doi: 10.1007/s00253-011-3403-3. [DOI] [PubMed] [Google Scholar]
- 47.Saul D.J., Williams L.C., Grayling R.A., Chamley L.W., Love D.R., Bergquist P.L. Celb, a gene coding for a bifunctional cellulase from the extreme thermophile “Caldocellum saccharolyticum”. Appl. Environ. Microbiol. 1990;56:3117–3124. doi: 10.1128/aem.56.10.3117-3124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris D.D., Reeves R.A., Gibbs M.D., Saul D.J., Bergquist P.L. Correction of the beta-mannanase domain of the Celc pseudogene from Caldocellulosiruptor saccharolyticus and activity of the gene product on Kraft pulp. Appl. Environ. Microbiol. 1995;61:2262–2269. doi: 10.1128/aem.61.6.2262-2269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luthi E., Jasmat N.B., Grayling R.A., Love D.R., Bergquist P.L. Cloning, sequence analysis, and expression in Escherichia coli of a gene coding for a beta-mannanase from the extremely thermophilic bacterium “Caldocellum saccharolyticum”. Appl. Environ. Microbiol. 1991;57:694–700. doi: 10.1128/aem.57.3.694-700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews G., Lewis D., Notey J., Kelly R., Muddiman D. Part I: Characterization of the extracellular proteome of the extreme thermophile Caldicellulosiruptor saccharolyticus by GeLC-MS2 (vol 398, pg 377, 2010) Anal. Bioanal. Chem. 2010;398:1837–1837. doi: 10.1007/s00216-010-4102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozdemir I., Blumer-Schuette S.E., Kelly R.M. S-Layer homology domain proteins Csac_0678 and Csac_2722 are implicated in plant polysaccharide deconstruction by the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol. 2012;78:768–777. doi: 10.1128/AEM.07031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanova G., Rakhely G., Kovacs K.L. Hydrogen production from biopolymers by Caldicellulosiruptor saccharolyticus and stabilization of the system by immobilization. Int.l J. Hydrogen Energy. 2008;33:6953–6961. doi: 10.1016/j.ijhydene.2008.08.058. [DOI] [Google Scholar]
- 53.VanFossen A.L., Verhaart M.R.A., Kengen S.M.W., Kelly R.M. Carbohydrate utilization patterns for the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus reveal broad growth substrate preferences. Appl. Environ. Microbiol. 2009;75:7718–7724. doi: 10.1128/AEM.01959-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Vrije T., Bakker R.R., Budde M.A., Lai M.H., Mars A.E., Claassen P.A. Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol. Biofuels. 2009;2:12. doi: 10.1186/1754-6834-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Vrije T., Budde M.A.W., Lips S.J., Bakker R.R., Mars A.E., Claassen P.A.M. Hydrogen production from carrot pulp by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy. 2010;35:13206–13213. doi: 10.1016/j.ijhydene.2010.09.014. [DOI] [Google Scholar]
- 56.De Vrije T., Mars A.E., Budde M.A.W., Lai M.H., Dijkema C., de Waard P., Claassen P.A.M. Glycolytic pathway and hydrogen yield studies of the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl. Microbiol. Biotechnol. 2007;74:1358–1367. doi: 10.1007/s00253-006-0783-x. [DOI] [PubMed] [Google Scholar]
- 57.Willquist K., van Niel E.W.J. Lactate formation in Caldicellulosiruptor saccharolyticus is regulated by the energy carriers pyrophosphate and ATP. Metab. Eng. 2010;12:282–290. doi: 10.1016/j.ymben.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Brown S.D., Guss A.M., Karpinets T.V., Parks J.M., Smolin N., Yang S.H., Land M.L., Klingeman D.M., Bhandiwad A., Rodriguez M., et al. Mutant alcohol dehydrogenase leads to improved ethanol tolerance in Clostridium thermocellum. Proc. Natl. Acad. Sci. USA. 2011;108:13752–13757. doi: 10.1073/pnas.1102444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng H., Wu G.G., Shao W.L. The aldehyde/alcohol dehydrogenase (AdhE) in relation to the ethanol formation in Thermoanaerobacter ethanolicus JW200. Anaerobe. 2008;14:125–127. doi: 10.1016/j.anaerobe.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Bielen A.A.M., Verhaart M.R.A., VanFossen A.L., Blumer-Schuette S.E., Stams A.J.M., van der Oost J., Kelly R.M., Kengen S.M.W. A thermophile under pressure:Transcriptional analysis of the response of Caldicellulosiruptor saccharolyticus to different H2 partial pressures. Int. J. Hydrogen Energy. 2012 in press. [Google Scholar]
- 61.Ma K., Hutchins A., Sung S.J.S., Adams M.W.W. Pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus, functions as a CoA-dependent pyruvate decarboxylase. Proc. Natl. Acad. Sci. USA. 1997;94:9608–9613. doi: 10.1073/pnas.94.18.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kannan V., Mutharasan R. Ethanol fermentation characteristics of Thermoanaerobacter ethanolicus. Enzyme. Microb. Technol. 1985;7:87–89. doi: 10.1016/0141-0229(85)90019-5. [DOI] [Google Scholar]
- 63.Desai S.G., Guerinot M.L., Lynd L.R. Cloning of L-lactate dehydrogenase and elimination of lactic acid production via gene knockout in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl. Microbiol. Biotechnol. 2004;65:600–605. doi: 10.1007/s00253-004-1575-9. [DOI] [PubMed] [Google Scholar]
- 64.Soboh B., Linder D., Hedderich R. A multisubunit membrane-bound [NiFe] hydrogenase and an NADH-dependent Fe-only hydrogenase in the fermenting bacterium Thermoanaerobacter tengcongensis. Microbiology. 2004;150:2451–2463. doi: 10.1099/mic.0.27159-0. [DOI] [PubMed] [Google Scholar]
- 65.Ma K.S., Adams M.W.W. An unusual oxygen-sensitive, iron- and zinc-containing alcohol dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 1999;181:1163–1170. doi: 10.1128/jb.181.4.1163-1170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verhaart M.R.A. Wageningen University; Wageningen, The Netherlands: 2010. Unpublished work. [Google Scholar]
- 67.DeLacey A.L., Stadler C., Fernandez V.M., Hatchikian E.C., Fan H.J., Li S.H., Hall M.B. IR spectroelectrochemical study of the binding of carbon monoxide to the active site of Desulfovibrio fructosovorans Ni-Fe hydrogenase. J. Biol. Inorg. Chem. 2002;7:318–326. doi: 10.1007/s00775-001-0301-7. [DOI] [PubMed] [Google Scholar]
- 68.Lemon B.J., Peters J.W. Binding of exogenously added carbon monoxide at the active site of the iron-only hydrogenase (CpI) from Clostridium pasteurianum. Biochemistry. 1999;38:12969–12973. doi: 10.1021/bi9913193. [DOI] [PubMed] [Google Scholar]
- 69.Heinonen J.K. Biological production of PPi. In: Heinonen J.K., editor. Biological Role of Inorganic Pyrophosphate. Kluwer Academic Publishers; Boston, MA, USA, Dordrecht, The Netherlands, London, UK: 2001. p. 264. [Google Scholar]
- 70.Chen J., Brevet A., Fromant M., Leveque F., Schmitter J.M., Blanquet S., Plateau P. Pyrophosphatase is essential for growth of Escherichia coli. J. Bacteriol. 1990;172:5686–5689. doi: 10.1128/jb.172.10.5686-5689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bielen A.A.M., Willquist K., Engman J., van der Oost J., van Niel E.W.J., Kengen S.W.M. Pyrophosphate as a central energy carrier in the hydrogen-producing extremely thermophilic Caldicellulosiruptor saccharolyticus. FEMS Microbiol. Lett. 2010;307:48–54. doi: 10.1111/j.1574-6968.2010.01957.x. [DOI] [PubMed] [Google Scholar]
- 72.Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991;285:1–5. doi: 10.1016/0014-5793(91)80711-B. [DOI] [PubMed] [Google Scholar]
- 73.Van Niel E.W.J., Claassen P.A.M., Stams A.J.M. Substrate and product inhibition of hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol. Bioeng. 2003;81:255–262. doi: 10.1002/bit.10463. [DOI] [PubMed] [Google Scholar]
- 74.Ljunggren M., Willquist K., Zacchi G., van Niel E.W.J. A kinetic model for quantitative evaluation of the effect of hydrogen and osmolarity on hydrogen production by Caldicellulosiruptor saccharolyticus. Biotechnol. Biofuels. 2011;4:31. doi: 10.1186/1754-6834-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willquist K., Pawar S.S., van Niel E.W.J. Reassessment of hydrogen tolerance in Caldicellulosiruptor saccharolyticus. Microb. Cell Fact. 2011;10:111. doi: 10.1186/1475-2859-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraemer J.T., Bagley D.M. Supersaturation of dissolved H2 and CO2 during fermentative hydrogen production with N2 sparging. Biotechnol. Lett. 2006;28:1485–1491. doi: 10.1007/s10529-006-9114-7. [DOI] [PubMed] [Google Scholar]
- 77.Bielen A.A.M. Wageningen University; Wageningen, The Netherlands: 2012. Unpublished work. [Google Scholar]
- 78.Kadar Z., de Vrijek T., van Noorden G.E., Budde M.A.W., Szengyel Z., Reczey K., Claassen P.A.M. Yields from glucose, xylose, and paper sludge hydrolysate during hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl. Biochem. Biotechnol. 2004;113–116:497–508. doi: 10.1385/abab:114:1-3:497. [DOI] [PubMed] [Google Scholar]
- 79.Willquist K., Claassen P.A.M., van Niel E.W.J. Evaluation of the influence of CO2 on hydrogen production by Caldicellulosiruptor saccharolyticus. Int. J. Hydrogen Energy. 2009;34:4718–4726. doi: 10.1016/j.ijhydene.2009.03.056. [DOI] [Google Scholar]
- 80.Amend J.P., Plyasunov A.V. Carbohydrates in thermophile metabolism: Calculation of the standard molal thermodynamic properties of aqueous pentoses and hexoses at elevated temperatures and pressures. Geochim. Cosmochim. Acta. 2001;65:3901–3917. doi: 10.1016/S0016-7037(01)00707-4. [DOI] [Google Scholar]
- 81.Amend J.P., Shock E.L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 2001;25:175–243. doi: 10.1111/j.1574-6976.2001.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 82.Burton K. Enthalpy change for reduction of nicotinamide-adenine dinucleotide. Biochem. J. 1974;143:365–368. doi: 10.1042/bj1430365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thauer R.K., Jungermann K., Decker K. Energy-conservation in chemotropic anaerobic bacteria. Bacteriol. Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watt G.D., Burns A. Thermochemical characterization of sodium dithionite, flavin mononucleotide, flavin-adenine dinucleotide and methyl and benzyl viologens as low-potential reductants for biological-systems. Biochem. J. 1975;152:33–37. doi: 10.1042/bj1520033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biegel E., Schmidt S., Gonzalez J.M., Muller V. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell. Mol. Life Sci. 2011;68:613–634. doi: 10.1007/s00018-010-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schut G.J., Adams M.W.W. The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: A new perspective on anaerobic hydrogen production. J. Bacteriol. 2009;191:4451–4457. doi: 10.1128/JB.01582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panagiotopoulos I.A., Bakker R.R., de Vrije T., Claassen P.A.M., Koukios E.G. Integration of first and second generation biofuels: Fermentative hydrogen production from wheat grain and straw. Bioresour. Technol. 2013;128:345–350. doi: 10.1016/j.biortech.2012.09.083. [DOI] [PubMed] [Google Scholar]
- 88.Panagiotopoulos I.A., Bakker R.R., de Vrije T., Claassen P.A.M., Koukios E.G. Dilute-acid pretreatment of barley straw for biological hydrogen production using Caldicellulosiruptor saccharolyticus. Int. J. Hydrogen Energy. 2012;37:11727–11734. [Google Scholar]
- 89.Panagiotopoulos I., Barker R., de Vrije T., Niel E.V., Koukios E., Claassen P. Exploring critical factors for fermentative hydrogen production from various types of lignocellulosic biomass. J. Jpn. Inst. Energy. 2011;90:363–368. doi: 10.3775/jie.90.363. [DOI] [Google Scholar]
- 90.Özgür E., Mars A.E., Peksel B., Louwerse A., Yücel M., Gündüz U., Claassen P.A.M., Eroğlu I. Biohydrogen production from beet molasses by sequential dark and photofermentation. Int. J. Hydrogen Energy. 2010;35:511–517. doi: 10.1016/j.ijhydene.2009.10.094. [DOI] [Google Scholar]
- 91.Mars A.E., Veuskens T., Budde M.A.W., van Doeveren P., Lips S.J., Bakker R.R., de Vrije T., Claassen P.A.M. Biohydrogen production from untreated and hydrolyzed potato steam peels by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy. 2010;35:7730–7737. [Google Scholar]
- 92.Herbel Z., Rakhely G., Bagi Z., Ivanova G., Acs N., Kovacs E., Kovacs K.L. Exploitation of the extremely thermophilic Caldicellulosiruptor saccharolyticus in hydrogen and biogas production from biomasses. Environ. Technol. 2010;31:1017–1024. doi: 10.1080/09593330.2010.484075. [DOI] [PubMed] [Google Scholar]
- 93.Panagiotopoulos J.A., Bakker R.R., de Vrije T., Urbaniec K., Koukios E.G., Claassen P.A.M. Prospects of utilization of sugar beet carbohydrates for biological hydrogen production in the EU. J. Cleaner Prod. 2010;18:S9–S14. doi: 10.1016/j.jclepro.2010.02.025. [DOI] [Google Scholar]
- 94.Panagiotopoulos I.A., Bakker R.R., de Vrije T., Koukios E.G., Claassen P.A.M. Pretreatment of sweet sorghum bagasse for hydrogen production by Caldicellulosiruptor saccharolyticus. Int. J. Hydrogen Energy. 2010;35:7738–7747. doi: 10.1016/j.ijhydene.2010.05.075. [DOI] [Google Scholar]
- 95.Yang S.J., Kataeva I., Hamilton-Brehm S.D., Engle N.L., Tschaplinski T.J., Doeppke C., Davis M., Westpheling J., Adams M.W.W. Efficient degradation of lignocellulosic plant biomass, without pretreatment, by the thermophilic anaerobe “Anaerocellum thermophilum” DSM 6725. Appl. Environ. Microbiol. 2009;75:4762–4769. doi: 10.1128/AEM.00236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panagiotopoulos I.A., Bakker R.R., Budde M.A.W., de Vrije T., Claassen P.A.M., Koukios E.G. Fermentative hydrogen production from pretreated biomass: A comparative study. Bioresour. Technol. 2009;100:6331–6338. doi: 10.1016/j.biortech.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 97.Ivanova G., Rakhely G., Kovacs K.L. Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int. J. Hydrogen Energy. 2009;34:3659–3670. doi: 10.1016/j.ijhydene.2009.02.082. [DOI] [Google Scholar]
- 98.Bagi Z., Acs N., Balint B., Horvath L., Dobo K., Perei K.R., Rakhely G., Kovacs K.L. Biotechnological intensification of biogas production. Appl. Microbiol. Biotechnol. 2007;76:473–482. doi: 10.1007/s00253-007-1009-6. [DOI] [PubMed] [Google Scholar]
- 99.Kadar Z., de Vrije T., Budde M.A., Szengyel Z., Reczey K., Claassen P.A. Hydrogen production from paper sludge hydrolysate. Appl. Biochem. Biotechnol. 2003;105–108:557–566. doi: 10.1385/abab:107:1-3:557. [DOI] [PubMed] [Google Scholar]
- 100.Willquist K., van Niel E.W.J. Growth and hydrogen production characteristics of Caldicellulosiruptor saccharolyticus on chemically defined minimal media. Int. J. Hydrogen Energy. 2012;37:4925–4929. doi: 10.1016/j.ijhydene.2011.12.055. [DOI] [Google Scholar]
- 101.Muddiman D., Andrews G., Lewis D., Notey J., Kelly R. Part II: Defining and quantifying individual and co-cultured intracellular proteomes of two thermophilic microorganisms by GeLC-MS(2) and spectral counting. Anal. Bioanal. Chem. 2010;398:391–404. doi: 10.1007/s00216-010-3929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Groenestijn J.W., Geelhoed J.S., Goorissen H.P., Meesters K.P., Stams A.J., Claassen P.A. Performance and population analysis of a non-sterile trickle bed reactor inoculated with Caldicellulosiruptor saccharolyticus, a thermophilic hydrogen producer. Biotechnol. Bioeng. 2009;102:1361–1367. doi: 10.1002/bit.22185. [DOI] [PubMed] [Google Scholar]
- 103.Van Niel E.W.J., Budde M.A.W., de Haas G.G., van der Wal F.J., Claasen P.A.M., Stams A.J.M. Distinctive properties of high hydrogen producing extreme thermophiles, Caldicellulosiruptor saccharolyticus and Thermotoga elfii. Int. J. Hydrogen Energy. 2002;27:1391–1398. doi: 10.1016/S0360-3199(02)00115-5. [DOI] [Google Scholar]
- 104.Ljunggren M., Wallberg O., Zacchi G. Techno-economic comparison of a biological hydrogen process and a 2nd generation ethanol process using barley straw as feedstock. Bioresour. Technol. 2011;102:9524–9531. doi: 10.1016/j.biortech.2011.06.096. [DOI] [PubMed] [Google Scholar]
- 105.Ljunggren M., Zacchi G. Techno-economic evaluation of a two-step biological process for hydrogen production. Biotechnol. Prog. 2010;26:496–504. doi: 10.1002/btpr.336. [DOI] [PubMed] [Google Scholar]
- 106.Nath K., Das D. Improvement of fermentative hydrogen production: Various approaches. Appl. Microbiol. Biotechnol. 2004;65:520–529. doi: 10.1007/s00253-004-1644-0. [DOI] [PubMed] [Google Scholar]
- 107.Kim D.H., Han S.K., Kim S.H., Shin H.S. Effect of gas sparging on continuous fermentative hydrogen production. Int. J. Hydrogen Energy. 2006;31:2158–2169. doi: 10.1016/j.ijhydene.2006.02.012. [DOI] [Google Scholar]
- 108.Kraemer J.T., Bagley D.M. Optimisation and design of nitrogen-sparged fermentative hydrogen production bioreactors. Int. J. Hydrogen Energy. 2008;33:6558–6565. doi: 10.1016/j.ijhydene.2008.08.033. [DOI] [Google Scholar]
- 109.Clark I.C., Zhang R.H.H., Upadhyaya S.K. The effect of low pressure and mixing on biological hydrogen production via anaerobic fermentation. Int. J. Hydrogen Energy. 2012;37:11504–11513. doi: 10.1016/j.ijhydene.2012.03.154. [DOI] [Google Scholar]
- 110.Lamed R.J., Lobos J.H., Su T.M. Effects of stirring and hydrogen on fermentation products of Clostridium thermocellum. Appl. Environ. Microbiol. 1988;54:1216–1221. doi: 10.1128/aem.54.5.1216-1221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Junghare M., Subudhi S., Lal B. Improvement of hydrogen production under decreased partial pressure by newly isolated alkaline tolerant anaerobe, Clostridium butyricum TM-9A: Optimization of process parameters. Int. J. Hydrogen Energy. 2012;37:3160–3168. doi: 10.1016/j.ijhydene.2011.11.043. [DOI] [Google Scholar]
- 112.Mandal B., Nath K., Das D. Improvement of biohydrogen production under decreased partial pressure of H2 by Enterobacter cloacae. Biotechnol. Lett. 2006;28:831–835. doi: 10.1007/s10529-006-9008-8. [DOI] [PubMed] [Google Scholar]
- 113.Sonnleitner A., Peintner C., Wukovits W., Friedl A., Schnitzhofer W. Process investigations of extreme thermophilic fermentations for hydrogen production: Effect of bubble induction and reduced pressure. Bioresour. Technol. 2012;118:170–176. doi: 10.1016/j.biortech.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 114.Fritsch M., Hartmeier W., Chang J.S. Enhancing hydrogen production of Clostridium butyricum using a column reactor with square-structured ceramic fittings. Int. J. Hydrogen Energy. 2008;33:6549–6557. doi: 10.1016/j.ijhydene.2008.07.070. [DOI] [Google Scholar]
- 115.Zeidan A.A., van Niel E.W.J. Developing a thermophilic hydrogen-producing co-culture for efficient utilization of mixed sugars. Int. J. Hydrogen Energy. 2009;34:4524–4528. doi: 10.1016/j.ijhydene.2008.07.092. [DOI] [Google Scholar]
- 116.Zeidan A.A., van Niel E.W.J. A quantitative analysis of hydrogen production efficiency of the extreme thermophile Caldicellulosiruptor owensensis OL(T) Int. J. Hydrogen Energy. 2010;35:1128–1137. doi: 10.1016/j.ijhydene.2009.11.082. [DOI] [Google Scholar]
- 117.Claassen P.A.M., de Vrije T. Non-thermal production of pure hydrogen from biomass: HYVOLUTION. Int. J. Hydrogen Energy. 2006;31:1416–1423. doi: 10.1016/j.ijhydene.2006.06.006. [DOI] [Google Scholar]
- 118.Liu H., Grot S., Logan B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005;39:4317–4320. doi: 10.1021/es050244p. [DOI] [PubMed] [Google Scholar]
- 119.Özkan E., Uyar B., Özgür E., Yücel M., Eroğlu I., Gündüz U. Photofermentative hydrogen production using dark fermentation effluent of sugar beet thick juice in outdoor conditions. Int. J. Hydrogen Energy. 2012;37:2044–2049. doi: 10.1016/j.ijhydene.2011.06.035. [DOI] [Google Scholar]
- 120.Özgür E., Afsar N., de Vrije T., Yücel M., Gündüz U., Claassen P.A.M., Eroğlu I. Potential use of thermophilic dark fermentation effluents in photofermentative hydrogen production by Rhodobacter capsulatus. J. Cleaner Prod. 2010;18:S23–S28. doi: 10.1016/j.jclepro.2010.02.020. [DOI] [Google Scholar]
- 121.Chung D., Farkas J., Huddleston J.R., Olivar E., Westpheling J. Methylation by a unique α-class N4-Cytosine methyltransferase is required for DNA transformation of Caldicellulosiruptor bescii DSM6725. PLoS One. 2012;7:e43844. doi: 10.1371/journal.pone.0043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bielen A.A.M. Wageningen University; Wageningen, The Netherlands: 2009. Unpublished work. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 (XLSX, 19 KB)
Supplementary Table 2 (XLSX, 24 KB)