Abstract
OBJECTIVE:
To examine whether there is a different clinical profile and severity of invasive pneumococcal disease (IPD) in children caused by nonvaccine types in the era of 13-valent pneumococcal conjugate vaccine (PCV13).
METHODS:
Observational study of childhood IPD in Massachusetts based on state public health surveillance data comparing pre-PCV13 (2007–2009) and post-PCV13 (2010–2012) eras.
RESULTS:
There were 168 pre-PCV13 cases of IPD and 85 post-PCV13 cases of IPD in Massachusetts children ≤5 years of age. PCV13 serotypes declined by 18% in the first 2 years after PCV13 use (P = .011). In the post-PCV13 phase, a higher proportion of children were hospitalized (57.6% vs 50.6%), and a higher proportion of children had comorbidity (23.5% vs 19.6%). Neither difference was statistically significant, nor were comparisons of IPD caused by vaccine and nonvaccine types. Children with comorbidities had higher rates of IPD caused by a nonvaccine type (27.6% vs 17.2%; P = .085), were more likely to be hospitalized (80.4% vs 50%; P < .0001), and were more likely to have a longer hospital stay (median of 3 days vs 0.5 days; P = .0001).
CONCLUSIONS:
Initial data suggest that nonvaccine serotypes are more common in children with underlying conditions, who have greater morbidity from disease. In the post-PCV13 era, a larger proportion of patients are hospitalized, but mortality rates are unchanged. Routine vaccination with PCV13 may not be enough to reduce the risk in patients with comorbidity.
Keywords: invasive pneumococcal disease, conjugate vaccine, children, comorbidity, severity
What’s Known on This Subject:
Invasive pneumococcal disease causes enormous morbidity in children. The spectrum and severity of illness caused by pneumococcal serotypes not present in the current vaccine, and whether the clinical profile and severity of disease have changed, are largely unknown.
What This Study Adds:
Initial data suggest that nonvaccine serotypes are more common in children with underlying conditions, who have greater morbidity from disease. In the post-PCV13 era, a larger proportion of patients are hospitalized, but mortality rates are unchanged.
Streptococcus pneumoniae is a pathogen that normally occupies the nasopharynx of healthy children but also causes otitis media and invasive syndromes such as meningitis, pneumonia, and sepsis.1 Invasive pneumococcal disease (IPD) causes 1 million deaths per year worldwide2,3; in the United States before the conjugate vaccine era, pneumococcal disease was estimated to cause 3000 cases of meningitis, 50 000 cases of bacteremia, and 500 000 cases of pneumonia annually, with bacteremia and meningitis occurring primarily in young children.4
The introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) led to a substantial decrease in IPD overall and specifically vaccine serotype (VT) disease; however, nonvaccine serotypes (NVTs) subsequently increased.5 In addition, the population at risk changed in the post-PCV7 era, with IPD affecting a greater proportion of children with comorbidity and chronic illness.6 One of the primary goals of introducing the broader spectrum 13-valent pneumococcal conjugate vaccine (PCV13) in February 2010 was to provide protection against the clinically relevant non-PCV7 serotypes, specifically 19A, 7F, and 6A, which caused a substantial burden of disease in the PCV7 era. We hypothesized that if the remaining non-PCV13 serotypes are intrinsically less virulent, IPD in the PCV13 era will be less severe.
The objective of the study was to describe the clinical features and outcomes of IPD in children ≤5 years of age in Massachusetts and to examine the epidemiology of IPD and differences in the clinical profile and severity of IPD before and after the introduction of PCV13.
Methods
This study was designed as a population-based observational study based on Massachusetts’ public health surveillance data on IPD in 2 periods: the pre-PCV13 era, from September 2007 to August 2009, and the post-PCV13 era, from September 2010 to August 2012. Cases were compared across age groups (<2 months, 2–23 months, and 2–5 years). A case of childhood IPD was defined by a positive culture for S. pneumoniae from normally sterile body fluid, from a Massachusetts resident ≤5 years of age. The outcomes compared were characteristics of IPD, including patient characteristics, clinical features, microbiologic features, comorbidities and other risk factors, and environmental factors.
In 2010, Massachusetts had a population of 6.6 million, of whom 1.4 million, or 22%, are children ≤18 years old.7 The percentage of children <5 years of age was 5.6%.8 The study population included all cases of IPD in children ≤5 years old who had an IPD diagnosis in Massachusetts.
Full-scale distribution of PCV7 according to guidelines began in July 2000 in Massachusetts, where all recommended childhood vaccines are purchased and distributed by the Massachusetts Department of Public Health (MDPH) to both public and private health care providers. Immunization uptake of ≥3 of PCV7 among children aged 19 to 35 months in Massachusetts in 2011 was 98%.9 In February 2010, PCV13 was approved for use in all children aged 6 weeks to 60 months, and up to 71 months of age in those with comorbidity, and replaced PCV7. All doses of PCV13 for both routine administration and supplemental doses are supplied universally by MDPH.
Increased statewide surveillance for IPD has been in place since October 2001. Each pediatric IPD case in Massachusetts is reportable, and clinical microbiology laboratories in Massachusetts are requested to provide all S. pneumoniae isolates recovered from sterile sites from children <18 years of age to MDPH. MDPH epidemiologists, in collaboration with local boards of health, collect a standardized set of data on age, gender, race, vaccination status, clinical presentation, and underlying medical conditions of the case and interview parents or guardians to document day care attendance and household information such as occupancy number.
Serotyping is performed on available isolates at the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, using the Quellung reaction with pneumococcal antisera (Statens Serum Institute, Copenhagen, Denmark). Antibiotic susceptibilities to penicillin, ceftriaxone, and azithromycin are determined by E-test, and minimum inhibitory concentration interpretations are based on current Clinical and Laboratory Standards Institute guidelines.
Statistical Methods
Patient characteristics were compared by using the Fisher’s exact test for categorical variables and the Wilcoxon or Kruskal–Wallis test for continuous variables. In analyzing length of hospitalization, we used the Wilcoxon test by ranking children who were not hospitalized as though they had the shortest stays and those who died during hospitalization as though they had the longest stays. We investigated the effect of VT on disease severity using logistic regression models, adjusting for gender, age group, and comorbidity as potential confounders. All data management was performed with Microsoft Excel 2011 version 14.3.6 (Microsoft Corporation, Redmond, WA), and statistical analyses were performed with SAS version 9.3 (SAS Institute, Inc, Cary, NC). The significant level for all tests was set at 5%. P values were not adjusted, but because numerous P values were calculated, all results were conservatively interpreted.
This study was approved by the institutional review boards of the University of Minnesota and the MDPH.
Results
Demographics and Patient Characteristics
There were 168 cases of IPD from 2007 to 2009 and 85 cases of IPD from 2010 to 2012 in Massachusetts children ≤5 years of age (Table 1). This was equivalent to incidence rates of 46 per 100 000 pre-PCV13 and 23 per 100 000 post-PCV13, with an estimated change in IPD rate being 23 per 100 000 (95% confidence interval, 14–31; P < .00001). In terms of absolute numbers, children 2 to 23 months of age formed the largest group with IPD in both pre- and post-PCV13 phases, comprising 53.0% and 56.5% of patients, respectively (Table 2). Caucasians were the predominant race, making up 47.0% of cases in the pre-PCV13 and 41.2% in the post-PCV13 phase (Table 1). In the pre-PCV13 phase, 59.5% were up to date on their PCV immunization, compared with 58.8% in the post-PCV13 phase. Comparing VT and NVT, 56.9% of patients with VT-IPD were up to date with their PCV immunization, compared with 67.8% of patients who had NVT-IPD, although this difference was not statistically significant (P = .145; Table 3).
TABLE 1.
Characteristics of Children With IPD in Pre-PCV13 and Post-PCV13 Periods
| Pre-PCV13, n (%) (n = 168) | Post-PCV13, n (%) (n = 85) | P | |
|---|---|---|---|
| Patient characteristics | |||
| Age group | .810 | ||
| <2 mo | 7 (4.2) | 2 (2.4) | |
| 2–23 mo | 89 (53.0) | 48 (56.5) | |
| 2–5 y | 72 (42.9) | 35 (41.2) | |
| Male | 93 (55.4) | 47 (55.3) | 1.000 |
| Race | .975 | ||
| White | 79 (47.0) | 35 (41.2) | |
| Black | 18 (10.7) | 11 (12.9) | |
| Asian | 7 (4.2) | 4 (4.7) | |
| American Indian | 1 (0.6) | 0 (0) | |
| Other | 18 (10.7) | 7 (8.2) | |
| Unknown | 45 (26.8) | 28 (32.9) | |
| Comorbidity | 33 (19.6) | 20 (23.5) | .510 |
| PCV immunization | 1.000 | ||
| Up to date | 100 (59.5) | 50 (58.8) | |
| PPV23 immunization | .552 | ||
| Received | 3 (1.8) | 0 (0) | |
| Clinical features | |||
| Site of isolation | .682 | ||
| Cerebrospinal fluid | 2 (1.2) | 1 (1.2) | |
| Blood | 159 (94.6) | 78 (91.8) | |
| Pleural fluid | 2 (1.2) | 3 (3.5) | |
| Synovial fluid | 1 (0.6) | 0 (0) | |
| Pancreatic fluid | 1 (0.6) | 0 (0) | |
| Symptoms | 72 (42.9) | 46 (54.1) | .109 |
| Fever | 49 (29.2) | 34 (40) | .090 |
| Microbiologic features | |||
| PCV7 serotype | |||
| 18C | 1 (0.6) | 1 (1.2) | 1.000 |
| 19F | 1 (0.6) | 0 (0) | 1.000 |
| PCV13 serotype | |||
| 3 | 0 (0) | 2 (2.4) | 1.000 |
| 7F | 16 (9.5) | 8 (9.4) | .823 |
| 19A | 67 (39.9) | 17 (20) | <.0001 |
| NVT | 40 (23.8) | 47 (55.3) | <.0001 |
| Outcomes | |||
| Hospitalized | 85 (50.6) | 49 (57.6) | .333 |
| Length of hospital stay, median [range, d] | 3 [0–42] | 4 [0–17] | .210 |
| Readmitted after discharge | 7 (4.2) | 2 (2.4) | .722 |
| Mortality | 6 (3.6) | 3 (3.5) | 1.000 |
PPV23, 23-valent pneumococcal polysaccharide vaccine.
TABLE 2.
Age-Related Counts of Children With IPD in Pre-PCV13 and Post-PCV13 Periods
| Pre-PCV13, n (%) (n = 168) | Post-PCV13, n (%) (n = 85) | |
|---|---|---|
| IPD | ||
| <2 mo | 7 (4.2) | 2 (2.4) |
| 2–23 mo | 89 (53.0) | 48 (56.5) |
| 2–5 y | 72 (42.9) | 35 (41.2) |
| VT-IPDa | ||
| <2 mo | 3 (1.8) | 0 (0) |
| 2–23 mo | 44 (26.2) | 14 (16.5) |
| 2–5 y | 41 (24.4) | 14 (16.5) |
| NVT-IPDa | ||
| <2 mo | 2 (1.2) | 1 (1.2) |
| 2–23 mo | 24 (14.3) | 27 (31.8) |
| 2–5 y | 14 (8.3) | 19 (22.4) |
Percentages do not add up to 100% because of incomplete data.
TABLE 3.
Characteristics of Children With VT-IPD and NVT-IPD
| VT-IPD, n (%) (n = 116) | NVT-IPD, n (%) (n = 87) | Unknown types-IPD, n (%) (n = 50) | P | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age group | .366 | |||
| <2 mo | 3 (2.6) | 3 (3.4) | 3 (6) | |
| 2–23 mo | 58 (50) | 51 (58.6) | 28 (56) | |
| 2–5 y | 55 (47.4) | 33 (37.9) | 19 (38) | |
| Male | 59 (50.9) | 51 (58.6) | 30 (60) | .320 |
| Race | .789 | |||
| White | 53 (45.7) | 38 (43.7) | 23 (46) | |
| Black | 13 (11.2) | 9 (10.3) | 6 (12) | |
| Asian | 6 (5.2) | 3 (3.4) | 2 (4) | |
| American Indian | 0 (0) | 1 (1.1) | 0 (0) | |
| Other | 14 (12.1) | 9 (10.3) | 3 (6) | |
| Unknown | 10 (8.6) | 9 (10.3) | 6 (12) | |
| No response | 20 (17.2) | 19 (21.8) | 10 (20) | |
| Comorbidity | 20 (17.2) | 24 (27.6) | 9 (18) | .085 |
| PCV immunization | .145 | |||
| Up to date | 66 (56.9) | 59 (67.8) | 25 (50) | |
| Clinical features | ||||
| Site of isolation | .147 | |||
| Cerebrospinal fluid | 1 (0.9) | 1 (1.1) | 0 (0) | |
| Blood | 108 (93.1) | 84 (96.6) | 47 (94) | |
| Pleural fluid | 4 (3.4) | 0 (0) | 1 (2) | |
| Synovial fluid | 1 (0.9) | 0 (0) | 0 (0) | |
| Pancreatic fluid | 0 (0) | 1 (1.1) | 0 (0) | |
| No response | 2 (1.7) | 1 (1.1) | 1 (2) | |
| Symptoms | 58 (50) | 38 (43.7) | 22 (44) | .397 |
| Fever | 41 (35.3) | 26 (29.9) | 16 (32) | |
| Outcomes | ||||
| Hospitalized | 66 (56.9) | 47 (54.0) | 21 (42) | .769 |
| Length of hospital stay, median (range, d) | 4 (0–16) | 3 (0–42) | 3 (0–15) | .519 |
| Readmitted after discharge | 6 (5.2) | 1 (1.1) | 2 (4) | .243 |
| Mortality | 5 (4.3) | 3 (3.4) | 1 (2) | 1.000 |
Clinical Features
The most common site of isolation of IPD was in the blood, 94.6% in the pre-PCV13 phase and 91.8% in the post-PCV13 phase. Although many different types of clinical infection were seen, and some patients had multiple types of infection (eg, clinical diagnoses of meningitis, otitis media, or cellulitis), there was no significant difference in the frequency of post-PCV13 compared with pre-PCV13 clinical diagnoses (data not shown). Fever was the most common symptom, 29.2% in the pre-PCV13 phase and 40% in the post-PCV13 phase, but this difference was not statistically significant (P = .09).
Microbiologic Features
The most common serotypes in the pre-PCV13 era were 19A (39.9%) and 7F (9.5%). PCV13 serotypes made up 51% of cases of IPD in the pre-PCV13 era and decreased to 33% in the first 2 years after PCV13. The estimated change in PCV13 serotypes was 17.7% (95% confidence interval, 4.2–31%; P = .0113). The most common serotype in the post-PCV13 era remained 19A (20%), although it decreased significantly (P < .001), followed by 7F (9.4%, P = .0823).
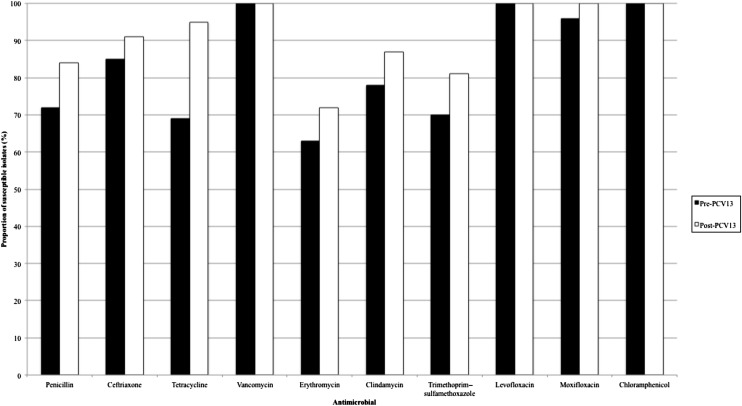
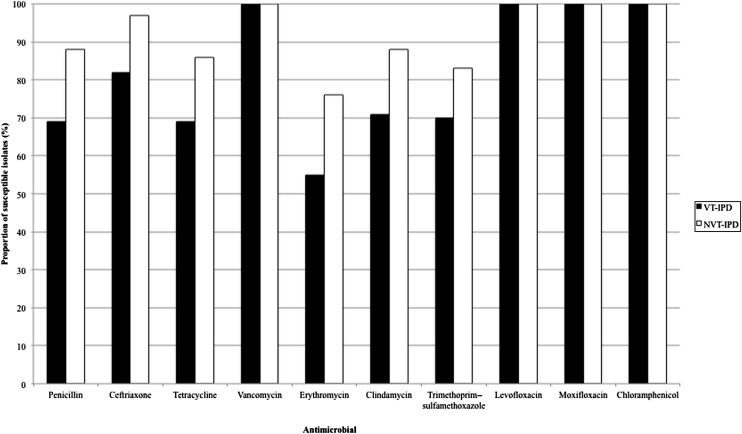
In analyzing antimicrobial susceptibility, we analyzed the isolates by using current Clinical and Laboratory Standards Institute breakpoints. Although a larger number of isolates were susceptible to penicillin, ceftriaxone, erythromycin, clindamycin, tetracycline, moxifloxacin, and trimethoprim–sulfamethoxazole in the post-PCV13 phase, the only statistically significant increase was in tetracycline (P = .028), although the increase in penicillin susceptibility also approached significance (P = .054; Fig 1). A larger proportion of NVTs were susceptible to all the antimicrobial agents tested; only penicillin, ceftriaxone, erythromycin, and trimethoprim–sulfamethoxazole had significant P values (P = .001, .002, .021, and .047, respectively; Fig 2).
FIGURE 1.
Antimicrobial susceptibility in pre-PCV13 and post-PCV13 periods.
FIGURE 2.
Antimicrobial susceptibility in VT-IPD and NVT-IPD.
Comorbidities
The most prevalent comorbidities listed were chronic lung disease, malignancy or immunosuppressive therapy, asthma, and sickle cell disease or asplenia (data not shown). In the pre-PCV13 phase, 19.6% of patients had comorbidity, compared with 23.5% in the post-PCV13 phase (P = .510); conversely, 17.2% of all patients with VT-IPD had a comorbidity, compared with 27.6% of patients with NVT-IPD (P = .085).
Outcomes
In the pre-PCV13 phase, 50.6% were hospitalized, compared with 57.6% in the post-PCV13 phase, and median length of hospital stay increased from 3 days to 4, although neither of the 2 variables was significantly different across the 2 phases (P = .333 and .210, respectively). Logistic regression revealed no significant association between vaccine phase and hospitalization when age and gender were controlled for (P = .241). We found that 80.4% of children with comorbidities were hospitalized, compared with 50% of children without comorbidities (P < .0001). After discharge, 4.2% in the pre-PCV13 phase and 2.4% in the post-PCV13 phase were readmitted (P = .722). Mortality for all children pre-PCV13 was 3.6% and post-PCV13 was 3.5% (P = 1.000). For children with comorbidities, data were underpowered to test the difference in mortality rates between the 2 phases.
When we compared hospitalization based on vaccine type from both time periods, 56.9% of patients with VT-IPD were hospitalized, compared with 54.0% of patients with NVT-IPD (P = .769; Table 3). When we controlled for age and gender, there was again no significant relationship between VT and hospitalization (P = .651), nor for VT and number of days hospitalized (P = .823). Children with comorbidities were hospitalized a median of 3 days and a range of 0 to 42 days, whereas children without comorbidities were hospitalized a median of 0.5 days and a range of 0 to 22 days (P = .0001). Readmission was 5.2% in the VT-IPD group and 1.1% in the NVT-IPD group (P = .243). Based on vaccine type, mortality was 4.3% of patients with VT-IPD and 3.4% in those with NVT-IPD (P = 1.000). Adjusting for comorbidity status, VT was not significantly associated with readmission, hospitalization, or mortality rates (P = .502, .140, and .241, respectively).
Discussion
Before the introduction of PCV7, S. pneumoniae caused ∼65 000 deaths in the United States, with highest rates in young children and older adults.4,10 The 7 pneumococcal serotypes chosen for the first conjugate vaccine accounted for >80% of invasive pneumococcal infections in children in the United States11 and also targeted the serotypes with the highest antimicrobial resistance rates.12 After licensure of PCV7 in 2000, rates of IPD fell from an average of 24.3 cases per 100 000 persons in 1998 and 1999 to 17.3 per 100 000 in 2001.13 There was a 76% decrease in IPD among children <5 years old, with the largest decline seen in children <2 years of age.13,14 However, IPD caused by non-PCV7 serotypes increased 140%.15 The most common non-PCV7 replacement serotype was serotype 19A, which increased 324% in children <5 years old,16 accounting for 28% of isolates among Alaskan Native children15 and 46% of non-PCV7 serotypes across 8 children’s hospitals nationwide.17
In addition, there has been a shift in the spectrum of disease caused by non-PCV7 serotypes. Before PCV7, serotype 19A was a common colonizer in the nasopharynx18 and the ninth most common cause of IPD in young children.17,19 After PCV7 was introduced, pneumococcal nasopharyngeal colonization from serotype 19A increased as children received more doses of PCV7, as did cases of otitis media caused by multidrug-resistant strains of serotype 19A.21 A 500% increase in cases of acute mastoiditis was attributed to serotype 19A.21 These cases were more likely to present with subperiosteal abscess and more likely to necessitate intraoperative mastoidectomy.21 Pneumococcal bacteremia caused by a vaccine-related serotype, part of the same serogroup but not same serotype in the vaccine, increased from 6% to 35% after PCV7 was introduced, with serotype 19A showing the greatest increase.22 The adaptation of pneumococci to the selective pressure of vaccination may result in a different clinical spectrum of disease.
The profile of children with IPD has also changed in the PCV7 era, where the mean age is higher than before, and the majority of these children are healthy, with no recognized risk factors.19,23 However, the percentage of children with IPD and an underlying comorbid condition increased from an average of 30% pre-PCV7 to >40%.17 In Massachusetts, a review of IPD cases over a 6-year period revealed that 16% of IPD cases occurred in children with comorbidities, with a 30% increase per year of life in the relative risk of having a high-risk condition in this population.6 In our study, the absolute numbers of pediatric patients with IPD decreased by 49%, but the proportion of patients with comorbidity increased by 17%, although these changes were not considered statistically significant. In addition, among patients with comorbidities who had IPD, a higher proportion of cases were caused by NVT isolates. This finding is similar to that of a study in England and Wales that showed NVT to be a more prevalent cause of IPD in children with chronic diseases and to be associated with greater mortality.24 The reason for this finding remains unknown, because NVT isolates are not considered to be as invasive or as prevalent as VT-IPD.25
Given that part of the aim in formulating PCV7 was to include serotypes associated with antimicrobial resistance, one would expect a decrease in antimicrobial resistance in pneumococcal isolates in the post-PCV era. We found a decrease in antimicrobial resistance among pneumococcal isolates, a result similar to those of other studies showing that antibiotic resistance among all isolates had decreased significantly overall.26 However, we found that NVT isolates were overall more susceptible than VT isolates, which is not consistent with the greater antimicrobial resistance noted among NVT isolates in a nationwide study involving 38 states in the post-PCV7 era.26,27
Serotype replacement in the nasopharynx since the introduction of PCV7 has led to disease caused by NVT isolates,28 whose associated features and morbidity have been less well described.29,30 However, taking hospitalization, length of hospitalization, readmission, and mortality as correlates of severity of disease, we were unable to show statistically significant differences in severity caused by serotype, although a trend toward lesser severity in NVT-IPD is visible.
This study is limited in that there were numerous missing data points, thereby making statistical analyses difficult. Because this study was restricted to a limited dataset, we were unable to examine additional variables; therefore, the quality of data depended on how completely and meticulously the health care workers filled out the case report form. In addition, the study phase was initiated within 6 months of introduction of PCV13 into the community, making it difficult to identify trends in such a short time span. We have not accounted for differences in clinical management or potential improvements in health care during this period, which could potentially confound the results.
Conclusions
We present population-based data that show a statistically significant decrease in rates of IPD and of IPD caused by vaccine-related serotypes since the introduction of PCV13. The data support a reduction in overall morbidity for invasive pneumococcal disease, with a trend toward children with underlying illness, and show that those with comorbid conditions have greater morbidity. Children with comorbidities were more likely to be hospitalized and to have longer hospital stays. Continued surveillance will reveal whether the trend toward less severe disease is sustained and whether morbidity is greater in patients with an underlying condition. Routine vaccination with PCV13 may not be sufficient to reduce the risk of invasive disease in patients with comorbidity, and administering 23-valent pneumococcal polysaccharide vaccine to all children with comorbidity at the earliest acceptable age should be considered. As health care continues to improve outcomes for children with chronic and comorbid conditions, preventive strategies targeting these at-risk populations must be considered.
Acknowledgments
We thank the MDPH staff for their work in collecting and compiling data for this study. We thank Molly Crockett, Noelle Cocoros, and Kerry O’Brien for their assistance on this project and Philippe Gaillard and Michael J. O’Connell for their assistance on statistical analysis.
Glossary
- IPD
invasive pneumococcal disease
- MDPH
Massachusetts Department of Public Health
- NVT
nonvaccine serotype
- PCV
pneumococcal conjugate vaccine
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- VT
vaccine serotype
Footnotes
Dr Iroh Tam conceptualized and designed the study and drafted the initial manuscript; Dr Madoff coordinated data collection at one site and critically reviewed the manuscript; Mr Coombes carried out the statistical analyses and reviewed the manuscript; Dr Pelton coordinated and supervised antimicrobial susceptibility testing of isolates at one site and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Iroh Tam received investigator-initiated grant support from Pfizer, Inc. (grant WS2420842) for this study. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award no. UL1TR000114). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Iroh Tam received investigator-initiated grant support from Pfizer for this study. Dr Pelton has received honoraria for advisory board service on pneumococcal vaccines from GSK Biologicals and Pfizer and has received investigator-initiated grants from Pfizer and Merck. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6(4):288–301 [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, et al. Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987 [DOI] [PubMed] [Google Scholar]
- 3.Rajaratnam JK, Marcus JR, Flaxman AD, et al. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet. 2010;375(9730):1988–2008 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). 1997. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/00047135.htm. Accessed May 1, 2010
- 5.Hsu KK, Shea KM, Stevenson AE, Pelton SI, Massachusetts Department of Public Health . Changing serotypes causing childhood invasive pneumococcal disease: Massachusetts, 2001–2007. Pediatr Infect Dis J. 2010;29(4):289–293 [DOI] [PubMed] [Google Scholar]
- 6.Hsu KK, Shea KM, Stevenson AE, Pelton SI, Members of the Massachusetts Department of Public Health . Underlying conditions in children with invasive pneumococcal disease in the conjugate vaccine era. Pediatr Infect Dis J. 2011;30(3):251–253 [DOI] [PubMed] [Google Scholar]
- 7.US Census Bureau. Age and sex composition: 2010. Available at: www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed April 23, 2013
- 8.US Census Bureau. QuickFacts from the US Census Bureau. 2011. Available at: http://quickfacts.census.gov/qfd/states/25000.html. Accessed April 23, 2013
- 9.Centers for Disease Control and Prevention (CDC). Estimated vaccination coverage with individual vaccines and selected vaccination series before 24 months of age by state and local area: US National Immunization Survey, Q1/2011–Q4/2011. 2011. Available at: www.cdc.gov/vaccines/stats-surv/nis/tables/11/tab09_24mo_iap_2011.pdf. Accessed April 23, 2013
- 10.Centers for Disease Control and Prevention (CDC). Provisional recommendations for the use of pneumococcal vaccines. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. 2008. Accessed May 2010
- 11.Feikin DR, Klugman KP. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis. 2002;35(5):547–555 [DOI] [PubMed] [Google Scholar]
- 12.Whitney CG, Farley MM, Hadler J, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network . Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343(26):1917–1924 [DOI] [PubMed] [Google Scholar]
- 13.Whitney CG, Farley MM, Hadler J, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network . Decline in invasive pneumococcal disease after the introduction of protein–polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–1746 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) . Invasive pneumococcal disease and 13-valent pneumococcal conjugate vaccine (PCV13) coverage among children aged ≤59 months: selected U.S. regions, 2010–2011. MMWR Morb Mortal Wkly Rep. 2011;60(43):1477–1481 [PubMed] [Google Scholar]
- 15.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–1792 [DOI] [PubMed] [Google Scholar]
- 16.Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network . Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125(3):429–436 [DOI] [PubMed] [Google Scholar]
- 18.Feikin DR, Davis M, Nwanyanwu OC, et al. Antibiotic resistance and serotype distribution of Streptococcus pneumoniae colonizing rural Malawian children. Pediatr Infect Dis J. 2003;22(6):564–567 [PubMed] [Google Scholar]
- 19.Robinson KA, Baughman W, Rothrock G, et al. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network . Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285(13):1729–1735 [DOI] [PubMed] [Google Scholar]
- 20.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–1778 [DOI] [PubMed] [Google Scholar]
- 21.Ongkasuwan J, Valdez TA, Hulten KG, Mason EO, Jr, Kaplan SL. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics. 2008;122(1):34–39 [DOI] [PubMed] [Google Scholar]
- 22.Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan KL. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin Infect Dis. 2006;42(7):907–914 [DOI] [PubMed] [Google Scholar]
- 23.Ampofo K, Pavia AT, Chris S, et al. The changing epidemiology of invasive pneumococcal disease at a tertiary children’s hospital through the 7-valent pneumococcal conjugate vaccine era: a case for continuous surveillance. Pediatr Infect Dis J. 2012;31(3):228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladhani SN, Andrews NJ, Waight P, Borrow R, Slack MP, Miller E. Invasive pneumococcal disease, comorbidities, and polysaccharide vaccine use in children aged 5–15 years in England and Wales. Clin Infect Dis. 2014;58(4):517–525 [DOI] [PubMed] [Google Scholar]
- 25.Pelton SI. The challenge of preventing invasive pneumococcal disease in children with comorbid illnesses. Clin Infect Dis. 2014;58(4):526–527 [DOI] [PubMed] [Google Scholar]
- 26.Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J. 2007;26(2):123–128 [DOI] [PubMed] [Google Scholar]
- 27.Mera R, Miller LA, Fritsche TR, Jones RN. Serotype replacement and multiple resistance in Streptococcus pneumoniae after the introduction of the conjugate pneumococcal vaccine. Microb Drug Resist. 2008;14(2):101–107 [DOI] [PubMed] [Google Scholar]
- 28.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196(9):1346–1354 [DOI] [PubMed] [Google Scholar]
- 29.Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46(2):174–182 [DOI] [PubMed] [Google Scholar]
- 30.Yildirim I, Stevenson A, Hsu KK, Pelton SI. Evolving picture of invasive pneumococcal disease in Massachusetts children: a comparison of disease in 2007–2009 with earlier periods. Pediatr Infect Dis J. 2012;31(10):1016–1021 [DOI] [PubMed] [Google Scholar]