Abstract
The current obesity epidemic has affected even the youngest children in our societies, including those in the first months of life. Animal experiments suggest that the early postnatal period may be critical to development of healthful energy homeostasis and thus prevention of obesity. In humans, observational studies and follow-up of randomized feeding trials show that rapid weight gain in the first half of infancy predicts later obesity and higher blood pressure. Despite the mounting consistency of results, several questions remain to be answered before clinical or public health implications are clear. These include the need for body composition data in infancy and data from the developing world to identify modifiable determinants of gain in adiposity in the early weeks of life, to mount interventions to modify these determinants, to examine tradeoffs of more vs. less rapid weight gain for different outcomes, and to incorporate any interventions that prove to be efficacious into clinical and public health practice in a cost-effective manner.
Obesity is now the most burdensome and costly nutritional condition worldwide. Childhood obesity not only presages adult obesity, diabetes, and heart disease, but it is also harmful to the child [1]. Overweight and obese children are at higher risk for developing asthma and orthopedic problems, they have worse cardiometabolic risk profiles, and they suffer psychosocial adversity. Once obesity is present, tenacious physiological processes resist weight loss [2]. By age 5 years, childhood obesity is fairly resistant to change throughout the remainder of childhood [3]. For these reasons, early childhood prevention of obesity is a key to avoiding myriad health problems. But how early in childhood?
Infancy is a period of rapid growth in stature and in neurocognitive, motor, and social development. Weight gain in the first 6 months is primarily gain in fat, whereas fat-free mass accumulates preferentially after that age [4]. Organs and systems are in developmentally plastic stages in which they may be particularly sensitive to perturbations. For example, in rat experiments from almost half a century ago, modification of energy intake in the first weeks of life had lifelong effects on weight gain even if normal energy intake was restored afterwards [5]. In contrast, energy reduction later in life had only a transient effect on weight gain. In a more recent rat model, administration of leptin postnatally abolished the otherwise permanent offspring metabolic effects of prenatal maternal energy restriction [6]. These and other animal experiments raise the possibility that the early postnatal period may be critical to development of healthful energy homeostasis and thus prevention of obesity and related conditions.
These issues are brought into sharper focus by the fact that the current obesity epidemic has affected even the youngest children in our societies. In a large study from a US-managed care population, from the early 1980s to the early 21st century the prevalence of overweight and obesity among 0- to 6-month-old infants rose from 10.4% to 17.0% [7]. Increases in older infants and preschoolers were more modest. Thus, questions naturally arise about infancy as a key period for development of obesity and its consequences.
Several studies now address the role of growth during infancy as a predictor of later adiposity. In 2005, for example, Baird et al. [8] published a systematic review of 10 studies that assessed the relation of infant weight gain with subsequent obesity. Relative risks of later obesity ranged from 1.17 to 5.70 among infants with more rapid weight gain in the first year of life. Associations were consistent for obesity at different ages and for people born over a period from 1927 to 1994.
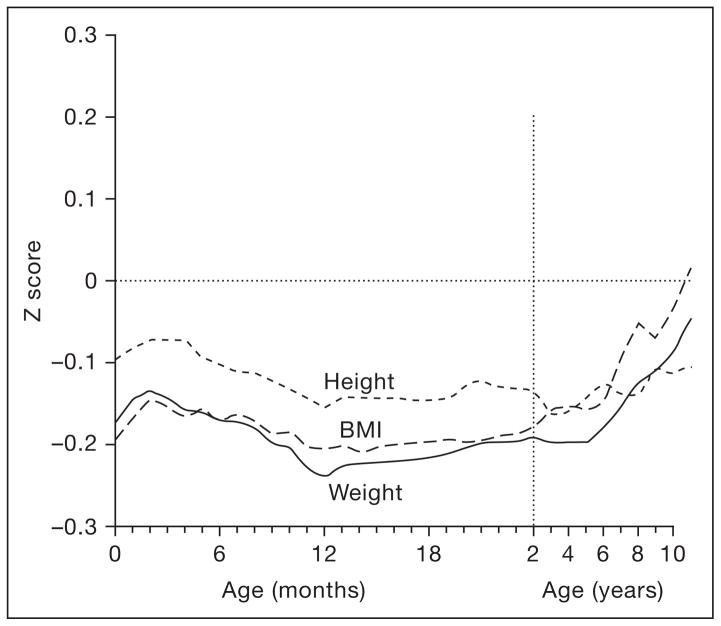
Since 2005, more observational studies have appeared, some with measured adiposity outcomes, not just body mass index (BMI). Yliharsila et al. [9] measured body composition with an 8-polar bioimpedance system among almost 2,000 Finnish adult men and women whose weights and heights were available from child welfare and school records. Gain in BMI from birth to age 1 year, or 1 to 2, was associated with later lean, but not fat, mass. The authors did not subdivide the first year of life further, but inspection of the published figures in Barker et al. [10] from the same cohort gives the impression that the BMI of Finnish men who eventually developed coronary heart disease increased in the first ~3 months before decreasing (fig. 1).
Fig. 1.
Mean z scores for height, weight, and body mass index in the first 11 years after birth among boys who had coronary heart disease as adults. The mean values for all boys are set at zero, with deviations from the mean expressed as standard deviations (z scores). Reproduced with permission from Barker et al. [10].
Among several hundred French boys and girls, Botton et al. [11] showed that weight gain velocity after the age of 3 years predicts fat and fat-free mass in adolescence, as measured by a foot-to-foot bioimpedance device. In that study, weight gain velocity at 3 and 6 months predicted adolescent fat mass better than weight gain velocity at 1 or 2 years. The data from the French cohort agree that weight gain velocity at age 1 or 2 years is a poor predictor of later fat mass.
Earlier data from the Finns [10] suggest that increasing BMI over the entire period from birth to 2 years does not predict higher (and may actually predict lower) risk of coronary heart disease as an adult, and data from Delhi on risk of impaired glucose intolerance appear to agree [12].
However, as in the Finnish cohort, early infancy in the Delhi cohort appeared to be a special period: gain in BMI in the first 6 months was related to both BMI and sum of skinfolds in adulthood [13].
Among 234 British 4- to 20-year-olds, Chomtho et al. [14] examined associations of early weight gain with fat mass and fat-free mass measured by the gold standard four-compartment model. They found that weight gain in the first 3 months of life predicted both fat mass and fat-free mass, weight gain from 3 to 6 months predicted fat mass only, and weight gain from 6 to 12 months predicted neither. Weight gain in the early months also predicted centrally deposited fat as indicated by waist circumference and (less so) by trunk fat mass estimated from dual X-ray absorptiometry.
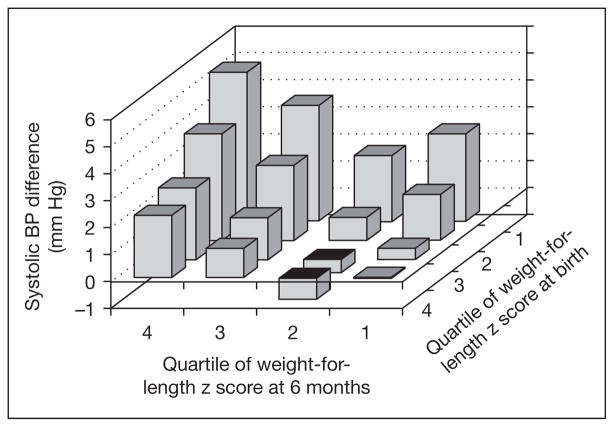
Some data are emerging on relations of infant weight gain with cardiometabolic risk factors. In the US cohort study Project Viva, gain in weight-for-length from 0 to 6 months predicted not only higher BMI and sum of skinfolds, but also blood pressure at age 3 years [15]. In a recent study from the UK’s Barry Caerphilly cohort, a steeper trajectory of weight gain in the first 5 months of life predicted higher blood pressure in adulthood [16]. In both of these studies, birthweight was inversely related to blood pressure level, in agreement with many other studies [17]. In the Viva cohort, the effect of infant growth on 3-year blood pressure was more pronounced in infants born small-for-dates (fig. 2), but no similar effect modification by fetal growth was evident for BMI and skinfold outcomes. In the SWEDES study, weight gain from 0 to 6 months predicted not only adiposity but also a metabolic risk score at age 17. Gain from 3 to 6 years did not predict this cluster of metabolic risk factors [18].
Fig. 2.
Predicted difference in systolic blood pressure at age 3 years according to quartile of weight-for-length z score at birth and age 6 months, adjusted for child age, sex, height, and blood pressure measurement conditions, and maternal income, education, race, ethnicity, and smoking status. Reproduced with permission from Belfort et al. [15].
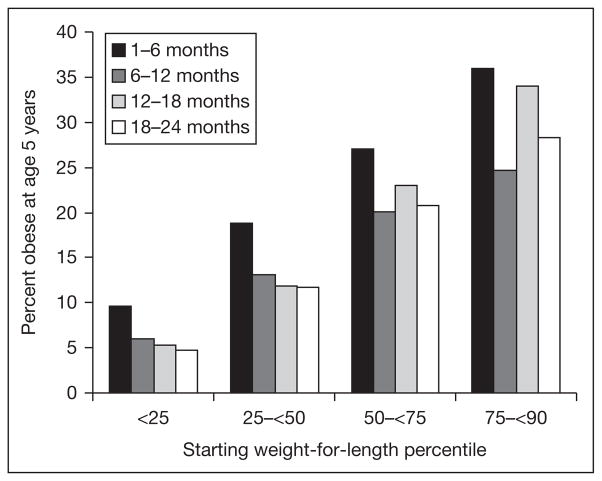
Some other studies also indicate that weight gain in the first half of infancy is more predictive of later obesity than is weight gain later in infancy or childhood. For example, in a formula-only fed population, Stettler et al. [19] showed that weight gain in the 1st week of life was directly associated with overweight in adulthood. In a cohort culled from electronic medical records of well-child visits in a managed care organization, we recently observed that upward crossing of 2 major weight-for-length centiles in the first 6 months was both common and predicted a high risk of obesity 5 years later. Upward crossing from 6 to 12, 12 to 18, or 18 to 24 months was less common and less predictive (fig. 3).
Fig. 3.
Prevalence of obesity for boys at age 5 years (BMI >95th percentile) predicted by crossing upwards two major percentile lines on the CDC growth charts from 1 to 6, 6 to 12, 12 to 18, and 18 to 24 months of age. Unpublished data from HMO Nutrition Surveillance System.
As reported elsewhere in this volume, Lucas and Singhal have published a series of observational follow-up studies of a subset of participants in feeding trials of premature infants. The findings suggest that weight gain in the first few weeks is directly associated with adolescent blood pressure and plasma insulin and leptin [20]. In more recent trials, term small-for-gestational age infants randomized to energy-enriched formula had more rapid weight gain from 0 to 9 months and higher fat mass and diastolic blood pressure at age 6–8 years [21].
Thus mounting evidence suggests that the first few months are critical for development of obesity and its related health conditions. This observation reveals a number of research imperatives:
The need for longitudinal body composition measures during infancy (the exposure period). Most studies have employed only weight measurements. Using weight-for-length is an improvement if length is measured by research standards [22]. Even the addition of length, however, is suboptimal. While weight and length are relevant for clinical decision making, relying on these as exposures in research studies does not provide sufficient information to investigate mechanism and determinants. For example, part of weight gain during infancy comprises lean mass rather than fat mass. Whether rapid increase in lean mass predicts adverse outcomes as well as fat mass is not known. Also in most of the existing literature, representation from developing countries, where stunting and wasting are still prevalent, is limited. The same is true for lower-income and racial/ethnic minority populations in western societies. More data are required to determine if, and why, associations differ across these and other populations.
The need to identify the modifiable determinants of gain in adiposity in the early weeks of life that also underlie long-term risks of obesity-related sequelae. Some determinants of infancy weight gain may predict later obesity, others not. Often we assume that mode of infant feeding must explain any association of infant weight gain with later obesity, but this assumption is not necessarily true. In Project Viva, while longer breastfeeding duration was associated with lower prevalence of obesity at age 3, this effect did not appear to be mediated by weight gain in the first 6 months (unpubl.). Also, in a seeming paradox, breastfeeding results in faster weight gain in the first few months than formula feeding; only later in infancy do breastfed infants have lower weights [23]. Perhaps overfeeding due to lack of responsiveness to infants’ satiety cues is more germane than just breast vs. bottle. It is also plausible that prenatal factors could play a role, factors such as maternal smoking, gestational weight gain, alterations in glucose-insulin homeostasis, or other nutrient-hormonal adaptations in the maternal-placental-fetal unit [24]. A preliminary analysis from Project Viva shows that gestational diabetes, as well as umbilical cord blood leptin concentration, is associated with less rapid gain in weight-for-length from birth to 6 months [25].
Once determinants are identified, the need to mount interventions to modify these determinants. As the nutritional, hormonal or other pathways that lead to harmful levels of weight gain are likely to be complex, so must any interventions to modify them take these complexities into account. In addition, interventions that improve some health outcomes may not do the same for others (see next section).
The need to examine tradeoffs of more vs. less rapid weight gain for different outcomes. At least among infants born preterm, more rapid weight gain in early infancy predicts better neurocognitive outcomes in childhood [26, 27]. Whether this same situation holds with term infants is less clear [28]. Thus, the amount of weight gain that optimizes both neurocognitive and cardiometabolic risk may differ by gestational age.
The need to educate clinicians, policy makers, and parents about the findings from these studies. It will not be enough to have pediatric clinicians identify rapid gainers from the usual growth charts, because the proper response is not yet known. For example, attempting to modify energy intake or expenditure among infants who are entrained by prenatal hormonal or genetic pathways to gain weight on a certain trajectory may cause at least as much harm as good. Should effective interventions be identified, a further challenge will be to incorporate such interventions into clinical and public health practice in a cost-effective manner.
‘How big should my baby be?’ is a question on the mind of most parents. Researchers, clinicians, and the public health community need to be able to answer that question. But they also need to address the follow-up challenge of how to achieve this optimal size for each infant. The answers to these questions hold great promise for prevention of obesity and related health outcomes.
Acknowledgments
This study was supported by a grant from NIH (K24 HL 068041).
References
- 1.Daniels SR. The consequences of childhood overweight and obesity. Future Child. 2006;16:47–67. doi: 10.1353/foc.2006.0004. [DOI] [PubMed] [Google Scholar]
- 2.Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 2008;32(suppl 7):S98–S108. doi: 10.1038/ijo.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner DS, Hosking J, Metcalf BS, et al. Contribution of early weight gain to childhood overweight and metabolic health: a longitudinal study (EarlyBird 36) Pediatrics. 2009;123:e67–e73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuis JD, Roemmich JN, Richmond EJ, et al. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27:101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 5.Widdowson EM, McCance RA. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond B Biol Sci. 1963;158:329–342. doi: 10.1098/rspb.1963.0051. [DOI] [PubMed] [Google Scholar]
- 6.Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes. 2007;14:17–22. doi: 10.1097/MED.0b013e328013da48. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Peterson KE, Scanlon KS, et al. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity (Silver Spring) 2006;14:1107–1112. doi: 10.1038/oby.2006.126. [DOI] [PubMed] [Google Scholar]
- 8.Baird J, Fisher D, Lucas P, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929–934. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yliharsila H, Kajantie E, Osmond C, et al. Body mass index during childhood and adult body composition in men and women aged 56 to 70 years. Am J Clin Nutr. 2008;87:1769–1775. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJP, Osmond C, Forsen TJ, et al. Trajectories of growth among children who later have coronary events. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 11.Botton J, Heude B, Maccario J, et al. Postnatal weight and height growth velocities at different ages between birth and 5 years and body composition in adolescent boys and girls. Am J Clin Nutr. 2008;87:1760–1768. doi: 10.1093/ajcn/87.6.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 14.Chomtho S, Wells JC, Williams JE, et al. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 15.Belfort MB, Rifas-Shiman SL, Rich-Edwards J, et al. Size at birth, infant growth, and blood pressure at three years of age. J Pediatr. 2007;151:670–674. doi: 10.1016/j.jpeds.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Shlomo Y, McCarthy A, Hughes R, et al. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth Study. Hypertension. 2008;52:638–644. doi: 10.1161/HYPERTENSIONAHA.108.114256. [DOI] [PubMed] [Google Scholar]
- 17.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ekelund U, Ong KK, Linne Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92:98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 19.Stettler N, Stallings VA, Troxel AB, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897–1903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 20.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363:1642–1645. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 21.Singhal A, Cole TJ, Fewtrell M, et al. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115:213–220. doi: 10.1161/CIRCULATIONAHA.106.617811. [DOI] [PubMed] [Google Scholar]
- 22.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, et al. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7:56. [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer MS, Guo T, Platt RW, et al. Feeding effects on growth during infancy. J Pediatr. 2004;145:600–605. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 24.Gillman MW, Rifas-Shiman SL, Kleinman KP, et al. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring) 2008;16:1651–1656. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker M, Rifas-Shiman SL, Belfort MB, et al. Pre- and peri-natal predictors of weight gain in early infancy (abstract]. Proc Pediatr Acad Soc Annu Meet; Baltimore. 2009; in press. [Google Scholar]
- 26.Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 27.Casey PH, Whiteside-Mansell L, Barrett K, et al. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics. 2006;118:1078–1086. doi: 10.1542/peds.2006-0361. [DOI] [PubMed] [Google Scholar]
- 28.Belfort MB, Rifas-Shiman SL, Rich-Edwards JW, et al. Infant growth and child cognition at 3 years of age. Pediatrics. 2008;122:e689–e695. doi: 10.1542/peds.2008-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]