Abstract
OBJECTIVE:
To ascertain the prevalence of and risk factors for obstructive sleep apnea syndrome (OSAS) in children with sickle cell anemia (SCA).
METHODS:
Cross-sectional baseline data were analyzed from the Sleep and Asthma Cohort Study, a multicenter prospective study designed to evaluate the contribution of sleep and breathing abnormalities to SCA-related morbidity in children ages 4 to 18 years, unselected for OSAS symptoms or asthma. Multivariable logistic regression assessed the relationships between OSAS status on the basis of overnight in-laboratory polysomnography and putative risk factors obtained from questionnaires and direct measurements.
RESULTS:
Participants included 243 children with a median age of 10 years; 50% were boys, 99% were of African heritage, and 95% were homozygous for βS hemoglobin. OSAS, defined by obstructive apnea hypopnea indices, was present in 100 (41%) or 25 (10%) children at cutpoints of ≥1 or ≥5, respectively. In univariate analyses, OSAS was associated with higher levels of habitual snoring, lower waking pulse oxygen saturation (Spo2), reduced lung function, less caretaker education, and non–preterm birth. Lower sleep-related Spo2 metrics were also associated with higher obstructive apnea hypopnea indices. In multivariable analyses, habitual snoring and lower waking Spo2 remained risk factors for OSAS in children with SCA.
CONCLUSIONS:
The prevalence of OSAS in children with SCA is higher than in the general pediatric population. Habitual snoring and lower waking Spo2 values, data easily obtained in routine care, were the strongest OSAS risk factors. Because OSAS is a treatable condition with adverse health outcomes, greater efforts are needed to screen, diagnose, and treat OSAS in this high-risk, vulnerable population.
Keywords: sickle cell anemia, obstructive sleep apnea, polysomnography, sleep disorders, epidemiology, cohort study, blood disorders, sleep medicine
What’s Known on This Subject:
Obstructive sleep apnea syndrome (OSAS) prevalence in children with sickle cell anemia is not well described. Although these children often experience nocturnal oxygen desaturation, it is unclear whether they are more likely to have OSAS.
What This Study Adds:
Children with sickle cell anemia have a high prevalence of OSAS with typical symptoms, beyond just nocturnal oxyhemoglobin desaturation. This study supports the need for increased efforts to screen for, diagnose, and treat OSAS in this vulnerable population.
Obstructive sleep apnea syndrome (OSAS) is a disorder of breathing during sleep in which episodic upper airway collapse disrupts ventilation and leads to oxyhemoglobin desaturation and poor sleep quality. This common pediatric disorder, with a prevalence of 1% to 5%, is associated with adverse health outcomes, such as behavioral problems, daytime sleepiness, cognitive deficits, cardiovascular changes, and reduced quality of life.1,2 In adults, untreated OSAS is associated with increased risk of incident cardiovascular disease and stroke.3 Adenotonsillar hypertrophy is the most commonly identified risk factor for childhood OSAS and adenotonsillectomy is effective treatment. Otherwise normal children with OSAS are more likely to be African-American,4–7 exposed to environmental tobacco smoke,7 born preterm,5 have undergone adenotonsillectomy,8 and live in disadvantaged neighborhoods.9,10 It is unknown whether these risk factors generalize to children with sickle cell anemia (SCA).
SCA affects 1 in 600 African-Americans and is characterized by chronic hemolytic anemia and complications related to recurrent vaso-occlusion. One of the strongest triggers for vaso-occlusion is oxyhemoglobin desaturation, which has been linked to several complications of SCA, such as increased pain,11 greater risk of central nervous system events,12 cognitive dysfunction,13 and history of acute chest syndrome.14 Oxyhemoglobin desaturation is a well-known phenomenon in SCA resulting from a combination of calibration of the pulse oximeter for hemoglobin A, and not hemoglobin S, a rightward shift of the oxyhemoglobin dissociation curve, and dyshemoglobins, which are elevated in the presence of intravascular hemolysis but incapable of transporting oxygen. Because desaturation is common to both SCA and OSAS, the relationship between SCA and OSAS is of great interest.
The prevalence of OSAS in children with SCA is not well defined, and there is uncertainty whether OSAS is more common in this disorder.15,16 Previously reported prevalence rates range from 5% to 79%. Except for 1 study,17,18 prevalence estimates have been based on samples clinically referred for OSAS symptoms19–23 or with desaturation.24,25 Furthermore, clinicians are uncertain whether the same symptoms, signs, and risk factors associated with OSAS in otherwise healthy children have clinical utility in screening for OSAS in children with SCA.1
We used data from participants in the prospective Sleep and Asthma Cohort Study who were unselected for OSAS symptoms to investigate the prevalence and risk factors for OSAS in children with SCA. We hypothesized that (1) children with SCA will have a higher than expected prevalence of OSAS for children of African heritage and (2) higher obstructive apnea hypopnea indices (OAHIs) will be associated with more OSAS symptoms and greater nocturnal oxyhemoglobin desaturation.
Methods
A detailed description of the testing procedures and statistical approach is provided in the Supplemental Information. In brief, we conducted a cross-sectional analysis of baseline data collected as part of the Sleep and Asthma Cohort Study, a prospective observational cohort of children ages 4 to 18 years with SCA (homozygous for sickle cell hemoglobin [HbSS] or compound heterozygotes for sickle β thalassemia zero [HbSβ0]), designed to evaluate the contribution of asthma and sleep abnormalities to SCA-related morbidity. Participants were recruited (66% participation rate) from 3 pediatric centers: St Louis, Missouri; Cleveland, Ohio; and London, United Kingdom. Children were enrolled without regard to past morbidity from or symptoms of sleep-disordered breathing or asthma. Children were ineligible for participation if they themselves were smokers, receiving long-term blood transfusions, or receiving long-term continuous positive airway pressure therapy at the time of enrollment; were participating in a clinical trial evaluating blood transfusion, oxygen, or hydroxyurea therapy; had chronic lung disease (other than asthma); or were HIV positive. Participants placed on transfusion therapy during the study were retained in the cohort. Institutional review boards for each site approved the study protocol. Written informed parental consent and patient assent were obtained for all participants.
Questionnaires and Definitions
Parents/primary caretakers of participants completed standardized questionnaires about demographic characteristics and medical history, including asthma, allergies, and sleep.26–29 Asthma was defined as a “yes” response to any of the following 3 questions: (1) “Has a doctor ever said that the participant has asthma?”, (2) “Does the participant take any asthma medications?”, and (3) “Does the participant still have asthma?” Hayfever was defined as a “yes” response to “Has a doctor ever said that the participant has hayfever?” Household tobacco smoke exposure was defined as a “yes” response to current exposure.
Habitual snoring was assessed by using the sleep questionnaire by response to “How many times did your child snore in the previous month?” and was defined as snoring ≥3 nights per week. Trouble breathing or witnessed apnea were defined when these symptoms occurred at least “sometimes” (1 to 2 times per week). Enuresis was defined as bedwetting at least 1 to 2 times per week. Previous tonsillectomy or adenoidectomy was indicated by a “yes” response to removal of tonsils or adenoids. Family history of OSAS was defined as positive if there were any “yes” responses to a family member either diagnosed with OSAS or using continuous positive airway pressure. Sleepiness was assessed by using the Epworth Sleepiness Scale modified for children, in which scores range from 0 to 24, with higher scores indicating greater daytime sleepiness.29
Physiologic assessments included standardized measurements of height, weight, BMI converted to age- and gender-adjusted z scores,30 and blood pressure converted to age-, gender-, and height-adjusted systolic and diastolic percentiles based on auscultatory normative data for children with SCA.31 Allergy skin tests using the prick puncture technique with the multitest (Lincoln Diagnostics, Decatur, IL) to 9 aeroallergens were performed as previously reported.32 Atopy was defined as having at least 2 positive skin tests.
Spirometry measures (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], and the ratio of FEV1 to FVC [FEV1:FVC]) were performed by study-certified technicians with the use of standardized protocols33 and compared with normative data for healthy black children.34,35 Waking pulse oxygen saturation (Spo2) values were collected by using a standardized 5-minute period. Other laboratory data were collected as part of clinical care.
Children underwent full channel, in-laboratory polysomnography by study-certified technicians with standardized protocols and equipment following American Academy of Sleep Medicine guidelines for data acquisition and scoring, except for carbon dioxide, which was not collected.36 Because there is no consensus about what OAHI threshold should be used to define OSAS status, OAHI data were summarized by using several commonly used pediatric cutpoints: OAHI ≥1,37 OAHI ≥1 plus habitual snoring,37,38 OAHI ≥1.5,39 OAHI ≥2,40 and OAHI ≥5.4–6,38 Primary snoring was defined as an OAHI value of <1 with habitual snoring 3 to 5 nights per week.
Statistical Analyses
Means, SDs, medians, and interquartile ranges were computed for continuous variables. Frequencies and percentages were computed for categorical variables. Continuous data were analyzed by using analysis of variance or the nonparametric equivalent for nonnormal distributions. Categorical data were analyzed with a χ2 test, and the Fisher’s exact test was used where expected cell counts did not meet minimum requirements. Logistic regression was used for the multivariable analyses to assess the association between potential risk factors and OSAS status. For analytic purposes, the dependent variable, OAHI, was categorized into 3 OSAS status groups: no OSAS (OAHI <1), mild OSAS (1 ≤ OAHI <5), and moderate to severe OSAS (OAHI ≥5). Associations between the risk factors and OSAS status were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). All tests for significance were 2-tailed. P values of <.05 were considered significant. All analyses were performed by using SPSS version 21 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
Results
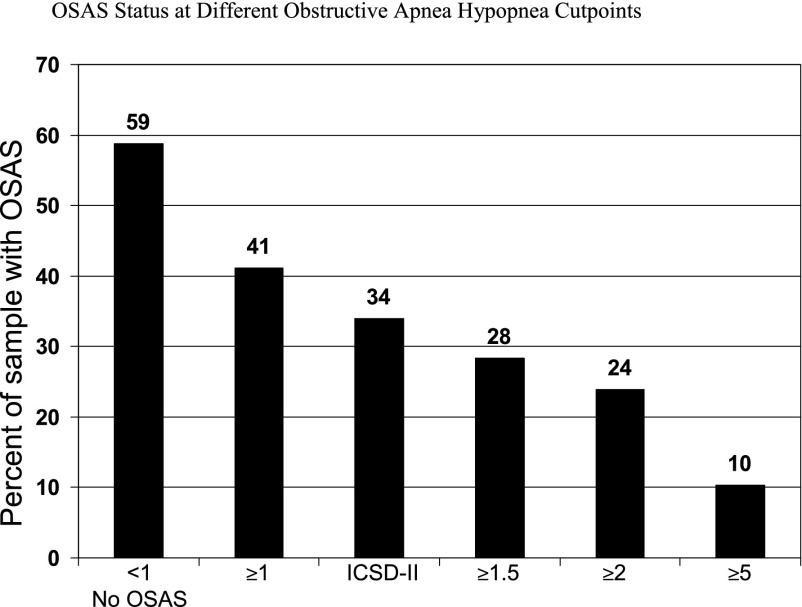
The final analytic sample included 243 children: 59 (24%) from Cleveland, 94 (39%) from London, and 90 (37%) from St Louis. The median age was 10 years, 50% were boys, 99% had African heritage, and 95% were homozygous for HbSS. The OAHI data were not normally distributed: mean (SD, range) = 2.1 (4.6, 0–37.2), whereas the median (interquartile range) was 0.6 (0.2–1.9). Figure 1 shows the prevalence of OSAS based on commonly used OAHI cutpoints and definitions: 41% for an OAHI ≥1, 34% for an OAHI ≥1 plus habitual snoring, 28% for an OAHI ≥1.5, 24% for an OAHI ≥2, and 10% for an OAHI ≥5. Among the 143 (59%) participants with OAHI values <1, 21% were habitual snorers (at least 3–5 nights per week) and 48% snored at least 1 to 2 nights per week.
FIGURE 1.
OSAS status at different OAHI cutpoints. ICSD-II, International Classification of Sleep Disorders II.
Participant Characteristics and OSAS Symptoms by OAHI Categories
Table 1 shows associations of participant characteristics and OSAS symptoms by OAHI categories. The frequency of OSAS symptoms (habitual snoring, trouble breathing, witnessed apnea, nocturnal enuresis) increased with higher OAHI values but not for nocturnal restlessness or daytime sleepiness. Only less caretaker education varied significantly with increasing OAHI values, whereas age, male gender, and household income did not. Lower waking Spo2 values were significantly associated with greater OAHI values, whereas lower hemoglobin values, higher BMI z scores, and elevated blood pressure were not. Participants with SCA were rarely obese. Greater OAHI values were significantly associated with reduced lung function (lower FEV1 and FEV1:FVC), but not with lower FVC. Preterm birth was associated with lower, not higher, apnea hypopnea index (OAHI) values. Other potential OSAS risk factors, such as family history of OSAS, previous adenotonsillectomy, and other allergic or inflammatory conditions (asthma, hayfever, atopy, and environmental tobacco smoke exposure), were not associated with increasing OAHI. Hydroxyurea prescription was caretaker reported for 10% of the sample but was not associated with lower OAHI (P = .46).
TABLE 1.
Distribution of Participant Characteristics and OSAS Symptoms by OAHI Categories
| All (N = 243) | OAHI <1 (n = 143; 59%) | OAHI ≥1 and <2 (n = 42; 17%) | OAHI ≥2 and <5 (n = 33; 14%) | OAHI ≥5 (n = 25; 10%) | P | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age, mean (SD), y | 10.6 (4.2) | 10.6 | 11.1 | 11.4 | 8.8 | .11 |
| Male gender, % | 50.2 | 47.6 | 50.0 | 48.5 | 68.0 | .31 |
| Caretaker education less than high school (n reported = 239), % | 39.3 | 33.1 | 50.0 | 57.6 | 32.0 | .03 |
| Household income <$30 000 (n reported = 207), % | 59.9 | 56.3 | 73.0 | 61.3 | 55.0 | .32 |
| Sleep and breathing symptoms | ||||||
| Habitual snoring ≥3 nights/week (n reported = 232), % | 34.0 | 21.1 | 36.8 | 50.0 | 80.0 | <.001 |
| Trouble breathing ≥1 night/week (n reported = 219), % | 19.2 | 14.0 | 13.2 | 25.9 | 48.0 | .001 |
| Witnessed apnea ≥1 night/week (n reported = 218), % | 9.2 | 5.6 | 2.6 | 14.3 | 32.0 | <.001 |
| Nocturnal enuresis, ≥1 night/week (n reported = 227), % | 30.0 | 27.6 | 20.0 | 32.9 | 56.0 | .016 |
| Restless, ≥1 night/week, % | 31.7 | 32.9 | 33.3 | 30.3 | 24.0 | .84 |
| Epworth Sleepiness Scalea (n reported = 237), mean (SD) | 7.1 (4.2) | 7.0 | 6.9 | 8.1 | 7.0 | .59 |
| Physiologic measurements | ||||||
| Hemoglobin, mean (SD), g/dL | 8.2 (1.2) | 8.4 | 8.1 | 8.0 | 7.9 | .10 |
| Waking Spo2 (n reported = 236), median (IQR), % | 97.1 (94.0 to 98.6) | 98.3 | 96.5 | 94.0 | 95.8 | <.001 |
| BMI z score (n reported = 241), median (IQR) | 0.02 (−0.71 to 0.82) | 0.06 | 0.04 | −0.10 | −0.02 | .90 |
| Systolic blood pressure >90th percentile (n reported = 241), % | 23.7 | 27.7 | 11.9 | 21.2 | 24.0 | .21 |
| Diastolic blood pressure >90th percentile (n reported = 240), % | 2.5 | 2.1 | 2.4 | 3.0 | 4.0 | .95 |
| Atopy (≥2 positive skin tests; n reported = 217), % | 26.3 | 25.0 | 26.3 | 37.9 | 18.2 | .41 |
| FVC (n reported = 241), % predicted | 92.2 (14.3) | 93.8 | 89.6 | 92.0 | 87.8 | .13 |
| FEV1 (n reported = 241), % predicted | 88.0 (13.8) | 90.7 | 84.3 | 83.6 | 84.4 | .003 |
| FEV1:FVC (n reported = 241) | 0.86 (0.07) | 0.87 | 0.85 | 0.81 | 0.87 | <.001 |
| Other medical and/or family history, % | ||||||
| Previous tonsillectomy or adenoidectomy (n reported = 240) | 19.6 | 17.6 | 28.6 | 16.1 | 20.0 | .43 |
| Household smoking exposure (n reported = 241) | 28.6 | 27.0 | 23.8 | 39.4 | 32.0 | .44 |
| Asthma | 27.6 | 23.1 | 35.7 | 33.3 | 32.0 | .30 |
| Hayfever (n reported = 196) | 18.9 | 20.0 | 15.8 | 18.5 | 18.8 | .95 |
| Preterm birth (n reported = 233) | 12.0 | 17.0 | 7.5 | 0.0 | 8.0 | .03 |
| Prescribed hydroxyurea at time of polysomnography (n reported = 218) | 10.3 | 12.6 | 9.5 | 6.1 | 4.0 | .46 |
| Family history of OSAS or CPAP use (n reported = 239) | 10.0 | 9.2 | 17.1 | 12.5 | 0.0 | .15 |
Continuous data with normal distributions are reported as means (SD), whereas data with nonnormal distributions are reported as medians (IQR). Categorical data are reported as a percentage of the entire sample (N = 243) or numbers (n) reported. IQR, interquartile range; CPAP, continuous positive airway pressure.
The range for the Epworth Sleepiness Scale score is 0 to 24, with higher scores indicating greater daytime sleepiness and normal values <11 in children.
Polysomnography Findings and Oximetry Metrics by Increasing OAHI Cutpoints
Polysomnography results stratified by OAHI cutpoints are shown in Table 2 for cardiorespiratory data and are shown in Table 3 for sleep data. Higher OAHI cutpoints were significantly associated with higher respiratory and desaturation indices and lower Spo2 metrics. Obstructive apneas had a negligible contribution to the overall OAHI at cutpoints <2. Central apneas tended to be more frequent with higher OAHIs, but remained within the normal range. Higher arousal index was the only sleep variable associated with higher OAHI cutpoints (P < .001).
TABLE 2.
Cardiorespiratory Polysomnography Variables by OAHI Diagnostic Cutpoints
| Characteristic | All (N = 243) | OAHI <1 (n = 143) | OAHI ≥1 (n = 100) | OAHI ≥2 (n = 58) | OAHI ≥5 (n = 25) | P |
|---|---|---|---|---|---|---|
| OAHI | ||||||
| In NREM, median (IQR) | 0.23 (0.0–1.18) | 0.0 | 0.85 | 1.68 | 4.62 | <.001 |
| In REM, median (IQR) | 2.0 (0.0–8.0) | 1.00 | 4.5 | 11.0 | 47.0 | <.001 |
| Median (IQR) | 0.0 | 0.0 | 0.0 | 0.13 | 0.71 | <.001 |
| Central apnea index, median (IQR) | 0.29 (0.12–0.68) | 0.24 | 0.50 | 0.35 | 0.38 | .005 |
| Sleep-wake Spo2 difference (n reported = 236), mean (SD), % | −0.85 (1.74) | −0.63 | −0.90 | −1.24 | −1.46 | .073 |
| Spo2, % | ||||||
| During sleep, median (IQR) | 96.4 (93.8–98.1) | 97.7 | 96.0 | 92.5 | 93.9 | <.001 |
| During NREM sleep, median (IQR) | 96.4 (94.0–98.8) | 97.5 | 95.9 | 92.4 | 94.4 | <.001 |
| In REM sleep (n reported = 242), median (IQR) | 96.7 (93.8–98.4) | 98.0 | 96.0 | 92.5 | 93.0 | <.001 |
| Minimum NREM Spo2, median (IQR), % | 92.0 (86.6–95.2) | 93.8 | 90.8 | 85.9 | 85.1 | <.001 |
| Minimum REM Spo2, median (IQR), % | 91.7 (84.8–95.0) | 93.3 | 90.0 | 84.0 | 76.0 | <.001 |
| 3% Desaturation index | ||||||
| Median (IQR) | 2.2 (0.6–6.3) | 0.9 | 3.2 | 7.0 | 15.9 | <.001 |
| In NREM, median (IQR) | 1.40 (0.39–4.44) | 0.70 | 2.35 | 4.90 | 8.33 | <.001 |
| In REM, median (IQR) | 3.79 (1.06–10.52) | 1.44 | 5.74 | 11.87 | 39.04 | <.001 |
| Sleep time with Spo2 <95%, median (IQR), % | 6.2 (0.2–81.2) | 0.4 | 20.2 | 88.9 | 68.2 | <.001 |
| Sleep time with Spo2 <92%, median (IQR), % | 0.07 (0–8.91) | 0.02 | 0.115 | 46.7 | 7.66 | <.001 |
| Sleep time with Spo2 <90%, median (IQR), % | 0.02 (0.0–0.6) | 0.0 | 0.03 | 1.74 | 1.91 | <.001 |
| Sleep time with Spo2 <85%, median (IQR), % | 0.0 (0.0–0.4) | 0.0 | 0.0 | 0.06 | 0.69 | <.001 |
| Heart rate asleep (n reported = 242), mean (SD), beats/min | 84.5 (10.7) | 82.9 | 83.7 | 86.6 | 92.3 | <.001 |
Continuous data with normal distributions are reported as means (SD), whereas data with nonnormal distributions are reported as medians (IQR). Categorical data are reported as a percentage of the entire sample (N = 243) or the numbers (n) of participants reported. IQR, interquartile range; NREM, nonrapid eye movement; REM, rapid eye movement.
TABLE 3.
Sleep Polysomnography Variables by OAHI Diagnostic Cutpoints
| Characteristic | All (N = 243) | OAHI <1 (n = 143) | OAHI ≥1 (n = 100) | OAHI ≥2 (n = 58) | OAHI ≥5 (n = 25) | P |
|---|---|---|---|---|---|---|
| Total sleep time, mean (SD), min | 438.7 (68.2) | 438.4 | 450.2 | 431.5 | 430.9 | .60 |
| Sleep latency, median (IQR), min | 20.5 | 20.0 | 24.8 | 20.5 | 15.0 | .88 |
| REM latency, median (IQR), min | 83.5 | 80.8 | 89.0 | 92.0 | 90.0 | .28 |
| Sleep efficiency, median (IQR), % | 85.5 (78.6–89.5) | 85.9 | 84.5 | 84.4 | 82.2 | .21 |
| NREM stage 1 sleep, mean (SD), % | 5.2 (2.7) | 5.0 | 5.0 | 5.9 | 5.8 | .22 |
| NREM stage 2 sleep, mean (SD), % | 45.6 (8.2) | 45.5 | 46.7 | 45.5 | 44.7 | .78 |
| NREM stage 3 sleep, mean (SD), % | 22.5 (8.1) | 22.4 | 20.9 | 22.7 | 25.7 | .13 |
| REM sleep, mean (SD), % | 26.6 (6.3) | 27.0 | 27.3 | 25.9 | 23.9 | .10 |
| Supine sleep time, mean (SD), % | 46 (0.26) | .47 | .42 | 41 | 57 | .09 |
| Arousal index, mean (SD) events/hr | 8.6 (3.0) | 8.2 | 8.3 | 9.1 | 10.9 | <.001 |
Continuous data with normal distributions are reported as means (SD), whereas data with nonnormal distributions are reported as medians (IQR). IQR, interquartile range; NREM, nonrapid eye movement; REM, rapid eye movement.
Risk Factors for OSAS: Multivariable Models Using 2 OSAS Severity Groups
Table 4 summarizes the final multivariable models in which the dependent variable, OSAS status, is categorized into 3 groups: no OSAS (OAHI <1), mild OSAS (1 ≤ OAHI <5), and moderate to severe OSAS (OAHI ≥5). When the β-value is negative, significant associations will have ORs <1, which means that a lower variable value is associated with a greater odds of being a member of the OSAS group. To improve clinical interpretability of the significant, but large, negative ORs, we “reformatted” waking Spo2 values <96% to present the effect with an OR >1 and recoded preterm birth as non–preterm birth.
TABLE 4.
Final Models of Logistic Regression Predicting OSAS at 2 Severity Levels
| β | OR | 95% CI | |
|---|---|---|---|
| No OSAS versus 1 ≤ OAHI <5 | |||
| Male gender | 0.331 | 1.39 | 0.66–2.92 |
| Age | 0.031 | 1.01 | 0.93–1.11 |
| Waking Spo2 values <96%a | −2.065a | 0.13 | 0.06–0.27 |
| 7.89a | 3.66–17.02a | ||
| Habitual snoring | 1.016 | 2.76 | 1.24–6.17 |
| FEV1, percent predicteda | −0.041 | 0.96 | 0.93–0.99 |
| Caretaker education < high school | 0.546 | 1.73 | 0.71–4.22 |
| Non–preterm birth | 1.606 | 4.98 | 1.18–20.97 |
| Environmental tobacco smoke exposure | 0.550 | 1.73 | 0.77–3.92 |
| No OSAS versus OAHI ≥5 | |||
| Male gender | 0.836 | 2.31 | 0.64–8.25 |
| Age | −0.122 | 0.89 | 0.77–1.02 |
| Waking Spo2 values <96%a | −1.706a | 0.18 | 0.05–0.61 |
| 5.51a | 1.631–18.61a | ||
| Habitual snoring | 2.829 | 16.93 | 4.98–57.50 |
| FEV1, % predicted | −0.320 | 0.97 | 0.93–1.01 |
| Caretaker education less than high school | 0.500 | 1.65 | 0.35–7.69 |
| Non–preterm birth | 1.026 | 2.79 | 0.41–18.18 |
| Environmental tobacco smoke exposure | −0.247 | 0.78 | 0.21–2.94 |
When the β-value is negative, significant associations will have ORs <1, which means that a lower variable value is associated with a greater odds of being a member of an OSAS group. To improve clinical interpretability of the significant but negative ORs, we “reformatted” some of them to present the effect with an OR >1.
There were 218 participants in the 2 groups: a no-OSAS group and a mild-OSAS group. In the screening model with 13 variables of interest, 186 of 218 participants had full data (loss of 14.7%) and the following 6 variables passed screening with P < .20: habitual snoring, waking Spo2 <96%, FEV1 percent predicted, environmental tobacco smoke exposure, caretaker education less than high school, and non–preterm birth. By using the 6 variables from the first step with the 186 participants to estimate a reduced model, multivariable analyses revealed that 4 variables were significantly and independently associated with a greater odds of mild OSAS: waking Spo2 values <96% (OR: 7.89; 95% CI: 3.66–17.02; P < .001), habitual snoring (OR: 2.76; 95% CI: 1.24–6.17; P = .013), lower FEV1 percent predicted (OR: 0.96; 95% CI: 0.93–0.99; P = .010), and non–preterm birth (OR: 4.98; 95% CI: 1.1–20.97; P = .028). The final model was 57.6% accurate at predicting mild OSAS in this sample (sensitivity = 0.78, specificity = 0.67; given a prevalence rate of 31% for mild OSAS in this sample, the positive predictive value was 0.52 and the negative predictive value was 0.87).
For no OSAS versus moderate to severe OSAS (OAHI ≥5), there were 168 participants. With the use of the 4 variables from the final model for mild OSAS (plus age and gender) in the model for moderate to severe OSAS (OAHI ≥5), there were 148 participants with full data (loss of 11.9%). Multivariable analyses revealed that only 2 factors significantly and independently associated with moderate to severe OSAS status: habitual snoring (OR: 16.93; 95% CI: 4.98–57.5; P < .001) and lower waking Spo2 <96% (OR: 5.51; 95% CI: 1.63–18.61; P = .006). This final model was 56% accurate at predicting moderate to severe OSAS status in this sample (sensitivity = 0.92, specificity = 0.78; given a prevalence rate of 10% for moderate to severe OSAS status, the positive predictive value was 0.32 and the negative predictive value was 0.99).
Discussion
This article summarizes clinical characteristics and polysomnography data from the largest sample of children with SCA, unreferred for signs or symptoms of OSAS. The OSAS prevalence rates, ranging from 41% at a cutpoint of an OAHI ≥1 to 10% at a cutpoint of an OAHI ≥5, are higher than those reported in otherwise normal children (1%–5% at a cutpoint ≥5),1,5,6 in African-American children (8.7% at a cutpoint ≥5),5 and in a Brazilian cohort of children with SCA (10.6% at a cutpoint ≥1).18
Habitual snoring was a strong risk factor for OSAS in children with SCA, although many of the previously reported risk factors for OSAS for otherwise healthy children were not observed, such as age, gender, obesity, hypertension, daytime sleepiness, asthma, hayfever, atopy, environmental tobacco smoke exposure, family history of OSAS, previous adenotonsillectomy, and preterm birth. The association of enuresis with OSAS was previously reported for this cohort.41 Lower caretaker education was a risk factor in univariate analyses, but not in multivariable analysis. Other unique risk factors to SCA were suggested by univariate analyses (lower waking Spo2 values and reduced lung function). However, in multivariable analyses, only lower waking Spo2 was retained at both OSAS categories. Lower FEV1 was associated with the mild OAHI category, but not at the higher OAHI category.
Higher OAHI values were not associated with asthma or hayfever. It is possible that our definitions of asthma and allergy, based on parent report of symptoms, were not reliable enough to pick up this association. A parent report of doctor-diagnosed asthma has been associated with an increased rate of pain and acute chest syndrome,42–46 so this definition has clinical relevance in this SCA sample. By using more quantitative measures of allergy and respiratory disease, we found that atopy was not associated with OSAS, but reduced FEV1 and FEV1:FVC were, suggesting an association of lower airway disease with OSAS. There was no evidence that hydroxyurea prescription was associated with a lower OSAS prevalence, but this study was not designed to evaluate whether hydroxyurea treatment decreases the risk of sleep-disordered breathing.
In otherwise healthy children, habitual snoring is sensitive but not very specific for predicting OSAS. Lower waking Spo2 had the strongest association with mild OSAS followed by habitual snoring, but this order was reversed for OAHI values ≥5, suggesting that OAHI values in the milder range may be less specific for OSAS. The models were only slightly more than 50% predictive of polysomnography-defined OSAS at either range, similar to the positive and predictive values of history and physical examination (65% and 46%, respectively) for the diagnosis of OSAS in otherwise healthy children, which led to the recommendation that objective testing such as polysomnography be used in the diagnosis of OSAS.2 This recommendation becomes even more important in children with SCA in whom other disturbances to oxygen delivery such as anemia, oxyhemoglobin desaturation, and other pulmonary dysfunction are often present.
Although we evaluated OSAS prevalence and risk factors on the basis of commonly used OAHI cutpoints for children, the gold standard for OSAS diagnosis is not polysomnography alone, but rather the skillful integration of clinical and polysomnography findings by experienced clinicians.16 The AHI is a popular metric to determine the presence of OSAS, but there is no consensus on how the AHI should be used to indicate disease severity. Previous studies have shown that the AHI was not useful in predicting either baseline neurobehavioral morbidity or response to OSAS treatment in children in the general population.40,47–53 Even “primary snoring,” that is snoring with an AHI <1, has been associated with neurobehavioral morbidity.54–56 For these reasons, we cannot identify an AHI threshold at which OSAS needs to be addressed. Because the study was based on cross-sectional data, the impact of OSAS or greater hypoxemia on health outcomes in children with SCA cannot be assessed. Further outcomes studies are required to determine what threshold is associated with SCA-related morbidity.
Although OSAS appears to be 1 contributor to sleep-disordered breathing in SCA, the oximetry metrics reveal that some children also experience more sustained nocturnal desaturation. Greater desaturation time was associated with higher OAHI cutpoints, but desaturation can also be related to comorbid lower respiratory tract pathology. In our sample with SCA, the oximetry metrics for sleep time of <95% and <92% were similar to the upper limits of normal reported for otherwise healthy children.57–59 Nevertheless, the oximetry and lung function findings suggest that a portion of the sleep-disordered breathing in children with SCA may be an indicator of lower respiratory tract problems rather than a specific marker of upper airway obstruction and OSAS.
The strengths of this study include participants who were unselected for symptoms of OSAS, the large multicenter sample, and objective assessment of sleep-disordered breathing by standardized full polysomnography scored centrally without clinical correlates. Nevertheless, several limitations are noted. With a participation rate of 66%, it is possible that the sample included families with greater concerns about sleep-disordered breathing, leading to higher OSAS prevalence rates. We recognize that questionnaire data and parent report of symptoms are imperfect measures of the presence and severity of OSAS2 and potential OSAS risk factors. On the other hand, in this cohort, parent report of wheezing symptoms was a better predictor of a physician diagnosis of asthma than lung function testing.60 In terms of data collection, tonsil size was not assessed, end-tidal carbon dioxide was not measured, and daytime behavioral problems were not evaluated, so the assessment of OSAS signs, symptoms, and consequences could have been more comprehensive. Finally, although validated in comparison with arterial saturation in patients with SCA,61 simple pulse oximetry may underestimate oxygenation status because the dyshemoglobins are not measured.62
Conclusions
This study confirms that children with SCA have a higher prevalence of sleep-disordered breathing consistent with OSAS, beyond greater nocturnal desaturation, and that these children experience typical nocturnal symptoms of snoring and breathing/sleep disturbances. Because OSAS is a treatable condition with adverse health outcomes, greater efforts are needed to implement and evaluate procedures for screening, diagnosing, and treating OSAS in this high-risk, vulnerable population.
Supplementary Material
Acknowledgments
We thank the following individuals at the 3 study sites and the coordinating site (Vanderbilt University, Nashville TN): St Louis site: Washington University: Joshua Field, MD, Lisa Garrett, RN, CCRP, Pamela Bates, CRT, RPFT, PRSGT, Rick Talbert, RPSGT, Sabrina Lockett, RPSGT, Valerie Morgan, RRT, Phillip Blanks, and Tinishia Greene. Cleveland site: Case Western Reserve University: Susan Surovec, BA, Nancy Scott, BS, REEG/EPT, RPSGT, REDT, CNIM, and Mary DeBarr, RN, BSN. London site: University College of London Institute of Child Health and Great Ormond Street Hospital: Jane Kirkby, BSc, Satwinder Sahota, BSc, Liam Welsh, PhD, Ursula Johnson, RN, Aidan Laverty, MSc, MBCS, and Anne O’Reilly; North Middlesex Hospital: Anne Yardumian, MD, FRCP, and Marilyn Roberts-Harewood, MRCPCH; University of York: Avijit Kumar Datta, MD, MRCP; Nashville: Ms Dionna O. Roberts, MPH, MS.
Glossary
- AHI
apnea hypopnea index
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HbS
sickle cell hemoglobin
- OAHI
obstructive apnea hypopnea index
- OSAS
obstructive sleep apnea syndrome
- SCA
sickle cell anemia
- Spo2
pulse oxygen saturation
Footnotes
Dr Rosen helped develop the data collection instruments for sleep, supervised sleep data collection at 1 site, designed the concepts for this manuscript, interpreted the data, created the initial draft, and revised the manuscript for final submission; Dr Debaun was the principal investigator for the National Institutes of Health–sponsored Sleep and Asthma Cohort (SAC) project and the principal investigator for 1 site and reviewed and helped revise the manuscript; Dr Strunk contributed to the development of the SAC project, study concepts, and procedures; supervised the quality assurance for pulmonary function testing; and reviewed and helped revise the manuscript; Dr Redline developed the procedures for sleep data collection and analysis, was the principal investigator for 1 site, oversaw the quality of the acquired sleep study data, and reviewed and helped revise the manuscript; Dr Seicean performed data acquisition and analysis at 1 site and reviewed and helped revise the manuscript; Dr Craven contributed as a coinvestigator at 1 site, supervised patient data collection, monitored patient safety, and reviewed and helped revise the manuscript; Ms Gavlak performed data acquisition and analysis at 1 site, monitored patient safety, and reviewed and helped revise the manuscript; Drs Wilkey, Inusa, and Roberts were principal investigators for 1 site, supervised patient data collection, monitored patient safety, and reviewed and helped revise the manuscript; Mr Goodpaster was responsible for sleep data analysis, management, and quality assurance and reviewed and helped revise the manuscript; Dr Malow supervised the sleep data analysis, management, and quality assurance and reviewed and helped revised the manuscript; Dr Rodeghier developed the statistical approach, performed those analyses, and reviewed the integrity of those analyses; Dr Kirkham was the principal investigator for 1 site, helped develop the concepts for this SAC project and this manuscript, analyzed and interpreted the data, contributed to the development of the initial draft, and reviewed/revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were supported in part by the National Heart, Lung, and Blood Institute (NIH 1R01HL079937 [Dr Debaun], UL1 RR024989 Case Western Reserve University Clinical Research Unit and by Research and Development in the National Health Service (United Kingdom). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584 [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3). Available at: www.pediatrics.org/cgi/content/full/130/3/e714 [DOI] [PubMed] [Google Scholar]
- 3.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728 [DOI] [PubMed] [Google Scholar]
- 4.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 pt 1):1527–1532 [DOI] [PubMed] [Google Scholar]
- 5.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142(4):383–389 [DOI] [PubMed] [Google Scholar]
- 6.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstock TG, Rosen CL, Marcus CL, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep. 2014;37(2):261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton S, Rosen C, Larkin E, Tishler P, Aylor J, Redline S. Predictors of sleep-disordered breathing in children with a history of tonsillectomy and/or adenoidectomy. Sleep. 2001;24(7):823–829 [DOI] [PubMed] [Google Scholar]
- 9.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr.2011;158(5):789–795, e781 [DOI] [PubMed]
- 10.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342–347 [DOI] [PubMed] [Google Scholar]
- 11.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101(3):846–848 [DOI] [PubMed] [Google Scholar]
- 12.Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357(9269):1656–1659 [DOI] [PubMed] [Google Scholar]
- 13.Hollocks MJ, Kok TB, Kirkham FJ, et al. Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. J Int Neuropsychol Soc. 2012;18(1):168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood. 1993;81(12):3422–3427 [PubMed] [Google Scholar]
- 15.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3):389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3):389–398 [DOI] [PMC free article] [PubMed]
- 17.Salles C, Ramos RT, Daltro C, Nascimento VM, Matos MA. Association between adenotonsillar hypertrophy, tonsillitis and painful crises in sickle cell disease. J Pediatr (Rio J). 2009;85(3):249–253 [DOI] [PubMed] [Google Scholar]
- 18.Salles C, Ramos RT, Daltro C, Barral A, Marinho JM, Matos MA. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. J Bras Pneumol. 2009;35(11):1075–1083 [DOI] [PubMed] [Google Scholar]
- 19.Brooks LJ, Koziol SM, Chiarucci KM, Berman BW. Does sleep-disordered breathing contribute to the clinical severity of sickle cell anemia? J Pediatr Hematol Oncol. 1996;18(2):135–139 [DOI] [PubMed] [Google Scholar]
- 20.Maddern BR, Reed HT, Ohene-Frempong K, Beckerman RC. Obstructive sleep apnea syndrome in sickle cell disease. Ann Otol Rhinol Laryngol. 1989;98(3):174–178 [DOI] [PubMed] [Google Scholar]
- 21.Samuels MP, Stebbens VA, Davies SC, Picton-Jones E, Southall DP. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67(7):925–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaleyias J, Mostofi N, Grant M, et al. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30(9):659–665 [DOI] [PubMed] [Google Scholar]
- 23.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6(4):374–381 [PMC free article] [PubMed] [Google Scholar]
- 24.Needleman JP, Franco ME, Varlotta L, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28(6):418–422 [DOI] [PubMed] [Google Scholar]
- 25.Spivey JF, Uong EC, Strunk R, Boslaugh SE, DeBaun MR. Low daytime pulse oximetry reading is associated with nocturnal desaturation and obstructive sleep apnea in children with sickle cell anemia. Pediatr Blood Cancer. 2008;50(2):359–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6 pt 2):1–120 [PubMed] [Google Scholar]
- 27.Childhood Asthma Management Program Research Group . The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999;20(1):91–120 [PubMed] [Google Scholar]
- 28.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051 [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545 [DOI] [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;Jun 8(314):1–27 [PubMed] [Google Scholar]
- 31.Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102(2):171–177 [DOI] [PubMed] [Google Scholar]
- 32.Field JJ, Stocks J, Kirkham FJ, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139(3):563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strunk RC, Szefler SJ, Phillips BR, et al. Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute . Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112(5):883–892 [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG, Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88 [DOI] [PubMed] [Google Scholar]
- 35.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968 [DOI] [PubMed] [Google Scholar]
- 36.Iber C, Ancoli-Israel S, Chesson A, Quan S, American Academy of Sleep Medicine The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 37.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005
- 38.Li AM, Au CT, Ng SK, et al. Natural history and predictors for progression of mild childhood obstructive sleep apnoea. Thorax. 2010;65(1):27–31 [DOI] [PubMed] [Google Scholar]
- 39.Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus CL, Moore RH, Rosen CL, et al. Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann GC, Bell TR, Kirkham FJ, et al. Enuresis associated with sleep disordered breathing in children with sickle cell anemia. J Urol. 2012;188(4 suppl):1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glassberg JA, Chow A, Wisnivesky J, Hoffman R, Debaun MR, Richardson LD. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br J Haematol. 2012;159(4):472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92(8):1115–1118 [DOI] [PubMed] [Google Scholar]
- 44.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glassberg J, Spivey JF, Strunk R, Boslaugh S, DeBaun MR. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. J Pediatr Hematol Oncol. 2006;28(8):481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leong MA, Dampier C, Varlotta L, Allen JL. Airway hyperreactivity in children with sickle cell disease. J Pediatr. 1997;131(2):278–283 [DOI] [PubMed] [Google Scholar]
- 47.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313–320 [DOI] [PubMed] [Google Scholar]
- 48.O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111(3):554–563 [DOI] [PubMed] [Google Scholar]
- 49.Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, Amin R. Neuropsychological effects of pediatric obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10(7):962–975 [DOI] [PubMed] [Google Scholar]
- 50.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458–464 [DOI] [PubMed] [Google Scholar]
- 51.Huang YS, Chen NH, Li HY, Wu YY, Chao CC, Guilleminault C. Sleep disorders in Taiwanese children with attention deficit/hyperactivity disorder. J Sleep Res. 2004;13(3):269–277 [DOI] [PubMed] [Google Scholar]
- 52.Mulvaney SA, Goodwin JL, Morgan WJ, Rosen GR, Quan SF, Kaemingk KL. Behavior problems associated with sleep disordered breathing in school-aged children—the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr Psychol. 2006;31(3):322–330 [DOI] [PubMed] [Google Scholar]
- 53.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–222 [DOI] [PubMed] [Google Scholar]
- 54.Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000;22(5):554–568 [DOI] [PubMed] [Google Scholar]
- 55.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44–49 [DOI] [PubMed] [Google Scholar]
- 56.Emancipator JL, Storfer-Isser A, Taylor HG, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160(2):203–210 [DOI] [PubMed] [Google Scholar]
- 57.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753 [DOI] [PubMed] [Google Scholar]
- 58.Tapia IE, Karamessinis L, Bandla P, et al. Polysomnographic values in children undergoing puberty: pediatric vs. adult respiratory rules in adolescents. Sleep. 2008;31(12):1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol. 2007;42(2):159–167 [DOI] [PubMed] [Google Scholar]
- 60.Strunk RC, Cohen RT, Cooper BP, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164(4):821–826, e821 [DOI] [PMC free article] [PubMed]
- 61.Fitzgerald RK, Johnson A. Pulse oximetry in sickle cell anemia. Crit Care Med. 2001;29(9):1803–1806 [DOI] [PubMed] [Google Scholar]
- 62.Caboot JB, Jawad AF, McDonough JM, et al. Non-invasive measurements of carboxyhemoglobin and methemoglobin in children with sickle cell disease. Pediatr Pulmonol. 2012;47(8):808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.