Abstract
Anosognosia is a complex symptom corresponding to a lack of awareness of one’s current clinical status. Anosognosia for cognitive deficits has frequently been described in Alzheimer’s disease (AD), while unawareness of current characteristics of personality traits has rarely been considered. We used a well-established questionnaire-based method in a group of 37 AD patients and in healthy controls to probe self- and hetero-evaluation of patients’ personality and we calculated differential scores between each participant’s and his/her relative’s judgments. A brain–behavior correlation was performed using 18-fluorodeoxyglucose positron emission tomography (FDG-PET) images. The behavioral data showed that AD patients presented with anosognosia for current characteristics of their personality and their anosognosia was primarily explained by impaired third perspective taking. The brain–behavior correlation analysis revealed a negative relationship between anosognosia for current characteristics of personality and dorsomedial prefrontal cortex (dMPFC) activity. Behavioral and neuroimaging data are consistent with the view that impairment of different functions subserved by the dMPFC (self-evaluation, inferences regarding complex enduring dispositions of self and others, confrontation of perspectives in interpersonal scripts) plays a role in anosognosia for current characteristics of personality in AD patients.
Keywords: dementia, neuroimaging, self, social cognition, unawareness
INTRODUCTION
Cognitive deficits constitute a major criterion in establishing a diagnosis of probable Alzheimer’s disease (AD) (McKhann et al., 1984). However, changes in personality are also frequently reported by caregivers (Strauss et al., 1993; Derouesne et al., 2001; Rankin et al., 2005; Talassi et al., 2007) and may occur even before measurable cognitive loss is detected (Balsis et al., 2005). Lack of awareness (or anosognosia) of cognitive impairment has frequently been observed in AD. Interestingly, anosognosia is relatively heterogeneous (Gil et al., 2001; Salmon et al., 2008) and does not necessarily affect all the impairments reported in AD. Only one recent study has examined self-awareness and personality changes in dementia (Rankin et al., 2005). Ten AD patients were shown to be able to evaluate most of their current personality facets, although they underestimated their unassuredness/submissiveness and overestimated their gregariousness/extraversion. In fact, the AD patients tended to describe their personality as it was before the onset of dementia, as if they had not updated their self-image (Mograbi et al., 2009).
Anosognosia for cognitive symptoms has been claimed to be related to both memory loss and executive dysfunction (Agnew and Morris, 1998). Many studies have shown that impaired retrieval monitoring processes or impaired metacognitive processes are more important than memory accuracy deficits in explaining cognitive anosognosia in AD (Cosentino et al., 2007; Gallo et al., 2007; Dodson et al., 2011). There have been very few direct investigations of anosognosia for current characteristics of personality traits in AD (Klein et al., 2003; Rankin et al., 2005). In a study focusing on perspective taking in AD, personality trait awareness (for the self and other people) was explored in AD patients (Ruby et al., 2009). In this experiment, AD patients and their relatives evaluated whether personality trait adjectives corresponded to their own or their relative’s personality, taking their own or their relative’s viewpoint in establishing the judgment. Discrepancy scores showed that the AD patients’ self-judgments differed from their relatives’ assessments of their personality and that the patients had impaired third perspective taking (inability to take the view point of the relative concerning their own personality traits).
Recent neuroimaging data have related anosognosia for cognitive deficits to medial temporal structures (Salmon et al., 2006), the orbitofrontal cortex (Salmon et al., 2006; Rosen et al., 2010), the ventromedial prefrontal and posterior cingulate cortex (Mimura and Yano, 2006; Ries et al., 2006; Zamboni et al., 2013), the superior frontal sulcus (SFS) and the temporoparietal junction (TPJ) (Salmon et al., 2006). When providing self-judgments about personality trait adjectives, AD patients also showed activation of the dorsomedial prefrontal cortex (dMPFC) (Ruby et al., 2009).
The most frequent hypothesis concerning anosognosia in AD is that patients base their judgments on past, non-updated information and not on their current situation (Mograbi et al., 2009). The first aim of this study was to reconsider this hypothesis (which was based on observations of few patients) and to precisely characterize unawareness of the current characteristics of personality traits in a population of 37 AD patients. Capitalizing on previous clinical (Klein et al., 2003; Ruby et al., 2007) and neuroimaging (Fossati et al., 2004; D'Argembeau et al., 2007; Ruby et al., 2009) studies, we used (in a new sample of participants) a well-established questionnaire-based method which consists in comparing patients’ perception of their personality traits with ratings given by their relatives. Second, we explored for the first time the brain metabolic correlates of unawareness of the current characteristics of personality traits in AD using resting 18-fluorodeoxyglucose positron emission tomography (FDG-PET). We anticipated correlation with regions that have been found to be associated with the representation of personal traits, i.e. the ventromedial prefrontal cortex (vMPFC), the dMPFC, the superior frontal gyrus and the TPJ.
MATERIALS AND METHODS
Subjects
Thirty-seven patients with probable AD (McKhann et al., 1984) were recruited from the memory clinic of the University Hospital of Liège. Diagnoses were based on interviews with the patient and a relative, neuropsychological assessment and laboratory and neuroimaging data. FDG-PET was used as a biomarker and the AD patients showed a typical pattern of parieto-temporal and posterior cingulate cortex hypometabolism (Herholz et al., 2002). Twenty-five healthy elderly control subjects (EC), without any medical history of cognitive decline, were included in the study. The University Hospital ethics committee approved the study. Informed consent was obtained from all subjects.
For each participant, a close relative (or a good friend, referred to here as a relative) was invited to take part in the study so we could obtain patient/relative discrepancy scores. Among the relatives of the AD patients, there were 15 spouses, 21 children and 1 friend. The relatives of the ECs included 14 spouses, 5 children and 6 friends. Relatives’ assessments have been found to have good validity in the literature (Salmon et al., 2006), regardless of the caregivers’ family ties with the patients (Strauss et al., 1993). They were taken as the best available proxy for daily reality in our clinical domain of interest, as the evaluation of personality traits cannot be objective (as neuropsychological performance can be). The term ‘subject’ (S) will be used to refer to AD patients and their matched ECs, whereas (R) refers to the relatives.
Demographic data are presented in Table 1. The AD patients were older and had a lower education level than the ECs. The AD patients were also more cognitively impaired than the ECs, as measured with the Mattis Dementia Rating Scale (Mattis, 1976). The proportion of males and females did not differ between the two groups and the score on the Geriatric Depression Scale (Yesavage et al., 1983) was similar between groups.
Table 1.
Demographic characteristics and clinical data on participants
| Variable | AD patients | ECs | Group comparisons |
|---|---|---|---|
| n | 37 | 25 | |
| Age, years | 78.22 (6.86) | 73.08 (6.99) | t = 2.87, P = 0.006 |
| Education, years | 9.92 (3.14) | 12.64 (2.94) | t = −3.43, P = 0.001 |
| Gender | |||
| Male | 12 | 9 | χ2 = 0.08, P = 0.78 |
| Female | 25 | 16 | |
| GDS | 3.27 (2.29) | 2.84 (1.34) | t = 0.84, P = 0.402 |
| Mattis | 119.76 (10.09) | 138.32 (6.08) | t = −823, P = 0.000 |
Numbers are means with standard deviations. Group comparisons were performed with Student’s t-test or χ2. GDS, Geriatric Depression Scale; Mattis, Mattis Dementia Rating Scale.
Depression was not screened with specific scores among relatives. However, none of the relatives in both groups reported depressive or anxious mood during data collection. AD patients’ relatives fulfilled Zarit’s burden interview (Zarit et al., 1980) during the testing. Among all patients’ relatives there was no ‘severe’ burden reported and only a few (4 in 37) reported a ‘moderate’ burden. The mean burden score for the AD relatives was 22.16 (which refers to a ‘light’ burden) with a standard deviation of 13.893.
Judgment of personality traits
The questionnaire used to assess personality traits was taken from a previous study on frontotemporal dementia (Ruby et al., 2007). The questionnaire is composed of 40 personality trait adjectives, taken from Kirby and Gardner’s publication (Kirby and Gardner, 1972). Subjects had to assess to what extent the adjectives corresponded to their own personality by choosing one of four possibilities (‘not at all’, ‘a little’, ‘quite well’ and ‘totally’). Moreover, the time period evaluated and the ability to take another person’s perspective were manipulated. First, the subjects (S) evaluated their own current personality (S1). For instance, they answered the question: ‘Currently, am I aggressive?’ Next, they judged their past personality (S1_before); ‘Ten years ago, was I aggressive?’ An interval of 10 years was chosen to evaluate the predementia stage in all patients. Finally, subjects had to take their relatives’ perspective (PP) by pretending to be the relative evaluating the subject’s personality (PP1). In this condition, they answered the question: ‘According to my relative, am I aggressive?’ Accordingly, AD patients in the clinic frequently tell that their spouse report daily forgetting, but that they exaggerate. Such an ‘third person perspective’ (PP1) was directly assessed in our study and it was feasible because our patients were in the early stages of AD. The questionnaire was done on paper, under supervision of the experimenter. The patients demonstrated a fair comprehension of the instructions when adding some comments on their spouse’s opinions.
The questionnaire was also completed by the relatives. They received the instruction to evaluate the subject’s current and past personality (R2 and R2_before). Concretely, they answered the questions: ‘Currently, is [subject’s name] aggressive?’ and ‘Ten years ago, was [subject’s name] aggressive?’
The subjects’ and relatives’ answers were scored from 1 to 4 (1: ‘not at all’, 2: ‘a little’, 3: ‘quite well’ and 4: ‘totally’). For each item, the difference between the answers provided by the subject and the relative was calculated and then the sum of the absolute values for all differences was calculated for the 40 personality trait adjectives. The discrepancy score was the sum of differences divided by 120 (maximum difference possible). A series of six discrepancy scores (described below) were obtained.
First, a measure of anosognosia was obtained by the difference between the subject’s answers for the current period and the relative’s answers for the current period (S1–R2). The higher the discrepancy score, the less aware subjects were of the current characteristics of their personality traits. The second discrepancy score indexed change over time, as perceived by the relative. This was calculated as the difference between the relative’s answers for the present and for the past (R2–R2_before). The third discrepancy score concerned self-evaluation of the change. The subject’s answers for the present and past were compared (S1–S1_before). The fourth discrepancy score evaluated dependency on the past and constituted the difference between the subject’s answers for the present period and the relative’s answers for the past period (S1–R2_before). A score close to zero indicated that the subject saw himself or herself as the relative thought he or she had been 10 years ago. The fifth discrepancy score concerned subjects’ perception of their past personality traits. Subjects’ answers for the past period (S1_before) were compared to their relatives’ answers for the same past period (R2_before). The final discrepancy score concerned the ability to take another person’s perspective. It consisted in the difference between the answers that the subject gave when taking a third-person perspective (PP1) and the relative’s answers (R2) for the present period. The closer the score was to zero, the better able the subject was to take the relative’s viewpoint of his/her personality.
Behavioral analyses
To examine self-assessment of personality in healthy elderly subjects, data for ECs were compared (using Student’s t-tests) to a ‘zero’ standard, corresponding to no difference between the values selected to obtain the given discrepancy score. The discrepancy scores indexing anosognosia and other clinical characteristics concerning personality traits in AD patients and ECs were compared with a one-way ANCOVA (group), in which age and years of education were included as covariates. Finally, to test the hypotheses that anosognosia is related to a perspective-taking deficit (Salmon et al., 2005) or to a crystallized self (Mograbi et al., 2009), we performed a multiple regression analysis, using the anosognosia discrepancy score as the dependent variable and dependency on the past and third person’s perspective as independent variables (i.e. two variables that were not significantly correlated, r = 0.22, P = 0.17).
It should be noted that the anosognosia scores for current characteristics of personality were significantly correlated with the personality change scores (i.e. patients who were the most anosognosic were those who had suffered the greatest personality change, according to their relatives). As expected from the literature, we although found a positive correlation between burden’s score and both anosognosia and personality changes over time (R2–R2 before). This suggests that patients’ unawareness increases the burden of the relative (Turró-Garriga et al., 2013), but this does not mean that the concerned relative is less reliable given the low burden scores in our population and the reported reliability of relatives in the literature (Strauss et al., 1993; Cacchione et al., 2003; Talassi et al., 2007).
FDG-PET acquisition
On the testing day, PET images were acquired in all subjects on a Siemens (ECAT EXACT HR) camera. Images of brain tracer distribution (scan duration 20 min) were obtained during quiet wakefulness with eyes closed, 30 min after an intravenous injection of 2-[18F]fluoro-2-deoxy-d-glucose (18FDG) (147–290 MBq) (Lemaire et al., 2004). Images were reconstructed using filtered backprojection including correction for measured attenuation and scatter using standard software.
Imaging processing and analyses
Image analyses were performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). The PET data underwent an affine and non-linear spatial normalization onto the SPM8 PET brain template. Then images were smoothed with a 12-mm full-width at half-maximum filter.
PET images of AD patients and ECs were compared using proportional scaling by cerebral global mean values to control for individual variation in global 18FDG uptake. Correlations between the anosognosia discrepancy score and resting brain 18FDG uptake were examined. The influence of age, severity of cognitive impairment and modification of personality traits over time was controlled for by including the respective values as confounding variables in a single design matrix. Personality change scores were orthogonalized with respect to anosognosia scores before being introduced as a covariate. Our main contrast of interest consisted in the negative brain-anosognosia correlation in AD patients and ECs. The maps were thresholded at P < 0.0005, and the cluster’s level of statistical significance was set at Pfwe < 0.05.
RESULTS
Judgment of personality traits
The discrepancy scores obtained by the AD patients and the ECs are presented in Table 2.
Table 2.
Discrepancy scores from personality assessment questionnaire in AD patients and comparison to healthy elderly controls
| Variable | AD patients | ECs | EC comparison to a standard | Group comparison |
|---|---|---|---|---|
| Anosognosia (S1–R2) | 0.244 (0.085) | 0.178 (0.053) | t = 17.493, P = 0.001 | F = 8.801, P = 0.004 |
| Change over time (R2–R2_before) | 0.182 (0.098) | 0.112 (0.059) | t = 9.519, P = 0.001 | F = 11.967, P = 0.001 |
| Self-evaluation of the change (S1–S1_before) | 0.111 (0.057) | 0.101 (0.046) | t = 10.970, P = 0.001 | F = 1.681, P = 0.199 |
| Dependency on the past (S1–R2_before) | 0.201 (0.066) | 0.200 (0.071) | t = 14.077, P = 0.001 | F = 0.110, P = 0.741 |
| Self-perception of the past (S1_before–R2_before) | 0.194 (0.064) | 0.192 (0.082) | t = 11.589, P = 0.001 | F = 0.177, P = 0.674 |
| PP according to the present (PP1–R2) | 0.231 (0.083) | 0.181 (0.051) | t = 16.170, P = 0.001 | F = 6.354, P = 0.014 |
Summary of analyses of discrepancy scores from the personality assessment questionnaire. Mean and standard deviation for each discrepancy score, for AD and ECs are presented in the second and third columns. Results of analyses comparing ECs’ discrepancy scores to the standard (t-test) are presented in the fourth column. Results of analyses comparing ECs’ and AD patients’ discrepancy scores (ANOVA with age and education as covariates) are presented in the fifth column. PP: third person’s perspective.
Anosognosia
The analysis of the discrepancy score measuring anosognosia (S1 vs R2) showed that even the ECs did not see themselves exactly as their relatives perceived them currently. The comparison between ECs and AD patients showed that anosognosia for current characteristics of personality traits was greater for AD patients than for ECs (P = 0.004). These results suggest that patients (as a group) were less aware of the current characteristics of their personality traits than ECs.
Personality changes
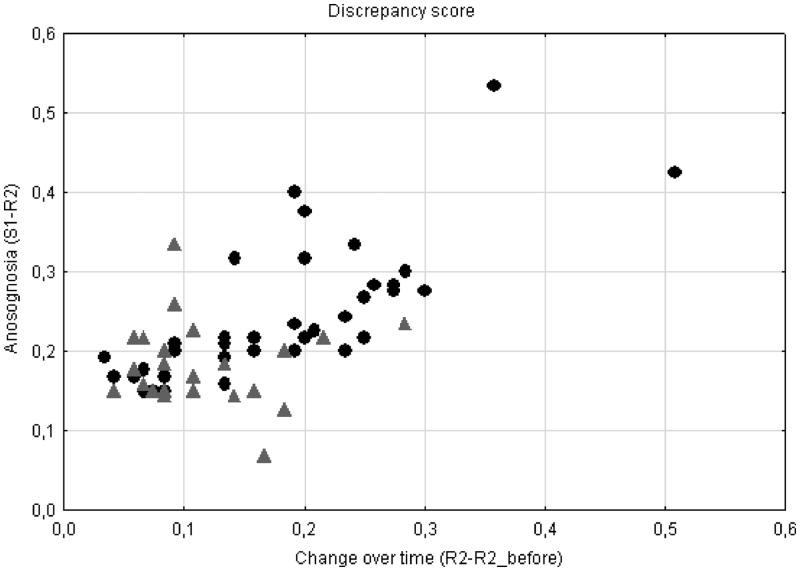
In ECs, the score indicating a modification of personality traits, as perceived by the relative (R2 vs R2_before), was different from zero, indicating that normal elderly subjects were likely to have experienced a change in personality during the past 10 years. The scores were significantly different for the AD patients and the ECs. AD patients had experienced greater personality changes over 10 years than ECs (P = 0.001), according to their respective relatives. As mentioned earlier, there was a positive correlation (r = 0.742, P < 0.001) between the discrepancy scores measuring anosognosia and personality changes, and we subsequently introduced the index of personality changes, after orthogonalization with respect to the anosognosia scores, as a confounding variable in the FDG-PET analysis. Figure 1 illustrates the distribution of the two discrepancy scores in AD and ECs, showing that some patients had considerable anosognosia for the current characteristics of their personality traits even when their personality changes over 10 years were mild. Accordingly, clinical practice shows that irritability may be an early and ‘constant’ behavioural characteristic in AD, while patients may become unable to recognize the daily importance of their irritability over time.
Fig. 1.
Distribution of anosognosia discrepancy scores and change over time discrepancy scores in AD patients and ECs. • = discrepancy score of AD patients, ▴ = discrepancy score of ECs.
Self-reported personality changes
The match between the ECs’ answers regarding the current and past characteristics of their personality (S1 vs S1_before) was not perfect, as revealed by the comparison with a zero standard. This was in keeping with the fact that their relatives reported personality changes, suggesting that the ECs also perceived some changes in their own personality over a 10-year period. The discrepancy scores for the AD patients and ECs did not differ (P = 0.199). In other words, AD patients reported a similar amount of change in their own personality as ECs did. This means that the AD patients did not report more changes in their personality traits than the ECs, even though their relatives actually observed more changes.
Dependency on the past
In keeping with the previous analysis, ECs did not rely on their previous personality to provide current judgments (S1 vs R2_before); that is, they did not evaluate their current personality as their relatives perceived them 10 years before. The discrepancy scores for AD patients did not differ from those for ECs (P = 0.741). Contrary to expectations that they would not engage in personal knowledge updating (Mograbi et al., 2009) these early-stage AD patients (as a group) did not describe the current characteristics of their personality as their relatives had seen them 10 years before.
Awareness of past personality
The ECs’ perception of their past personality did not completely match their relatives’ view for the same period. The comparison of AD patients with the ECs showed no group difference (P = 0.674). Thus, the AD patients’ judgments of their past personality traits (S1_before vs R2_before) were comparable to those of the ECs. This demonstrates that patients were able to perform the judgment task accurately for their past personality traits.
Perspective taking
As revealed by the comparison of the ECs’ discrepancy score (PP1 vs R2) with a standard, the ECs were unable to take their relatives’ viewpoint completely accurately. The AD patients’ perspective taking was impaired compared to that of the ECs (P = 0.014), suggesting that they had significant difficulties taking their relatives’ perspective to assess the current characteristics of their own personality traits.
Regression analysis
When we tested the hypotheses that anosognosia is related to perspective-taking deficit or to a crystallized self, the independent variables included in the regression analysis explained 79.1% of the variance of anosognosia [R2 = 0.791, F (2, 34) = 64.41, P < 0.0001]. Both impaired perspective taking and dependency on the past significantly predicted anosognosia in patients (perspective taking β = 0.77, P < 0.0001 and dependency on the past β = 0.29, P < 0.001).
FDG-PET results
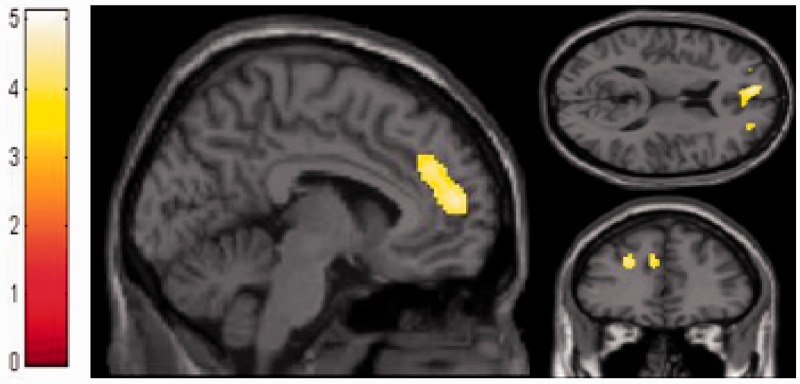
The results of the brain–behavior correlations are presented in Table 3. The anosognosia discrepancy score of AD patients negatively correlated with a large cluster in the dMPFC, extending to the vMPFC and to the left SFS. We did not see any significant negative brain–behavior correlation in the ECs. To demonstrate that the negative correlation was specific to AD patients, it was directly contrasted to that in ECs. Compared to the ECs, the AD patients’ anosognosia discrepancy score was confirmed to be negatively correlated with metabolism in the dMPFC and the left SFS (Figure 2). For the sake of completeness, we also looked for positive correlations but we did not obtain any significant results.
Table 3.
Brain metabolic correlation of participants’ anosognosia discrepancy scores
| Contrast | Anatomical region | Coordinates |
Cluster voxel Z-score | Cluster-level P-value (corrected) | Number of voxels in cluster | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| AD | dMPFC | 12 | 56 | 20 | 4.2 | 0.002 | 903 |
| dMPFC | −6 | 54 | 16 | ||||
| vMPFC | −8 | 60 | 8 | ||||
| AD < EC | dMPFC | −5 | 40 | 28 | 4.00 | 0.03 | 314 |
| dMPFC | −5 | 54 | 14 | ||||
| SFS | −24 | 44 | 26 | 4.5 | 0.14 | 167 | |
The stereotactic coordinates refer to the MNI space. AD and EC refer to Alzheimer’s patients and elderly control subjects. The SPM{T} maps were thresholded at P < 0.0005 at peak level and Pfwe < 0.05 at cluster level.
Fig. 2.
Brain–behavior correlation between anosognosia discrepancy scores and FDG-PET images in AD patients compared to elderly controls shows significant hypometabolism in the dMPFC. Images are displayed in the Montreal Neurological Institute (MNI) space. SPM displayed at P < 0.0005 uncorrected.
DISCUSSION
We capitalized on certain well-established procedures for judgments concerning personality traits (Klein et al., 2003; D’Argembeau et al., 2007) to provide discrepancy scores (Ruby et al., 2007) that comprehensively describe various aspects of personality trait knowledge in the early stages of AD. Patients were able to make judgments since, like the healthy controls, they were aware of their past personality and they reported some changes in personality over 10 years. On the other hand, the AD patients showed anosognosia for the current characteristics of their personality traits and for personality changes over 10 years (as assessed by relatives), and impaired capacity to take their relative’s perspective. Investigation of the cerebral metabolic impairment (measured by FDG-PET) related to anosognosia in AD showed that the dMPFC is less active in patients who are less aware of the current characteristics of their personality traits.
These findings support the earlier observation that anosognosia in AD does affect non-cognitive domains such as personality (Klein et al., 2003; Rankin et al., 2005; Ruby et al., 2009; Zamboni et al., 2013) and they shed some light on the mechanisms underlying this deficit. A recent hypothesis that has been proposed to explain anosognosia for clinical symptoms in AD patients is that it results, at least in part, from impaired third perspective taking (Salmon et al., 2005; Ruby et al., 2009). In keeping with this view, our results showed that AD patients had significantly more difficulties than ECs in taking their relative’s perspective on the current characteristics of their personality traits. Moreover, a regression analysis showed that perspective taking was the best predictor of anosognosia for current characteristics of personality. The ability to take another person’s perspective is important in forming a self-perception (Pfeifer et al., 2009). The perspective-taking deficit in AD patients suggests that they cannot take their relative’s viewpoint to modulate the assessment of their own personality by acknowledging the observations made by the relative.
Another recent hypothesis in the literature suggests that anosognosia is due to a lack of updating of personal information in memory (Klein et al., 2003; Graham et al., 2005; Rankin et al., 2005; Mograbi et al., 2009). In that context, AD patients would base their judgment for the current characteristics of their personality on past information. Although our regression analysis showed that dependency on the past explained a significant part of the anosognosia score, the comparison between the AD patients’ and ECs’ discrepancy score based on the personality questionnaire does not fully support this hypothesis. Our data show that AD patients were able to assess their past personalities as well as the ECs did (S1_before–R2_before), suggesting that they were able to access information about their past self. More importantly, however, they did not depend on the past to provide their current judgments, and the score for self-reported changes suggests that they did discriminate past from present information regarding their personality.
The degree of anosognosia for current characteristics of personality traits was highly correlated with dMPFC metabolism (extending to the vMPFC and the left SFS) in our AD sample. Recent reports have essentially linked anosognosia for cognitive deficits in neurodegenerative dementia to vMPFC activity (Mimura and Yano, 2006; Ries et al., 2006; Rosen et al., 2010; Zamboni and Wilcock, 2010; Zamboni et al., 2013).
The combined interest of our behavioral analysis is that it demonstrated that the differential score for ‘anosognosia’ was primarily explained by impaired perspective taking in our AD population. Accordingly, the correlation is very consistent with a previous fMRI study, in which dMPFC activation was characterized by an interaction between third perspective taking and the self, in an experimental situation where participants had to take a close relative’s perspective on their own personality (D’Argembeau et al., 2007).
The differential anosognosia score for current characteristics of personality traits was also related to superior frontal metabolism in our AD population. Impaired superior frontal activity has previously been shown to be related to anosognosia in AD (Starkstein et al., 1995; Salmon et al., 2006; Sedaghat et al., 2010), and it may be involved in self-(un)awareness (Wicker et al., 2003; Schmitz et al., 2004; Goldberg et al., 2006). A preferential relationship between the dMPFC and SFS during self-appraisal has also been previously reported (Schmitz and Johnson, 2006).
In a previous brain–behavior correlation study, anosognosia for cognitive deficits in a large cohort of patients with mild to moderate AD was related to TPJ metabolism (Salmon et al., 2006). Those observations and the current ones would suggest that the differential score for patients’ and their relatives’ judgments, reflecting anosognosia, is essentially related to regions (the dMPFC and the TPJ) involved in the evaluation of alternative (and possibly conflicting) perspectives (Mitchell, 2008; Van Overwalle, 2009). The difference between the current study and the previous one is consistent with a recent meta-analysis, which found the TPJ to be involved in inferring concrete, temporary states (such as evaluating recent cognitive functioning), while the dMPFC was involved in inferring people’s complex, enduring dispositions (such as the evaluation of personality traits) and interpersonal scripts (Mitchell, 2008).
Finally, anosognosia remains a complex syndrome rooted in the (dys)function of different, entangled neural networks taking part in self- and other-referential processing, memory and executive functions and social and emotional abilities. Specific defects in the interaction between different networks, combined with the specific maintenance of interactions that stabilize the self, might explain different forms and degrees of anosognosia.
Acknowledgments
This work was supported by the French Speaking Community Concerted Research Action (ARC-06/11-340). D.F. was funded by ARC 06/11-340. C.B. is a researcher in a Belgian InterUniversity Attraction Pole (IUAP6/29 and IUAP7/11). H.J. is a research fellow, A.D. is a research associate, F.C. is a senior research associate and M.A.B. is a logistic collaborator at the National Fund for Scientific Research (FNRS).
REFERENCES
- Agnew SK, Morris RG. The heterogeneity of anosognosia for memory impairment in Alzheimer’s disease: a review of the literature and a proposed model. Aging and Mental Health. 1998;2:7–19. [Google Scholar]
- Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. Journal of Gerontology Series B Psychological Sciences and Social Sciences. 2005;60:P98–101. doi: 10.1093/geronb/60.2.p98. [DOI] [PubMed] [Google Scholar]
- Cacchione PZ, Powlishta KK, Grant EA, Buckles VD, Morris JC. Accuracy of collateral source reports in very mild to mild dementia of the Alzheimer type. Journal of the American Geriatrics Society. 2003;51(6):819–23. doi: 10.1046/j.1365-2389.2003.51263.x. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex. 2007;43:1004–19. doi: 10.1016/s0010-9452(08)70697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective-taking. Journal of Cognitive Neuroscience. 2007;19:935–44. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Derouesne C, Piquard A, Thibault S, Baudouin-Madec V, Lacomblez L. Noncognitive symptoms in Alzheimer’s disease. A study of 150 community-dwelling patients using a questionnaire completed by the caregiver. Revue Neurologique (Paris) 2001;157:162–77. [PubMed] [Google Scholar]
- Dodson CS, Spaniol M, O’Connor MK, Deason RG, Ally BA, Budson AE. Alzheimer’s disease and memory-monitoring impairment: Alzheimer’s patients show a monitoring deficit that is greater than their accuracy deficit. Neuropsychologia. 2011;49:2609–18. doi: 10.1016/j.neuropsychologia.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Chen JM, Wiseman AL, Schacter DL, Budson AE. Retrieval monitoring and anosognosia in Alzheimer’s disease. Neuropsychology. 2007;21:559–68. doi: 10.1037/0894-4105.21.5.559. [DOI] [PubMed] [Google Scholar]
- Gil R, Arroyo-Anllo EM, Ingrand P, et al. Self-consciousness and Alzheimer’s disease. Acta Neurologica Scandinavica. 2001;104:296–300. doi: 10.1034/j.1600-0404.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–39. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Graham DP, Kunik ME, Doody R, Snow AL. Self-reported awareness of performance in dementia. Cognitive Brain Research. 2005;25:144–152. doi: 10.1016/j.cogbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–16. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Gardner RC. Ethnic stereotypes: norms on 208 words typically used in their assessment. Canadian Journal of Psychology. 1972;26:140–54. [Google Scholar]
- Klein SB, Cosmides L, Costabile KA. Preserved knowledge of self in a case of Alzheimer’s dementia. Social Cognition. 2003;21:157–65. [Google Scholar]
- Lemaire C, Damhaut P, Lauricella B, Mosdzianowski C, Morelle JL, Monclus M. Fast [18F]FDG synthesis by alkaline hydrolysis on a low polarity solid phase support. Journal of Labelled Compounds and Radiopharmaceuticals. 2004;45:435–47. [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patients. In: Bellack L, Karasu B, editors. Geriatric Psychiatry. New York: Grune & Stratton; 1976. pp. 77–121. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mimura M, Yano M. Memory impairment and awareness of memory deficits in early-stage Alzheimer’s disease. Reviews in the Neurosciences. 2006;17:253–66. doi: 10.1515/revneuro.2006.17.1-2.253. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2008;18:262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Brown RG, Morris RG. Anosognosia in Alzheimer’s disease—the petrified self. Consciousness and Cognition. 2009;18:989–1003. doi: 10.1016/j.concog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development. 2009;80:1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL. Self awareness and personality change in dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:632–9. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries ML, Schmitz TW, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage. 2006;29:485–92. doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O, Rothlind J, et al. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49:3358–64. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Collette F, D’Argembeau A, et al. Perspective taking to assess self-personality: what’s modified in Alzheimer’s disease? Neurobiology of Aging. 2009;30:1637–51. doi: 10.1016/j.neurobiolaging.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Ruby P, Schmidt C, Hogge M, D’Argembeau A, Collette F, Salmon E. Social mind representation: where does it fail in frontotemporal dementia? Journal of Cognitive Neuroscience. 2007;19:671–83. doi: 10.1162/jocn.2007.19.4.671. [DOI] [PubMed] [Google Scholar]
- Salmon E, Perani D, Collette F, et al. A comparison of unawareness in frontotemporal dementia and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:176–9. doi: 10.1136/jnnp.2007.122853. [DOI] [PubMed] [Google Scholar]
- Salmon E, Perani D, Herholz K, et al. Neural correlates of anosognosia for cognitive impairment in Alzheimer’s disease. Human Brain Mapping. 2006;27:588–97. doi: 10.1002/hbm.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E, Ruby P, Perani D, et al. Two aspects of impaired consciousness in Alzheimer’s disease. Progress in Brain Research. 2005;150:287–98. doi: 10.1016/S0079-6123(05)50021-9. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. Neuroimage. 2006;30:1050–8. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Sedaghat F, Dedousi E, Baloyannis I, et al. Brain SPECT findings of anosognosia in Alzheimer’s disease. Journal of Alzheimers Disease. 2010;21:641–7. doi: 10.3233/JAD-2010-090631. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Vazquez S, Migliorelli R, Teson A, Sabe L, Leiguarda R. A single-photon emission computed tomographic study of anosognosia in Alzheimer’s disease. Archives of Neurology. 1995;52:415–20. doi: 10.1001/archneur.1995.00540280105024. [DOI] [PubMed] [Google Scholar]
- Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychology and Aging. 1993;8:475–80. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- Talassi E, Cipriani G, Bianchetti A, Trabucchi M. Personality changes in Alzheimer’s disease. Aging and Mental Health. 2007;11:526–31. doi: 10.1080/13607860601086603. [DOI] [PubMed] [Google Scholar]
- Turró-Garriga O, Garre-Olmo J, Vilalta-Franch J, Conde-Sala JL, de Gracia Blanco M, López-Pousa S. Burden associated with the presence of anosognosia in Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2013;28(3):291–7. doi: 10.1002/gps.3824. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Research Brain Research Reviews. 2003;43:224–30. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Drazich E, McCulloch E, et al. Neuroanatomy of impaired self-awareness in Alzheimer’s disease and mild cognitive impairment. Cortex. 2013;3:668–78. doi: 10.1016/j.cortex.2012.04.011. doi: 10.1016/j.cortex.2012.04.011. Epub 2012 May 8. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Wilcock G. Lack of awareness of symptoms in people with dementia: the structural and functional basis. International Journal of Geriatric Psychiatry. 2010;26:783–92. doi: 10.1002/gps.2620. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–55. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]