Abstract
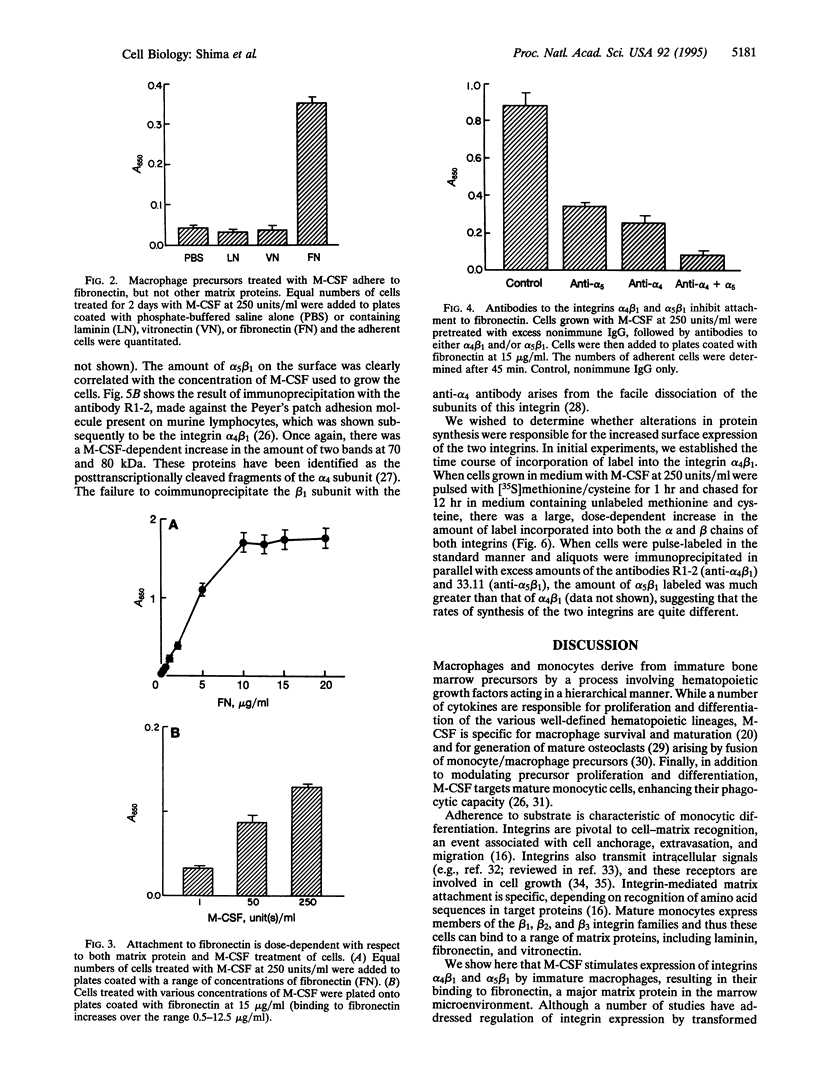
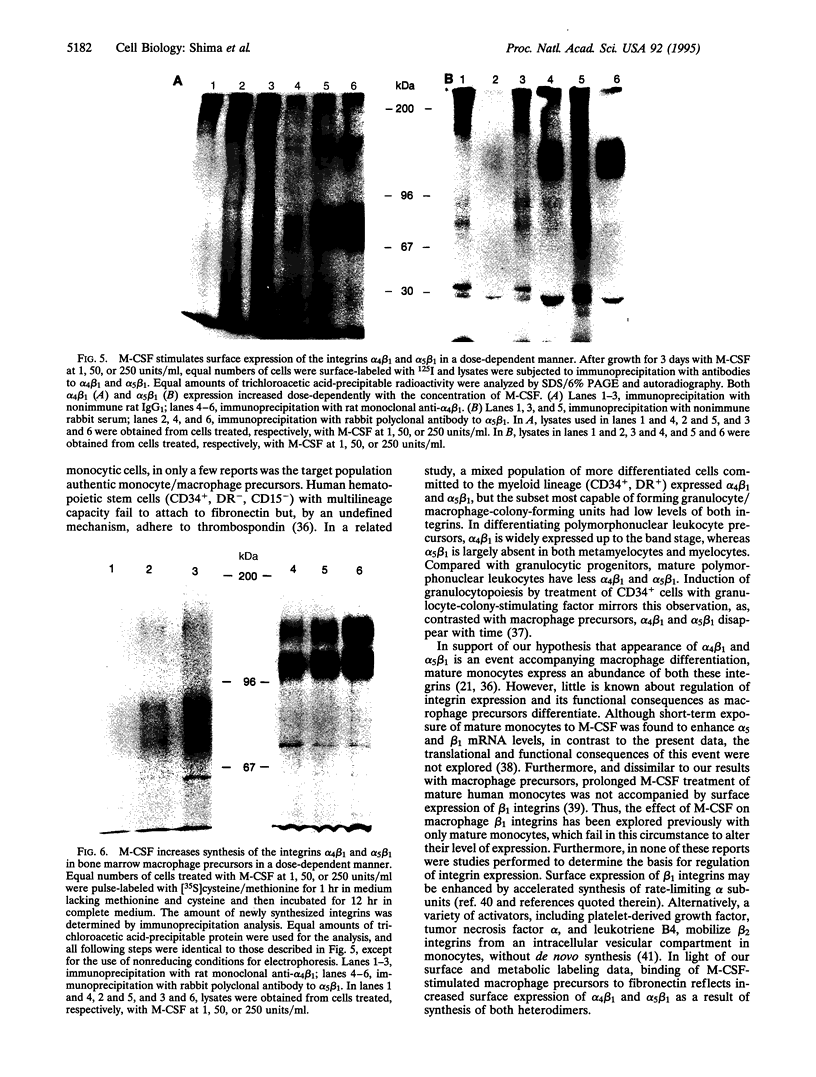
We observed that when monocyte/macrophage precursors derived from murine bone marrow were treated with macrophage-colony-stimulating factor (M-CSF), there was a dose-dependent increase in both the number of adherent cells and the degree to which the cells were highly spread. Attachment was supported by fibronectin, but not by vitronectin or laminin, suggesting that the integrins alpha 4 beta 1 and/or alpha 5 beta 1 might mediate this event. Binding to fibronectin was blocked partially by antibodies to either integrin, and inhibition was almost complete when the antibodies were used in combination. By a combination of surface labeling with 125I and metabolic labeling with [35S]methionine and [35S]cysteine, we demonstrated that M-CSF treatment led to increased synthesis and surface expression of the two beta 1 integrins. Since attachment to fibronectin and/or stromal cells plays an important role in the maturation of other hematopoietic lineages, we propose that the action of M-CSF in the differentiation of immature monocytes/macrophages includes stimulated expression of the integrins alpha 4 beta 1 and alpha 5 beta 1, leading to interactions with components of the marrow microenvironment necessary for cell maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Kahn A. J., Teitelbaum S. L. Defective binding of macrophages to bone in rodent osteomalacia and vitamin D deficiency. In vitro evidence for a cellular defect and altered saccharides in the bone matrix. J Clin Invest. 1983 Aug;72(2):526–534. doi: 10.1172/JCI111000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelmez S. H., Sacca R., Stanley E. R. Lineage specific receptors used to identify a growth factor for developmentally early hemopoietic cells: assay of hemopoietin-2. J Cell Physiol. 1985 Mar;122(3):362–369. doi: 10.1002/jcp.1041220305. [DOI] [PubMed] [Google Scholar]
- Bednarczyk J. L., Szabo M. C., McIntyre B. W. Post-translational processing of the leukocyte integrin alpha 4 beta 1. J Biol Chem. 1992 Dec 15;267(35):25274–25281. [PubMed] [Google Scholar]
- Campbell A. D., Long M. W., Wicha M. S. Haemonectin, a bone marrow adhesion protein specific for cells of granulocyte lineage. Nature. 1987 Oct 22;329(6141):744–746. doi: 10.1038/329744a0. [DOI] [PubMed] [Google Scholar]
- Clohisy D. R., Bar-Shavit Z., Chappel J. C., Teitelbaum S. L. 1,25-Dihydroxyvitamin D3 modulates bone marrow macrophage precursor proliferation and differentiation. Up-regulation of the mannose receptor. J Biol Chem. 1987 Nov 25;262(33):15922–15929. [PubMed] [Google Scholar]
- Coulombel L., Vuillet M. H., Leroy C., Tchernia G. Lineage- and stage-specific adhesion of human hematopoietic progenitor cells to extracellular matrices from marrow fibroblasts. Blood. 1988 Feb;71(2):329–334. [PubMed] [Google Scholar]
- Dalton S. L., Marcantonio E. E., Assoian R. K. Cell attachment controls fibronectin and alpha 5 beta 1 integrin levels in fibroblasts. Implications for anchorage-dependent and -independent growth. J Biol Chem. 1992 Apr 25;267(12):8186–8191. [PubMed] [Google Scholar]
- De Nichilo M. O., Burns G. F. Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate alpha v integrin expression on cultured human macrophages. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2517–2521. doi: 10.1073/pnas.90.6.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Holers V. M., Ruff T. G., Parks D. L., McDonald J. A., Ballard L. L., Brown E. J. Molecular cloning of a murine fibronectin receptor and its expression during inflammation. Expression of VLA-5 is increased in activated peritoneal macrophages in a manner discordant from major histocompatibility complex class II. J Exp Med. 1989 May 1;169(5):1589–1605. doi: 10.1084/jem.169.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B., McIntyre B. W., Weissman I. L. Identification of a murine Peyer's patch--specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989 Jan 13;56(1):37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Imschenetzky M., Puchi M., Pimentel C., Bustos A., Gonzales M. Immunobiochemical evidence for the loss of sperm specific histones during male pronucleus formation in monospermic zygotes of sea urchins. J Cell Biochem. 1991 Sep;47(1):1–10. doi: 10.1002/jcb.240470102. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerst J. M., Sanders J. B., Slaper-Cortenbach I. C., Doorakkers M. C., Hooibrink B., van Oers R. H., von dem Borne A. E., van der Schoot C. E. Alpha 4 beta 1 and alpha 5 beta 1 are differentially expressed during myelopoiesis and mediate the adherence of human CD34+ cells to fibronectin in an activation-dependent way. Blood. 1993 Jan 15;81(2):344–351. [PubMed] [Google Scholar]
- Kerst J. M., Sanders J. B., Slaper-Cortenbach I. C., Doorakkers M. C., Hooibrink B., van Oers R. H., von dem Borne A. E., van der Schoot C. E. Alpha 4 beta 1 and alpha 5 beta 1 are differentially expressed during myelopoiesis and mediate the adherence of human CD34+ cells to fibronectin in an activation-dependent way. Blood. 1993 Jan 15;81(2):344–351. [PubMed] [Google Scholar]
- Kim Y. R., Abraham N. G., Lutton J. D. Mechanisms of differentiation of U937 leukemic cells induced by GM-CSF and 1,25(OH)2 vitamin D3. Leuk Res. 1991;15(6):409–418. doi: 10.1016/0145-2126(91)90050-4. [DOI] [PubMed] [Google Scholar]
- Kodama H., Nose M., Niida S., Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991 May 1;173(5):1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine F. M., Dedhar S., Lima G. M., Eaves C. J. Transformation-associated alterations in interactions between pre-B cells and fibronectin. Blood. 1990 Dec 1;76(11):2311–2320. [PubMed] [Google Scholar]
- Long M. W., Briddell R., Walter A. W., Bruno E., Hoffman R. Human hematopoietic stem cell adherence to cytokines and matrix molecules. J Clin Invest. 1992 Jul;90(1):251–255. doi: 10.1172/JCI115844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi A., Alvarez J., Greenfield E. M., Teti A., Grano M., Colucci S., Zambonin-Zallone A., Ross F. P., Teitelbaum S. L., Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991 Oct 25;266(30):20369–20374. [PubMed] [Google Scholar]
- Mufson R. A. Induction of immediate early response genes by macrophage colony-stimulating factor in normal human monocytes. J Immunol. 1990 Oct 1;145(7):2333–2339. [PubMed] [Google Scholar]
- Nojima Y., Humphries M. J., Mould A. P., Komoriya A., Yamada K. M., Schlossman S. F., Morimoto C. VLA-4 mediates CD3-dependent CD4+ T cell activation via the CS1 alternatively spliced domain of fibronectin. J Exp Med. 1990 Oct 1;172(4):1185–1192. doi: 10.1084/jem.172.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., O'Shea K. S., Campbell A. D., Wicha M. S., Long M. W. Fetal expression of hemonectin: an extracellular matrix hematopoietic cytoadhesion molecule. Blood. 1990 Jan 15;75(2):357–364. [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Byrne M., Krane S. M. 1,25-Dihydroxyvitamin D3 induces collagen binding to the human monocyte line U937. J Clin Invest. 1987 Oct;80(4):962–969. doi: 10.1172/JCI113189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Stimulation of macrophage tumoricidal activity by the growth and differentiation factor CSF-1. Cell Immunol. 1987 Apr 1;105(2):270–279. doi: 10.1016/0008-8749(87)90076-1. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Rosemblatt M., Vuillet-Gaugler M. H., Leroy C., Coulombel L. Coexpression of two fibronectin receptors, VLA-4 and VLA-5, by immature human erythroblastic precursor cells. J Clin Invest. 1991 Jan;87(1):6–11. doi: 10.1172/JCI115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. H., Nuccie B. L., Abboud C. N., Winslow J. M. Vascular cell adhesion molecule-1 and the integrin VLA-4 mediate adhesion of human B cell precursors to cultured bone marrow adherent cells. J Clin Invest. 1991 Sep;88(3):995–1004. doi: 10.1172/JCI115403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson-Johannes A., Carlino J. A. Enhancement of human monocyte tumoricidal activity by recombinant M-CSF. J Immunol. 1988 Nov 15;141(10):3680–3686. [PubMed] [Google Scholar]
- Sheppard D., Cohen D. S., Wang A., Busk M. Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem. 1992 Aug 25;267(24):17409–17414. [PubMed] [Google Scholar]
- Simmons P. J., Masinovsky B., Longenecker B. M., Berenson R., Torok-Storb B., Gallatin W. M. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood. 1992 Jul 15;80(2):388–395. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J. Methods for the purification, assay, characterization and target cell binding of a colony stimulating factor (CSF-1). J Immunol Methods. 1981;42(3):253–284. doi: 10.1016/0022-1759(81)90156-3. [DOI] [PubMed] [Google Scholar]
- Symington B. E. Fibronectin receptor modulates cyclin-dependent kinase activity. J Biol Chem. 1992 Dec 25;267(36):25744–25747. [PubMed] [Google Scholar]
- Teixidó J., Hemler M. E., Greenberger J. S., Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992 Aug;90(2):358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S., Patel V., Beaumont E., Lodish H. F., Nathan D. G., Sieff C. A. Differential binding of erythroid and myeloid progenitors to fibroblasts and fibronectin. Blood. 1987 Jun;69(6):1587–1594. [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Verfaillie C. M., McCarthy J. B., McGlave P. B. Differentiation of primitive human multipotent hematopoietic progenitors into single lineage clonogenic progenitors is accompanied by alterations in their interaction with fibronectin. J Exp Med. 1991 Sep 1;174(3):693–703. doi: 10.1084/jem.174.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillet-Gaugler M. H., Breton-Gorius J., Vainchenker W., Guichard J., Leroy C., Tchernia G., Coulombel L. Loss of attachment to fibronectin with terminal human erythroid differentiation. Blood. 1990 Feb 15;75(4):865–873. [PubMed] [Google Scholar]
- Weinstein R., Riordan M. A., Wenc K., Kreczko S., Zhou M., Dainiak N. Dual role of fibronectin in hematopoietic differentiation. Blood. 1989 Jan;73(1):111–116. [PubMed] [Google Scholar]
- Williams D. A., Rios M., Stephens C., Patel V. P. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991 Aug 1;352(6334):438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]