Abstract
Introduction
Patients with inflammatory bowel diseases (IBD) have an increased risk of clostridium difficile infection (CDI). Cathelicidins are anti-microbial peptides that attenuate colitis and inhibit the effect of clostridial toxins. Plasma 25(OH)D stimulates production of cathelicidins.
Aim
To examine the association between plasma 25(OH)D and CDI in patients with IBD
Methods
From a multi-institutional IBD cohort, we identified patients with at least one measured plasma 25(OH)D. Our primary outcome was development of CDI. Multivariate logistic regression models adjusting for potential confounders were used to identify independent effect of plasma 25(OH)D on risk of CDI.
Results
We studied 3,188 IBD patients whom 35 patients developed CDI. Patients with CDI-IBD were older and had greater co-morbidity. The mean plasma 25(OH)D level was significantly lower in patients who developed CDI (20.4ng/ml) compared to non-CDI IBD patients (27.1ng/mL) (p=0.002). On multivariate analysis, each 1ng/ml increase in plasma 25(OH)D was associated with a 4% reduction in risk of CDI (OR 0.96, 95% CI 0.93 – 0.99, p = 0.046). Compared t o individuals with vitamin D > 20ng/mL, patients with levels < 20ng/mL were more likely to develop CDI (OR 2.27, 95% CI 1.16 – 4.44). The mean plasma 25(OH)D in patients with CDI who subsequently died was significantly lower (12.8+8.1ng/ml) compared to those who were alive at the end of follow-up (24.3+13.2ng/ml) (p=0.01).
Conclusion
Higher plasma 25(OH)D is associated with reduced risk of C difficile infection in patients with IBD. Further studies of therapeutic supplementation of vitamin D in IBD-CDI patients may be warranted.
Keywords: Crohn’s disease, ulcerative colitis, Clostridium difficile, vitamin D
INTRODUCTION
Clostridium difficile infection (CDI) is an important cause of morbidity and mortality in patients with inflammatory bowel diseases (IBD; Crohn’s disease (CD), ulcerative colitis (UC))1–5. It is associated with four-fold excess mortality, and increased need for hospitalization, emergency room visits, and colectomy up to 5 years after the initial episode3, 5–7. Both age and comorbidity are recognized risk factors for CDI. However, compared to non-IBD patients with CDI, CDI-IBD patients are often younger and have fewer predisposing non-IBD related co-morbidities 5, 8. Furthermore, extrinsic risk factors identified in the general population such as antibiotic or proton pump inhibitor (PPI) use are less frequently reported in CDI-IBD patients. This suggests that other mechanisms may exist to explain the association between the two conditions1, 9.
There is growing interest in the immunologic role of vitamin D beyond its well recognized effects on bone and mineral metabolism10–14. Mice deficient in vitamin D or with knockout of the vitamin D receptor (VDR) demonstrate greater susceptibility to chemical models of colitis that can be ameliorated through administration of 1,25-dihydroxy cholecalciferol (1,25(OH)2D3)12, 15. An emerging body of literature supports a role for vitamin D deficiency in the pathogenesis16 as well as natural history of IBD13, 17, 18 through its effect on innate immunity. Given the important role of innate immunity in antimicrobial defense and the effect of vitamin D on pathogen sensing and other innate immune responses,10–12, 15 it is conceivable that vitamin D deficiency may play a role in susceptibility to CDI. Indeed it was recently demonstrated that cathelicidin, an antimicrobial peptide whose production is stimulated by vitamin D, inhibits the effect of C difficile toxin19. Furthermore, a prior study from our group examining genetic risk factors for CDI demonstrated an association with a single nucleotide polymorphism (SNP) on chromosome 7 associated with CREB5, a member of the cyclic AMP responsive element-binding protein family associated with expression of the cathelicidin promoter sequence 20, 21.
Consequently, we performed this study to examine whether plasma 25-hydroxy vitamin D [25(OH)D] is associated with risk of subsequent CDI in a large multi-institutional cohort of patients with CD and UC. Identifying such an association will provide important insights into pathogenesis of CDI as well as suggest a role for vitamin D in the treatment of CDI.
METHODS
Study Population
The data source for our study was a multi-institutional cohort of patients with established CD or UC. The establishment of our cohort has been described in previous publications6, 17, 22, 23. In brief, we utilized electronic medical record (EMR) data from patients seeking care at one of two tertiary referral hospitals, Massachusetts General Hospital and Brigham and Women’s Hospital, in the greater Boston metropolitan area serving a population of over 3 million people. All patients with an International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) code for CD (555.x) or UC (556.x) were identified from the EMR (n = 24,182). A classification algorithm was developed using codified data or narrative free text concepts identified through natural language processing. Our final algorithm had high specificity and positive predictive value of 97%, and yielded a cohort of 5,506 patients who were classified as having CD and 5,522 classified as having UC22.
Variables and Outcomes
This study included 3,188 patients who had at least one measured plasma 25(OH)D as part of their routine clinical care. As described previously, patients with measured plasma 25(OH)D were similar in age, but more likely to be female, use immunosuppressive therapy, or require hospitalization for their IBD17, 23. Plasma 25(OH)D was measured using radioimmunoassay prior to 2008 and high-performance liquid chromatography with mass spectrometry since then with similar ranges for normal values across both time periods and acceptable coefficients of variance (7.5%–12.5%).
Our primary outcome of interest was the development of CDI identified through the presence of the appropriate ICD-9-CM code (008.45). The ICD-9-CM code for CDI has been validated previously, and extensively used to define burden of disease and secular trends in infection6, 24–26. Plasma 25(OH)D values measured after the diagnosis of C difficile were excluded from the analysis.
Information was obtained on age at entry into our cohort, gender, race, and co-morbidity (prior to CDI or end of follow-up) assessed using the widely used and validated Charlson co-morbidity index27. The type of IBD as well as use of immunosuppressive or corticosteroids for treatment of disease was ascertained using the electronic prescription function of our EMR. Corticosteroid use was defined as a prescription within 90 days prior to the diagnosis of CDI or the end of follow-up. We also assessed the use of antibiotics, statins, and proton-pump inhibitors within 90 days prior to development of CDI or end of follow-up, as well as hospitalization within 90 days prior to development of CDI.
Statistical Analysis
All analysis was performed using Stata SE 12.1 (StataCorp, College Station, TX). Continuous variables were summarized using means and standard deviations and compared using t-tests, while categorical variables were expressed as proportions and compared using the chi-square tests with fisher’s exact modification when appropriate. Plasma 25(OH)D was modeled as a continuous variable in our primary analysis. In secondary analyses, it was model as a dichotomous (> 20ng/ml; < 20ng/mL) or a stratified (<20ng/ml, 20–29.9ng/ml, > 30ng/ml) variable. Univariate logistic regression with development of CDI as the outcome was performed, and variables significant in the univariate analysis at p < 0.10 were selected for inclusion in the final multivariate model where a 2-sided p-value < 0.05 indicated independent statistical significance. To examine if the effect of vitamin D was mediated through its association with disease severity, we additionally adjusted for C-reactive protein or erythrocyte sedimentation rates where available. To account for the fact that patients who have their vitamin D level measured may be sicker than those who do not have an available vitamin D measure, we additional constructed a propensity score estimate the likelihood a plasma 25(OH)D vitamin D was obtained. Though our study population included only patients with at least 1 measured vitamin D level, we additional adjusted for this propensity score in a sensitivity analysis to examine the robustness of our results.
Ethical approval was obtained from the Institutional Review Board of Partners Healthcare.
RESULTS
Our study included 3,188 patients with CD or UC who had at least one measured plasma 25(OH)D among whom 35 patients developed C difficile infection (CDI). Table 1 presents the characteristics of included patients with and without a history of CDI. Patients who developed CDI were older (mean age 60.5 years vs. 48.7 years) and more likely to have a Charlson co-morbidity index > 3. There was no difference in race, gender, or IBD type between the two groups. Patients with IBD-CDI nearly universally had a history of hospitalization within 90 days prior to C difficile infection, or had used antibiotic therapy. Immunosuppression (immunomodulator or anti-TNF biologics) was more common among those with CDI, while recent prescription of steroids was less frequent in those with CDI. The mean plasma 25(OH)D level was significantly lower in patients who developed CDI (20.4ng/ml) compared to non-CDI IBD patients (27.1ng/mL) (p=0.002) (Figure 1). Examining vitamin D status as a dichotomous variable, levels below 20ng/mL were associated with a two-fold increase in risk of CDI (Odds ratio(OR) 2.27, 95% confidence interval (CI) 1.16 – 4.44). The median interval between the measured plasma 25(OH)D and CDI in the case population was 228 days (interquartile range (IQR) 56 days – 752 days).
Table 1.
Characteristics of patients with inflammatory bowel disease, stratified by diagnosis of clostridium difficile infection
| Characteristic | With C difficile infection (n = 35) % |
Without C difficile infection (n = 3,153) % |
p-value |
|---|---|---|---|
| Mean age (SD) (in years) |
60.5 (16.9) | 48.7 (18.0) | 0.0001 |
| Female | 63 | 61 | 0.80 |
| Non-white | 11 | 13 | 0.82 |
| Charlson co-morbidity | < 0.001 | ||
| 0–2 | 8 | 47 | |
| ≥ 3 | 94 | 53 | |
| IBD type | 0.47 | ||
| Ulcerative colitis | 51 | 45 | |
| Crohn’s disease | 49 | 55 | |
| Recent hospitalization or surgery† |
95 | 55 | 0.009 |
| Antibiotic use† | 95 | 75 | 0.009 |
| Statin† | 31 | 17 | 0.02 |
| Steroids† | 29 | 53 | 0.003 |
| Proton pump inhibitor† |
63 | 48 | 0.08 |
| Immunosuppressive therapy |
20 | 7 | 0.003 |
| Mean plasma 25(OH)D (SD) (in ng/ml) |
20.4 (12.8) | 27.1 (12.7) | 0.002 |
- within 90 days of C difficile infection for cases
Figure 1.
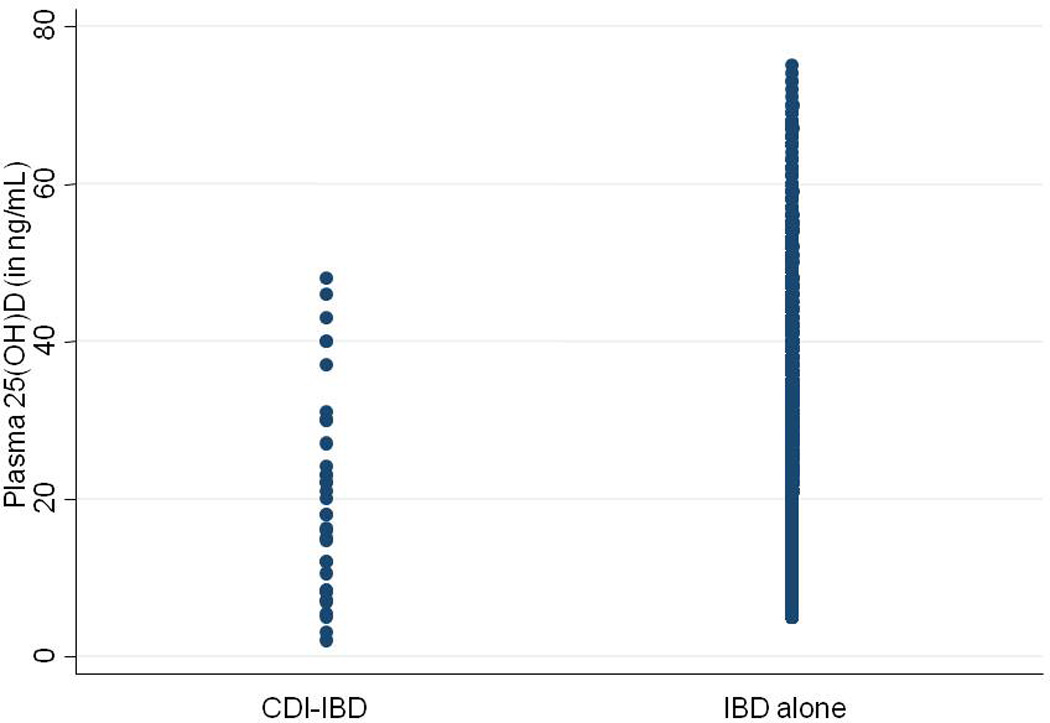
Plasma 25-hydroxy vitamin D levels in patients with inflammatory bowel disease, stratified by history of clostridium difficile infection
We performed multivariate analysis examining predictors of CDI (Table 2). Plasma 25(OH)D level was an independent predictor of CDI after adjusting for potential confounders. Each 1ng/ml increase in plasma 25(OH)D was associated with a 4% reduction in risk of CDI (OR 0.96, 95% CI 0.93 – 0.99, p = 0.046). Greater co-morbidity, recent hospitalization, and immunosuppressive use were also associated with increased risk of CDI while steroid use was inversely associated with disease risk. Antibiotic proton pump inhibitor, or statin use was not associated with risk of CDI in our cohort. Adjusting for a propensity score estimating likelihood of measuring plasma 25(OH)D did not alter our estimates.
Table 2.
Multivariate analysis of predictors of clostridium difficile infection
| Characteristic | Adjusted odds ratio† | 95% confidence interval |
|---|---|---|
| Charlson co-morbidity | ||
| 0–2 | 1.0 | |
| ≥ 3 | 6.06 | 1.34 – 27.47 |
| Recent surgery or hospitalization |
||
| No | 1.0 | |
| Yes | 8.84 | 1.98 – 39.46 |
| Steroid use | ||
| No | 1.0 | |
| Yes | 0.16 | 0.07 – 0.36 |
| Immunosuppressive use | ||
| No | 1.0 | |
| Yes | 3.82 | 1.46 – 9.99 |
| Serum 25(OH)D | 0.96 | 0.93 – 0.99 |
Additionally adjusted for age, gender, race, season of measurement, IBD type, antibiotic use within 90 days, and duration of follow-up
One-quarter of patients with CDI-IBD (26%) subsequently underwent surgery, and one-third died during follow-up (34%) compared to corresponding rates of 16% (p=0.13) and 5% (p < 0.001) among those without CDI. The risk of surgery was not different by vitamin D status. However, the mean plasma 25(OH)D in patients with CDI who subsequently died was significantly lower (12.8+8.1ng/ml) compared to those who were alive at the end of follow-up (24.3+13.2ng/ml) (p=0.01) (Figure 2).
Figure 2.
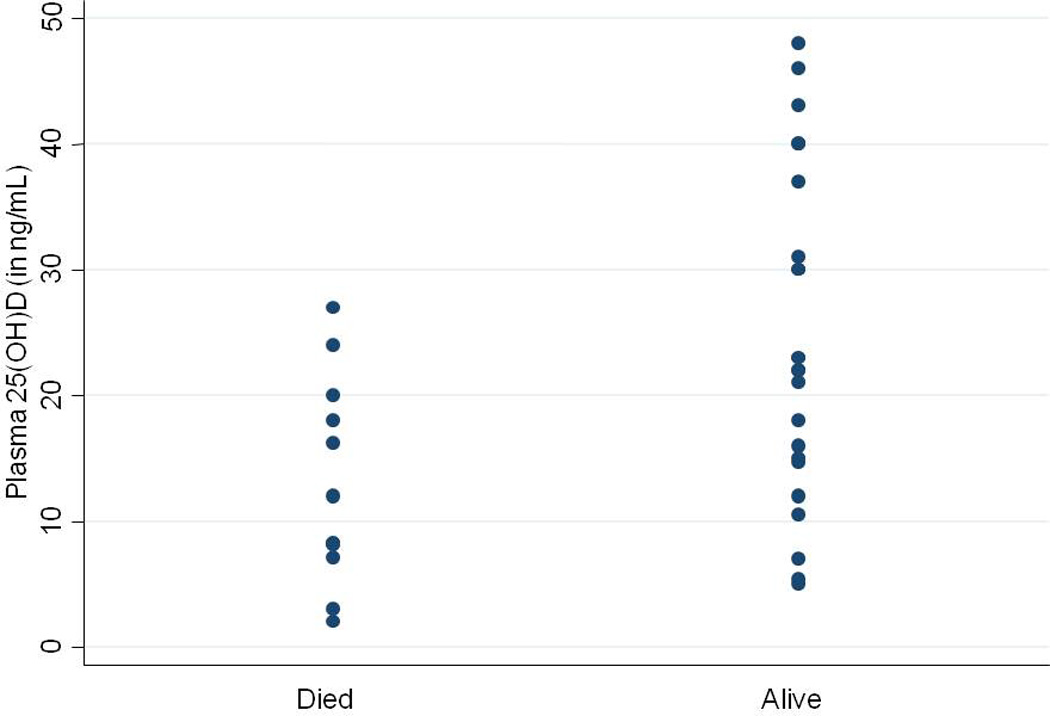
Plasma 25(OH)D and long-term mortality in IBD patients with Clostridium difficile infection
DISCUSSION
Clostridium difficile infection is an important source of morbidity in patients with IBD1–5. The association between IBD and CDI could be related to co-occurrence of risk factors; however, this does not adequately explain disease risk in a large proportion of patients1, 4. Using a large multi-institutional IBD cohort, we demonstrate that higher vitamin D levels were independently associated with a reduced risk for CDI in patients with IBD. Secondly, IBD-CDI patients with low vitamin D levels had higher mortality when compared to those with sufficient levels.
Our findings intriguingly suggest a role for vitamin D in the pathogenesis of CDI, particularly in patients with IBD. Several pieces of experimental evidence support our results. Cathelicidin is a key antimicrobial peptide in mammals that is found at sites of inflammation and is involved in host defense against bacteria and other pathogens20. 1,25(OH)2D3 regulates its production by binding to the vitamin D receptor (VDR), and subsequently through the vitamin D response element (VDRE) at the cathelicidin promoter sequence, thereby regulating its production28. Cathelicidin is expressed widely in neutrophils, natural killer cells, keratinocytes, lymphocytes, dendritic cells, macrophages, as well as intestinal epithelium, and has antibacterial activity against a spectrum of bacterial pathogens20, 29. Intracolonic administration of the mouse cathelicidin is associated with amelioration of colitis30. In an elegant study by Hing et al., wild type and cathelicidin deficient mice were treated with antibiotics and infected orally with C difficile. Exogenously administered cathelicidin was associated with reduced colonic tumor necrosis factor and myeloperoxidase levels and reduced histologic damage in both the colon and ileum19. In a previous study, our group demonstrated an association between a SNP on chromosome 7 associated with the gene CREB5 and increased risk for CDI in UC patients21. The CREB protein competes for binding to the cathelicidin promoter sequence and regulates its expression. In an earlier study, human alpha-defensins inhibited the effect of C difficile toxin B31; adequate levels of 25(OH)D are essential for production of human defensins, further supporting a mechanistic association between 25(OH)D and CDI. Vitamin D and cathelicidin have also been associated with risk of other infections including tuberculosis, influenza A, hepatitis C, and Helicobacter pylori29. Low levels of cathelicidin were associated with higher infection related mortality in patients on hemodialysis32.
A few recent studies have examined this association between vitamin D levels and CDI in non-IBD cohorts33–36. In a study from the Veterans affairs population, Youssef et al. identified a five-fold increase in risk of health care costs associated with CDI in individuals who were deficient in vitamin D compared to those with sufficient levels33. Wang et al. similarly demonstrated that individuals with CDI who were deficient in vitamin D were less likely to resolve infection36, consistent with data from van der Wilden et al. who demonstrated an inverse correlation between severity of CDI and plasma vitamin D levels35. Only one study previously examined vitamin D levels as a risk factor for hospital acquired CDI and identified an increase in risk of CDI in individuals with plasma 25(OH)D levels below 20ng/mL34.
Other independent predictors of CDI in our cohort were higher burden of co-morbid illness and immunosuppressive therapy consistent with prior studies1, 5, 8, 37. Whether immunosuppressive therapy itself confers risk or is a marker for more active colonic disease38 which may be associated with increased risk for CDI is unclear. Adjusting for C-reactive protein levels as a marker of inflammation did not influence the magnitude of association between immunosuppression and CDI suggesting that there may indeed be an elevated risk associated with therapy. Interestingly, we observed an inverse association between corticosteroid use and CDI which is in contrast to prior data37, 39. This may in part be due to the overall greater severity of disease in our cohort with higher frequency of immunosuppression than in some prior studies. As well, use of corticosteroids tends to be intermittent and of varying duration; consequently electronic prescription dates may be inadequate markers of dose and duration of use. Consistent with known epidemiology of CDI, recent hospitalization or surgery remains one of the strongest risk factors for CDI. We observed no association between use of antibiotics, proton pump inhibitors, or statin and risk of CDI. While studies in non-IBD patients have demonstrated the former two to be risk factors40–42, and the latter to be inversely associated with CDI risk43, 44, data from IBD cohorts has not consistently demonstrated independent associations with medication use and risk of CDI1, 9, and our findings confirm this lack of association.
There are a few implications to our findings. In IBD patients, prior studies have demonstrated low predicted plasma 25(OH) vitamin D levels to be associated with increased risk for CD16, greater likelihood of IBD related hospitalizations, surgery13, 17, 45, and increased risk of malignancy, particularly colorectal cancer23. The present study explores another immunologic consequence of vitamin D deficiency in patients with IBD, and suggests that low vitamin D levels may be associated with an increased risk of CDI, an effect possibly mediated through reduced cathelicidin production. A study in patients with eczema demonstrated that high dose oral vitamin D3 is associated with a significant increase in plasma cathelicidin levels46, suggesting that there is merit in future studies examining the role of vitamin D supplementation as an adjunct therapy in CDI-IBD. There is also an important need for studies examining the role of vitamin D in the pathogenesis of CDI in non-IBD cohorts.
There are several limitations to our study. First, the number of patients with a measured plasma 25(OH)D level who subsequently developed CDI was small. Though this is the first and largest study to date examining this association, it is important to replicate this association in additional cohorts. Second, since plasma 25(OH)D was measured as part of routine clinical care, it was not available for all patients. Those with available 25(OH)D levels differed from those without any 25(OH)D available. Third, since our cohorts were based at a tertiary referral center, it is possible that there exists a bias towards more severe disease. However as both the CDI-IBD cases and non-CDI IBD controls were drawn from the same pool, it is unlikely that the bias differentially affects our association. Fourth, it is possible that sicker patients are at higher risk for CDI and as well for vitamin D deficiency owing to reduced physical activity and time spent outdoors. However, that our findings were robust to adjustment for immunosuppressive and steroid use, recent hospitalization, surgery, and co-morbidity suggests that this confounding is unlikely to explain our association. Fifth, the median interval between measured vitamin D and CDI was 228 days. It is possible that in the interim, some individuals who were identified to be deficient received vitamin D supplementation. However, such non-differential misclassification of exposure would bias results towards the null making our estimates more conservative. Finally, it is possible that some patients with CDI were misclassified as controls. However, such misclassification would bias the effect towards the null, making our results a conservative estimate.
In conclusion, we demonstrate that higher vitamin D levels were associated with a reduced risk of CDI in patients with IBD. This suggests a potential role for vitamin D in the pathogenesis of CDI. As well, it raises interesting possibilities of whether vitamin D may have a role as adjunct therapy for CDI. Further studies in IBD and non-IBD cohorts are essential to replicate our findings, investigate the mechanisms of this association, and its implications for patient care.
Acknowledgments
Sources of Funding: The study was supported by NIH U54-LM008748. A.N.A is supported by funding from the American Gastroenterological Association and from the US National Institutes of Health (K23 DK097142). K.P.L. is supported by NIH K08 AR060257 and the Harold and Duval Bowen Fund. E.W.K is supported by grants from the NIH (K24 AR052403, P60 AR047782, R01 AR049880).
Footnotes
Financial conflicts of interest: None
Specific author contributions:
An author may list more than one contribution, and more than one author may have contributed to the same element of the work
Study concept – Ananthakrishnan,
Study design – Ananthakrishnan
Data Collection – Ananthakrishnan, Gainer, Cagan, Cai, Cheng, Churchill, Kohane, Shaw, Liao, Szolovits, Murphy
Analysis – Ananthakrishnan, Cai, Cheng,
Preliminary draft of the manuscript – Ananthakrishnan
Approval of final version of the manuscript – Ananthakrishnan, Gainer, Cagan, Cai, Cheng, Churchill, Kohane, Shaw, Liao, Szolovits, Murphy
REFERENCES
- 1.Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:345–351. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Jen MH, Saxena S, Bottle A, et al. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:1322–1331. doi: 10.1111/j.1365-2036.2011.04661.x. [DOI] [PubMed] [Google Scholar]
- 3.Murthy SK, Steinhart AH, Tinmouth J, et al. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther. 2012;36:1032–1039. doi: 10.1111/apt.12073. [DOI] [PubMed] [Google Scholar]
- 4.Ananthakrishnan AN, Issa M, Binion DG. Clostridium difficile and inflammatory bowel disease. Gastroenterol Clin North Am. 2009;38:711–728. doi: 10.1016/j.gtc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:789–795. doi: 10.1111/j.1365-2036.2012.05022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jodorkovsky D, Young Y, Abreu MT. Clinical Outcomes of Patients with Ulcerative Colitis and Co-existing Clostridium difficile Infection. Dig Dis Sci. 2009 doi: 10.1007/s10620-009-0749-9. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen GC, Kaplan GG, Harris ML, et al. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–1450. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 9.Arif M, Weber LR, Knox JF, et al. Patterns of proton pump inhibitor use in Inflammatory Bowel disease and concomitant risk of Clostridium difficile infection. Gastroenterology. 2007;132:A513. [Google Scholar]
- 10.Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11–20. [PubMed] [Google Scholar]
- 11.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 12.Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 13.Narula N, Marshall JK. Management of inflammatory bowel disease with vitamin D: beyond bone health. J Crohns Colitis. 2012;6:397–404. doi: 10.1016/j.crohns.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Garg M, Lubel JS, Sparrow MP, et al. Review article: vitamin D and inflammatory bowel disease - established concepts and future directions. Aliment Pharmacol Ther. 2012;36:324–344. doi: 10.1111/j.1365-2036.2012.05181.x. [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT, Munsick C, Bemiss C, et al. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 16.Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin d status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012;142:482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Normalization of Plasma 25-Hydroxy Vitamin D Is Associated with Reduced Risk of Surgery in Crohn's Disease. Inflamm Bowel Dis. 2013;19:1921–1927. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–315. doi: 10.1038/ncpgasthep0215. [DOI] [PubMed] [Google Scholar]
- 19.Hing TC, Ho S, Shih DQ, et al. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut. 2013;62:1295–1305. doi: 10.1136/gutjnl-2012-302180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandamme D, Landuyt B, Luyten W, et al. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Ananthakrishnan AN, Oxford EC, Nguyen DD, et al. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment Pharmacol Ther. 2013;38:522–530. doi: 10.1111/apt.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananthakrishnan AN, Cai T, Savova G, et al. Improving Case Definition of Crohn's Disease and Ulcerative Colitis in Electronic Medical Records Using Natural Language Processing: A Novel Informatics Approach. Inflamm Bowel Dis. 2013;19:1411–1420. doi: 10.1097/MIB.0b013e31828133fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananthakrishnan AN, Cheng SC, Cai T, et al. Association Between Reduced Plasma 25-hydroxy Vitamin D and Increased Risk of Cancer in Patients with Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubberke ER, Reske KA, McDonald LC, et al. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis. 2006;12:1576–1579. doi: 10.3201/eid1210.060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmiedeskamp M, Harpe S, Polk R, et al. Use of International Classification of Diseases, Ninth Revision, Clinical Modification codes and medication use data to identify nosocomial Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:1070–1076. doi: 10.1086/606164. [DOI] [PubMed] [Google Scholar]
- 26.Shaklee J, Zerr DM, Elward A, et al. Improving surveillance for pediatric Clostridium difficile infection: derivation and validation of an accurate case-finding tool. Pediatr Infect Dis J. 2011;30:e38–e40. doi: 10.1097/INF.0b013e3182027c22. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty K, Maity PC, Sil AK, et al. cAMP stringently regulates human cathelicidin antimicrobial peptide expression in the mucosal epithelial cells by activating cAMP-response element-binding protein, AP-1, and inducible cAMP early repressor. J Biol Chem. 2009;284:21810–21827. doi: 10.1074/jbc.M109.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 30.Tai EK, Wu WK, Wong HP, et al. A new role for cathelicidin in ulcerative colitis in mice. Exp Biol Med (Maywood) 2007;232:799–808. [PubMed] [Google Scholar]
- 31.Giesemann T, Guttenberg G, Aktories K. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology. 2008;134:2049–2058. doi: 10.1053/j.gastro.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Gombart AF, Bhan I, Borregaard N, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48:418–424. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youssef D, Bailey B, El Abbassi A, et al. Healthcare costs of Staphylococcus aureus and Clostridium difficile infections in veterans: role of vitamin D deficiency. Epidemiol Infect. 2010;138:1322–1327. doi: 10.1017/S0950268809991543. [DOI] [PubMed] [Google Scholar]
- 34.Quraishi SA, Litonjua AA, Moromizato T, et al. Association Between Prehospital Vitamin D Status and Hospital-Acquired Clostridium difficile Infections. JPEN J Parenter Enteral Nutr. 2014 doi: 10.1177/0148607113511991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Wilden GM, Fagenholz PJ, Velmahos GC, et al. Vitamin D Status and Severity of Clostridium difficile Infections: A Prospective Cohort Study in Hospitalized Adults. JPEN J Parenter Enteral Nutr. 2014 doi: 10.1177/0148607113519129. [DOI] [PubMed] [Google Scholar]
- 36.Wang WJ, Gray S, Sison C, et al. Low vitamin D level is an independent predictor of poor outcomes in Clostridium difficile-associated diarrhea. Therap Adv Gastroenterol. 2014;7:14–19. doi: 10.1177/1756283X13502838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeweiss S, Korzenik J, Solomon DH, et al. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther. 2009;30:253–264. doi: 10.1111/j.1365-2036.2009.04037.x. [DOI] [PubMed] [Google Scholar]
- 38.Powell N, Jung SE, Krishnan B. Clostridium difficile infection and inflammatory bowel disease: a marker for disease extent? Gut. 2008;57:1183–1184. author reply 1184. [PubMed] [Google Scholar]
- 39.Issa M, Weber LR, Skaros S, et al. Decreasing rates of colectomy despite high rates of hospitalization in C difficie. infection IBD patients: A tertiary referral center experience. Gastroenterology. 2007;132:A663. [Google Scholar]
- 40.Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. Cmaj. 2004;171:33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. Jama. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 42.Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17–26. doi: 10.1038/nrgastro.2010.190. [DOI] [PubMed] [Google Scholar]
- 43.Nseir W, Bishara J, Mograbi J, et al. Do statins protect against the development of Clostridium difficile-associated diarrhoea? J Antimicrob Chemother. 2013;68:1889–1893. doi: 10.1093/jac/dkt101. [DOI] [PubMed] [Google Scholar]
- 44.Motzkus-Feagans CA, Pakyz A, Polk R, et al. Statin use and the risk of Clostridium difficile in academic medical centres. Gut. 2012;61:1538–1542. doi: 10.1136/gutjnl-2011-301378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph AJ, George B, Pulimood AB, et al. 25 (OH) vitamin D level in Crohn's disease: association with sun exposure & disease activity. Indian J Med Res. 2009;130:133–137. [PubMed] [Google Scholar]
- 46.Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


