Abstract
BACKGROUND AND OBJECTIVES:
Adenotonsillectomy for obstructive sleep apnea syndrome (OSAS) may lead to weight gain, which can have deleterious health effects when leading to obesity. However, previous data have been from nonrandomized uncontrolled studies, limiting inferences. This study examined the anthropometric changes over a 7-month interval in a randomized controlled trial of adenotonsillectomy for OSAS, the Childhood Adenotonsillectomy Trial.
METHODS:
A total of 464 children who had OSAS (average apnea/hypopnea index [AHI] 5.1/hour), aged 5 to 9.9 years, were randomized to Early Adenotonsillectomy (eAT) or Watchful Waiting and Supportive Care (WWSC). Polysomnography and anthropometry were performed at baseline and 7-month follow-up. Multivariable regression modeling was used to predict the change in weight and growth indices.
RESULTS:
Interval increases in the BMI z score (0.13 vs 0.31) was observed in both the WWSC and eAT intervention arms, respectively, but were greater with eAT (P < .0001). Statistical modeling showed that BMI z score increased significantly more in association with eAT after considering the influences of baseline weight and AHI. A greater proportion of overweight children randomized to eAT compared with WWSC developed obesity over the 7-month interval (52% vs 21%; P < .05). Race, gender, and follow-up AHI were not significantly associated with BMI z score change.
CONCLUSIONS:
eAT for OSAS in children results in clinically significant greater than expected weight gain, even in children overweight at baseline. The increase in adiposity in overweight children places them at further risk for OSAS and the adverse consequences of obesity. Monitoring weight, nutritional counseling, and encouragement of physical activity should be considered after eAT for OSAS.
Keywords: BMI, height, weight
What’s Known on This Subject:
Growth failure has been frequently reported in children who have obstructive sleep apnea syndrome (OSAS) owing to adenotonsillar hypertrophy. Adenotonsillectomy (AT) has been reported to accelerate weight gain in children who have OSAS in nonrandomized uncontrolled studies.
What This Study Adds:
This randomized controlled trial of AT for pediatric OSAS demonstrated significantly greater weight increases 7 months after AT in all weight categories. AT normalizes weight in children who have failure to thrive, but increases risk for obesity in overweight children.
Adequate growth trajectory is an important measure of wellness in children. Growth failure has been frequently reported (27%–56%) in children who have obstructive sleep apnea syndrome (OSAS).1–5 Adenotonsillar hypertrophy is the primary cause of OSAS in children, and is usually treated with adenotonsillectomy (AT). AT has been reported to accelerate weight6–14 in children with baseline failure to thrive (FTT),1,3,4,15 normal weight patients,9,11,14,16–20 obese individuals,9,13,16,21,22 and infants.10 The majority of studies also have demonstrated an increase in the height growth rate after AT for OSAS,3,6,11,17,23,24 but other studies reported no significant differences.9,12 Whereas accelerated weight gain post-AT is likely beneficial in the setting of baseline FTT, an exaggerated increase in adiposity in overweight children could increase their risk for OSAS recurrence and obesity-related morbidity.
The current study uses longitudinal anthropometric data from a large-scale, randomized controlled trial of AT for polysomnographically verified OSAS in a diverse sample of prepubertal children. The primary aim of the study is to determine if AT for OSAS leads to weight gain in children across a wide range of BMI. The secondary goal is to assess the influence of race, baseline weight, OSAS severity, and residual OSAS on growth after AT. Identifying children at risk for obesity after AT has considerable importance owing to the adverse consequences of childhood obesity.25
Methods
Study Sample and Recruitment
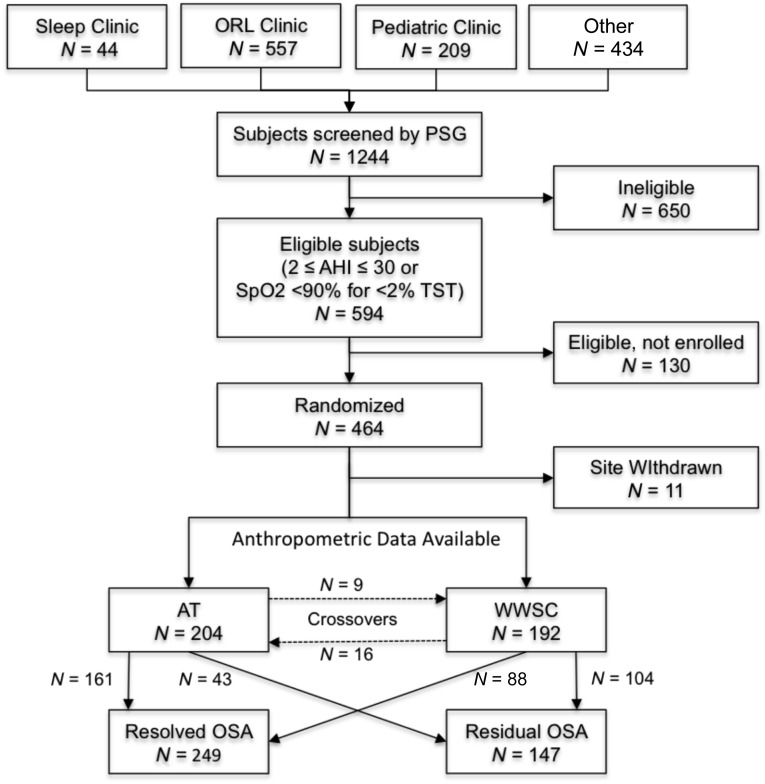
A detailed description of this multicenter, single-blind, randomized controlled trial of AT for OSAS in children has been published26 and the primary cognitive and behavioral outcomes have been reported.27 The influence of AT on growth was an a priori secondary outcome for this study. Briefly, children referred for evaluation of OSAS, tonsillar hypertrophy, or frequent snoring were recruited primarily from general pediatric, sleep, and otolaryngology clinics, as well as other community sources from January 2008 to September 2011 (Fig 1). Children were eligible for study entry if they were 5 to 9.9 years of age, had a history of snoring, tonsillar hypertrophy, and were considered to be surgical candidates for AT by an otolaryngologist. Exclusion criteria included a history of recurrent tonsillitis, extreme obesity (BMI z score ≥3), therapy for failure to thrive, medications for psychiatric or behavioral disorders (including attention deficit hyperactivity disorder), developmental delays requiring school accommodations, and known genetic, craniofacial, neurologic, or psychiatric conditions likely to affect the airway, cognition, or behavior. Children were screened further by standardized polysomnography (PSG). Children who had OSAS, defined as an obstructive apnea-hypopnea index (AHI) between 2 and 30/hour or an obstructive apnea index between 1 and 20/hour, and without prolonged oxygen desaturation time (arterial oxygen saturation [SpO2] <90% that was <2% of total sleep time) were eligible for study participation.
FIGURE 1.
Flow diagram of subject enrollment for whom anthropometric data were available.
Children were randomized to either early adenotonsillectomy (eAT; surgery within 4 weeks of randomization) or to Watchful Waiting with Supportive Care (WWSC). Repeat PSG and anthropometry were performed at approximately 7 months after randomization. The study was approved by the Institutional Review Board of each institution. Informed consent was obtained from caregivers, and assent from children ≥7 years of age.
Protocol
Anthropometric measurements were obtained at baseline and at 7-month follow-up using a standardized protocol by centrally trained and certified personnel. Measurements were made by a 2-member team that included a “measurer” and a “recorder.” All children underwent full, in-laboratory PSG by study-certified technicians according to a standardized protocol, using similar sensors, and following American Academy of Sleep Medicine guidelines.28 The AHI was defined as the numbers of obstructive apnea and hypopneas per hour of sleep. The arousal index was defined as the number of electrocortical arousals per hour of sleep. The oxygen desaturation index (ODI) was defined as the number of 3% oxygen desaturation per hour of sleep. The sleep duration and physical activity levels of each child were determined by parental questionnaire at the baseline visit. Weight classification definitions were based on percentiles for age and gender as follows: FTT, <5th percentile; normal, ≥5th and <85th; overweight, ≥85th and <95th; and obese, ≥95th.29
Statistical Considerations
Comparisons of demographic, sleep, activity, and polysomnographic data within and between groups were conducted by using unpaired t tests or χ2 and Fisher’s exact tests. The primary outcome was change in BMI z score, with secondary analyses examining change in absolute BMI, weight, weight z score, height, height z score, and BMI and Weight velocities (change in variable per time in years). The primary analysis was an intention to treat analysis comparing anthropometric outcomes in children randomized to eAT versus WWSC (noted as interval change between groups). Analyses were adjusted for factors that included site, age (5 to 7 vs 8 to 9 years), race (African American versus other), baseline weight status (overweight versus non-overweight), gender, season, and baseline AHI. A series of multivariable regression models were used to also consider the possible influences of physical activity, sleep duration, and various polysomnographic indices. Secondary analyses also examined groups defined according to therapy received (eAT versus WWSC) and according to resolution of OSAS at follow-up (AHI <2/hours and obstructive apnea index <1/hour) and tested for the presence of effect modification of treatment group with race, age, weight status, and gender. Analyses were conducted for the raw and z scores for weight, height, and BMI. Group differences were analyzed 3 ways; as an intention to treat analysis, as an analysis based on actual treatment received, and according to resolution of OSAS. Variables with highly skewed distributions were log transformed for analysis. Exploratory analyses were performed by using the reported sleep duration, daily running duration, and polysomnographic variables. Owing to the large number of 0 values, the percentage of time with an oxygen saturation <90% was included in the models as a binary variable (0 vs >0). Analyses were performed by using SAS 9.3 (SAS Institute, Inc, Cary, NC).
Results
Figure 1 demonstrates the flow of participants. Baseline anthropometric, sleep, and activity characteristics were not significantly different between intervention groups (Table 1). Approximately half of the subjects were overweight or obese. Follow-up anthropometric data were available for 98% of participants. Only 14 children were considered FTT at baseline (7 eAT, 7 WWSC). Initial analyses indicated that patterns of growth change were similar for FFT and normal weight children, and for overweight and obese children. Therefore, the weight classification data are reported as a binary variable, not overweight (<85th percentile) and overweight or obese (≥85th percentile).
TABLE 1.
Demographic, Sleep, and Activity Data
| eAT (n = 204) | WWSC (n = 192) | P value | |
|---|---|---|---|
| Age (y) | 7.03 (1.41) | 6.99 (1.39) | .73 |
| Gender (% female) | 54 | 49 | .12 |
| Race (% African American) | 55 | 54 | .74 |
| Failure to thrive (%) | |||
| Baseline | 3.4 | 3.6 | .58 |
| Follow-up | 0.9 | 2.2 | .25 |
| Interval change P value | .055 | .766 | .38 |
| Overweight and obese (%) | |||
| Baseline | 47.4 | 46.7 | .89 |
| Follow-up | 51.8 | 48.7 | .51 |
| Interval change P value | .34 | .67 | .15 |
| Obese (%) | |||
| Baseline | 32.7 | 33.5 | .87 |
| Follow-up | 36.7 | 35.0 | .69 |
| Interval change P value | .37 | .74 | .57 |
| Sleep duration (h) | |||
| Baseline | 9.46 (1.54) | 9.59 (1.39) | .40 |
| Follow-up | 9.38 (1.28) | 9.56 (1.30) | .17 |
| Interval change P value | .48 | .98 | .64 |
| Running (min/d) | |||
| Baseline | 5.22 (11.33) | 7.38 (12.40) | .05 |
| Follow-up | 6.76 (11.94) | 7.62 (12.82) | .49 |
| Interval change P value | .07 | .63 | .44 |
Mean (SD).
Baseline polysomnographic data were not significantly different between intervention groups (Table 2). At follow-up, the eAT group had greater reductions compared with the WWSC group in the AHI, arousal index, rapid eye movement (REM) ODI, and the percentage of sleep time <95% oxygen saturation (Table 2).
TABLE 2.
Polysomnographic Data
| eAT (n = 204) | WWSC (n = 192) | P value | |
|---|---|---|---|
| Apnea/hypopnea index (events/h) | |||
| Baseline | 5.22 (2.05) | 5.00 (2.12) | .46 |
| Follow-up | 0.71 (4.22) | 2.12 (5.47) | <.0001 |
| Interval change P value | <.0001 | <.0001 | <.0001 |
| Arousal index (events/h) | |||
| Baseline | 8.08 (1.43) | 7.85 (1.45) | .30 |
| Follow-up | 6.69 (1.42) | 7.69 (1.57) | .0007 |
| Interval change P value | <.0001 | .64 | <.0001 |
| Slow wave sleep (% TST) | |||
| Baseline | 31.5 (7.2) | 31.6 (7.6) | .84 |
| Follow-up | 29.9 (7.0) | 30.9 (6.8) | .14 |
| Interval change P value | .01 | .08 | .48 |
| REM sleep (% TST) | |||
| Baseline | 18.6 (4.2) | 18.2 (4.3) | .24 |
| Follow-up | 18.7 (4.0) | 17.8 (4.2) | .04 |
| Interval change P value | .85 | .36 | .61 |
| ODI in REM ≤3% (events/h) | |||
| Baseline | 10.6 (3.3) | 9.1 (3.6) | .21 |
| Follow-up | 3.9 (6.0) | 6.5 (3.9) | <.0001 |
| Interval change P value | <.0001 | .0008 | <.0001 |
| Oxygen saturation ≤95% (% of total sleep time) | |||
| Baseline | 1.8 (5.8) | 1.7 (5.8) | .73 |
| Follow-up | 0.8 (6.0) | 1.4 (5.5) | .004 |
| Interval change P value | <.0001 | .023 | .012 |
Mean (SD).
Weight/BMI
The weight, weight z scores, BMI, and BMI z scores all increased during the study interval in both the eAT and WWSC groups (Table 3). After adjusting for baseline weight status and other covariates, regression modeling demonstrated that eAT was associated with a significantly larger increase in the weight, weight velocity, weight z scores, BMI, BMI velocity, and BMI z scores, compared with the WWSC group. Multivariable regression modeling furthermore showed that BMI z score change was independently and positively associated with eAT, baseline BMI <85% percentile, and baseline but not follow-up AHI. After considering these variables, BMI z score change was not associated with age, gender, or race (Table 4). Exploratory models did not identify BMI z score change to be associated with reported duration of sleep or daily running activity. Of the polysomnographic measures, only the baseline REM ODI and decrease in REM ODI had a significant positive relationship to the interval change in the BMI z score (after adjusting for baseline AHI). There was no evidence of interactions between intervention arm and baseline weight status, race, age, or gender. The findings for the weight z score were generally similar to the BMI z score in all regression models.
TABLE 3.
Anthropometric Measures in the Early Adenotonsillectomy Compared With the Watchful Waiting Group at Baseline and Follow-up
| eAT (n = 204) | WWSC (n = 192) | Unadjusted P | P value 1 | P value 2 | |
|---|---|---|---|---|---|
| Wt (kg) | |||||
| Baseline | 31.21 (12.96) | 30.45 (12.37) | .524 | ||
| Follow-up | 34.58 (14.11) | 32.76 (12.60) | .175 | ||
| P value | <.0001 | <.0001 | |||
| Interval change between groups | .005 | .004 | .013 | ||
| Wt (z score) | |||||
| Baseline | 1.02 (1.32) | 0.99 (1.23) | .748 | ||
| Follow-up | 1.20 (1.22) | 1.03 (1.16) | .152 | ||
| P value | <.0001 | <.0001 | |||
| Interval change between groups | .003 | .001 | .001 | ||
| BMI (kg/m2) | |||||
| Baseline | 19.10 (5.02) | 18.92 (4.80) | .682 | ||
| Follow-up | 19.98 (5.27) | 19.27 (4.72) | .157 | ||
| P value | <.0001 | <.0001 | |||
| Interval change between groups | .015 | .014 | .026 | ||
| BMI (z score) | |||||
| Baseline | 0.87 (1.35) | 0.87 (1.25) | .998 | ||
| Follow-up | 1.18 (1.21) | 1.00 (1.27) | .163 | ||
| P value | <.0001 | <.0001 | |||
| Interval change between groups | .004 | .003 | .003 | ||
| Height (cm) | |||||
| Baseline | 125.5 (11.30) | 124.8 (10.76) | .503 | ||
| Follow-up | 129.2 (11.17) | 128.5 (10.57) | .479 | ||
| P value | <.0001 | <.0001 | |||
| Interval change between groups | .113 | .068 | .070 | ||
| Height (z score) | |||||
| Baseline | 0.69 (1.02) | 0.62 (0.99) | .445 | ||
| Follow-up | 0.74 (1.02) | 0.62 (0.96) | .235 | ||
| P value | .0022 | .2612 | |||
| Interval change between groups | .412 | .371 | .295 |
P value 1 adjusts for site, race (African American vs non-African American), age (5–7 vs 8–10 y), and weight (<85th vs ≥85th percentile).
P value 2 adjusts for site, race (African American vs non-African American), age (5–7 vs 8–10 y), and weight (<85th vs ≥85th percentile), gender, season (August to November vs other), baseline Log (AHI), and baseline value of outcome variable.
Mean (SD)
TABLE 4.
Regression Modeling to Predict the Change in BMI z score
| Variable | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | |
| eAT | 0.121 | 0.04 | .0031 | 0.116 | 0.04 | .0039 | 0.136 | 0.04 | .0019 |
| Race (African American) | 0.26 | 0.04 | .545 | 0.005 | 0.04 | .9141 | 0.021 | 0.04 | .629 |
| Weight <85% | 0.206 | 0.04 | <.0001 | 0.211 | 0.04 | <.0001 | 0.206 | 0.04 | <.0001 |
| Age (5 to 7 y) | 0.054 | 0.05 | .281 | 0.05 | 0.05 | .308 | 0.055 | 0.05 | .272 |
| Gender | −0.024 | 0.04 | .563 | ||||||
| Baseline AHI | 0.081 | 0.03 | .004 | ||||||
| Follow-up AHI | 0.012 | 0.01 | .397 | ||||||
Recruitment site was not a significant variable (not shown). Age variable was 5 to 7 vs 8 to 10 years.
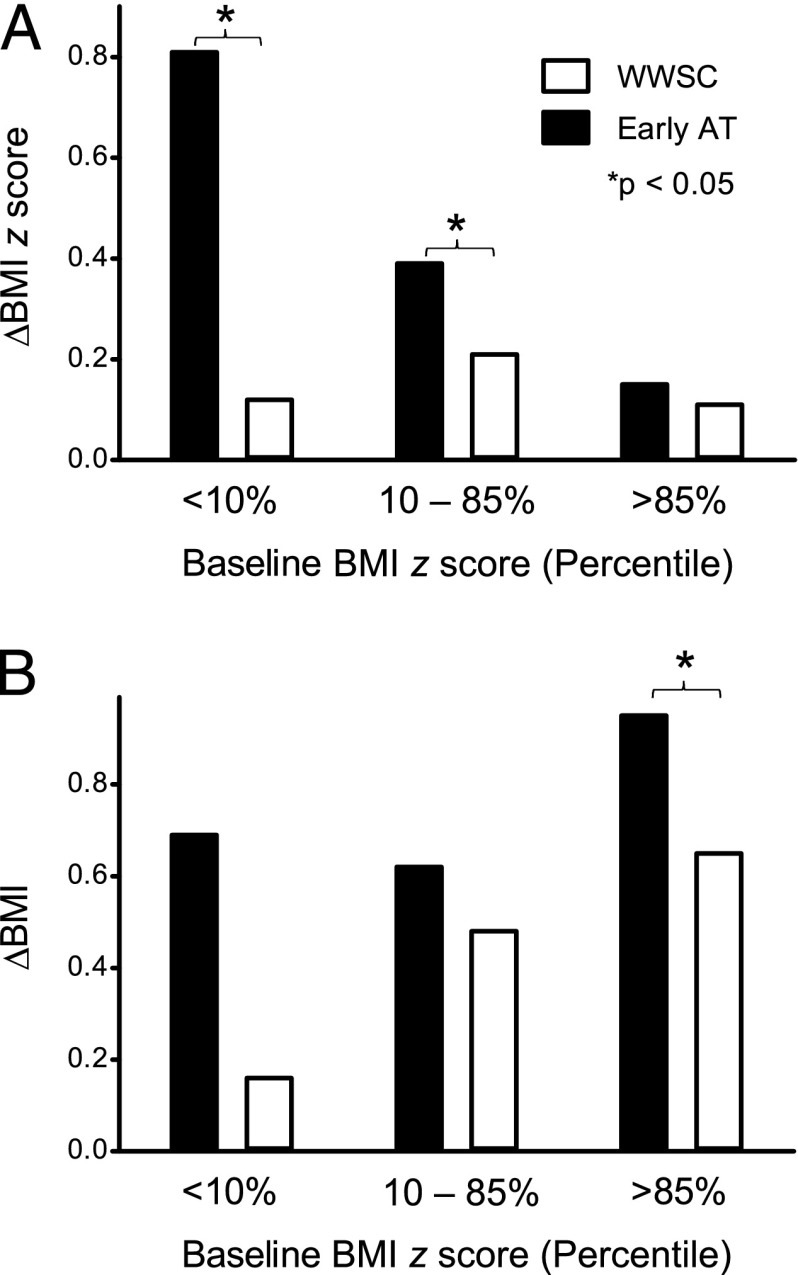
There were 14 children who were defined as FTT at baseline (7 eAT and 7 WWSC). In the eAT group, all 7 of these children increased their weight z scores at follow-up (P < .05), and entered the normal range. In the WWSC group, 5/7 of the FTT children increased their weight z score, 3 of whom entered the normal range (P = .13). Considering children who had a normal BMI, 16 children (15%) in the eAT group became overweight at follow-up, compared with 17 (17%) in the WWSC group (P = .72). Considering only children who were overweight at baseline, 14 (52%) in the eAT group became obese at follow-up, compared with only 5 (21%) in the WWSC group (P < .05). Both children <10th percentile and between the 10th and 85th percentile had a significant increase in the BMI z score in the eAT group compared with the WWSC group (Fig 2A). Children who were overweight at baseline and randomized to eAT had a larger absolute BMI change compared with comparable children randomized to WWSC (Fig 2B). Table 5 further shows the absolute weight change as a function of age, treatment group, and baseline weight.
FIGURE 2.
Change in the A, BMI z score, and B, absolute BMI for both treatment groups as a function of baseline BMI z score percentile. The change in BMI z score for children who had a baseline BMI z score either <10% or between the 10th and 85th percentile was significantly increased in the eAT group compared with the WWSC group. The absolute change in BMI for children who had a baseline BMI z score >85th percentile was significantly greater in the eAT group compared with the WWSC group.
TABLE 5.
Average Weight (kg) Gain Over 7-Month Study Interval
| Age (y) | eAT (n = 204) | WWSC (n = 192) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 5 | 6 | 7 | 8 | 9 | |
| FTT | 2.4 | 2.8 | NA | 0 | NA | 1.1 | 1.4 | NA | NA | NA |
| <10th | 2.2 | 2.3 | 2.2 | 2.6 | NA | 1.2 | 1.6 | 1.7 | 1 | 3.1 |
| Normal | 2.5 | 2.4 | 2.9 | 2.4 | 3 | 2 | 2.2 | 2.5 | 1.7 | 3 |
| Overweight | 3.6 | 2.5 | 3.9 | 2.7 | 6.8 | 1.6 | 1.7 | 4.1 | 3.9 | 3.9 |
| Obese | 4 | 5.1 | 4.5 | 4 | 4.7 | 2.6 | 3.4 | 4.7 | 4.3 | 4 |
FTT, <5th percentile; <10th, weight less than the 10th percentile; NA, not available.
Height
An increase in height over the 7-month follow-up period was observed in both the eAT and WWSC groups. The follow-up height z score was slightly but significantly higher in the eAT group (Table 3). However, the interval changes in height and height z score, as well as the height velocity measures (data not shown) were not significantly different between the eAT and WWSC groups. Height change was not associated with age, race, gender, treatment arm, site, weight status, baseline AHI, or follow-up AHI.
Other Secondary Analyses
Approximately 5% of children did not receive the assigned intervention do to parental preferences or treatment failure. There were no significant differences between the intention-to-treat analysis and that based on actual intervention received. Analyzing the changes in height, weight, and BMI as a “velocity” (expressed as changes over the individual time intervals between measurements) was comparable to the primary analyses. In an alternative analysis, children whose OSAS resolved did not differ in regard to change in anthropometric variables compared with children who did not have resolution of OSAS.
Discussion
This randomized controlled trial of eAT for polysomnographically confirmed pediatric OSAS revealed significantly greater increases in weight and BMI z score 7 months after AT as compared with WWSC. After adjusting for demographic variables and overweight status at baseline, eAT was associated with an average increase in BMI z score of 0.12 U compared with WWSC. Furthermore, we observed no evidence of a significant interaction between intervention group and baseline overweight status on change in BMI, indicating that BMI increases associated with eAT occurred in both overweight and non-overweight children. However, overweight but not normal weight children randomized to eAT were more likely to become obese at follow-up compared with children randomized to WWSC. Overweight and obese children also had an increase in the absolute BMI in the eAT compared with the WWSC group. Although not statistically significant, children who were initially classified as FFT tended to be more likely to develop a normal weight when treated with eAT as compared with WWSC. There was no evidence that the influence of eAT varied by gender, race, age, or baseline OSAS severity. Thus, these findings are consistent in demonstrating greater increases in weight in the 7 months after eAT compared with WWSC, and suggest that eAT results in a small overall increase in weight in children regardless of their baseline weight. Thus, in children who are initially FFT, eAT may have a positive effect on reaching targeted weight goals. In contrast, in children who are overweight at baseline, eAT may increase the short-term likelihood of developing obesity.
Several previous studies have also reported excessive weight gain post-AT in obese and non-obese children.16,21,22 Weight gain measured using population z scores has been reported to increase after AT in some uncontrolled studies,12 but not others.30–32 However, the observation that untreated children in the WWSC group also significantly increased their weight and BMI z scores during the 7-month follow-up interval underscores the importance of the randomized controlled design of the study in quantifying treatment effects. Previous longitudinal population-based anthropometric studies have observed that school-aged children are increasing their BMI z score over time.33 The explanation for the increasing incidence of obesity has been attributed to a shift toward sedentary lifestyles and high caloric food choices. Nevertheless, children in the eAT group had greater increases in weight and BMI z scores compared with WWSC controls over the study interval, suggesting that AT has an independent effect on weight gain in this population. Analyses showed that non-obese children had the greatest increases in BMI z score after AT, consistent with previous studies.34 Nevertheless, increases in the absolute BMI were also observed in the overweight and obese children, and overweight children treated with eAT were the ones most likely to develop obesity. Thus, the risk for worsening overweight and obesity after AT should be incorporated into the preoperative counseling for at-risk children.
Significant increases in height z scores after adenotonsillectomy for pediatric OSAS have been reported in many studies,3,11,14,16,18 but not others.9,12 Our results demonstrated no significant differences between the eAT and WWSC groups with regard to postoperative height, although in the eAT group there was a significant increase in the height z score after 7 months. Linear height is generally more resistant to changes in nutrition and growth hormone dysregulation than body weight. Also, 1 study reported that an increase in height post-AT was observed in the second 6-month postoperative period, but not the first.14 Furthermore, a study with a 5-year follow-up demonstrated a significantly increased height post-AT.35 Nevertheless, the observation that only the eAT group had a statistically significant increase in the height z scores over the study interval suggests that perhaps an association would be observed in a larger population of children, with more severe OSAS, or over a longer postoperative interval.
The baseline AHI was positively correlated with increases in weight and BMI z scores during the study interval regardless of treatment group or baseline BMI. There are 2 broad mechanisms by which OSAS could mediate alterations in growth. First, the intermittent hypoxemia associated with OSAS may result in metabolic compensation that aims to maintain adequate growth. With improvement of OSAS severity (which was seen in both treatment arms), this metabolic adaption may predispose toward excessive weight gain. We indeed observed a relationship between the baseline REM ODI and change in the REM ODI with growth. Second, children who have OSAS may consume excessive calories in the setting of disrupted metabolism or insufficient sleep.36 Once the OSAS has been treated, the hormonal dysregulation resolves in the setting of continued high caloric intake. The mechanisms by which AT results in increased weight gain in children who have OSAS include increased caloric intake,3 unhealthy food choices,7 decreased caloric expenditure owing to lower work of breathing, resolution of intermittent hypoxemia, and increased growth hormone secretion. Hyperactivity and total daily activity are also reported to decrease after AT, thus potentially contributing to a higher BMI z score. Differences in the work of breathing resulting in changes in energy expenditure over the course of the study may also explain the greater weight gain in children who had a higher baseline AHI. Finally, several studies have reported increases in growth velocity after AT in children who had recurrent adenotonsillitis.8,35 The decreased number of tonsillitis episodes post-AT may reduce inflammation, thereby improving growth.12 However, it is possible that some of the children in these studies with recurrent infection also had unrecognized OSAS. Alternatively, chronic inflammation per se may mediate the growth-inhibiting effects of adenotonsillar enlargement.
Amin et al reported that 1 year after AT for OSAS, the BMI increased more in the children who had recurrence of OSA after resolution of their apnea measured 6 weeks after AT.25 In our study, children who had a higher AHI at baseline, and in particular those who had an elevated REM ODI, had greater postoperative increases in their ponderal indices 7 months after AT. However, there was no significant association between changes in any anthropometric measure and follow-up AHI, or between children with or without OSAS resolution. This paradox may be explained by several mechanisms. First, the AHI may not fully define the severity of OSAS. More precise measures of respiratory effort, such as esophageal manometry, were not made during this study and therefore airflow limitation unassociated with obstruction may have been missed. Secondly, changes in AHI and BMI are correlated, which may limit the ability to discern longitudinal associations between changes in those measures.37 Third, Amin et al observed a significant increase in the AHI from the 6-month to the 12-month time point, whereas our study followed children only 6 months postoperatively.
There are several limitations of the study that may have influenced our interpretation of the results. First, the follow-up study interval was limited to only 7 months and therefore it is possible that greater changes in anthropometric measures, especially height, would have been seen with a longer follow-up period. Conversely, it is unknown whether the observed increases in weight z scores will be sustained long-term. Second, we primarily used BMI z scores, which may lead to a “ceiling effect” for children who have high baseline BMI in longitudinal studies.38 That is, for children who have a high BMI z score at baseline, large increases in BMI result in small additional increases in the BMI z score. We thus performed an additional analysis using absolute BMI changes along with age in the regression model to establish that excessive weight gain was also observed in obese children.
Conclusions
This is the first study to evaluate the effect of eAT for OSAS on anthropometric variables using a randomized controlled design including laboratory-based PSG. eAT resulted in greater increases in weight and BMI z scores in generally healthy 5- to 9.9-year-old children who had OSAS than did WWSC. Particularly, increases in the BMI z score were observed after AT in children who had FTT, normal weight, and overweight. Notably, 51% of overweight children randomized to eAT became obese after eAT over the study interval. OSAS has been shown to have important adverse effects on energy balance and metabolism, and this study suggests that these changes are at least partially reversible after treatment. However, the observation that increases in the BMI z score were observed even in overweight children after AT suggests that monitoring weight, nutritional counseling, and encouragement of physical activity are important considerations after surgical intervention for OSAS in children.
Acknowledgments
We thank Xiaoling Hou and Yutuan Gao for their assistance with SAS programming.
Glossary
- AHI
apnea/hypopnea index
- AT
adenotonsillectomy
- eAT
early adenotonsillectomy
- FTT
failure to thrive
- ODI
oxygen desaturation index
- OSAS
obstructive sleep apnea syndrome
- PSG
polysomnography
- WWSC
Watchful Waiting and Supportive Care
Footnotes
Dr Katz participated in the collection and interpretation of the data and drafted and edited the manuscript; Dr Moore was primarily responsible for analyzing and interpreting the data and editing the manuscript; Drs Rosen, Mitchell, Amin, Arens, Muzumdar, Marcus, Paruthi, and Willging participated in the collection and interpretation of the data and edited the manuscript; Dr Chervin participated in the study design, oversight of data collection, interpretation of the data, and editing of the manuscript; Dr Redline designed the study, participated in the interpretation of the data, and edited the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00560859), Childhood Adenotonsillectomy Study for Children With OSAS (CHAT).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health grants HL083075, HL083129, UL1 RR024134, and UL1 RR024989. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Chervin has served as a consultant for Zansors and received educational grants through the University of Michigan from Respironics/Phillips and Fisher-Paykel. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100(1):31–40 [DOI] [PubMed] [Google Scholar]
- 2.Bonuck K, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int J Pediatr Otorhinolaryngol. 2006;70(5):769–778 [DOI] [PubMed] [Google Scholar]
- 3.Selimoğlu E, Selimoğlu MA, Orbak Z. Does adenotonsillectomy improve growth in children with obstructive adenotonsillar hypertrophy? J Int Med Res. 2003;31(2):84–87 [DOI] [PubMed] [Google Scholar]
- 4.Williams EF, Woo P. MIller R, Kellman RM. The effects of adenotonsillectomy on growth in children. Otolaryngol Head Neck Surg. 1991;104:509–516 [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung. 1981;159(5):275–287 [DOI] [PubMed] [Google Scholar]
- 6.Fernandes AA, Alcântara TA, D’Avila DV, D’Avila JS. Study of weight and height development in children after adenotonsillectomy. Braz J Otorhinolaryngol. 2008;74(3):391–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gkouskou KK, Vlastos IM, Hajiioannou I, Hatzaki I, Houlakis M, Fragkiadakis GA. Dietary habits of preschool aged children with tonsillar hypertrophy, pre- and post-operatively. Eur Rev Med Pharmacol Sci. 2010;14(12):1025–1030 [PubMed] [Google Scholar]
- 8.Kiris M, Muderris T, Celebi S, Cankaya H, Bercin S. Changes in serum IGF-1 and IGFBP-3 levels and growth in children following adenoidectomy, tonsillectomy or adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2010;74(5):528–531 [DOI] [PubMed] [Google Scholar]
- 9.Bar A, Tarasiuk A, Segev Y, Phillip M, Tal A. The effect of adenotonsillectomy on serum insulin-like growth factor-I and growth in children with obstructive sleep apnea syndrome. J Pediatr. 1999;135(1):76–80 [DOI] [PubMed] [Google Scholar]
- 10.Greenfeld M, Tauman R, DeRowe A, Sivan Y. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol. 2003;67(10):1055–1060 [DOI] [PubMed] [Google Scholar]
- 11.Ahlqvist-Rastad J, Hultcrantz E, Melander H, Svanholm H. Body growth in relation to tonsillar enlargement and tonsillectomy. Int J Pediatr Otorhinolaryngol. 1992;24(1):55–61 [DOI] [PubMed] [Google Scholar]
- 12.Aydogan M, Toprak D, Hatun S, Yüksel A, Gokalp AS. The effect of recurrent tonsillitis and adenotonsillectomy on growth in childhood. Int J Pediatr Otorhinolaryngol. 2007;71(11):1737–1742 [DOI] [PubMed] [Google Scholar]
- 13.Camilleri AE, MacKenzie K, Gatehouse S. The effect of recurrent tonsillitis and tonsillectomy on growth in childhood. Clin Otolaryngol Allied Sci. 1995;20(2):153–157 [DOI] [PubMed] [Google Scholar]
- 14.Ersoy B, Yücetürk AV, Taneli F, Urk V, Uyanik BS. Changes in growth pattern, body composition and biochemical markers of growth after adenotonsillectomy in prepubertal children. Int J Pediatr Otorhinolaryngol. 2005;69(9):1175–1181 [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz MD, Hoşal AS, Oğuz H, Yordam N, Kaya S. The effects of tonsillectomy and adenoidectomy on serum IGF-I and IGFBP3 levels in children. Laryngoscope. 2002;112(5):922–925 [DOI] [PubMed] [Google Scholar]
- 16.Soultan Z, Wadowski S, Rao M, Kravath RE. Effect of treating obstructive sleep apnea by tonsillectomy and/or adenoidectomy on obesity in children. Arch Pediatr Adolesc Med. 1999;153(1):33–37 [DOI] [PubMed] [Google Scholar]
- 17.Marcus CL, Carroll JL, Koerner CB, Hamer A, Lutz J, Loughlin GM. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr. 1994;125(4):556–562 [DOI] [PubMed] [Google Scholar]
- 18.Vontetsianos HS, Davris SE, Christopoulos GD, Dacou-Voutetakis C. Improved somatic growth following adenoidectomy and tonsillectomy in young children. Possible pathogenetic mechanisms. Hormones (Athens). 2005;4(1):49–54 [DOI] [PubMed] [Google Scholar]
- 19.Conlon BJ, Donnelly MJ, McShane DP. Improvements in health and behaviour following childhood tonsillectomy: a parental perspective at 1 year. Int J Pediatr Otorhinolaryngol. 1997;41(2):155–161 [DOI] [PubMed] [Google Scholar]
- 20.Nieminen P, Löppönen T, Tolonen U, Lanning P, Knip M, Löppönen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109(4). Available at: www.pediatrics.org/cgi/content/full/109/4/e55 [DOI] [PubMed] [Google Scholar]
- 21.Roemmich JN, Barkley JE, D’Andrea L, et al. Increases in overweight after adenotonsillectomy in overweight children with obstructive sleep-disordered breathing are associated with decreases in motor activity and hyperactivity. Pediatrics. 2006;117(2). Available at: www.pediatrics.org/cgi/content/full/117/2/e200 [DOI] [PubMed] [Google Scholar]
- 22.Hashemian F, Farahani F, Sanatkar M. Changes in growth pattern after adenotonsillectomy in children under 12 years old. Acta Med Iran. 2010;48(5):316–319 [PubMed] [Google Scholar]
- 23.Bonuck KA, Freeman K, Henderson J. Growth and growth biomarker changes after adenotonsillectomy: systematic review and meta-analysis. Arch Dis Child. 2009;94(2):83–91 [DOI] [PubMed] [Google Scholar]
- 24.Stradling JR, Thomas G, Warley AR, Williams P, Freeland A. Effect of adenotonsillectomy on nocturnal hypoxaemia, sleep disturbance, and symptoms in snoring children. Lancet. 1990;335(8684):249–253 [DOI] [PubMed] [Google Scholar]
- 25.Amin R, Anthony L, Somers V, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177(6):654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus CL, Moore RH, Rosen CL, et al. Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson AI, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine; 2007
- 29.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in children under 3 years. Otolaryngol Head Neck Surg. 2005;132(5):681–684 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell RB, Kelly J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol Head Neck Surg. 2004;131(1):104–108 [DOI] [PubMed] [Google Scholar]
- 32.Apostolidou MT, Alexopoulos EI, Chaidas K, et al. Obesity and persisting sleep apnea after adenotonsillectomy in Greek children. Chest. 2008;134(6):1149–1155 [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Lee D, Guo G, Harris KM. Trends in body mass index in adolescence and young adulthood in the United States: 1959-2002. J Adolesc Health. 2011;49(6):601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177(10):1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JM, Auo HJ, Yoo YH, Cho JH, Kim BG. Changes in serum levels of IGF-1 and in growth following adenotonsillectomy in children. Int J Pediatr Otorhinolaryngol. 2008;72(7):1065–1069 [DOI] [PubMed] [Google Scholar]
- 36.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33(9):1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 pt 1):1527–1532 [DOI] [PubMed] [Google Scholar]
- 38.Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol. 2007;17(1):44–50 [DOI] [PubMed] [Google Scholar]