Abstract
Aims
We reported that patients with small aortic valve area (AVA) and low flow despite preserved left ventricular ejection fraction (LVEF), i.e. ‘paradoxical’ low flow (PLF), have worse outcomes compared with patients with normal flow (NF), although they generally have a lower mean gradient (MG). The aortic valve weight (AVW) excised at the time of valve replacement is a flow-independent marker of stenosis severity. The objective of this study was to compare the AVW of patients with PLF and MG<40 mmHg with the AVW of patients with NF and MG≥40 mmHg.
Methods and results
We recruited 250 consecutive patients undergoing valve replacement (Cohort A) for severe stenosis. Among them, 33 (13%) were in PLF [LVEF > 50% but stroke volume index (SVi) ≤ 35 mL/m2] with MG < 40 mmHg (PLF-LG group) and 105 (42%) were in NF (LVEF > 50% and SVi > 35 mL/m2) with MG ≥ 40 mmHg (NF-HG group). Despite a much lower MG (29 ± 7 vs. 53 ± 10 mmHg; P < 0.0001), patients in the PLF-LG group had a similar AVA (0.73 ± 0.12 vs. 0.69 ± 0.13; P = 0.19) compared with those in the NF-HG group. The AVW [median (interquartile): 1.90 (1.63–2.50) vs. 2.60 (1.66–3.32)] and prevalence of bicuspid phenotype (15 vs. 42%) were lower in the PLF-LG group than in the NF-HG group. However, AVWs analysed separately in the tricuspid and bicuspid valves were similar in both groups [tricuspid valves: 1.80 (1.63–2.50) vs. 2.30 (1.58–3.00) g; P = 0.26 and bicuspid valves: 2.72 (1.73–3.61) vs. 2.60 (2.10–3.55) g; P = 0.93]. When using cut-point values of AVW established in another series of non-consecutive patients (n = 150, Cohort B) with NF and concordant Doppler-echocardiographic findings, we found that the percentage of patients with evidence of severe stenosis in Cohort A was 70% in patients with PLF-LG and 86% in patients with NF-HG.
Conclusion
The aortic valve weight data reported in this study provide evidence that a large proportion of patients with PLF and low-gradient have a severe stenosis and that the gradient may substantially underestimate stenosis severity in these patients. A multi-parametric approach including all Doppler-echocardiographic parameters of valve function as well as other complementary diagnostic tests may help correctly identify these patients.
Keywords: Aortic stenosis, Low flow, Echocardiography, Surgery
Introduction
A previous paradigm was that patients with severe aortic stenosis (AS) and preserved left ventricular ejection fraction (LVEF) should necessarily have a high transvalvular gradient. However, we previously reported that a substantial proportion of patients with severe AS on the basis of aortic valve area (AVA) (i.e.≤1.0 cm2 and indexed AVA ≤ 0.6 cm2/m2) may develop a restrictive physiology and myocardial dysfunction resulting in lower LV outflow and thus often lower than expected transvalvular gradients despite the presence of a preserved LVEF, and this clinical entity was labelled ‘paradoxical’ low-flow (PLF) AS. Several studies reported that patients with PLF AS have a 40–50% lower referral to aortic valve replacement (AVR) compared with those with the more classical normal-flow (NF), high-gradient pattern of AS, likely due to underestimation of stenosis severity in light of the relatively low gradient.1–3 Yet, these patients generally have worse prognosis compared with those with NF and high gradient.1,2,4,5 A sub-study from the SEAS trial, however, suggested that patients with PLF AS have similar outcomes compared with those with moderate AS and NF.6 Hence, there are some uncertainties and debates regarding the actual severity of the stenosis and the relevance of AVR in the subset patients with PLF low-gradient (PLF-LG) AS. The weight of the valve explanted at the time of AVR has been proposed as a flow-independent marker that can be used a posteriori to corroborate stenosis severity.7,8
The objectives of this study were thus: (i) to determine the most appropriate cut-point values of the aortic valve weight (AVW) to identify haemodynamically severe stenosis in men and women; (ii) to compare the AVW in patients with PLF-LG AS vs. those with normal flow and high gradient (NF-HG), i.e. patients with known severe AS; and (iii) to assess AS severity of patients with PLF-LG AS with the use of the AVW.
Methods
Among 250 consecutive patients undergoing AVR (Cohort A), we analysed the clinical, Doppler-echocardiographic, natriuretic peptides, and AVW data in patients with PLF-LG and patients with NF-HG. The primary indication for surgery was the presence of severe AS documented by an AVA ≤ 1.0 cm2 and indexed AVA ≤ 0.6 cm2/cm2. Patients with a history of rheumatic disease, endocarditis, and inflammatory diseases as well as patients with aortic valve regurgitation, mitral valve regurgitation, or stenosis grade > mild were excluded.
We also analysed the Doppler-echocardiographic and AVW data in a group of 150 non-consecutive patients (Cohort B) with moderate or severe AS, normal LVEF, and NF undergoing AVR ± coronary artery bypass graft surgery. This cohort served as a reference group to determine the optimal cut-point values of AVW for the identification of haemodynamically severe AS.
Clinical data
Clinical data included age, gender, documented diagnosis of hypertension [patients receiving antihypertensive medications or having known, but untreated, hypertension (blood pressure ≥140/90 mm Hg)], obesity (body mass index ≥30 kg/m2), dyslipidaemia (patients receiving cholesterol lowering medication or, in the absence of such medication, documentation of plasma LDL cholesterol level >6.2 mmol/L), diabetes (fasting glucose ≥7 mmol/L), and coronary heart disease (history of myocardial infarction, coronary artery stenosis on preoperative, or previous coronary angiography). The aortic valve phenotype (i.e. bicuspid vs. tricuspid) was assessed by the surgeon at the time of AVR.
Laboratory data
Blood samples were drawn before surgery and plasma NT pro-BNP was determined by a commercially available enzyme-linked immunosorbent assay (USCN Life Science Inc., Wuhan, China). Creatinine was also measured.
Doppler echocardiography
All patients underwent a comprehensive Doppler-echocardiographic exam within 3 months of AVR. Left ventricular dimensions were measured according to the recommendations of the American Society of Echocardiography. Left ventricular ejection fraction was measured with the use of the Simpson biplane method. Doppler echocardiographic measurements included the LV stroke volume, AVA using the continuity equation, and transvalvular gradients using the modified Bernoulli equation. We paid particular attention to search for the highest peak transvalvular velocity with the use of multi-window continuous-wave Doppler interrogation. The Doppler-echocardiographic measurement of LV outflow tract stroke volume was corroborated by the 2D volumetric method.
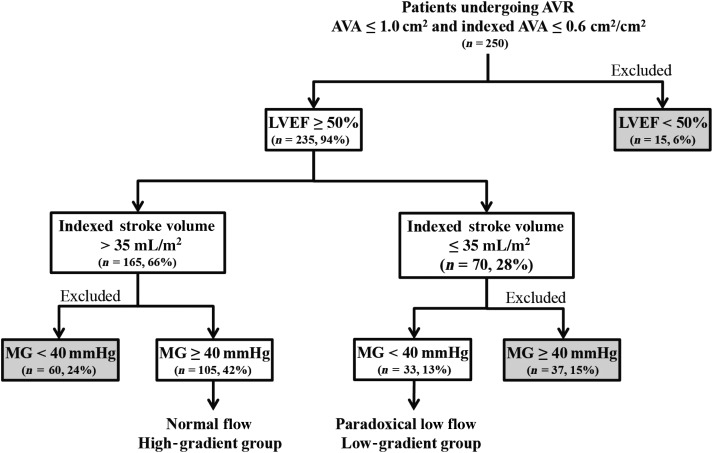
From patients in Cohort A (250 patients, 164 men, 86 women), we identified 105 patients with normal LVEF (≥50%), NF [stroke volume index (SVi) > 35 mL/m2] and high mean gradient (MG) (≥40 mmHg) (NF-HG group) and 33 patients with normal LVEF, low flow (SVi ≤ 35 mL/m2) and low MG (<40 mmHg) (PLF-LG group) (Figure 1).
Figure 1.
Flow chart for study Cohort A. AVA, aortic valve area; LVEF, LV ejection fraction; MG, mean gradient; AVR, aortic valve replacement.
Systemic arterial compliance was calculated as the ratio of SVi to the pulse pressure. As a measure of global LV afterload, we calculated the valvulo-arterial impedance by dividing the sum of systolic blood pressure and mean transvalvular gradient by the SVi.
All patients in Cohort B had normal LVEF (≥50%) and flow (SVi > 35 mL/m2). Among the 150 patients in this cohort, 127 (88 men and 39 women) underwent AVR for severe AS defined as AVA ≤ 1 cm2, indexed AVA ≤ 0.6 cm2/m2, and MG ≥ 40 mmHg, and 23 (15 men and 8 women) underwent coronary artery bypass grafting (CABG) with concomitant AVR of moderate AS defined as AVA>1 cm2, AVA > 0.6 cm2/m2, and MG <40 mmHg.
Weight of explanted aortic valves
Each valve excised at the time of surgery was analysed by one of three pathologists. As part of an ongoing research protocol (PROGRESSA Study, ClinicalTrials.gov Identifier: NCT01679431), two cusps (or one cusp if bicuspid valve) were placed in a container filled with HEPES solution and one cusp was placed in RNA later (Ambion Inc., Austin, TX, USA) for subsequent analyses. The cusps and small fragments (if any) in HEPES solution were removed from the container, placed on absorbing paper, and then weighed the same laboratory scale that has an accuracy of ±0.01 g. The weight of the cusp placed in RNA later was obtained by subtracting the known weight of the empty container and RNA later solution from the measured total weight of the container + solution + cusp. The total AVW was then determined by adding the weights of the cusps/fragments in HEPES and in RNA later. We used the data of Cohort B to determine the optimal cut-point values of AVW to identify severe AS and we then applied these cut-point values in Cohort A to corroborate the severity of AS.
Statistical analysis
Results are expressed as mean ± SD, median (interquartile), or percentages when appropriate. Continuous variables were tested for normality by the Shapiro–Wilk test. Aortic valve weight, NT pro-BNP, and creatinine were not normally distributed. We used a natural logarithm transformation to normalize AVW and NT pro-BNP. Differences between NF-HG and PLF-LG groups were analysed with the use of t-test or Wilcoxon rank sum test for continuous variables as appropriate, and the Chi-square test or the Fisher exact tests for categorical variables as appropriate. All subsequent analyses of AVW or NT pro-BNP as continuous variables were performed with the use of logarithm-transformed variables. The relationship between AVW and echocardiographic parameters of stenosis severity was assessed with the use of the Pearson correlation coefficient. Receiver operating characteristic (ROC) curves were used to determine the best cut-point values of AVW to identify severe AS. A P-value <0.05 was considered statistically significant. Statistical analyses were performed with the use of JMP 9.0.0 software.
Results
Preoperative data (Cohort A)
There was no difference in baseline clinical characteristics between the NF-HG and PLF-LG groups, except for the prevalence of dyslipidaemia and coronary artery disease that were higher in PLF-LG patients (Table 1). Notwithstanding a normal LVEF, stroke volume and flow rate were lower in the PLF-LG patients than in the NF-HG patients. Patients in the PLF-LG group also had smaller LV end-diastolic volume index and LV mass index. As expected, patients in the PLF-LG group had lower gradients despite a similar AVA and indexed AVA compared with patients in the NF-HG group. The valvulo-arterial impedance was higher in the PLF-LG group than in the NF-HG group (Table 1). Although they had a lower gradient, patients in PLF-LG had a similar (P = 0.82) level of NT pro-BNP compared with those in the NF-HG group [863 (584–1224) vs. 778 (535–1295); respectively], even after adjustment for coronary artery disease and creatinine level (P = 0.21).
Table 1.
Preoperative and operative data in patients with normal-flow, high-gradient (NF-HG) and those with paradoxical low-flow, low-gradient (PLF-LG) aortic stenosis
| PLF-LG group (n = 33) | NF-HG group (n = 105) | P-value | |
|---|---|---|---|
| Clinical data | |||
| Age, years | 72 ± 8 | 69 ± 10 | 0.12 |
| Male gender | 21 (64) | 64 (61) | 0.78 |
| Height, m | 1.68 ± 0.09 | 1.64 ± 0.10 | 0.18 |
| Weight, kg | 80 ± 20 | 76 ± 14 | 0.15 |
| Body surface area, m2 | 1.9 ± 0.3 | 1.8 ± 0.2 | 0.13 |
| Body mass index, kg/m2 | 28.3 ± 5.6 | 27.8 ± 4.2 | 0.59 |
| Systolic blood pressure, mmHg | 125 ± 19 | 127 ± 18 | 0.80 |
| Diastolic blood pressure, mmHg | 73 ± 11 | 74 ± 10 | 0.96 |
| Hypertension, n (%) | 26 (79) | 74 (70) | 0.34 |
| Dyslipidaemia, n (%) | 33 (100) | 86 (82) | 0.0008 |
| Diabetes, n (%) | 12 (36) | 28 (27) | 0.29 |
| Obesity, n (%) | 11 (33) | 29 (28) | 0.53 |
| Coronary artery disease, n (%) | 19 (58) | 37 (36) | 0.03 |
| Laboratory data | |||
| NT-proBNP, pg/mL median (25–75%) | 863 (584–1224) | 778 (535–1295) | 0.82 |
| Creatinine, µmol/L median (25–75%) | 95 (80–105) | 86 (70–98) | 0.04 |
| Doppler-echocardiographic data | |||
| Aortic annulus diameter, mm | 20.7 ± 1.5 | 21.4 ± 2.0 | 0.07 |
| LV end-diastolic diameter index, mm/m2 | 23.7 ± 3.0 | 25.1 ± 3.4 | 0.04 |
| LV end-diastolic volume index, mL/m2 | 48 ± 9 | 53 ± 14 | 0.06 |
| Interventricular septum thickness, mm | 12.4 ± 1.7 | 13.6 ± 2.6 | 0.02 |
| Left ventricular posterior wall thickness, mm | 10.5 ± 2.1 | 12.0 ± 2.3 | 0.003 |
| LV mass index, g/m2 | 98 ± 24 | 123 ± 33 | 0.0002 |
| Relative wall thickness ratio | 0.52 ± 0.09 | 0.57 ± 0.13 | 0.04 |
| LV ejection fraction, % | 63 ± 8 | 64 ± 8 | 0.57 |
| Stroke volume, mL | 57 ± 10 | 76 ± 11 | <0.0001 |
| Stroke volume index, mL/m2 | 30 ± 4 | 42 ± 5 | <0.0001 |
| Transvalvular flow rate, mL/s | 186 ± 36 | 240 ± 42 | <0.0001 |
| Peak aortic jet velocity, cm/s | 348 ± 49 | 463 ± 45 | <0.0001 |
| Peak gradient, mmHg | 49 ± 11 | 86 ± 17 | <0.0001 |
| Mean gradient, mmHg | 29 ± 7 | 53 ± 10 | <0.0001 |
| Doppler velocity index | 0.22 ± 0.04 | 0.19 ± 0.04 | 0.003 |
| Aortic valve area, cm2 | 0.73 ± 0.12 | 0.69 ± 0.13 | 0.19 |
| Indexed aortic valve area, cm2/m2 | 0.39 ± 0.06 | 0.38 ± 0.07 | 0.56 |
| Systemic arterial compliance, mL/mmHg/m2 | 0.61 ± 0.05 | 0.82 ± 0.22 | 0.0005 |
| Valvulo-arterial impedance, mmHg/mL/m2 | 5.1 ± 0.7 | 4.4 ± 0.1 | 0.0001 |
| Operative data | |||
| Concomitant CABG, n (%) | 18 (55) | 37 (35) | 0.05 |
| Bicuspid valve, n (%) | 5 (15) | 44 (42) | 0.003 |
| Aortic valve weight, g | |||
| Whole cohort | 1.90 (1.63–2.50) | 2.60 (1.66–3.32) | 0.03 |
| Men | 2.30 (1.80–2.55) | 3.00 (2.30–3.72) | 0.001 |
| Women | 1.60 (1.27–2.03) | 1.60 (1.30–2.40) | 0.81 |
| Tricuspid valve | 1.80 (1.63–2.50) | 2.30 (1.58–3.00) | 0.26 |
| Bicuspid valve | 2.72 (1.73–3.61) | 2.60 (2.10–3.55) | 0.93 |
Operative data (Cohort A)
The AVW was similar (P = 0.28) in patients who underwent concomitant CABG [2.30 (1.55–2.80) g] vs. those who did not [2.40 (1.63–3.30) g]. There was a strong trend (P = 0.05) for higher proportion of CABG in the PLF-LG patients (55%) compared with NF-HG patients (35%).
The AVW was higher (P = 0.004) in bicuspid vs. tricuspid valves [2.60 (2.10–3.55) vs. 2.02 (1.60–2.68) g respectively]. The proportion of patients with bicuspid aortic valve was higher (P = 0.003) in the NF-HG group (42%) than in the PLF-LG group (15%) (Table 1).
The AVW was significantly (P = 0.02) higher in the NF-HG group compared with the PLF-LG group but with important overlap between the two groups (Table 1 and Figure 2A). After further dichotomization by gender, the difference in AVW remains significant in men [NF-HG: 3.00 (2.30–3.72) vs. PLF-LG: 2.30 (1.80–2.55) g; P = 0.001] but not in women [NF-HG: 1.60 (1.30–2.40) vs. PLF-LG: 1.60 (1.27–2.03) g; P = 0.82] (Figure 2A). However, after dichotomization by the bicuspid/tricuspid valve phenotype, the difference in AVW did not reach the significance in both bicuspid [NF-HG: 2.60 (2.10–3.55) vs. PLF-LG: 2.72 (1.73–3.61) g; P = 0.93] and tricuspid [NF-HG: 2.30 (1.55–3.00) vs. PLF-LG: 1.80 (1.63–2.50); P = 0.26] valve subsets (Figure 2B). After adjustment for gender (P < 0.0001) and bicuspid phenotype (P = 0.001), the AVW was similar in the NF-HG and PLF-LG groups (P = 0.16). Furthermore, NT pro-BNP levels were similar in the PLF-LG vs. NF-HG groups in the whole cohort as well as in the subsets of men and women (Figure 3).
Figure 2.
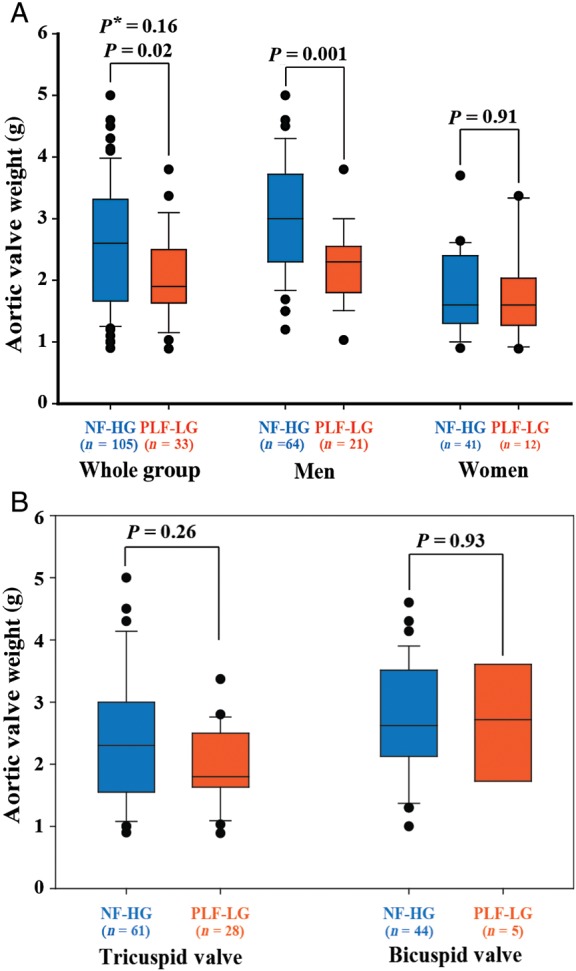
Comparison of aortic valve weight between patients with paradoxical low-flow, low-gradient (PLF-LG), and those with normal-flow high gradient (NF-HG). (A) The data in the whole Cohort A and in the subsets of men and women. (B) The data in patients with bicuspid valve and those with tricuspid valve. P* is adjusted for gender and bicuspid phenotype.
Figure 3.
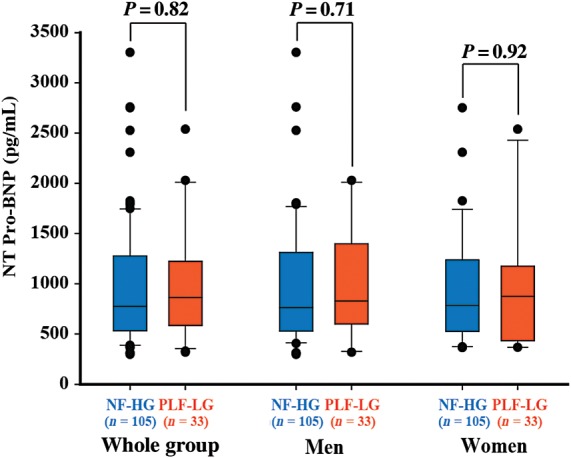
Comparison of NT pro-BNP between patients with paradoxical low-flow low gradient (PLF-LG), and normal-flow high gradient (NF-HG) in the whole Cohort A and in the subsets of men and women.
Identification of severe aortic stenosis with the use of aortic valve weight (Cohort B)
The AVW correlated with the parameters of stenosis severity in the whole Cohort B (n = 150) as well as in men (n = 103) and women (n = 47) (all r ≥ |0.40|; all P ≤ 0.0002) (Table 2). Table 3 presents the area under the ROC curve (AUC) for the AVW as well as the best cut-point values and their sensitivity and specificity to differentiate severe from moderate AS. The best accuracy to identify severe AS was obtained with an AVW ≥2.0 g for men and ≥1.2 g for women.
Table 2.
Correlation between logarithm of aortic valve weight and hemodynamic markers of aortic stenosis severity in a cohort of 150 patients with normal flow (Cohort B)
| Mean gradient |
Peak gradient |
Peak aortic jet velocity |
Indexed aortic valve area |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Whole cohort (n = 150) | 0.50 | <0.0001 | 0.54 | <0.0001 | 0.46 | <0.0001 | −0.41 | <0.0001 |
| Men (n = 103) | 0.54 | <0.0001 | 0.59 | <0.0001 | 0.54 | <0.0001 | −0.40 | <0.0001 |
| Women (n = 47) | 0.52 | 0.0002 | 0.61 | <0.0001 | 0.57 | <0.0001 | −0.60 | <0.0001 |
Table 3.
Accuracy and criteria of aortic valve weight to identify severe aortic stenosis in patients with normal left ventricular outflow (Cohort B)
| Aortic valve weight, g | AUC (95%CI) | Cut-off | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) |
|---|---|---|---|---|
| Men | 0.94 (0.89–0.98) | 2.0 | 86 (77–93) | 93 (68–100) |
| Women | 0.90 (0.89–0.98) | 1.2 | 85 (70–94) | 88 (47–100) |
AUC, area under the curve; 95% CI, 95% confidence interval.
Confirmation of severe aortic stenosis based on aortic valve weight (Cohort A)
When applying the cut-off values of AVW predetermined in Cohort B to the patients of Cohort A, 19 (70%) of PLF-LG patients and 63 (86%) of NF-HG patients had evidence of severe AS on the basis of AVW (P = 0.08) (Figure 4). When this analysis was dichotomized by gender, the percentage of severe AS was 89% in men with NF-HG vs. 65% in men with PLF-LG (P = 0.03) and 81% in women with NF-HG vs. 80% in women with PLF-LG (P = 0.92) (Figure 4).
Figure 4.
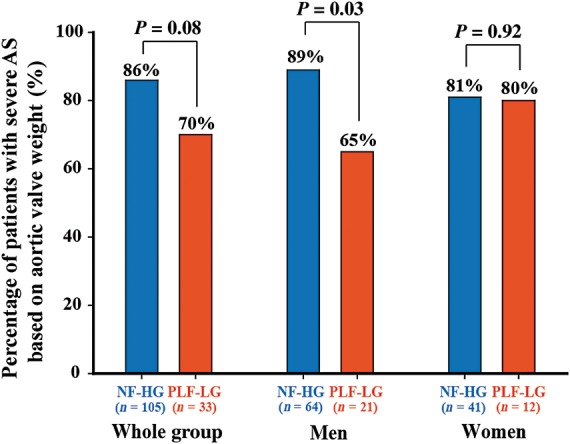
Comparison of the percentage of patients with evidence of severe aortic stenosis as documented by aortic valve weight (AVW>1.2 g in women and >2.0 g in men) in the patients with paradoxical low-flow, low-gradient (PLF-LG) and those with normal-flow and high-gradient (NF-HG) of the Cohort A.
Discussion
The main findings of this study are: (i) patients with PLF-LG AS have lower AVW compared with patients with NF-HG. However, after adjustment for gender and bicuspid valve phenotype, the difference between the two groups is no longer significant. (2) Among patients with PLF-LG, 70% have evidence of severe AS on the basis of AVW and this percentage is similar to that of patients with NF and high gradient (86%). These findings suggest that the gradient (or peak aortic jet velocity) may underestimate stenosis severity in patients with PLF-LG and may thus lead to underutilization or inappropriate delay of AVR in some patients. The low flow state may indeed result in pseudo-normalization of the transvalvular gradient despite the presence of severe AS.1,9 In this regard, the present study reveals that 13% of the patients had a PLF-LG and the analysis of excised valve weight independently corroborated the presence of severe stenosis in 70% of these patients. However, the significant difference in AVW observed in men between PLF LG and NF HG groups, as well as the significant lower proportion of severe AS observed in men (65%) vs. women (80%) according to the cut-off value of AVW suggests that identification of true severe AS may be more difficult in men than in women in the presence of PLF LG.
Nevertheless, the results of this study are consistent with the conclusion that a large proportion of patients with PLF-LG have a severe AS, when using AVW as a reference. And these findings thus lend support to the recommendation (Class IIa, Evidence C) included in the 2012 ESC/EACTS guidelines, which states that AVR should be considered in symptomatic patients with PLF-LG AS after careful confirmation of AS severity.10
The patients with PLF-LG included in this study presented the typical Doppler-echocardiographic features reported in previous studies,1,4,9,11,12 including small LV cavity size, reduced arterial compliance, and increased LV global hemodynamic load as reflected by high valvulo-arterial impedance. Other recent studies1,4,11,12 also revealed that, compared with patients with NF and high gradient, patients with PLF and low-gradient have more myocardial fibrosis and a markedly reduced LV longitudinal systolic function, which is not evidenced by LVEF. All these factors contribute to the reduced LV outflow and transvalvular gradient and to the worse outcomes observed in these patients.1,4,5,11,12
The presence of a low gradient in the context of a preserved LVEF may lead to underestimation of AS severity and therefore underutilization or inappropriate delay of AVR. Yet, several retrospective or prospective studies reported that these patients generally have better survival when treated surgically than conservatively, even after adjustment for differences in the baseline risk profile.5,9,13–17 However, a sub-study of the SEAS trial6 reported that patients with severe AS on the basis of AVA<1.0 cm2 but having a low gradient (<40 mmHg) despite preserved LVEF have similar outcomes compared with those with moderate AS, thereby suggesting that these patients may not benefit of AVR. As highlighted in subsequent publications,4,18 this subset of patients with discordant AVA-gradient findings is highly heterogeneous and includes patients with PLF-LG AS as well as patients with measurements errors, small body size, and/or inconsistency in the AVA/gradient criteria proposed in the guidelines to define severe stenosis.18
These observations further underline the importance of ruling out measurement errors and making meticulous differential diagnosis when confronted to discordance between AVA (small) and gradient (low) in patients with preserved LVEF. As emphasized in the recent ESC-EACTS guidelines,10 patients with PLF should receive a particular attention and undergo further investigation to confirm stenosis severity.
The main advantage of the AVW measured at AVR is that it provides a flow-independent measure of stenosis severity. And in this regard, the correlations we obtained between AVW and parameters of stenosis severity in the present study were similar to those reported by Roberts and Ko7 and the best correlations were obtained with the peak gradient. However, the main limitation of the AVW from a clinical standpoint is that this parameter can only be used a posteriori, i.e. after AVR. Quantification of aortic valve calcification by multidetector computed tomography (MDCT) could be useful to identify the PLF patients who likely have a severe stenosis despite the presence of a low gradient. In this regard, Cueff et al.19 initially reported that a valve calcium score >1650 Agatston Unit (AU) provides the best accuracy to identify severe AS. However, a recent study also suggested that different cut-point values of MDCT valve calcium scores should be applied in women (≥1274 AU) vs. men (≥2065 AU),20 which is consistent with the AVW results reported in the present study. A recent multicentre study also demonstrated the usefulness of stress echocardiography for the confirmation of stenosis severity in patients with PLF-LG AS. Further studies are needed to confirm the usefulness of MDCT and stress echocardiography in patients with PLF-LG AS.21
Lancellotti et al.4 reported that patients with PLF-LG AS have higher plasma BNP levels compared with those with NF low-gradient but similar levels compared with those with NF high-gradient. Consistently in the present study, there was also no significant difference in the NT-proBNP levels between the PLF-LG and NF-HG groups (Table 1 and Figure 3). This finding might be related to the fact that BNP is secreted in response to myocardial stretch and that for any given level of pressure overload, LV wall tension is generally lower in patients with smaller LV cavities, such as in patients with PLF. Since BNP synthesis is also influenced by LV geometry and function as well as the presence of concomitant hypertension and coronary artery disease, this biomarker may have limited utility to corroborate stenosis severity in patients with PLF-LG AS.
Study limitations
All patients in this study underwent AVR, which necessarily introduced a treatment bias. In particular, the PLF patients referred to AVR are those who had substantial evidence of severe AS on the basis of the different diagnostic tests performed prior to operation. Hence, the patients' baseline characteristics as well as the prevalence of non-severe, i.e. ‘pseudo-severe’ reported in this study may not necessarily reflect that observed in the general population with PLF-LG AS.
The AVW may underestimate the stenosis severity in patients with a small aortic annulus and overestimate the severity in patients with a large annulus. The utilization of different cut-point values of AVW in men vs. women has partially contributed to overcome this limitation. A more robust option would have been to index the AVW for the cross-sectional area of the aortic annulus after excision of the valve. However, the intraoperative measurement of the aortic annulus with an independent sizer was available only in a small proportion of the patients included in this study.
The present study was focused on PLF-LG AS. However, a substantial proportion of patients has a small AVA, a NF but a low-gradient (Figure 1). Recent studies suggest that this normal-flow, low-gradient entity is highly heterogeneous and includes patients with measurements errors, small body size, and discordances due to inconsistencies in the guidelines criteria.22–24 These studies also suggest that this entity is generally associated with better prognosis compared with PLF-LG AS. Nonetheless, further studies are needed to determine what is the proportion of patients with normal-flow, low-gradient AS who really have severe AS and who may thus benefit from AVR.
Conclusion
These AVW data provide evidence that a large proportion of patients with PLF-LG have a severe stenosis and that the gradient may substantially underestimate stenosis severity in these patients. These findings emphasize the importance to correctly identify patients with PLF AS and to confirm stenosis severity in those having a low gradient. A multi-parametric evaluation including all Doppler-echocardiographic parameters of valve function as well as other complementary diagnostic tests, such as stress echocardiography or valve calcium quantification by MDCT might help identify the patients with PLF-LG severe AS who may benefit of AVR. However, further studies are needed to validate this approach and to assess the impact of AVR on the outcome of these patients.
Funding
This study was funded by a grant (# MOP-57745 and #MOP-126072) from the Canadian Institutes of Health Research (CIHR), Ottawa, Ontario, Canada. P.P. holds the Canada Research Chair in Valvular Heart Diseases supported by CIHR, Ottawa, Ontario, Canada. M.-A.C. was supported by a Vanier Canada Graduate Scholarship from CIHR and currently holds a postdoctoral fellowship from CIHR . P.M. is a research scholar from the Fonds de Recherches en Santé du Québec, Montreal, Canada.
Conflict of interest: none declared.
References
- 1.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 2.Barasch E, Fan D, Chukwu EO, Han J, Passick M, Petillo F, Norales A, Reichek N. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis. 2008;17:81–88. [PubMed] [Google Scholar]
- 3.Malouf J, Le TT, Pellikka P, Sundt TM, Scott C, Schaff HV, Enriquez-Sarano M. Aortic valve stenosis in community medical practice: determinants of outcome and implications for aortic valve replacement. J Thorac Cardiovasc Surg. 2012;144:1421–1427. doi: 10.1016/j.jtcvs.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 4.Lancellotti P, Magne J, Donal E, Davin L, O'Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis. Insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–243. doi: 10.1016/j.jacc.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 5.Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–1267. doi: 10.1016/j.jacc.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Skjaerpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Barwolf C. Outcome of patients with low-gradient ‘severe’ aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 7.Roberts WC, Ko JM. Relation of weights of operatively excised stenotic aortic valves to preoperative transvalvular peak systolic pressure gradients and to calculated aortic valve areas. J Am Coll Cardiol. 2004;44:1847–1855. doi: 10.1016/j.jacc.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 8.Côté N, Couture C, Pibarot P, Després JP, Mathieu P. Angiotensin receptor blockers are associated with lower remodelling of stenotic aortic valves. Eur J Clin Invest. 2011;41:1172–1179. doi: 10.1111/j.1365-2362.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 9.Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. doi: 10.1093/eurheartj/ehp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De BM, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann S, Stork S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G, Weidemann F. Low-gradient aortic valve stenosis: myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, Sportouch-Dukhan C, Réant P, Laffitte S, Cade S, Le Dolley Y, Thuny F, Avierinos JF, Lancellotti P, Habib G. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle tracking echocardiography: A multicenter study. Circ Cardiovasc Imaging. 2012;5:27–35. doi: 10.1161/CIRCIMAGING.111.967554. [DOI] [PubMed] [Google Scholar]
- 13.Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg. 2008;86:1781–1789. doi: 10.1016/j.athoracsur.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Tarantini G, Covolo E, Razzolini R, Bilato C, Frigo AC, Napodano M, Favaretto E, Fraccaro C, Isabella G, Gerosa G, Iliceto S, Cribier A. Valve replacement for severe aortic stenosis with low transvalvular gradient and left ventricular ejection fraction exceeding 0.50. Ann Thorac Surg. 2011;91:1808–1815. doi: 10.1016/j.athoracsur.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 15.Ozkan A, Hachamovitch R, Kapadia SR, Tuzcu EM, Marwick TH. Impact of aortic valve replacement on outcome of symptomatic patients with severe aortic stenosis with low gradient and preserved left ventricular ejection fraction. Circulation. 2013;128:622–631. doi: 10.1161/CIRCULATIONAHA.112.001094. [DOI] [PubMed] [Google Scholar]
- 16.Eleid MF, Nishimura RA, Sorajja P, Borlaug BA. Systemic hypertension in low gradient severe aortic stenosis with preserved ejection fraction. Circulation. 2013;128:1349–1353. doi: 10.1161/CIRCULATIONAHA.113.003071. [DOI] [PubMed] [Google Scholar]
- 17.Mohty D, Magne J, Deltreuil M, Aboyans V, Echahidi N, Cassat C, Pibarot P, Laskar M, Virot P. Outcome and impact of surgery in paradoxical low-flow, low-gradient severe aortic stenosis and preserved left ventricular ejection fraction: a cardiac catheterization study. Circulation. 2013;128:S235–S242. doi: 10.1161/CIRCULATIONAHA.112.000031. [DOI] [PubMed] [Google Scholar]
- 18.Dumesnil JG, Pibarot P. Letter by Dumesnil and Pibarot regarding article, ‘Outcome of patients with low-gradient ‘severe’ aortic stenosis and preserved ejection fraction. Circulation. 2011;124:e360. doi: 10.1161/CIRCULATIONAHA.111.038497. [DOI] [PubMed] [Google Scholar]
- 19.Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A, Messika-Zeitoun D. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–726. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 20.Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal S, Malouf J, Araoz P, Michelena H, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 21.Clavel MA, Ennezat PV, Maréchaux S, Dumesnil JG, Capoulade R, Hachicha Z, Mathieu P, Bellouin A, Bergeron S, Meimoun P, Arsenault M, Le Tourneau T, Pasquet A, Couture C, Pibarot P. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. J Am Coll Cardiol Img. 2013;6:175–183. doi: 10.1016/j.jcmg.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. doi: 10.1016/j.jacc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Mehrotra P, Jansen K, Flynn AW, Tan TC, Elmariah S, Picard MH, Hung J. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur Heart J. 2013;34:1906–1914. doi: 10.1093/eurheartj/eht094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. doi: 10.1161/CIRCULATIONAHA.113.003695. [DOI] [PMC free article] [PubMed] [Google Scholar]