Abstract
Iron oxide nanoparticle (IONP) hyperthermia is a novel therapeutic strategy currently under consideration for the treatment of various cancer types. Systemic delivery of IONP followed by non-invasive activation via a local alternating magnetic field (AMF) results in site-specific energy deposition in the IONP-containing tumor. Targeting IONP to the tumor using an antibody or antibody fragment conjugated to the surface may enhance the intratumoral deposition of IONP and is currently being pursued by many nanoparticle researchers. This strategy, however, is subject to a variety of restrictions in the in vivo environment, where other aspects of IONP design will strongly influence the biodistribution. In these studies, various targeted IONP are compared to non-targeted controls. IONP were injected into BT-474 tumor-bearing NSG mice and tissues harvested 24hrs post-injection. Results indicate no significant difference between the various targeted IONP and the non-targeted controls, suggesting the IONP were prohibitively-sized to incur tumor penetration. Additional strategies are currently being pursued in conjuncture with targeted particles to increase the intratumoral deposition.
Keywords: iron oxide, magnetite, magnetic, nanoparticle, hyperthermia, biodistribution, in vivo, antibody, targeting
1. INTRODUCTION
While direct injection of starch-coated IONP into tumor tissues has shown clinical success, reasonable doses of intravenously-delivered IONP have yet to achieve intratumoral concentrations producing the same levels of heating. The development of IONP as an MRI contrast for applications in liver cancer has resulted in clinically-accepted IONP designs (crystal core with a dextran or starch-based coating) heavily favoring sequestration by macrophages in the reticuloendothelial system (RES) (1, 2, 3). Using this IONP design as a basis for treating other cancer types, such as breast, has resulted in minimal IONP deposition in the tumor tissue and subsequently the inability to elevate tumor temperatures with hysteretic heating.
In general, tumor tissue is known to have abnormal vessel and lymphatic networks with high variability in extracellular matrix (ECM) density (4). While the EPR effect may favor some nanoparticle designs in this situation, not all particle designs will benefit. In order to penetrate solid tumor tissue, IONP must first maintain a serum half-life long enough to diffuse into the tumor. IONP must also be small or flexible enough, notwithstanding opsonized proteins, to diffuse through tumor vasculature as well as through ECM (5). Various sizes, shapes and functionally-derived variants of IONP exist that may help to mitigate the low concentration problem, notably antibody-targeted and PEG-ylated IONP (6, 7, 8, 9, 10). PEG-ylation can increase serum half life of a therapeutic by evading protein opsonization and macrophage detection (11).
Antibody targeting may allow nanoparticles to interact with specific epitopes characteristic to the tumor environment, resulting in less diffusion out of the tumor or even the activation of targeted pathways such as internalization (12). However, a comprehensive picture of how these variables affect starch-base coated IONP biodistribution in multiple in vitro and solid mouse tumor models is not available, though literature summaries have been attempted (13). The most comprehensive set of studies available, from Chouly et al. at Laboratoire de Biophysique in France, tests variants of superparamagnetic dextran-coated IONP with different sizes (33-90.6 nm) and surface charges (-30 - +20mV) as well as a copolymer coating modification, however do not include tumor tissue in their mouse model (14).
The following study attempted to assess the ability of tumor targeting and PEG-ylation, separately, to increase tumor deposition of IONP following intravenous injection. An additional smaller IONP group was also tested in one mouse to explore the difference between base particle sizes.
2. METHODOLOGY
2.1 Mice used for study
All mice are cared for according to approved IACUC animal protocol. Female mice of the strain NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ were obtained from The Jackson Laboratory (Bar Harbor, Maine 04609 USA) or from an in-house stock propagated from breeders originally from The Jackson Laboratory. At 8-11 weeks old, mice were implanted in mammary fat pad 10 with 5 million cells in 100μl of a three part mixture of rat tail collagen I (BD Biosciences), Matrigel™ basement membrane matrix (BD Biosciences) and serum-free DMEM-F12 50/50 using a 1ml syringe and a 30G needle. Mice were monitored every three days until the tumor reaches 50mm3, upon which mice were measured once every two days. Mice were put on study once the tumor volume reaches between 100-200mm3 as measured by calipers and calculated using an ellipsoid approximation.
2.2 Particles used for the studies
All nanoparticles used for these studies were purchased from Micromod Partikeltechnologie GmbH (18119 Rostock-Warnemuende, GERMANY) or BioPal (80 Webster Street, Worcester, MA). 20nm (BioPal, aminodextrancoated) and 50nm SPIO (Micromod Partikeltechnologie GmbH, dextran-coated) were obtained and characterized using ferrozine assay, ICP-MS and the Malvern Zetasizer. Micromod IONP were additionally available with PEG-200 functionalization. Stock iron concentrations vary from batch to batch and were validated in house before utilizing a given batch. Batches were stored at 4°C and used within the reported shelf life. Additional modified particles, specifically protein-targeted (TNP) and maleimide-amine-functionalized (MSPIO) associated control, were synthesized by Warren Kett.
2.3 IONP administration
Once a mouse reaches treatment size, IONP dose was calculated using mouse body mass (g). All other iron concentrations were normalized to the lowest concentration to ensure equal volume scaling. IONP stocks were made isotonic with the addition of salts (PBS, NaCl) prior to in vivo use. A 1ml syringe with 30g needle was loaded with the full injection dose and half the volume marked off. Mice were anesthetized using isoflurane and secured on a heated, vented surface for injection. Half the IONP dose was then injected intravenously into the tail vein; the mouse was then allowed to wake up and return to her cage. Two hours following the first injection, the syringe was drawn back to recoup any void volume in the needle and the needle was replaced with a fresh tip. The mouse was re-anesthetized and injected with the second half of the IONP dose. Two hours was chosen in order to allow the large injection volume and subsequent spike in blood pressure to normalize, as after one hour injections were noted to be challenging and incur excessive bleeding at the injection site after needle withdrawal. Timepoints are read after the second injection. An equal volume PBS control injection group was completed to evaluate background tissue iron concentrations.
2.4 Tissue harvesting
At a given timepoint following the second injection, mice were anesthetized using an overdose of isoflurane. At least 10 seconds after respiration is no longer observed, the mouse was checked for pain stimulus response by pinching the leg before collecting 100-400μl of blood via intracardial blood draw. The tumor was then removed and placed on a pre-weighed weigh boat for massing. Remaining organs of interest, including heart, lungs, liver, spleen and kidneys, were removed and placed in a large weigh boat containing PBS to prevent tissue drying and blood clotting on the surface. Organs are removed from the PBS bath, blot-dried and massed. After weighing each whole organ, a scalpel was used to reserve part of the tissue for histology, with the piece reserved being consistently from the same region of each organ type. The histology sample was placed in a glass vial containing an excess of 10% formalin; the remaining tissue is placed in a pre-weighted 15ml conical tube (Sarstedt) and post-weighed to determine tissue mass. Fixed tissues are allowed to fix for at least 24 hours at room temperature before preparing cassettes and submitting to the Pathology Translation Research Core at Dartmouth.
2.5 Inductively Coupled Plasma Mass Spectrometry (ICP-MS) digestion protocol
The following digestion procedure occurs in a fume hood due to ammonia production. A 1:3 volumetric mixture of stock concentration trace metal grades HCl to nitric acid (Fisher Scientific) was prepared in a 50ml conical tube. The acid mixture was immediately added to the post-weighed conical tubes containing harvested tissues. A DORM-3 standard, tumor, spleen, heart and lung tissues received at least 2ml of the acid mixture while blood and kidney tissues received at least 5ml and livers receive at least 7ml. The tissues were allowed to digest at room temperature, caps vented, for at least one hour before being placed on a heat block and incubated at 60°C for at least two hours. Vials were sealed and inverted at least twice during the heating process. Following heating, samples were allowed to cool a minimum of overnight before caps were sealed and all vials weighed and numbered. Sample data was logged in a spreadsheet and samples are submitted to the Dartmouth Trace Elements core for iron content analysis.
3. RESULTS
Table 1 shows a summary of size and surface charge characteristics for the various IONP used. All IONP displayed near-neutral zeta potentials, however varied significantly in size depending on functionalization. The non-functionalized plain 50nm IONP had an average size of ~78nm in diameter. Adding amination and maleimide functionalization resulted in an increase in average size to 88-89 nm in diameter. Attaching the targeting moiety further increased the average size to ~95nm, as expected from the 2-3nm estimated length of the targeting protein. The PEG-ylation process, which includes base functionalization of the plain IONP variant before attachment of PEG-200, increased the average size to ~103nm, making this the largest variant tested of the set.
Table 1.
IONP size (hydrodynamic diameter), polydispersity index (PDI) and zeta potential (mV) as determined using a Malvern zetasizer instrument. Samples were diluted to 1 mg Fe/ml in Millipore-filtered H2O with 10mM NaCl. Data for 20nm non-targeted IONP is presented as a reference. Targeted 20nm IONP were functionalized using a comparable process to 50nm IONP.
| Particle Type | Hydrodynamic diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| MSPIO Dextran 50nm | 88.5 | 0.184 | -0.978 |
| TNP Dextran 50nm | 95.48 | 0.177 | -1.032 |
| Plain Dextran 50nm | 77.88 | 0.19 | -0.59267 |
| PEG200 Dextran 50nm | 102.96 | 0.247 | -0.62003 |
| Plain Dextran 20nm | 25.14 | 0.197 | -0.19143 |
As is evident from Figure 7, though all particle types tested displayed tumor iron values above background, only the Plain and PEG200-ylated variants were statistically different from the other IONP types (P ≤ 0.035), though not from one another. The promising single point data from the 20nm targeted particle showed high intratumoral iron even with more than 30% less injected dose. 20nm targeted IONPs may also demonstrate less liver accumulation at the 24 hour timepoint, though additional mice are required to verify either of these observations. Liver concentrations of all other particle groups are not statistically significantly different with the exception of the PEG200-ylated variant, which is different from all other groups (P ≤ 0.035), and Plain from MSPIO (P ≤ 0.03), with all others being above P = 0.05. That the PEG-ylated variant shows slightly lower liver iron concentrations than all other groups may be expected from PEG’s effect on circulation, and 24 hour blood iron concentrations for the PEG group are statistically different from background (P ≤ 0.035).
Figure 7.
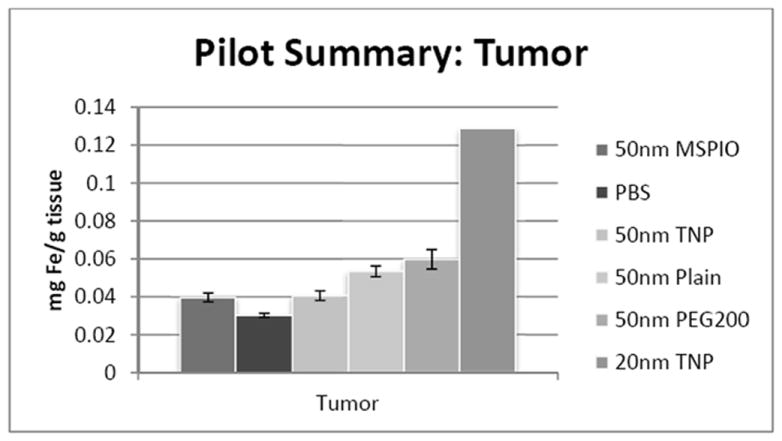
ICP-MS-determined iron concentrations for mouse tumor tissue samples in mg iron per gram tissue mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error.
All 50nm IONP-injected mouse liver samples are significantly above background, with additional mice necessary to assess the 20nm particle types (Figure 1). The difference in PEG-ylated IONP concentrations in the liver may be due to the PEG modification discouraging rapid macrophage sequestration. PEG-ylated 50nm IONP may then reside in the blood compartment longer; a hypothesis partially supported by the slightly elevated iron levels noticed in the PEG-ylated IONP group blood samples at 24 hours (Figure 4).
Figure 1.
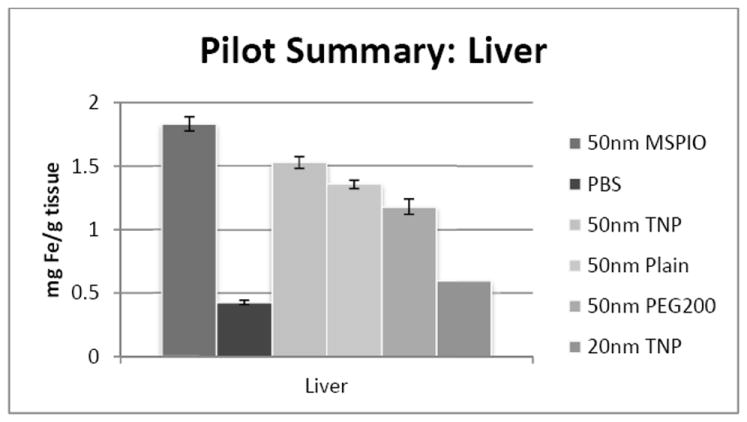
ICP-MS-determined iron concentrations for mouse liver tissue samples in mg iron per gram tissue mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error.
Figure 4.
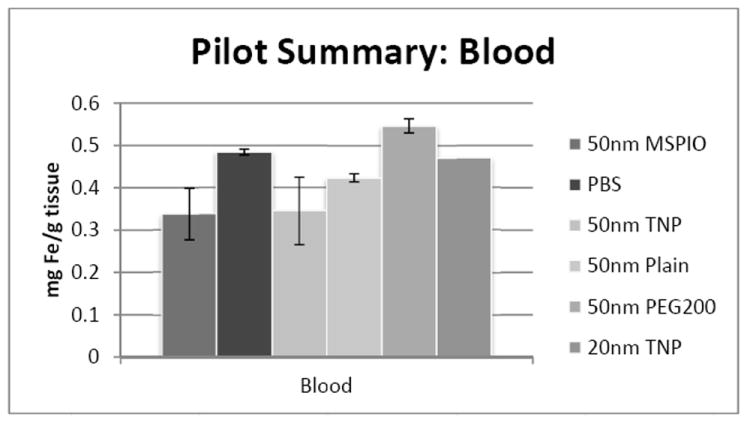
ICP-MS-determined iron concentrations for mouse blood samples in mg iron per gram blood mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error
Kidney iron concentrations overall did not show a significant difference of any 50nm IONP type from all other 50nm types (Figure 2). Splenic iron concentrations also did not show any significant differences between any two IONP types (Figure 3).
Figure 2.
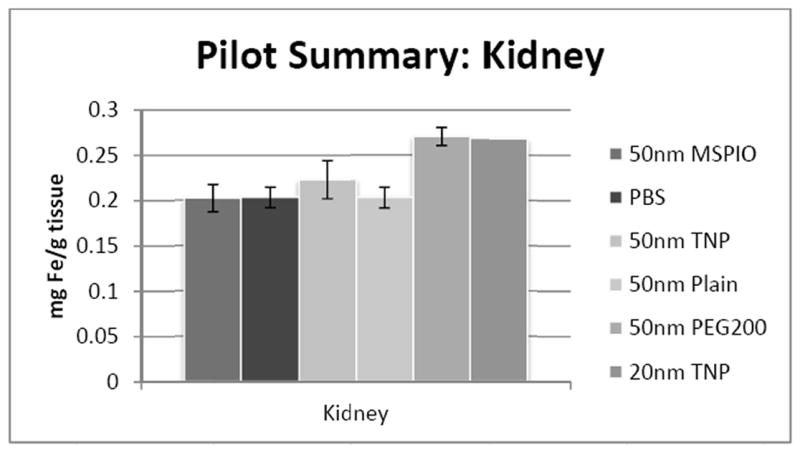
ICP-MS-determined iron concentrations for mouse kidney tissue samples in mg iron per gram tissue mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error.
Figure 3.
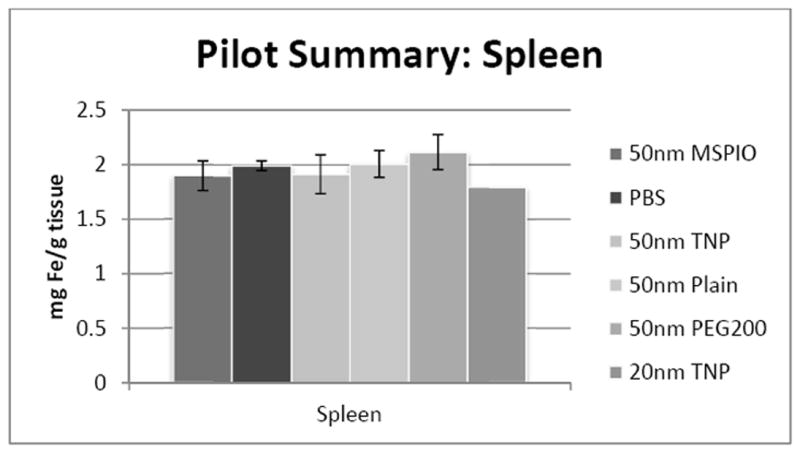
ICP-MS-determined iron concentrations for mouse spleen tissue samples in mg iron per gram tissue mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error.
Blood iron concentrations were below background for all 50nm IONP types except the PEG-ylated variant. No statistically-significant difference has yet been observed for either heart or lung tissues for any of the 50nm IONP types vis-à-vis all the other 50nm flavors. Additional mice are necessary to clarify the 20nm IONP results in the lung, though preliminary data may suggest an increase in iron concentration here.
Tumor tissue results indicate Plain and PEG200-ylated IONP injections result in statistically different intratumoral iron as compared to the other IONP types (P ≤ 0.035), though not from one another. The single point 20nm targeted IONP data suggests almost an order of magnitude higher intratumoral iron than any of the 50nm groups. These results were obtained with a lower dose of injected iron, so are not directly comparable to the 50nm groups.
4. DISCUSSION
Pilot results of IONP distribution do not show statistically-significant differences in tumor iron concentrations for 50nm targeted IONP as compared to its non-targeted MSPIO control. This may be due to rapid sequestration of both base and targeted particle types by the RES, and both plain and PEG-ylated 50nm IONP types appear to have higher intratumoral IONP than the targeted and base variants, as well as lower liver iron in the PEG group and, for the MSPIO, in the plain group. The differences in IONP size and surface characteristics (functionalization, presence of surface proteins) may be accountable for the difference in sequestration rate, clearance from the blood compartment or tumor penetrability of targeted and base-functionalized IONP variants. Additional data exploring the kinetics of uptake and serum half life would be necessary to verify this hypothesis, though blood data at the 24 hour timepoint suggests detectable levels of PEG-ylated IONP still in circulation. A near neutral zeta potential is thought to be favorable to increased circulation time, and all the IONP characterized have very comparable near-neutral surface charges (Table 1).
The 20nm targeted particle pilot displays relatively promising initial data as compared to the 50nm particle flavors, which all have an average hydrodynamic diameter above 70nm (Table 1). Since these results were obtained using a significantly lower iron dose of 20nm IONP than with the 50nm IONP, additional data comparing equal iron doses is necessary for a direct comparison, however it is hypothesized from previous studies that increased dose will result in increased deposition (15).
5. CONCLUSIONS AND FUTURE DIRECTIONS
Initial results suggest that in order for targeting to be effective, additional IONP design variables must be considered including size and surface characteristics. If targeted IONP experience rapid sequestration or the blocking of targeting moieties by serum proteins, they lose the opportunity to interact with intratumoral targets. Smaller IONP and PEG-ylation may both enhance intratumoral IONP deposition and allow for increased exposure of IONP targeting moieties to the appropriate tumor cell surface antigens. Additional work is necessary with PEG-ylated targeted 50nm and 20nm IONP and all associated controls to see whether these variables can favorably affect targeted IONP tumor uptake. Future work includes the completion of 50nm PEG-ylated and targeted as well as 20nm targeted, PEG-ylated and both targeted and PEG-ylated IONP in order to address this hypothesis.
Figure 5.
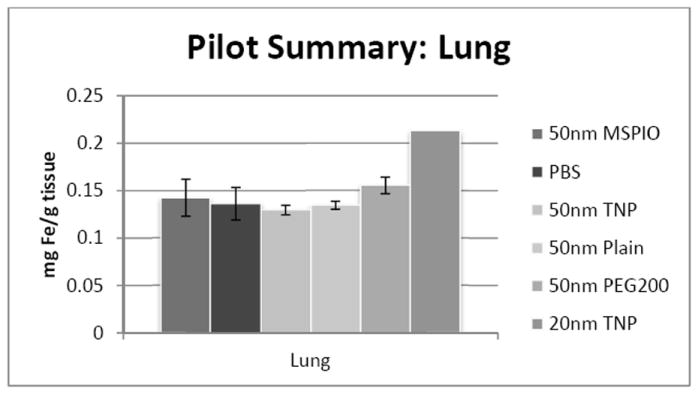
ICP-MS-determined iron concentrations for mouse lung tissue samples in mg iron per gram tissue mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error.
Figure 6.
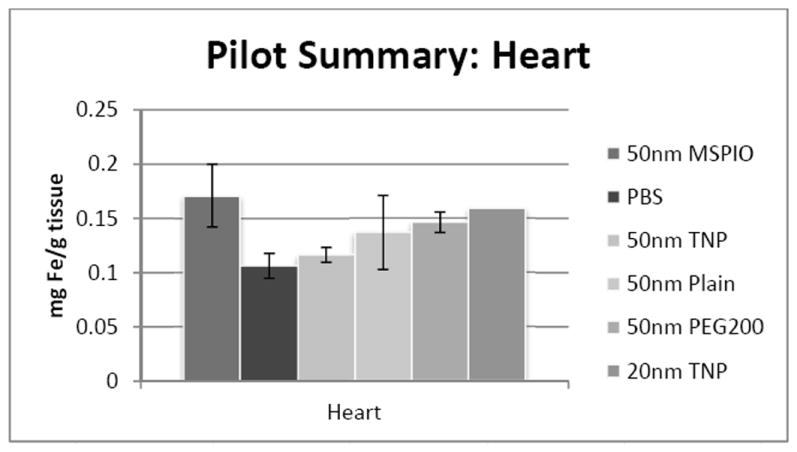
ICP-MS-determined iron concentrations for mouse heart tissue samples in mg iron per gram tissue mass. Mice were injected intravenously over a period of two injections, two hours apart, with 0.08 mg Fe/g mouse of the above variants of SPIO IONP and tissues harvested 24 hours post-second injection. All groups have N = 5-7 mice with the exception of the 20nm targeted nanoparticle data, where N = 1 and injected dose was lower at 0.05 mg Fe/g mouse. Error bars are standard error.
Acknowledgments
Supported by the Dartmouth Center for Cancer Nanotechnology Excellence: NCI-CCNE U54CA151662-03
References
- 1.Bourrinet P, et al. Preclinical Safety and Pharmacokinetic Profile of Ferumoxtran-10, an Ultrasmall Superparamagnetic Iron Oxide Magnetic Resonance Contrast Agent. Investigative radiology. 2006;41(3):313–24. doi: 10.1097/01.rli.0000197669.80475.dd. Web. [DOI] [PubMed] [Google Scholar]
- 2.Jain TK, et al. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Molecular pharmaceutics. 2008;5(2):316–27. doi: 10.1021/mp7001285. Web. [DOI] [PubMed] [Google Scholar]
- 3.Majumdar S, Zoghbi SS, Gore JC. Pharmacokinetics of Superparamagnetic Iron-Oxide MR Contrast Agents in the Rat. Investigative radiology. 1990;25(7):771–7. doi: 10.1097/00004424-199007000-00004. Web. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK, Stylianopoulos T. Delivering Nanomedicine to Solid Tumors. Nature Reviews Clinical Oncology. 2010;7(11):653–64. doi: 10.1038/nrclinonc.2010.139. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeil Scott E. Nanoparticle Therapeutics: A Personal Perspective. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1(3):264–71. doi: 10.1002/wnan.6. Web. [DOI] [PubMed] [Google Scholar]
- 6.Pouliquen D, et al. Iron Oxide Nanoparticles for use as an MRI Contrast Agent: Pharmacokinetics and Metabolism. Magnetic resonance imaging. 1991;9(3):275–83. doi: 10.1016/0730-725x(91)90412-f. Web. [DOI] [PubMed] [Google Scholar]
- 7.Schipper Meike L, et al. Particle Size, Surface Coating, and PEGylation Influence the Biodistribution of Quantum Dots in Living Mice. Small. 2009;5(1):126–34. doi: 10.1002/smll.200800003. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan VP, et al. Fluorescent Nanorods and Nanospheres for Real-Time in Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angewandte Chemie (International ed in English) 2011;50(48):11417–20. doi: 10.1002/anie.201104449. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaur Upasna, et al. Biodistribution of Fluoresceinated Dextran using Novel Nanoparticles Evading Reticuloendothelial System. International journal of pharmaceutics. 2000;202(1–2):1–10. doi: 10.1016/s0378-5173(99)00447-0. Web. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, et al. Macromolecular MRI Contrast Agents with Small Dendrimers: Pharmacokinetic Differences between Sizes and Cores. Bioconjugate chemistry. 2003;14(2):388–94. doi: 10.1021/bc025633c. Web. [DOI] [PubMed] [Google Scholar]
- 11.Owens DonaldE, III, Peppas Nicholas A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. International journal of pharmaceutics. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. Web. [DOI] [PubMed] [Google Scholar]
- 12.Lee Jae-Hyun, et al. Artificially Engineered Magnetic Nanoparticles for Ultra-Sensitive Molecular Imaging. Nature medicine. 2007;13(1):95–9. doi: 10.1038/nm1467. Web. [DOI] [PubMed] [Google Scholar]
- 13.Almeida JPM, et al. In Vivo Biodistribution of Nanoparticles. Nanomedicine. 2011;6(5):815–35. doi: 10.2217/nnm.11.79. Web. [DOI] [PubMed] [Google Scholar]
- 14.Chouly C, et al. Development of Superparamagnetic Nanoparticles for MRI: Effect of Particle Size, Charge and Surface Nature on Biodistribution. Journal of microencapsulation. 1996;13(3):245–55. doi: 10.3109/02652049609026013. Web. [DOI] [PubMed] [Google Scholar]
- 15.Tate J, et al. Toxicity and biodistribution of activated and non-activated intravenous iron oxide nanoparticles. Proc SPIE. 2009;7181(71810-L) doi: 10.1117/12.809830. [DOI] [PMC free article] [PubMed] [Google Scholar]


