Abstract
Background
U.S. lung cancer incidence rates overall are declining in the United States. We investigated the trends by histologic type and demographic characteristics.
Methods
Surveillance, Epidemiology, and End Results (SEER) program rates of microscopically-confirmed lung cancer overall and squamous cell, small cell, adenocarcinoma, large cell, other, and unspecified carcinomas among U.S. whites and blacks diagnosed 1977–2010 and white non-Hispanics, Asian/Pacific Islanders and white Hispanics diagnosed 1992–2010 were analyzed by gender and age.
Results
Squamous and small cell carcinoma rates declined since the 1990s, although less rapidly among females than males. Adenocarcinoma rates decreased among males and only through 2005, after which they then rose during 2006–2010 among every racial/ethnic/gender group; rates for unspecified type declined. Male/female rate ratios declined among whites and blacks more than among other groups. Recent rates among young females were higher than among males for adenocarcinoma among all racial/ethnic groups and for other specified carcinomas among whites.
Conclusions
U.S. lung cancer trends vary by gender, histologic type, racial/ethnic group, and age, reflecting historical cigarette smoking rates, duration, cessation, cigarette composition, and exposure to other carcinogens. Substantial excesses among males have diminished and higher rates of adenocarcinoma among young females have emerged as rates among males declined more rapidly. The recognition of EGFR mutation and ALK rearrangements that occur primarily in adenocarcinomas are the primary basis for the molecular revolution that has transformed lung cancer diagnosis and treatment over the past decade, and these changes have affected recent type-specific trends.
Introduction
Overall lung cancer age-adjusted incidence rates in the United States peaked in 1984 among men and have stabilized in recent years among women [1]. Rates and trends for total lung cancer and by histologic type vary by gender and race [2–5]. The dominant risk factor is cigarette smoking. Although exposure to factors such as radon, secondhand smoke (SHS), asbestos, arsenic, and various other chemicals, diet, genetic predisposition, hormonal factors, and infections and inflammatory processes also contribute to risk, about 95% of lung cancers among men and 90% among women in the United States are attributable to smoking [5–8]. Earlier U.S. studies had data pertaining only to blacks and whites [2–4]. More recent data now allow analysis of rates among Asian/Pacific Islanders (Asian/PIs) and Hispanics (1, 5). We have used Surveillance, Epidemiology, and End Results (SEER) program data to investigate recent lung cancer incidence according to histologic type, gender, race/ethnicity, age, and year of birth. Furthermore, we have compiled prevalence data on ever and current cigarette smoking to compare the smoking and lung cancer patterns.
Materials and Methods
SEER data were used to analyze trends among whites and blacks during 1977 to 2010 in the nine original registries of Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah [9], and among white non-Hispanics, Asian/PIs and Hispanic whites in the 13 registries: the SEER9 registries plus Los Angeles, San Jose-Monterey, rural Georgia, and Alaska Natives during 1992–2010 [10].
Site recode in SEER*stat software version 8.1.2[11] was used to select microscopically-confirmed lung and bronchus cancer cases. Histologic groupings were created using ICD-O-3 [12] morphology codes. Six main histologic type categories were formed: squamous cell carcinoma, small cell carcinoma, adenocarcinoma, large cell carcinoma, other specified carcinoma, and unspecified types. The morphology codes were: squamous cell carcinoma (8051-2, 8070-6, 8078, 8083-4, 8090, 8094, 8120, 8123); small cell carcinoma (8002, 8041-5); adenocarcinoma (8015, 8050, 8140-1, 8143-5, 8147, 8190, 8201, 8211, 8250-5, 8260, 8290, 8310, 8320, 8323, 8333, 8401, 8440, 8470-1, 8480-1, 8490, 8503, 8507, 8550, 8570-2, 8574, 8576); large cell carcinoma (8012-4, 8021, 8034, 8082); other specified carcinoma (8003-4, 8022, 8030-3, 8035, 8200, 8240-1, 8243-6, 8249, 8430, 8525, 8560, 8562, 8575); and unspecified malignant neoplasms (carcinoma not otherwise specified [NOS] 8010-1, 8020, 8230; non-small cell carcinoma 8046 ; malignant neoplasm NOS 8000-1). We omitted cases specified as a non-carcinoma (8580-9999) or that appeared to be a metastasis (8005, 8095, 8124, 8130, 8146, 8160, 8170, 8231, 8247, 8263, 8312, 8340-1, 8350, 8370, 8441, 8460, 8500, 8501, 8510, 8524, 8530, 8551). A code for non-small cell carcinoma (8046) was added to ICD-O-3 in 2001, which was also used for some cases diagnosed prior to 2001.
We calculated incidence counts, rates per 100,000 person-years, rate ratios (IRRs) and 95% CIs by histologic type, period, gender, racial/ethnic group, and 5-year age group. Rates were age-adjusted using the 2000 US standard for all ages combined and for 10-year age groups 25–34 to 75–84 and 85+ years for calendar years 1977–1981, 1982–1986, 1987–1991, 1992–1996, 1997–2000, 2001–2005, and 2006–2010, with each 5 years except for the 4-year interval 1997–2000 to allow assessment of the change to ICD-O-3 in 2001. Year of birth was estimated by subtracting the age group mid-year from the diagnosis period mid-year. All rates were plotted using the period mid-year and a semi-logarithmic scale such that a change of 1% per year was depicted by a slope of 10 degrees [13], achieved by having one y-axis log cycle the same length as 40 years on the x-axis.
The prevalence of ever smoking at age 35 by year of birth through 1950–54 was available from 1885–89 for whites and from 1900–04 for blacks in the Smoking and Tobacco Control Monograph 8 [14]. More recent ever smoking data for the age group 35–44 were based on National Health Interview Data for each 5th year 1990–2010 [15]. The prevalence of current cigarette smoking aged 18 and older by race/ethnicity and sex were from several National Health Interview Surveys [16]. Rates among whites and blacks were from surveys conducted every 5 years 1965 – 2005 and 2008, 2009, and 2010, with rates for the three years averaged and plotted at their mid-point, 2009.5. Asian and Hispanic rates were for 3-year period averages, 1990–1992, 1999–2001, and 2008–2010. Rates were not readily available for ever smoking among Asians or Hispanics or current smoking among Asians. Temporal trends in ever and current smoking prevalence were plotted as were the incidence rates, so that the slopes of the curves are comparable [13].
Results
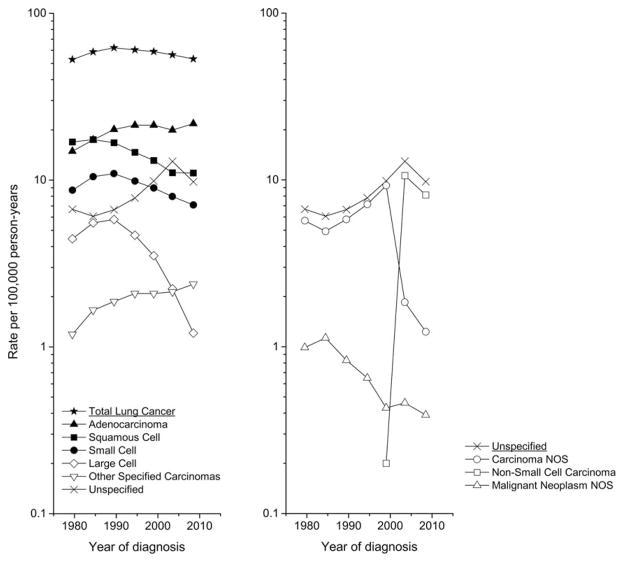
During 1977–2010 in the SEER 9, a total of 503,611 lung and bronchus cancers were diagnosed, of which 90.1% were microscopically confirmed. After omitting the few cases that were specified non-carcinomas or a likely metastatic type, 452,714 cases were available for analysis. The lung cancer incidence rate peaked in the early 1990s before declining from 62 to 53 per 100,000 person-years. (Figure 1, left panel) Squamous cell carcinoma rates peaked in the mid-1980s and then declined from 17 to 11 per 100,000 person-years during 2006–10. Small cell carcinoma rates rose through the mid-1980s to 11 before declining to 7 cases per 100,000 person-years. Adenocarcinoma rates rose from 15 to more than 20 cases per 100,000 person-years during the 1990s, before declining through 2004. Large cell carcinoma rates decreased markedly from a peak of 6 around 1990 to about 1 case per 100,000 person-years, whereas the rate for other specified carcinomas rose more rapidly during the early years than subsequently. During the most recent time period 2006–2010, the rates for adenocarcinoma and other specified carcinoma rose, and the squamous cell carcinoma rate declined less rapidly than earlier. The unspecified rate steadily rose until the early 2000s after which it declined. Among the unspecified cancers (right panel), the carcinoma NOS rate plummeted during the 2000s, the non-small cell carcinoma rate initially rose and then decreased, and the malignant neoplasms NOS rate declined steadily over the years, making consideration of these types individually untenable. For the rest of the analysis, we focused on total lung cancer and the six major types.
Figure 1.
Trends in lung cancer incidence rates (age-adjusted 2000 US standard) from 1977 to 1981 through 2006 to 2010 in the SEER 9 registries by histologic type.
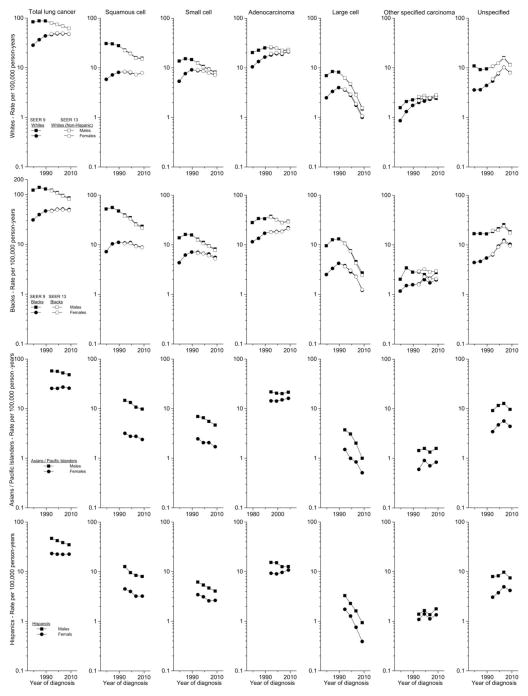
Four more registries that included 86,678 additional cases were added to the SEER 9, providing a total of more than a half million lung cancer cases for analysis. Among males, total lung carcinoma incidence peaked in the mid-1980s before declining from 88 to 62 per 100,000 person-years among whites and from 140 to 86 among blacks; rates among Asian/PIs and Hispanics were considerably lower, but also declined during the past two decades (Figure 2). Squamous cell carcinoma rates also peaked in the mid-1980s among blacks and earlier among whites, declined continually among all groups, with some recent leveling off. Small cell carcinoma trends mirrored those for total lung cancer. Adenocarcinoma rates rose until 1992–96 when they peaked among both whites and blacks; since then, they declined only modestly or plateaued among each of the four racial/ethnic groups, with upturns during the most recent period in every group. In recent years the adenocarcinoma rate uniformly has been higher than the squamous cell carcinoma. Large cell carcinoma rates declined drastically since the late 1980s, while the other specified carcinoma rates were generally steady in recent years. The unspecified cancer rates rose until 2001–05 when they decreased dramatically among each of the groups.
Figure 2.
Trends in lung cancer incidence rates (age-adjusted 2000 US standard) by histologic type and sex in the SEER 9 registries from 1977 to 1981 through 2006 to 2010 among whites and blacks, and in the SEER 13 registries 1992 to 1996 through 2006 to 2010 among whites (non-Hispanic), Asian/Pacific Islanders, and Hispanics (white).
Among females, total lung cancer rates among whites and blacks rose steadily until stabilizing in recent years; rates among Asian/PIs and Hispanics also did not change greatly since the 1990s. Squamous cell carcinoma rates among whites and blacks plateaued earlier than the total rates with a recent increase in white females, and decreases are apparent among Asian/PIs and Hispanics. Small cell carcinoma rates rose during the early years, peaked in the early-1990s, and declined in recent years among all groups. Adenocarcinoma rates rose among whites and blacks more rapidly during the earlier years; rates also increased among all groups in recent years. Large cell carcinoma rates declined dramatically since the late 1980s, while rates for other specified carcinomas rose in recent years. Rates for unspecified cancers rose over the study period until the most recent time period when they declined universally.
Rates among males and females have been converging, as decreases among males have been more pronounced. The male/female incidence rate ratios (MF IRR) among whites decreased from 2.95 during 1977–81 to 1.29 during 2006–10 and from 3.94 to 1.71 among blacks. Rates also converged for squamous cell carcinoma (from 5.28 to 1.97 among whites and 7.29 to 2.64 among blacks), small cell carcinoma (from 2.55 to 1.12 among whites and 3.17 to 1.48 among blacks), adenocarcinoma (from 1.94 to 1.08 among whites and 2.43 to 1.35 among blacks), large cell carcinoma (from 2.80 to 1.47 among whites and 3.79 to 2.22 among blacks), and the unspecified cancers (from 3.05 to 1.44 among whites and 3.82 to 1.78 among blacks). Notably, gender parity is approaching, with recent MF IRRs approaching one among whites for both small cell carcinoma and adenocarcinoma and 0.89 for the other specified carcinomas. The MF IRRs for adenocarcinoma also decreased among Asian/PIs from 1.51 during 1992–96 to 1.33 during 2006–10 and among Hispanics from 1.65 to 1.17. Trends among Asian/PIs and Hispanics for the other types were generally parallel among males and females, with IRRs decreasing modestly from 4.59 to 4.09 and from 2.81 to 2.73 for squamous and small cell carcinomas among Asian/PIs and from 2.82 to 2.49 and from 1.79 to 1.54 among Hispanics, respectively.
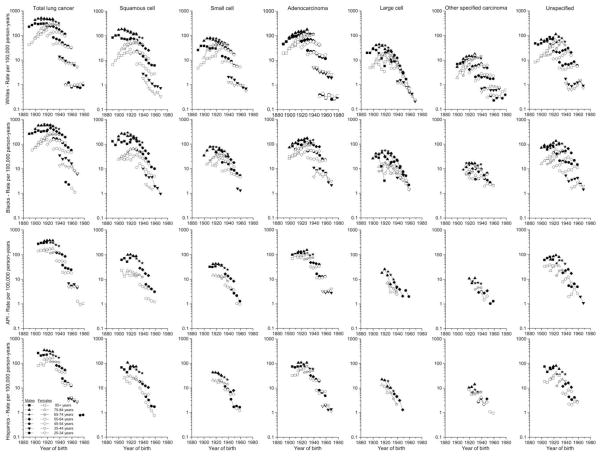
The numbers of cases were considerably larger in the SEER 13 than SEER 9, providing more stable rates, and the point estimates were generally quite similar, so we used the age-specific SEER 13 data for white non-Hispanics and blacks for 1992–2010 in Figure 3, along with the SEER 9 data for 1977–1992. Among whites and blacks, age-specific rates generally increased among those born in earlier years before peaking and declining among the more recently-born cohorts for total lung cancer and by type, although the timing of the peaks varied by gender and type (Figure 3). The peaks for total lung cancer occurred earlier among white males (born prior to about 1925) than females (born around 1930–40). The rates for squamous cell carcinomas peaked earliest (males born prior to 1905 and females born 1925–30), for small and large cell carcinomas intermediate (peaks among males 1910–25 and females 1930–40), and for adenocarcinomas most recently (males 1925–30 and females 1935–40). The other specified carcinoma rates peaked among those born during the late 1930s, were similar among males and females, and have declined less rapidly among the recently-born than the rates for other types. The unspecified cancer rates peaked among older ages for those born earlier and among younger ages among those born more recently. Large cell carcinoma rates also declined across most of the birth cohorts. Patterns among blacks were similar, although the peaks in adenocarcinoma rates are distributed more broadly before 1925. Peaking of the age-specific rates generally is not as clear among Asian/PIs and Hispanics due to fewer years of data available.
Figure 3.
Trends in age-specific lung cancer incidence rates (age-adjusted 2000 US standard) by histologic type and sex in the SEER 9 registries from 1977 to 1981 through 1987 to 1991 among whites and blacks, and in the SEER 13 registries from 1992 to 1996 through 2006 to 2010 among whites (non-Hispanic), blacks, Asian/Pacific Islanders, and Hispanics (white) by year of birth. Rates based on <16 cases are not shown.
Historically, rates have been higher among males than females within each group, but those differences have been diminishing. The overall MF IRRs among whites were most pronounced at older ages, decreasing among those ages 75+ from more than five to less than two. Among whites, blacks, and Hispanics born since about 1960, female rates have exceeded those for males. The largest MF IRR was 13 among those 85+ diagnosed with squamous cell carcinoma during the earliest years. Across all age groups, the MF IRRs have been highest for squamous cell, intermediate for small cell, and lowest for adenocarcinoma. Adenocarcinoma rates have been higher in females than males among whites born since 1940, Hispanics since 1950, and blacks and Asian/PIs since 1960. Among whites, other specified carcinoma rates have been higher among females than males among those born since 1940.
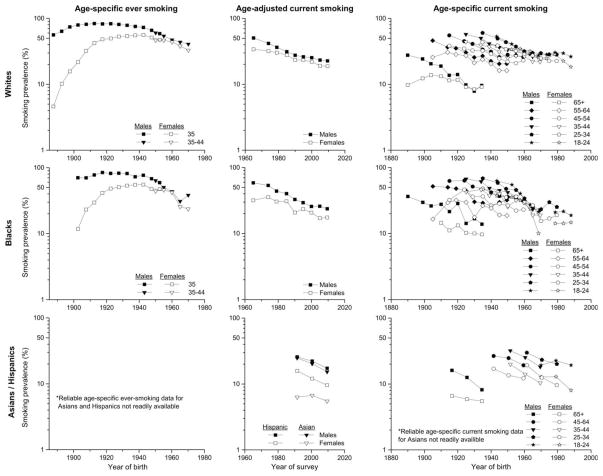
Among whites born during the late 1880s, 56% of US males and 5% of females were ever smokers by age 35 years, which rose to more than 80% among males born during 1905–29 before declining by half to 41% among those born around 1970 and among females rose to more than 55% among the 1935–44 birth cohort and subsequently declined to 33% (Figure 4). Among blacks, the ever-smoking prevalence among the early 1900s cohort was 70% among males and only 12% among females, which rose among males to 84% among the 1915–19 cohort before declining to 38% among the 1970 cohort and among females increased to 55% among the 1935–44 cohort before declining rapidly to 24% in the most recent cohort. From 1965 to 2010, the age-adjusted percent of whites 18 and older who currently smoked decreased from 50% to 21% among males and from 34% to 18% among females; among blacks, the corresponding figures were from almost 60% to 23% among males and from 32% to 17% among females. From 1990–92 to 2008–10, the smoking prevalence decreased among Hispanic males from 26% to 17%, Hispanic females from 16% to 10%, Asian males from 25% to 15%, and ranged between 6–7% among Asian females Gender differences in smoking prevalence have been diminishing among all groups except Hispanics. Among whites, age-specific smoking rates declined through the 1950s cohorts among males and through the 1960s cohorts among females, after initial increases, and have plateaued since then. The age-specific birth cohort patterns among blacks are similar to those among whites. Smoking rates among Hispanics also declined steadily.
Figure 4.
Cigarette smoking by race/ethnicity and sex: age-specific prevalence of ever-smoking by year of birth, age-adjusted prevalence of current smoking by year of survey, and age-specific prevalence of current smoking by year of birth [14,15].
Discussion
Our updated and expanded analysis of US lung cancer incidence patterns overall and by histologic type and cigarette smoking that spans a century of birth cohorts has confirmed a number of previous observations as well as identifying several new observations [2–5]. Overall rates have been declining for several decades among males of each racial/ethnic group; rates among females have plateaued. Squamous and small cell carcinoma rates have been decreasing among both males and females, more rapidly among white and black males than females. A new finding is that decreases in squamous and small cell carcinoma have been fairly parallel among male and female Asian/PIs and Hispanics, in contrast to converging rates among whites and blacks. Of note, adenocarcinoma rates are continuing to climb among females while generally declining among males. In the most recent time period, the unspecified carcinoma rates decreased consistently and notably among every gender/racial/ethnicity group while they rose for adenocarcinomas and the other specified carcinomas, and flattened for squamous cell carcinoma.
The age-specific cancer rates by year of birth, which span more than a century, clearly show strong cohort effects not only among whites and blacks but also Asian/PIs and Hispanics, with declining overall rates among males born since the 1920s and females born since the 1930s. Rates for squamous cell carcinoma were higher among cohorts born earlier and for adenocarcinoma among those born more recently. Adenocarcinoma excesses among females at younger ages have emerged as the rates have dropped more rapidly among males. The trends for large cell and unspecified carcinomas reflect period effects that occurred earlier for large cell and more recently for the unspecified carcinomas, likely related to changing diagnostic conventions. Trends in ever-smoking prevalence rates strikingly resemble the subsequent age-adjusted lung cancer rates, while the age-specific current smoking and lung cancer patterns are generally similar across the birth cohorts.
The annual US per capita cigarette consumption among adults ≥ 18 years of age rose from virtually zero in the early 1900s to more than 4,000 during the 1960s before declining to nearly 1,700 in 2006, a level not seen since 1935 [17]. Lung cancer risks have been found to be similar among men and women at each level of cigarettes smoked [18]. The converging overall lung cancer rates among white and black males and females reflect converging ever-smoking prevalences. Cigarette smoking among males declined rapidly since 1965, and lung cancer rates have decreased since the mid-1980s. Declines in smoking among females have been less rapid, and rates have decreased notably only for small cell carcinomas. Smoking prevalence differed by gender more for other race/ethnic groups, consistent with the persisting gender differences in cancer rates. Within a particular cohort, the prevalence of current smoking will rise as persons adopt smoking and then also decrease with cessation. In many instances, the older age-specific current smoking prevalence peaks occurred among earlier cohorts and the younger peaks occurred among more recent cohorts, suggesting period effects related to changing cigarettes and to smoking cessation. The lack of declines in current smoking prevalence among whites born since the early 1960s is already reflected in plateauing cancer rates among the 25–34 years age group. The prevalence of moderate-heavy smoking has declined since the 1990s, that of very light smoking has increased, and the average daily consumption decreased [19].
All lung carcinoma histologies are associated with smoking [20], with the association strongest for squamous and small cell carcinoma and more modest for adenocarcinoma. Early cigarettes were non-filtered, and the smoke composition discouraged deep inhalation, primarily exposing the trachea and bronchus and producing squamous cell carcinomas [17]. When filters were introduced, smaller particles were dispersed deeper into the respiratory tree, resulting in adenocarcinomas with a more peripheral distribution [17]. Nicotine is more readily absorbed at a more alkaline pH [17, 21], which can be achieved by changing the chemical composition of tobacco via ammonia and other compounds that increase pH. Degree of inhalation, cigarettes per day, number of years smoking, and smoking cessation, all influence lung cancer risk and may affect histology in different ways [17, 21]. However, changing tobacco blends decreased nicotine but increased nitrate and N-nitrosamines [5], and genetic selection of tobacco plants resulted in deeper inhalation [17,22–23], possibly contributing to the decrease in squamous and small cell carcinomas of the central airways and increase in peripheral adenocarcinomas as smokers inhale more deeply [18,24–25]. The summation of these complex changes may result in a less rapid decline for adenocarcinoma than for the other types. Squamous cell carcinomas among males were the most frequent type among whites until 1992 and blacks until 2002. Adenocarcinoma has been more frequent than either squamous or small cell carcinoma consistently among women and among men in recent years. Risk declines with smoking cessation, more rapidly for squamous cell carcinomas and less rapidly for adenocarcinomas [26], also likely contributing to the changing cell type relative frequencies. Most newly-diagnosed cases in recent years have been among former smokers [27].
Exposure to SHS increases lung cancer risk and represents a major risk factor among never smokers [28]. It has been difficult to assess lung cancer risk among never smokers or to determine gender differences in light of their potential exposure to SHS at home, occupationally, or elsewhere or to other pulmonary carcinogens [29]. Other environmental exposures associated with lung cancer include arsenic, asbestos, and radon, which are known carcinogens [5].
The geographic patterns of lung cancer mortality rates have changed substantially over time and differ by gender and race [30]. The converging SEER incidence and emerging female predominance at ages <55 years contrast with the persisting male predominance in national smoking rates. In the past, lung cancer mortality rates were higher in the NCI-surveyed areas than the total United States, but they crossed as the national rates continued to increase more rapidly [31]; in recent years the rates have been higher nationally than in the SEER areas. These observations suggest that the SEER incidence patterns may be predicting future national rates and that SEER gender differences in smoking rates may have diminished more sharply than nationally.
With the addition of the code for non-small cell carcinoma in 2001, the rate for carcinoma NOS dropped precipitously, suggesting that trying to differentiate between non-small cell and carcinoma NOS is futile. Major improvements in histologic subtyping and especially classification of adenocarcinoma have occurred that, in concert with the identification of specific mutations, are offering new treatment possibilities [32]. Updates in the classification [33] and more facile mutation testing will improve treatment options and place clinical trials and TMN classification on a more rational basis. Advances in whole genome sequencing (WGS) of lung cancer tumors have allowed the guidance of cancer care to a personal level [34] and will eventually determine the effectiveness of targeted therapies. The precipitous drop in the unspecified carcinomas since the early 2000s corresponds to when immunostaining for TTF-1 was introduced into clinical practice by pathologists. This is a marker for adenocarcinoma differentiation as well as lung origin. The decreases in the unspecified type rates coincide with a reversal of the previous decline in adenocarcinoma rates. The recent increases in adenocarcinoma rates among both males and females of all racial/ethnic groups likely reflect these improvements in classification of histologic type. Immunohistochemical markers for squamous differentiation such as p63 and more recently p40 have been introduced into routine clinical practice by pathologists [32–35]. This may explain in part the increase in rates for squamous cell carcinoma in white females and the moderation in the decline in white males. The other specified carcinoma group is comprised of carcinoids (33%), adenosquamous carcinomas (34%), neuroendocrine carcinomas (19%), sarcomatoid carcinomas (12%), and miscellaneous other types (2%); further investigation of these rare cancers is needed to reveal which types contributed to the recent upturn in rates.
In the large National Lung Screening Trial of persons aged 55 to 74 years with a smoking history of at least 30 pack years who were either current smokers or former smokers who quit less than 15 years ago, lung cancer incidence increased and mortality declined among those screened with low-dose computed tomography (CT) compared to those screened only with chest radiography [36] . Recent guidelines have been issued for the implementation of low-dose CT screening [37, 38]. These guidelines are too new to have an impact on our data, and notably there are no recent increases in incidence rates among those aged 55 to 74 years of age in Figure 3. Improvements in lung cancer risk models will be required to refine the identification of those at highest risk to achieve maximum benefit from screening [37]. As these models evolve, it will be imperative to monitor lung cancer trends among older individuals to determine whether CT screening may have a differential impact by histologic type.
In summary, this analysis supports previous findings of the US declining total lung cancer rates among males and stabilizing rates among females. Lung carcinoma rates remain higher among males than females, although rates have been converging and there are emerging excesses of adenocarcinoma and other specified carcinomas among young women. Among males of each racial/ethnic group, rates declined sharply for smoking-related types (squamous and small cell carcinomas); however, the declines were not as striking for adenocarcinoma. Among white and black females, squamous and small cell carcinoma rates have not decreased as rapidly as among males, although among Asian/PIs and Hispanics the declines were similar between the sexes. Although it is not possible to directly relate the changes in smoking prevalence to the observed rates of lung cancer, the timing of cessation and the rise of cigarettes with a different composition likely have changed the magnitude and range of risks observed for the various histologic types. These findings will require renewed clinical awareness and surveillance and will guide future studies of cancer risk and control. In addition, the advances in determination of histologic type and molecular targeted therapy will hopefully improve patient survival.
Acknowledgments
The authors thank Ms. Lois Dickie, Certified Tumor Registrar, Surveillance Research Program, National Cancer Institute, for consultation regarding lung cancer histologies.
Funding: None.
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Apr, 2013. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends declining for most histologic types among males, increasing among females. Cancer. 1996;77:2464–2470. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Travis WD, Tarone RE, et al. Lung cancer rates convergence in young men and women in the United States: Analysis by birth cohort and histologic type. Int J Cancer. 2003;105:101–107. doi: 10.1002/ijc.11020. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 5.Spitz MR, Wu X, Wilkinson A, Wei Q. Cancer of the lung. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology & Prevention. 3. New York: Oxford University Press; 2006. pp. 638–658. [Google Scholar]
- 6.Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. Amer J Roentgenol. 2011;196:287–295. doi: 10.2214/AJR.10.5412. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos A, Guida F, Cenee S, et al. Cigarette smoking and lung cancer in women: results of the French ICARE case-control study. Lung cancer. 2011 doi: 10.1016/j.lungcan.2011.04.013. In press. [DOI] [PubMed] [Google Scholar]
- 8.Field RW, Wither BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33:681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 9 Regs Research Data, Nov 2012 Sub (1973–2010) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- 10.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2012 Sub (1992–2010) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- 11.Seer*Stat software: Surveillance Research Program, National Cancer Institute SEER*Stat software. 2013 Sep 18; ( seer.cancer.gov/seerstat) version 8.1.2 up.
- 12.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 13.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 14.Smoking and Tobacco Control Monograph No. 8. Bethesda, MD: National Cancer Institute; 1997. pp. 305–382. (NIH publication no. 97-4213) [Google Scholar]
- 15.National Centers for Health Statistics. National Health Interview Survey, 1990, 1995, 2000, 2005, 2010 machine readable datafile and documentation. Hyattsville, MD: National Center for Health Statistics; [accessed January 2013]. [Google Scholar]
- 16.National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville, MD: 2012. pp. 219–222. Smoking prevalence tables 60 and 62. [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014. [Google Scholar]
- 18.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-Year Trends in Smoking-Related Mortality in the United States. NEJM. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce JB, White MM, Messer K. Changing Age-Specific Patterns of Cigarette Consumption in the United States, 1992–2002: Association with Smoke-free Homes and State-level Tobacco Control Activity. Nicotine and Tobacco Res. 2009;11:171–177. doi: 10.1093/ntr/ntp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta- analysis. Lung cancer. 2001;31(2–3):139–148. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 21.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-Century Hazards of Smoking and Benefits of Cessation in the United States. NEJM. 2013;368:342–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 22.Burns D, Benowitz N. Risks associated with smoking cigarettes with low machine-measured tar and nicotine. Smoking and tobacco control monograph no. 15. Bethesda, MD: National Cancer Institute; 2001. Public health implication of changes in cigarette design and marketing; pp. 1–12. (NIH publication no. 02-5074) [Google Scholar]
- 23.Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 2010. [PubMed] [Google Scholar]
- 24.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21:7307–7025. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 25.Thun MJ, Laily CA, Flannery JT, et al. Cigarette smoking and changes in the histopathology of lung cancer. JNCI. 1997;89:1580–1586. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 26.Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest. 2001;120:1577–1583. doi: 10.1378/chest.120.5.1577. [DOI] [PubMed] [Google Scholar]
- 27.Tota JE, Ramanakumar AV, Franco EL. Lung cancer screening: Review and performance comparison under different risk scenarios. Lung. 2013:191. doi: 10.1007/s00408-013-9517-x. Published online 24 October 2013. [DOI] [PubMed] [Google Scholar]
- 28.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008 Sep;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devesa SS, Grauman DJ, Blot WJ, Fraumeni JF., Jr Cancer surveillance series: Changing geographic patterns of lung cancer mortality in the United States, 1950 through 1991. JNCI. 1999;91:1040–1050. doi: 10.1093/jnci/91.12.1040. [DOI] [PubMed] [Google Scholar]
- 31.Devesa SS, Silverman DT, Young JL, Jr, Pollack ES, Brown CC, Horm JW, Percy CL, Myers MH, McKay FW, Fraumeni JF., Jr Cancer incidence and mortality trends among whites in the United States, 1947–1984. JNCI. 1987;79:701–770. [PubMed] [Google Scholar]
- 32.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society International multidisciplinary classification of lung adenocarcinoma. J Thor Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. JCO. 2013;46 doi: 10.1200/JCO.2012.46.9270. epub ahead of print. [DOI] [PubMed] [Google Scholar]; 31:992–1001. [Google Scholar]
- 34.Daniels MG, Bowman RV, Yang IA, Govindan R, Fong KM. An emerging place for lung cancer genomics in 2013. J Thorac Dis. 2013;(Suppl 5):S491–S497. doi: 10.3978/j.issn.2072-1439.2013.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 36.The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tammemagi MC, Katki HA, Hocking WG, Church TR, Caporaso N, et al. Selection criteria for lung-cancer screening. N Eng J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Eng J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]