Abstract
OBJECTIVE:
To test in the primary care setting the short- and long-term efficacy of a behavioral intervention that simultaneously targeted an overweight child and parent versus an information control (IC) targeting weight control only in the child.
METHODS:
Two- to 5-year-old children who had BMI ≥85th percentile and an overweight parent (BMI >25 kg/m2) were randomized to Intervention or IC, both receiving diet and activity education over 12 months (13 sessions) followed by 12-month follow-up (3 sessions). Parents in the Intervention group were also targeted for weight control and received behavioral intervention. Pediatricians in 4 practices enrolled their patients with the assistance of embedded recruiters (Practice Enhancement Assistants) who assisted with treatment too.
RESULTS:
A total of 96 of the 105 children randomized (Intervention n = 46; IC n = 50) started the program and had data at baseline. Children in the Intervention experienced greater reductions in percent over BMI (group × months; P = .002) and z-BMI (group × months; P < 0.001) compared with IC throughout treatment and follow-up. Greater BMI reduction was observed over time for parents in the Intervention compared with IC (P < .001) throughout treatment and follow-up. Child weight changes were correlated with parent weight changes at 12 and 24 months (r = 0.38 and 0.26; P < .001 and P = .03).
CONCLUSIONS:
Concurrently targeting preschool-aged overweight and obese youth and their parents in primary care with behavioral intervention results in greater decreases in child percent over BMI, z-BMI, and parent BMI compared with IC. The difference between Intervention and IC persists after 12 months of follow-up.
Keywords: overweight, obesity, patient centered medical home, percent over BMI, preschool children, expert committee recommendations
What’s Known on This Subject:
Pediatricians need to treat overweight in early childhood. Family-based interventions in specialized clinics are efficacious in children age 8 years and older. Data regarding treatment of younger children are limited in specialty clinics and primary care.
What This Study Adds:
This study shows that a 12-month family-based behavioral intervention in primary care is more efficacious compared with Control condition with a child-only focus. Weight outcome differences between Intervention and Control persist in children and parents after a 12-month follow-up.
Overweight starts in early childhood and 22.8% of 2- to 5-year-old children have a BMI over the 85th percentile.1 However, there are limited data on treatment of overweight for preschool-aged children in specialty clinics and primary care.2–7 Pediatricians are tasked with implementing the Expert Committee Recommendations to prevent and treat overweight and obesity, but they often do not have the training/resources to comply with this mandate. Programs that can be implemented in primary care are needed to treat overweight young children to reduce the risk for overweight in adulthood and prevent obesity comorbidities.8–11 Buffalo Healthy Tots (BHT) was designed to test the efficacy of treating 2- to 5-year-old overweight children with either a traditional approach focused only on the child (Information Control [IC]), or a behavioral Intervention jointly targeting the child and parent in pediatric primary care practices. At 6 months BHT Intervention reduced child percent over BMI (%OBMI) and z-BMI and parent BMI compared with IC.5 However, maintenance of treatment effects represents a major challenge in obesity treatment.12–14 The aim of this study was to test in the primary care setting the hypothesis that joint treatment of parents and preschool-aged children for weight control and behavioral modification would lead to greater initial (6- and 12-month) and sustained (18- and 24-month) reductions in %OBMI and BMI changes in children and parents, respectively, compared with the traditional approach focusing on the child alone.
Methods
A summary of the protocol previously described is given herein.5 The study took place in 4 pediatric Patient Centered Medical Homes (PCMH), which followed children from diverse socioeconomic and ethnic backgrounds. Pediatricians recruited study participants and introduced the parent to the Practice Enhancement Assistant (PEA), who was embedded part-time in each PCMH to assist in recruitment and study implementation. PEAs held a Master/Bachelor Degree in Psychology, Nutrition, Exercise Science, or equivalent, or were Registered Dieticians. Children who had a BMI over the 85th percentile for age and gender and having a parent who had a BMI >25 kg/m2 were included. The main exclusion criteria were: small for gestational age, short stature, and child/parent inability to perform physical activity. Families were recruited in cohorts of 12 and then stratified by gender of the targeted child and randomized to Intervention or IC using a random number generator. This approach allowed pediatricians to recruit subjects when children attended office visits and limited personnel to 3 part-time PEAs and the Project Coordinator (Ecker). One PEA and the project coordinator were group leaders during the sessions. The study was approved by the Institutional Review Board of the Women and Children’s Hospital of Buffalo and was conducted in concordance with the Declaration of Helsinki.
Summary of Protocol Common to Both Groups
Parents attended thirteen 60-minute group sessions over the 12-month treatment period (4 weekly, 2 biweekly, 4 monthly, and 3 at 8- to 10-week intervals), followed by a 12-month follow-up (3 meetings at month 16, 20, and 24). The Intervention and IC groups were held on different evenings. A PEA assigned to each family telephoned the parent between scheduled meetings 10 times during treatment and 3 times during follow-up. The intervention was delivered through the parents. PEAs cared for the children while parents attended the sessions. Both groups received dietary, physical, and sedentary activity guidelines in keeping with the Expert Committee Recommendations. The child’s weight goal was 0.5 to 1 pound/week weight loss. Parents were instructed on the appropriate number of servings for their child from each food group to provide 1000 to 1200 daily kilocalories depending on age,15 and to avoid food with >5 g of fat/serving, high in sugar, or containing artificial sweeteners because they habituate the child to a high sugary taste and in adults have been shown to increase the risk for metabolic syndrome and type 2 diabetes.16,17 The threshold of 5 g of fat/serving was adapted from the validated Traffic Light Diet.18 Efforts to limit high-sugar foods focused mainly on sugar-sweetened drinks and breakfast food such as commercial cereals (>5 g of sugars/serving). The child’s pediatrician reviewed %OBMI changes every 6 months. Between the 6-month visits the PEA prepared a letter outlining the child’s progress for the families.
Summary of Protocol Components Pertinent Only to the Intervention
In the Intervention behavior modification and education on parenting techniques (ie, positive reinforcement, modeling healthy diet and activity, and stimulus control) were delivered by the group leader during the group meetings and by a PEA, assigned to each family, during brief individual sessions held the same evenings as the group meetings. Parents were instructed to monitor their child and their own weight twice a week and received dietary (1500 and 1800 kcals/day for mothers and fathers, respectively), physical, and sedentary activity guidelines with the goal of a minimum of 1 pound/week weight loss.15 A list of foods with portion sizes and energy content information was provided. Parents recorded intake and activity for their child and themselves in a diary by crossing off icons detailing the different food groups and physical and sedentary activity. The number of icons was tailored to the child and parent so that shaping up/down of targeted behaviors could be individualized.
Measures
The protocol for weight and height monitoring has been described.5 Several measures have been proposed to follow BMI/weight changes in pediatric obesity treatment19–21 and weight control trials in youth who have diabetes.22 We are reporting %OBMI and z-BMI. Percent OBMI is defined as [(child’s BMI − 50th percentile BMI)/50th percentile BMI] * 100.19,23
Sample Size Considerations
Based on a decrease in %OBMI of 6.5% (SD, 21%) in a 12-week pilot study, we calculated that a sample of 108 subjects was required to provide a power of >80% to detect the treatment difference, if a difference of ≥8.7% was maintained between Intervention and IC groups throughout the study.5,24 Recruitment was halted at 105 families because preliminary analyses indicated efficacy of the primary outcome. The first cohort started treatment in October 2008 and the last cohort completed the 12-month follow-up in June 2013.
Statistical Analysis
Group baseline characteristics between groups were compared using analysis of variance or Pearson’s X2 test as appropriate. We performed intention to treat analysis using all subjects for whom we had baseline data (n = 96; 46 Intervention and 50 Control). The primary outcomes were analyzed by using the mixed model analysis of covariance, which can handle missing data. In an effort to choose a reasonably fitted model satisfying the model assumptions, the residual plots and the residual-based fit statistics such as Akaike's Information for various covariance structures were examined. The models included treatment, time and their interaction as class variables, and baseline values of the respective outcome variable as covariates with autoregressive covariance structures to incorporate the clustering effect within participants. Because the effect of clustering by clinical site was negligible (altering estimates at the 3rd/4th decimal places), it was not included as a random effect in the final models. A completers analysis was also performed by using mixed effects analysis of covariance. Mixed models tested for differences in treatment groups over time in the study’s primary endpoints of child %OBMI and parent BMI. In addition, planned comparisons tested for group differences from baseline to each follow-up assessment (6, 12, 18, and 24 months), as well as comparisons within each group or each period based on the fitted mixed models.
Participating parent’s gender, baseline BMI, and concordance/discordance of parent-child genders were tested separately as moderators of child %OBMI change by interacting each variable with the group × month effects. The correlations between child %OBMI change and parent BMI change were analyzed by using Pearson correlation coefficients at 6, 12, 18, and 24 months. Statistical software SAS (SAS Institute, Inc, Cary, NC; Version 9.3) was used for all analyses.
Results
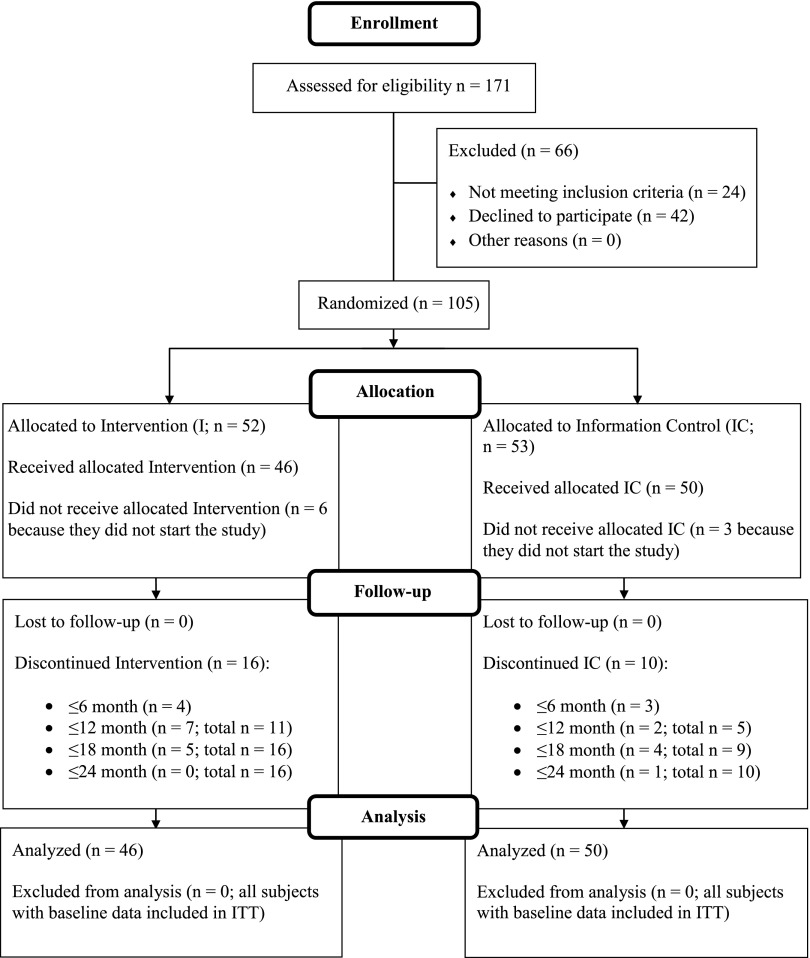
Data are expressed as mean ± SEM. Figure 1 shows the study participants’ disposition throughout the study. One-hundred five child-parent dyads were randomized, but 9 did not attend any sessions and did not have baseline data. The main reasons were work schedule changes, parental separation, and family moving out of the area. Of the 96 subjects who started the study and received any treatment, 83% completed the 12-month treatment and nearly 73% completed the 24-month follow-up. Attrition did not differ between groups at any time point (P = 0.61, 0.07, 0.06, and 0.10 at 6, 12, 18, and 24 months, respectively). For as long as families were in the study they received 100% of the planned curriculum (missed sessions were always rescheduled). Parent and child demographic and anthropometric parameters were similar between groups (Table 1). The sample included 27% minorities with a mean yearly income of all families of $65 729 ± $3068 (8.3% families <$20 000). Children’s %OBMI and parents’ BMI of families who dropped during the first 6 months of the intervention did not differ compared with completers (P = .09 and P = .63, respectively). However, children who dropped out at 12 months were heavier at baseline (%OBMI 47.7 ± 4.6 vs 27.7 ± 2.1; P < .001) and had parents who had higher BMI (42.4 ± 1.8 vs 35.6 ± 0.8; P = .001). Child or parent age did not differ among drop-outs and completers. The most common reason for discontinuation was change in work schedule, followed by family moving out of state or feeling the program was not what they expected/disagreeing on the study/coping with ADHD diagnosis, 1 pregnancy, and 1 bariatric surgery (despite mother having lost weight in the program).
FIGURE 1.
This diagram summarizes the flow of study participants throughout the 12-month treatment period and 12-month follow-up. All participants who had data at baseline and received any of the allocated Intervention or Information control were included in the analysis.
TABLE 1.
Baseline Characteristics of Children and Parents Assigned to the Intervention and Information Control Conditions
| Children | Parents | |||
|---|---|---|---|---|
| Intervention | IC | Intervention | IC | |
| N | 46 | 50 | 46 | 50 |
| Age, y | 4.6 ± 0.2 | 4.4 ± 0.2 | 37.2 ± 0.7 | 36.4 ± 0.7 |
| Sex, F/M | 31/15 | 33/17 | 33/13 | 39/11 |
| Height, cm | 109.3 ± 1.4 | 107.5 ± 1.3 | 168.3 ± 1.4 | 166.1 ± 1.3 |
| Weight, kg | 24.8 ± 1.0 | 23.5 ± 0.8 | 105.8 ± 3.9 | 99.8 ± 2.8 |
| BMI | 20.4 ± 0.5 | 20.1 ± 0.4 | 37.2 ± 8.3 | 36.2 ± 6.9 |
| Ethnic/minority status | ||||
| Non-Hispanic white | 33 | 37 | 38 | 42 |
| Non-Hispanic black | 7 | 4 | 5 | 5 |
| Hispanic | 5 | 4 | 1 | 3 |
| Asian | 1 | 0 | 0 | 0 |
| Other | 0 | 5 | 0 | 2 |
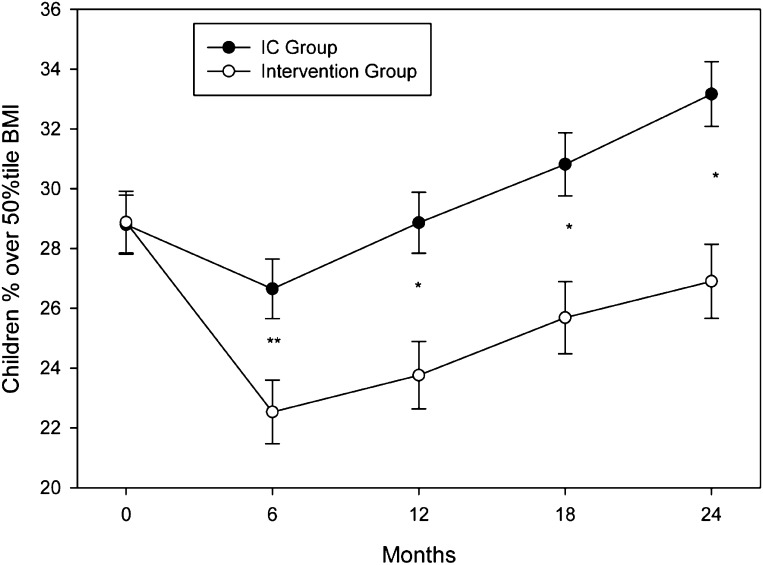
Changes in %OBMI are shown in Fig 2. There were differential group changes across time (P = .002). The Intervention group had greater decreases compared with the IC group from baseline to 6 (P < 0.001), 12 (P = .005), 18 (P = .005), and 24 months (P = .001). Similar effects were seen for z-BMI (Table 2; group × month; P < 0.001), with greater decreases at 6 (P < 0.001), 12 (P < 0.001), 18 (P < 0.01), and 24 months (P < .007) for Intervention versus IC. Child height, weight, and z-BMI estimates over the 24-month study period are shown in Table 2. Smaller weight increases were observed in the Intervention compared with the IC group with an overall group × months effect (P < .004), and greater decreases for the Intervention at 6 (P < .002), 12 (P < 0.002), 18 (P < .001), and 24 months (P < .001). Height growth velocity over 24 months was within normal limits in both groups (Intervention 12.6 ± 0.5 cm; IC 13.5 ± 0.0.3 cm). However, a slower increase in height was observed in the Intervention compared with the IC group with an overall group × months effect (P < .02), with differences during the follow-up period at 18 (P < .001) and 24 months (P < .02).
FIGURE 2.
Child %OBMI values (mean ± SEM) throughout the 12-month treatment period and 12-month follow-up were lower in the Intervention (open circles) compared with the IC group (solid circles). **P < 0.001; *P < 0.01.
TABLE 2.
Child Height, Weight, and z-BMI Estimates Over the 24-Month Study Period
| Months | |||||
|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | |
| Weight Intervention, kg | 23.4 ± 0.3 | 23.5 ± 0.3 | 25.1 ± 0.3 | 27.0 ± 0.3 | 28.9 ± 0.3 |
| Weight IC, kg | 23.5 ± 0.3 | 24.4 ± 0.3 | 26.4 ± 0.3 | 28.5 ± 0.3 | 30.6 ± 0.3 |
| Height Intervention, cm | 108.0 ± 0.2 | 111.1 ± 0.3 | 114.3 ± 0.2 | 117.3 ± 0.3 | 120.5 ± 0.3 |
| Height IC, cm | 107.9 ± 0.2 | 111.1 ± 0.2 | 114.8 ± 0.2 | 118.4 ± 0.2 | 121.4 ± 0.2 |
| z-BMI Intervention | 2.11 ± 0.05 | 1.69 ± 0.05 | 1.66 ± 0.05 | 1.66 ± 0.06 | 1.61 ± 0.06 |
| z-BMI IC | 2.11 ± 0.05 | 1.93 ± 0.05 | 1.90 ± 0.05 | 1.86 ± 0.05 | 1.86 ± 0.05 |
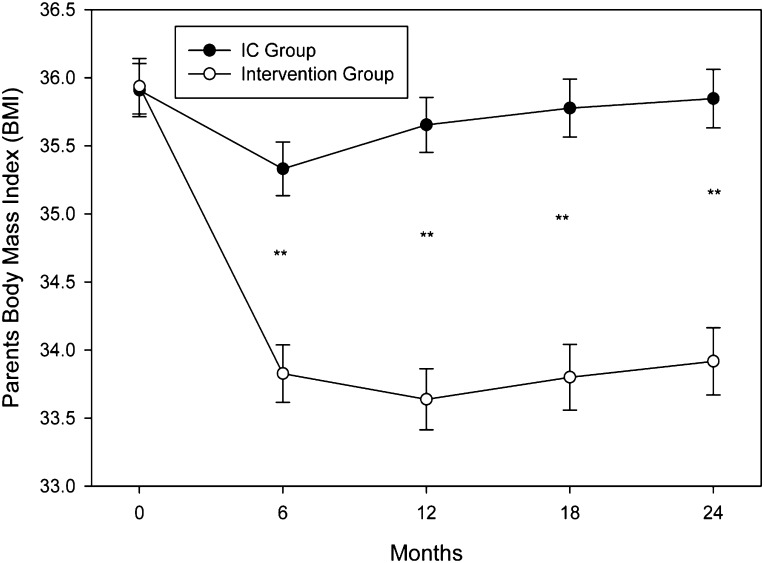
Greater (P < 0.001) BMI reduction over time was observed in parents assigned to Intervention compared with those in IC. Model adjusted means of parental BMI changes are displayed in Fig 3. The Intervention had greater (P < .001 at all time points) decreases compared with the IC group from baseline to 6, 12, 18, and 24 months. Parents in the Intervention had greater decreases in weight at 6, 12, 18, and 24 months (P < .001 at all time points). Weight estimates decreased in the Intervention (101.5 ± 0.6, 94.7 ± 0.6, and 95.5 ± 0.7 kg at baseline, 12 months, and 24 months, respectively), whereas they were essentially unchanged in the IC group throughout the study period (101.2 ± 0.6, 100.6 ± 0.6, and 101.9 ± 0.6 kg at baseline,12 months, and 24 months, respectively).
FIGURE 3.
Parent BMI values (mean ± SEM) throughout the 12-month treatment period and 12-month follow-up were lower in the Intervention (open circles) compared with the IC group (solid circles). *P < 0.001.
Mixed model analysis of variance of study completers (n = 70) also demonstrated differences (P < 0.01) in child %OBMI between Intervention and IC groups, baseline to 6 (P < .03), 12 (P < .02), 18 (P = .05), and 24 months (P < .003). Parent BMI was different between Intervention and IC (P < .001 at all data points).
Percent OBMI and parent BMI changes were correlated from baseline to 12 months (r = 0.38; P < 0.001), 18 months (r = 0.35; P = .004), and 24 months (r = 0.26; P < .03). Baseline values of child age, gender, and %OBMI, were not significant moderators of group responses in %OBMI over time. Also, participating parent’s gender or BMI or concordance or discordance of parent-child genders were not significant moderators of child %OBMI changes.
Discussion
We have shown that concurrent treatment of overweight parents and children in the primary care setting produces greater initial and sustained improvement in %OBMI and z-BMI in the child and BMI decrease in the parents compared with the traditional focus on the child only without behavioral intervention. Our study adds to limited data in preschool-aged children, is longer in duration than other studies, and documents the weight outcomes after a 12- month follow-up. At a time of focus on care coordination among pediatricians and specialists, BHT represents a move forward from the paradigm of referral of the child to co-management by the pediatrician and specialists trained in weight management and behavior modification. Our program overcame the pediatricians’ challenge to implement the Expert Committee Recommendations25,26 by embedding PEAs within practices, and demonstrated that pediatricians are willing to partner in research that is relevant to their patients’ health.27,28 Having PEAs in the PCMH creates the opportunity for “structured programs,” which are usually carried out in specialized clinics, to be translated to the primary care setting, where they are more accessible to youth and represent an ongoing resource for the child and family.3,4,8,29 The PEAs flagged overweight/obese children in the electronic health records, helping pediatricians to identify overweight young children who often do not look overweight, because they are still growing relatively fast in height.30 However, these tools have to be coupled with the willingness to reorganize the flow and operation of the practice in a manner similar to that successfully implemented in the practice participating in BHT and in the Academic Innovation Collaborative.31,32
Simplifying delivery of treatment programs is necessary to overcome time and logistics involved in attending programs in specialized clinics while maintaining optimal quality and dose of treatment. Despite the fact BHT decreased the frequency of attended sessions from 20 in the specialized clinic4 to 13 in the pediatrician office, time was cited as the main reason for declining to enroll in the study. Yet the US Preventive Services Task Force recommends a treatment dose of at least 30 hours across a 2-year period.33,34
Concomitant treatment of children and parents leads to weight changes in both,35 preventing early obesity comorbidities in the child and ameliorating/reverting obesity comorbidities already set in the obese parent.36,37 Cost effectiveness analyses of this approach have to be performed, while considering that the United States spends the majority of heath care dollars attempting to treat chronic conditions, many of which are linked to or worsened by obesity, such as diabetes and cardiovascular disease.38 The validity of a family approach is corroborated by the correlation between parent and child weight changes. Parents in the Intervention group were coached on parenting, modeling, and maintaining a healthy eating and activity environment to promote weight control for their children. Indeed, children have better weight control if their parents are committed to change and model healthier behaviors,35,39 suggesting that one approach to improving child weight loss may be to emphasize parental weight loss.40
Children in both groups grew normally in height. Children in IC had a greater height velocity than children in Intervention, likely owing to greater ongoing weight gain driving growth in height.41,42 As such the reduction in %OBMI and z-BMI in Intervention is even more relevant from a clinical standpoint, because %OBMI of children in IC was lower than baseline only at 6 months and greater than baseline at 24 months. Within the Intervention group, %OBMI was lower compared with baseline and at 6 and 12 months, with some relapse at 18 and 24 months, at which time a 2.1% decrease in %OBMI from baseline still remained. Parents in the Intervention maintained their weight loss not only throughout the treatment, but also during follow-up.
It is not known if maintaining/lowering %OBMI is needed to prevent/improve obesity comorbidities in young children. In older children only small changes in weight are needed to normalize %OBMI as children are growing, and this effect may be more pronounced in younger children.43 We have shown that, compared with children who have normal weight, 2- to 5-year-old overweight children have greater LDL cholesterol (36% exhibiting a LDL level >110 mg/dL),44 suggesting that early intervention may indeed be important for preventing later diseases.
Strengths of the study include long follow-up, control for attention across groups, and ability to implement a less intensive treatment in the primary care setting. Although children’s %OBMI and parents’ BMI was not different in those who dropped out by 6 months compared with completers, children and parents who dropped out by 12 months were heavier than completers at baseline. Because 8/13 treatment sessions occurred in the first 6 months, this suggests that more intensive treatment is needed for children and parents who are heavier at outset. Although this could not be implemented in our study (to control for attention between groups), closer follow-up could be implemented by pediatricians in their practice. Two additional issues need to be discussed. First, parents in IC were not targeted for weight loss. However, it is unlikely that instructing parents to lose weight would promote child weight loss. Secondly, parents were able to maintain better weight loss than their children, without relapse over the full 12-month follow-up, which is a highlight of the study, because most adult weight control programs show relapse beginning at 6 months.45–47 It is likely that, different from older children who learn along with parents more self-control methods and techniques to maintain healthy behaviors, younger children must rely more on parenting to maintain changes.48
Conclusions
The results of this study demonstrate that effective treatment of overweight can be implemented in primary care, substituting the traditional concept of referral to a specialty clinic the new concept of “co-management,” requiring collaboration among health care providers with different sets of expertise. By relying on PEAs, our program can be applied on a larger scale than previous weight control programs. The PCMH in which the child is followed by the pediatrician into adulthood may be the ideal setting for the implementation of family-based treatments such as BHT, as well as maintenance programs. Further studies are needed to replicate our findings and validate our approach.
Acknowledgments
We are deeply thankful to the children and families who participated in this study. We are also grateful to the Pediatricians, Allied Health Personnel, and Office Staff who actively partnered with us in this study. We thank our expert Practice Enhancement Assistants Amanda Ayler, MPH; Elissa Ortolani, BA, medical student; Megan Russell, MS, CAS; and Lisa Rychlicki, RD. We are indebted to Sherry Ortiz for her assistance with manuscript preparation.
Glossary
- %OBMI
percent over BMI
- BHT
Buffalo Healthy Tots
- IC
information control
- PCMH
Patient Centered Medical Home
- PEA
Practice Enhancement Assistant
- z-BMI
z score body mass index
Footnotes
Drs Quattrin and Roemmich contributed to study design, trial organization and execution, collection and analysis of data, and interpretation of the data and manuscript preparation; Mr Paluch contributed to study design, data management and analysis, interpretation of the data, and manuscript preparation; Drs Yu and Epstein contributed to study design, data analysis, and interpretation of the data and manuscript preparation; Ms Ecker contributed to trial organization, study design and execution of both the pilot study and the study published herein, development of study protocol, manuscript preparation with particular attention to the Methods section, and data collection, management, and interpretation; all authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01029834).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health grant 1R01HD053773-01, Principal Investigator, Teresa Quattrin. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Epstein consulted for and has equity in Kurbo, a company designed to provide online support for pediatric weight control; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26(5):521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373–383 [DOI] [PubMed] [Google Scholar]
- 4.Epstein LH, Valoski A, Koeske R, Wing RR. Family-based behavioral weight control in obese young children. J Am Diet Assoc. 1986;86(4):481–484 [PubMed] [Google Scholar]
- 5.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker MA. Efficacy of family-based weight control program for preschool children in primary care. Pediatrics. 2012;130(4):660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saelens BE, Liu L. Clinician’s comment on treatment of childhood overweight meta-analysis. Health Psychol. 2007;26(5):533–536 [DOI] [PubMed] [Google Scholar]
- 7.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10(1):22–32 [DOI] [PubMed] [Google Scholar]
- 8.Roemmich JN, Liu EY, Rogol AD, Epstein LH, Quattrin T. Diminished insulin resistance with weight loss in severely overweight youth. Metab Syndr Relat Disord. 2004;2(3):160–168 [DOI] [PubMed] [Google Scholar]
- 9.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–480 [DOI] [PubMed] [Google Scholar]
- 10.Toprak D, Toprak A, Chen W, Xu JH, Srinivasan S, Berenson GS. Adiposity in childhood is related to C-reactive protein and adiponectin in young adulthood: from the Bogalusa Heart Study. Obesity (Silver Spring). 2011;19(1):185–190 [DOI] [PubMed] [Google Scholar]
- 11.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91(5):1499S–1505S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1 suppl):5–16 [DOI] [PubMed] [Google Scholar]
- 13.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571 [DOI] [PubMed] [Google Scholar]
- 14.Svetkey LP, Stevens VJ, Brantley PJ, et al. Weight Loss Maintenance Collaborative Research Group . Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148 [DOI] [PubMed] [Google Scholar]
- 15.USDA. Estimated calorie needs per day by age, gender, and physical activity level. Available at: www.cnpp.usda.gov/publications/usdafoodpatterns/estimatedcalorieneedsperdaytable.pdf. Accessed June 12, 2014
- 16.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR, Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2009;32(4):688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488 [DOI] [PubMed] [Google Scholar]
- 18.Epstein LH, Kazdin AE, Weisz JR. Development of evidence-based treatments for pediatric obesity. In: Kazdin AE, Weisz JR, eds. Evidence-Based Psychotherapies for Children and Adolescents. New York: Guilford Publications, Inc; 2003:374–388 [Google Scholar]
- 19.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59(3):419–425 [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000; (314):1–27 [PubMed] [Google Scholar]
- 21.Flegal AR, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 Growth Charts. Natl Health Stat Rep. 2013;63:1–4 [PubMed] [Google Scholar]
- 22.Zeitler P, Hirst K, Pyle L, et al. TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. Am J Hum Biol. 2007;19(4):487–494 [DOI] [PubMed] [Google Scholar]
- 24.Quattrin T, Roemmich J, Epstein L, Gambino M, Roman A. Treating overweight youth and parents in the pediatrician’s office: results of a pilot study. Presented at the Pediatric Academic Societies Annual Meeting, San Francisco, CA, April 29–May 2, 2006 [Google Scholar]
- 25.Benson L, Baer HJ, Kaelber DC. Trends in the diagnosis of overweight and obesity in children and adolescents: 1999-2007. Pediatrics. 2009;123(1). Available at: www.pediatrics.org/cgi/content/full/123/1/e153 [DOI] [PubMed] [Google Scholar]
- 26.Dorsey KB, Wells C, Krumholz HM, Concato J. Diagnosis, evaluation, and treatment of childhood obesity in pediatric practice. Arch Pediatr Adolesc Med. 2005;159(7):632–638 [DOI] [PubMed] [Google Scholar]
- 27.Nagykaldi Z, Mold JW, Aspy CB. Practice facilitators: a review of the literature. Fam Med. 2005;37(8):581–588 [PubMed] [Google Scholar]
- 28.Mold JW, Peterson KA. Primary care practice-based research networks: working at the interface between research and quality improvement. Ann Fam Med. 2005;3(suppl 1):S12–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight-loss interventions: a systematic review of randomised trials. Int J Obes Relat Metab Disord. 2003;27(9):987–1005 [DOI] [PubMed] [Google Scholar]
- 30.Quattrin T, Liu E, Shaw N, Shine B, Chiang E. Obese children who are referred to the pediatric endocrinologist: characteristics and outcome. Pediatrics. 2005;115(2):348–351 [DOI] [PubMed] [Google Scholar]
- 31.Cheng JK, Cox JE, Taveras EM. Patient-centered approaches to childhood obesity care. Child Obes. 2013;9(2):85–88 [DOI] [PubMed] [Google Scholar]
- 32.Taveras EM, Hohman KH, Price SN, et al. Correlates of participation in a pediatric primary care-based obesity prevention intervention. Obesity (Silver Spring). 2011;19(2):449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton M, US Preventive Services Task Force . Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125(2):361–367 [DOI] [PubMed] [Google Scholar]
- 34.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e396 [DOI] [PubMed] [Google Scholar]
- 35.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Arch Pediatr Adolesc Med. 2004;158(4):342–347 [DOI] [PubMed] [Google Scholar]
- 36.Epstein LH, Kuller LH, Wing RR, Valoski A, McCurley J. The effect of weight control on lipid changes in obese children. Am J Dis Child. 1989;143(4):454–457 [DOI] [PubMed] [Google Scholar]
- 37.Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010;95(4):256–261 [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Overweight and obesity causes and consequences 2012. Accessed April 27, 2012. Available at: www.cdc.gov/obesity/adult/causes/index.html/
- 39.Sonneville KR, Rifas-Shiman SL, Kleinman KP, Gortmaker SL, Gillman MW, Taveras EM. Associations of obesogenic behaviors in mothers and obese children participating in a randomized trial. Obesity (Silver Spring). 2012;20(7):1449–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boutelle KN, Cafri G, Crow SJ. Parent predictors of child weight change in family based behavioral obesity treatment. Obesity (Silver Spring). 2012;20(7):1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forbes GB. Nutrition and growth. J Pediatr. 1977;91(1):40–42 [DOI] [PubMed] [Google Scholar]
- 42.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101(3 pt 2):518–525 [PubMed] [Google Scholar]
- 43.Goldschmidt AB, Wilfley DE, Paluch RA, Roemmich JN, Epstein LH. Indicated prevention of adult obesity: how much weight change is necessary for normalization of weight status in children? JAMA Pediatr. 2013;167(1):21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bethin K, Rafalson L, Creighton P, et al. Higher BMI and dental decay may be linked. The Endocrine Society's 92nd Annual Meeting, San Diego CA, June 19–22, 2010 [Google Scholar]
- 45.Kassirer JP, Angell M. Losing weight—an ill-fated New Year’s resolution. N Engl J Med. 1998;338(1):52–54 [DOI] [PubMed] [Google Scholar]
- 46.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84(2):441–461, vii [vii.] [DOI] [PubMed] [Google Scholar]
- 47.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. Int J Obes. 1989;13(2):123–136 [PubMed] [Google Scholar]
- 48.Epstein LH, Valoski AM, Kalarchian MA, McCurley J. Do children lose and maintain weight easier than adults: a comparison of child and parent weight changes from six months to ten years. Obes Res. 1995;3(5):411–417 [DOI] [PubMed] [Google Scholar]